Abstract
The iron-regulatory peptide hepcidin exhibits antimicrobial activity. Having previously shown hepcidin expression in the kidney, we addressed its role in urinary tract infection (UTI), which remains largely unknown. Experimental UTI was induced in wild-type (WT) and hepcidin-knockout (Hepc−/−) mice using the uropathogenic Escherichia coli CFT073 strain. Compared with infected WT mice, infected Hepc−/− mice showed a dramatic increase in renal bacterial load. Moreover, bacterial invasion was significantly dampened by the pretreatment of WT mice with hepcidin. Infected Hepc−/− mice exhibited decreased iron accumulation in the renal medulla and significant attenuation of the renal inflammatory response. Notably, we demonstrated in vitro bacteriostatic activity of hepcidin against CFT073. Furthermore, CFT073 repressed renal hepcidin, both in vivo and in cultured renal cells, and reduced phosphorylation of SMAD kinase in vivo, suggesting a bacterial strategy to escape the antimicrobial activities of hepcidin. In conclusion, we provide new mechanisms by which hepcidin contributes to renal host defense and suggest that targeting hepcidin offers a strategy to prevent bacterial invasion.
Keywords: pyelonephritis, renal epithelial cell, renal protection, cell and transport physiology, cell signaling, kidney disease
Urinary tract infection (UTI), one of the most common infectious diseases of humans, is mainly caused by uropathogenic Escherichia coli (UPEC).1,2 When E. coli, the intestinal inhabitant, become pathogenic and acquire virulence factors, they reach the urinary tract and affect a wide range of cellular processes.3,4 Indeed, the urinary tract is sterile and armed with host defenses such as reduced availability of iron, antimicrobial peptides, the acidic pH of urine, and inflammatory responses mediating the clearance of acute UPEC infection. Iron is particularly deficient in the urine because iron is permanently bound to transferrin in the plasma, which limits its glomerular filtration. This situation may explain why UPEC, such as the prototypical pyelonephritis-associated CFT073 strain, overexpress several iron acquisition systems during UTI including chelate iron siderophores.5,6 We have recently shown that renal iron content and the renal iron carriers are under the control of hepcidin, the major peptide that regulates iron homeostasis.7 Hepcidin has been discovered in 2000 as an antimicrobial peptide8,9 and in 2001 as a hyposideremic peptide.10,11 Hepcidin is mainly produced by hepatocytes in response to increased serum and tissue iron,10,11 and inhibits iron efflux from duodenal enterocyte and macrophage stores. Hepcidin binds to ferroportin, the sole iron exporter in mammalian cells, and limits the iron efflux.12,13 In absorptive enterocytes, we and other authors showed that hepcidin reinforces the control over iron absorption by additionally downregulating the iron importer DMT1 present at the apical plasma membrane.14–17 Hepcidin is regulated by iron through a complex of integral hemochromatosis proteins, i.e., HFE, HJV (hemojuvelin) and transferrin receptor 2. These proteins tightly coordinate signaling through the BMP6/HJV/SMAD pathway.18–20
Hepcidin was observed to have additionally an in vitro antimicrobial activity and a structure reminiscent of four disulfide defensins.8,9 Hepcidin synthesis is induced by inflammatory signals such as IL-6, allowing it to play a major role in the anemia associated with chronic diseases and inflammation.21–23 Hepcidin is also induced in the liver in response to LPS through activin B and Smad1/5/8-signaling24 and in macrophages through Toll-like receptor 4 (TLR4) signaling.25 Notably, hepcidin expression was recently identified in several epithelial barriers that are frequently confronted by pathogen infection, including renal distal nephron.7,26–28 However, whether hepcidin is required for resistance to epithelial barrier infection has received little attention. Here, we provide evidence that hepcidin may represent an effective defense system against UPEC infection and more interestingly that UPEC represses local hepcidin to evade renal host defenses during UTI.
Results
Expression and Localization of Hepcidin in the Kidney
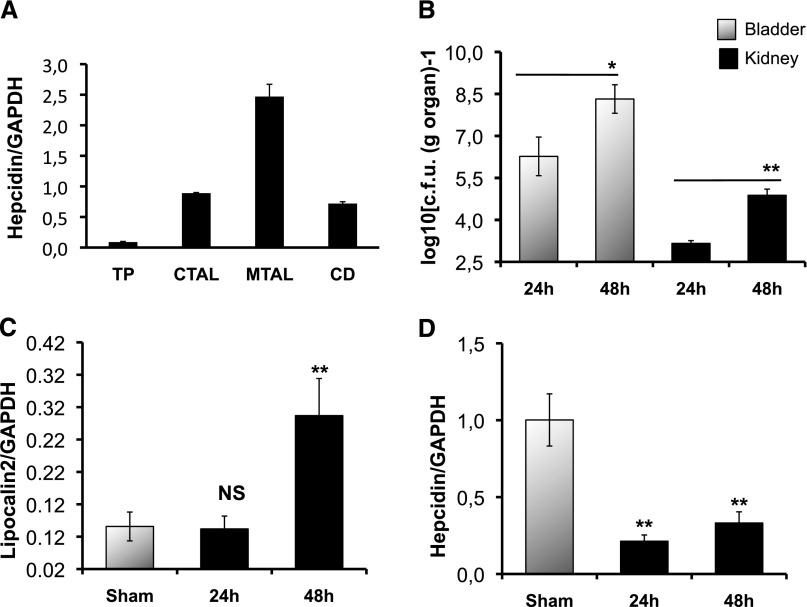
Quantitative RT-PCR was performed to analyze the relative levels of hepcidin-mRNA in isolated proximal tubules, the cortical and medullary thick ascending limb, and the collecting duct microdissected from wild-type (WT) kidneys. Figure 1A evidenced a large and preferential expression of hepcidin in the distal nephron, as previously described.29 The hepcidin transcript level was considerably higher in the medullary thick ascending limb but was nearly undetectable in the proximal tubules.
Figure 1.
Hepcidin expression and bacterial invasion. (A) Hepcidin mRNA quantification in isolated microdissected renal tubules. TP, proximal tubule; CTAL, cortical thick ascending limb; MTAL, medullary thick ascending limb; CD, collecting duct. (B) Bacterial invasion in bladder and kidneys. CBA/J WT mice were infected with 109 colony-forming units of CFT073, and bacterial counts were performed on bladder and kidneys at 24 and 48 hours postinfection. (Sham) means unifected mice. Gray bars are bacterial counts in the bladder, and black bars are bacterial counts in the kidneys. (C) and (D) Hepcidin and lipocalin-2 mRNA quantifications in the WT kidneys at 24 and 48 hours postinfection, respectively. All mRNA quantifications were determined by quantitative RT-PCR. Data were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mRNA levels. The results are the mean±SEM of at least six individual mice in each group. *P<0.05; **P<0.001. NS, nonsignificant.
Decreased Renal Hepcidin Expression in UPEC Infection
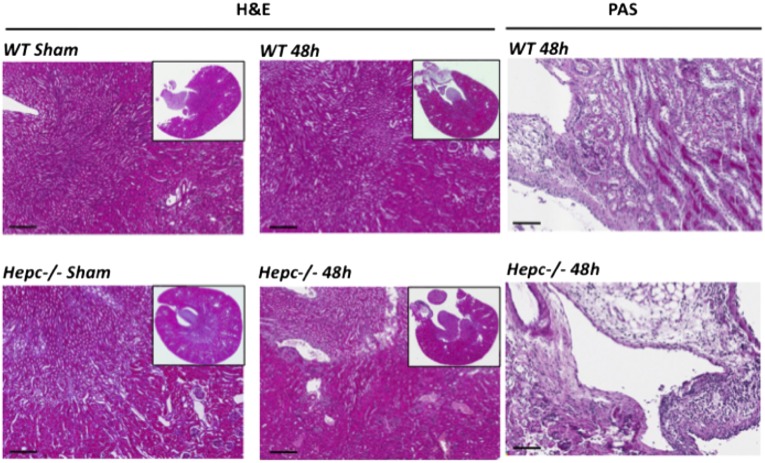
The medullary collecting ducts represent the preferential site of UPEC colonization,30 we therefore assessed the impact of CFT073 infection on local synthesis of hepcidin. We measured bladder and kidney-associated titers of CFT073 following inoculations of adult CBA/j female mice. CFT073 colony-forming unit was significantly greater in the 48 hours postinfected than 24 hours bladder and kidneys (Figure 1B). In these experiments, there was no bacterial sepsis evaluated by a CFT073 count in the liver homogenates. The mRNA level of lipocalin-2, the siderophore chelator used as a positive control, was greatly increased at 48 hours postinfection while hepcidin-mRNA abundance was significantly decreased at both 24 and 48 hours postinfection (approximately 5-fold decrease, P<0.001) (Figure 1, C and D). Hepcidin repression was dominant despite a significant local increase of TLR4 and IL-6 transcripts (Supplemental Figure 1). This effect appeared to be kidney-specific because the expression of hepcidin in liver and in serum (measured by liquid chromatography-tandem mass spectrometry) was either not increased or increased at 24 and 48 hours postinfection, respectively (Supplemental Figure 2). Kidney damage by CFT073 infection was evaluated by the examination of hematoxylin and eosin (H&E), periodic acid–Schiff (PAS), and picrosirus red -stained sections. Renal tissue integrity was conserved and the infected kidneys exhibited only a minor epithelial proliferation 48 hours postinfection with no evidence of fibrosis or tubular dilatation (Figure 2 and Supplemental Figure 3). We additionally quantified the mRNA expression of Na+/H+ exchanger 3 (NHE3), the Na+/K+/2Cl- co-transporter (BSC1) and the water channel aquaporin-2 as well as the tight junction protein ZO-1. No change was observed in any of these genes (Supplemental Figure 4), suggesting that the loss of hepcidin was not because of structural damage of the tubular epithelium.
Figure 2.
Histologic analysis in the kidney. WT and Hepc−/− mice were infected with 109 colony-forming units of CFT073. After 48 hours postinfection, kidney sections were stained with hematoxylin and eosin staining (H&E) and with periodic acid–Schiff staining (PAS). (Sham) means uninfected mice. Scalebars represents 200 µm for H&E and 80 µm for PAS.
Involvement of Hepcidin in Opposing UPEC Infection
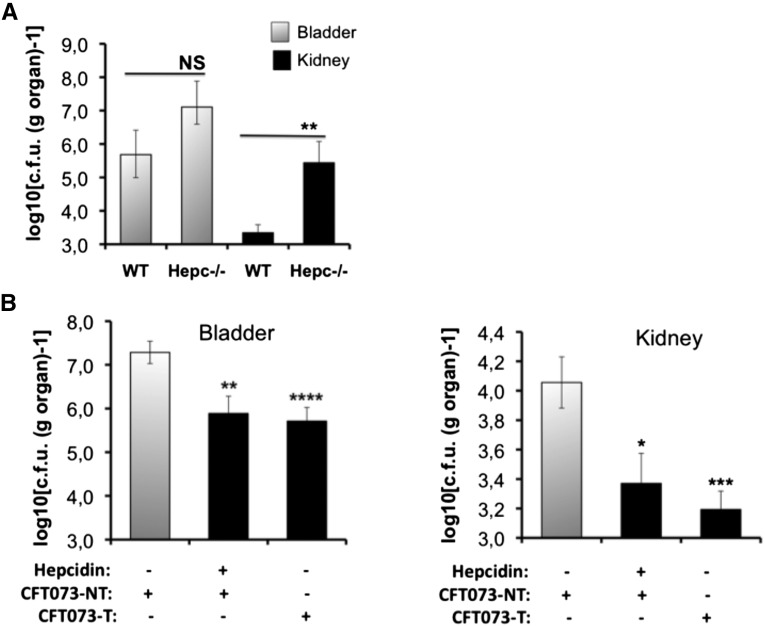
We then determined the effect of CFT073 in Hepc−/− mice. These mice were of C57BL/6 genetic background. Therefore, for these experiments, we used as control animals the C57BL/6 WT littermates. As shown in Figure 3A, at 48 hours postinfection, significantly greater bacterial loads were detected in kidneys from Hepc−/− mice than in their WT counterparts. CFT073 colony-forming unit was also but not significantly greater in the bladder of Hepc−/− compared with WT mice. In addition, renal bacterial loads were significantly reduced when WT mice were intraperitoneally injected with synthetic murine hepcidin peptide 1 hours before infection (Figure 3B). A similar effect was observed when CFT073 were pretreated with hepcidin before inoculation to WT mice (Figure 3B). These data provide in vivo evidence that hepcidin improves renal host protection against bacterial infection and strongly suggest that hepcidin may represent one of the targets for UPEC to evade renal host defenses.
Figure 3.
Involvement of hepcidin against CFT073 infection. (A) C57BL/6 WT and Hepc−/− mice were infected with 109 colony-forming units (c.f.u.s) of CFT073; bacterial counts were performed on bladder and kidneys at 48 hours postinfection. Gray bars are bacterial counts in the bladder, and black bars are bacterial counts in the kidneys. (B) C57BL/6 WT mice were intraperitoneally injected either with NaCl 0.9% (hepcidin –) or with 5 mg/kg hepcidin (hepcidin +). After 1 hour, the two groups were inoculated with 109 c.f.u.s of non-treated CFT073 (CFT073-NT). In a third group (CFT073-T), hepcidin (200 µg/ml) was added to the CFT073 suspension 1 hour prior to WT mice inoculation. At 48 hours postinfection, bacterial counts in the bladder and in the kidneys were determined. The results are the mean±SEM of at least six individual mice in each group. *P<0.05; **P<0.03; ***P<0.02; ****P<0.001. NS, nonsignificant.
Iron Availability Regulated by Hepcidin to Oppose UPEC Infection
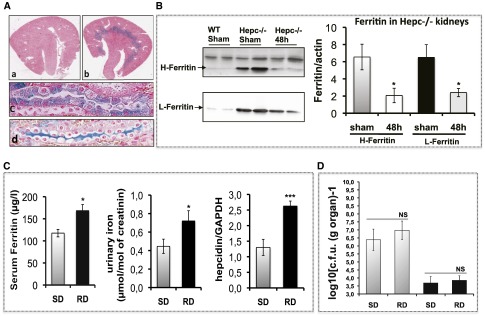
We then investigated the benefit barriers for UPEC in the absence of hepcidin. Compared with WT mice, Hepc−/− mice exhibited significant iron overload in renal medulla and in the urine (Figure 4A, and Moulouel et al.).7 Iron excess was mainly sequestered by increased ferritin but the protein levels of both H-ferritin and L-ferritin were significantly reduced in Hepc−/− kidneys following infection, suggesting that in the absence of hepcidin, iron is easily consumed by UPEC (Figure 4B). In WT mice, the basal level of ferritin was quite low, rendering it impossible to be quantified before and after infection. We tested the bacterial load of CFT073 in WT mice previously fed with an iron-rich diet (RD mice). Figure 4C showed that RD mice exhibited high serum ferritin and considerable excretion of iron in urine. However, this urinary iron bioavailability was not advantageous for CFT073 growth (Figure 4D), probably because endogenous hepcidin is already increased in these mice (Figure 4C).
Figure 4.
Iron content in the kidney. (A) Tissue iron content was determined by Perl’s staining of the kidney sections. The upper panel (a) and (b) are representative images of WT (a) and Hepc−/− (b) kidneys. Iron deposition was observed only in Hepc−/− kidneys, mostly in the medulla with a minor presence in the cortical region. At higher magnification (the two lower panels), iron was observed in thick ascending limb cells (c) and in the lumen of the collecting duct (d). (B) Protein expression of H-ferritin and L-ferritin in the kidney. WT and Hepc−/− mice were infected with 109 colony-forming units (CFUs) of CFT073, and the protein expression of H-ferritin and L-ferritin in the kidneys was determined by western blot at 48 hours postinfection. The amounts of both proteins were normalized to β-actin. (C) Iron parameters in mice fed with an iron-rich diet (RD) compared with control mice with standard died (SD), were evaluated by the measure of serum ferritin, urinary iron and hepatic level of hepcidin-mRNA. (D) SD and RD mice were infected with 109 CFUs of CFT073, and bacterial counts were performed on bladder and kidneys at 24 and 48 hours postinfection. (Sham) means uninfected mice. Gray bars are bacterial counts in the bladder, and black bars are bacterial counts in the kidneys. The results are the mean±SEM of at least six individual mice in each group. *P<0.05; ***P<0.003. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, nonsignificant.
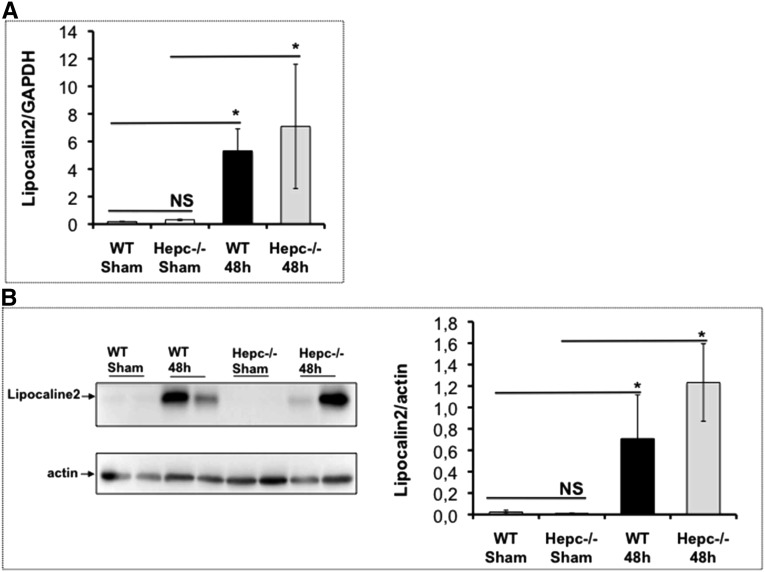
We also explored the expression of lipocalin-2 in Hepc−/− kidneys. Indeed, lipocalin-2 has recently been reported to induce growth arrest of CFT073 by chelation of their siderophores.31 The expression level of lipocalin-2 was not increased in uninfected Hepc−/− kidneys (Figure 5). However, following UPEC infection, both mRNA and protein levels of lipocalin-2 were induced in WT and Hepc−/− infected mice, and to the same extent, suggesting that lipocalin-2 is not influenced by the lack of hepcidin nor by iron overload in urine and thick ascending limb cells (Figure 5). Thus, for UTI protection, hepcidin and lipocalin-2 are likely to be complementary and have non-redundant functions.
Figure 5.
mRNA and protein expression of lipocaline-2 in Hepc−/−: WT and Hepc−/− mice were infected with 109 colony-forming units of CFT073 during 48 hours. (A) the mRNA level of lipocalin-2 was quantified by quantitative RT-PCR in uninfected (sham) and infected (48 h) kidneys. (B) Protein level of lipocalin-2 was determined by western blot and its amount was normalized to β-actin. (Sham) means uninfected mice. *P<0.05. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NS, nonsignificant.
Inflammatory Response of the Kidneys Triggered by Hepcidin Following CFT073 UTI
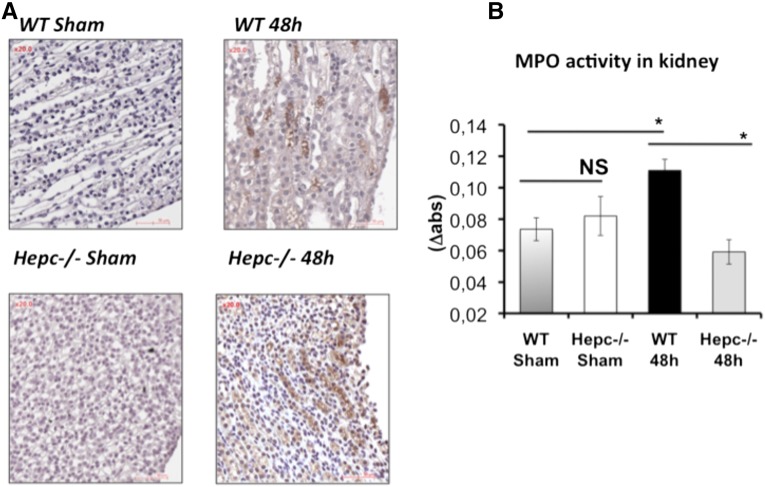
We examined histologic specimens from Hepc−/− and WT kidneys following CFT073 UTI (Figure 2, Supplemental Figure 3). We noted a greater inflammatory infiltrate at the papilla and urinary tract (bordered by transitional epithelium) in Hepc−/− compared with WT mice. At higher magnification, the H&E and PAS staining showed that the inflammatory infiltrate in Hepc−/− mice was rather composed of lymphocytes and plasma cells than of neutrophils. We next analyzed the expression levels of inflammatory cytokines. IL-6, IL-1α, TNF-α, IFN-γ, CXCL2, and CCL5/RANTES were significantly increased in the infected WT mice compared with controls (Table 1). However, for the infected Hepc−/− mice, except IL-6 and IFN-γ that were increased, the expression levels of the other cytokines showed little or no change compared with sham WT mice. Also, TNF-α and IL-1α were induced in sham Hepc−/− animals but they were highly reduced after UTI (Supplemental Table 1). We also investigated macrophage and neutrophil recruitment to the kidney site of infection. In Figure 6A, the positive immunostaining of F4/80 and quantification indicated a similar amount of macrophage infiltrate in the medullary region of both WT and Hepc−/− infected mice. However, myeloperoxidase activity was significantly decreased in Hepc−/−compared with WT infected mice (Figure 6B), confirming the reduced neutrophil influx observed by PAS renal sections staining. Altogether these results suggest that a lack of hepcidin is significantly associated with attenuated inflammatory response to CFT073.
Table 1.
Expression profile of inflammatory cytokines in WT and Hepc−/− kidneys prior to and after CFT073 infection
| Cytokines | Sham (ng/ml) | WT | Hepc−/− 48 h (ng/ml) | Hepc−/− versus WT in 48 h P Value | |
|---|---|---|---|---|---|
| 48 h (ng/ml) | P Value | ||||
| IL-6 | 15.9±0.7 | 70.5±15.8 | 0.01 | 87.1±41.4 | NS |
| ifn-γ | 51±3.1 | 124.8±13.2 | 0.001 | 105.2±15.3 | NS |
| TNF-α | 2.3±0.3 | 4.7±0.02 | 0.02 | 1.9±0.2 | 0.05 |
| IL-lα | 272±12.3 | 696±40.2 | 0.001 | 356±51.2 | 0.002 |
| CXCL2 | 12.6±1.6 | 61.5±14.2 | 0.001 | 10.5±1.1 | 0.02 |
| CCL5 | 58.1±9.6 | 304.6±50.8 | 0.001 | 50±12.5 | 0.01 |
The results are the mean±SEM. NS indicates nonsignificant.
Figure 6.
Inflammatory response of kidney to CFT073 infection. WT and Hepc−/− mice were infected with 109 colony-forming units of CFT073. (A) The macrophage marker F4/80 was stained in the kidneys. Positive immuno-staining (brown) was observed in both groups. (B) The myeloperoxidase (MPO) activity, a marker of polymorphonuclear neutrophil granules, was assessed for WT and Hepc−/− infected kidneys according to the Bradley method. The results are the mean±SEM of at least six individual mice in each group. (Sham) means uninfected mice. *P<0.05. NS, nonsignificant.
Hepcidin Triggers Urinary Acidic pH
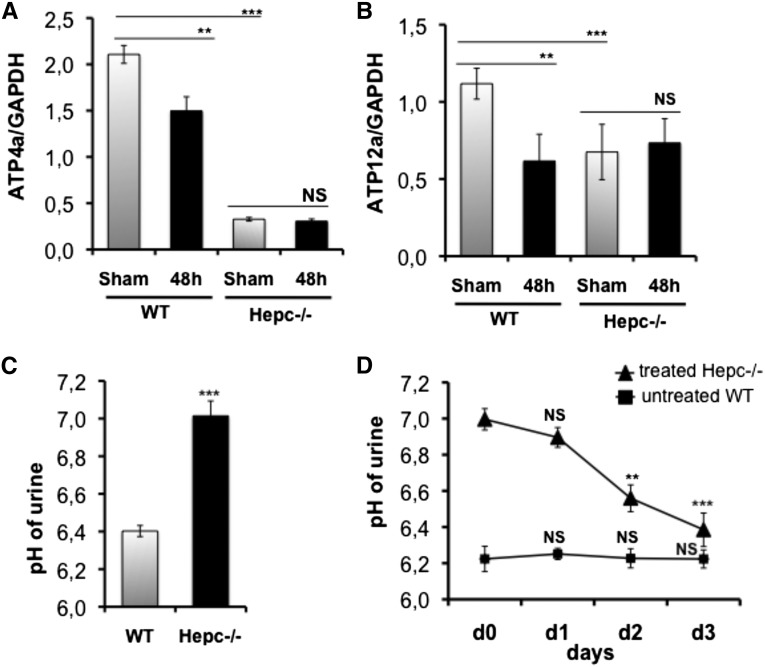
In the collecting duct, the apical H+/K+-ATPase pumps largely mediate acid secretion. Moreover, Schwarz et al.28 reported that the Atp4a pump is regulated by hepcidin via the autocrine/paracrine pathway in gastric parietal cells. Therefore, we attempted to evaluate the mRNA expression of both Atp4a and Atp12a pumps in Hepc−/− kidneys. Compared with WT mice, the level of the two pumps was significantly lower in the Hepc−/− mice with a pronounced effect on the Atp4a transcript (Figure 7B). The degree of reduction of both pump transcripts was correlated with a significant increase of the pH value of the urine (Figure 7C). To investigate further whether hepcidin modulates the urinay pH, we treated Hepc−/− mice intravenously with the hepcidin peptide and measured the pH of urine daily. Figure 6D showed that treated Hepc−/− mice exhibited significant recovery of acidic pH by d 2 of hepcidin injection. A normal range of pH value was attained as soon as d 3 (6.39±0.09 versus 7.00±0.06 at d 0; P=0.001). Because CFT073 inhibited hepcidin synthesis in the kidney, we analyzed the expression of Atp4a and Atp12a pumps in WT mice at 48 hours postinfection and found that their transcripts were reduced (Figure 7, A and B). The mRNA levels of NHE3 and BSC1 that are sensitive to the body acid-base balance were not affected (Supplemental Figure 4).32–35
Figure 7.
Hepcidin triggering of urinary acidic pH. (A) and (B) The kidneys of sham and 48-h-infected WT and Hepc−/− mice were harvested, and the mRNA levels of both ATP4a (A) and ATP12a (B) pumps were quantified by quantitative RT-PCR. Data were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels. (Sham) means uninfected mice. (C) The urine of noninfected WT and Hepc−/− mice was collected and the pH values measured. (D) Hepc−/− mice were intravenously injected with 3 µg of hepcidin peptide daily. Then the urine of untreated WT and treated Hepc−/− mice was collected and the pH values measured. The results are the mean±SEM of at least six individual mice in each group. **P<0.02; ***P<0.001. NS, nonsignificant.
Hepcidin as an Antimicrobial Peptide Active Against CFT073
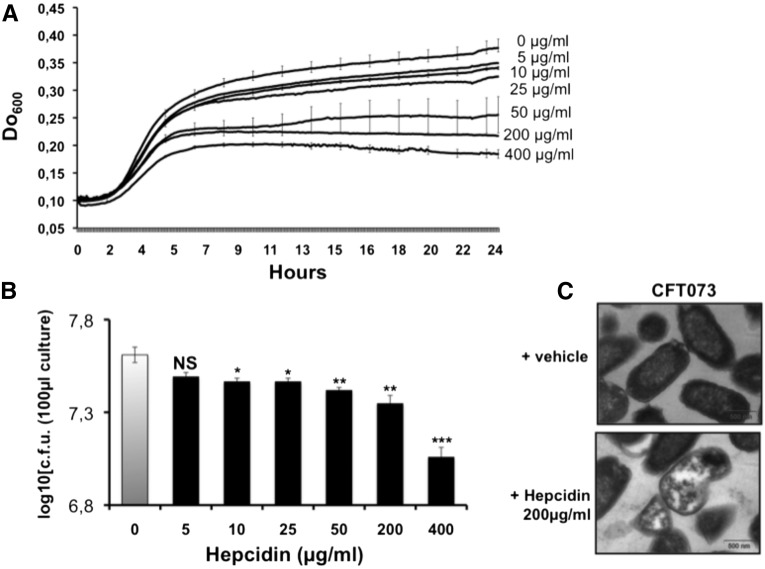
We finally analyzed hepcidin antibacterial activity on UPEC in vitro. We first treated CFT073 that were cultured in healthy adult male sterile urine containing increasing concentrations of hepcidin peptide, and found that the growth kinetics of CFT073 were significantly reduced in a dose-dependent fashion (Figure 8A). At the end of the treatment we analyzed UPEC survival on lysogeny broth (LB) agar plates. Figure 8B showed that treated CFT073 started to grow again, suggestive of a bacteriostatic action of hepcidin. We then determined the structural architecture of CFT073 by electron microscopy analysis, and observed that within 2 hours of incubation with 200 µg/ml of hepcidin, about 41% of CFT073 (compared with 3.5% in controls) exhibited disruption of their envelopes with obvious cell lysis (Figure 8C), indicating that high concentrations of hepcidin may also act as bactericidal CFT073.
Figure 8.
Antimicrobial peptide activity of hepcidin against CFT073. CFT073 were incubated for 24 hours with increasing concentrations of hepcidin as indicated in the methods section. (A) The growth kinetics of treated CFT073 in urine. (B) treated CFT073 were plated in lysogeny broth agar and bacterial counts were performed. (C) A representative transmission electron microscopy photograph of control CFT073 (vehicle) and treated CFT073 (hepcidin). Bacterial growth was inhibited in dose-dependent manner. Hepcidin led to disruption of normal architecture and to loss of bacterial cytosol.
Mechanism by which CFT073 Targets Hepcidin in the Kidneys
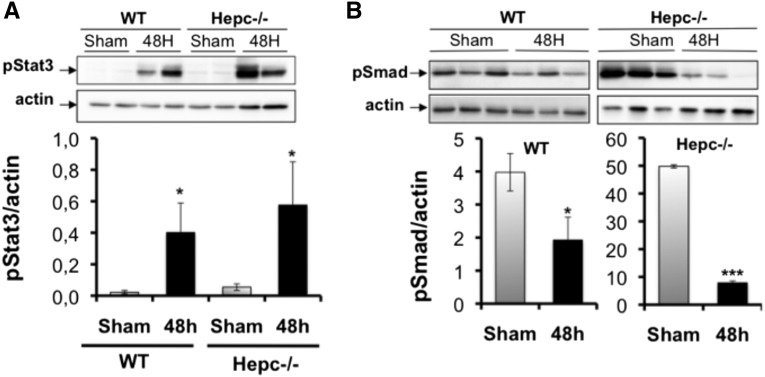
We examined both BMP6/SMAD and IL-6/Stat3 pathways in the kidneys of WT and Hepc−/− mice at 48 hours postinfection. Consistent with the induction of IL-6 in our experiments, Stat3 phosphorylation was increased in infected mice compared with the control group but there was no difference in the extent of Stat3 induction between the two groups (Figure 9A). However, the level of SMAD3/5/8 phosphorylation we reduced in WT mice at 48 hours postinfection (Figure 9B). This SMAD inactivation was more obvious in Hepc−/− mice in which the steady-state level of phosphorylated SMAD kinase was already high. Thus, CFT073 may decrease renal hepcidin expression by acting on the BMP6/SMAD pathway.
Figure 9.
Mechanism by which CFT073 targets renal hepcidin. WT and Hepc−/− mice were infected with 109 colony-forming units of CFT073. Stat3 and SMAD signaling in the kidney was analyzed by western blot at 48 hours postinfection. (A) Induced level of phospho–Stat-3 (pStat3) in both infected WT and Hepc−/− kidneys. (B) Reduced level of phospho-Smad1/5/8 (pSmad) in both infected WT and Hepc−/− kidneys. The amounts of phospho-proteins were normalized to β-actin. The results are the mean±SEM of at least six individual mice in each group. *P<0.05; ***P<0.003.
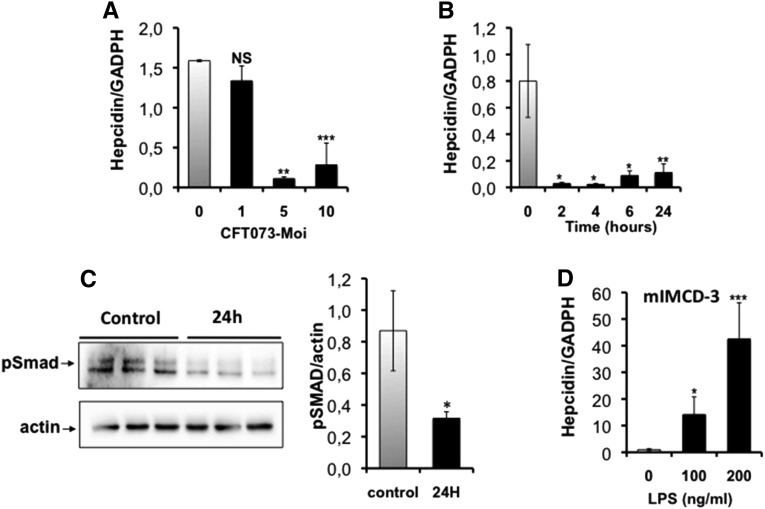
We then infected both medullary collecting duct mIMCD-3 cells and hepatocyte HepG2 cells with formaldehyde (PFA)-fixed CFT073 and examined hepcidin mRNA expression at different multiplicities of infection (MOIs; number of bacteria per number of monolayer cells) and different incubation times. Figure 10A shows that an MOI of 5:1 was sufficient to reduce by 4-fold the mRNA level of hepcidin in mIMCD-3 cells compared with controls. Full suppression was observed by 2 hours postinfection, persisting up to 24 hours postinfection (Figure 10B). Reduced SMAD phosphorylation was also confirmed in inoculated mIMCD-3 cells (Figure 10C). In contrast, we found that 4 hours postinfection with LPS led to a significant increase in hepcidin mRNA in mIMCD-3 cells (Figure 10D). Furthermore, lipocalin-2 was significantly induced in mIMCD-3 cells, and hepcidin mRNA reduced in HepG2 cells, following CFT073 incubation (Supplemental Figure 5).
Figure 10.
Effect of CFT073 on hepcidin expression in cultured renal mIMCD-3 cells. mIMCD-3 cells were infected with PFA-fixed CFT073, and the mRNA level of hepcidin was explored at different multiplicities of infection (MOIs; number of bacteria per number of monolayer cells) and different incubation times. (A) The effect of different MOIs on hepcidin mRNA abundance. (B) The time-course effect using an MOI of 5:1. (C) SMAD signaling in mIMCD-3 infected during 24 hours with PFA-fixed CFT073 (MOI of 5:1), was analyzed by western blot. The left panel is a representative western blot of three independent cell cultures, and the right panel is the quantification of pSmad1/5/8 levels. The data were normalized to β-actin. (D) mIMCD-3 cells were incubated with different concentrations of LPS, and the mRNA level of hepcidin was explored by quantitative RT-PCR. All mRNA quantifications were determined by quantitative RT-PCR. Data were normalized by glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-mRNA levels. The results are the mean±SEM of at least six individual mice in each group. *P<0.05; **P<0.03; ***P<0.001. NS, nonsignificant.
Discussion
In the present study, we provide evidence that hepcidin is an effective mediator of renal defense systems against UPEC infection. Indeed, a total lack of hepcidin was found to alter renal host barriers, including urinary iron restriction, acidic pH of urine, renal inflammatory response and antimicrobial activity. Consequently, CFT073 UPEC infection and growth were favored within the urinary tract. Our data also suggest that renal hepcidin may be one of the targets that UPEC triggers to become more pathogenic and evade host defenses during UTI.
Renal Inhibition of Hepcidin by UPEC
Numerous studies have recently reported direct links between local synthesis of hepcidin and pathogen infection in several epithelial barriers, although no study has been performed in the kidney so far. For example, hepcidin produced by the gastric parietal cells and by the retina was reported to be upregulated following Helicobacter pylori and Staphylococcus aureus infections, respectively.28,36 Our study revealed that renal hepcidin is also regulated on UTI but in an opposite way. Indeed, renal hepcidin was unexpectedly downregulated 24 hours postinfection, whereas hepatic hepcidin increased, despite a delayed response (at 48 hours postinfection). Furthermore, as both hepatic and renal hepcidin transcripts were repressed when renal mIMCD-3 and hepatic HEPG2 cells were infected with CFT073, we assumed that a direct contact of host cells with UPEC is required to repress hepcidin. Bacterial LPS was shown to induce hepcidin mRNA in mIMCD-3 cells, thereby excluding this endotoxin from the repression mechanism of hepcidin and suggesting the involvement of an unknown bacterial compound during this process. Repression of hepcidin has previously been described in a mouse model of gastric mucosal infection with Helicobacter felis.37 The authors attributed this effect to the reduced number of parietal cells that synthesize hepcidin. However, in our UTI model, renal epithelium remained intact up to 48 hours postinfection. Moreover, because the decline of hepcidin was detected earlier following infection, we believe that UPEC may directly modulate hepcidin expression and that discrepant effects of pathogens on hepcidin must result either from the nature of the pathogenic strain or from the host environment. Indeed, UPEC differs from Helicobacter by the high degree of virulence.38 And consistent with the influence of the host environment are the results from Gaddy et al.39 showing in gerbils that a high-salt diet led to reverse from induction to repression the effect of H. pylori on hepcidin expression. This observation may also explain our results with UPEC UTI because the kidneys are the exclusive desalting sites with high osmolarity of urine and of the renal medulla counterpart.
Another notable finding in our study is the UPEC-mediated signaling pathway leading to hepcidin repression. We observed that SMAD1/5/8 phosphorylation, the primary BMP6-induced pathway that controls hepcidin expression in the liver, was preferentially inhibited by UPEC in both WT and Hepc−/− infected kidneys. The IL-6-induced Stat3 phosphorylation was not affected by the UTI infection. Because renal hepcidin was repressed in this model, we concluded that UPEC might block BMP signaling as well as inflammatory IL-6 and LPS/TLR4 signaling by targeting the SMAD pathway. Our results support evidence showing that IL-6 and LPS-dependent activin B pathways may tightly overlap with the BMP/SMAD pathway to induce hepcidin.19,24 Interestingly, recent studies from Arosio’s group have shown that heparin, acting through SMAD signaling alteration, is a potent inhibitor of hepatic hepcidin.40,41 This mechanism process may explain the inhibition of hepcidin in CFT073-infected kidney because UPEC may express heparin-like molecules at their cell envelope, as observed in the urinary tract-infective E. coli O10:K5:H4 and O4:K12:H-.42,43 Further studies are in progress to investigate this mechanism in our UTI model.
The Role of Hepcidin in Renal Host Defense Barriers
In this study, we have highlighted several mechanisms by which hepcidin protects against UPEC. Our data show that a lack of hepcidin in Hepc−/− mice was associated with iron overload in the kidney. Reduced protein levels of ferritin on infection is indicative of rapid invasion and iron acquisition. Lack of hepcidin was also associated with significant reduction of proinflammatory cytokines and chemokines (Figure 5, Table 1), which dampened the host immune system against CFT073. Our findings share similarities with studies described in the lung using the hepcidin-deficient Hfe−/− model, which exhibited impaired neutrophil recruitment in response to inflammation.44 Since hepcidin deficiency is associated with iron release from macrophages, we postulate that reduced inflammatory response in both Hepc−/− and Hfe−/− models may result from a reduced iron pool in the immune monocytes/macrophages, as was recently suggested.45,46 Hepcidin also suppressed bacterial infection by acting directly on UPEC because we observed that simple pre-incubation of CFT073 with hepcidin was sufficient to prevent UTI. Indeed, we found that hepcidin conserved bacteriostatic and bactericidal actions reminiscent of the mechanism by which defensins exert their antibacterial activity.8,9 Finally, we observed that hepcidin increased the acidic pH of urine by acting on the expression of the Atp4a and Atp12a pumps. Thus, as in gastric parietal cells, an autocrine/paracrine regulation of Atp4a and Atp12a by hepcidin may occur in renal tubular cells.
We provided new insights into the regulatory networks of the antimicrobial peptide hepcidin to prevent UPEC pathogenesis in the kidneys. Hepcidin appears to be a bridge between innate and adaptive immunity in this organ. The targeting of renal endogenous hepcidin by UPEC appears to be beneficial for these pathogens, especially at the initial stages of invasion. Our data may lead to the identification of new therapeutic strategies that target hepcidin or specific pathways regulating this peptide to enhance host defense or prevent maladaptive inflammatory responses leading to kidney injury in a variety of infectious and non-infectious conditions.
Concise Methods
Animals
Seven-week-old CBA/J female mice were purchased from the Janvier-Europe laboratory and acclimated in our animal facility for 1 wk. Hepcidin-deficient C57BL/6 mice (Hepc−/−) were generated by Sophie Vaulont (Institut Cochin, Paris, France). All experimental procedures involving animals were performed in compliance with the French and European regulations on animal welfare and public health service recommendations.
Bacterial Strains
The E. coli strain used was the uropathogenic CFT073 (O6 :K2 :H1) isolated from a urosepsis episode and belongs to the B2 phylogenetic group.
Ascending UTI Model
The mouse model of ascending unobstructed UTI was used as described in Labat et al.47 Briefly, female mice were anesthetized using a xylazine/ketamine mixture before inoculation into the bladder of 50 µl of 0.9% NaCl containing 109 bacteria. The urethral catheter used was immediately removed after inoculation. Sham-infected mice were infused with 50 µl of 0.9% NaCl. Animals were killed 24 hours or 48 hours later. The kidneys, bladder and liver were aseptically removed and frozen at –80°C for further study. For bacterial counts, half of the infected kidneys, the bladder and part of the liver were homogenized, and bacterial cultures were performed on LB agar plates. Bacterial count was expressed as log10[c.f.u.(g organ)-1]. Six mice per group were infected and UTI experiments were repeated at least twice.
Biochemical Analyses
For urine collection, mice were individually maintained in metabolic cages and were allowed free access to water and food. Their body weight and water and food intakes were systematically measured before and after housing experiments. They showed no difference between groups. Collected urine was aliquoted and frozen at –20°C until used. For serum collection, mice were anesthetized by intraperitoneal injection of pentobarbital (Ceva Santé Animale, Paris, France), and blood was then collected by puncture of the vena cava. Serum and urine non-heme iron, ferritin, and transferrin levels were measured using an AU400 automate (Olympus, Tokyo, Japan). Serum hepcidin was measured with a previously validated liquid chromatography-tandem mass spectrometry method48 with modifications.
Cytokine and Chemokine Assays
Screening for cytokines and chemokines in renal extract was performed using MILLIPLEX MAP Mouse Cytokine (EMD Millipore, Saint-Quentin-en-Yvelines, France) according to the manufacturer’s instructions. For renal extracts, the kidneys were homogenized in a lysis buffer (50 mM HEPES pH 7.0, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, and 1× protease inhibitor cocktail ((EDTA Complete; Thermo Fisher Scientific, Paris France), and 10 µL/mL phosphatase inhibitor cocktail 2 (Sigma-Aldrich, Saint-Quentin Fallavier, France)). The homogenate was centrifuged at 100g at 4°C for 30 s, and the supernatants were transferred to a fresh tube. Ten per cent NP-40 was added to a final concentration of 0.05%, and the tubes were mixed by gentle inversion before an additional centrifugation at 1000g for 7 min. This final supernatant was used for cytokine and chemokine measurements. Eight-point standard curves were generated for each cytokine using the Luminex bead technology.
Myeloperoxidase Activity
Myeloperoxidase activity was assessed in kidney tissues by the spectrophotometeric assay based on myeloperoxidase-catalyzed oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) by H2O2. Samples were homogenized in a potassium phosphate buffer (50 mM, pH 6.0) and then centrifuged at 10,000g for 15 min at 4°C. The pellets were resuspended again in an equivalent volume of phosphate buffer (50 mmol/l, pH 6). Protein concentration was determined using the Bradford assay. Each sample of 100 µg of protein was resuspended in hexadecyl trimethylammonium bromide (HTAB) buffer (0.5% w/v, in 50 mM potassium phosphate buffer, pH 6.0), sonicated at 4°C, and centrifuged again at 10,000g for 15 min at 4°C. Fifty microliters of the supernatants were combined with 150 µl H2O2 (3 mM diluted in citrate), and 150 µl TMB solution (3 mM). In this assay, TMB was oxidized to a blue product that absorbs at 655 nm. The enzyme activity was measured continuously by monitoring the absorbance changes at 655 nm with a spectrophotometer.
Histologic Examination
The kidneys were fixed in 4% formaldehyde, embedded in paraffin and sections of 4 µm were then stained with Perls’ Prussian blue, H&E and picrosirus red. Images were acquired using a ScanScope digital scanner (Aperio, TRIBVN, France). Morphologic assessments were conducted by two independent renal pathologists who were uninformed about the treatments and mouse phenotypes.
Immunohistochemistry
Antigen retrieval from kidney sections was performed in citrate buffer at pH 6 (Vector Laboratories, Burlingame, CA) in a steamer. Endogenous peroxidase activity was blocked with 3% H2O2, and nonspecific protein binding was blocked with serumfree protein blocking solution (RTU Kit; Vector Laboratories). Kidney sections were first incubated overnight at 4°C with primary anti-F4/80 monoclonal antibody (AbD Serotec, a Bio-Rad company, Marnes-la-Coquette, France) applied at 1:50 dilution and incubated. The signal was revealed by a secondary antibody already labeled with horseradish peroxidase-conjugated streptavidin (Dako, Les Ulis, France), and visualized using Nova Red substrate kit (Vector Laboratories). The sections were then stained with hematoxylin.
Quantitative RT-PCR
Total RNA was isolated using the SV Total RNA isolation system (Promega, Charbonnières-les-Bains, France), and subsequent cDNA synthesis was performed with SuperScript II reverse transcription kit (Invitrogen, Life Technologies, Saint Aubin, France) according to the manufacturer’s instructions. Real-time PCR was performed in duplicate in a Light Cycler II LC 480 (Roche Diagnostics, Meylan, France) using the SYBR Green PCR mix (Roche Diagnostics, Meylan, France). The primers used were as shown in Table 2.
Table 2.
Primers sequences
| Gene | Forward | Reverse |
|---|---|---|
| Mouse Hepc1 | CGATACCAATGCAGAAGAGAAGG | TTTGCAACAGATACCACACTGGG |
| Mouse Atp4a | AGCACCAGGCACCATGGGGAAG | CACCAGGGCCAGACCCCAGTT |
| Mouse Atp12a | GGCAGCCGCCACCGAAAGAT | CTGGCGTGCTGAGGTGAGCC |
| Mouse Tlr4 | ACCAGGAAGCTTGAATCCCTGCA | GGAATGTCATCAGGGACTTTGCTGA |
| Mouse Ngal | CTGAATGGGTGGTGAGTGTG | GATGGAGTGGCAGACAGA |
| Mouse BSC1 | CCGTCCCCAAGATTGAA | TCACATTCTTAGCCAGCTGC |
| Mouse Aq2 | CCGCCATCCTCCATGAGATT | GGAAGAGCTCCACAGTCACC |
| Mouse NHE3 | GGAGGCCACCAACTATGAA | GGAGAACACGGGATTATCAA |
| Mouse GAPDH | TGAAGCAGGCATCTGAGGG | CGAAGGTGGAAGAGTGGGAG |
| Human HEPC1 | TGACAGCAGCCGCAGCAGAA | GGCCAGCTGGATGCCCATGT |
| Human B2M | TGCTGTCTCCATGTTTGATGTATCT | TCTCTGCTCCCCACCTCTAAGT |
| Mouse Aq2 | CCGCCATCCTCCATGAGATT | GGAAGAGCTCCACAGTCACC |
To validate experiments examining the mRNA expression of hepcidin, liver RNA was systemically used as a positive control, and RNA from kidneys of hepcidin knockout mice (Hepc−/−, in which hepcidin is fully absent) was used as a negative control.
Protein Extraction and Western Blotting
Both tissues and cell pellets (approximately 40 mg) were harvested in a lysis buffer (150 mM NaCl, 50 mM Tris/HCl pH 7.6, 1% Triton, 0.1% SDS and 26.6 mg/ml 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride) containing a 1× protease inhibitor cocktail (EDTA Complete; Thermo Fisher Scientific). The homogenate was incubated in the same buffer for 60 min at 4°C. After centrifugation at 16,000g for 15 min, the supernatant containing total proteins was transferred to a fresh tube and stored at –80°C until used. Protein concentrations were determined using the Bradford protein assay with bovine albumin standards.
Proteins were separated by electrophoresis on 8% or 10% SDS-polyacrylamide gels depending on the molecular weight of the target proteins. Primary antibodies were used at a 1:6000 dilution for anti-FPN (a kind gift from Dr. D. Haile, San Antonio, TX), 1:1000 for anti-TFR1 (Zymed, Invitrogen, Life Technologies, Saint Aubin, France), 1:1000 for anti-H-ferritin or anti-L-ferritin (a kind gift from Paolo Arosio, Brescia, Italy), 1:1000 for anti-P-SMAD1/5/8 (EMD Millipore, Saint-Quentin-en-Yvelines, France), 1:2000 for anti-P-stat3 (Cell Signaling-Ozyme; Cell Signaling Technology, Danvers, Massachusetts), 1:1500 for anti-Lcn2 (R&D Systems), 1:2000 for anti-ZO-1 (Sigma-Aldrich, Saint-Quentin Fallavier, France) and 1:10,000 for anti-b-actin mouse monoclonal antibody (Sigma-Aldrich Fine Chemicals), which was used as an additional control to check for equal loading and transfer onto the nitrocellulose membranes. Immunoreactive bands were revealed by horseradish peroxidase-conjugated secondary antibodies using Amersham enhanced chemiluminescence.
Bacterial Growth Measurement
E. coli CFT073 was cultured in 4 ml of LB medium for 16 hours at 37°C. The E. coli were then centrifuged at 2500g for 10 min, washed with 20 ml of isotonic saline, recentrifuged and washed twice with 10 ml of sterile urine (see below). Cultures were diluted to obtain a starting concentration of 5×105 bacteria/ml. The growth experiments in the absence or presence of increasing concentrations of hepcidin (0, 5, 10, 25, 50, 200, 400 µg/ml) were assayed using 96-well plates, and OD600 was measured at intervals of 5 min over 24 hours with a Tecan Infinite M200 plate reader. Each experiment was performed in triplicate and repeated in three different cultures.
Sterile urine was collected over 24 hours from three healthy adult male volunteers with no history of UTIs or antibiotic use during the previous 6 months. The urine samples were pooled, filtered (0.22 μm pore size) and used within 48 hours.
Cell Culture and Infection
The mIMCD-3 cell line derived from a mouse inner medullary collecting duct (ATCC CRL-2123) was cultured in DMEM:F-12 medium supplemented by 10% FBS (ATCC with LGC Standard Co., Molsheim, France). HEPG2 cells derived from human hepatocellular carcinoma (ATCC CRL-2123) were cultured in DMEM with 10% FBS. For cell infection, the CFT073 bacterial strain was grown overnight in a LB medium and then centrifuged and resuspended in 2% formaldehyde (PFA; Electron Microscopy Sciences, Hatfield, PA) and incubated for 20 min at 37°C. Fixed bacteria were centrifuged again, washed twice and resuspended in sterile PBS. They were then added to confluent mIMCD-3 and HEPG2 cells at a concentration of 1, 5, or 10 bacteria per cell. Infected cell lines were harvested after 2, 4, 6, and 24 hours after inoculation. Cells treated with PBS or LPS (LPS-EK Ultrapure; Invivogen, France) were used as controls. Fixed bacteria showed no further growth on LB agar plates.
Electronic Microscopy Analysis
E. coli CFT073 bacteria were cultured in 4 ml of Luria-Bertani medium for 16 hours at 37°C. They were then centrifuged at 2500g for 10 min and washed twice with 20 ml of isotonic saline. Cultures were then incubated with or without hepcidin (200 µg/ml). After 1 hour, the cell suspensions were centrifuged at 10,000g for 15 min, washed with PBS, resuspended in fixative (3% glutaraldehyde and 4% PFA in 0.1 M cacodylate buffer at pH 7.2) for 24 hours at 4°C, postfixed in osmium tetroxide, dehydrated with ethanol, and embedded in Epon. Ultrathin sections stained with lead citrate were examined on a Jeol 1010 electron microscope. Images were captured by a digital imaging system.
Chemical Synthesis of Mouse Hepcidin Analog
The mouse hepcidin 1 analog was manually synthesized as described.49 Briefly, a linear precursor with protected side chains was assembled manually by solid-phase peptide synthesis on Wang resin low load, preloaded with a threonine substituted at 0.27 mmol.g-1 using Fmoc chemistry. After deprotection of Fmoc groups, using 20% piperidine/DMF (v/v), coupling was performed using 1:1:1:2 amino acid/PyBOP/HOBt/DIEA in 5 ml of DMF (30 min) for all amino acids, except Fmoc-Cys(Trt)-OH, Fmoc-Cys(Acm)-OH, and Fmoc-Cys(tBu)-OH, for which collidine was used instead of DIEA. The resin bound peptide was cleaved from resin support and the side-chain protecting groups were removed by treatment with tri-fluoro-acetic acid. After cleavage from the resin, the peptide was dissolved in a mixture of DMSO/H2O (1/2, v/v) and air oxidized to allow the Cys7-Cys23 disulfide bond formation. The oxidized peptide was extensively diluted in water (10 v/v) and lyophilized to dryness and kept at –20°C as a white powder until use. The resulting peptide was analyzed by mass spectrometry and its biologic activity was systematically checked for its ability to promote ferroportin degradation on J774 macrophages.
Statistical Analysis
For statistical analyses of quantitative variables, Student’s t test was performed. A finding of P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Dr. Jamel El Benna for helping in myeloperoxidase activity measurements, Sara Dion for helping in UTI and the renal pathologists Dr. Laurence Choudat and Dr. Jean-baptiste Gibier for the morphologic assessments, and Alain Grodet for electronic microscopy analysis.
INSERM and the Université Paris Diderot, France supported this work.
Part of this work is funded by the program ‘University of Sorbonne Paris Cité, Excellence Initiative, IDEX’, reference KIRON.
Dounia Houamel was supported by the Université Paris Diderot, the Société Française d'Hématologie and by the Laboratory of excellence, GR-Ex, Paris, France.
The labex GR-Ex, reference ANR-11-LABX-0051 is funded by the program ‘Investissements d’avenir’ of the French National Research Agency, reference ANR-11-IDEX-0005-02.
Boualem Moulouel was supported by a grant from the Fondation pour la Recherche Médicale Française.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014101035/-/DCSupplemental.
References
- 1.Kaper JB, Nataro JP, Mobley HL: Pathogenic Escherichia coli. Nat Rev Microbiol 2: 123–140, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Totsika M, Moriel DG, Idris A, Rogers BA, Wurpel DJ, Phan MD, Paterson DL, Schembri MA: Uropathogenic Escherichia coli mediated urinary tract infection. Curr Drug Targets 13: 1386–1399, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Foxman B: Urinary tract infection syndromes: occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect Dis Clin North Am 28: 1–13, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Brown P: Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 17: 227–241, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Snyder JA, Haugen BJ, Buckles EL, Lockatell CV, Johnson DE, Donnenberg MS, Welch RA, Mobley HL: Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect Immun 72: 6373–6381, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu BC, Garcia-Herrero A, Johanson TH, Krewulak KD, Lau CK, Peacock RS, Slavinskaya Z, Vogel HJ: Siderophore uptake in bacteria and the battle for iron with the host; a bird’s eye view. Biometals 23: 601–611, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Moulouel B, Houamel D, Delaby C, Tchernitchko D, Vaulont S, Letteron P, Thibaudeau O, Puy H, Gouya L, Beaumont C, Karim Z: Hepcidin regulates intrarenal iron handling at the distal nephron. Kidney Int 84: 756–766, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Krause A, Neitz S, Mägert HJ, Schulz A, Forssmann WG, Schulz-Knappe P, Adermann K: LEAP-1, a novel highly disulfide-bonded human peptide, exhibits antimicrobial activity. FEBS Lett 480: 147–150, 2000 [DOI] [PubMed] [Google Scholar]
- 9.Park CH, Valore EV, Waring AJ, Ganz T: Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806–7810, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Nicolas G, Bennoun M, Devaux I, Beaumont C, Grandchamp B, Kahn A, Vaulont S: Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci U S A 98: 8780–8785, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigeon C, Ilyin G, Courselaud B, Leroyer P, Turlin B, Brissot P, Loréal O: A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem 276: 7811–7819, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J: Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Delaby C, Pilard N, Gonçalves AS, Beaumont C, Canonne-Hergaux F: Presence of the iron exporter ferroportin at the plasma membrane of macrophages is enhanced by iron loading and down-regulated by hepcidin. Blood 106: 3979–3984, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Yamaji S, Sharp P, Ramesh B, Srai SK: Inhibition of iron transport across human intestinal epithelial cells by hepcidin. Blood 104: 2178–2180, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Mena NP, Esparza A, Tapia V, Valdés P, Núñez MT: Hepcidin inhibits apical iron uptake in intestinal cells. Am J Physiol Gastrointest Liver Physiol 294: G192–G198, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Chaston T, Chung B, Mascarenhas M, Marks J, Patel B, Srai SK, Sharp P: Evidence for differential effects of hepcidin in macrophages and intestinal epithelial cells. Gut 57: 374–382, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Brasse-Lagnel C, Karim Z, Letteron P, Bekri S, Bado A, Beaumont C: Intestinal DMT1 cotransporter is down-regulated by hepcidin via proteasome internalization and degradation. Gastroenterology 140: 1261–1271, e1, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ, Andrews NC, Lin HY: Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38: 531–539, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Wang RH, Li C, Xu X, Zheng Y, Xiao C, Zerfas P, Cooperman S, Eckhaus M, Rouault T, Mishra L, Deng CX: A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab 2: 399–409, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Silvestri L, Guillem F, Pagani A, Nai A, Oudin C, Silva M, Toutain F, Kannengiesser C, Beaumont C, Camaschella C, Grandchamp B: Molecular mechanisms of the defective hepcidin inhibition in TMPRSS6 mutations associated with iron-refractory iron deficiency anemia. Blood 113: 5605–5608, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Ganz T, Nemeth E: Iron sequestration and anemia of inflammation. Semin Hematol 46: 387–393, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicolas G, Chauvet C, Viatte L, Danan JL, Bigard X, Devaux I, Beaumont C, Kahn A, Vaulont S: The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110: 1037–1044, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T: IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 113: 1271–1276, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Besson-Fournier C, Latour C, Kautz L, Bertrand J, Ganz T, Roth MP, Coppin H: Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood 120: 431–439, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Peyssonnaux C, Zinkernagel AS, Datta V, Lauth X, Johnson RS, Nizet V: TLR4-dependent hepcidin expression by myeloid cells in response to bacterial pathogens. Blood 107: 3727–3732, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frazier MD, Mamo LB, Ghio AJ, Turi JL: Hepcidin expression in human airway epithelial cells is regulated by interferon-γ. Respir Res 12: 100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gnana-Prakasam JP, Martin PM, Mysona BA, Roon P, Smith SB, Ganapathy V: Hepcidin expression in mouse retina and its regulation via lipopolysaccharide/Toll-like receptor-4 pathway independent of Hfe. Biochem J 411: 79–88, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz P, Kübler JA, Strnad P, Müller K, Barth TF, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G, Kulaksiz H: Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut 61: 193–201, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Kulaksiz H, Theilig F, Bachmann S, Gehrke SG, Rost D, Janetzko A, Cetin Y, Stremmel W: The iron-regulatory peptide hormone hepcidin: expression and cellular localization in the mammalian kidney. J Endocrinol 184: 361–370, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Chassin C, Tourneur E, Bens M, Vandewalle A: A role for collecting duct epithelial cells in renal antibacterial defences. Cell Microbiol 13: 1107–1113, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Paragas N, Kulkarni R, Werth M, Schmidt-Ott KM, Forster C, Deng R, Zhang Q, Singer E, Klose AD, Shen TH, Francis KP, Ray S, Vijayakumar S, Seward S, Bovino ME, Xu K, Takabe Y, Amaral FE, Mohan S, Wax R, Corbin K, Sanna-Cherchi S, Mori K, Johnson L, Nickolas T, D’Agati V, Lin CS, Qiu A, Al-Awqati Q, Ratner AJ, Barasch J: α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest 124: 2963–2976, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karim Z, Attmane-Elakeb A, Sibella V, Bichara M: Acid pH increases the stability of BSC1/NKCC2 mRNA in the medullary thick ascending limb. J Am Soc Nephrol 14: 2229–2236, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Karim Z, Szutkowska M, Vernimmen C, Bichara M: Recent concepts concerning the renal handling of NH3/NH4+. J Nephrol 19[Suppl 9]: S27–S32, 2006 [PubMed] [Google Scholar]
- 34.Attmane-Elakeb A, Mount DB, Sibella V, Vernimmen C, Hebert SC, Bichara M: Stimulation by in vivo and in vitro metabolic acidosis of expression of rBSC-1, the Na+-K+(NH4+)-2Cl- cotransporter of the rat medullary thick ascending limb. J Biol Chem 273: 33681–33691, 1998 [DOI] [PubMed] [Google Scholar]
- 35.Bobulescu IA, Moe OW: Na+/H+ exchangers in renal regulation of acid-base balance. Semin Nephrol 26: 334–344, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh PK, Shiha MJ, Kumar A: Antibacterial responses of retinal Müller glia: production of antimicrobial peptides, oxidative burst and phagocytosis. J Neuroinflammation 11: 33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomson MJ, Pritchard DM, Boxall SA, Abuderman AA, Williams JM, Varro A, Crabtree JE: Gastric Helicobacter infection induces iron deficiency in the INS-GAS mouse. PLoS ONE 7: e50194, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muotiala A, Helander IM, Pyhälä L, Kosunen TU, Moran AP: Low biological activity of Helicobacter pylori lipopolysaccharide. Infect Immun 60: 1714–1716, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM, Jr, Algood HM, Cover TL: High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect Immun 81: 2258–2267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poli M, Asperti M, Naggi A, Campostrini N, Girelli D, Corbella M, Benzi M, Besson-Fournier C, Coppin H, Maccarinelli F, Finazzi D, Arosio P: Glycol-split nonanticoagulant heparins are inhibitors of hepcidin expression in vitro and in vivo. Blood 123: 1564–1573, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poli M, Girelli D, Campostrini N, Maccarinelli F, Finazzi D, Luscieti S, Nai A, Arosio P: Heparin: a potent inhibitor of hepcidin expression in vitro and in vivo. Blood 117: 997–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Vann WF, Schmidt MA, Jann B, Jann K: The structure of the capsular polysaccharide (K5 antigen) of urinary-tract-infective Escherichia coli 010:K5:H4. A polymer similar to desulfo-heparin. Eur J Biochem 116: 359–364, 1981 [DOI] [PubMed] [Google Scholar]
- 43.Schmidt MA, Jann K: Structure of the 2-keto-3-deoxy-D-manno-octonic-acid-containing capsular polysaccharide (K12 antigen) of the urinary-tract-infective Escherichia coli O4:K12:H-. Eur J Biochem 131: 509–517, 1983 [DOI] [PubMed] [Google Scholar]
- 44.Benesova K, Vujić Spasić M, Schaefer SM, Stolte J, Baehr-Ivacevic T, Waldow K, Zhou Z, Klingmueller U, Benes V, Mall MA, Muckenthaler MU: Hfe deficiency impairs pulmonary neutrophil recruitment in response to inflammation. PLoS ONE 7: e39363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nairz M, Schleicher U, Schroll A, Sonnweber T, Theurl I, Ludwiczek S, Talasz H, Brandacher G, Moser PL, Muckenthaler MU, Fang FC, Bogdan C, Weiss G: Nitric oxide-mediated regulation of ferroportin-1 controls macrophage iron homeostasis and immune function in Salmonella infection. J Exp Med 210: 855–873, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nairz M, Fritsche G, Crouch ML, Barton HC, Fang FC, Weiss G: Slc11a1 limits intracellular growth of Salmonella enterica sv. Typhimurium by promoting macrophage immune effector functions and impairing bacterial iron acquisition. Cell Microbiol 11: 1365–1381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Labat F, Pradillon O, Garry L, Peuchmaur M, Fantin B, Denamur E: Mutator phenotype confers advantage in Escherichia coli chronic urinary tract infection pathogenesis. FEMS Immunol Med Microbiol 44: 317–321, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Li H, Rose MJ, Tran L, Zhang J, Miranda LP, James CA, Sasu BJ: Development of a method for the sensitive and quantitative determination of hepcidin in human serum using LC-MS/MS. J Pharmacol Toxicol Methods 59: 171–180, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Khemtemourian L, Desbenoit N, Mahesh P, Chatterjee S, Deschemin JC, Vaulont S, Tomas A, Sari MA, Artaud I: Synthesis and biological activity of mouse hepcidin peptide analogs containing three disulfide bridges: manual and microwave-assisted solid-phase peptide synthesis. Protein Pept Lett 19: 219–227, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.