Abstract
Background
Acute kidney injury (AKI) has been characterized in young high-risk inpatients, in whom AKI is frequent and associated with increased mortality, morbidity, and length of stay. The incidence of AKI among patients not requiring intensive care is unknown.
Study Design
Retrospective cohort study
Setting & Participants
13,914 noncritical admissions during 2011–2012 at our tertiary referral pediatric hospital were evaluated. Patients <28 days or >21 years of age, or with chronic kidney disease (CKD), were excluded. Admissions with ≥2 serum creatinine measurements were evaluated.
Factors
Demographic features, laboratory measurements, medication exposures, and length of stay.
Outcome
AKI defined by increased serum creatinine in accordance with KDIGO (Kidney Disease: Improving Global Outcomes) criteria. Based on time of admission, time interval requirements were met in 97% of cases, but KDIGO time window criteria were not strictly enforced to allow implementation using clinically-obtained data.
Results
Two or more creatinine measurements (one baseline before or during admission, and a second during admission) in 2,374 of 13,914 (17%) patients allowed for AKI evaluation. A serum creatinine difference of ≥0.3 mg/dL or ≥1.5 times baseline was seen in 722 of 2,374 (30%) patients. A minimum of 5% of all noncritical inpatients without CKD in pediatric wards have an episode of AKI during routine hospital admission.
Limitations
Urine output, glomerular filtration rate, and time interval criteria for AKI were not applied secondary to study design and available data. The evaluated cohort was restricted to patients with ≥2 clinically obtained serum creatinine measurements, and baseline creatinine may have been measured after the AKI episode.
Conclusions
AKI occurs in at least 5% of all non-critically ill hospitalized children, adolescents, and young adults without known CKD. Physicians should increase their awareness of AKI and improve surveillance strategies with serum creatinine measurements in this population so that exacerbating factors such as nephrotoxic medication exposures may be modified as indicated.
Index words: Acute Kidney Injury (AKI); acute renal failure (ARF); serum creatinine; incidence; Nephrotoxicity; medication exposure; Inpatient Pediatrics; children, adolescents, young adults, Electronic Medical Records (EMRs); KDIGO AKI criteria
Clinical manifestations of acute kidney injury (AKI) encompass a spectrum from subtle changes in glomerular filtration rate (GFR) to symptomatic end organ failure. In hospitalized children, AKI is associated with increased mortality, length of stay, permanent loss of kidney function, and risk for future chronic kidney disease (CKD).1–5 Previous studies reporting the incidence and sequelae of AKI in the young focused on high-risk patient populations. Cohorts have been limited to those with nephrotoxic medications,3,6–8 cardiac surgery/bypass,9,10 sepsis,11,12 or admission to an intensive care unit.3,4,13–15 Due to patient selection, these may not accurately convey overall AKI incidence.
Case definitions for AKI have been based on diagnostic codes, laboratory data, and/or estimated GFR. International expert consensus definitions assess the degree of change from baseline in serum creatinine or estimated creatinine clearance and some consider urinary output.4,16,17 The KDIGO (Kidney Disease: Improving Global Outcomes) definition requires a serum creatinine concentration increase of ≥0.3 mg/dL, or ≥1.5-fold increase from baseline. This increase in creatinine serves as a proxy for significant change in true kidney function from baseline. The KDIGO definition also includes specific threshold and time window requirements for the change in creatinine (increase by 0.3 mg/dL in 48 hours or increase by 1.5 fold in 7 days), urine output cutoffs, and estimated GFRs for establishing the diagnosis of AKI. The incidence of AKI by the KDIGO criteria in non-critically ill young patients has not been established.
Using a large, retrospective analysis of electronic medical records (EMRs) of admissions to a tertiary care children’s hospital over a two-year period, we sought to determine the incidence of AKI in non-critically ill children, adolescents, and young adults using KDIGO serum creatinine criteria with modifications to allow ascertainment from clinically-obtained EMR data. We also characterized the features that have been associated with AKI in other settings such as nephrotoxic drug exposure, length of stay, and contrast exposure.
Methods
This study was approved by the Vanderbilt University institutional review board; individual parental consent was waived.
Population
The most recent admission of patients aged 28 days through 21 years admitted to the Monroe Carell Jr. Children’s Hospital at Vanderbilt from 1/1/2011 through 12/31/2012 were considered for this study. Exclusion criteria were intensive care during the admission or pre-existing CKD defined as two or more ICD-9 or CPT codes preceding admission (Table S1, available as online supplementary material). Although young people with CKD are also at risk for superimposed AKI, we sought to assess patients without pre-existing kidney disease. Patients were included irrespective of their indication for admission or admitting service.
Clinical Data
Demographic data included age, sex, race, and ethnicity, as recorded in the patient’s EMR. Clinical parameters included length of hospital admission, weight, inpatient medications (except those administered during operations), and clinical laboratory values. Weight is represented as a z-score, or as standard deviations from the mean for age and sex.18 Nephrotoxic medications were categorized using a modified Delphi method as follows: high risk, group 1: nephrotoxin as single agent; or moderate risk, group 2: nephrotoxin in at-risk clinical situation or in conjunction with additional agent by their relative contribution to the development of AKI (Table S2). The discharge diagnosis, based on ICD-9 code, for each admission was obtained from the hospital administrative database. Data outliers underwent manual verification, and filters were implemented to ensure data validity. Specifically, all weight measurements with negative values were excluded. Weight z-scores above 5 and below −5 were excluded. Laboratory values with nonnumeric values were manually reviewed to ensure that no valid results were contained in the entry. Laboratory values that were below or above the limits of the assay were assigned the numeric minimum or maximum, as indicated (Table S3). Extreme values for reported laboratory results were manually verified in the EMR. Comparisons between groups were conducted with a Wilcoxon test for continuous data or a Pearson Chi-Squared test for categorical data. Comparisons between the stages of AKI were conducted with a proportional odds test. When proportional odds assumptions were violated, the Cochran-Armitage trend test for proportions or Pearson chi-squared test was used.
AKI Definition and Severity
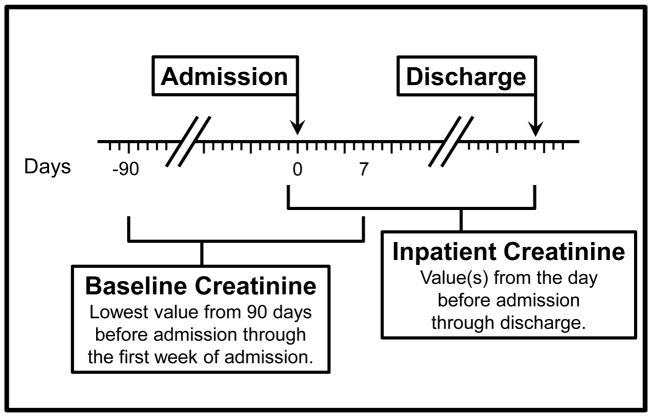
For a patient to qualify for AKI evaluation, at least two creatinine measurements were required, one during the baseline time window and a second on a different day during the admission (Figure 1). The baseline was defined as the lowest creatinine measured 90 days before admission through the first week of admission. This baseline measurement may have occurred before or after the increased creatinine measurement leading to the AKI classification. This allowed evaluation of AKI status for patients in whom no serum creatinine was measured until after the onset of AKI. Inpatient creatinine measurements were defined as those obtained in the 24 hours prior to admission through discharge. In our dataset, the admission time reflected the entry of the admission order into the EMR. By including the 24 hours prior to the admission order, laboratory evaluations performed in the emergency department or outpatient clinic portion of the patient’s hospitalization were also captured. Patients with one creatinine measurement during the admission and no additional creatinine measurements in the 90 days prior to admission were not evaluated for AKI status. Each patient with a baseline (measured up to 90 days prior to admission through the first 7 days of admission) and an inpatient creatinine value measured on a different date was evaluated and further classified as no AKI, or assigned an AKI stage. Per KDIGO serum creatinine criteria,16 a patient was classified as having AKI if any inpatient creatinine measurement was ≥0.3 mg/dL greater than the baseline value or ≥1.5-fold above the baseline value. The KDIGO criteria also specifies time windows for the increase in creatinine values, which were not imposed due to the paucity of clinically obtained creatinine measurements in our dataset.
Figure 1.
Schematic representation of methodology used to determine AKI status based on modified KDIGO consensus criteria. The lowest value 90 days before through 7 days after admission defined the baseline serum creatinine. The highest value 1 day before admission through discharge defined the peak serum creatinine.
AKI severity was defined using KDIGO stages 1–3 (Table S4), based on the baseline and maximum inpatient creatinine measurement. Because urine output data were not consistently available in this noncritical population, AKI was defined solely using the changes in measured serum creatinine. Additionally, we did not estimate the GFR for patients, as this calculation requires an accurate height measurement during the admission.
Results
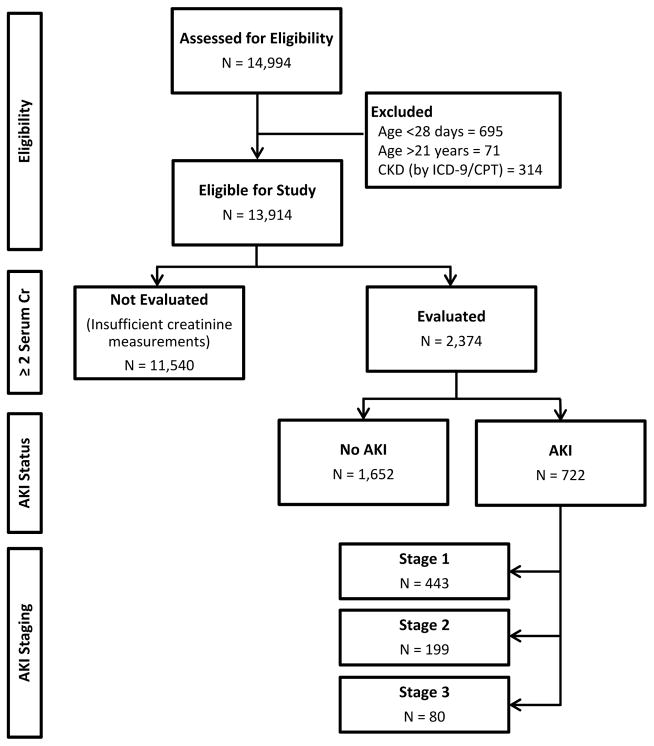
Over the two year study period, admissions for 13,914 unique patients met inclusion and exclusion criteria (Figure 2). Of these, 2374 (17%) had both a baseline and inpatient creatinine value to allow for AKI evaluation (Table 1). The 11,540 patients not evaluated for AKI included 3,704 with one serum creatinine measurement during hospitalization. Also included were 7,836 (56% of all patients) with no inpatient serum creatinine measurement, of which 840 had a measurement in the 90 days prior to admission. Those not evaluated for AKI showed some difference from evaluated patients with regard to race (74% and 77% white, respectively; p-value <0.001) and ethnicity (9% and 7% Hispanic or Latino; p-value <0.001; nonmissing = 11,748), but were similar in sex (55% and 54% male; p-value = 0.2). Patients not evaluated for AKI were younger than evaluated patients (median age, 5.8 vs 8.8 years; p-value <0.001) and had shorter lengths of stay (median, 1 vs 3 days; p-value <0.001). In the 24 hours before and first 48 hours of admission, the patients not evaluated for AKI also had fewer total unique medication exposures (median, 2 vs 4 medications; p-value <0.001), and fewer not evaluated patients had nephrotoxic drug exposures (median, 0 vs 1; p-value <0.001) than evaluated patients.
Figure 2.
Flow diagram of the cohorts evaluated in this study. Of the 13,914 unique individuals eligible for inclusion, 2374 had sufficient creatinine measurements to allow AKI evaluation. Of these, 722 met criteria for AKI and 1652 did not.
Table 1.
Demographic and clinical characteristics in 24 hours prior and 48 hours after admission of patients not evaluated for AKI vs evaluated patients and the evaluated patients with and without AKI.
| Total Eligible Population | Evaluated Population | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Not Evaluated (n= 11,540) | Evaluated (n = 2,374) | P value | No AKI (n = 1,652) | AKI (n = 722) | P value | |
|
| ||||||
| Age (y) | 5.8 (1.8 – 11.9) | 8.8 (2.6 – 14.8) | < 0.0011 | 10.5 (3.3 – 15.2) | 5.3 (2.0 – 12.4) | < 0.0011 |
|
| ||||||
| Male sex | 6348 (55%) | 1271 (54%) | 0.22 | 858 (52%) | 413 (57%) | 0.022 |
|
| ||||||
| Race | < 0.0012 | 0.72 | ||||
| White | 8570 (74%) | 1830 (77%) | 1268 (77%) | 562 (78%) | ||
| Black | 1981 (17%) | 400 (17%) | 287 (17%) | 113 (16%) | ||
| Other# | 289 (3%) | 51 (2%) | 33 (2%) | 18 (2%) | ||
| Unknown | 700 (6%) | 93 (4%) | 64 (4%) | 29 (4%) | ||
|
| ||||||
| Hispanic/Latino | 1004 (9%) | 164 (7%) | < 0.0012 | 101 (6%) | 63 (9%) | 0.022 |
|
| ||||||
| Weight z-score | 0.3 (−0.6 – 1.1) | −0.1 (−1.1 – 0.8) | < 0.0011 | 0.0 (−0.9 – 0.9) | −0.4 (−1.4 – 0.6) | < 0.0011 |
|
| ||||||
| No. of Scr measurements during Admission* | 0: 7836 (68%); 1: 3704 (32%) | 2 (1 – 3) | < 0.0011 | 2 (1 – 2) | 3 (2 – 5) | < 0.0011 |
|
| ||||||
| Baseline Scr (mg/dL) | – | 0.37 (0.25 – 0.54) | – | 0.42 (0.29 – 0.58) | 0.26 (0.18 – 0.41) | < 0.0011 |
|
| ||||||
| Scr (mg/dL) | – | 0.50 (0.35 – 0.70) | – | 0.49 (0.34 – 0.68) | 0.53 (0.37 – 0.77) | < 0.0011 |
|
| ||||||
| WBC count (×103/μL) | 10.6 (7.7 – 14.7) | 9.2 (6.1 – 13.3) | < 0.0011 | 9.2 (6.4 – 12.8) | 9.3 (5.5 – 14.4) | 0.81 |
|
| ||||||
| Hematocrit (%) | 35 (31 – 38) | 33 (29 – 38) | < 0.0011 | 34 (29 – 38) | 33 (29 – 37) | 0.11 |
|
| ||||||
| Platelet count(×103/μL) | 288 (229 – 359) | 268 (195 – 359) | < 0.0011 | 271 (201 – 359) | 263 (176 – 360) | 0.091 |
|
| ||||||
| Total Medication Exposures | 2 (1 – 4) | 4 (2 – 7) | < 0.0011 | 4 (2 – 6) | 4 (2 – 7) | 0.0041 |
|
| ||||||
| Nephrotoxic Medication Exposures | 0 (0 – 1) | 1 (0 – 1) | < 0.0011 | 1 (0 – 1) | 1 (0 – 1) | 0.051 |
|
| ||||||
| Contrast Exposure | 777 (7%) | 200 (8%) | 0.0042 | 158 (10%) | 42 (6%) | 0.0042 |
|
| ||||||
| Hospital Length of Stay (d) | 1 (1 – 2) | 3 (2 – 6) | < 0.0011 | 3 (2 – 6) | 4 (2 – 8) | < 0.0011 |
Note: Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range]. Conversion factor for serum creatinine in mg/dL to μmol/L, ×88.4.
AKI, acute kidney injury; Scr, serum creatinine; WBC, white blood cell
Other includes Asian, American Indian/Alaska Native and Native Hawaiian or Other Pacific Islander.
Wilcoxon test
Pearson test
Of the 2374 patients evaluated for AKI, 722 (30% of the evaluated patients, 5% of all eligible patients) met criteria for AKI. Compared to the 1652 evaluated patients with no AKI (Table 1), patients with AKI were younger (median age, 5.3 vs. 10.5 years; p-value <0.001), had lower weight-for-age (z-score, −0.4 vs. 0.0; p-value <0.001; nonmissing = 2321), lower baseline serum creatinine (0.26 vs. 0.42 mg/dL; p-value <0.001), and higher maximum serum creatinine (0.53 vs. 0.49 mg/dL; p-value <0.001). In laboratory measurements performed in the 24 hours before and first 48 hours of admission, these patients had similar white blood cell counts (9.3 vs. 9.2 ×103/μL; p-value = 0.8; nonmissing = 1875), minimum platelet counts (263 vs. 271 ×103/μL; p-value = 0.09; nonmissing = 1877), and minimum hematocrit measurements (33% vs. 34%; p-value = 0.1; nonmissing = 2031) compared to those without AKI. Those with and without AKI had similar numbers of total medication exposures per day (median, 4 vs. 4; p-value =0.004) and nephrotoxic medication exposures (median, 1 vs. 1; p-value = 0.05). Those with AKI had the lower rates of contrast exposure (6% vs. 10%; p-value = 0.004) and longer median length of hospital stay (4 vs. 3 days; p-value <0.001). The mortality in our cohort was quite low with only 1 (0.06%) death among those without AKI and 4 (0.6%) deaths in those with AKI (Table S5).
The definition for baseline serum creatinine used in this study allowed for the nadir value to be measured before or after the peak serum creatinine measurement. To determine whether nadir serum creatinine measurements after AKI were systematically lower or higher than those before the event, we performed additional analysis of the 225 (31%) patients with AKI who had measured creatinine values both before and after their AKI. The median difference between the lowest creatinine measured before and after the onset of AKI indicated that minimal, if any, systematic increase or decrease occurred (median decrease, 0.04 [interquartile range, decrease of 0.11 to increase of 0.03] mg/dL). Similarly, in the 225 of 1652 (14%) who had no AKI, but had at least one measured creatinine value in the 90 days before admission to compare to the creatinine measures during admission, there was no apparent difference in the serum creatinine measured before vs. during admission (median difference, 0.00 [interquartile range, −0.07 to 0.10] mg/dL).
We then examined clinical factors more closely in patients who met criteria for AKI, categorizing them by KDIGO stage (Table 2). Of the 722 patients with AKI per our study criteria, 443 (61%) met KDIGO criteria stage 1; 199 (28%), stage 2; and 80 (11%) stage 3. Among patients with AKI, those with stages 2 and 3 were younger (median age for stage 1: 6.4 years; stage 2, 4.1 years; stage 3, 4.2 years; p-value <0.001) and had longer hospital stays (4, 5, and 5 days, respectively; p-value <0.001). Across AKI stages, the proportion of patients exposed to group 1 nephrotoxic medications in the 24 hours before and 48 hours after admission were similar (42%, 40% and 36% for stages 1, 2 and 3, respectively; p-value = 0.7), but the proportion of patients exposed to group 2 nephrotoxic drugs increased across KDIGO stages (21%, 31% and 34%, respectively; p-value = 0.001). The median of the tally of total unique medications per day was similar across AKI stages (4, 5, and 4; p-value = 0.006). The proportion of patients exposed to contrast decreased with increasing AKI stage, but without statistically significant difference (7%, 5%, and 4%, respectively; p-value = 0.2). The distribution of common discharge diagnoses was rather broad, with acute kidney failure as the second most common in the patients with stage 3, but this diagnosis was not common in the other stages (Table S5).
Table 2.
Inpatient characteristics by AKI stage from 24 hours prior to until 48 hours after admission.
| Stage 1 (n=443) | Stage 2 (n=199) | Stage 3 (n=80) | P Value | |
|---|---|---|---|---|
|
| ||||
| Age (y) | 6.4 (2.0 – 13.7) | 4.1 (2.0 – 8.8) | 4.2 (1.8 – 10.4) | < 0.0011 |
|
| ||||
| Male sex | 243 (55%) | 119 (60%) | 51 (64%) | 0.092 |
|
| ||||
| Hospital Length of Stay (d) | 4 (2 – 7) | 5 (3 – 10) | 5 (2 – 10) | 0.0011 |
|
| ||||
| Weight z-score | −0.2 (−1.2 – 0.8) | −0.8 (−1.9 – 0.2) | −0.3 (−1.3 – 0.7) | 0.0041 |
|
| ||||
| Total no. of Unique Medications | 4 (2 – 7) | 5 (2 – 7) | 4 (2 – 9) | 0.11 |
|
| ||||
| No. of group 1* medications given | 0.83 | |||
|
| ||||
| 0 | 257 (58%) | 119 (60%) | 51 (64%) | |
| 1 | 134 (30%) | 60 (30%) | 20 (25%) | |
| ≥2 | 52 (12%) | 20 (10%) | 9 (11%) | |
|
| ||||
| No. of group 2* medications given | <0.0011 | |||
|
| ||||
| 0 | 0: 349 (79%) | 0: 138 (69%) | 0: 53 (66%) | |
| 1 | 1: 79 (18%) | 1: 40 (20%) | 1: 16 (20%) | |
| ≥2 | =2: 15 (3%) | =2: 21 (11%) | =2: 11 (14%) | |
|
| ||||
| Contrast Given in Prior 7 Days | 30 (7%) | 9 (5%) | 3 (4%) | 0.21 |
Note: Values for categorical variables are given as number (percentage); for continuous variables, as median [interquartile range].
P values compare stages 1, 2, and 3 by the 1Proportional odds test, 2Cochran-Armitage trend test, or 3Pearson chi-squared test.
Groups 1 and 2 are nephrotoxic medications; see Methods.
Discussion
This study reports the incidence and severity of AKI among hospitalized, noncritically ill children, adolescents, and young adults based upon a modified KDIGO definition able to be implemented using clinically-obtained EMR data. There was AKI observed in 30% of non-critically ill inpatients in pediatric wards who had at least two creatinine measurements, which accounts for 5% of all non-critically ill inpatients in pediatric wards during the period of study. This 5% incidence represents a minimum estimate of the occurrence of AKI in the total population because our methods required patients to have at least two serum creatinine measurements available, such that the change from baseline to maximum serum creatinine could be calculated to determine AKI status. The requisite serum creatinine measurements were obtained in only 17% of admissions to pediatric wards included in this study. In 83% of patients, AKI status was unable to be assigned due to an insufficient number of creatinine measurements. Thus, the true incidence of AKI in our population was above 5%, but we are unable to provide a more precise estimate.
Previous studies describing AKI incidence have also used creatinine-based classifications, but predominantly in cohorts of critically ill children requiring renal replacement therapy after cardiac surgery with cardiopulmonary bypass,3,4,10,13,14,19,20 or in children receiving nephrotoxic medications without reference to severity of illness.6–8,21,22 In our cohort, the true incidence of AKI is most accurately described as ≥5% because of the large number of patients with insufficient creatinine measurements to allow evaluation of AKI status. This limitation reflects a lack of creatinine monitoring in non-critically ill young people and the otherwise healthy young population. The minimum AKI incidence of 5% indicates that AKI is a common event among hospitalized children, adolescents, and young adults outside of intensive care units, affecting at least one in twenty of these admissions.
Methods for defining baseline creatinine or kidney function directly affect the detection and staging of AKI. Prior studies have either not clearly stated how the baseline creatinine was determined6,7,22 or used various methods to estimate baseline creatinine/GFR.3,4,8,20 In contrast, we required a measured baseline creatinine value to be included for an evaluated patient because of the inherent error when baseline GFR is estimated.1 This is complicated by the paucity of baseline data in the healthy young population, but our decision not to use estimated creatinine clearance stems from the unavailability and inaccuracy of height measurements for inpatients in pediatric wards and poor precision of this estimation methodology with a large margin of error and validity only in steady state conditions.1 We instead elected to use the lowest creatinine level available during the 90 days before through the first week of admission as an indicator of baseline kidney function.23 This definition resulted in some baseline creatinine measurements, 401 of 722 (56%) occurring after the AKI event. The decreased serum creatinine measurement after the AKI event either is a reflection of the return to baseline kidney function or could represent dilution of the serum creatinine due to fluid administration. Based on an estimated corrected serum creatinine calculation, administration of a 20 mg/kg bolus would lower the measured serum creatinine by 0.03 mg/dL.14 In a non-critically ill population in pediatric wards, patients were unlikely to receive fluid resuscitation to the degree that would significantly affect the measured serum creatinine. Additionally, evaluation of the subset of patients with serum creatinine measurements both before and after their AKI episode supports our use of recovery creatinine values to approximate baseline creatinine function.
Comparisons within the evaluated cohort revealed that patients with AKI were younger, smaller for age, and had lower baseline serum creatinine measurements than those without AKI. These parameters may be a proxy for chronic illness, prematurity, and/or poor nutritional status, all of which increase the risk for AKI. The lower baseline serum creatinine measurements in those with AKI may be due to the younger and smaller patients in this group. Lower creatinine baselines also bias toward AKI classification, as a smaller absolute change in serum creatinine measurement is required to meet the AKI criteria of 1.5-fold increase. These small changes in creatinine concentration seen in routine care of young patients may represent measurement error inherent in laboratory testing, and fluctuating creatinine measurements may remain within the normal reference range for the patient. The majority of patients with and without AKI had no exposures to nephrotoxic medications which can serve as a clinically recognizable risk factor. The methodology of this study does not distinguish between community-acquired and iatrogenic AKI, which may account for the lack of difference for known iatrogenic risk factors. The lower incidence of contrast exposure in patients with AKI, although not statistically significant, may reflect appropriate avoidance of this well recognized nephrotoxic agent by clinical caregivers and the lower frequency of studies in younger children. This difference is unlikely to represent any ascertainment bias, as exposures were extracted from EMRs as a single dataset without regard to AKI status. While patients with contrast and nephrotoxic medication exposures are known to have increased risk for AKI and merit close monitoring, broadening surveillance to include patients without these specific risk factors will allow for improved detection, prevention, and minimization of further kidney damage in many additional young patients with AKI.
Our comparison of patients across AKI stages revealed that patients with the higher stages of AKI were younger and had lower weights. Investigation of the medication and contrast exposures across AKI stages did not find significant differences in group 1 nephrotoxic medications, but exposures to group 2 medications were more common among patients with stages 2 and 3. This observational data cannot determine causality, but increasing awareness of AKI among providers may lead to more careful consideration of nephrotoxic drug exposures and other insults, resulting in the sparing of kidney function.
The median length of stay for patients with multiple creatinine measurements was longer than those with insufficient data for evaluation. This may indicate a selection bias for creatinine measurement, as patients with longer hospitalizations are more likely to have additional laboratory studies. However, among patients with ≥2 serum creatinine measurements, those with AKI had a longer length of stay compared to patients without AKI. We also observed longer length of stay with increasing severity of AKI. Patients may require longer inpatient stays because of their AKI, or those with lengthy hospitalization may have higher risk for AKI due to prolonged exposure to inpatient risk factors or greater severity of illness.
Our use of EMRs as our data source leads to several study limitations. The data used for this study were derived from a single tertiary care hospital and examined retrospectively. All data were recorded in the course of clinical care without research purposes in mind. Thus, our AKI definition was based on the KDIGO criteria for changes in serum creatinine, but did not include urine output or an estimation of glomerular filtration rate. In addition, due to the limited serum creatinine measurements obtained clinically in young patients, baseline creatinine measurements were defined as the lowest values in the 90 days preceding admission through the first week of admission. These baseline values were not required to be measured before the elevated creatinine values indicating the AKI episode. We believe that these creatine measurements taken after the AKI episode represent renal recovery and serve as an accurate surrogate for baseline kidney function in this non-intensive care cohort from pediatric wards. Additionally, these baseline values may not have been measured in the 48 hours or 7 days before AKI, as specified by the KDIGO guidelines, but the vast majority of AKI episodes occurred early in the admission. Only prospective, systematic, serial collection of serum creatinine measurements and urine output data would definitively and precisely determine the incidence of AKI among young inpatients. Further analysis of the risk factors contributing to AKI is beyond the scope of this study, but identification of such factors in future work may facilitate targeted AKI screening.
The ultimate goal of early detection and prevention of AKI requires more comprehensive screening of young people at risk and increased clinical recognition. In this cohort, even patients with previously described risk factors such as dehydration and nephrotoxic medication use were not universally screened. One strategy that has met success is the routine screening of children based on specific parameters around nephrotoxic medication use.21,22 Based on the data presented herein, not only are these children at risk for AKI, but also those in a broader cohort which includes at least 5% of patients cared for in non-critical inpatient pediatric settings. Physicians should have a lower threshold for screening young people for AKI with a serum creatinine measurement, especially in the setting of modifiable factors such as nephrotoxic medications, contrast exposure, and fluid management.
Supplementary Material
Acknowledgments
The authors thank James C. Gay, MD, MMHC, for his assistance in obtaining discharge diagnoses for the cohort.
Support: This work was supported by Clinical and Translational Science Award (CTSA) UL1 TR000445, request VR5924.1, from the Vanderbilt Institute for Clinical and Translational Research; Dr Van Driest is supported by the PhRMA Foundation Early Career Award and by CTSA award KL2 TR000446 from the National Center for Advancing Translational Sciences; Dr McGregor is supported by the National Institutes of Health/National Institute of Child Health and Human Development (K23 HD000001). These funding sources were not involved in design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Research idea and study design: TLM, DPJ, LW, ID, BCB, GMF, JS-R, DB, SLV; data acquisition: TLM, ID, JS-R, LC; data analysis/interpretation: TLM, DPJ, LW, ID, BCB, GMF, DB, SLV; statistical analysis: TLM, LW, DB; supervision or mentorship: DPJ, DB. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring the questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. TLM and SLV take responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Table S1: ICD-9 and CPT codes used to exclude admissions of patients with pre-existing CKD.
Table S2: Nephrotoxic medications classified by relative contribution to AKI development into groups 1 and 2.
Table S3: Data conversions to allow for analysis.
Table S4: AKI definition for staging patients who met criteria for AKI during admission.
Table S5: Discharge diagnoses and mortality.
Note: The supplementary material accompanying this article (doi:_______) is available at www.ajkd.org
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). ICD-9 and CPT codes used to exclude admissions of patients with pre-existing CKD.
Supplementary Table S2 (PDF). Nephrotoxic medications classified by relative contribution to AKI development into groups 1 and 2.
Supplementary Table S3 (PDF). Data conversions to allow for analysis.
Supplementary Table S4 (PDF). AKI definition for staging patients who met criteria for AKI during admission.
Supplementary Table S5 (PDF). Discharge diagnoses and mortality.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011 Apr;7(4):201–208. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005 Nov;16(11):3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008 Jul;3(4):948–954. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007 May;71(10):1028–1035. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 5.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int. 2006 Jan;69(1):184–189. doi: 10.1038/sj.ki.5000032. [DOI] [PubMed] [Google Scholar]
- 6.McKamy S, Hernandez E, Jahng M, Moriwaki T, Deveikis A, Le J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J Pediatr. 2011 Mar;158(3):422–426. doi: 10.1016/j.jpeds.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011 Apr;6(4):856–863. doi: 10.2215/CJN.08110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in noncritically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011 Jan;26(1):144–150. doi: 10.1093/ndt/gfq375. [DOI] [PubMed] [Google Scholar]
- 9.Moffett BS, Goldstein SL, Adusei M, Kuzin J, Mohan P, Mott AR. Risk factors for postoperative acute kidney injury in pediatric cardiac surgery patients receiving angiotensin-converting enzyme inhibitors. Pediatr Crit Care Med. 2011 Sep;12(5):555–559. doi: 10.1097/PCC.0b013e31820ac40a. [DOI] [PubMed] [Google Scholar]
- 10.Lex DJ, Toth R, Cserep Z, et al. A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg. 2014 Jan;97(1):202–210. doi: 10.1016/j.athoracsur.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 11.Joo EJ, Peck KR, Ha YE, et al. Impact of acute kidney injury on mortality and medical costs in patients with meticillin-resistant Staphylococcus aureus bacteraemia: a retrospective, multicentre observational study. J Hosp Infect. 2013 Apr;83(4):300–306. doi: 10.1016/j.jhin.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Basu RK, Zappitelli M, Brunner L, et al. Derivation and validation of the renal angina index to improve the prediction of acute kidney injury in critically ill children. Kidney Int. 2014 Mar;85(3):659–667. doi: 10.1038/ki.2013.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mammen C, Al Abbas A, Skippen P, et al. Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012 Apr;59(4):523–530. doi: 10.1053/j.ajkd.2011.10.048. [DOI] [PubMed] [Google Scholar]
- 14.Basu RK, Andrews A, Krawczeski C, Manning P, Wheeler DS, Goldstein SL. Acute kidney injury based on corrected serum creatinine is associated with increased morbidity in children following the arterial switch operation. Pediatr Crit Care Med. 2013 Jun;14(5):218–224. doi: 10.1097/PCC.0b013e3182772f61. [DOI] [PubMed] [Google Scholar]
- 15.Cao Y, Yi ZW, Zhang H, Dang XQ, Wu XC, Huang AW. Etiology and outcomes of acute kidney injury in Chinese children: a prospective multicentre investigation. BMC Urol. 2013;13:41. doi: 10.1186/1471-2490-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter Suppl. 2012;2:1–138. [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention, National Center for Health Statistics. CDC growth charts: United States. 2000 http://www.cdc.gov/growthcharts/
- 19.Hui-Stickle S, Brewer ED, Goldstein SL. Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis. 2005 Jan;45(1):96–101. doi: 10.1053/j.ajkd.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Plotz FB, Bouma AB, van Wijk JA, Kneyber MC, Bokenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008 Sep;34(9):1713–1717. doi: 10.1007/s00134-008-1176-7. [DOI] [PubMed] [Google Scholar]
- 21.Downes KJ, Rao MB, Kahill L, Nguyen H, Clancy JP, Goldstein SL. Daily serum creatinine monitoring promotes earlier detection of acute kidney injury in children and adolescents with cystic fibrosis. J Cyst Fibros. 2014 Jul;13(4):435–441. doi: 10.1016/j.jcf.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein SL, Kirkendall E, Nguyen H, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013 Sep;132(3):e756–767. doi: 10.1542/peds.2013-0794. [DOI] [PubMed] [Google Scholar]
- 23.Menon S, Kirkendall ES, Nguyen H, Goldstein SL. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr. 2014 Sep;165(3):522–527. doi: 10.1016/j.jpeds.2014.04.058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.