Abstract
Background
Dual antiplatelet therapy (DAPT) is recommended for ≥12 months following coronary drug-eluting stents (DES) to reduce risk of major adverse ischemic events. Randomized trials suggest an abbreviated DAPT duration (≤6 months) is adequately protective. However, these trials are individually underpowered to detect differences in rare but serious events such as stent thrombosis (ST).
Objectives
We performed a meta-analysis of published randomized trials to define the impact of abbreviated DAPT (≤6 months) on death, myocardial infarction (MI), stent thrombosis(ST) and bleeding complications compared to standard-duration DAPT (≥12 months).
Methods
Seven randomized controlled trials comparing abbreviated vs. standard DAPT regimens following DES use were identified by 2 independent investigators. Study characteristics were reviewed and clinical endpoint data were abstracted and analyzed in aggregate using fixed and random-effects models.
Results
The 7 trials included 15,874 randomized patients. Second-generation DES were used in most patients. Compared to standard-duration DAPT, abbreviated DAPT was not associated with an increase in mortality (OR 0.93; CI: 0.73 to 1.17; p = 0.52), MI (OR 1.14; CI: 0.89 to 1.45; p = 0.30) or ST (OR 1.25; CI: 0.81 to 1.93; p = 0.31). Abbreviated DAPT was associated with significantly fewer major bleeding complications (OR 0.52; CI: 0.34 to 0.82; p = 0.005). The results were consistent between fixed and random-effects models, with no heterogeneity. Sensitivity analyses adjusting for inclusion of bare metal stents, 1st generation DES and/or abbreviated DAPT regeimens of 3 months resulted in similar conclusions.
Conclusions
In a meta-analysis of >15,000 patients primarily treated with second-generation DES, abbreviated-duration DAPT (≤6 months) was associated with a significant reduction in major bleeding complications with no evidence of a significant increase in risk of death, MI or ST. Accordingly, abbreviated DAPT should be strongly considered for patients receiving second generation DES.
Keywords: meta-analysis, drug-eluting stents, dual antiplatelet therapy, percutaneous coronary intervention
INTRODUCTION
Dual antiplatelet therapy (DAPT) is required to prevent stent thrombosis (ST) following coronary stent implantation.(1) Interruption of DAPT is the primary etiology underlying ST in the early months following coronary stent implantation.(2) Given the serious clinical consequences of ST, antiplatelet therapy following stenting has been the subject of intense clinical research over the last two decades. The duration of mandatory DAPT was relatively well defined with use of bare metal stents (BMS), but variable delays in endothelialization following use of drug-eluting stents (DES) has rendered the optimal duration of DAPT following DES to be uncertain. In 2006, a US Food and Drug Administration (FDA) advisory panel recommended extending the duration of DAPT following DES to 12 months.(3) While there was some evidence suggesting this duration to be associated with reduced risk,(4-6) there were no randomized clinical trial data to support such a recommendation. Nonetheless, given the concerns for higher ST rates with early DES, the 12 months of DAPT became the standard adopted in national practice guidelines.(7,8) Defining the optimal duration is further complicated by the guideline recommendation of using DAPT for one year in acute coronary syndrome patients regardless of type of stent,(9,10) and the recent results of a large randomized trial demonstrating a reduction in ischemic adverse outcomes, albeit with increased risk of bleeding if DAPT is used for >30 months post-stenting.(11)
Over the last 5 years, several randomized trials examined the possibility of a shorter DAPT duration and its impact on the major adverse ischemic events (MACE), specifically on the risk of ST.(12-18) In these trials, in which second-generation DES were predominantly used, abbreviated DAPT duration was not associated with an increased risk of ST or MACE overall. However, these studies were individually underpowered to detect differences between abbreviated and standard DAPT durations, particulary with rare events such as ST.
In this study, we sought to review and perform a meta-analysis of the randomized trials comparing abbreviated DAPT duration (≤6months) to the current standard duration DAPT (≥12months) following use of DES in coronary interventions.
METHODS
Review question and study protocol
Our analysis sought to answer the following question: Is an abbreviated-duration (≤6 months) DAPT regimen as safe and effective in reducing major adverse outcomes following use of contemporary DES as a standard-duration (≥12 months) DAPT? We report this protocol-driven systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).(19)
Eligibility criteria
Two reviewers (K.M.Z. and A.A.L.) independently judged the eligibility of all studies. Eligible studies included randomized controlled trials (RCTs) examining the use of an abbreviated-duration (≤6 months) vs. standard-duration (≥12 months) DAPT after coronary implantation of DES. Non-randomized studies and case reports were excluded.
Search Strategy
We searched MEDLINE (January 1980 to February 2015), the Cochrane databases (February 2015), EMBASE (January 1980 to February 2015), CINAHL (January 1982 to February 2015), the US Food and Drug Administration Web site (http://www.fda.gov), and BIOSIS Previews (January 1980 to February 2015) using the following database-appropriate MESH terms: percutaneous coronary intervention, balloon angioplasty, stenting, drug-eluting stents, duration, dual antiplatelet therapy and clinical outcomes. We identified additional studies by reviewing the reference lists of eligible studies, relevant review articles, and the published abstracts of the American Heart Association, the American College of Cardiology, the European Society of Cardiology, and the Trans-catheter Cardiovascular Therapeutics annual meetings.
Data Abstraction
Two reviewers (K.M.Z. and A.A.L.) working in duplicate and independently used a standardized form to abstract the data from each study. Disagreements were resolved by consensus. For each outcome, absolute event numbers were included and results were expressed as proportions among total participants with complete follow up in corresponding study arms. The longest follow-up data available were used for each study.
Quality assessment
We used the criteria by Juni et al to ascertain the methodological quality and the potential for bias of included randomized trials.(20) Briefly, the authors evaluated the study quality based on the following criteria: adequacy of allocation, appropriate description of randomization method, similarity of groups at the onset of the study, blinding for both participants and caregivers, blind ascertainment of outcomes, attrition and intention-to-treat analysis. The authors’ statements regarding blinding and other methods in the original manuscripts were accepted verbatim.
Data Analysis
We performed a meta-analysis of the RCTs comparing clinical outcomes of patients treated with abbreviated- (≤6 months) vs standard- (≥12 months) duration DAPT following coronary interventions using DES. The pre-specified outcomes of our analyses were all-cause mortality, cardiac mortality, myocardial infarction (MI), stent thrombosis (ST), stroke, target vessel revascularization (TVR), major bleeding and combined study endpoint. Of note, cardiac mortality was reported in 6 and TVR in 5 of the 7 included studies.
Concordant with the low observed heterogeneity among studies’ findings, we conducted fixed-effects meta-analyses to obtain estimated odds ratios (OR) for the pre-specified main clinical outcomes of patients treated with abbreviated- vs. standard-duration DAPT and their associated 95% confidence intervals (CI). The estimated OR from separate studies were combined according to both the Mantel-Haenszel(21) and the Peto(22) methods. Due to variability in patient populations, indications for stenting, endpoints and duration of therapy, the meta-analysis was also performed using random-effects modeling according to DerSimonian-Laird method.(23)
Additional senstivity analyses were performed to assess the possible interaction of use of bare metal stents (BMS) or 1st generation DES in some studies and use of 3 vs. 6 months in the abbreviated-duration DAPT regimens.
When a statistically significant difference between the abbreviated and standard duration DAPT regimens was detected, we calculated the number needed to treat (NNT) and the number needed to harm (NNH) to assess clinical relevance of the results. The NNT and NNH are the reciprocal of the estimated risk difference (RD) calculated based on the fixed-effect model using the Mantel-Haenzel method. NNT denotes the number of patients that would need to be treated with abbreviated-duration instead of extended-duration DAPT to prevent one adverse event, whereas NNH denotes the number of patients that would need to be treated with standard-duration DAPT instead of abbreviated-duration DAPT to cause one adverse outcome in this analysis. We estimated the proportion of between-study inconsistency due to true differences between studies (rather than differences due to chance) using the I2 statistic,(24) with values of 25%, 50%, and 75% considered low, moderate, and high, respectively. Funnel plots were graphically explored for evidence of publication bias. The Review Manager software (RevMan version 5.1.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011) was used for these analyses.
RESULTS
Search Results
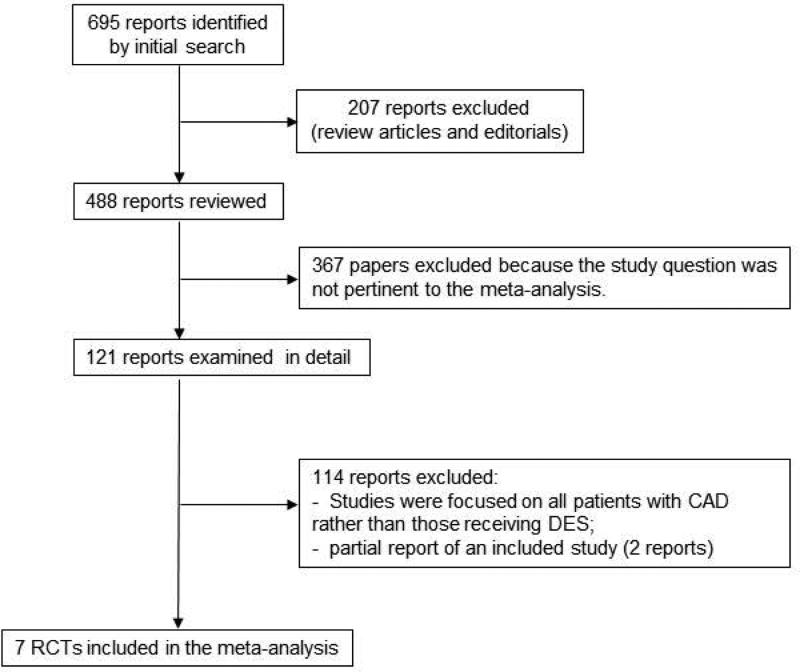
Of 695 articles retrieved during the initial search (Figure 1), 207 were not original investigations (review articles and editorials); 367 papers were not pertinent to the study question (studies of embolic protection devices, covered stents, and brachytherapy); and 114 other reports were also excluded (not pertinent to the meta-analysis question or were partial reports). Seven randomized controlled trials with a total of 15,874 patients (7,918 received abbreviated-duration DAPT and 7,956 received standard-duration DAPT) were eligible for review. (12-18) The inter-reviewer agreement on study eligibility was 100%. Of note, 3 randomized controlled trials addressing DAPT duration of therapy were not included because the shortest duration of DAPT therapy among the randomized patients was 12 months i.e. these studies did not include an abbreviated (≤6 months) DAPT regimen.(11,25,26)
Figure 1.
Selection of trials for inclusion in meta-analysis.
Study characteristics
Table 1 summarizes the clinical characteristics of the included studies. In each of the included randomized trials, there were no significant differences in baseline characteristics between the patient group receiving abbreviated- vs standard-duration DAPT. The average age, female participation and diabetic status were similar between both treatment groups. All studies allowed enrollment of patients with acute coronary syndromes (mean 53% and median 52% among all patients treated with abbreviated- and standard-duration DAPT). However, in 2 trials, inclusion was limited to stable patients and those with low-risk acute coronary syndrome.(15,27) Patients undergoing primary PCI for ST-segment elevation MI were included in only one trial.(13)
Table 1.
Patient and Study Characteristics
| ITALIC (18) | SECURITY (17) | RESET (12) | PRODIGY (13) | OPTIMIZE (15) | ISAR-SAFE (16) | EXCELLENT (14) | ||
|---|---|---|---|---|---|---|---|---|
| PATIENT CHARACTERISTICS | ||||||||
| Number of Patients | 2031 | 1399 | 2117 | 2013 | 3119 | 4005 | 1443 | |
| Mean Age (years) | Abbreviated DAPT | 61.7 | 65.5 | 62.4 | 67.9 | 61.3 | 67.2 | 63.0 |
| Standard DAPT | 61.5 | 64.9 | 62.4 | 67.8 | 61.9 | 67.2 | 62.4 | |
| Female (%) | Abbreviated DAPT | 19.2 | 22.4 | 35.6 | 24.0 | 36.5 | 19.3 | 34.9 |
| Standard DAPT | 20.8 | 23.2 | 36.1 | 22.6 | 36.9 | 19.5 | 36.1 | |
| Diabetes (%) | Abbreviated DAPT | 36.3 | 31.1 | 29.8 | 23.7 | 35.4 | 24.8 | 37.7 |
| Standard DAPT | 37.8 | 31.4 | 28.8 | 24.7 | 35.3 | 24.2 | 38.6 | |
| PCI for ACS/UA (%) | Abbreviated DAPT | 58.9 | 38.4* | 55.5 | 74.6 | 40.2* | 39.8 | 51.1 |
| Standard DAPT | 58.5 | 38.4* | 53.7 | 74.2 | 41.4* | 40.3 | 52.0 | |
| Primary PCI for STEMI | Excluded | Excluded | Excluded | Included | Excluded | Excluded | Excluded | |
| STUDY CHARACTERISTICS | ||||||||
| Primary composite endpoint | All-cause mortality MI Stroke Major Bleeding TVR |
Cardiac mortality MI Stroke Major bleeding ST |
Cardiac mortality MI Stroke ST TVR |
All-cause mortality MI Stroke |
All-cause mortality MI Stroke Major Bleeding |
All-cause mortality MI Stroke Major Bleeding ST |
Cardiac mortality MI TVR |
|
| DAPT Regimen | ASA + Clopidogrel (98.5%) | ASA + Clopidogrel (99%) | ASA + Clopidogrel (100%) | ASA + Clopidogrel (100%) | ASA + Clopidogrel (100%) | ASA + Clopidogrel (100%) | ASA + Clopidogrel (100%) | |
| Duration of DAPT (months) | Abbreviated DAPT | 6 | 6 | 3 | 6 | 3 | 6 | 6 |
| Standard DAPT | 24 | 12 | 12 | 24 | 12 | 12 | 12 | |
| Timing of Randomization | At time of index stenting | At time of index stenting | At time of index stenting | 1 month after index stenting | 3 months after index stenting | 6 months after index stenting | At time of index stenting | |
| Follow up (months) | 36 | 24 | 12 | 24 | 12 | 15 | 12 | |
| BMS/1st Gen DES/2nd Gen DES (%) | Abbreviated DAPT | 0/0/100 | 0/0/100 | 0/0/100 | 25/25/50 | 0/0/100 | 0.4/11/88 | 0/25/75 |
| Standard DAPT | 0/0/100 | 0/0/100 | 0/28/72 | 25/25/50 | 0/0/100 | 0.3/10/89 | 0/25/75 | |
| NOTABLE ANGIOGRAPHIC CHARACTERISTICS | ||||||||
| 3 Vessel Disease (%) | Abbreviated DAPT | 19.5 | 11.3 | 15.6 | 30.4 | 25.3 | 30.8 | 20.6 |
| Standard DAPT | 18 | 11.4 | 15.3 | 29.6 | 26.4 | 31 | 19.8 | |
| Left Main Disease (%) | Abbreviated DAPT | 1.5 | 0 | 0 | 5.7 | 1.2 | 0.5 | 0 |
| Standard DAPT | 0.9 | 0 | 0 | 5.6 | 1.5 | 0.2 | 0 | |
| Saphenous Vein Graft (%) | Abbreviated DAPT | 6.5 | 0 | N/A | 1.7 | 0 | 1.5 | N/A |
| Standard DAPT | 4.3 | 0 | N/A | 2.3 | 0 | 1.3 | N/A | |
ACS, acute coronary syndrome; ASA; aspirin; BMS, bare metal stent; DAPT, dual antiplatelet therapy; DES, drug eluting stent; MI, myocardial infarction; TVR, target vessel revascularization; UA, unstable angina.
The percentage of three-vessel coronary artery disease (mean 26% and median 20% in study-specific percentages among both arms) and left main disease (mean 2.1% and median 1.4% in study specific percentages among both arms) were relatively low. Most studies excluded PCI of saphenous vein graft lesions (Table 1).
The majority of the studies used second-generation DES, although first- generation DES represented 28% of the stents used in the standard-duration DAPT in 1 trial,(12) and roughly 25% of patients in both treatment arms in 2 trials.(14,15) BMS compromised approximately 25% of the entire study population in one trial.(13) Aspirin and clopidogrel were the components of DAPT for almost all patients in all studies, with very infrequent use of newer-generation P2Y12 inhibitors.
In 4 of the 7 included trials, randomization to abbreviated- vs standard-duration DAPT occurred at the time of the index procedure (i.e. patients were not randomized at a time distant to stent implantation). In the other 3 trials, randomization occurred after the patients received DAPT for a period of time ranging between 1 and 6 months (Table 1). The combined endpoint varied slightly among the studies; the majority of them included all-cause or cardiac mortality, myocardial infarction, stroke and major bleeding. Stent thrombosis was reported in accordance with the Academic Research Consortium (ARC) criteria.(28) We included definite and probable stent thrombosis that was recorded in all the studies. Two studies reported possible stent thrombosis, but this was not included in the meta-analysis. Major bleeding complications were defined according to the TIMI classification in 5 trials,(12-14,18,29) BARC in 2 (13,29) and a combination of the REPLACE-2 and the GUSTO criteria in 1 trial.(15)
Study Quality
Supplemental Table 1 describes the methodological quality of the included RCTs. Most studies ascertained the outcomes adequately through independent committees that were blinded to subject allocation. The follow-up was complete in the majority of included studies, except in 1 study, where loss to follow-up reached approximately 19% of the study population.(27) However, a sensitivity analysis excluding this study showed no qualitative effect on the meta-analysis results. The inter-reviewer agreement on these quality domains was greater than 90%.
Primary Meta-analysis
The primary analyses were first performed using the fixed-effects model according to the Mantel-Haenszel method.(21) The results for each of the individual outcomes were as follows:
Mortality
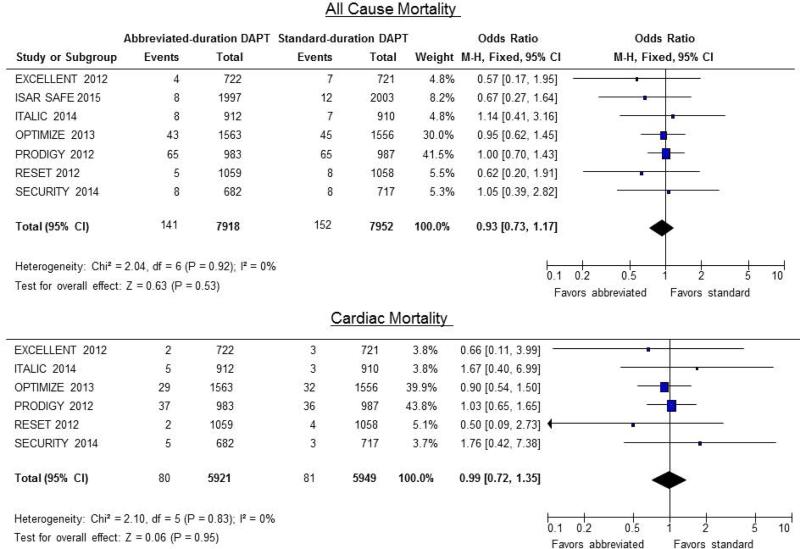
The risk of death due to any cause was not significantly different between the patients receiving abbreviated and standard duration DAPT (1.8% vs. 1.9%, OR 0.93; CI: 0.73 to 1.17; P = 0.52). Likewise, there was no significant differences in the risk of cardiac mortality (1.35% vs. 1.4%, OR 0.97; CI: 0.71 to 1.32; P = 0.83) (Figure 2).
Figure 2.
Odds ratio for all-cause mortality (top) and cardiac mortality (bottom). Forest plots of unadjusted odds ratio (OR, with 95% CIs) for all-cause and cardiac mortality in patients receiving abbreviated- vs. standard-DAPT regimens. There was no significant difference in the odds of all-cause or cardiac mortality between the 2 groups (OR 0.93; CI: 0.73 to 1.17; P = 0.52 and OR 0.97; CI: 0.71 to 1.32; P = 0.83 respectively).
Myocardial Infarction
The risk of MI was not significantly different between the patients receiving abbreviated and standard duration DAPT (1.8% vs. 1.6%, OR 1.14; CI: 0.89 to 1.45; P = 0.30)
Stent thrombosis
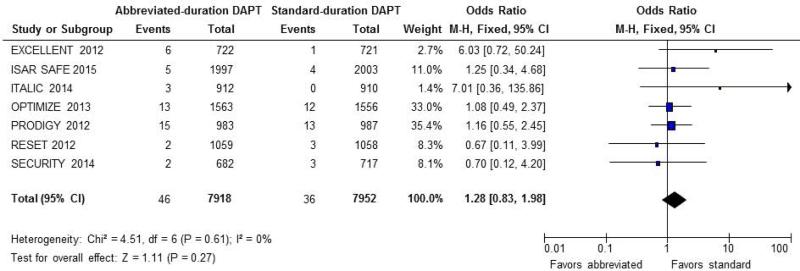
There was no significant increase in the risk of ST associated with abbreviated-compared to standard-duration DAPT (0.60% vs. 0.45%, OR 1.28; CI: 0.83 to 1.98; P = 0.31) (Figure 3).
Figure 3.
Odds ratio for stent thrombosis (ST). Forest plot of unadjusted odds ratio (OR, with 95% CIs) for definite or probable ST in patients receiving abbreviated- vs. standard-DAPT regimens. There was no significant difference in the odds of stent thrombosis between the 2 groups (OR 1.25; CI: 0.81 to 1.93; P = 0.31).
Major Bleeding
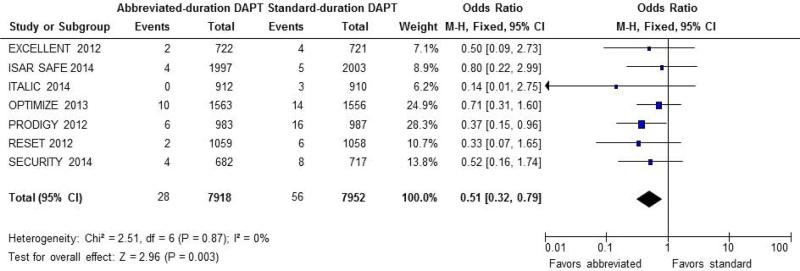
The risk of major bleeding was substantially lower in patients treated with abbreviated DAPT compared to those who received standard-duration DAPT (0.35% vs. 0.70%, OR 0.52; CI: 0.34 to 0.82; P = 0.005) (Figure 4). In the case of major bleeding, the RD was statistically significant (RD = −0.35%, 95% CI, −0.13 to −0.58%, NNT of 286).
Figure 4.
Odds ratio for major bleeding. Forest plot of unadjusted odds ratio (OR, with 95% CIs) for major bleeding in patients receiving abbreviated- vs. standard-DAPT regimens. There was a significant reduction in the estimated OR of major bleeding in patients treated with abbreviated DAPT (OR 0.52; CI: 0.34 to 0.82; P = 0.005).
Stroke
No significant differences were noted in the estimated odds of stroke among patients treated with abbreviated vs. standard-duration DAPT (0.52% vs. 0.60%, OR 0.84; CI: 0.56 to 1.27; P = 0.42)
Other Endpoints
Patients treated with abbreviated DAPT had similar odds of the study-defined composite endpoint as those in the standard duration DAPT group. (4.6% vs. 4.6%, OR 0.99; CI: 0.85 to 1.15; P = 0.89) Similarly, TVR was not different between the groups (2.7% vs. 2.2%, OR 1.18; CI: 0.91 to 1.53; P = 0.22)
The data was re-examined using the Peto method (22) and random-effects model using the DerSimonian-Laird method.(23) As shown in Supplemental Table 2, the results of these analyses were qualitatively and quantitatively quite similar, thus not influencing the conclusions reached from fixed-effects modeling.
Additional Analyses
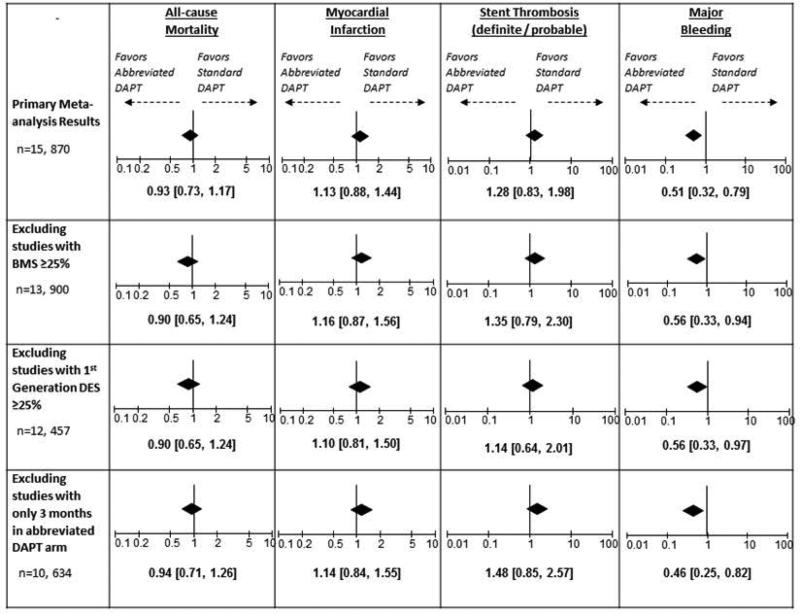
There was no significant interaction or appreciable impact on the estimated odds ratios and the conclusions of the primary analysis when studies including BMS use ≥25%, 1st generation DES use ≥25% and/or 3 months of DAPT in the abbreviated arm were excluded (Supplemental Tables 3-5). Figure 5 summarises the findings of the various secondary analyses and the impact on the estimated odds ratios of the major clinical endpoints. Similar to the primary analysis findings, there was no significant increase in the risk of death, MI or ST, while there was a significant reduction in major bleeding events associated with the abbreviated DAPT duration.
Figure 5.
Odds ratios and confidence intervals for major adverse outcomes according to the primary meta-analysis and the secondary analyses adjusting for important variations in study methodologies. Conclusions were consistent among all secondary analyses and similar to the primary analysis conclusions.
We drew funnel plots to seek evidence of publication bias (Supplemental Figure 1). Upon visual inspection, funnel plots looked symmetrical with no suggestion of significant publication bias. The funnel plots for major bleeding and stroke appeared to be somewhat asymmetrical due to data points from the ITALIC study.(18) However, given the low weight of this study in our analyses, this should not affect the overall conclusions of our meta-analysis.
Heterogeneity Analysis
Tests for heterogeneity were done for each of the clinical endpoints using the I2 statistic. There was no significant heterogeneity (I2 = 0%) noted for all of the the outcomes tested.
DISCUSSION
This meta-analysis of >15,000 patients from 7 controlled randomized trials comparing abbreviated (≤6 months) vs. standard-duration (≥12 months) DAPT following use of second-generation DES demonstrates no significant differences in the risk of death, MI, ST or stroke between the two DAPT regimens. Importantly, the abbreviated regimen was associated with a substantial reduction in major bleeding. These findings support the use of abbreviated DAPT following implantation of second-generation DES, and can provide the basis for reconsideration of current practice guidelines at least in a number of patient subgroups.
The findings of this meta-analysis differ from the earlier observations, upon which DAPT was recommended for ≥12 monthsafter DES. Several factors contributed to this difference. The initial observational studies that demonstrated an increased risk of ST and death with DES were performed with first-generation DES,(4,5) whereas patients in the more contemporary trials received second-generation DES. There is robust evidence that the safety of second-generation DES has significantly improved compared to the first-generation devices. In a network meta-analysis of >50,000 patients included in 49 trials, the second-generation everolimus-eluting stent (EES) was associated with a significantly lower risk of ST compared to all first-generation DES and even to bare metal stents.(30) In the direct comparison between EES and first-generation paclitaxel-eluting stents(PES) in patients with multi-vessel and/or multi-lesion intervention, the incidence of MI related to the target vessel was reduced by >60% and target lesion revascularization by >50% with EES.(31) Recently, the DAPT trial (that included patients receiving 4 different types of DES) suggested a much longer duration of DAPT (30 months) is associated with reduced risk of ST.(11) Although only 38% of the DAPT trial patients received first-generation DES (including 27% of all study patients receiving PES), the absolute number of ST events in those receiving PES represented 57% of all ST events. A significant interaction was measured between the degree of risk reduction with extended DAPT and the type of stent used, favoring second-generation devices (hazard ratio 0.89 with EES and 0.53 with PES, p=0.05 for the interaction).(32) These differences in outcomes may be related to specific characteristics of second-generation DES, such as reduced strut thickness or modified polymer coating.
Differences other than DES type may have contributed to the improved safety profile noted in contemporary trials. It is concievable that 10 years ago, interventional operators where on a steep learning curve regarding patient selection, technique of stent implantation and accumulating knowledge regarding higher doses and pretreatment loading of DAPT. The maturation of knowledge regarding these variables plausibly contributed to improved patient outcomes
A significant limitation of the individual randomized trials included in this meta-analysis is the lack of statistical power to detect differences in rare adverse events such as ST. Despite including >15000 patients in this meta-analysis, it remains difficult to ascertain that the risk of ST was not influenced by abbreviating the duration of DAPT primarily due to the paucity of ST (0.5% of all patients). We can, however, make some conclusions about ST based on this analysis. First, the wide confidence interval around the point estimate (0.83 to 1.98 in this case) suggests that no meaningful difference can be detected between the two DAPT regimens in this relatively large patient sample. Second, the sample size needed to detect statistically significant differences in ST between abbreviated- and standard-duration DAPT would be greater than 7-fold the sample included in this meta-analysis (ie, >100,000 patients). These calculations reflect the rarity of ST in contemporary practice and near identical rates of such events in patients receiving DAPT for 6 or 12 months.
The other key finding of this meta-analysis is the reduced risk of major bleeding complications with the abbreviated DAPT duration. Excess bleeding with DAPT has been shown previously,(33) and understandably, the risk of such bleeding events increases with increased duration of therapy.(11,34) Although these events are not always considered by some to be as serious as acute thrombotic events, it is important to note that major bleeding is associated with serious clinical consequences. Indeed, the risk of death is increased by >70% with access site bleeding and is approximately 3-fold higher with non-access site bleeding following coronary intervention.(35)
Two other meta-analyses of randomized trials have been published recently.(36,37) The analysis by Stefanini et al reached similar conclusions, but included a smaller number of trials and an overall patient population (<9000 patients) which raised the concern of statistical power to detect differences in rare events.(37) The more recent analysis by Giustino et al addressed the randomized trials of DAPT therapy in general, while we focused on the issue of abbreviating DAPT duration to ≤6 months. Thus, Giustino et al included trials in which there was no abbreviated DAPT arm, whereas those were excluded in this study. Nonetheless, the conclusions of both analyses demonstrated a clear disadvantage of extending DAPT duration in regards to serious bleeding complications. Giustino et al also demonstrated a statistically significant difference in risk of stent thrombosis favoring second generation DES.(36)
As is expected with any meta-analysis, there are important limitions that need to be considered. While abbreviating the duration of DAPT to ≤6 months following second-generation DES appears safe, it may seem to contradict current recommendations and findings of other studies. In patients with acute coronary syndromes, current guidelines recommend extending the duration of DAPT to 12 months regardless of DES use.(9,10) Patients with acute coronary syndromes were well represented in randomized trials included in this meta-analysis (>50% of total cohort), but it is not clear that guideline recommendations can be abandoned solely on the basis of these data. Yet, it is conceivable that 12 months of DAPT may not be needed in all patients receiving second-generation DES due to improved safety of these devices. In fact, a recent report from the Swedish Registry demonstrates a clear and significant reduction in early, late and very late ST with second-generation DES compared to first-generation devices among patients presenting with ST-segment elevation MI.(38) An analysis of a potential interaction between acute presentation and safety of abbreviated vs extended DAPT duration was not feasible without the availability of patient-level data. In addition, we cannot apply these conclusions to specific patient subsets, such as those who were underrepresented or excluded, namely ST-segment elevation MI, left main and saphenous vein graft interventions (Table 1). Absence of patient level data did not allow us to directly study the impact of using first generavation vs. second generation DES vs. BMS, but sensitivity analyses suggest that the conclusions of the primary analysis hold true even when studies with a significant number of those patients are excluded (Figure 5, Supplemental Tables 3-5). The defintions used for major bleeding events varied among the included trials, but the differences between the various published bleeding definitions are mostly in defining minor bleeding i.e. major bleeding events (which were used for the purpose of this analysis) are always clinically significant. Overall, it is important to note that subtle differences among various clinical scenarios, nuances of clinical and procedural variables, and an assessment of bleeding risk in every patient may require an individualized decision regarding the most appropriate length of DAPT following coronary intervention.
In conclusion, based on a meta-analysis including >15,000 patients primarily treated with second-generation DES, an abbreviated (≤6 months) duration of dual antiplatelet therapy was associated with a significant reduction in major bleeding complications with no evidence of an increase in risk of death, MI, stent thrombosis or stroke. Reconsideration of the current practice of ≥12 months of dual antiplatelet therapy following use of second-generation DES and individualization of the duration of therapy based on risk/benefit may be warranted.
Supplementary Material
ACKNOWLEDGMENT
Dr. Abdel-Latif is supported by the University of Kentucky Clinical and Translational Science Pilot Award (UL1TR000117), the UK COBRE Early Career Program (P20 GM103527), and NIH Grant R56 HL124266.
Footnotes
The authors have no conflict of interest to report in relation to this manuscript
REFERENCES
- 1.Colombo A, Hall P, Nakamura S, et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation. 1995;91:1676–88. doi: 10.1161/01.cir.91.6.1676. [DOI] [PubMed] [Google Scholar]
- 2.Iakovou I, Schmidt T, Bonizzoni E, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. Jama. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 3.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents: report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8, 2006. Circulation. 2007;115:2352–7. doi: 10.1161/CIRCULATIONAHA.107.688416. [DOI] [PubMed] [Google Scholar]
- 4.Lagerqvist B, James SK, Stenestrand U, et al. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. The New England journal of medicine. 2007;356:1009–19. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 5.Pfisterer M, Brunner-La Rocca HP, Buser PT, et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. Journal of the American College of Cardiology. 2006;48:2584–91. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 6.Eisenstein EL, Anstrom KJ, Kong DF, et al. Clopidogrel use and long-term clinical outcomes after drug-eluting stent implantation. Jama. 2007;297:159–68. doi: 10.1001/jama.297.2.joc60179. [DOI] [PubMed] [Google Scholar]
- 7.Grines CL, Bonow RO, Casey DE, Jr., et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Journal of the American College of Cardiology. 2007;49:734–9. doi: 10.1016/j.jacc.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124:e574–651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 9.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;64:e139–228. doi: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 10.O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:529–55. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 11.Mauri L, Kereiakes DJ, Yeh RW, et al. Twelve or 30 Months of Dual Antiplatelet Therapy after Drug-Eluting Stents. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim BK, Hong MK, Shin DH, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). Journal of the American College of Cardiology. 2012;60:1340–8. doi: 10.1016/j.jacc.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Valgimigli M, Campo G, Monti M, et al. Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation. 2012;125:2015–26. doi: 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 14.Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. 2012;125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 15.Feres F, Costa RA, Abizaid A, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. Jama. 2013;310:2510–22. doi: 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 16.Schulz-Schupke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. European heart journal. 2015;36:1252–63. doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 17.Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. Journal of the American College of Cardiology. 2014;64:2086–97. doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Gilard M, Barragan P, Noryani AA, et al. 6- Versus 24-Month Dual Antiplatelet Therapy After Implantation of Drug-Eluting Stents in Patients Nonresistant to Aspirin: The Randomized, Multicenter ITALIC Trial. Journal of the American College of Cardiology. 2015;65:777–86. doi: 10.1016/j.jacc.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Juni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. Bmj. 2001;323:42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22:719–48. [PubMed] [Google Scholar]
- 22.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Progress in cardiovascular diseases. 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28:105–14. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collet JP, Silvain J, Barthelemy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet. 2014;384:1577–85. doi: 10.1016/S0140-6736(14)60612-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation. 2014;129:304–12. doi: 10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 27.Colombo A, Chieffo A, Frasheri A, et al. Second Generation Drug-Eluting Stents Implantation Followed by Six Versus Twelve-Month - Dual Antiplatelet Therapy- The SECURITY Randomized Clinical Trial. Journal of the American College of Cardiology. 2014 doi: 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Cutlip DE, Windecker S, Mehran R, et al. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–51. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 29.Schulz-Schupke S, Byrne RA, Ten Berg JM, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 versus 12 months of clopidogrel therapy after drug-eluting stenting. European heart journal. 2015 doi: 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 30.Palmerini T, Biondi-Zoccai G, Della Riva D, et al. Stent thrombosis with drug-eluting and bare-metal stents: evidence from a comprehensive network meta-analysis. Lancet. 2012;379:1393–402. doi: 10.1016/S0140-6736(12)60324-9. [DOI] [PubMed] [Google Scholar]
- 31.Kereiakes DJ, Sudhir K, Hermiller JB, et al. Comparison of everolimus-eluting and paclitaxel-eluting coronary stents in patients undergoing multilesion and multivessel intervention: the SPIRIT III (A Clinical Evaluation of the Investigational Device XIENCE V Everolimus Eluting Coronary Stent System [EECSS] in the Treatment of Subjects With De Novo Native Coronary Artery Lesions) and SPIRIT IV (Clinical Evaluation of the XIENCE V Everolimus Eluting Coronary Stent System in the Treatment of Subjects With De Novo Native Coronary Artery Lesions) randomized trials. JACC Cardiovascular interventions. 2010;3:1229–39. doi: 10.1016/j.jcin.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Colombo A, Chieffo A. Dual Antiplatelet Therapy after Drug-Eluting Stents - How Long to Treat? The New England journal of medicine. 2014;371:2225–2226. doi: 10.1056/NEJMe1413297. [DOI] [PubMed] [Google Scholar]
- 33.Yusuf S, Zhao F, Mehta SR, et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The New England journal of medicine. 2001;345:494–502. doi: 10.1056/NEJMoa010746. [DOI] [PubMed] [Google Scholar]
- 34.Tada T, Natsuaki M, Morimoto T, et al. Duration of dual antiplatelet therapy and long-term clinical outcome after coronary drug-eluting stent implantation: landmark analyses from the CREDO-Kyoto PCI/CABG Registry Cohort-2. Circulation Cardiovascular interventions. 2012;5:381–91. doi: 10.1161/CIRCINTERVENTIONS.111.967463. [DOI] [PubMed] [Google Scholar]
- 35.Ndrepepa G, Neumann FJ, Richardt G, et al. Prognostic value of access and non-access sites bleeding after percutaneous coronary intervention. Circulation Cardiovascular interventions. 2013;6:354–61. doi: 10.1161/CIRCINTERVENTIONS.113.000433. [DOI] [PubMed] [Google Scholar]
- 36.Giustino G, Baber U, Sartori S, et al. Duration of Dual Antiplatelet Therapy Following Drug-Eluting Stent Implantation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of the American College of Cardiology. 2015 doi: 10.1016/j.jacc.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 37.Stefanini GG, Siontis GC, Cao D, Heg D, Juni P, Windecker S. Short versus long duration of DAPT after DES implantation: a meta-analysis. Journal of the American College of Cardiology. 2014;64:953–4. doi: 10.1016/j.jacc.2014.06.1158. [DOI] [PubMed] [Google Scholar]
- 38.Sarno G, Lagerqvist B, Nilsson J, et al. Stent thrombosis in new-generation drug-eluting stents in patients with STEMI undergoing primary PCI: a report from SCAAR. Journal of the American College of Cardiology. 2014;64:16–24. doi: 10.1016/j.jacc.2014.04.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.