Abstract
Purpose
To identify and reduce image artifacts in non-contrast-enhanced velocity-selective (VS) magnetization-prepared peripheral magnetic resonance angiography (MRA) at 3T.
Methods
To avoid signal loss in the arteries, double and quadruple refocused VS excitation pulse sequences were designed which were robust to a wide range of B0 and B1 offset. To suppress stripe artifact and background signal variation, we successively applied two VS preparations with excitation profiles shifted by half the period of the stripes. VS-MRA using single, double and quadruple refocused VS preparations was tested in healthy subjects and a patient.
Results
In the regions of large B0 and B1 offsets, arterial signal loss was yielded by single refocused VS preparation, but was avoided with double or refocused preparations. Compared to single VS preparation, the two consecutive preparations with shifted excitation profiles substantially reduced the stripe artifact and background signal variation, as demonstrated by increased mean and decreased standard deviation of relative contrast ratio. The proposed VS-MRA well identified multi-level disease in the femoral arteries of the patient, as validated by digital subtraction angiography.
Conclusion
Two multi-refocused VS magnetization preparations with shifted excitation profiles yield artifact-free peripheral angiograms at 3T.
Keywords: velocity-selective magnetization preparation, non-contrast-enhanced MR angiography at 3T, peripheral angiography
Introduction
Non-contrast-enhanced (NCE) MR angiography (MRA) has been explored as a promising diagnostic tool for peripheral artery disease (PAD) due to the inherent safety of the technique which is devoid of ionizing radiation as well as gadolinium contrast, and therefore serves to provide a preferential advantage over more traditional methods (1). The elimination of the risk for nephrogenic systemic fibrosis associated with gadolinium-based contrast agents used in CE MRA is of importance in imaging patients with PAD many of whom have concomitant chronic kidney disease (2,3).
A number of NCE MRA techniques have been developed for peripheral applications. Quiescent-interval single-shot unenhanced angiography (QISS) is an inflow-based 2D multi-slice method and has advantages of ease of use and robust performance over diverse flow patterns (4). A minor drawback is limited spatial resolution in the superior-inferior direction due to the limited minimum thickness of 2D slice excitation. Another group of methodologies enables 3D encoded MRA with arbitrary high 3D spatial resolution without relying on inflow effect. The core strategy common in these methods is to subtract an artery-suppressed image from a reference image with the same background signal while the approach to suppress arterial signal includes spin-echo readout (5,6) and flow-sensitive magnetization preparation (7,8). Although the subtractive nature offers excellent background suppression, the requirement of two acquisitions remains a drawback.
Another recently introduced NCE MRA method uses velocity-selective (VS) excitation as magnetization preparation which saturates all tissues but arterial blood based on their velocities (9). While VS-MRA shares the advantage of high 3D resolution and large 3D FOV with the subtractive methods, it generates positive angiographic contrast directly in a single acquisition by allocating arterial blood and background tissues to velocity passband and stopband, respectively. Another advantage is that the velocity sensitization is utilized during systolic phases only, making the technique robust to cardiac arrhythmia which alters the diastole significantly but the systole minimally (10,11).
While VS-MRA has demonstrated great potential at 1.5 T, availability of its 3 T version would be advantageous in scenarios where adjunctive MR studies are performed for improving the evaluation of PAD. For example, gadolinium-enhanced peripheral angiography or perfusion imaging can achieve enhanced image contrast at 3T due to long native T1 (12,13), and arterial spin labeling (ASL) perfusion imaging needs 3 T nearly mandatorily due to inherently low SNR of ASL (14). However, peripheral VS-MRA has been deemed challenging at 3 T due to the sensitivity of VS excitation to B0 and B1 variation which tends to be large in the corresponding volume of interest. In this study, we first show that the effects of B0 and B1 offsets on VS magnetization preparation are manifested as arterial signal loss, stripe artifact and background signal variation.
We develop multiple-refocused VS excitation pulse sequences to avoid arterial signal loss and propose successive applications of two VS preparations with shifted excitation profiles to suppress stripe artifact and background signal variation. The proposed strategies are validated in numerical simulations and human subjects including a PAD patient.
Methods
Single refocused VS excitation
An off-resonance-robust VS excitation pulse sequence can be designed by inserting 180° refocusing pulses in the middle of bipolar gradients which are repeatedly interleaved with a train of rectangular RF pulses (15). From the excitation k-space perspective (16), this applies the B1 field at increments of the velocity Fourier variable (kv) which is proportional to the 1st moment of the gradient waveform while erasing the undesired phase accrued by off-resonance. Figure 1a shows a refocused VS 90° excitation pulse sequence generated with the design parameters of velocity FOV (FOVv) = 82 cm/s, full-width-half-maximum excitation bandwidth = 18 cm/s and number of rectangular RF pulses = 5. A minor difference from the design used in Ref. (15) is the application of Malcolm-Levitt (MLEV)-4 phase cycling (17,18) for the refocusing pulses instead of iterative numerical optimizations for further enhancement of B0 and B1 immunity.
Figure 1.
VS saturation pulse sequence with single refocusing (a), simulated longitudinal magnetization Mz over the plane of velocity versus off-resonance (b) and the plane of velocity versus B1 scale (= the ratio of actual B1 to prescribed B1 values) (c). In the plots of Mz, a positive velocity indicates the direction of arterial flow in the legs (i.e., in the superior-to-inferior direction). The excitation profiles get severely distorted with large B0 offsets beyond ±100 Hz or large B1 offsets beyond ±20%.
Figures 1b and 1c show Bloch simulations of the excitation profile over velocity, off-resonance and B1 offset. The excitation profile is robust to off-resonance to some extent, but suffers from severe distortion in the presence of large B1 and/or B0 inhomogeneity, which can be attributed largely to two types of errors. First, the error of 180° refocusing pulses caused by both B0 and B1 inhomogeneity, prevents spins from being accurately dephased based on their velocities during each velocity encoding step enclosed by two rectangular RF pulses. While affecting the excitation profile over the entire range of velocity, this error is particularly serious due to the distortion of the velocity passband profile and therefore loss of arterial signal. Second, the error of rectangular RF pulses causes inaccurate excitation flip angle in the velocity stopband and therefore suboptimal background suppression. This error is caused exclusively by B1 variation but not by off-resonance due to the very brief period of each rectangular pulse.
Multiple refocused VS excitation
The refocusing error can be further reduced by increasing the number of 180° pulses (Nrefoc) in each velocity encoding step (Fig. 2) (19). The underlying rationale is similar to the one for the established T2 preparation sequences in that the phase of the transverse magnetization made by the preceding rectangular RF pulse is barely changed by B0 and B1 offsets after multiple refocusing pulses weighted by MLEV phase cycling schemes (20). We consider double refocusing (Nrefoc = 2) (21) as well as quadruple refocusing (Nrefoc = 4). Each velocity encoding step consists of a unipolar gradient, Nrefoc refocusing pulses interleaved with (Nrefoc -1) bipolar gradients each of which is made of two of the unipolar gradient, followed by another unipolar gradient with the opposite polarity to the initial unipolar gradient (Figs 2a and 2b). Each unipolar gradient is assumed to be of triangle shape since the target velocity FOV is large enough not to require trapezoidal shape.
Figure 2.
Schematic diagrams of multiple refocused VS excitation pulse sequences. One velocity encoding step enclosed by two rectangular RF pulses (black bars) is shown in cases of double refocusing (a) and quadruple refocusing (b). Each encoding step begins with a unipolar gradient, contains a series of bipolar gradients interleaved with 180° refocusing pulses, and ends with a unipolar gradient with the opposite polarity to the initial unipolar. An equivalent gradient waveform in the absence of refocusing pulses can be obtained by flipping the unipolars that experience an odd number of refocusing pulses (c and d). The size of unipolar (Tuni) is decided such that the equivalent gradient waveform yields the desired first moment for given velocity FOV and duration of refocusing pulse (Eq. 1).
A note of caution should be mentioned with regards to the determination of the size of unipolar gradient. First, an effective configuration of gradient pulses should be formed in a way that yields the same 1st moment in the absence of the refocusing pulses. This is done by flipping the polarities of the unipolar gradients that experience odd numbers of refocusing pulses through the entire pulse sequence, as shown in Figs 2c and 2d for Nrefoc = 2 and Nrefoc = 4, respectively. Then, the size of the unipolar gradient should be determined such that each velocity encoding step generates kv increment of the inverse of the velocity FOV. Cubic equations for the time duration of a unipolar gradient, Tuni, are derived in Appendix and summarized as follow.
| [1] |
where constant variables include Trefoc = time duration of the refocusing pulse, FOVv = velocity FOV, γ = gyromagnetic ratio, and Sm = maximum slew rate of gradient field. After the size of each unipolar gradient is determined, the polarities of all the unipolar gradients should return back to the original states.
Figure 3 contains VS excitation pulse sequences for double refocusing (Fig. 3a) and quadruple refocusing (Fig. 3d). The design parameters were the same as those for the single refocused VS pulse sequence (Fig. 1) except that the quadruple design used a single hard pulse instead of the 90°-180°-90° composite pulse in order to limit the total sequence duration and specific absorption rate (SAR). MLEV-8 and MLEV-16 phase cycling schemes were used for the double and quadruple refocused designs, respectively. The Bloch simulations show that both designs well preserve the pass-band Mz profile over a wide range of B0 and B1 offsets due to the improved compensation for the refocusing errors (Figs. 3b, 3c, 3d and 3f). The stopband signal, however, remains to vary in proportion to the B1 scale that directly affects the rectangular RF pulses.
Figure 3.
VS saturation pulse sequence with double refocusing (a), simulated Mz over the plane of velocity versus off-resonance (b) and the plane of velocity versus B1 scale (c). The pulse sequence and simulated Mz profiles for quadruple refocused design are shown in the same format in d through f. Single 180° hard RF pulse is used for quadruple refocusing to limit the duration of the entire pulse sequence, whereas 90°-180°-90° composite pulse train is used for double refocusing as in the single refocused design. Both double and quadruple refocused designs significantly improve the robustness to B0 and B1 offsets compared to the single refocused design.
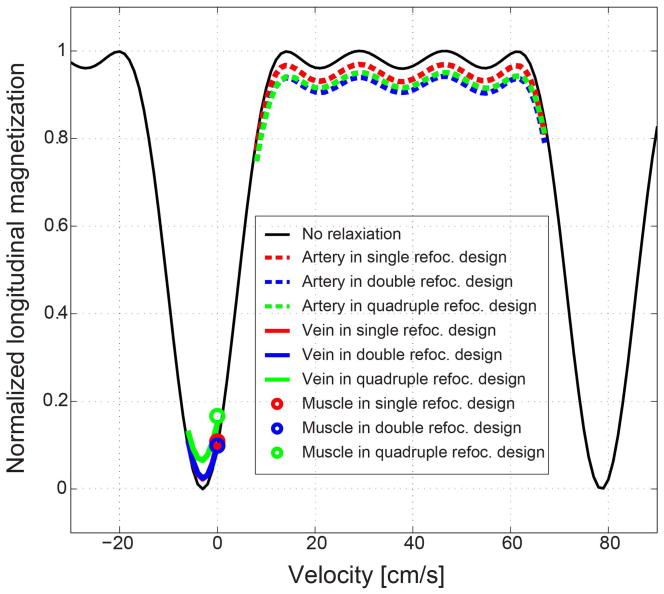
Figure 4 shows Bloch simulation of the effect of tissue relaxation which is expected to be larger in multi-refocused VS excitation than single refocused excitation due to the longer pulse duration. The following relaxation times were chosen from literature: T1/T2 = 1930/164 msec for arterial blood, 1930/70 msec for venous blood (assuming 70% oxygen level), and 1412/50 msec for muscle (22,23). In all of single (red), double (blue) and quadruple (green) refocused designs, tissue relaxation causes decrease in arterial blood magnetization (dotted lines) and increase in venous blood (thick solid lines) and muscle (circles) magnetization but to a limited extent. Compared to single refocused design, double and quadruple refocused designs reduce both artery-vein contrast and artery-muscle contrast by less than 10%.
Figure 4.
Effect of T1 and T2 relaxation of tissues on the excitation profiles of single and multi-refocused VS preparation pulses. Tissue relaxation decreases arterial blood magnetization (dotted lines) while increasing venous blood (thick solid lines) and muscle (circles) magnetizations but only marginally. Compared to single refocused design, double and quadruple refocused designs reduce both artery-vein contrast and artery-muscle contrast by less than 10%.
Improvement of background suppression
While the imperfect 180° refocusing was shown to distort the desired spin’s response to velocity, the refocusing error also causes image artifacts in stationary tissues. In each velocity encoding step (Fig. 2), stationary spins are dephased by the 1st unipolar gradient at a spatial frequency of ω that is proportional to the area of the unipolar, and rephased by the 2nd unipolar gradient at the end if the refocusing pulse in the middle works perfectly. However, imperfect 180° rotation alters the configuration of the dephased spins by the 1st unipolar gradient and leaves a fraction of spins still dephased after the 2nd unipolar gradient. With the undesired dephasing repeated over the entire sequence, the residual modulation of longitudinal magnetization at the end of the sequence would have the fundamental spatial frequency kG defined as (γ/2π)∫Guni(τ)dτ (where Guni(τ) is unipolar gradient) as well as higher harmonics the strength of which depends primarily on the degree of refocusing error (24). The 1st row in Fig. 5 shows simulated Mz of stationary tissues (i.e. with zero velocity) over the plane of spatial position versus off-resonance for B1 scales of 0.75, 1.0 and 1.25. In both double refocused (Fig. 5a) and quadruple refocused (Fig. 5d) designs, the Mz profiles are nearly constant along the spatial position with small B0 and B1 offsets, but show periodic fluctuation when B0 offset and particularly B1 error are large. The stripe artifact is approximately sinusoidal with period of λG that is the inverse of the fundamental frequency created by each unipolar gradient.
Figure 5.
Simulated Mz profiles of multiple-refocused VS preparation pulses in the plane of spatial location versus off-resonance with 3 representative B1 scales (0.75, 1.0 and 1.25). Due to the imperfect refocusing between the preceding and subsequent unipolar gradients, VS excitation creates approximately sinusoidal fluctuation of Mz the period of which is proportional to the inverse of the area of the unipolar gradient (Eq. 2) (a and d). When an additional VS preparation is applied with excitation profiles shifted by half the period of the sinusoidal fluctuation (b and e), the Mz response becomes much flatter along the spatial dimension (c and f).
| [2] |
Note that the period of the stripe artifact is shorter in the double refocused design due to the larger size of the unipolar gradient than the quadruple refocused design.
Based on the sinusoidal approximation of the stripe artifact, the longitudinal magnetization of stationary tissues (normalized by the equilibrium level M0) can be modeled as:
| [3] |
where x is spatial position in the direction of the VS gradient; C is determined as the cosine of an actual flip angle of VS excitation, and varies from -0.6 to 0.6 for B1 variation of ±40%; c represents the amplitude of the sinusoidal fluctuation and varies approximately from 0.0 to 0.4 according to Bloch simulation based on B0 variation of ±100 Hz and B1 variation of ±40% for both double and quadruple refocused designs.
As a simple solution to suppress the stripe artifact, we apply another VS preparation pulse sequence with the same excitation profile as the original sequence except being shifted in the spatial dimension by half λG. This additional VS sequence can be obtained by adding an RF phase waveform θ(t) that is proportional to the area of a gradient waveform and the desired amount of shifting (i.e. 0.5 λG) to the original RF waveform B1(t).
| [4] |
where T and G(t) are the duration and gradient waveform of the entire VS excitation pulse sequence. The longitudinal magnetization after successive application of the two VS pulse sequences can be written as:
| [5] |
The amplitude of the sinusoidal fluctuation is reduced from c to 0.5c2, and the amplitude of the constant component is reduced from C to C2+0.5c2 where 0.0 < c < 0.4 and -0.6 < C < 0.6.
The 2nd row of Fig. 5 shows simulated Mz of the additional VS preparation pulse sequence for the same conditions of B0 and B1 offsets as the 1st row for the original VS pulse sequence. The 3rd row of Fig. 5 shows the resultant Mz after the successive application of the two VS sequences. The stripe artifact and overall background signal level are significantly reduced compared to the single VS preparation in either 1st or 2nd row.
In-vivo Experiments
In vivo experiments were performed on a 3 T whole-body MR scanner (Tim-TRIO; Siemens Medical Solutions, Erlangen, Germany) equipped with maximum RF amplitude of 0.016 mT, gradient amplitude of 40 mT/m and gradient slew rate of 150 mT/m/ms. A 6-channel body matrix coil and a spine coil are used for signal reception. Eight healthy subjects were scanned after written consent forms approved by the University of Maryland institutional review board were obtained. For each subject, the proposed NCE VS-MRA as well as B0 and B1 mappings were applied to the calf (n=4) or the pelvis (n=4). Single, double and quadruple refocused VS preparation pulse sequences were tested for comparisons. For each of the 3 VS preparations, single preparation and two preparations with shifted excitation profiles were applied, which resulted in six cases in total for each subject. Velocity-selective MRA with quadruple refocused preparation was performed in the pelvis and thigh of a patient who was referred for a digital subtraction angiography (DSA) examination due to suspected PAD. The vendor-provided 2nd order B0 shimming was used with “standard” shim mode for all scans. All in-vivo studies were performed under SAR constraint of 4.0 W/kg averaged over whole body.
The NCE MRA pulse sequence was cardiac triggered by ECG gating and consisted of a VS saturation pulse, fat saturation pulses immediately before and after the VS pulse, and a 3D segmented RF-spoiled gradient recalled echo (GRE) readout with centric view order. This set of pulse sequences was repeated every two cardiac cycles. The imaging parameters were coronal imaging orientation, flip angle = 15°, spatial resolution = 1.1×1.1×1.2 mm3 / 1.3×1.3×2.7 mm3 (calf / pelvis), FOV = 36×36×9.6 cm3/ 40×40×21.6 cm3 (calf / pelvis), flow-compensation gradient in the frequency encoding (superior-inferior) direction, TE = 5.4 ms, TR = 9.4 ms, readout bandwidth = 200 Hz/pixel, view per segment = 62, and scan time = 5.3 min (based on 60 beats per minute). Three-fold iterative self-consistent parallel imaging with 24 self-calibration lines was used for scan acceleration (25). The VS preparation pulse was played at the time of peak systolic flow by adjusting a trigger delay based on prior phase-contrast flow measurements, as described in (9,15). The nominal velocity pass-band (defined as the velocity region for Mz > 0.8M0) and saturation-bands (for Mz < 0.2M0) designed for the calf was [8.2, 67.6] cm/s and [−7.4, 1.4] cm/s, respectively, where positive velocity indicates the direction of arterial flow. For imaging the pelvis, the pass- and suppression-bands were widened by 35 % (i.e., [12.6, 104] cm/s and [−11.4, 2.2] cm/s, respectively) by scaling down the VS gradient waveforms at the same rate. B0 maps were obtained using two GRE acquisitions with an echo delay of 2.3 ms. B1 maps were obtained using the Bloch-Siegert phased-based method with an 8-msec Fermi saturation pulse (26). Both B0 and B1 mappings used the same 3D FOV as the subsequent MRA scans for convenient post-analyses. The imaging parameters for the B0 and B1 mappings and MRA are summarized in Table 1.
Table 1.
Imaging parameters for B0 and B1 measurements and VS-MRA.
| Calf | Pelvis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| B0 mapping | B1 mapping | MRA | B0 mapping | B1 mapping | MRA | |
| FOV (mm3) | 360×360×96 | 360×360×96 | 360×360×96 | 400×400×216 | 400×400×216 | 400×400×216 |
| Resolution (mm3) | 2.8×2.8×4.0 | 3.8×3.8×6.0 | 1.1×1.1×1.2 | 3.1×3.1×4.9 | 4.2×4.2×13.5 | 1.3×1.3×2.7 |
| Flip angle (°) | 15 | 15 | 15 | 15 | 15 | 15 |
| TR (ms) | 11 | 100 | 9.4 | 11 | 100 | 9.4 |
| Readout bandwidth (Hz/pixel) | 640 | 660 | 200 | 640 | 660 | 200 |
| View per segment | N/A* | N/A* | 62 | N/A* | N/A* | 62 |
| Scan time (min) | 2 | 5 | 5.3** | 2 | 5 | 5.3** |
Note:
Non-gated continuous acquisition.
Based on 60 beats per minute.
Since contrast-to-noise-ratio cannot be calculated in parallel imaging reconstructed images due to spatially varying noise, relative signal contrast ratio was calculated, defined as (Sa – Sm) / Sa where Sa is the signal intensity of arterial blood and Sm is the signal intensity of muscle (27). After in-plane interpolation of raw images for a voxel size of 0.5×0.5×1.2 mm3 (calf) or 0.5×0.5×2.7 mm3 (pelvis), ROI analysis was performed on 14 equally spaced axial slices. On each slice, two in-plane ROIs were specified for each of arterial blood and muscle and copied to 6 neighboring slices (3 superior and 3 inferior slices), which yielded a total of 14×2×7 =196 ROIs for each tissue and thus 196 contrast ratio measures.
Results
MRA scans with single, double and quadruple refocused VS preparations were successfully completed in all subjects without exceeding the SAR constraint (<4 W/kg). The relative SAR of the three types of VS preparations were 100% (single), 194% (double) and 194% (quadruple), respectively based on numerical integration of the square of the pulse magnitude. The quantitative SAR of the entire MRA sequence ranged 1.1 – 1.5 W/kg for double and quadruple refocused VS preparations with the two-preparation option.
Figure 6 shows representative calf angiograms reformatted through curved maximum-intensity-projection (MIP) of 3D raw data with a projection width of 5 cm as well as B0 and B1 maps reformatted through average intensity projection (AIP) on the same volume of interest as used for the MIP operation of angiograms. The angiogram obtained from single refocused VS preparation (a) shows signal loss in the right popliteal artery due to low B1 field combined with large off-resonance (open arrowhead) and in the left anterior tibial artery due to high B1 field although with small off-resonance (solid arrowheads). Background suppression is sub-optimal, being spatially variant depending on actual B1 values (red and blue circles). Two successive VS preparations make background signal intensity lower, and more uniform, but worsen the arterial signal loss (b). Double refocused and quadruple refocused VS preparations yield uniformly high arterial signal without signal loss due to the improved robustness to B0 and B1 offsets, but create stripes that are repeated along the direction of the VS gradient (i.e. superior-to-inferior direction) (c and e). The periods of the stripes are 3.9 mm and 4.6 mm for the double and quadruple refocused preparations, respectively, as determined by the unipolar areas of 6.1 mT·ms/m and 5.1 mT·ms/m, and Eq. 2. The background signal spatially varies in the same pattern as in the single refocusing case since the B1 offset affects the VS flip angle in the same way regardless of the type of refocusing (red versus blue circles). With additional VS preparations with shifted excitation profiles, the stripe artifacts and the background signal variation are substantially reduced (d and f).
Figure 6.
Representative calf angiograms obtained using single, double and quadruple refocused VS preparations with single and two consecutive preparation schemes. Single refocused VS preparation yields arterial signal loss in the regions of large B0 and B1 offsets (open and solid arrowheads) and background signal variation (red versus blue circles) (a). Double and quadruple refocused designs avoid the arterial signal loss but create stripe artifacts as well as the same background signal variation as in the single refocusing case (c and e). Two consecutive VS preparations with shifted excitation profiles well suppress the stripe artifact as well as background signal variation (d and f).
Figure 7 shows representative pelvis angiograms obtained using the same 6 combinations of VS preparations as in Fig. 6 as well as B0 and B1 field maps. Single refocused VS preparation yields mild (open arrowheads) and severe (solid arrowheads) signal loss in the left iliac arteries. With fairly small off-resonance frequencies in the corresponding regions (arrowheads in Fig. 7g), the arterial signal loss can be attributed to excessively low B1 field (arrowheads in Fig. 7h). Whereas, both double and quadruple refocused VS preparations well avoid the arterial signal loss in the same regions of low B1 field (Figs. 7c and 7e) and suppress the stripe artifacts as well as the variation in background signal through two successive preparations with spatially shifted excitation profiles (Figs. 7d and 7f). Note that the periods of the stripes in Figs. 7c and 7e are longer than those of Figs. 6c and 6e by 35% due to the reduced areas of unipolar gradients at the same rate.
Figure 7.
Representative pelvis angiograms obtained using single, double and quadruple refocused VS preparations with single and two consecutive preparation schemes. Arterial signal loss is caused by single refocused VS preparation (a), but is avoided by double and quadruple refocused VS preparations (c and e). The stripe artifact created by double or quadruple refocused VS preparation is well suppressed by successive application of two preparations with shifted excitation profiles (d and f). The two-preparation scheme reduces spatial variation in background signal as well.
Figure 8 shows peripheral VS-MRA in a 76-year-old female patient, compared with digital subtraction angiography (DSA). The MR angiogram shows good correlation with the DSA image in identifying bilateral, multi-level disease in the superficial femoral arteries. The occlusion of the right superficial femoral artery at the level of the adductor canal is exaggerated in the MRA presumably due to suppression of slow flow around the occlusion.
Figure 8.
Lower extremity VS-MRA in a 76-year-old female patient, compared with digital subtraction angiography. The MRA accurately depicts multi-level disease in the superficial femoral arteries (SFA) bilaterally, with severe segmental disease at the adductor canal level. The occlusion of the right SFA is exaggerated by MRA presumably due to suppression of slow flow below the lower bound of the velocity passband.
The artery-to-muscle relative contrast ratio, field map and B1 map measurements are summarized in Table 2. In calf MRA (n=4), the relative contrast ratio (in the format of mean ± standard deviation) is 0.69±0.14 and 0.68±0.15 for double and quadruple refocused VS preparations, respectively and is 0.85±0.08 and 0.83±0.07 when additional preparations with shifted excitation profiles are applied. With the two-preparation scheme, the mean of relative contrast ratio increases due to the reduced signal in the muscle while the standard deviation decreases primarily due to the reduction of the stripe artifacts. Similarly in pelvis MRA (n=4), the relative contrast ratio is increased from 0.77±0.11 and 0.78±0.10 (with one VS preparation) to 0.89±0.06 and 0.86±0.07 (with two VS preparations) for double and quadruple refocused designs, respectively. The 95% confidence intervals of B0 and B1 distribution are [−83.2, 92.0] Hz and [0.58, 1.28] in the calf, and [−100.5, 102.5] Hz and [0.57, 1.41] in the pelvis.
Table 2.
Measurements of relative contrast ratio, off-resonance and B1 scale
| Subject | D1 | D2 | Q1 | Q2 | B0 offset [Hz] | B1 scale |
|---|---|---|---|---|---|---|
| C1 | 0.70±0.11 | 0.87±0.03 | 0.68±0.12 | 0.84±0.04 | [−68.8,84.8] | [0.55, 1.24] |
| C2 | 0.72±0.13 | 0.86±0.06 | 0.71±0.13 | 0.87±0.05 | [−84.2,89.5] | [0.54,1.36] |
| C3 | 0.69±0.15 | 0.84±0.11 | 0.65±0.18 | 0.83±0.08 | [−97.5,126.6] | [0.66,1.23] |
| C4 | 0.65±0.16 | 0.81±0.10 | 0.67±0.15 | 0.79±0.09 | [−84.1,77.3] | [0.61,1.25] |
| All | 0.69±0.14 | 0.85±0.08 | 0.68±0.15 | 0.83±0.07 | [−83.2,92.0] | [0.58,1.28] |
|
| ||||||
| P1 | 0.76±0.09 | 0.90±0.07 | 0.77±0.10 | 0.88±0.08 | [−109.2,97.2] | [0.63,1.37] |
| P2 | 0.80±0.11 | 0.90±0.05 | 0.81±0.10 | 0.87±0.07 | [−102.3,96.1] | [0.61,1.43] |
| P3 | 0.78±0.11 | 0.88±0.04 | 0.78±0.11 | 0.84±0.07 | [−103.8,116.1] | [0.51,1.43] |
| P4 | 0.72±0.12 | 0.86±0.06 | 0.75±0.09 | 0.85±0.05 | [−83.5,97.1] | [0.56,1.39] |
| All | 0.77±0.11 | 0.89±0.06 | 0.78±0.10 | 0.86±0.07 | [−100.5,102.5] | [0.57,1.41] |
Note: C = Calf, P = Pelvis, D1 = One double refocused VS preparation, D2= Two double refocused VS preparations, Q1 = One quadruple refocused VS preparation, Q2 = Two quadruple refocused VS preparations. B0 and B1 ranges are represented by 95% confidence intervals
Discussion
Possible image artifacts in VS-MRA at 3 T can be divided into signal loss in the arteries, high frequency fluctuation of background signal (stripe artifact) and globally large background signal. The arterial signal loss is typically due to the distortion of the passband excitation profile caused by both B0 and B1 offsets, and represents the most serious issue for angiography as it may mimic arterial stenosis. We have shown that multiple refocused VS excitation is robust to a sufficiently wide range of B0 and B1 variation enough to avoid arterial signal loss in the calf and pelvis at 3 T. The stripe artifact is associated with the error of 180° refocusing which makes the effects of the preceding and subsequent unipolar gradients asymmetric. Under the approximation of sinusoidal signal fluctuation, we implemented two successive VS preparations with shifted excitation profiles by half of the approximated sinusoidal period and were able to significantly reduce the artifact. The globally large background signal is due to the B1 error in hard RF pulses and could be mitigated by the two successive VS preparations.
While both double and quadruple refocused VS preparations were superior to single refocused design in avoiding arterial signal loss, they were both roughly comparable with regards to observed vessel contrast. The mean of relative contrast ratio was higher with the double refocused preparation but very marginally (0.85 vs. 0.83 in the calf and 0.89 vs. 0.86 in the pelvis), and the standard deviation was nearly the same (0.08 vs. 0.07 in the calf and 0.06 vs. 0.07 in the pelvis). The numerical simulation showed slightly weaker stripe artifact with quadruple refocused excitation (Figs. 4c vs. 4f), but did not result in smaller standard deviation of the relative contrast ratio in vivo. While it would be the most appropriate to conclude at the moment that the two approaches perform equally well, further comparisons would be warranted in a larger cohort of subjects including PAD subjects. Compared to single refocused design, multi-refocused VS excitation pulse sequences have drawbacks of higher SAR and longer pulse durations. Despite 98% increase of SAR compared to the single refocused design, the entire VS-MRA sequence with either double or quadruple refocused VS preparation yielded quantitative SAR values well below the FDA guideline (< 4 W/kg averaged over the whole body). Relaxation effects have been found to be only marginal (less than 10% decrease in both artery-vein and artery-muscle contrast). Another consequence of the longer duration is increased sensitivity to higher order motion beyond constant velocity. Although numerical simulations in previous studies revealed that the effect of acceleration is marginal in a 16-ms long design without refocusing pulses (9), the effects of acceleration and higher-order motion on the presented multi-refocused designs need to be further investigated.
In addition to B0 and B1 inhomogeneity, eddy currents are another possible source of the errors of VS excitation. Eddy currents may worsen arterial signal loss by distorting the unipolar and bipolar gradient waveforms and thus the pass-band excitation profile. In this sense, the reduced arterial signal loss in multi-refocused VS excitation than single-refocused excitation could have been partially due to smaller unipolar gradient which involves less eddy currents. The background stripe artifact could have been also partially due to eddy currents, as implied by imperfect correlation between B0 and B1 maps, and the degree of the stripe artifact particularly in calf angiograms. We have observed that the period of the stripes well followed the theoretical calculation based on the area of each unipolar gradient (Eq. 2), which implies marginal distortion of the unipolar gradient and supports the validity of the proposed two-preparation scheme with shifted excitation profiles. Nonetheless, systemic investigation of the degree and effect of eddy currents would be warranted to maximize the accuracy of VS excitation.
Balanced steady-state free precession (bSSFP) has been the acquisition method of the choice for many NCE MRA techniques at 1.5 T due to the favorable T2/T1 contrast for high arterial signal, but poses the risk of the well-known banding artifacts in the presence of large field inhomogeneity expected at high field. We chose RF-spoiled GRE acquisitions to eliminate any potential issues on the acquisition side, focusing solely on the effects of VS magnetization preparation pulse sequences. However, GRE readouts are sub-optimal with regards to SNR efficiency due to the dephasing of transverse magnetization by spoiler gradients, which necessitates the use of low readout bandwidth (200 Hz/pixel) and therefore increases readout TR (9.4 ms). Furthermore, the dephasing of magnetization results in very small longitudinal magnetization at the end of the acquisition, which combined with large T1 values of tissues at 3T, necessitates long repetition peirod of 2 cardiac cycles for sufficient Mz recovery until the next magnetization preparation (vs. 1 cardiac cycle in the previous studies at 1.5 T). In fact, retrospective analysis of the field map measurement revealed a smaller range of distribution (approximately [-100,100] Hz based on 95% confidence interval) than we had expected presumably owing to successful application of the 2nd order shimming used in this study. As it is feasible to achieve as short TR as 3 ms which can cover an off-resonance range of [-166.7, 166.7] Hz without banding artifacts, it may be worthwhile to investigate bSSFP readouts to improve arterial SNR and shorten scan time.
There arises a concern that the use of 1D velocity selectivity along the superior-inferior direction may suppress arterial segments oriented along the other orthogonal dimensions. Nonetheless, our recent clinical studies in PAD patients using the previous work at 1.5T (15) have found that horizontally oriented vessel segments are visualized without severe signal loss, as seen in example angiograms (arrows in Supporting Figure S1). We presume that a significant portion, though not all, of arterial blood in horizontal vessel segments still has vertical velocity component due to the curvy nature of nearby vessels. In fact, the weak dependence of the direction of velocity selectivity has been observed in other independent studies. Velocity-selective arterial spin labeling was found to produce nearly the same perfusion measurements using any of three orthogonal directions only if the cut-off velocity is reasonably small (28). Very recently, application of VS-MRA for the cerebral arteries yielded only marginal difference in resultant angiograms obtained using VS preparations along each of three orthogonal directions (29).
The main limitation of this study is the lack of sufficient clinical validation in PAD patients. The performance of the proposed VS preparation schemes may vary over patients depending on the pattern of arterial flow. Arterial blood magnetization in very slow or turbulent flow in the diseased vasculatures may be undesirably suppressed by the VS preparation. The performance will also be affected by the extent of B0 and B1 offsets which vary depending the size and shape of the subject’s body. Although clinical feasibility was shown by good correlation with DSA in one PAD patient, the proposed techniques needs to be further investigated in a large cohort of patients.
Conclusions
We have identified image artifacts associated with NCE peripheral VS-MRA at 3T, including signal loss in the arteries, stripe artifact and spatial variation in the background signal, and proposed a strategy to reduce the artifacts with in-vivo demonstrations in healthy subjects and a PAD patient. The arterial signal loss can be avoided by using double or quadruple refocused VS excitation pulse sequences, which are immune to a wide range of B0 and B1 inhomogeneity expected at 3T. The stripe artifact and background signal variation can be suppressed by successive application of two VS preparations with excitation profiles spatially shifted by half the period of the stripes, as validated by increased mean and decreased standard deviation of relative contrast ratio.
Supplementary Material
Supporting Figure S1: Example VS-MR angiograms obtained using the previous work at 1.5 T (Ref 15). Horizontally oriented arterial segments are well visualized with VS magnetization preparation applied in the superior-inferior direction presumably due to the curvy nature of nearby vessels.
Acknowledgments
Grant Sponsors:
NIH K25 HL121192 (QQ), NIH R21 EB019206 (TS)
Appendix
Design of gradient waveform in multiple refocused VS excitation
The goal in designing gradient waveform is to generate the first moment m1 for the desired kv increment (Δkv) which should be the inverse of velocity field of view (FOVV).
| [A1] |
The gradient waveform in each velocity encoding step consists of 2Nrefoc unipolar pulses which are termed gi(t) (i = 1, … , 2Nrefoc) (Figs. 2c and 2d). The 1st moment of the gradient waveform can be calculated as the summation of the 1st moment of gi(t) due to the linearity of moment calculation. Using the property M1[ f(t-t0) ] = t0 A[ f(t) ] + M1[ f(t) ], where M1[·] and A[·] represent the calculation of the 1st moment and area, respectively, the 1st moment of each unipolar can be written as,
| [A2] |
where ci is 1 or -1 depending on the polarity of gi(t), and Ti is the start time of gi(t). Assuming a unipolar pulse of triangular shape with duration of Tuni and maximum slew rate of Sm, the largest possible area and first moment are calculated as ¼ Sm Tuni2 and ⅛ Sm Tuni3, respectively. Then Eq. A2 becomes
| [A3] |
After expressing Ti as a function of Tuni and Trefoc (≡ the fixed duration of a refocusing pulse) appropriately for each i, the 1st moment of G(t) can be written as:
| [A4] |
Equations for Tuni are given by equating Eq. [A4] to Eq. [A1].
| [A5] |
References
- 1.Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008;248:20–43. doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 2.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 3.HST NSF: Still relevant. J Magn Reson Imaging. 2014;40:11–12. doi: 10.1002/jmri.24422. [DOI] [PubMed] [Google Scholar]
- 4.Edelman RR, Sheehan JJ, Dunkle E, Schindler N, Carr J, Koktzoglou I. Quiescent-interval single-shot unenhanced magnetic resonance angiography of peripheral vascular disease: Technical considerations and clinical feasibility. Magn Reson Med. 2010;63:951–958. doi: 10.1002/mrm.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meuli RA, Wedeen VJ, Geller SC, Edelman RR, Frank LR, Brady TJ, Rosen BR. MR gated subtraction angiography: evaluation of lower extremities. Radiology. 1986;159:411–418. doi: 10.1148/radiology.159.2.3961174. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H. Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging. 2000;12:776–783. doi: 10.1002/1522-2586(200011)12:5<776::aid-jmri17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Fan Z, Sheehan J, Bi X, Liu X, Carr J, Li D. 3D noncontrast MR angiography of the distal lower extremities using flow-sensitive dephasing (FSD)-prepared balanced SSFP. Magn Reson Med. 2009;62:1523–1532. doi: 10.1002/mrm.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priest AN, Graves MJ, Lomas DJ. Non-contrast-enhanced vascular magnetic resonance imaging using flow-dependent preparation with subtraction. Magn Reson Med. 2012;67:628–637. doi: 10.1002/mrm.23040. [DOI] [PubMed] [Google Scholar]
- 9.Shin T, Worters PW, Hu BS, Nishimura DG. Non-contrast-enhanced renal and abdominal MR angiography using velocity-selective inversion preparation. Magn Reson Med. 2013;69:1268–1275. doi: 10.1002/mrm.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharib AM, Herzka DA, Ustun AO, Desai MY, Locklin J, Pettigrew RI, Stuber M. Coronary MR angiography at 3T during diastole and systole. J Magn Reson Imaging. 2007;26:921–926. doi: 10.1002/jmri.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissler AM, Harris WS, Schoenfeld CD. Systolic time intervals in heart failure in man. Circ. 1968;37:149–159. doi: 10.1161/01.cir.37.2.149. [DOI] [PubMed] [Google Scholar]
- 12.Berg F, Bangard C, Bovenschulte H, Hellmich M, Nijenhuis M, Lackner K, Gossmann A. Feasibility of peripheral contrast-enhanced magnetic resonance angiography at 3. 0 Tesla with a hybrid technique: comparison with digital subtraction angiography. Invest Radiol. 2008;43:642–649. doi: 10.1097/RLI.0b013e31817e90ba. [DOI] [PubMed] [Google Scholar]
- 13.Isbell DC, Epstein FH, Zhong X, DiMaria JM, Berr SS, Meyer CH, Rogers WJ, Harthun NL, Hagspiel KD, Weltman A, Kramer CM. Calf muscle perfusion at peak exercise in peripheral arterial disease: measurement by first-pass contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2007;25:1013–1020. doi: 10.1002/jmri.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pollak AW, Meyer CH, Epstein FH, Jiji RS, Hunter JR, Dimaria JM, Christopher JM, Kramer CM. Arterial spin labeling MR imaging reproducibly measures peak-exercise calf muscle perfusion: a study in patients with peripheral arterial disease and healthy volunteers. JACC Cardiovasc Imaging. 2012;5:1224–1230. doi: 10.1016/j.jcmg.2012.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin T, Hu BS, Nishimura DG. Off-resonance-robust velocity-selective magnetization preparation for non-contrast-enhanced peripheral MR angiography. Magn Reson Med. 2013;70:1229–1240. doi: 10.1002/mrm.24561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauly J, Nishimura DG, Macovski A. A k-space analysis of small-tip-angle excitation. J Magn Reson. 1989;81:43–56. doi: 10.1016/j.jmr.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Levitt M, Freeman R, Frenkiel T. Broadband heteronuclear decoupling. J Magn Reson. 1982;47:328–330. [Google Scholar]
- 18.Shaka AJ, Rucket SP, Pines A. Iterative Carr-Purcell trains. J Magn Reson. 1988;77:606–612. [Google Scholar]
- 19.Qin Q, van Zijl PC. Velocity-selective inersion prepared arterial spin labeling for 3D whole-brain perfusion measurement. Proceeding of ISMRM, 22nd Annual Meeting; Milan, Italy. 2014. p. 420. [Google Scholar]
- 20.Brittain JH, Hu BS, Wright GA, Meyer CH, Macovski A, Nishimura DG. Coronary angiography with magnetization-prepared T2 contrast. Magn Reson Med. 1995;33:689–696. doi: 10.1002/mrm.1910330515. [DOI] [PubMed] [Google Scholar]
- 21.Qin Q, Shin T, Schar M, Guo H, Ciao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3T. ISMRM Workshop on Non-contrast Cardiovascular MRI; Long Beach. 2015. [Google Scholar]
- 22.Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, Henkelman RM. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 23.Lee T, Stainsby JA, Hong J, Han E, Brittain J, Wright GA. Blood relaxation properties at 3T - effects of blood oxygen saturation. Proceeding of ISMRM, 11th Annual Meeting; Toronto. 2003. p. 131. [Google Scholar]
- 24.Scheffler K. A pictorial description of steady-states in rapid magnetic resonance imaging. Concepts Magn Reson. 1999;11:291–304. [Google Scholar]
- 25.Lustig M, Pauly JM. SPIRiT: Iterative self-consistent parallel imaging reconstruction from arbitrary k-space. Magn Reson Med. 2010;64:457–471. doi: 10.1002/mrm.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sacolick LI, Weisinger F, Hancu I, Vogel MW. B1 mapping by Bloch-Siegert shift. Magn Reson Med. 2010;63:1315–1322. doi: 10.1002/mrm.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atanasova IP, Kim D, Lim RP, Storey P, Kim S, Guo H, Lee VS. Noncontrast MR angiography for comprehensive assessment of abdominopelvic arteries using quadruple inversion-recovery preconditioning and 3D balanced steady-state free precession imaging. J Magn Reson Imaging. 2011;33:1430–1439. doi: 10.1002/jmri.22564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong EC, Cronin M, Wu W-C, Inglis B, Frank LR, Liu TT. Velocity-selective arterial spin labeling. Magn Reson Med. 2006;55:1334–1341. doi: 10.1002/mrm.20906. [DOI] [PubMed] [Google Scholar]
- 29.Qin Q, Shin T, Schar M, Guo H, Chen H, Qiao Y. Velocity-selective magnetization-prepared non-contrast-enhanced cerebral MR angiography at 3T. Magn Reson Med. 2015 doi: 10.1002/mrm.25764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1: Example VS-MR angiograms obtained using the previous work at 1.5 T (Ref 15). Horizontally oriented arterial segments are well visualized with VS magnetization preparation applied in the superior-inferior direction presumably due to the curvy nature of nearby vessels.