Abstract
Factors associated with HIV associated neurocognitive disorders (HAND) include CD4+ nadir and count, HIV RNA level, and HIV-1 subtype. Here, we investigated demographical and clinical markers with respect to HAND in a homogenous Chinese population. Individuals with HAND (Global Deficit Score ≥ 0.5) had lower nadir (p<0.01) and CD4+ counts (p=0.03). HAND was also associated with AIDS (p<0.01), but subtype was not (p=0.198). Furthermore, worse impairment correlated with higher viral diversity (r=0.16,p<0.01), lower nadir (r=−0.17,p<0.01) and CD4+ counts (r=−0.11,p=0.01). These remained significant even when correcting for subtype. Our findings suggest that subtype does not have a major impact on HAND.
Keywords: HIV-associated, neurocognitive disorders, CD4 nadir, neurocognitive impairment, HIV subtype
Introduction
HIV-1 can cause neurocognitive impairment in infected individuals, even among those taking effective antiretroviral therapy (ART) (Heaton et al, 2010). The underlying mechanisms by which HIV associated neurocognitive disorder (HAND) develops are not fully understood, but HAND has been associated with infecting HIV-1 subtype, nadir and current CD4+ T cell count, diversity in HIV-1 proteins Env and Tat, and blood and cerebrospinal fluid (CSF) HIV RNA levels (Ellis et al, 2011; Kaul et al, 2001; Munoz-Moreno et al, 2008).
A major challenge in understanding how HIV infection causes neurocognitive impairment is distinguishing the influence of HIV from the influence of factors in neurocognitive performance. Studies demonstrating an association of HIV-1 subtype with HAND have been performed in heterogeneous populations, and therefore may not have accounted for the influence of host genetics (Munoz-Moreno et al, 2008). When evaluating the influence of HIV-1 subtype on HAND, comparisons of study participants in the United States, where subtype B predominates, to individuals in India, where subtype C predominates, may be confounded by factors such as host genetics, endemic co-infections, education, risk factors, and other characteristics. The best approach to accounting for these factors may be to study a more homogeneous population in which more than one HIV-1 subtypes co-circulates. For this reason, we evaluated the association of HIV-1 subtype (B, C, and B/C) with HAND in two ethnically similar populations in China. This study focused on two HIV-1 genes, Env and Tat, which are known to have neurotoxic properties (Albini et al, 1998; Kruman et al, 1998; Li et al, 2009).
Materials & Methods
Study Participants and Specimens
The HIV Neurobehavioral Research Center (HNRC) at the University of California San Diego (UCSD) performed this study in collaboration with research groups in Anhui and Yunnan provinces, China. Blood-derived HIV-1 Env and Tat sequences as well as standardized, comprehensive neurocognitive (NC) evaluations with population-specific normative corrections were obtained from all participants.
HIV RNA extraction and sequencing
HIV RNA was extracted from blood plasma using the QIAmp Viral RNA Mini Kit (Qiagen, Valencia, CA) and RT-PCR performed using Transcriptor Universal cDNA Master (Roche) according to the manufacturers’ instructions. The RT-PCR used a single step continuous RT-PCR method followed by a nested PCR amplification of either HIV-1 Tat exon 1 or the C2V3 region of Env. PCR products were purified using the QIAquick PCR Purification Kit (Qiagen), and population-based sequencing was performed on an ABI Sequencer, as previously described (Heaton et al, 2010).
Neuropsychological Assessments
Study participants underwent a detailed NC assessment that has been used in several international studies and is similar to one used in the United States (Heaton et al, 2010), as previously described (Cysique et al, 2010; Gupta et al, 2014; Heaton et al, 2008). The tests were validated in Mandarin-speaking individuals within each province, using population-specific norms corrected for age, education, and sex. The Global Deficit Score (GDS) was calculated as described (Heaton et al, 2010). Participants with a GDS < 0.5 were classified as “not impaired”, whereas those with GDS ≥ 0.5 were classified as “impaired”.
Sequence Analysis
Sequences were aligned using Clustal X contained within the Bioedit software suite (Larkin et al, 2007; Thompson et al, 1994). Mixed bases in both sequences were counted to determine a mixed base index (MBI) and were resolved manually based on electropherograms (highest peak). To determine HIV-1 subtype classification, intersubtype recombination, Shannon entropy, and N-glycosylation sites we used the tools Recombinant Identification Program (RIP) 3.0 and Treemaker, Entropy, and Glycosite tools, which are offered on the Los Alamos National Laboratory (LANL) HIV Sequence Database website (http://www.hiv.lanl.gov/). Infecting HIV-1 subtype was determined based on both Tat and Env coding regions. Participants were classified as B/C if the two regions identified differently through RIP, and as either B or C in all other cases.
Statistical Analysis
We evaluated HIV-1 subtype, MBI, and clinical and demographic variables in the context of HAND using fixed-effects regression analyses. GDS was used as both a continuous and dichotomous variable. After identifying key predictors of HAND, we performed multivariate analyses and corrected for subtype in order to determine possible interaction effects. Longitudinal data were analyzed using a linear mixed-effects model adjusting for repeated measures. In the multivariate models, predictors of HAND were selected by step-wise regression, eliminating each predictor or interaction effect that were not significant as part of the model.
Results
Study cohort and characteristics
NC assessment was performed on 308 HIV-infected subjects in Anhui and Yunnan, China (n = 124 and 184, respectively). Each patient had up to five repeat visits (n=1117 total visits). For each subject, infection duration and time since initial diagnosis were reported. Infection duration was estimated to be the time since an identified exposure point. Initial HIV diagnosis refers to the time since an identified exposure point to the virus.
Of these participants, 226 (73.4%) exhibited NC impairment, and 82 (26.6%) had a GDS consistent with HAND. Based on Env and Tat sequence analyses, 111 participants were infected with subtype B (all from Anhui), 75 with subtype B/C (13 from Anhui, 62 from Yunnan), and 122 with subtype C (all from Yunnan). Most of the subjects were men (64.6%), and the median age of the study population was 36 years. At baseline, the median CD4+ T cell count was 392 cells/µL, the median nadir CD4+ count was 260 cells/µL, and the median HIV-1 RNA level was 3.9 log10 copies/mL. Most participants (76%) had a detectable viral load and were co-infected with Hepatitis C (HCV, 91%), with a majority (63%) not receiving ART (Table 1).
Table 1.
Participant Baseline Characteristics
| Characteristic | Overall (n = 308) | Not impaired (n=226) |
Impaired (n=82) |
p- value |
|---|---|---|---|---|
| Region (% Yunnan) | 184 (60%) | 134 (62%) | 41 (51%) | 0.09 |
| Age (years) | 36 (32 – 40) | 36 (33 – 36.75) | 36 (31 – 39.75) | 0.53 |
| Education (years) | 9 (6 – 9) | 9 (6 – 9) | 9 (4 – 9) | 0.09 |
| Subtype (%) | ||||
| B | 111 (36%) | 75 (33%) | 36 (44%) | |
| C | 122 (40%) | 95 (42%) | 27 (33%) | |
| B/C | 75 (24%) | 56 (25%) | 19 (23%) | 0.20 |
| Sex, no. (%) male | 199 (65%) | 148 (65%) | 51 (62%) | 0.60 |
| AIDS Status (% with) | 131 (43%) | 84 (37%) | 47 (57.3%) | <0.01 |
| ART Status (%) | ||||
| On | 114 (37%) | 75 (33%) | 39 (48%) | |
| Off | 195 (63%) | 150 (67%) | 43 (52%) | 0.02 |
| HCV Status (%) | ||||
| Positive | 281 (91%) | 206 (91%) | 75 (91%) | |
| Negative | 13 (4%) | 7 (3%) | 6 (7%) | |
| Unknown | 14 (5%) | 13 (6%) | 1 (1%) | 0.07 |
| Mixed Base Index (MBI) | 0.18 (0.16 – 0.19) | 0.17 (0.16 – 0.19) | 0.18 (0.17 – 0.19) | 0.05 |
| CD4+ nadir (cells/µL) | 260 (163.5 – 425.5) | 287.0 (185.5 – 451.5) | 197.5 (117.5 – 265.3) | <0.01 |
| CD4+ count (cells/µL) | 392.0 (247.8 – 536.5) | 407.5 (270.0 – 536.2) | 359.5 (199.8 – 515.8) | 0.03 |
| HIV RNA levels (log10 copies/µL) | 3.9 (2.3 – 4.5) | 4.0 (2.7 – 4.5) | 3.6 (1.8 – 4.4) | 0.19 |
| Viral Load (% detectable) | 235 (76%) | 174 (77%) | 61 (74%) | 0.67 |
| Infection Duration (years) | 8.1 (3.7 – 12.4) | 7.4 (3.3 – 12.3) | 9.3 (5.4 – 12.6) | 0.06 |
| Initial HIV Diagnosis (years) | 1.8 (0.8 – 2.9) | 1.9 (0.7 – 2.9) | 2.1 (0.96 – 3.1) | 0.11 |
Baseline characteristics of our study participants. Medians with IQR or values with percentages are shown for the entire group and by groups defined by neurocognitive status. P-values were calculated using two-tailed Mann-Whitney or Fisher tests. P-values are significant at the 0.05 level are bolded and those significant at the 0.10 level are in red.
HIV subtype associations with HAND
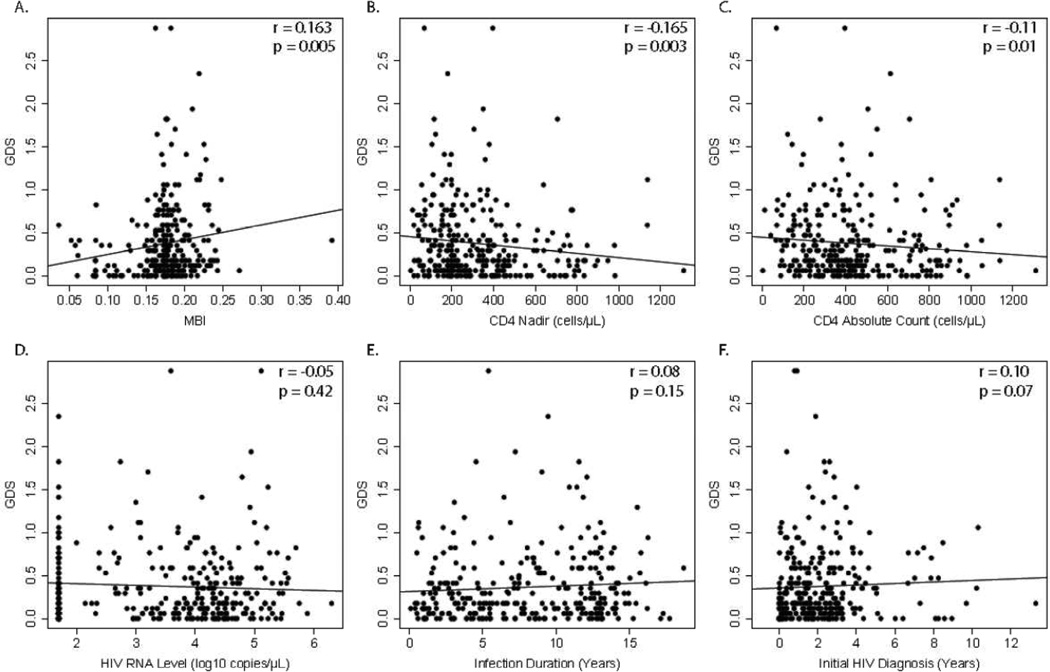
Impaired and not impaired groups did not differ in sex, age, education, HIV RNA levels, or initial HIV diagnosis at baseline (Table 1). HIV-1 subtypes were not associated with prevalence of HAND (Fisher exact test, p = 0.198). However, HAND was associated with AIDS (57% vs. 37%, p = 0.002), ART status (48% vs. 33%, p = 0.02), viral diversity (0.1744 vs. 0.1742 bases/unit length, p = 0.05), and lower nadir (265.3 vs. 345.3 cells/µL, p = 0.001) and current CD4+ cell count (390.5 vs. 445.4 cells/µL, p = 0.034) (Table 1). Analyzing GDS as a continuous variable, worse NC performance correlated with greater viral diversity (r = 0.16, p = 0.005), longer time since initial HIV diagnosis (r = 0.10, p = 0.06), and lower CD4+ nadir (r = −0.17, p = 0.003) and current CD4+ counts (r = −0.11, p = 0.01) at baseline (Figure 1).
Figure 1.
Worse neurocognitive performance was associated with higher HIV diversity, lower CD4+ counts, and lower CD4 nadir+. There was no association of neurocognitive performance with HIV RNA levels, estimated infection duration, and time since initial HIV diagnosis.
When stratifying analyses by geographical region, greater viral diversity (r = 0.22, p = 0.004) and lower CD4+ nadir (r = −0.195, p = 0.008) remained associated with worse NC performance in Yunnan. In this sub-analysis of a larger, previously published analysis, no clinical variables were associated with NC performance in Anhui (Heaton et al, 2008). In our multivariate analyses by step-wise regression including all variables, we found that higher MBI (p = 0.01), lower CD4+ nadir (p = 0.02), and HCV (p = 0.02) status were predictors of HAND when correcting for geographical region. HIV-1 status was not a significant predictor for HAND. Furthermore, in a sub-analysis including all visits from all participants, higher viral diversity (MBI, p = 0.0001) and ART status (p = 0.02) were significantly correlated to lower GDS scores, while CD4+ nadir became not significant. CD4+ nadir, initial diagnosis, and estimated duration of infection exhibited a significant interaction effect (p < 0.01) as well as including MBI (p < 0.01) in the interaction effect.
Discussion
Multiple factors have been associated with HAND in adults infected with HIV-1 B and non-B subtype infections, and infecting subtypes have been implicated in both the frequency and the severity of HAND (de Almeida et al, 2013). Studies of HIV-1 subtype, however, often evaluated non-homogeneous populations, usually from different countries, an approach that may have introduced confounding factors due to the many inherent differences between such populations.
Our neuropsychological assessments were normed on the basis of large HIV-uninfected control groups from each province and with the same risk factors (former plasma donors in Anhui and injection drug users in Yunnan). Among HIV-infected subjects, we did observe those with AIDS were more likely to be impaired than those without AIDS, using data from the HIV-uninfected population to generate region-specific normative data.
Impairment rates did vary slightly by HIV-1 subtype, with subtypes B being slightly more impaired than the C and B/C groups (31.8%, 23.9%, and 25.3%, respectively), but unlike other studies comparing subtype and impairment, the differences in HAND prevalence between the different subtypes circulating in these Chinese cohorts were not statistically significant. Furthermore, the observed impairment level in the population (26.6%) was considerably lower than in other studies (40% to 60%) (de Almeida et al, 2013; Gupta et al, 2007; Yepthomi et al, 2006).
Our study identified key, previously reported biomarkers that have been associated with HAND, including CD4+ T cell count, CD4+ nadir, and viral diversity (Ellis et al, 2011; Hightower et al, 2012). Two of these, nadir CD4+ T cell count and viral diversity, remained significantly correlated to GDS, even when correcting for subtype. This means that the HIV-1 infecting subtype likely did not play a significant role in the biological determination of impairment in these relatively homogeneous populations.
In conclusion, HIV-1 infecting subtype was not associated with different rates of HAND in an ethnically homogeneous Chinese population with multiple circulating HIV-1 subtypes. Our findings support a previously published report, which demonstrated that previous AIDS diagnoses, lower CD4+ count, lower CD4+ nadir, and higher viral diversity were significantly associated with worse neurocognitive impairment (Hightower et al, 2012). These findings indicate that advanced HIV infection and progression to AIDS have a higher impact on neurocognitive function than HIV-1 infecting subtype.
Acknowledgements
Thank you to all participants of the study and to the collaborating authors for all of their hard work and support.
Financial Disclosure
This work was supported by the Department of Veterans Affairs and grants from the National Institutes of Health: AI100665, MH097520, DA034978, MH083552, AI036214, MH062512; the James B. Pendleton Charitable Trust.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci U S A. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Letendre SL, Ake C, Jin H, Franklin DR, Gupta S, Shi C, Yu X, Wu Z, Abramson IS, Grant I, Heaton RK group HIVNRC. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010;24:983–990. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida SM, Ribeiro CE, de Pereira AP, Badiee J, Cherner M, Smith D, Maich I, Raboni SM, Rotta I, Barbosa FJ, Heaton RK, Umlauf A, Ellis RJ. Neurocognitive impairment in HIV-1 clade C- versus B-infected individuals in Southern Brazil. J Neurovirol. 2013;19:550–556. doi: 10.1007/s13365-013-0215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Badiee J, Vaida F, Letendre S, Heaton RK, Clifford D, Collier AC, Gelman B, McArthur J, Morgello S, McCutchan JA, Grant I, Group C. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25:1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta JD, Satishchandra P, Gopukumar K, Wilkie F, Waldrop-Valverde D, Ellis R, Ownby R, Subbakrishna DK, Desai A, Kamat A, Ravi V, Rao BS, Satish KS, Kumar M. Neuropsychological deficits in human immunodeficiency virus type 1 clade C-seropositive adults from South India. J Neurovirol. 2007;13:195–202. doi: 10.1080/13550280701258407. [DOI] [PubMed] [Google Scholar]
- Gupta S, Iudicello JE, Shi C, Letendre S, Knight A, Li J, Riggs PK, Franklin DR, Jr, Duarte N, Jin H, Hampton Atkinson J, Yu X, Wu Z, Grant I, Heaton RK Group HCC. Absence of neurocognitive impairment in a large Chinese sample of HCV-infected injection drug users receiving methadone treatment. Drug Alcohol Depend. 2014;137:29–35. doi: 10.1016/j.drugalcdep.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Cysique LA, Jin H, Shi C, Yu X, Letendre S, Franklin DR, Ake C, Vigil O, Atkinson JH, Marcotte TD, Grant I, Wu Z San Diego HIVNRCG. Neurobehavioral effects of human immunodeficiency virus infection among former plasma donors in rural China. J Neurovirol. 2008;14:536–549. doi: 10.1080/13550280802378880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower GK, Wong JK, Letendre SL, Umlauf AA, Ellis RJ, Ignacio CC, Heaton RK, Collier AC, Marra CM, Clifford DB, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Grant I, Little SJ, Richman DD, Kosakovsky Pond SL, Smith DM, Group CS. Higher HIV-1 genetic diversity is associated with AIDS and neuropsychological impairment. Virology. 2012;433:498–505. doi: 10.1016/j.virol.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res. 2009;16:205–220. doi: 10.1007/s12640-009-9047-8. [DOI] [PubMed] [Google Scholar]
- Munoz-Moreno JA, Fumaz CR, Ferrer MJ, Prats A, Negredo E, Garolera M, Perez-Alvarez N, Molto J, Gomez G, Clotet B. Nadir CD4 cell count predicts neurocognitive impairment in HIV-infected patients. AIDS Res Hum Retroviruses. 2008;24:1301–1307. doi: 10.1089/aid.2007.0310. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepthomi T, Paul R, Vallabhaneni S, Kumarasamy N, Tate DF, Solomon S, Flanigan T. Neurocognitive consequences of HIV in southern India: a preliminary study of clade C virus. J Int Neuropsychol Soc. 2006;12:424–430. doi: 10.1017/s1355617706060516. [DOI] [PubMed] [Google Scholar]