Abstract
The aim of the present study was to examine a potential mechanism of action of gabapentin to manage cannabis-use disorders by determining the interoceptive effects of gabapentin in cannabis users discriminating Δ9-THC using a pharmacologically selective drug-discrimination procedure. Eight cannabis users learned to discriminate 30 mg oral Δ9-THC from placebo and then received gabapentin (600 and 1200 mg), Δ9-THC (5, 15 and 30 mg) and placebo, alone and in combination. Self-report, task performance and physiological measures were also collected. Δ9-THC served as a discriminative stimulus, produced positive subjective effects, elevated heart rate and impaired psychomotor performance. Both doses of gabapentin substituted for the Δ9-THC discriminative stimulus and engendered subjective and performance-impairing effects that overlapped with those of Δ9-THC when administered alone. When administered concurrently, gabapentin shifted the discriminative-stimulus effects of Δ9-THC leftward/upward, and combinations of Δ9-THC and gabapentin generally produced larger effects on cannabinoid-sensitive outcomes relative to Δ9-THC alone. These results suggest that one mechanism by which gabapentin might facilitate cannabis abstinence is by producing effects that overlap with those of cannabinoids.
Keywords: drug-discrimination, cannabis, marijuana, subjective effects, repeated acquisition task, digit-symbol-substitution task, cardiovascular, human
Introduction
Gabapentin, a γ-aminobutyric acid (i.e., GABA) analog that is indicated for the treatment of neuropathic pain and seizures, has recently emerged as a promising candidate for management of cannabis-use disorder in adults. In an initial pilot study, 50 individuals seeking treatment for cannabis dependence received 1200 mg/day gabapentin across a 12-week randomized, double-blind, placebo-controlled clinical efficacy trial (Mason et al., 2012). Gabapentin treatment decreased cannabinoid (CB) metabolite levels in the urine, self-reported cannabis use, scores and on craving and depression questionnaires, and improved performance on tests of executive function, relative to placebo. In light of these positive findings, and considering that there are no currently approved pharmacotherapies for cannabis-use disorder, exploration of the mechanisms by which gabapentin functioned as an effective pharmacotherapeutic is needed to inform future medication development efforts.
The pharmacological mechanism of action for gabapentin has been linked primarily to voltage-dependent calcium channels (VDCCs), specifically those containing α2δ subunits (Sills, 2006). VDCCs are found both centrally and throughout the periphery, and the α2δ subunit is found across the different VDCC types (Arikkath and Campbell, 2003). Although the consequences of ligand binding to the α2δ subunit have not been fully established, there appears to be a direct impact on calcium conductance, as well as VDCC trafficking, with the overall result being a dampening of neuronal activity, which in turn impacts the release of various neurotransmitters (see Gale and Houghton, 2011). Cannabinoid agonists also affect VDCC function. One of the main consequences of CB-receptor mediated G-protein activation is the inhibition of VDCCs (Howlett et al., 2010). In addition, there is evidence for a CB-receptor-independent modulation of VDCCs by CB ligands, either through interaction with the plasma membrane lipid bilayer or by direct interaction with a binding site on the ion channel (Lozovaya et al., 2009). Also worth noting is that a primary role of endogenous CBs is to modulate the release of other neurotransmitters (Szabo and Schlicker, 2005), which is also a well-characterized function of VDCCs.
Only one preclinical study appears to have examined the potential use of a VDCC ligand for cannabis-use disorders (Aracil-Fernández et al., 2013). In that murine study, the effects of the high-efficacy CB agonist CP-55,940 on brain gene transcription and motor and anxiety-like behavior were determined during spontaneous CB withdrawal. Pregabalin, considered a “next-generation” VDCC ligand due to its improved pharmacokinetic profile and greater bioavailability, decreased anatomically specific changes in the synthesis of certain proteins thought to be involved in cannabis dependence (i.e., tyrosine hydroxylase in the ventral tegmental area and CB1 receptors in the nucleus accumbens) and attenuated the motor and anxiety-like responses induced by cannabinoid agonist cessation. Other research that has concurrently evaluated cannabinoids and gabapentinoids using in vivo methods is limited and has consisted mainly of studies using preclinical pain models. Those studies have generally found that gabapentin, pregabalin, cannabinoid direct agonists and endogenous cannabinoid metabolic enzyme inhibitors function as analgesics under the same experimental pain conditions (e.g., Hasnie et al., 2007; Luszczki and Florek-Luszcki, 2012; Wallace et al., 2007).
One clinical study (Bestard and Toth, 2011) directly compared nabilone, a non-selective CB agonist, with gabapentin in an open label study in neuropathic pain patients. Relative to baseline, pain, sleep and anxiety outcomes were similarly improved by gabapentin and nabilone following 3- and 6-months of treatment. Cross-study comparisons have also demonstrated the ability of CB agonists and VDCC ligands to improve outcomes related to pain, sleep and anxiety (e.g., de-Paris et al., 2003; Hindmarch et al., 2005; Karst et al., 2010; Nakano et al., 1978; Roth et al., 2012; Saldaña et al, 2012; Ware et al., 2010). Important to note is that sleep difficulties, anxiety and physical discomfort are commonly observed during cannabis abstinence and are often reported as reasons for continued use (e.g., Budney et al., 2008; Copersino et al., 2006).
Taken together, these studies demonstrate substantial neuropharmacological and therapeutic overlap between VDCC ligands and cannabinoids that might contribute to the ability of gabapentin to manage cannabis-use disorders. To further investigate potential similarities, particularly as they relate to the abuse-related interoceptive effects of cannabis, the present study determined the separate and combined effects of gabapentin and Δ9-tetrahydrocananbinol (i.e., Δ9-THC, the primary active constituent of cannabis) using pharmacologically selective drug-discrimination procedures (Lile et al., 2009, 2010, 2012, 2014). Gabapentin was hypothesized to occasion Δ9-THC-like discriminative-stimulus alone and shift the discriminative-stimulus effects of Δ9-THC leftward/upward when administered concurrently. Secondary outcomes were also collected and included subject-rated, psychomotor performance, cardiovascular and thermoregulatory measures.
Methods
Subjects
Adult men and women reporting weekly cannabis use were recruited from the local community. Potential subjects completed demographic, drug-use and medical history questionnaires, as well as medical screens. Individuals with current or past histories of Axis I disorders according to DSM-IV criteria (American Psychiatric Association, 2000), including substance dependence other than tobacco, were excluded from participating. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document.
Eight subjects (4 white females, 4 white males), reporting cannabis use between 2 to 7 days/week (mean = 5), completed the protocol. Subjects were between 18 and 31 years old (median = 23 years) and weighed between 60 to 92 kg (median = 71 kg). Five reported regular alcohol consumption (range = 1–18 drinks per week). Six subjects reported tobacco use (cigarettes, n = 5; smokeless n = 1) 1 or 2 times per day (n = 3), 1 or 2 times per week (n = 2) or 15–20 per day (n = 1). Other drug use was endorsed, but rarely in the month prior to screening. During screening, subjects provided a urine sample that was assessed for recent use of amphetamine, cocaine, cannabis, methamphetamine, opioids, phencyclidine, benzodiazepines, barbiturates, methadone and 3,4-methylenedioxy-N-methamphetamine (Integrated E-Z Split Cup II, Acon Laboratories, San Diego, CA); all subjects provided a urine sample positive for recent use of cannabis and negative for other substance use prior to beginning the study.
General Procedures
Subjects completed two drug-free practice sessions prior to 21 to 26 (mean = 23) experimental sessions conducted over 8 to 18 weeks (mean = 11).
Subjects were informed that they would receive orally administered placebo, Δ9-THC and gabapentin, alone or in combination, but were blind to the dose and order of administration. They were asked to refrain from cannabis, caffeine or food intake on the morning of each experimental session, and alcohol for 12 h prior to and following each experimental session. Subjects were also asked to abstain from illicit drugs other than cannabis or medications, other than non-steroidal anti-inflammatory analgesics, for the duration of the experiment. Urine toxicology screening was positive for recent drug use other than cannabis on two occasions; the subjects were discharged from that session, but were permitted to continue study participation once a urine sample negative for all non-cannabinoid drugs was provided. The subject who smoked tobacco cigarettes daily was also asked to abstain from smoking the morning of each session, but was allowed to smoke a single tobacco cigarette upon arrival to the laboratory.
Experimental sessions lasting 6.5 h were conducted at a fixed time, Monday through Friday, with 1 to 5 sessions occurring per week. At the beginning of each session, subjects completed a questionnaire that asked about recent drug use, health and changes in daily routine. Breath (Alcolyzer, AK Solutions USA, Palisades Park, NJ) and urine tests to assess drug use (described above) and pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK) were conducted. Subjects also completed field sobriety assessments and were observed by the research staff for signs of cannabis intoxication (e.g., bloodshot, glassy eyes); no cannabis intoxication was detected during intake throughout the study. Subjects were reassessed at the end of the session for possible intoxication and/or residual drug effects, and were required to report no further drug effects, prior to release.
Drug-Discrimination Procedure
A well-established “Drug X” (i.e., 30 mg Δ9-THC) versus “Not Drug X” (i.e., placebo) drug-discrimination procedure was used (e.g., Lile et al., 2009; Rush et al., 1998). Oral Δ9-THC was chosen as the training condition to eliminate the external cues and/or expectations associated with smoked cannabis. In a previous study in which human subjects learned to discriminate smoked cannabis containing an active concentration of Δ9-THC (2.7%) from placebo cannabis, subjects based their discrimination responding, at least in part, on the taste or harshness of the cannabis cigarette (Chait et al., 1988). Importantly, although smoked cannabis is more typically used, research that directly compared smoked cannabis with oral Δ9-THC demonstrated that, although the time course of the drug effects differed, the profile of behavioral and physiological effects was similar (Chait and Zacny, 1992; Hart et al., 2002; Wachtel et al., 2002).
Sampling Phase
During two sampling sessions, subjects ingested capsules that contained a total of 30 mg Δ9-THC. The capsules were identified by a letter code (e.g., Drug X; a unique letter code was used for each subject); subjects were not informed that the capsules contained Δ9-THC, but were instructed to associate drug effects with the letter code.
Control Phase
A double-blind control phase, lasting between 4 and 12 sessions, was conducted to determine whether subjects could discriminate 30 mg Δ9-THC from placebo. The order of administration of the two control conditions (i.e., 30 mg Δ9-THC and placebo) was randomized in 4-session blocks containing two sessions of each control condition. Sessions were identical to the sampling phase, except that subjects were not informed which drug condition (i.e., Drug X or Not Drug X) was administered until the end of the session. The criterion for having acquired the discrimination was ≥ 80% correct responding (i.e., percent of total number of responses allocated to the correct response option) during the final 5-h assessment on the drug-discrimination task described below, for four consecutive sessions.
Test Phase
A test phase was conducted in which placebo, Δ9-THC (5, 15 and 30 mg) and gabapentin (600 and 1200 mg) were tested, alone and in combination, under double-blind conditions. Each drug dose and dose combination was administered once for a total of 12 sessions. Four control sessions (i.e., 30 mg Δ9-THC or placebo) were also included in the test phase to monitor drug-discrimination performance and provide feedback to subjects regarding their performance. The order of drug administration for the 12 test sessions and four control sessions was initially randomized and then edited to avoid administering active drug dose on more than three consecutive sessions (i.e., placebo was administered at least every three sessions), and to ensure that the highest doses of Δ9-THC (30 mg) and gabapentin (1200 mg) were not administered together before a lower dose combination was tested. If a subject responded incorrectly on a control session (i.e., < 80% correct responding), additional control sessions were scheduled until the subject accurately identified both of the training conditions once each across consecutive sessions.
Outcome measures
Data were collected in fixed order, immediately prior to drug administration, and 1, 2, 3, 4 and 5 h after Δ9-THC administration, with the following exceptions: The drug-discrimination task was completed at only the 3 to 5 h time points because of the slow onset of the effects of oral Δ9-THC (e.g., Lile et al., 2013); and a non-contingent Multiple-Choice Procedure (see below) was completed at the end of the 5-h assessment.
Drug-Discrimination Task
Two circles labeled Drug X and Not Drug X, and associated counters, were displayed on a computer screen. Button presses increased the counter for a particular circle according to a fixed-interval 1-sec schedule for 60 s. At the end of the final assessment, subjects were informed whether it was a control or a test session. During control sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.28/point (up to approximately $50.00/session). During test sessions, when drugs and/or doses other than the control conditions were administered, subjects earned the average from all previous sessions in which control conditions were tested. The dependent variable for this task was the percent responding on the drug-appropriate option at the 5-h time point.
Subject-Rated Questionnaires
Visual Analog Scale (VAS) Subject-Rated Drug-Effect Questionnaire:. Subjects rated 20 items (see Lile et al., 2012) that assessed general “positive” (e.g., Good Effects, Like Drug) and “negative” (e.g., Bad Effects, Nauseated) drug effects, as well as items specific to cannabis intoxication (e.g., Stoned). Items were presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”.
Multiple-Choice Procedure:. This task provided a hypothetical assessment of the monetary value of each dose condition (e.g., Greenwald et al., 2008). Subjects made a series of nine discrete choices between the drug dose received during that session and ascending amounts of money. The dollar value increased across the choices (USD $0.10, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00 and 32.00). The dependent measure on the Multiple-Choice Procedure was the maximum dollar value at which subjects chose drug over money (i.e., “crossover point”).
Performance Tasks
Repeated Acquisition of Response Sequences Task (RA task): During the initial acquisition component, subjects pressed 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a 10-response sequence (a “chain”) for 180 s. A new chain was randomly generated each time the task was presented, both within and across sessions. When a correct key in the sequence was pressed, a “position” counter on the screen increased by 1. When the tenth and final key in the sequence was pressed, a “points” counter increased by one, and the position counter reset. A 60-s performance component of this task, in which the 10-response sequence remained the same across all presentations of the task throughout the experiment, followed the acquisition component. The primary dependent measures for this task were the number of chains completed (i.e., accuracy) and the total number of responses emitted (i.e., response rate).
Digit-Symbol-Substitution Test (DSST): A modified version of the computerized DSST was used. Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns identified on a given trial for 90 s. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct; accuracy) and the total number of patterns entered (i.e., trials completed; response rate).
Time Reproduction Task: Four time periods, 3, 30, 60 and 180s were presented. Subjects responded to start a timer, and held down the response key until they believed that the interval had elapsed.
Physiological Indices
Cardiovascular: Heart Rate and Blood Pressure were recorded using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
Temperature: An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. An electronic thermometer was used to measure oral temperature.
Drug Administration
Subjects consumed a low-fat snack approximately 20 minutes prior to drug administration. Doses of Δ9-THC were prepared by encapsulating commercially available capsules of Marinol® (Δ9-THC in sesame oil, Solvay Pharmaceuticals Inc., Marietta, GA) and a generic formulation of gabapentin (Ascend Laboratories, Montvale, NJ) in opaque green size 00 capsules. For reference, the acute recommended Δ9-THC dosing range in adults for appetite stimulation and the prevention of nausea and vomiting is 2.5 to 20 mg (Marinol® product information, 2006). The recommended starting therapeutic oral dose of gabapentin is 300–400 mg, administered three times a day, and titrated up to a clinically effective maintenance dose of 1800–3600 mg/day (Gabapentin product information, 2011). However, single-dose clinical research studies have administered 1200 mg, the maximum gabapentin dose administered in this study, on an acute basis without dose titration (e.g., Gordi et al., 2008). Gabapentin and Δ9-THC were administered concurrently, consistent with the onset of their peak concentrations following oral dosing (Gidal et al., 1998; Lile et al., 2013). The use of oral Δ9-THC and gabapentin in this study was for investigational purposes.
Data Analyses
Drug-discrimination data were analyzed as percent drug-appropriate responding using two-factor, repeated-measure analysis of variance (ANOVA; JMP, SAS Institute Inc., Cary, NC) with Δ9-THC and gabapentin as the factors. For the 30 mg Δ9-THC and placebo conditions, data were averaged across the sessions in which these conditions were presented during the test phase. Raw data from the self-reported drug-effect questionnaires, performance tasks and physiological measures were analyzed for each drug as the peak-effect (i.e., the mean of the maximum or minimum value observed for each subject 1 to 5 h after drug administration). Crossover point data from the Multiple-Choice Procedure were first subjected to a square-root transformation because of violations in the assumptions of ANOVA. For all measures, effects were considered significant for p ≤ 0.05. If a main effect of Δ9-THC attained statistical significance, contrast statements were used to compare active drug doses to placebo; if a main effect of gabapentin, or an interaction of Δ9-THC and gabapentin, attained statistical significance, each dose of Δ9-THC alone was compared to that dose of Δ9-THC administered in combination with gabapentin.
Results
Drug-discrimination task
Subjects met the discrimination criterion in an average of 6 sessions (range = 4 to 9). During the final four sessions of the control phase, subjects reported an average of 0.0 (SEM = 0.0) percent Δ9-THC-appropriate responding on the drug-discrimination task during placebo sessions and 99.5 (SEM = 0.5) percent drug-appropriate responding during sessions when the training dose of Δ9-THC (i.e., 30 mg) was administered.
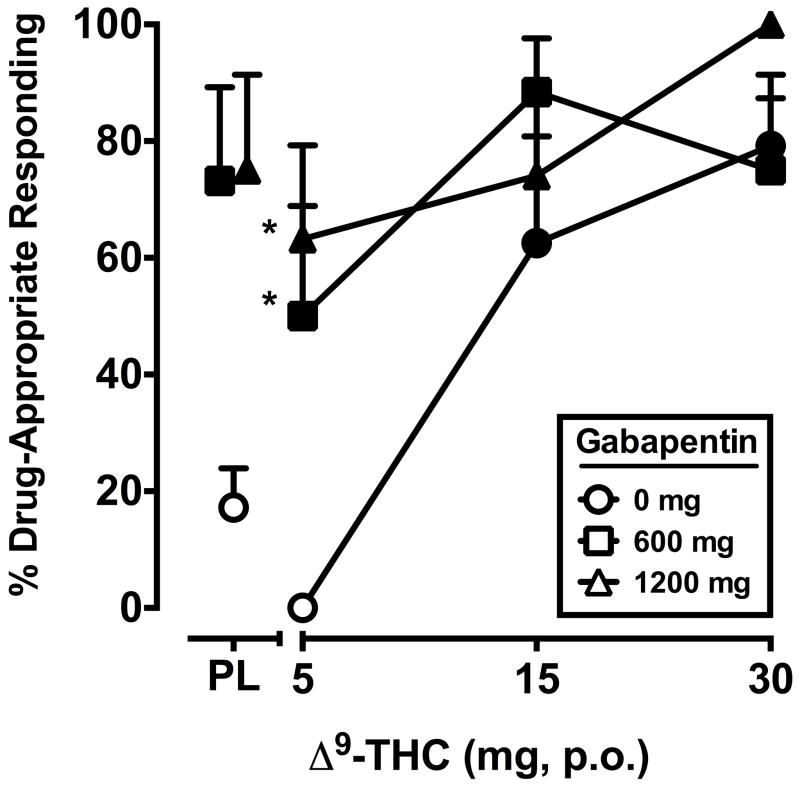
The two-factor, repeated-measure ANOVA revealed a significant interaction of Δ9-THC and gabapentin (F6,42 = 2.7, p < 0.05) for percentage of Δ9-THC-appropriate responding. The discriminative-stimulus effects of Δ9-THC, alone and in combination with gabapentin, are shown in Figure 1. During the test phase, placebo and the training dose of Δ9-THC occasioned an average of 17.2 (SEM = 6.7) and 79.2 (SEM = 8.1) percent Δ9-THC-appropriate responding, respectively. Δ9-THC alone dose-dependently increased drug-appropriate responding on the drug-discrimination task. The 600 and 1200 mg doses of gabapentin alone also significantly increased drug-appropriate responding, and significantly shifted the discriminative-stimulus effects of the 5 dose of Δ9-THC leftward/upward.
Figure 1.
Separate and combined effects of Δ9-THC and gabapentin on Δ9-THC-appropriate responding on the drug-discrimination task. Filled symbols indicate values that are significantly different from placebo. Asterisks indicate combinations of Δ9-THC and gabapentin that are significantly different from that dose of Δ9-THC alone. The x-axis represents the Δ9-THC dose in mg; PL denotes placebo. Data points show means of 8 subjects. Uni-directional brackets indicate 1 SEM.
Subjective Ratings
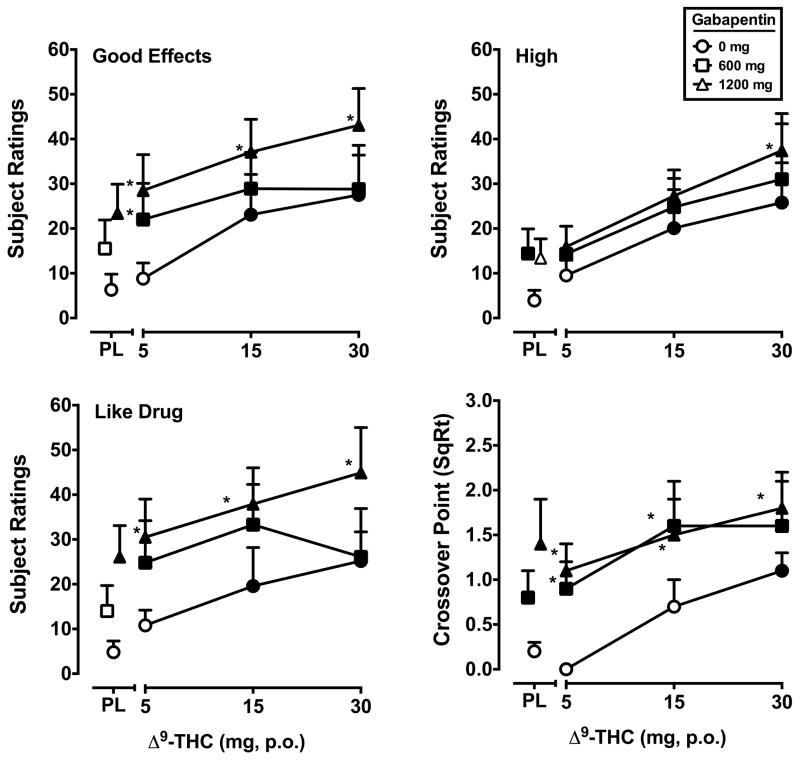
Signficant main effects of Δ9-THC (F’s3,21 = 3.6–6.7, p’s < 0.05) and gabapentin (F’s2,14 = 6.2–20.9, p’s < 0.05) were detected for five VAS items: Any Effect, Good Effects*, High*, Like Drug* and Stoned (data from items marked with an asterisk* are presented in Figure 2). In general, at least one dose of both drugs alone increased ratings on these items, and the effects of the combination of Δ9-THC and gabapentin were significantly greater compared to Δ9-THC alone. The subject-rated effects of the combinations were frequently a function of dose (i.e., the combination of 1200 mg tiagabine and Δ9-THC produced larger effects than 600 mg tiagabine combined with the same Δ9-THC dose). In addition, the VAS item Thirsty was significantly increased by Δ9-THC (F3,21 = 5.8, p’s < 0.01) regardless of gabapentin administration.
Figure 2.
Peak (maximum value) Visual Analog Scale ratings for Δ9-THC and gabapentin, alone and in combination, on the drug-effect questionnaire items Good Effects, High, Like Drug and crossover point values (square root transformation) from a Multiple Choice Procedure. All other details are as in Figure 1.
Signiifcant main effects of gabapentin only (F’s2,14 = 3.8–8.1, p’s < 0.05) were found for the items Sedated, Take Again, Pay For, Dizzy/Lightheaded and Forgetful. For the items Sedated, Take Again and Pay For, at least one dose of gabapentin alone increased subject ratings relative to placebo, and the combined effects of gabapentin and Δ9-THC were significantly larger than those of that dose of Δ9-THC alone. For the item Dizzy/Lightheaded, both gabapentin doses increased subjective ratings when combined with 5 mg Δ9-THC, but did not engender a subjective response when administered alone. Despite a significant main effect of gabapentin, ratings of Forgetful appeared unrelated to gabapentin dose.
Multiple-Choice Procedure
Significant main effects of Δ9-THC (F3,21 = 10.6, p < 0.001) and gabapentin (F2,14 = 7.6, p < 0.01) were found for the crossover point. Δ9-THC alone increased crossover point relative to placebo at the 30 mg dose. The mean dollar value associated with the crossover point at the 30 mg Δ9-THC was USD $1.6 ($0.4 SEM) compared to $0.1 ($0.1) for placebo. The 600 and 1200 mg dose of gabapentin, alone and combined with Δ9-THC, significantly increased crossover point compared to placebo. Combinations of Δ9-THC and gabapentin also increased crossover point relative to Δ9-THC alone under most dose conditions. The mean dollar value associated with the crossover point for 600 mg gabapentin, alone and when administered with each Δ9-THC dose, in escalating order, was $1.1 ($0.5), $1.4 ($0.6), $4.0 ($2.0) and $4.1 ($1.9). The mean dollar value associated with the crossover point for 1200 mg gabapentin, alone and when administered with each Δ9-THC dose, in escalating order, was $3.6 ($2.0), $2.0 ($1.0), $3.2 ($1.1) and $4.1 ($1.2).
Performance
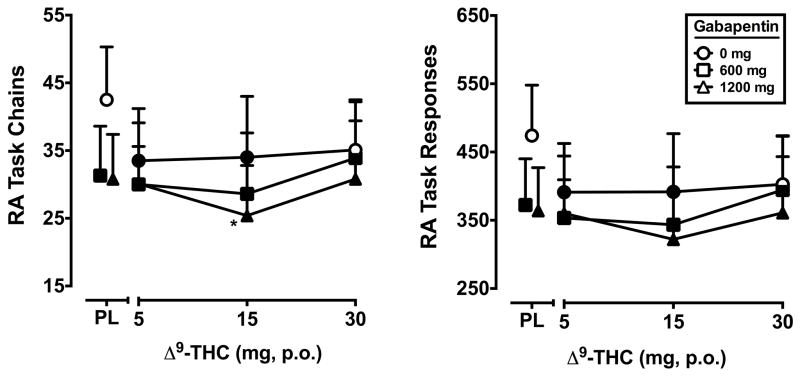
Significant main effects of Δ9-THC (F3,21 = 3.8, p ≤ 0.05) and gabapentin (F2,14 = 4.5, p < 0.01) were found for the number of chains completed on the acquisition component of the RA task. Likewise, significant main effects of Δ9-THC (F3,21 = 3.3, p ≤ 0.05) and gabapentin (F2,14 = 4.2, p < 0.05) were found for the total number of responses emitted. Relative to placebo, performance on these outcomes was impaired by the 600 and 1200 mg doses of gabapentin alone, the 5 and 15 mg doses of Δ9-THC alone, and by all active Δ9-THC doses when combined with gabapentin. The 1200 mg dose of gabapentin alone also reduced response rate and accuracy relative to placebo and further impaired performance when combined with the 15 mg Δ9-THC dose. The effects of Δ9-THC and gabapentin on rate and accuracy on the acquisition component of the RA task are presented in Figure 3. Rate and accuracy on the performance component of the RA task were not significantly impacted by Δ9-THC or gabapentin (data not shown).
Figure 3.
Peak number of chains completed and total responses during the acquisition component of the repeated acquisition task (minimum value) for Δ9-THC and gabapentin, alone and in combination. All other details are as in Figure 1.
ANOVA revealed a significant main effect only of gabapentin (F2,14 = 4.7, p < 0.05) to reduce the number of correct trials on the DSST (data not shown). The 1200 mg dose of gabapentin impaired accuracy alone and when combined with every active dose of Δ9-THC, compared to placebo. In addition, accuracy was impaired following administration of 600 mg gabapentin combined with 15 or 30 mg Δ9-THC. A significant main effect only of Δ9-THC (F3,21 = 4.3, p < 0.05) was observed for DSST trials completed (data not shown). The 1200 mg gabapentin + 15 mg Δ9-THC condition was the only dose combination that impaired rate relative to placebo.
Gabapentin resulted in an under-reproduction of the 3-s (600 and 1200 mg; F2,14 = 7.4, p < 0.01) and 30-s (1200 mg; F2,14 = 4.5, p < 0.05) time intervals (data not shown). Significant main effects of gabapentin (F2,14 = 10.7, p < 0.01) and Δ9-THC (F3,21 = 3.7, p < 0.05) were found for the 60-s interval (data not shown). At that interval, administration of the 1200 mg dose of gabapentin alone and in combination with all Δ9-THC doses, as well as the 600 mg gabapentin + 30 mg Δ9-THC combination, resulted in an under-reproduction of time, compared to when placebo was administered. Neither gabapentin or Δ9-THC affected reproduction of the 180-s time interval (data not shown).
Heart Rate, Blood Pressure and Temperature
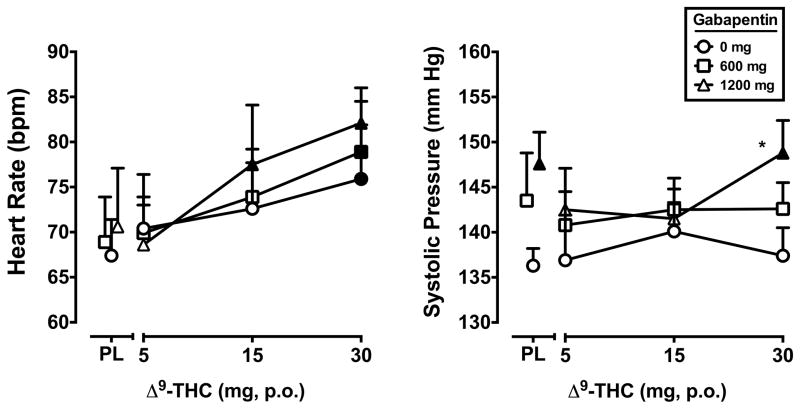
A significant main effect of Δ9-THC was found for heart rate (F3,21 = 9.9, p < 0.001). The 30 mg dose of Δ9-THC increased heart rate regardless of gabapentin dose, and the 15 mg dose of Δ9-THC + 1200 mg gabapentin dose condition increased heart rate, relative to placebo (Figure 4). Systolic blood pressure was significantly elevated by gabapentin (F2,14 = 7.3, p < 0.01; Figure 4). The 1200 mg dose of gabapentin increased systolic blood pressure alone and when combined with the 30 mg dose of Δ9-THC, relative to placebo. In addition, the 1200 mg gabapentin + 30 mg Δ9-THC dose combination significantly elevated systolic blood pressure relative to that dose of Δ9-THC alone. Diastolic blood pressure was not influenced by Δ9-THC or gabapentin (data not shown).
Figure 4.
Peak (maximum value) heart rate and systolic blood pressure for Δ9-THC and gabapentin, alone and in combination. All other details are as in Figure 1.
A significant interaction of gabapentin and Δ9-THC was found for index finger skin temperature (F6,42 = 3.1, p < 0.05), but did not appear to be a function of dose. Compared to placebo, the 1200 mg gabapentin dose alone and the 15 mg Δ9-THC dose alone decreased index finger skin temperature (data not shown). Oral temperature was not influenced by Δ9-THC or gabapentin (data not shown).
Discussion
The present study determined the discriminative-stimulus effects of gabapentin in subjects who had learned to discriminate Δ9-THC. Gabapentin alone substituted for the Δ9-THC discriminative stimulus, and shifted the drug discrimination dose-response functions for Δ9-THC leftward and upward when combined, indicative of a possible role for VDCCs in the interoceptive cue produced by Δ9-THC. These results are concordant with the ability of CB agonists to impact VDCC function via CB1 receptor mediated G-protein activation as well as CB-receptor-independent mechanisms (Howlett et al., 2010; Lozovaya et al., 2009). In previous studies, drug discrimination procedures like those used here revealed that the cannabinoid agonist nabilone substituted for Δ9-THC, but the dopamine/norepinephrine reuptake inhibitor methylphenidate, the opioid agonist hydromorphone and the GABAA positive allosteric modulators triazolam and diazepam did not, demonstrating the pharmacological selectivity of the Δ9-THC discrimination (Lile et al., 2009, 2010, 2014). The present results support this selectivity by demonstrating Δ9-THC stimulus generalization to a compound having a common neurobiological target.
Because the endogenous CB system and VDCCs modulate the activity of neurotransmitters, other systems are also likely involved in the discriminative-stimulus effects of Δ9-THC and gabapentin. In a recent study using similar methodology, we found that the GABA reuptake inhibitor tiagabine shared discriminative-stimulus effects with Δ9-THC, suggesting that elevated GABA is an aspect of the neuropharmacological response to Δ9-THC administration that engenders its interoceptive cue in humans (Lile et al., 2012). In vivo imaging and in vitro electrophysiology studies have demonstrated that gabapentin also elevates human brain GABA levels (Cai et al., 2012; Errante et al., 2002; Petroff et al., 2000), which might have contributed to the substitution of gabapentin for the Δ9-THC cue. A shared involvement of GABA in the discriminative-stimulus effects of Δ9-THC, gabapentin and tiagabine is consistent with the overlap in their therapeutic effects. Like gabapentin, tiagabine and other GABAergic compounds are indicated for the treatment of seizures, and emerging evidence also supports the use of cannabinoid agonists for seizure control (Dos Santos et al., 2014; Szaflarski and Bebin, 2014). In addition, human studies have demonstrated that CB agonists and VDCC ligands alleviate pain and anxiety and promote sleep, as described above, which are also effects produced by tiagabine (e.g., Flowers et al., 2006; Schwartz and Nihalani, 2006; Steiger, 2007; Todorov et al., 2005).
In addition to shared discriminative-stimulus effects, gabapentin produced a Δ9-THC-like profile of responses on subject-rated questionnaire items (e.g., increases in Good Drug Effects, High, Like Drug) and combinations of gabapentin and Δ9-THC produced larger “positive” subject-rated effects relative to Δ9-THC alone, which is suggestive of a potential for gabapentin abuse. Based on the present results, the abuse potential of gabapentin appears similar to that of oral Δ9-THC, which is generally considered to have lower abuse potential than smoked cannabis (e.g., Calhoun et al., 1998; Mendelson and Mello, 1984). Nonetheless, a recent review of the available data on the abuse potential of gabapentin and its analog pregabalin identified signals of abuse from surveys of the general population and addiction treatment clinics, particularly in individuals with a history of substance use problems (Schifano, 2014). In addition, a qualitative overview of Internet forum discussions on the effects of these gabapentinoids described reports of drug experiences that were consistent with those of other psychoactive drugs, using routes of administration to facilitate more rapid absorption, taking supratherapeutic doses, and combining gabapentin and pregabalin with other substances (Schifano et al., 2011). Despite these signals of gabapentinoid abuse, clinical trials and human laboratory studies that enrolled drug-dependent individuals in order to evaluate the potential for gabapentin maintenance to treat dependence on cocaine (e.g., Bisaga et al., 2006), alcohol (Mason et al., 2009, 2014) and cannabis (Mason et al., 2012) found no indication that gabapentin possessed abuse liability characteristics. The reasons for the discrepancies across studies on the apparent abuse potential of gabapentinoids in individuals with substance use histories is unknown, but could be due to dose, assessment methods and/or the use of dose escalation and maintenance procedures in the studies aimed at screening gabapentinoids for drug dependence. Importantly, the misuse of gabapentinoids administered therapeutically appears minimal and therefore the continued pursuit of these drugs for the management of substance use disorders, including cannabis, is warranted, especially considering that few effective pharmacotherapies exist. Furthermore, any positive subjective effects of gabapentin and pregabalin might be advantageous, as they would likely enhance medication compliance.
Other effects of gabapentin assessed here that would be considered side effects in the context of therapeutic use are self-reported sedation, psychomotor performance task impairment and changes in vital signs. Following administration of gabapentin alone, subjective ratings of Sedated were increased, accuracy on the DSST and both rate and accuracy on the acquisition component of the RA task were impaired, and systolic pressure was elevated. Although these findings might be of concern, these results could be due to the administration of doses above what is recommended at the start of treatment, and without titration. Based on its regulatory approval and subsequent post-marketing surveillance and use in controlled clinical studies, gabapentin therapy with recommended doses appears to have an acceptable safety profile. In fact, several studies have demonstrated that maintenance on even large daily gabapentin doses (≥ 2400 mg/day) had relatively minor, if any, impact on cognitive task performance across various domains in healthy individuals (e.g., Salinsky et al., 2002) and in epilepsy patients (e.g., Leach et al., 1997). Similarly, in the clinical trial conducted by Mason and colleagues (2012), scores on tests of executive function actually improved in cannabis users during treatment on gabapentin, although whether the cognitive improvement in treatment-seeking cannabis users was a direct consequence of gabapentin administration or a result of reduced cannabis use is not clear. The Mason et al. (2012) study also did not report any serious cardiovascular events associated with gabapentin administration in cannabis users, consistent results from the abovementioned trials evaluating gabapentin for cocaine and alcohol dependence (Bisaga et al., 2006; Mason et al., 2009, 2014).
The initial rationale for conducting a controlled clinical trial to evaluate gabapentin for cannabis-use disorders was based, in large part, on its ability to modulate brain stress systems (Mason et al., 2014). More specifically, gabapentin was found to normalize dysregulated interactions between GABA and corticotropin releasing factor in the amygdala associated with anxiety-related behavior in rodents (Roberto et al., 2008). The present results suggest that an additional mechanism through which gabapentin might facilitate cannabis abstinence is by producing interoceptive effects that are similar to those of cannabinoids. Also, as described above, cannabinoids and gabapentinoids alleviate sleep disturbances, pain and anxiety, which reportedly make cannabis abstinence difficult (e.g., Budney et al., 2008). The ability of gabapentin to produce overlapping effects with cannabinoids is consistent with an agonist replacement approach, which has proven successful for dependence on other substances (Bonhomme et al., 2012; Fant et al., 2009; Stoops and Rush, 2013). Worth noting, however, is that the use of a drug such as gabapentin, which operates through different neuropharmacological pathways, represents an improved strategy relative to traditional substitution pharmacotherapies, in that it likely has less potential for diversion and abuse, its efficacy might be less influenced by drug use history (i.e., tolerance) and it might be more acceptable to treatment providers.
Acknowledgments
We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We also thank Shanna Babalonis, Beth Eaves, Cleeve Emurian, Sarah Ingebrand, Dustin Lee, Jillian O’Rourke, Glenn Robbins and Sheila Rutherford for expert technical assistance.
This research and the preparation of this manuscript were supported by grants awarded to Dr. Joshua Lile (National Institute on Drug Abuse grants K02 DA031766, R01 DA025605 and R01 DA036550) as well as the University of Kentucky Center for Clinical and Translational Science (National Center for Advancing Translational Sciences grant UL1TR000117).
Footnotes
The authors declare no conflict of interest.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. American Psychiatric Association; Washington, DC: 2000. text revision. [Google Scholar]
- Aracil-Fernández A, Almela P, Manzanares J. Pregabalin and topiramate regulate behavioural and brain gene transcription changes induced by spontaneous cannabinoid withdrawal in mice. Addiction Biology. 2013;18:252–262. doi: 10.1111/j.1369-1600.2011.00406.x. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Current Opinion in Neurobiology. 2003;13:298–307. doi: 10.1016/s0959-4388(03)00066-7. [DOI] [PubMed] [Google Scholar]
- Bestard JA, Toth CC. An open-label comparison of nabilone and gabapentin as adjuvant therapy or monotherapy in the management of neuropathic pain in patients with peripheral neuropathy. Pain Practice. 2011;11:353–368. doi: 10.1111/j.1533-2500.2010.00427.x. [DOI] [PubMed] [Google Scholar]
- Bisaga A, Aharonovich E, Garawi F, Levin FR, Rubin E, Raby WN, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug and Alcohol Dependence. 2006;81:267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Bonhomme J, Shim RS, Gooden R, Tyus D, Rust G. Opioid addiction and abuse in primary care practice: A comparison of methadone and buprenorphine as treatment options. National Medical Assocation. 2012;104:342–350. doi: 10.1016/s0027-9684(15)30175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: Severity and contribution to relapse. Journal of Substance Abuse Treatment. 2008;35:362–368. doi: 10.1016/j.jsat.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: A 7T 1H-MRS study. Neuropsychopharmacology. 2012;37:2764–2771. doi: 10.1038/npp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun SR, Galloway GP, Smith DE. Abuse potential of dronabinol (Marinol) J Psychoactive Drugs. 1998;30:187–186. doi: 10.1080/02791072.1998.10399689. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology. 1992;107:255–262. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Chait LD, Evans SM, Grant KA, Kamien JB, Johanson CE, Schuster CR. Discriminative stimulus and subjective effects of smoked marijuana in humans. Psychopharmacology. 1988;94:206–212. doi: 10.1007/BF00176846. [DOI] [PubMed] [Google Scholar]
- Copersino ML, Boyd SJ, Tashkin DP, Huestis MA, Heishman SJ, Dermand JC, Simmons MS, Gorelick DA. Cannabis withdrawal among non-treatment-seeking adult cannabis users. American Journal of Addiction. 2006;15:8–14. doi: 10.1080/10550490500418997. [DOI] [PubMed] [Google Scholar]
- de-Paris F, Sant’Anna MK, Vianna MR, Barichello T, Busnello JV, Kapczinski F, et al. Effects of gabapentin on anxiety induced by simulated public speaking. Journal of Psychopharmacology. 2003;17:184–188. doi: 10.1177/0269881103017002006. [DOI] [PubMed] [Google Scholar]
- Dos Santos RG, Hallak JE, Leite JP, Zuardi AW, Crippa JA. Phytocannabinoids and epilepsy. Journal of Clinical Pharmacy and Therapeutics. 2014 doi: 10.1111/jcpt.12235. e-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Errante LD, Williamson A, Spencer DD, Petroff OA. Gabapentin and vigabatrin increase GABA in the human neocortical slice. Epilepsy Research. 2002;49:203–210. doi: 10.1016/s0920-1211(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Fant RV, Buchhalter AR, Buchman AC, Henningfield JE. Pharmacotherapy for tobacco dependence. Handbook of Experimental Pharmacology. 2009;192:487–510. doi: 10.1007/978-3-540-69248-5_17. [DOI] [PubMed] [Google Scholar]
- Flowers CM, Racoosin JA, Kortepeter C. Seizure activity and off-label use of tiagabine. New England Journal of Medicine. 2006;354:773–774. doi: 10.1056/NEJMc055301. [DOI] [PubMed] [Google Scholar]
- Gabapentin product information. Ascend Laboratories; Montvale, NJ: 2011. [Google Scholar]
- Gale JD, Houghton LA. Alpha 2 Delta (α(2)δ) ligands, gabapentin and pregabalin: What is the evidence for potential use of these ligands in irritable bowel syndrome. Frontiers in Pharmacology. 2011;2:28. doi: 10.3389/fphar.2011.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidal BE, Maly MM, Kowalski JW, Rutecki PA, Pitterle ME, Cook DE. Gabapentin absorption: Effect of mixing with foods of varying macronutrient composition. Annals of Pharmacotherapy. 1998;32:405–409. doi: 10.1345/aph.17281. [DOI] [PubMed] [Google Scholar]
- Gordi T, Hou E, Kasichayanula S, Berner B. Pharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive- extended-release and immediate-release tablets: A randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjects. Clinical Therapeutics. 2008;30:909–916. doi: 10.1016/j.clinthera.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Greenwald MK. Behavioral economic analysis of drug preference using multiple choice procedure data. Drug Alcohol Depend. 2008;93:103–110. doi: 10.1016/j.drugalcdep.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Ward AS, Haney M, Comer SD, Foltin RW, Fischman MW. Comparison of smoked marijuana and oral delta 9-tetrahydrocannabinol in humans. Psychopharmacology. 2002;164:407–415. doi: 10.1007/s00213-002-1231-y. [DOI] [PubMed] [Google Scholar]
- Hasnie FS, Breuer J, Parker S, Wallace V, Blackbeard J, Lever I, et al. Further characterization of a rat model of varicella zoster virus-associated pain: Relationship between mechanical hypersensitivity and anxiety-related behavior, and the influence of analgesic drugs. Neuroscience. 2007;144:1495–1508. doi: 10.1016/j.neuroscience.2006.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187–193. doi: 10.1093/sleep/28.2.187. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Blume LC, Dalton GD. CB(1) cannabinoid receptors and their associated proteins. Current Medicinal Chemistry. 2010;17:1382–1393. doi: 10.2174/092986710790980023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karst M, Wippermann S, Ahrens J. Role of cannabinoids in the treatment of pain and (painful) spasticity. Drugs. 2010;70:2409–2438. doi: 10.2165/11585260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Leach JP, Girvan J, Paul A, Brodie MJ. Gabapentin and cognition: A double blind, dose ranging, placebo controlled study in refractory epilepsy. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;62:372–376. doi: 10.1136/jnnp.62.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile for Δ9-THC, triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-THC. Psychopharmacology. 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Substitution profile of the cannabinoid agonist nabilone in humans discriminating Δ9-THC. Clinical Neuropharmacology. 2010;33:235–242. doi: 10.1097/WNF.0b013e3181e77428. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABA reuptake inhibitor tiagabine and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2012;122:61–69. doi: 10.1016/j.drugalcdep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Charnigo RJ, Stinchcomb AL, Hays LR. Pharmacokinetic and pharmacodynamics profile of supratherapeutic oral doses of Δ9-THC in cannabis users. Journal of Clinical Pharmacology. 2013;53:680–690. doi: 10.1002/jcph.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABAA positive modulator diazepam and Δ9-THC in humans discriminating Δ9-THC. Drug and Alcohol Dependence. 2014;143:141–148. doi: 10.1016/j.drugalcdep.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozovaya N, Min R, Tsintsadze V, Burnashev N. Dual modulation of CNS voltage-gated calcium channels by cannabinoids: Focus on CB1 receptor-independent effects. Cell Calcium. 2009;46:154–162. doi: 10.1016/j.ceca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Luszczki JJ, Florek-Łuszczki Synergistic interaction of pregabalin with the synthetic cannabinoid, WIN 55,212–2 mesylate in the hot-plate test in mice: An isobolographic analysis. Pharmacological Reports. 2012;64:723–732. doi: 10.1016/s1734-1140(12)70867-8. [DOI] [PubMed] [Google Scholar]
- Marinol® product information. Solvay Pharmaceuticals, Inc; Marietta, GA: 2006. [Google Scholar]
- Mason BJ, Light JM, Williams LD, Drobes DJ. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: Effects of gabapentin. Addiction Biology. 2009;14:73–83. doi: 10.1111/j.1369-1600.2008.00133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: Effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37:1689–1698. doi: 10.1038/npp.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: A randomized clinical trial. JAMA Internal Medicine. 2014;174:70–77. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Reinforcing properties of oral delta 9-tetrahydrocannabinol, smoked marijuana, and nabilone: influence of previous marijuana use. Psychopharmacology. 1984;83:351–356. doi: 10.1007/BF00428544. [DOI] [PubMed] [Google Scholar]
- Nakano S, Gillespie HK, Hollister LE. A model for evaluation of antianxiety drugs with the use of experimentally induced stress: Comparison of nabilone and diazepam. Clinical Pharmacology and Therapeutics. 1978;23:54–62. doi: 10.1002/cpt197823154. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41:675–680. doi: 10.1111/j.1528-1157.2000.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. The Journal of Neuroscience. 2008;28:5762–5771. doi: 10.1523/JNEUROSCI.0575-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: A randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care and Research. 2012;64:597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion and triazolam in d-amphetamine trained humans. Experimental and Clinical Psychopharmacology. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Saldaña MT, Pérez C, Navarro A, Masramón X, Rejas J. Pain alleviation and patient-reported health outcomes following switching to pregabalin in individuals with gabapentin-refractory neuropathic pain in routine medical practice. Clinical Drug Investigation. 2012;32:401–412. doi: 10.2165/11599400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Salinsky MC, Binder LM, Oken BS, Storzbach D, Aron CR, Dodrill CB. Effects of gabapentin and carbamazepine on the EEG and cognition in healthy volunteers. Epilepsia. 2002;43:482–490. doi: 10.1046/j.1528-1157.2002.22501.x. [DOI] [PubMed] [Google Scholar]
- Schifano F. Misuse and abuse of pregabalin and gabapentin: Cause for concern? CNS Drugs. 2014;28:491–496. doi: 10.1007/s40263-014-0164-4. [DOI] [PubMed] [Google Scholar]
- Schifano F, D’Offizi S, Piccione M, Corazza O, Deluca P, Davey Z, et al. Is there a recreational misuse potential for pregabalin? Analysis of anecdotal online reports in comparison with related gabapentin and clonazepam data. Psychotherapy and Psychosomatics. 2011;80:118–122. doi: 10.1159/000321079. [DOI] [PubMed] [Google Scholar]
- Schwartz TL, Nihalani N. Tiagabine in anxiety disorders. Expert Opinion on Pharmacotherapy. 2006;7:1977–1987. doi: 10.1517/14656566.7.14.1977. [DOI] [PubMed] [Google Scholar]
- Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current Opinion in Pharmacology. 2006;6:108–113. doi: 10.1016/j.coph.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Steiger A. Neurochemical regulation of sleep. Journal of Psychiatric Research. 2007;41:537–552. doi: 10.1016/j.jpsychires.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Agonist replacement for stimulant dependence: A review of clinical research. Current Pharmaceutical Design. 2013;19:7026–7035. doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handbook of Experimental Pharmacology. 2005;168:327–365. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Bebin EM. Cannabis, cannabidiol and epilepsy: From receptors to clinical response. Epilepsy & Behavior. 2014;41:277–282. doi: 10.1016/j.yebeh.2014.08.135. [DOI] [PubMed] [Google Scholar]
- Todorov AA, Kolchev CB, Todorov AB. Tiagabine and gabapentin for the management of chronic pain. Clinical Journal of Pain. 2005;21:358–361. doi: 10.1097/01.ajp.0000110637.14355.77. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology. 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Wallace VCJ, Segerdahl AR, Lambert DM, Vandevoorde S, Blackbeard J, Pheby T, et al. The effect of the palmitoylethanolamide analogue, palmitoylallylamide (L-29) on pain behaviour in rodent models of neuropathy. British Journal of Pharmacology. 2007;151:1117–1128. doi: 10.1038/sj.bjp.0707326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware MA, Fitzcharles MA, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesthesia and Analgesia. 2010;110:604–610. doi: 10.1213/ANE.0b013e3181c76f70. [DOI] [PubMed] [Google Scholar]