Abstract
Chronic renal insufficiency inexorably progresses in patients, such as it does after partial renal ablation in rats. However, the progression of renal diseases can be delayed by angiotensin II blockers that stabilize renal function or increase GFR, even in advanced phases of the disease. Regression of glomerulosclerosis can be induced by angiotensin II antagonism, but the effect of these treatments on the entire vascular tree is unclear. Here, using microcomputed tomography and scanning electron microscopy, we compared the size and extension of kidney blood vessels in untreated Wistar rats with those in untreated and angiotensin II antagonist–treated Munich Wistar Frömter (MWF) rats that spontaneously develop kidney disease with age. The kidney vasculature underwent progressive rarefaction in untreated MWF rats, substantially affecting intermediate and small vessels. Microarray analysis showed increased Tgf-β and endothelin-1 gene expression with age. Notably, 10-week inhibition of the renin-angiotensin system regenerated kidney vasculature and normalized Tgf-β and endothelin-1 gene expression in aged MWF rats. These changes were associated with reduced apoptosis, increased endothelial cell proliferation, and restoration of Nrf2 expression, suggesting mechanisms by which angiotensin II antagonism mediates regeneration of capillary segments. These results have important implications in the clinical setting of chronic renal insufficiency.
Keywords: angiotensin–converting enzyme inhibitors, renal progression, vascular disease
The progression of renal diseases leads invariably to end stage organ failure in patients as well as experimental models.1,2 This translates into one half million patients reaching ESRD each year globally and >700,000 deaths.2 The scale of the problem has been underestimated, whereas the costs for RRT are becoming problematic, even for wealthy nations. In the last 10 years, we and others have provided evidence that this progression can be significantly delayed and even reversed in rats and humans by the use of drugs that interfere with the renin-angiotensin system (RAS).3 However, despite important clinical implications, the mechanisms by which RAS inhibition induces reversal of renal lesions have not been established so far.
In this study, using imaging of rat kidney by microcomputed tomography (microCT) and scanning electron microscopy (SEM), we analyzed extension and organization of micro- and macrovasculature in Munich Wistar Frömter (MWF) rats, a model of progressive glomerular injury, and normal control Wistar rats. We also investigated the effect of RAS inhibition on kidney vasculature in MWF rats, treatments known to induce regression of renal structural and functional changes that develop spontaneously with age in these animals.4 Other than morphologic and morphometric evaluations of kidney vasculature, we finally elucidated the potential molecular mechanisms by gene and protein expression that could account for loss of vascular segments and changes in glomerular capillary (GC) organization in MWF rats and successful regeneration of kidney vasculature obtained by RAS inhibition.
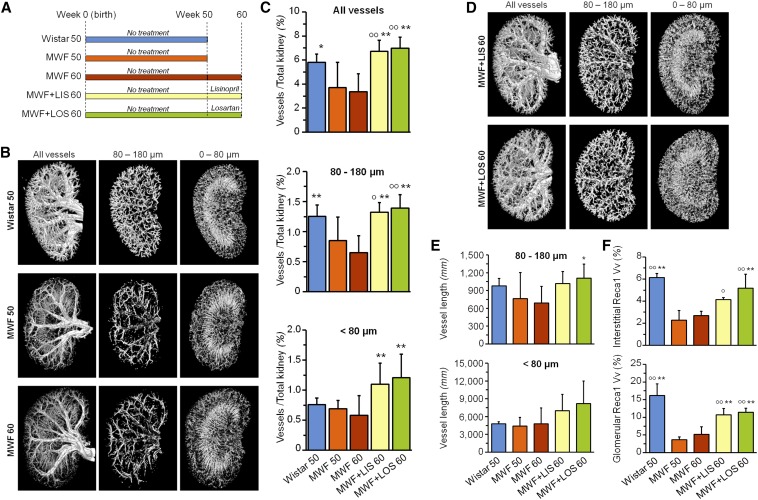
We investigated kidney vasculature in normal Wistar and MWF rats at different ages and with different treatments as reported in the diagram in Figure 1A. In MWF rats, the spatial density of arteries and veins was importantly reduced as kidney disease progressed at 50 weeks of age, when histologic damage at glomerular and tubular levels was already marked (Supplemental Figure 1, Supplemental Table 1) and important proteinuria developed, compared with Wistar rats (444±89 versus 6.6±1.5 mg/24 h in MWF50 versus Wistar50, respectively). The same pattern was observed for small arterial and venous vessels as well as the microcirculation (Figure 1B). Thus, the volume density of intermediate-sized vessels (80–180 µm diameter) was significantly reduced in aged MWF rats compared with controls (Figure 1, B and C). Both angiotensin–converting enzyme inhibitor (ACEi) and angiotensin II receptor antagonist (AIIRA) treatments markedly improved renal histology (with regression of glomerular and tubular damage) (Supplemental Figure 1, Supplemental Table 1), significantly reduced proteinuria (499±216 MWF60, 69±36 MWF+LIS60, and 101±42 mg/24 h MWF+LOS60), and robustly regenerated kidney vasculature (Figure 1D). Of note, both treatments importantly increased vasculature extension (Figure 1, C and D), which was higher than in control animals at same age. The regeneration of new vascular segments is also shown by the statistically significant increase in estimated length of small caliber blood vessels (Figure 1E). We also verified (as reported in Supplemental Material) that angiotensin–converting enzyme inhibition did not affect the volume density and estimated capillary length in two additional groups of Wistar rats (data not shown). Vascular rarefaction in MWF rats and regeneration after RAS inhibition were also confirmed by evaluating capillary volume density with rat endothelial cell antigen (Recal) staining in both interstitial and glomerular areas (Figure 1F).
Figure 1.
RAS blockade regenerates kidney vasculature. (A) Diagram of the experimental design. (B) Representative three–dimensional views of kidney vasculature by ex vivo microCT in 50- and 60-week-old MWF rats compared with 50-week-old Wistar rats and representation of intermediate– and small–sized capillary beds. (C) Quantification of vascular and kidney volume (top panel), vessels with diameter ranging from 80 to 180 μm (middle panel), and vessels with diameter <80 μm (bottom panel). (D) Representation of kidney vasculature in MWF rats on treatment with lisinopril and losartan. (E) Equivalent vessel length of blood vessels with diameters ranging from 80 to 180 μm (upper panel) and <80 μm (lower panel). (F) Quantification of capillary volume density (Vv) as a percentage of Reca1 positivity in interstitial (upper panel) and glomerular (lower panel) areas. Values are means±SDs; n=5 for Wistar and MWF50 rats, n=10 for untreated MWF60 rats and MWF rats treated from 50 to 60 weeks of age with lisinopril, and n=7 for MWF rats treated from 50 to 60 weeks of age with losartan. *P<0.05 versus MWF60; **P<0.01 versus MWF60; °P<0.05 versus MWF50; °°P<0.01 versus MWF50. LIS, lisinopril; LOS, losartan; Reca1, rat endothelial cell antigen 1.
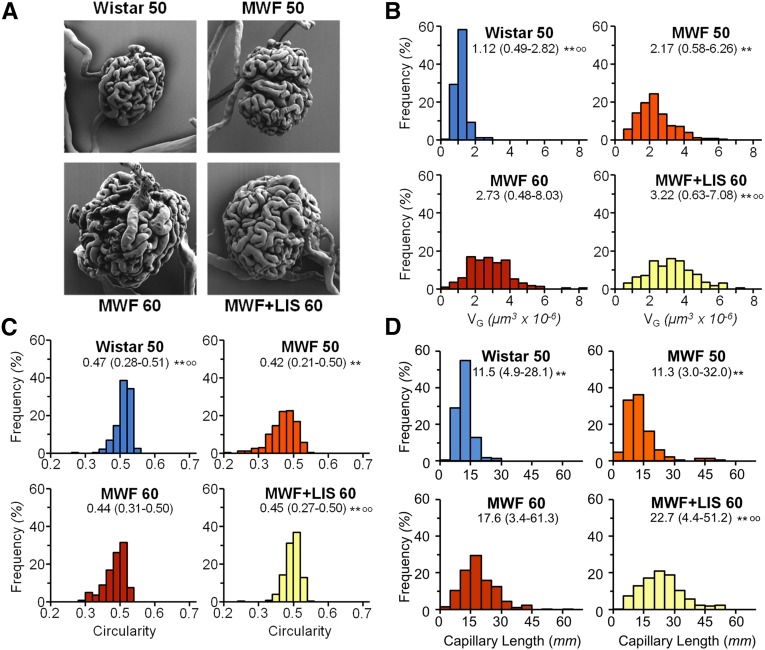
Vascular rarefaction is expected to translate in GC injury, which resulted in proteinuria, glomerulosclerosis, and loss of kidney function.5 We also reported that RAS inhibition induced regression of glomerular lesions and enhanced the tuft area devoid of sclerotic lesions.4 Here, we have now characterized the effects of ACEi and AIIRA on aged MWF rats at the level of GC ultrastructure by virtue of quantitative SEM. As shown in Figure 2A (and more extensively in Supplemental Figure 2), major changes characterized the glomerular population in MWF rats. Thus, mean GC volume (VG) increased with age compared with that in Wistar rats, with remarkable broadening of VG distribution (Figure 2B), likely for a compensatory mechanism. Similarly, the shape of the GC tuft in MWF rats was more irregular compared with that in Wistar rats, which was shown by the distribution of the circularity parameter (Figure 2C). Morphometric estimation of GC length (Figure 2D) showed a more heterogeneous distribution in the MWF50 group, with some glomeruli characterized by very long (up to 32 mm) and short (<3 mm) capillaries. Of interest, the mean GC diameter was higher in MWF rats compared with Wistar rats (10.9±0.3 versus 7.4±0.4 µm). RAS inhibition in MWF rats by lisinopril did not reduce but actually, increased the glomerular enlargement and heterogeneity observed with age in these rats. Actually, VG was higher in lisinopril–treated MWF rats than in untreated animals, with a higher number of glomeruli with large volume (up to 8 µm3×10−6). The same was observed for GC length (median [range]; 22.7 [4–51] in MWF+LIS60 versus 17.6 [3–61] mm in MWF60), with a larger fraction of glomeruli characterized by longer capillary length. On the contrary, capillary diameter remained constant (9.8±0.7 versus 9.4±0.6 µm). This detailed analysis of GC structure suggests that the kidney disease in this model modifies (to a major extent) the structure of functioning glomerular capillaries and ACEi treatment, likely affecting the remaining functional nephrons and further increasing the heterogeneity of the glomerular tufts with formation of new capillary segments in already enlarged capillary tufts. These changes likely provide higher volumes of functional GC segments and consequently, increased filtering surface area. Our results of increased mean diameter as well as segment length indicate that hydraulic resistance of GC networks may not be changed by treatments. Actually, enlargement of capillary diameter is expected to reduce hydraulic resistance, whereas elongation of capillary segments is expected to increase it. These two effects may cancel each other.
Figure 2.
ACE inhibition enhances GC volume and length. (A) Representative SEM images of GC lumen in Wistar and MWF rats. (B) VG distribution histograms in Wistar and MWF rats. (C) Distribution histogram of glomerular circularity. (D) Distribution histogram of estimated individual capillary length. Values are median (range; n=225 glomeruli per group). **P<0.01 versus MWF60; °°P<0.01 versus MWF50. LIS, lisinopril.
To clarify the molecular mediators underlying the regeneration of the kidney vasculature elicited by RAS blockade, we first analyzed by PCR array the renal expression profile of 84 genes involved in the modulation of the biologic processes of angiogenesis (Figure 3A). Unexpectedly, gene expression of vascular growth–promoting factors, such as Vegf and related receptors or angiopoietin-1 and -2, did not change between MWF60 and Wistar rats, whereas genes related to fibrosis, inflammation, and extracellular matrix remodeling were differentially expressed between the two strains. This framework is consistent with hallmarks of renal scarring that characterize advanced nephropathy in MWF rats when vasculature rarefaction was strongly evident. Upregulated profibrotic genes included the three Tgf-β isoforms, with Tgf-β2 being the most expressed, and endothelin-1 (Et-1). Validation of the array data by quantitative RT-PCR (Figure 3B) confirmed renal overexpression of Tgf-β2 and Et-1 genes in MWF rats. Of interest is the observation that abnormal expression of such genes was almost normalized by both ACEi and AIIRA. These findings are in line with previous evidence showing that renoprotection induced by ACEi in MWF rats was associated with normalization of TGF-β protein in glomeruli and the cortical interstitium and paralleled the reduction in urinary excretion of ET-1, which likely reflects the renal synthesis of the peptide.4,6,7 ET-1, synthesized predominantly (although not exclusively) in endothelial cells (ECs), exerts proinflammatory, mitogenic, and profibrotic effects through the ETA receptor (ETAR).8,9 At the level of glomerular microcirculation, it has been reported that podocyte-specific activation of TGF-β signaling results in the release of ET-1 by visceral epithelial cells that act as paracrine stimulus for EC dysfunction through ETAR activation, setting in motion a vicious cycle that leads to podocyte depletion that eventuates in segmental glomerular damage.10 This evidence prompted us to investigate the role of endothelial deregulation of ET-1/ETAR signaling in MWF rats, a well known model of progressive endothelial and podocyte loss.5,11 To identify the cellular sources of ET-1, we performed multiple immunostaining and found that ET-1 protein was highly expressed by both ECs and podocytes, which were documented by costaining of Reca1 and α-actinin4, in kidneys of MWF compared with Wistar rats (Supplemental Figure 3A). Renal ETAR expression in the vascular endothelium of MWF rats was also increased in a time-dependent manner (Supplemental Figure 3B). Angiotensin II inhibition markedly reduced ET-1 renal expression to control levels, particularly in cortical interstitium (Supplemental Figure 3A), and normalized ETAR endothelial expression, which was indicated by reduced ETAR staining in Reca1-positive cells (Supplemental Figure 3B).
Figure 3.
RAS blockade downregulates profibrotic genes and inhibits endothelial-to-mesenchymal transition. (A) Expression profile by PCR array of genes involved in angiogenesis in Wistar and MWF rats treated or not treated with lisinopril. Gene expression changes were detected comparing the normalized expression of individual genes between Wistar and MWF rats as well as between MWF60 rats and MWF rats treated from 50 to 60 weeks of age with lisinopril or losartan. The lines on the scatter plots indicate the 2-fold boundary used for selecting genes with differential expression. (B) TGF-β2 and ET-1 mRNA expressions normalized to GAPDH in MWF rats with respect to Wistar rats, which were used as physiologic reference. (C) Immunostaining for Reca1 (green) and αSMA (red) showing the effect of both lisinopril and losartan on EndMT. (D) Quantitative assessment expressed as the percentage of Reca1/αSMA-positive area on total Reca1–positive area per ×40 field at glomerular (left panel) and interstitial (right panel) levels. (E) Evaluation of interstitial fibrotic vessels, which were identified as large vessels with abnormal αSMA accumulation on total Reca1+ large vessels, in untreated and lisinopril– and losartan–treated MWF rats. Values are means±SDs (n=3). Scale bar, 50 μm. **P<0.01 versus MWF60; °P<0.05 versus MWF50; °°P<0.01 versus MWF50. Ct, cycle threshold; EndMT, endothelial-to-mesenchymal transition; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LIS, lisinopril; LOS, losartan; Reca1, rat endothelial cell antigen 1.
Both TGF-β2 and ET-1 promote the endothelial-to-mesenchymal transition, which generates matrix-producing fibroblasts and/or myofibroblasts, while directly leading to EC loss.12–15 Although we cannot exclude the contribution of pericyte detachment from EC to scar-forming myofibroblasts, to assess the contribution of the endothelial-to-mesenchymal transition to vascular rarefaction, we evaluated the expression of the mesenchymal marker α-smooth muscle actin (αSMA) at the vascular level by means of colocalization with the Reca1 antigen. Glomerular and interstitial peritubular capillaries of MWF rats strongly expressed αSMA, whereas only occasional staining was observed in Wistar rats. RAS blockade by ACEi and AIIRA significantly reduced αSMA expression in kidney microvasculature of MWF rats to an equal extent (Figure 3, C and D). A similar mechanism seems to operate at the level of larger vessels to the extent that αSMA staining progressively increased, reaching a peak at 60 weeks in MWF rats. RAS blockade did not simply lower αSMA accumulation in large vessels but even normalized it in most animals (Figure 3E).
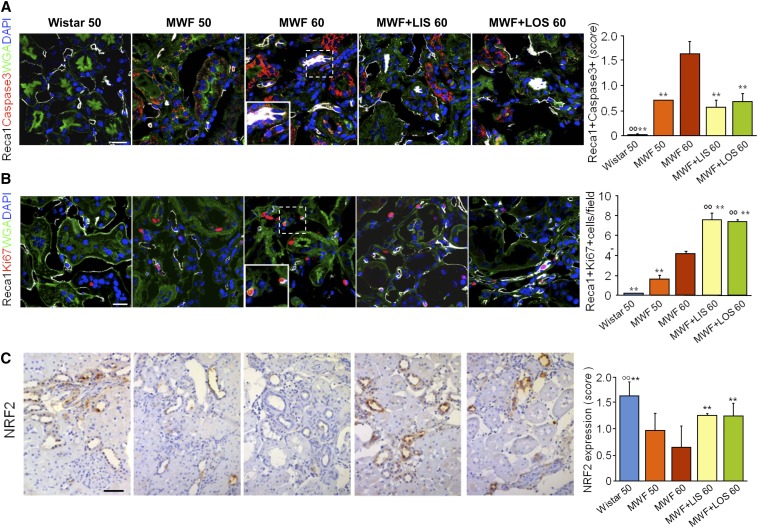
Collectively, these data indicate that inhibition of TGF-β and ET-1/ETAR signaling likely explains the beneficial effect of RAS blockade on the regeneration of kidney vasculature in advanced nephropathy. Because on RAS blockade, new vessel segment and capillary formation occurs, we further explored how the balance between apoptosis and proliferation at the EC level could account for vessel regeneration. Activated caspase3 was strongly upregulated in Reca1-positive ECs in MWF rats at 50 weeks of age and even more at 60 weeks of age (Figure 4A). However, ECs were induced to proliferate, which was confirmed by the increased number of Ki67-positive cells in the renal vascular compartment, possibly to counterbalance the onset of the apoptotic events (Figure 4B). Angiotensin II inhibition significantly reduced the number of apoptotic cells and sustained the compensating protective mechanism fostering EC proliferation, which could account for the important increase in the density and length of the microvessels observed by microCT (Figure 1, C and D) in treated MWF rats. NF-E2–related factor2 (Nrf2) has been recently recognized as a critical intracellular regulator of EC dynamics, governing angiogenic sprouting and vascular branching.16 Such pathways can be theoretically targeted therapeutically to counteract the effects of oxidative stress and TGF-β/Smad–mediated renal fibrosis.17–20 Finding here that RAS blockade rescues Nrf2 expression (as shown in Figure 4C), which was significantly decreased in untreated MWF rats, is consistent with this possibility.
Figure 4.
RAS blockade reduces EC apoptosis, while increases proliferation, and restores Nrf2 expression. (A) Immunostaining for Reca1 (white) and caspase3 (red). Apoptotic ECs were evaluated by semiquantitative score (from zero to three in each field). (B) Immunostaining and quantification of Reca1 (white) and Ki67 (red) expression in untreated and lisinopril– or losartan–treated MWF rats. Nuclei were stained with DAPI (blue), and renal structures were stained with WGA (green). The enlarged details are shown in Insets in A and B. (C) Immunostaining for Nrf2 in formalin–fixed, paraffin–embedded kidney sections by immunoperoxidase technique. Nrf2 expression was evaluated by semiquantitative score (from zero to three in each field). Values are means±SDs (A and B, n=3; C, n=4). Scale bar, 50 μm. **P<0.01 versus MWF60; °°P<0.01 versus MWF50. DAPI, 4′,6-diamidin-2-fenilindolo LIS, lisinopril; LOS, losartan; Reca1, rat endothelial cell antigen1; WGA, wheat germ agglutinin.
In conclusion, our findings of vascular rarefaction in progressive renal disease in the MWF rat and vascular regeneration on RAS inhibition disclose a new paradigm for renal disease progression and offer room for therapeutic interventions. Vascular regeneration by angiotensin II antagonism, documented here for the first time, encompasses the entire vascular tree and is apparently accomplished by halting TGF-β– and ET-1–mediated damage. This phenomenon enhances the intrinsic reparative capability of the kidney by balancing regeneration and fibrosis and promoting angiogenesis, Nrf2–dependent vascular remodeling, and EC proliferation. These effects translate into improvement of tissue blood perfusion and filtration capacity and regression of kidney fibrosis. These results have obvious important implications for human medicine, showing effective improvement of an important clinical problem2 that has costs are becoming problematic, even for wealthier nations.
Concise Methods
Animal Studies
The study design is graphically reported in Figure 1A, and more detailed information is reported in Supplemental Material. Briefly, one group of Wistar rats was studied at 50 weeks of age, and four groups of MWF rats were studied at 50 and 60 weeks of age with and without treatment with lisinopril or losartan. Kidney vasculature was investigated by microCT imaging with the use of a radio-opaque resin (Microfil; Flow Tech) injected in the kidney under general anesthesia (Supplemental Material). Imaging by microCT was obtained by three–dimensional numeric reconstruction using the NRecon software (Bruker-MicroCT), and quantification of vasculature components was performed using CTAnalyser software (Bruker-MicroCT). Details on calculations and statistics of geometric parameters of kidney blood vessels are reported in Supplemental Material. Glomerular microcirculation was investigated by morphologic and quantitative SEM using a casting technique and imaging on a scanning electron microscope (Cross-Beam 1540EsB; Carl Zeiss). Morphometric analysis was performed on digital images to estimate GC dimensions as described in Supplemental Material.
Gene Expression Analysis and Immunohistochemistry
Total RNA was extracted with the Qiagen RNeasy Mini Kit and reverse transcribed with the RT2 First-Strand Kit (Qiagen). The Rat Angiogenesis Pathway RT2 Profiler PCR Array (Qiagen) was used to determine the differentially expressed genes. Validation of Tgf-β2 and Et-1 gene expression was performed by quantitative RT-PCR. Reca1, αSMA, Ki67, caspase3, ETAR, ET-1, and α-actinin4 protein expression was evaluated by immunofluorescence experiments, whereas Nrf2 expression was detected by the immunoperoxidase technique, which is specified in Supplemental Material.
Statistical Analyses
Data are expressed as means±SDs or medians and ranges as specified. Statistical analysis was performed using ANOVA (Prism 6.0; GraphPad Software Inc., San Diego, CA). Bonferroni post hoc analysis was adopted to estimate statistical significance of between groups’ comparisons. Statistical significance was defined as P<0.05.
Study Approval
Animal care and treatment were conducted in accordance with the institutional guidelines and compliance with national (DL 116, GU 18/2/1992, Circ. 8, and G.U 14/7/1994) and international laws and policies (Dir. 2010/63/EU and 9/22/2010). All animal studies were approved by the Institutional Animal Care and Use Committee of Istituto di Ricerche Farmacologiche Mario Negri.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Dr. Lucia Condorelli, Dr. Katia Passera, and Daniela Cavallotti for excellent assistance during scanning electron microscopy and image processing.
We also thank the Associazione Ricerca sulle Malattie Rare Research Association for supporting P.R. with a research fellowship.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014100971/-/DCSupplemental.
References
- 1.Brenner BM, Meyer TW, Hostetter TH: Dietary protein intake and the progressive nature of kidney disease: The role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med 307: 652–659, 1982 [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA, 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Remuzzi A, Gagliardini E, Sangalli F, Bonomelli M, Piccinelli M, Benigni A, Remuzzi G: ACE inhibition reduces glomerulosclerosis and regenerates glomerular tissue in a model of progressive renal disease. Kidney Int 69: 1124–1130, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Macconi D, Bonomelli M, Benigni A, Plati T, Sangalli F, Longaretti L, Conti S, Kawachi H, Hill P, Remuzzi G, Remuzzi A: Pathophysiologic implications of reduced podocyte number in a rat model of progressive glomerular injury. Am J Pathol 168: 42–54, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remuzzi A, Benigni A, Malanchini B, Bruzzi I, Foglieni C, Remuzzi G: ACE inhibition prevents renal failure and death in uninephrectomized MWF/Ztm rats. Kidney Int 47: 1319–1326, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Benigni A, Perico N, Gaspari F, Zoja C, Bellizzi L, Gabanelli M, Remuzzi G: Increased renal endothelin production in rats with reduced renal mass. Am J Physiol 260: F331–F339, 1991 [DOI] [PubMed] [Google Scholar]
- 8.Gagliardini E, Buelli S, Benigni A: Endothelin in chronic proteinuric kidney disease. Contrib Nephrol 172: 171–184, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Remuzzi G, Perico N, Benigni A: New therapeutics that antagonize endothelin: Promises and frustrations. Nat Rev Drug Discov 1: 986–1001, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, Yu L, D’Agati V, Schlondorff D, Kriz W, Haraldsson B, Bottinger EP: Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest 124: 1608–1621, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macconi D, Sangalli F, Bonomelli M, Conti S, Condorelli L, Gagliardini E, Remuzzi G, Remuzzi A: Podocyte repopulation contributes to regression of glomerular injury induced by ACE inhibition. Am J Pathol 174: 797–807, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chrobak I, Lenna S, Stawski L, Trojanowska M: Interferon-γ promotes vascular remodeling in human microvascular endothelial cells by upregulating endothelin (ET)-1 and transforming growth factor (TGF) β2. J Cell Physiol 228: 1774–1783, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Xu Y, Koya D, Kanasaki K: Role of the endothelial-to-mesenchymal transition in renal fibrosis of chronic kidney disease. Clin Exp Nephrol 17: 488–497, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Medici D, Potenta S, Kalluri R: Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling. Biochem J 437: 515–520, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei Y, Gong J, Thimmulappa RK, Kosmider B, Biswal S, Duh EJ: Nrf2 acts cell-autonomously in endothelium to regulate tip cell formation and vascular branching. Proc Natl Acad Sci U S A 110: E3910–E3918, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujita H, Fujishima H, Morii T, Sakamoto T, Komatsu K, Hosoba M, Narita T, Takahashi K, Takahashi T, Yamada Y: Modulation of renal superoxide dismutase by telmisartan therapy in C57BL/6-Ins2(Akita) diabetic mice. Hypertens Res 35: 213–220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kang SJ, You A, Kwak MK: Suppression of Nrf2 signaling by angiotensin II in murine renal epithelial cells. Arch Pharm Res 34: 829–836, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Aminzadeh MA, Reisman SA, Vaziri ND, Shelkovnikov S, Farzaneh SH, Khazaeli M, Meyer CJ: The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores endothelial function impaired by reduced Nrf2 activity in chronic kidney disease. Redox Biol 1: 527–531, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oh CJ, Kim JY, Choi YK, Kim HJ, Jeong JY, Bae KH, Park KG, Lee IK: Dimethylfumarate attenuates renal fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. PLoS ONE 7: e45870, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.