Abstract
Background
Plant protein intake is associated with lower production of uremic toxins and lower serum phosphorus levels. Therefore, at a given total protein intake, a higher proportion of dietary protein from plant sources might be associated with lower mortality in CKD.
Study Design
Observational study
Settings & Participants
14,866 NHANES III participants aged 20 years or older without missing data for plant and animal protein intake and mortality.
Predictors
Plant protein–total protein ratio and total plant protein intake. Patients were stratified by eGFR < or ≥ 60 ml/min/1.73 m2.
Outcomes
All-cause mortality.
Measurements
Plant and total protein intakes were estimated from 24h dietary recalls. Mortality was ascertained by probabilistic linkage with National Death Index records through 12/31/2000.
Results
The mean of plant protein intake and plant protein–total protein ratio were 24.6 ± 13.2 (SD) g/d and 33.0% ± 14.0%, respectively. The prevalence of eGFR < 60 ml/min/1.73 m2 was 4.9%. There were 2,163 deaths over an average follow-up of 8.4 years. Adjusted for demographics, smoking, alcohol use, comorbidity, BMI, calorie and total protein intake and physical inactivity, each 33% increase in plant protein–total protein ratio was not associated with mortality (HR, 0.88; 95% CI, 0.74-1.04) in the eGFR ≥60 mL/min/1.73 m2 subpopulation, but was associated with lower mortality risk (HR, 0.77; 95% CI, 0.61-0.96) in the eGFR < 60 mL/min/1.73 m2 subpopulation. In sensitivity analyses, results were similar in those with eGFR < 60 mL/min/1.73 m2 defined by serum cystatin C.
Limitations
Whether the results are related to plant protein itself or to other factors associated with more plant-based diets is hard to establish.
Conclusions
A diet with higher proportion of protein from plant sources is associated with lower mortality in those with eGFR < 60 mL/min/1.73 m2. Future studies are warranted to determine the causal role of plant protein intake in reducing mortality in those with eGFR < 60 mL/min/1.73 m2.
Keywords: plant protein, animal protein, diet, protein intake, dietary recall, nutrition, chronic kidney disease (CKD), mortality, decreased renal function, estimated glomerular filtration rate (eGFR), disease progression, NHANES (National Health and Nutrition Examination Survey)
It is estimated that 14% of US adults have chronic kidney disease (CKD).1 Mortality remains unusually high even in patients with non-dialysis-dependent CKD, with the annual rate surpassing 9% in patients with CKD stage 4.2 Thus, CKD is extremely common, carrying a significant health burden. As high protein intake might accelerate CKD progression,3 the current national recommendations for dietary protein intake is 0.60 g protein/kg/d for individuals with CKD,4,pS58 and 1.2 g protein/kg/d for clinically stable maintenance hemodialysis patients.4,pS40 However, CKD patients tend to be at high risk for protein malnutrition because of their inadequate protein intake, acidemia-induced muscle breakdown, hyper-catabolism from a chronic inflammatory state, associated illnesses, and, when applicable, dialysis procedures. 6-12 Thus, the optimal protein intake in individuals with non–dialysis-dependent CKD should minimize the risk of CKD progression, yet avoid malnutrition.
High-protein, plant-based diets have been investigated as an approach to decrease CKD progression as well as maintaining adequate nutrition. Some studies suggest that plant proteins do not accelerate CKD progression as animal proteins do. In the Nurses’ Health Study,13 high animal protein intake in women with mild CKD was associated with significantly greater decline in estimated glomerular filtration rate (eGFR) than high plant protein intake. Furthermore, animal and human models suggest that plant protein diets may provide nutritional adequacy.14-18 In general healthy populations, studies found an inverse association between plant protein intake and all-cause mortality.19,20
To our knowledge, the associations of plant protein with mortality in the CKD population have not been studied. Therefore, we hypothesized that at a given total protein intake, a higher proportion of dietary protein from plant sources is associated with lower risk of mortality in CKD. We tested this hypothesis by examining the association of plant protein intake with all-cause mortality.
Methods
Study Population
The NHANES (National Health and Nutrition Examination Survey) III was conducted from 1988 to 1994 by the Centers for Disease Control and Prevention. The study design of NHANES was a multistage, stratified, clustered probability sampling of the United States’ civilian population.21 NHANES III was approved by the Institutional Review Board at the Centers for Disease Control and Prevention, and written informed consents were obtained from all study participants.
Data on demographics (age, sex and race), history of comorbid conditions (myocardial infarction, stroke, congestive heart failure and cancer), current or past cigarette smoking, alcohol use (had at least 12 drinks in the last 12 months), and leisure-time physical inactivity (those with no self-reported leisure time physical activity) were collected in home interviews conducted by trained personnel. Anthropometric measurements, blood pressure readings, 24-hour dietary recall, blood and urine specimens, and other physical measurements were obtained at a mobile examination center.
In NHANES III, serum creatinine was measured using a kinetic rate Jaffe method, and recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory (Cleveland, Ohio) as standard creatinine =(0.960 × serum creatinine) – 0.184. 22 Kidney function was estimated by creatinine-based eGFR, calculated using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation23.
Nutrition Intake Assessment
Dietary interviews were performed by an NHANES examiner in the mobile examination center for each subject. Dietary Data Collection System, a computer-based 24-h dietary recall program developed by the University of Minnesota's Nutrition Coordinating Center (NCC; Regents of the University of Minnesota), was used to assist the interviewer in the survey. The NHANES calculated the plant and animal protein intakes (variables “NCPNVPRO” and “NCPNAPRO”)24 using the US Department of Agriculture Survey Nutrient Database. The dietary recall data files were also coded to the NCC foods and nutrient composition database. 25 The NCC nutrient database has 165 nutrients, nutrient ratios and other food components, including total protein, animal protein and plant protein. Protein values were calculated from the analyzed nitrogen content of a food. 26 Plant protein is defined as the amount of protein contributed by plant products, including grains, fruits, vegetables, legumes, nuts and seeds. Animal protein sources include meats, eggs and dairy foods. The total protein in a food equals the sum of plant and animal protein in that food. 27 Details about dietary assessment methodology have been published elsewhere.21
Follow-up Data
The National Center for Health Statistics performed a probabilistic match between NHANES III and National Death Index death records to create a mortality file. Details about the NHANES III mortality linkage methodology have been provided elsewhere. 28. In this study, we used the follow-up mortality data from the date of survey participation (1988-1994) to December 31, 2000.
Statistical Analysis
As NHANES is based on a complex probability sample design, several aspects must be taken into account in data analysis. We used the svy suite of commands in Stata 12 (StataCorp LP, College Station, TX). Following the analytical guidelines for NHANES data, primary sampling units, pseudo strata (“SDPSTR6”) and appropriate sampling weights were used to calculate the expected means and proportions of adults aged 20 and older in the US non-institutionalized civilian population.29
Univariate descriptive statistics including means, standard deviations, and designated quantiles including medians, 25th and 75th percentiles were obtained for numeric variables; proportions were obtained to describe distributions of categorical variables.
Plant protein ratio was defined as plant protein–total protein ratio. In order to test whether a higher proportion of dietary protein from plant sources was associated with lower risk of mortality at a given total protein intake, we examined the mortality associations of plant protein ratio adjusted for total protein intake in eGFR ≥ 60 and < 60 mL/min/1.73 m2 subpopulations in Cox regression models. These regression models were first adjusted for age, gender, race, smoking, alcohol use, calorie intake, and physical inactivity. In additional analyses, body mass index (BMI), hypertension, cancer, myocardial infarction (MI), congestive heart failure (CHF), stroke and diabetes were added as covariates. Calorie and fat intakes were highly correlated (r = 0.87). In order to avoid colinearity, only calorie intake was included in the models. In sensitivity analysis, fat intake was substituted for calorie intake.
In the above analyses, the hazard ratios (HRs) relating mortality to the plant protein ratio were expressed relative to a 33% increase in the plant protein ratio. We also examined the associations of quartiles of plant protein ratio with mortality using the lowest quartile as the reference.
To illustrate the shape of the relationship between the HRs of mortality and plant protein ratio, we also applied restricted cubic splines in Cox regression models with 4 knots and the 10th percentile of plant protein ratio as reference.
We evaluated whether the relationship between mortality and the plant protein ratio differed between the eGFR < 60 and ≥ 60 mL/min/1.73 m2 subgroups by fitting the Cox regression analyses relating mortality to the plant protein ratio separately in the 2 eGFR groups, then computing the ratio of the difference in the Cox regression coefficients between the 2 groups to the standard error of this difference, and finally by comparing this ratio to quantiles of the standard normal distribution.
In sensitivity analyses, the associations of plant protein ratio with mortality in the subpopulation with cystatin C–based eGFR (eGFRcys; calculated by the CKD-EPI cystatin C equation29a) < 60 mL/min/1.73 m2 as well as CKD stages 1 and 2 (eGFR ≥ 60 ml/min/1.73 m2 and urinary albumin-creatinine ratio > 30 mg/g) were examined.
The assumption of proportional hazards was examined by comparing the logarithm of the HR for each predictor variable in the first 36 months of follow-up to the logarithm of the HR of the predictor variables after 36 months. No deviation from proportional hazards was detected with respect to plant protein ratio in any of the Cox regression models.
Results
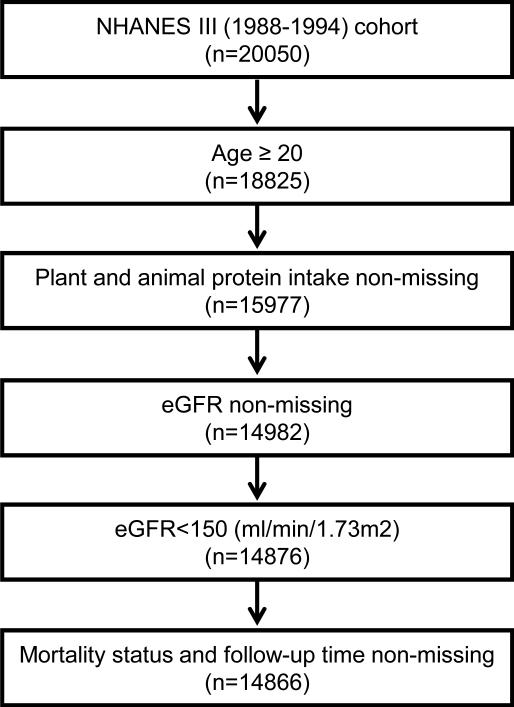
The study population included 14,866 adult (≥20 years) participants with eGFR <150 ml/min/1.73m2, with non-missing data for plant protein and total protein intake, as well as mortality status in those participants (Figure 1). The prevalence of eGFR < 60 mL/min/1.73 m2 was 4.9%. The mean age was 44.8±15.8 (standard deviation) years, 48% were men, and 10% were African Americans. The means of plant protein intake and plant protein ratio were 24.6 ± 13.2 g/d and 33.0%± 14.0%, respectively.
Figure 1.
Participant flow chart
Baseline characteristics by plant protein ratio quartiles in the entire cohort are summarized in table 1. Older age and presence of cardiovascular conditions (MI, CHF, or stroke) or lower eGFR were associated with higher plant protein ratio, whereas being male or African American, smoking, alcohol use and higher BMI were associated with a lower plant protein ratio. Those with higher plant protein ratio also had higher fiber and carbohydrate intake but lower total calorie, total fat, and saturated fat intake.
Table 1.
Baseline characteristics of entire cohort according to plant protein ratio quartiles
| Q1: <22.4% (n=3781) | Q2: 22.4%-30.6% (n=3611) | Q3: 30.7%-40.9% (n=3632) | Q4: >40.9% (n=3842) | P | |
|---|---|---|---|---|---|
| Plant protein ratio (%) | 16.6 ± 4.2 | 26.5 ± 2.1 | 35.3 ± 2.7 | 53.5 ± 11.4 | |
| Plant protein intake (g/d) | 16.6 ± 8.3 | 22.7 ± 10.2 | 26.7 ± 12.0 | 32.5 ± 16.5 | <0.001 |
| Total protein intake (g/d) | 101.9 ± 47.8 | 85.9 ± 38.3 | 76.0 ± 34.2 | 62.5 ± 30.9 | <0.001 |
| Age (y) | 42.6 ± 15.0 | 43.7 ± 15.3 | 45.8 ± 16.2 | 46.8 ± 16.7 | <0.001 |
| Male sex | 53.5 | 48.6 | 44.8 | 43.4 | <0.001 |
| African American | 14.1 | 11.2 | 9.4 | 8.0 | <0.001 |
| MI | 3.1 | 3.0 | 4.0 | 3.7 | 0.2 |
| CHF | 1.7 | 1.7 | 2.4 | 2.7 | 0.02 |
| Stroke | 1.2 | 2.1 | 2.4 | 2.1 | 0.05 |
| Diabetes mellitus | 7.4 | 7.0 | 6.8 | 7.3 | 0.9 |
| Cancer | 3.8 | 2.5 | 4.2 | 4.9 | 0.002 |
| Current smoker | 36.3 | 31.5 | 24.7 | 20.6 | <0.001 |
| Alcohol use | 59.9 | 54.7 | 52.1 | 48.8 | <0.001 |
| BMI (kg/m2) | 26.9 ± 5.4 | 26.6 ± 5.1 | 26.6 ± 5.3 | 26.1 ± 5.0 | 0.001 |
| Leisure-time physical inactivity | 14.5 | 15.6 | 14.1 | 14.9 | 0.7 |
| Calorie intake (kcal/d) | 2289 ± 1069 | 2266 ± 1005 | 2184 ± 939 | 2032 ± 901 | <0.001 |
| Saturated fat intake (g) | 32.7 ± 19.7 | 30.5 ± 17.8 | 27.3 ± 14.9 | 22.0 ± 13.3 | <0.001 |
| Carbohydrate intake (g) | 234 ± 115 | 269± 119 | 279 ± 123 | 285 ± 126 | <0.001 |
| Fiber intake (g) | 12.8 ± 7.2 | 16.1 ± 8.0 | 18.7 ± 9.3 | 22.1 ± 11.8 | <0.001 |
| Total fat intake (g/d) | 94.3 ± 53.3 | 89.5 ± 47.9 | 83.1 ± 43.7 | 72.1 ± 42.5 | <0.001 |
| Animal protein intake (g/d) | 83.8 ± 41.0 | 62.0 ± 28.2 | 48.2 ± 22.3 | 29.2 ± 17.0 | <0.001 |
| eGFR (ml/min/1.73m2) | 101 ± 20 | 100 ± 20 | 98 ± 20 | 97 ± 20 | <0.001 |
| eGFR < 60 ml/min/1.73 m2 | 3.8 | 4.6 | 5.3 | 5.9 | 0.002 |
| Urinary ACR ≥ 30 mg/g (%) | 8.1 | 7.9 | 8.9 | 9.0 | 0.5 |
Note: Values for categorical variables are given as percentage; for continuous variables, as mean ± standard deviation.
Abbreviations: MI, myocardial infarction; CHF, congestive heart failure; eGFR, estimated glomerular filtration rate; ACR, albumin-creatinine ratio; BMI, body mass index. Q, quartlle
Out of 14,866 NHANES III participants included in the analysis, there were 2,163 deaths (14.6%) over an average follow-up of 8.4 years. The eGFR < 60 mL/min/1.73 m2 subpopulation consisted of 1,065 participants with 633 deaths (59.4%) over an average follow-up of 6.2 years. The eGFR ≥ 60 subpopulation of 13,801 participants had 1,537 deaths (11.1%) over an average follow-up of 8.6 years.
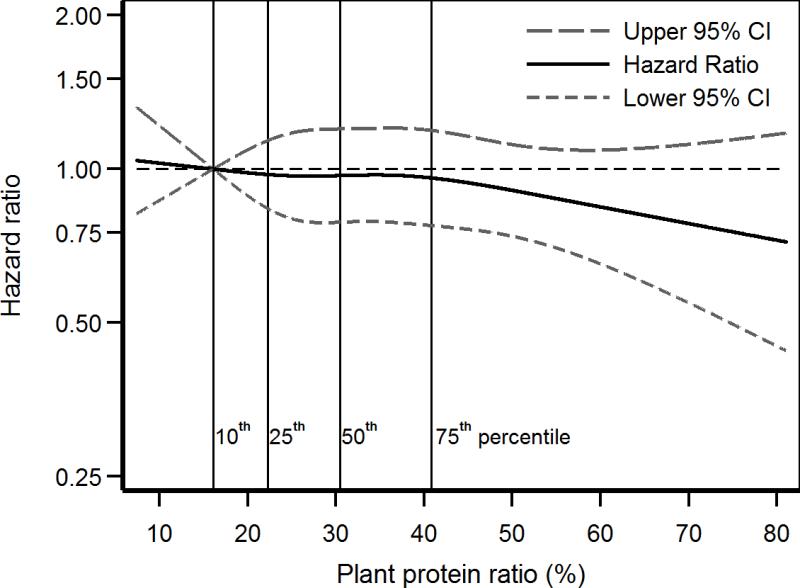
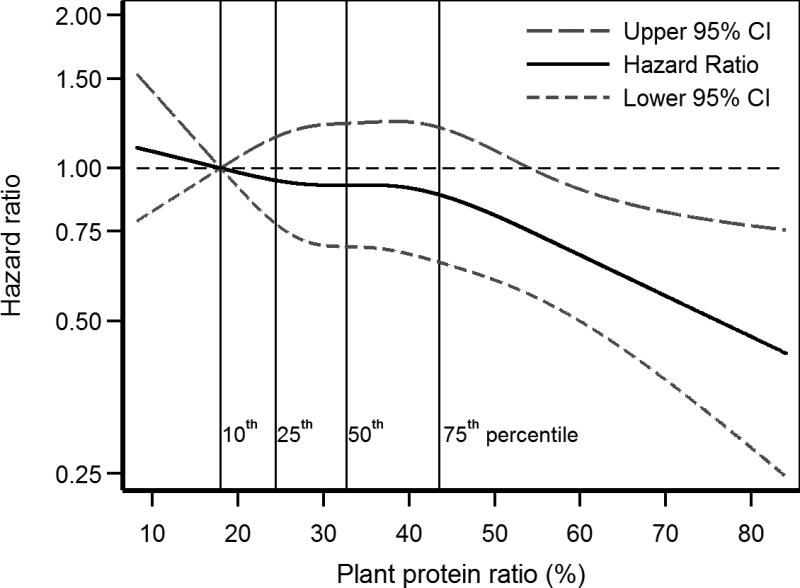
After adjustment for age, gender, race, smoking, alcohol use, physical inactivity, and calorie intake, each 33% increase the in plant protein ratio had a statistically non-significant lower risk of death (HR, 0.87; 95% confidence interval [CI], 0.74-1.03) in the eGFR ≥ 60 mL/min/1.73 m2 subpopulation but a significantly lower risk of death (HR, 0.81; 95% CI, 0.66-0.99) in the eGFR < 60 subpopulation (Table 2). These results were consistent in spline regression analyses in the eGFR ≥ 60 (Figure 2) and < 60 mL/min/1.73 m2 (Figure 3) subpopulations. Further adjustment for BMI, hypertension, stroke, cancer, MI, CHF, and diabetes resulted in a stronger association of plant protein ratio with lower mortality in the eGFR < 60 mL/min/1.73 m2 subpopulation but not in the eGFR ≥ 60 mL/min/1.73 m2 subpopulation (Table 2). When these analyses were repeated with adjustment for fat intake instead of calories, each 33% increase in the plant protein ratio was not associated with mortality (HR, 0.88; 95% CI, 0.72-1.07) in the eGFR ≥ 60 mL/min/1.73 m2 subpopulation, but with lower risk of death (HR, 0.79; 95% CI, 0.62-1.00) in the eGFR < 60 mL/min/1.73 m2 subpopulation.
Table 2.
Associations of plant protein ratio with all-cause mortality in normal and eGFR < 60 mL/min/1.73 m2 subpopulations in continuous and categorical models
| Model 1* | Model 2$ | Model 3# | |
|---|---|---|---|
| eGFR ≥ 60 subpopulation (n =14801) | |||
| Continuous model: per each 33% increase in plant protein ratio | 0.89 (0.75,1.04) | 0.87 (0.74,1.03) | 0.88 (0.74,1.04) |
| Categorical model | |||
| Q1: < 22.3% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 22.3% - 30.5% | 0.93 (0.72,1.21) | 0.98 (0.77,1.27) | 0.98 (0.78,1.24) |
| Q3: 30.6% - 40.8% | 1.04 (0.83,1.30) | 1.00 (0.81,1.24) | 0.99 (0.79,1.24) |
| Q4: >40.8% | 0.87 (0.69,1.09) | 0.87 (0.70,1.08) | 0.89 (0.71,1.12) |
| eGFR < 60 subpopulation (n =1065) | |||
| Continuous model: per each 33% increase in plant protein ratio | 0.94 (0.77,1.14) | 0.81 (0.66,0.99) | 0.77 (0.61,0.96) |
| Categorical model | |||
| Q1: < 24.4% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 24.4% - 32.7% | 0.96 (0.65,1.43) | 0.86 (0.58,1.27) | 0.80 (0.55,1.17) |
| Q3: 32.8% - 43.5% | 1.12 (0.81,1.54) | 1.00 (0.69,1.45) | 0.93 (0.66,1.29) |
| Q4: > 43.5% | 0.85 (0.61,1.20) | 0.73 (0.50,1.06) | 0.67 (0.46,0.96) |
Note: Associations are given as hazard ratio (95% confidence interval).
eGFR, estimated glomerular filtration rate; Q, quartile
Model 1: adjusted for total protein intake
Model 2: Model 1 + age, gender, race, smoking, alcohol use, calorie intake and physical inactivity;
Model 3: Model 2 + body mass index, hypertension, cancer, myocardial infarction, congestive heart failure, stroke and diabetes
Figure 2.
Restricted cubic spline of mortality association of plant protein ratio in eGFR ≥ 60 mL/min/1.73 m2 subpopulation
Figure 3.
Restricted cubic spline of mortality association of plant protein ratio in eGFR < 60 mL/min/1.73 m2 subpopulation
Compared to the lowest quartile of plant protein ratio (Table 2), the highest quartile had lower risk of mortality in the eGFR < 60 mL/min/1.73 m2 subpopulation (HR, 0.67; 95% CI, 0.46-0.96) but not in the eGFR ≥ 60 mL/min/1.73 m2 subpopulation (HR, 0.89; 95% CI, 0.71-1.12; Table 2).
Regression coefficients relating plant protein ratio with mortality in the eGFR ≥ 60 and < 60 mL/min/1.73 m2 subpopulations were not significantly different (p > 0.05) suggesting that reduced eGFR might not modify the associations of plant protein intake with mortality.
In sensitivity analyses, higher plant protein ratio was associated with lower mortality in those with eGFRcys < 60 ml/min/1.73 m2 (Table 3) but not in those with CKD stages 1 or 2 (eGFR ≥ 60 ml/min/1.73 m2 and urinary albumin-creatinine ratio > 30 mg/g; Table 4).
Table 3.
Associations of plant protein ratio with mortality in eGFRcys < 60 mL/min/1.73 m2 subpopulation in continuous and categorical models
| Model 1* | Model 2$ | Model 3# | |
|---|---|---|---|
| Continuous model: per each 33% increase in plant protein ratio | 0.75 (0.58,0.96) | 0.75 (0.60,0.94) | 0.73 (0.58,0.92) |
| Categorical model | |||
| Q1: < 25.3% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 25.3% - 33.3% | 1.05 (0.77,1.44) | 1.01 (0.75,1.36) | 0.96 (0.71,1.29) |
| Q3: 33.4% - 43.5% | 1.05 (0.82,1.35) | 1.02 (0.77,1.35) | 0.98 (0.78,1.23) |
| Q4: >43.5% | 0.69 (0.49,0.96) | 0.70 (0.51,0.96) | 0.67 (0.49,0.92) |
Note: n=1543. Associations are given as hazard ratio (95% confidence interval).
eGFRcys,cystatin C–based estimated glomerular filtration rate
Model 1: adjusted for total protein intake
Model 2: Model 1 + age, gender, race, smoking, alcohol use, calorie intake and exercise level;
Model 3: Model 2 + body mass index, hypertension, cancer, myocardial infarction, congestive heart failure, stroke and diabetes
Table 4.
Associations of plant protein ratio with mortality in CKD stages 1 and 2
| Model 1* | Model 2$ | Model 3# | |
|---|---|---|---|
| Continuous model: per each 33% increase in plant protein ratio | 0.70 (0.49,1.00) | 0.85 (0.60,1.20) | 0.79 (0.54,1.16) |
| Categorical model | |||
| Q1: < 22.8% | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2: 22.8% - 31.7% | 1.02 (0.58,1.77) | 1.26 (0.75,2.14) | 1.17 (0.68,2.00) |
| Q3: 31.8% - 41.3% | 0.73 (0.41,1.31) | 0.93 (0.54,1.59) | 0.93 (0.51,1.72) |
| Q4: >41.3% | 0.83 (0.49,1.42) | 1.01 (0.58,1.77) | 0.90 (0.49,1.66) |
Note: n=1347. CKD stages 1 and 2 refers to persons with estimated glomerular filtration rate ≥60 mL/min/1.73 m2 and urinary albumin-creatinine ratio >30 mg/g. Associations given as hazard ratio (95% confidence interval).
CKD, chronic kidney disease; Q, quartile
Model 1: adjusted for total protein intake
Model 2: Model 1 + age, gender, race, smoking, alcohol use, calorie intake and exercise level;
Model 3: Model 2 + body mass index, hypertension, cancer, myocardial infarction, congestive heart failure, stroke and diabetes
Discussion
In the general population, the 2010 Dietary Reference Intake recommends an individual daily protein intake of 0.8 g/kg.30 However, in the typical American diet, the average daily protein intake is approximately 1.2 g/kg.31 Popular weight-loss diets today recommend a high-protein, low-carbohydrate diet, which has been shown to promote weight loss in obese patients. 32 Since obesity is a major risk factor for kidney damage,33,34 it might be reasonable to use a high protein diet in obese individuals for weight loss. On the other hand, there are concerns about whether a high protein diet poses particular problems for CKD patients, because high protein intake might cause nephrotoxicity and accelerate CKD progression.3,35,36
The results of this study suggest that at a given total protein intake, a higher proportion of protein from plant sources is associated with lower mortality in CKD. The following discussion summarizes the current literature and interprets the current study.
Most studies on plant protein intake are food based, and thus cannot isolate the protein effect without considering other factors. However, various mechanisms have been proposed for the potential benefits of high plant protein intake in CKD.
The differences in amino acid composition of plant protein and animal protein might explain their different effects on kidney function. Individuals with high plant protein and low animal protein intake consume greater proportions of cysteine, proline, glutamic acid, phenylalanine, and serine, and smaller proportions of the other 13 amino acids versus individuals with lower plant protein and higher animal protein intake.37
Plant proteins are also found to have a significant influence on cholesterol metabolism. High intakes of plant protein from gluten are shown to lower oxidized low-density lipoprotein (LDL) cholesterol, serum triacylglycerol, and uric acid.38 Similarly, high intake of soy protein is found to significantly decrease serum total cholesterol, LDL cholesterol and triglycerides.39 By decreasing these serum lipids levels, plant protein may help attenuate oxidized-lipoprotein–induced glomerular damage and CKD progression.40
Another explanation for the benefits of plant protein involves the predominance of acid precursors in animal protein 41 compared to the base precursors (e.g., potassium bicarbonate) found in plant protein.42 Imbalance between these precursors may cause a chronic net dietary acid load that contributes to complications and disease progression in CKD. In the African American Study of Kidney Disease and hypertension (AASK) cohort of more advanced CKD, results showed that a diet high in animal sources of protein may lead to higher estimated net endogenous acid production, which is associated with decreased serum bicarbonate 43 and greater loss of measured GFR.44 In the same cohort, each 1-mmol/l increase in serum bicarbonate within the normal range was associated with decreased risk of a composite of death, dialysis, or GFR event.45
A plant protein–rich diet is also associated with decreased production of uremic toxins such as p-cresyl sulfate and indoxyl sulfate,13,46 which have been implicated in CKD progression, cardiovascular disease, and mortality in kidney disease patients.47-51 Since these uremic toxins accumulate in more advanced CKD, a diet high in plant sources might be more beneficial in preventing or managing advanced CKD.
In a Western diet, more than half of dietary phosphorus comes from animal proteins.52 Unlike the phosphorus in animal proteins, which is made up of organic phosphates, easily hydrolyzed and quickly absorbed, phosphorus in plant protein is mostly in the form of phytic acid or phytate.53 Therefore, the bioavailability of phosphorus from plant food sources is fairly low (typically <50%).54 In a crossover trial in nine CKD patients with a mean eGFR of 32 ml/min, 1 week of predominantly plant protein diet prepared by clinical research staff with nutrients equivalent to a meat diet led to decreased serum phosphorus levels and reduced fibroblast growth factor 23 levels.55 Thus, theoretically, prescribing CKD patients a diet high in plant protein may not only meet their nutrition requirements but also lead to lower body phosphorus burden.
Despite plentiful evidence from observational research on the benefit of plant protein in decreasing cancer,56-58 cardiovascular mortality,19,20,56,59 blood pressure 60-62 and diabetes 63, caution is needed in interpreting these results. Whether the results are related to the plant protein itself or to the higher polyunsaturated fatty acid and lower saturated fatty acid or increased fiber levels associated with more plant-based diets is difficult to establish without intervention trials that increase solely plant protein. Therefore, even with observational studies suggesting benefit, such a result might not be seen in intervention trials.
There are other limitations to the current study. The eGFR was calculated based on a single measurement of serum creatinine, which may not account for changes in creatinine levels over time. Moreover, residual confounding due to unmeasured confounders cannot be ruled out. Finally, no method of dietary intake assessment is perfect. The misclassification of food sources may lead to underestimation or overestimation of the diet impact on mortality. In addition, a single 24-h-diet recall may not represent the usual or long-term dietary behavior of the patient. The 24-h-diet recall also has other disadvantages such as recall bias, patients being unable to remember everything, and difficulty estimating portion size and ingredients, etc. However, it provides good estimates of group means.
In conclusion, this study suggests that a higher proportion of protein from plant sources is associated with lower mortality in CKD patients.
Further studies should continue to explore different effects of plant protein and animal protein on kidney function, and establish the mechanisms of these associations. The causal role of plant protein in decreasing mortality in the CKD population should be established with intervention trials.
Acknowledgements
Support: This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (RO1-DK077298 and RO1-DK078112) and the University of Utah Study Design and Biostatistics Center (funded in part from the Public Health Services research grant numbers UL1-RR025764 and C06-RR11234 from the National Center for Research Resources). The funding sources had no role in the design, analysis, and interpretation of the data, nor the preparation, approval, or decision to submit the manuscript for review.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Contributions: Study concept and design: XC, SB, TG, TJ, JM; acquisition, analysis, or interpretation of data: all authors; statistical analysis: GW, TG; supervision: SB, TG, TJ, JM. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. SB takes responsibility that this study has been reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Peer Review: Evaluated by 2 external peer reviewers, a Statistical Editor, a Co-Editor, and the Editor-in-Chief.
References
- 1.Collins AJ, Foley RN, Chavers B, et al. US Renal Data System 2013 annual data report. Am J Kidney Dis. 2014;63(1)(suppl 1):e1–e420. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Archives of internal medicine. 2004 Mar 22;164(6):659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 3.Kasiske BL, Lakatua JD, Ma JZ, Louis TA. A meta-analysis of the effects of dietary protein restriction on the rate of decline in renal function. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1998 Jun;31(6):954–961. doi: 10.1053/ajkd.1998.v31.pm9631839. [DOI] [PubMed] [Google Scholar]
- 4.National Kidney Foundation KDOQI Clinical Practice Guidelines for Nutrition in Chronic Renal Failure. 2000;35(6)(suppl 1):S1–S140. doi: 10.1053/ajkd.2000.v35.aajkd03517. [DOI] [PubMed] [Google Scholar]
- 5
- 6.Kalantar-Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition-inflammation complex syndrome in dialysis patients: causes and consequences. Am J Kidney Dis. 2003;42(14582032):864–881. doi: 10.1016/j.ajkd.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 7.Siew ED, Pupim LB, Majchrzak KM, Shintani A, Flakoll PJ, Ikizler TA. Insulin resistance is associated with skeletal muscle protein breakdown in non-diabetic chronic hemodialysis patients. Kidney Int. 2007 Jan;71(2):146–152. doi: 10.1038/sj.ki.5001984. [DOI] [PubMed] [Google Scholar]
- 8.May RC, Kelly RA, Mitch WE. Mechanisms for defects in muscle protein metabolism in rats with chronic uremia. Influence of metabolic acidosis. J Clin Invest. Apr. 1987;79(4):1099–1103. doi: 10.1172/JCI112924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikizler TA, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole-body protein loss and alters substrate oxidation. American Journal Physiology - Endocrinology and Metabolism. 2002;282:E107–E116. doi: 10.1152/ajpendo.2002.282.1.E107. [DOI] [PubMed] [Google Scholar]
- 10.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). Journal of Renal Nutrition. 2013;23(2):77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 12.Raj DS, Sun Y, Tzamaloukas AH. Hypercatabolism in dialysis patients. Current opinion in nephrology and hypertension. 2008;17(6):589–594. doi: 10.1097/MNH.0b013e32830d5bfa. [DOI] [PubMed] [Google Scholar]
- 13.Knight EL, Stampfer MJ, Hankinson SE, Spiegelman D, Curhan GC. The impact of protein intake on renal function decline in women with normal renal function or mild renal insufficiency. Ann Intern Med. 2003 Mar 18;138(6):460–467. doi: 10.7326/0003-4819-138-6-200303180-00009. [DOI] [PubMed] [Google Scholar]
- 14.Ogborn MR, Bankovic-Calic N, Shoesmith C, Buist R, Peeling J. Soy protein modification of rat polycystic kidney disease. The American journal of physiology. 1998 Mar;274(3 Pt 2):F541–549. doi: 10.1152/ajprenal.1998.274.3.F541. [DOI] [PubMed] [Google Scholar]
- 15.Tomobe K, Philbrick DJ, Ogborn MR, Takahashi H, Holub BJ. Effect of dietary soy protein and genistein on disease progression in mice with polycystic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1998 Jan;31(1):55–61. doi: 10.1053/ajkd.1998.v31.pm9428452. [DOI] [PubMed] [Google Scholar]
- 16.Aukema HM, Housini I, Rawling JM. Dietary soy protein effects on inherited polycystic kidney disease are influenced by gender and protein level. Journal of the American Society of Nephrology : JASN. 1999 Feb;10(2):300–308. doi: 10.1681/ASN.V102300. [DOI] [PubMed] [Google Scholar]
- 17.Ogborn MR, Nitschmann E, Weiler HA, Bankovic-Calic N. Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney international. 2000 Jan;57(1):159–166. doi: 10.1046/j.1523-1755.2000.00835.x. [DOI] [PubMed] [Google Scholar]
- 18.Trujillo J, Ramirez V, Perez J, et al. Renal protection by a soy diet in obese Zucker rats is associated with restoration of nitric oxide generation. American journal of physiology. Renal physiology. 2005 Jan;288(1):F108–116. doi: 10.1152/ajprenal.00077.2004. [DOI] [PubMed] [Google Scholar]
- 19.Kelemen LE, Kushi LH, Jacobs DR, Jr., Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. American journal of epidemiology. 2005 Feb 1;161(3):239–249. doi: 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 20.Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Annals of internal medicine. 2010 Sep 7;153(5):289–298. doi: 10.1059/0003-4819-153-5-201009070-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-94. Series 1: programs and collection procedures. Vital Health Stat 1. Jul;1994(32):1–407. [PubMed] [Google Scholar]
- 22.Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988-1994, 1999-2004. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2007 Dec;50(6):918–926. doi: 10.1053/j.ajkd.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Center for Health Statistics, Centers for Disease Control and Prevention NHANES III Total Nutrient Intakes File Documentation [Google Scholar]
- 25. [4/24, 2015];NHANES III Total Nutrient Intake File Documentation. Series 11. 1998 ftp://ftp.cdc.gov/pub/health_statistics/nchs/nhanes/nhanes3/2A/examdr acc.pdf.
- 26.Schakel SF, Buzzard IM, SGebhardt SE. Procedures for Estimating Nutrient Values for Food Composition Databases. J. Food Comp. Anal. 1996 Jun;10(2):102–114. [Google Scholar]
- 27.Abrass CK, Raugi GJ, Gabourel LS, Lovett DH. Insulin and insulin-like growth factor I binding to cultured rat glomerular mesangial cells. Endocrinology. 1988;123(3049050):2432–2439. doi: 10.1210/endo-123-5-2432. [DOI] [PubMed] [Google Scholar]
- 28.National Center for Health Statistics, Centers for Disease Control and Prevention NCHS Data Linked to Mortality Files. http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm.
- 29.National Center for Health Statistics, Centers for Disease Control and Prevention: Analytic and reporting guidelines The Third National Health and Nutrition Examination Survey, NHANES III (1988-94) http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf.
- 29a.Inker LA, Schmid CH, Tighiouart H, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. New England Journal of Medicine. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. [10/14/2013];Protein and Amino Acids 2006. 2013 http://books.nap.edu/openbook.php?record_id=11537&page=144.
- 31.Friedman AN. High-protein diets: potential effects on the kidney in renal health and disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2004 Dec;44(6):950–962. doi: 10.1053/j.ajkd.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 32.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. Jama. 2007 Mar 7;297(9):969–977. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 33.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006 Jan 3;144(1):21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 34.Chagnac A, Weinstein T, Herman M, Hirsh J, Gafter U, Ori Y. The effects of weight loss on renal function in patients with severe obesity. Journal of the American Society of Nephrology : JASN. 2003 Jun;14(6):1480–1486. doi: 10.1097/01.asn.0000068462.38661.89. [DOI] [PubMed] [Google Scholar]
- 35.Frank H, Graf J, Amann-Gassner U, et al. Effect of short-term high-protein compared with normal-protein diets on renal hemodynamics and associated variables in healthy young men. The American journal of clinical nutrition. 2009 Dec;90(6):1509–1516. doi: 10.3945/ajcn.2009.27601. [DOI] [PubMed] [Google Scholar]
- 36.Skov AR, Toubro S, Bulow J, Krabbe K, Parving HH, Astrup A. Changes in renal function during weight loss induced by high vs low-protein low-fat diets in overweight subjects. Int J Obes Relat Metab Disord. 1999 Nov;23(11):1170–1177. doi: 10.1038/sj.ijo.0801048. [DOI] [PubMed] [Google Scholar]
- 37.Elliott P, Stamler J, Dyer AR, et al. Association between protein intake and blood pressure: the INTERMAP Study. Archives of internal medicine. 2006 Jan 9;166(1):79–87. doi: 10.1001/archinte.166.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkins DJ, Kendall CW, Vidgen E, et al. High-protein diets in hyperlipidemia: effect of wheat gluten on serum lipids, uric acid, and renal function. The American journal of clinical nutrition. 2001 Jul;74(1):57–63. doi: 10.1093/ajcn/74.1.57. [DOI] [PubMed] [Google Scholar]
- 39.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. The New England journal of medicine. 1995 Aug 3;333(5):276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 40.Tovar AR, Murguia F, Cruz C, et al. A soy protein diet alters hepatic lipid metabolism gene expression and reduces serum lipids and renal fibrogenic cytokines in rats with chronic nephrotic syndrome. The Journal of nutrition. 2002 Sep;132(9):2562–2569. doi: 10.1093/jn/132.9.2562. [DOI] [PubMed] [Google Scholar]
- 41.Frassetto LA, Todd KM, Morris RC, Jr., Sebastian A. Estimation of net endogenous noncarbonic acid production in humans from diet potassium and protein contents. The American journal of clinical nutrition. 1998 Sep;68(3):576–583. doi: 10.1093/ajcn/68.3.576. [DOI] [PubMed] [Google Scholar]
- 42.Remer T, Manz F. Potential renal acid load of foods and its influence on urine pH. Journal of the American Dietetic Association. 1995 Jul;95(7):791–797. doi: 10.1016/S0002-8223(95)00219-7. [DOI] [PubMed] [Google Scholar]
- 43.Scialla JJ, Appel LJ, Astor BC, et al. Estimated net endogenous acid production and serum bicarbonate in African Americans with chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2011 Jul;6(7):1526–1532. doi: 10.2215/CJN.00150111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scialla JJ, Appel LJ, Astor BC, et al. Net endogenous acid production is associated with a faster decline in GFR in African Americans. Kidney international. 2012 Jul;82(1):106–112. doi: 10.1038/ki.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raphael KL, Wei G, Baird BC, Greene T, Beddhu S. Higher serum bicarbonate levels within the normal range are associated with better survival and renal outcomes in African Americans. Kidney international. 2011 Feb;79(3):356–362. doi: 10.1038/ki.2010.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandberg AS, Andersson H, Kivisto B, Sandstrom B. Extrusion cooking of a high-fibre cereal product. 1. Effects on digestibility and absorption of protein, fat, starch, dietary fibre and phytate in the small intestine. The British journal of nutrition. 1986 Mar;55(2):245–254. doi: 10.1079/bjn19860031. [DOI] [PubMed] [Google Scholar]
- 47.Wu IW, Hsu KH, Lee CC, et al. p-Cresyl sulphate and indoxyl sulphate predict progression of chronic kidney disease. Nephrol Dial Transplant. 2011 Mar;26(3):938–947. doi: 10.1093/ndt/gfq580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niwa T, Ise M. Indoxyl sulfate, a circulating uremic toxin, stimulates the progression of glomerular sclerosis. The Journal of laboratory and clinical medicine. 1994 Jul;124(1):96–104. [PubMed] [Google Scholar]
- 49.Motojima M, Hosokawa A, Yamato H, Muraki T, Yoshioka T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney international. 2003 May;63(5):1671–1680. doi: 10.1046/j.1523-1755.2003.00906.x. [DOI] [PubMed] [Google Scholar]
- 50.Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to mesenchymal transition. PLoS One. 2012;7(3):e34026. doi: 10.1371/journal.pone.0034026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adijiang A, Niwa T. An oral sorbent, AST-120, increases Klotho expression and inhibits cell senescence in the kidney of uremic rats. Am J Nephrol. 2010;31(2):160–164. doi: 10.1159/000264634. [DOI] [PubMed] [Google Scholar]
- 52.Pecoits-Filho R. Dietary protein intake and kidney disease in Western diet. Contributions to nephrology. 2007;155:102–112. doi: 10.1159/000101003. [DOI] [PubMed] [Google Scholar]
- 53.Bohn L, Meyer AS, Rasmussen SK. Phytate: impact on environment and human nutrition. A challenge for molecular breeding. Journal of Zhejiang University. Science. B. 2008 Mar;9(3):165–191. doi: 10.1631/jzus.B0710640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lei XG, Porres JM. Phytase enzymology, applications, and biotechnology. Biotechnology letters. 2003 Nov;25(21):1787–1794. doi: 10.1023/a:1026224101580. [DOI] [PubMed] [Google Scholar]
- 55.Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2011 Feb;6(2):257–264. doi: 10.2215/CJN.05040610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clifton PM. Protein and coronary heart disease: the role of different protein sources. Current atherosclerosis reports. 2011 Dec;13(6):493–498. doi: 10.1007/s11883-011-0208-x. [DOI] [PubMed] [Google Scholar]
- 57.Bosetti C, La Vecchia C, Talamini R, et al. Energy, macronutrients and laryngeal cancer risk. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2003 Jun;14(6):907–912. doi: 10.1093/annonc/mdg251. [DOI] [PubMed] [Google Scholar]
- 58.Mayne ST, Risch HA, Dubrow R, et al. Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2001 Oct;10(10):1055–1062. [PubMed] [Google Scholar]
- 59.Preis SR, Stampfer MJ, Spiegelman D, Willett WC, Rimm EB. Dietary protein and risk of ischemic heart disease in middle-aged men. The American journal of clinical nutrition. 2010 Nov;92(5):1265–1272. doi: 10.3945/ajcn.2010.29626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alonso A, Beunza JJ, Bes-Rastrollo M, Pajares RM, Martinez-Gonzalez MA. Vegetable protein and fiber from cereal are inversely associated with the risk of hypertension in a Spanish cohort. Archives of medical research. 2006 Aug;37(6):778–786. doi: 10.1016/j.arcmed.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Stamler J, Liu K, Ruth KJ, Pryer J, Greenland P. Eight-year blood pressure change in middle-aged men: relationship to multiple nutrients. Hypertension. 2002 May;39(5):1000–1006. doi: 10.1161/01.hyp.0000016178.80811.d9. [DOI] [PubMed] [Google Scholar]
- 62.Dong JY, Tong X, Wu ZW, Xun PC, He K, Qin LQ. Effect of soya protein on blood pressure: a meta-analysis of randomised controlled trials. The British journal of nutrition. 2011 Aug;106(3):317–326. doi: 10.1017/S0007114511000262. [DOI] [PubMed] [Google Scholar]
- 63.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. The American journal of clinical nutrition. 2008 Feb;87(2):339–346. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]