Abstract
Pulmonary hypertension (PH) is associated with poor outcomes in the dialysis and general populations, but its effect in CKD is unclear. We evaluated the prevalence and predictors of PH measures and their associations with long–term clinical outcomes in patients with nondialysis-dependent CKD. Chronic Renal Insufficiency Cohort (CRIC) Study participants who had Doppler echocardiography performed were considered for inclusion. PH was defined as the presence of estimated pulmonary artery systolic pressure (PASP) >35 mmHg and/or tricuspid regurgitant velocity (TRV) >2.5 m/s. Associations between PH, PASP, and TRV and cardiovascular events, renal events, and all-cause mortality were examined using Cox proportional hazards models. Of 2959 eligible participants, 21% (n=625) had PH, with higher rates among those with lower levels of kidney function. In the multivariate model, older age, anemia, lower left ventricular ejection fraction, and presence of left ventricular hypertrophy were associated with greater odds of having PH. After adjusting for relevant confounding variables, PH was independently associated with higher risk for death (hazard ratio, 1.38; 95% confidence interval, 1.10 to 1.72) and cardiovascular events (hazard ratio, 1.23; 95% confidence interval, 1.00 to 1.52) but not renal events. Similarly, TRV and PASP were associated with death and cardiovascular events but not renal events. In this study of patients with CKD and preserved left ventricular systolic function, we report a high prevalence of PH. PH and higher TRV and PASP (echocardiographic measures of PH) are associated with adverse outcomes in CKD. Future studies may explain the mechanisms that underlie these findings.
Keywords: CKD, pulmonary hypertension, mortality, heart failure
CKD is present in over 25 million Americans, and it is associated with an increased risk of ESRD, cardiovascular disease, and mortality.1,2 Factors contributing to such high risk of adverse outcomes in CKD remain poorly defined. Interventions to lower cardiovascular risk in CKD by targeting the traditional cardiovascular risk factors have been only modestly successful at best.3–6 Therefore, there is an urgent need to evaluate novel risk factors for cardiovascular disease and mortality in CKD that may eventually serve as therapeutic targets.
There has been increasing interest recently in studying the association between pulmonary hypertension (PH) and CKD.7–10 PH, defined by elevated pulmonary arterial pressure, is rare in the general population but seen in 30%–40% of patients with ESRD (on the basis of echocardiographic studies).11,12 After it is established, PH is often progressive and associated with high morbidity and mortality.13 Risk factors for PH, such as left ventricular hypertrophy (LVH) and diastolic dysfunction, are common in patients with CKD and may predispose them to PH.14 However, there are very limited data available evaluating the prevalence of PH in nondialysis-dependent CKD, factors that contribute to PH in this setting, and more importantly, whether the presence of PH is associated with a greater risk of adverse outcomes in this population.7,8,15–18
Therefore, we examined the prevalence, predictors, and associations between PH parameters and clinical outcomes in participants enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study. We hypothesized that there is a high prevalence of PH in those with nondialysis-dependent CKD and that the presence of PH (measured using estimated pulmonary artery systolic pressure [PASP] and tricuspid regurgitant velocity [TRV]) is associated with an increased risk for cardiovascular and renal events as well as death.
Results
Prevalence and Patient Characteristics
PH was present in 21.1% (625 of 2959) of the study population. Prevalence of PH was 5.88%, 10.9%, 20.97%, 21.85%, 26.5%, and 32.8% in those with stages 1, 2, 3a, 3b, 4, and 5, respectively. Participants with PH were older, were more likely to be of non–Hispanic black origin, and had lower eGFR. In addition, participants with PH were more likely to have underlying comorbid conditions and use cardiovascular medications, including statins, β-blockers, and diuretics (Table 1). Participants with PH had lower left ventricular ejection fraction (LVEF), higher left ventricular mass index, and higher prevalence of LVH than those without PH (Table 1). There were also significant differences between participants who did not have PH measures performed compared with those with PH measures who are included in this analysis (Supplemental Table 1).
Table 1.
Clinical characteristics of CRIC Study participants with and without PH (PASP>35 mmHg and/or TRV>2.5 m/s)
| Variables | With PH (n=625; Mean [SD] or %) | Without PH (n=2334; Mean [SD] or %) | P Value |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 63.2 (9.1) | 58.6 (10.8) | <0.001 |
| Sex (men/women; %) | 52.2/47.8 | 54.3/45.7 | 0.34 |
| Race (%) | |||
| Non-Hispanic white | 38.9 | 47.9 | <0.001 |
| Non-Hispanic black | 52.3 | 43.0 | |
| Hispanic | 5.1 | 4.7 | |
| Other | 3.7 | 4.5 | |
| Comorbid conditions | |||
| Cardiovascular disease (%) | 46.1 | 34.5 | <0.001 |
| Stroke (%) | 13.4 | 10.3 | 0.02 |
| Congestive heart failure (%) | 16.0 | 9.6 | <0.001 |
| Peripheral vascular disease (%) | 8.8 | 7.5 | 0.26 |
| COPD (%) | 7.9 | 4.3 | <0.001 |
| Systolic BP (mmHg) | 130.9 (22.3) | 125.2 (21.0) | <0.001 |
| Diastolic BP (mmHg) | 67.6 (12.5) | 70.3 (12.7) | <0.001 |
| Diabetes (%) | 53.1 | 47.0 | <0.01 |
| Body mass index (kg/m2) | 32.0 (7.3) | 32.0 (7.8) | 0.97 |
| Smoking (%) | 10.7 | 13.1 | 0.10 |
| Laboratory parameters | |||
| eGFR (ml/min per 1.73 m2) | 38.8 (15.2) | 43.8 (17.4) | <0.001 |
| BUN | 33.8 (17.5) | 30.0 (15.3) | <0.001 |
| 24-h Proteinuria (g) | 0.2 (0.1–0.9) | 0.2 (0.1–0.8) | 0.10 |
| Serum phosphorous (mg/dl) | 3.7 (0.6) | 3.7 (0.7) | 0.06 |
| Serum bicarbonate (mg/dl) | 24.4 (3.4) | 24.4 (3.2) | 0.68 |
| Serum uric acid (mg/dl) | 7.6 (1.9) | 7.3 (1.9) | <0.001 |
| Serum LDL cholesterol (mg/dl) | 93.6 (32.4) | 100.8 (34.8) | <0.001 |
| Serum HDL cholesterol (mg/dl) | 48.7 (15.6) | 49.0 (15.8) | 0.75 |
| Hs C–reactive protein | 5.9 (10.9) | 5.2 (8.9) | 0.08 |
| BNP | 152.3 (337.0) | 71.0 (193.6) | <0.001 |
| Medication use (%) | |||
| Angiotensin–converting enzyme inhibitors/angiotensin receptor use | 70.7 | 69.0 | 0.40 |
| Statins use | 67.5 | 57.6 | <0.001 |
| β-Blocker use | 64.5 | 47.5 | <0.001 |
| Diuretic use | 67.8 | 57.5 | <0.001 |
| Echo findings | |||
| Ejection fraction (%) | 53.1 (10.6) | 54.6 (8.0) | <0.001 |
| PASP (mmHg) | 34.9 (8.9) | 22.6 (4.1) | <0.001 |
| TRV (m/s) | 2.81 (0.29) | 2.25 (0.15) | <0.001 |
| Right atrial pressure (mmHg) | 2.8 (3.4) | 2.0 (3.0) | <0.001 |
| LVH (%) | 64.8 | 47.7 | <0.001 |
| Left ventricular mass index (g/m2.7) | 55.9 (15.1) | 50.2 (13.4) | <0.001 |
COPD, chronic obstructive pulmonary disease; Hs, highly sensitive; BNP, brain natriuretic peptide.
Cross-Sectional Association of Clinical Characteristics with PH
In the cross–sectional multivariable adjusted analyses, older age, anemia (hemoglobin <10 g/dl), lower LVEF, and presence of LVH were independently associated with higher odds of PH (Table 2). Supplemental Table 2 shows the results of a multivariable adjusted analysis, in which diastolic relaxation was used as a marker of diastolic function (instead of LVH).
Table 2.
Cross-sectional association between clinical and demographic factors and prevalent PH in CRIC Study participants
| Variable | PH Odds Ratio (95% Confidence Interval)a |
|---|---|
| Age (per 5-yr increase) | 1.25 (1.18 to 1.32) |
| Men | 0.99 (0.80 to 1.23) |
| Race | |
| Non-Hispanic black versus non-Hispanic white | 1.24 (0.99 to 1.55) |
| Hispanic versus non-Hispanic white | 1.18 (0.73 to 1.90) |
| Other versus non-Hispanic white | 0.98 (0.58 to 1.68) |
| eGFR (per SD decrease) | 1.11 (0.97 to 1.26) |
| Proteinuria (per SD increase) | 1.06 (0.96 to 1.18) |
| Diabetes | 0.92 (0.74 to 1.14) |
| Hypertension | 1.24 (0.83 to 1.85) |
| Hyperlipidemia | 0.99 (0.71 to 1.39) |
| Chronic obstructive pulmonary disease | 1.43 (0.93 to 2.19) |
| Smoking | 0.75 (0.54 to 1.05) |
| Body mass index (kg/m2) | |
| <18.5 versus 18.5–24.9 | 1.46 (0.45 to 4.68) |
| 25–29.9 versus 18.5–24.9 | 1.08 (0.78 to 1.50) |
| >30 versus 18.5–24.9 | 0.85 (0.61 to 1.17) |
| LVEF (each SD decrease) | 1.12 (1.01 to 1.23) |
| LVH | 1.55 (1.22 to 1.95) |
| Hemoglobin (g/dl) | |
| <10 versus 10–12 | 1.60 (1.05 to 2.45) |
| 12 versus 10–12 | 0.76 (0.60 to 0.97) |
COPD, chronic obstructive pulmonary disease.
Model adjusted for age, sex, race, eGFR, proteinuria, diabetes, hypertension, smoking, COPD, body mass index, LVEF, LVH, and hemoglobin levels.
Associations between PH Measures and Clinical Outcomes
PH and Outcomes
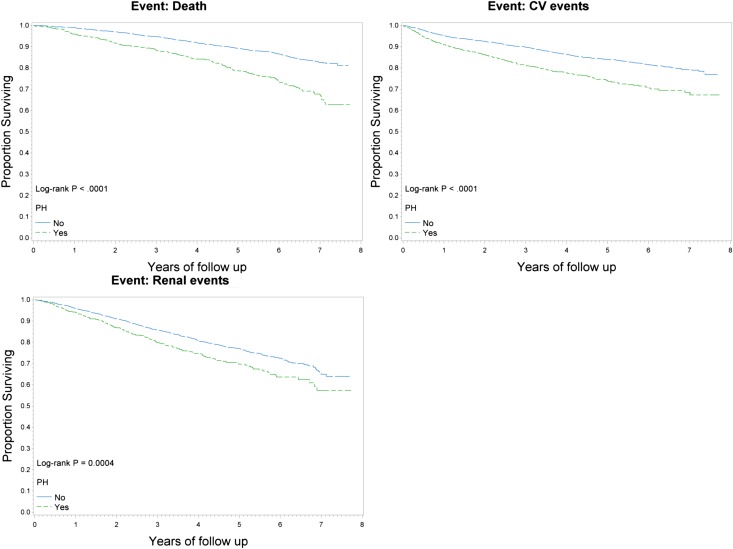
After a median follow-up of 4.06 years, 497 participants died, 575 cardiovascular events occurred, and 705 renal events occurred. Participants with PH had higher rates of renal events, cardiovascular events, and all-cause mortality (log-rank P<0.001) (Figure 1). After adjusting for relevant confounding variables, the presence of PH was independently associated with a 38% and a 23% increased risk for all-cause mortality and cardiovascular events, respectively, but was not associated with an increased risk for renal events (Table 3).
Figure 1.
Clinical outcomes in those with and without Pulmonary Hypertension. Kaplan-Meier curve for mortality, cardiovascular (CV) events, and renal events for those with and without PH (defined as PASP>35 mmHg and/or TRV>2.5 m/s).
Table 3.
Associations between PH (defined as PASP>35 mmHg and/or TRV>2.5 m/s), TRV, PASP, and outcomes
| Outcome | Unadjusted | Model Aa | Model Bb | Model Cc | Model Dd | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| PH versus no PH | ||||||||||
| All-cause mortality | 2.13 (1.77 to 2.57) | <0.001 | 1.68 (1.38 to 2.05) | <0.001 | 1.48 (1.21 to 1.81) | <0.001 | 1.40 (1.12 to 1.74) | 0.003 | 1.38 (1.10 to 1.72)e | 0.004e |
| Cardiovascular events | 1.71 (1.42 to 2.05) | <0.001 | 1.45 (1.20 to 1.75) | <0.001 | 1.34 (1.11 to 1.62) | 0.002 | 1.31 (1.07 to 1.61) | 0.01 | 1.23 (1.00 to 1.52)e | 0.04e |
| Renal events | 1.38 (1.15 to 1.65) | <0.001 | 1.31 (1.09 to 1.58) | 0.003 | 1.13 (0.93 to 1.36) | 0.21 | 1.12 (0.91 to 1.37) | 0.28 | 1.16 (0.95, 1.43) | 0.14 |
| CHF | 2.08 (1.69 to 2.56) | <0.001 | 1.77 (1.42 to 2.19) | <0.001 | 1.59 (1.28 to 1.97) | <0.001 | 1.47 (1.16 to 1.86) | 0.001 | 1.38 (1.09 to 1.77)e | <0.01e |
| MI | 1.33 (0.96 to 1.82) | 0.08 | 1.12 (0.80 to 1.55) | 0.51 | 1.02 (0.73 to 1.42) | 0.91 | 1.07 (0.75 to 1.52) | 0.71 | 1.04 (0.73 to 1.49) | 0.80 |
| CVA | 1.33 (0.83 to 2.13) | 0.23 | 1.16 (0.71 to 1.89) | 0.54 | 1.07 (0.65 to 1.76) | 0.78 | 1.04 (0.61 to 1.75) | 0.89 | 0.99 (0.58 to 1.70) | 0.98 |
| TRV (per 1-SD increase)e | ||||||||||
| All-cause mortality | 1.58 (1.44 to 1.73) | <0.001 | 1.45 (1.31 to 1.60) | <0.001 | 1.28 (1.16 to 1.42) | <0.001 | 1.19 (1.06 to 1.33) | 0.003 | 1.18 (1.05 to 1.32)e | <0.01e |
| Cardiovascular events | 1.69 (1.53 to 1.86) | <0.001 | 1.61 (1.45 to 1.78) | <0.001 | 1.41 (1.27 to 1.56) | <0.001 | 1.31 (1.17 to 1.47) | <0.001 | 1.26 (1.12 to 1.42)e | <0.001e |
| Renal events | 1.36 (1.24 to 1.50) | <0.001 | 1.32 (1.19 to 1.46) | <0.001 | 1.16 (1.03 to 1.29) | 0.01 | 1.14 (1.00 to 1.29) | 0.04 | 1.12 (0.99 to 1.27) | 0.06 |
| CHF | 1.85 (1.67 to 2.06) | <0.001 | 1.78 (1.59 to 1.99) | <0.001 | 1.51 (1.34 to 1.69) | <0.001 | 1.36 (1.19 to 1.55) | <0.001 | 1.30 (1.14 to 1.49)e | <0.001e |
| MI | 1.31 (1.11 to 1.54) | 0.001 | 1.22 (1.02 to 1.46) | 0.03 | 1.09 (0.91 to 1.30) | 0.36 | 0.99 (0.82 to 1.20) | 0.95 | 0.97 (0.81 to 1.18) | 0.79 |
| CVA | 1.46 (1.15 to 1.86) | 0.002 | 1.34 (1.04 to 1.72) | 0.03 | 1.20 (0.94 to 1.55) | 0.14 | 1.30 (0.97 to 1.74) | 0.07 | 1.29 (0.95 to 1.74) | 0.10 |
| PASP (per 1-SD increase)e | ||||||||||
| All-cause mortality | 1.50 (1.37 to 1.64) | <0.001 | 1.41 (1.28 to 1.55) | <0.001 | 1.25 (1.12 to 1.38) | <0.001 | 1.15 (1.02 to 1.29) | 0.02 | 1.15 (1.02 to 1.29)e | 0.02e |
| Cardiovascular events | 1.69 (1.54 to 1.86) | <0.001 | 1.64 (1.48 to 1.81) | <0.001 | 1.42 (1.28 to 1.58) | <0.001 | 1.34 (1.19 to 1.51) | <0.001 | 1.28 (1.13 to 1.45)e | <0.001e |
| Renal events | 1.27 (1.15 to 1.40) | <0.001 | 1.24 (1.11 to 1.38) | <0.001 | 1.08 (0.95 to 1.22) | 0.24 | 1.07 (0.93 to 1.23) | 0.34 | 1.10 (0.95 to 1.27) | 0.18 |
| CHF | 1.85 (1.68 to 2.04) | <0.001 | 1.81 (1.62 to 2.01) | <0.001 | 1.55 (1.38 to 1.75) | <0.001 | 1.40 (1.22 to 1.61) | <0.001 | 1.35 (1.17 to 1.56)e | <0.001e |
| MI | 1.32 (1.12 to 1.56) | 0.001 | 1.24 (1.03 to 1.48) | 0.01 | 1.1 (0.92 to 1.34) | 0.26 | 1.03 (0.84 to 1.26) | 0.78 | 1.00 (0.82 to 1.22) | 0.98 |
| CVA | 1.46 (1.18 to 1.82) | <0.001 | 1.36 (1.08 to 1.71) | <0.01 | 1.20 (0.94 to 1.52) | 0.13 | 1.30 (0.98 to 1.71) | 0.06 | 1.25 (0.94 to 1.68) | 0.12 |
Each SD increase in TRV is 0.35 m/s, and each SD increase in PAP is 8.8 mmHg. CHF, congestive heart failure; MI, myocardial infarction; CVA, cerebrovascular accidents; COPD, chronic obstructive pulmonary disease; RAS, renin-angiotension system; BNP, brain natriuretic peptide; HR, hazard ratio; 95% CI, 95% confidence interval.
Model A: adjusted for age, sex, race, and clinical center.
Model B: adjusted for model A plus smoking, diabetes, hypertension, previous cardiovascular disease, COPD, chronic obstructive pulmonary disease, RAS: Renin-angiotension system; BNP - brain natriuretic peptide, body mass index, albumin, hemoglobin, LDL cholesterol, use of RAS blockers, diuretics, statins, β-blockers, and BNP.
Model C: adjusted for model B plus LVEF and LVH.
Model D: adjusted for model C plus eGFR and urine albumin to creatinine ratio.
Each SD increase in TRV 0.35 m/sec and SD increase in PAP 8.8 mm Hg.
TRV and Outcomes
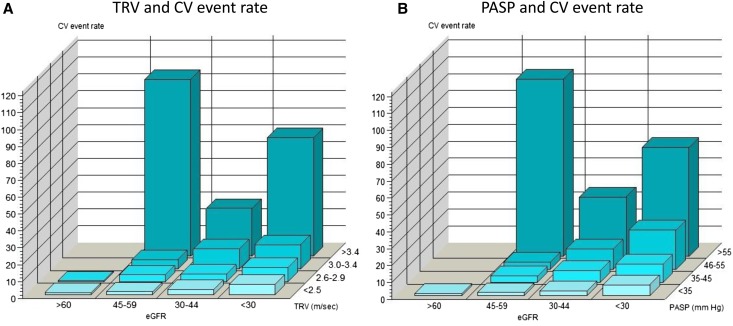
Participants with TRV>2.5 m/s had higher rates of renal events, cardiovascular events, and all-cause mortality than those with TRV<2.5 m/s (log-rank P<0.001) (Supplemental Figure 1). We did not find evidence against linearity of TRV as a predictor of the outcomes and therefore, treated it as a single continuous variable in the primary analyses. After adjusting for confounding variables, each SD increase in TRV was associated with an 18% increased risk for death and a 26% increased risk for composite cardiovascular events (Table 3). Among the individual cardiovascular events, each SD increase in TRV was associated with higher risk for congestive heart failure (CHF) but not myocardial infarction (MI) or cerebrovascular accidents (CVA) (Table 3). There was a graded association between TRV and composite cardiovascular events at all levels of eGFR; participants with TRV>3.4 m/s had almost a 3-fold higher rate of composite cardiovascular outcomes compared with those with TRV<2.5 m/s (Figure 2A, Supplemental Table 3). Although there was a trend toward significance, there was no significant association between TRV and renal events, except for in those with TRV>3.0 m/s (Table 3, Supplemental Figure 2).
Figure 2.
Cardiovascular (CV) event rates. (A) TRV and CV event rates among various stages of CKD. (B) PASP and CV event rates among various stages of CKD.
PASP and Outcomes
Participants with higher PASP had higher rates of composite cardiovascular outcomes (Figure 2B). The Kaplan-Meier analysis showed significant differences in all-cause mortality and cardiovascular and renal events for patients with CKD and PASP>35 versus <35 mmHg (Supplemental Figure 3). After adjusting for confounding variables, each SD increase in PASP was associated with a 15% and a 28% increased risk for death and cardiovascular events, respectively, but was not associated with renal events. Each SD increase in PASP was associated with increased risk of CHF but not MI or CVA. Mortality and cardiovascular risk were significantly higher for those with PASP>55 mmHg (Supplemental Table 4).
Sensitivity Analyses
Associations between PH, TRV, and PASP and congestive heart failure were consistent in sensitivity analyses, which excluded participants who had a history of cardiovascular disease at the year 1 CRIC Study visit (Supplemental Table 5). Although the effect estimate suggested higher risk for composite cardiovascular events with PH and each SD increase in TRV and PASP, it was not statistically significant (Supplemental Table 5). In another sensitivity analysis, presence of PH and each SD increase in TRV were associated with the ESRD/death composite (Supplemental Table 6). Results were also consistent by subgroups of age, ethnicity, and eGFR at baseline (interaction P values ≥0.05 for all subgroups), except that the associations between PH and mortality differed between men and women (hazard ratio for men, 1.05; 95% confidence interval, 0.76 to 1.45 versus hazard ratio for women, 1.92; 95% confidence interval, 1.32 to 2.79) (Supplemental Figures 4 and 5).
Discussion
In this large, prospective study of patients with CKD and preserved left ventricular systolic function, we report a high prevalence of PH and note that the prevalence of PH increases with lower levels of kidney function. We show, for the first time, that the presence of PH is an independent predictor of mortality and cardiovascular events in patients with CKD. We also show that TRV and PASP (echocardiographic measures of PH) are also associated with mortality and cardiovascular events (primarily heart failure).
Echocardiography is commonly used for the initial evaluation of PH in clinical practice and in epidemiologic studies. Using Doppler–based echocardiographic measures, we report that the prevalence of PH is 21% in a large cohort of patients with nondialysis-dependent CKD. Comparison of prevalence of PH across study populations is limited by the varying definitions of PH used in different studies. Our findings are consistent with previous study results in patients with sickle cell that reported a prevalence of 25%–30% PH using a similar definition.19 However, it is important to point out that the sensitivity and specificity of echocardiography to diagnose PH are modest (83% and 72%, respectively).20,21 This suggests that confirmation by right heart catheterization is needed for additional clinical evaluation. However, as discussed below, the predictive value of the echocardiography-based measures shown in our study is an important advance and highlights the need for additional study in this area.
In the CRIC Study, the likelihood of prevalent PH increased with older age, presence of anemia, and lower levels of LVEF and LVH. In the general population, PASP assessed using Doppler echocardiography increased with age.22 LVH and systolic dysfunction are important factors contributing to the development of PH, particularly with the underlying volume overload observed in this population. It is important to note that most CRIC Study participants had preserved LVEF, and patients with preexisting New York Heart Association (NYHA) classes III and IV heart failure were excluded. However, LVEF was still associated with PH in our study.23 However, diastolic dysfunction (measured as LVH) or possibly, higher rates of precapillary hypertension (pulmonary arterial hypertension) with underlying vascular calcification noted in CKD may be contributing factors. Furthermore, CKD is associated with endothelial dysfunction, arterial stiffness, and elevated parathyroid hormone, factors implicated in the development of PH.24–27 Our ability to identify the etiology of PH was limited in this large epidemiologic study. Hence, future mechanistic studies, possibly including right heart catheterization, may offer additional insights.
PH is associated with a high morbidity and mortality burden in the general population, those with sickle cell disease, chronic lung diseases, or ambulatory heart failure, and those on dialysis.19,22,28–30 Consistent with these studies, we now show that the presence of PH and higher TRV and PASP values are independent risk factors for mortality and cardiovascular events in patients with CKD. Interestingly, the risk for cardiovascular events and mortality observed in our study (in those without heart failure) is similar to what was noted in other cohorts with heart failure, highlighting the importance of PH in the CKD population. Furthermore, the stage of CKD did not modify the associations between PH measures and cardiovascular outcomes, suggesting that the risk for adverse outcomes begins even with mild to moderate CKD. We noted that the associations between PH and mortality are different for men and women, and additional studies are needed to explain these sex differences. Because most patients with early stages of CKD are cared for by primary care physicians, these results emphasize the need for them to understand the implications of PH in kidney disease.
Another important finding in our study was the association between PH, TRV, PASP, and the risk for heart failure. With left ventricular dysfunction, an increased left ventricular filling pressure leads to elevated left atrial pressure, which in turn, raises pulmonary venous pressure. This hemodynamic alteration that decreases filling pressure leads to pulmonary vascular remodeling in the long term, with resultant worsening of underlying PH and pulmonary edema. Our findings of increased hazard for CHF (but not MI or CVA in the cardiovascular event composite) with higher TRV are consistent with this sequence leading to heart failure in patients with CKD and PH. Furthermore, PH and TRV were also associated with incident cardiovascular events (in those without prior cardiovascular disease), strengthening these findings. In contrast to other cohorts, a higher proportion of CRIC Study participants had greater left ventricular mass index and LVH but with preserved LVEF. Future studies should clarify if these CHF events were related to right–sided heart failure caused by PH or congestive heart failure caused by left ventricular systolic or diastolic dysfunction.
The principal strength of this study is the availability of comprehensive clinical data and echocardiographic assessment of cardiac function using a standard protocol in a large, well characterized, contemporary cohort of patients with CKD without preexisting NYHA class III or IV heart failure. In addition, long-term follow-up, availability of adjudicated cardiovascular and renal events, and adequate adjustment for confounding variables in the analysis enhance the quality of the findings. However, there are important limitations in our study. The lack of a standardized definition of PH on the basis of echocardiographic data is a limitation in this field. To address this, we also considered PASP and TRV as continuous measures in addition to the categorical definition of PH. Although some guidelines suggest the use of TRV>2.7 m/s as possible PH, we used a lower cutoff, which was used by other studies.19,31 However, our categorical analysis confirms higher risk for cardiovascular events and death in those with TRV>2.9 m/s. It is well known that Doppler echocardiograms tend to overestimate PH measures, but right heart catheterization is not feasible in such large cohorts. Given the nature and details collected in the parent study, we did not have information about vasculitis, chronic lung disease, details of AKI and duration of CKD, obstructive sleep apnea and continuous positive airway pressure use, and evaluation for other etiologies of PH, precluding us from adjusting for and classifying our patients into the diagnostic categories recommended by the World Health Organization.32 Because of the nature of the cohort (followed in health care systems), additional studies should confirm these findings. Although pulse–wave velocity measures (to assess arterial stiffness) were performed in the CRIC Study, the differences in timing of these studies limited our availability to evaluate the association between arterial stiffness and PH.
In summary, the prevalence of PH (defined as PASP>35 mmHg and/or TRV>2.5 m/s) was high in CRIC Study participants on the basis of echocardiographic assessment. PH, TRV, and PASP are independent predictors of cardiovascular events and mortality in this CKD cohort without preexisting heart failure. Importantly, additional studies are warranted to examine the mechanisms, perhaps including right heart catheterization, which might explain these associations, and explore the potential therapeutic implications.
Concise Methods
Study Design and Population
The CRIC Study enrolled 3939 individuals ages 21–74 years old with eGFRs of 20–70 ml/min per 1.73 m2 from June of 2003 to December of 2008 at seven clinical centers across the United States (Ann Arbor, MI; Baltimore, MD; Chicago, IL; Cleveland, OH; New Orleans, LA; Philadelphia, PA; and Oakland, CA). Study design and baseline participant characteristics were previously published.23,33 Major exclusion criteria included prior dialysis lasting >1 month, NYHA class III/IV heart failure, polycystic kidney disease or other primary renal diseases requiring active immunosuppression, HIV, and pregnancy. Participants underwent annual study visits and telephone follow-up two times per year. Study participants provided written informed consent and authorized release of medical records for study purposes, and they were followed annually under protocols approved by institutional review boards at each of the CRIC Study clinical centers. Patients who underwent Doppler echocardiography study at the year 1 visit of the study with available data relating to PH (TRV and PASP) were included in this analysis (Supplemental Figure 6).
Doppler Echocardiogram
Doppler echocardiography was performed 1 year after enrollment according to the American Society of Echocardiography guidelines to assess heart structure and function.14 Data were sent to a core echocardiography laboratory for measurement and analysis (University of Pennsylvania). The peak difference between right ventricular and right atrial pressure was assessed from the continuous-wave signal of tricuspid regurgitation. Right atrial pressure was estimated by visual inspection of the inferior vena caval diameter through several respiratory cycles. This was added to the right atrial/right ventricular pressure gradient obtained from the tricuspid regurgitation velocity to yield the PASP. Left ventricular mass and LVH were assessed as described previously.14 Left ventricular diastolic function was categorized into normal or mildly, moderately, or severely abnormal (corresponding to normal and grades 1–3 diastolic dysfunction, respectively).34 Multiple reproducibility, inter- and intrareader reliability, and reader drift analyses were performed throughout the course of this study on a 2% random sample of the entire cohort each year. The intraclass correlation coefficients (and κ-statistics) for the echocardiographic measures are LVH=0.759 (κ=0.61), TRV=0.97 (κ not available), and LVEF=0.854 (κ not applicable).
PH Definition
PH was defined as the presence of PASP>35 mmHg and/or TRV>2.5 m/s in the echocardiogram obtained during the year 1 CRIC Study visit.19 We also examined TRV and PASP, key PH measures, as a continuous measure (each SD higher). Tricuspid regurgitant Doppler was graded for quality as not done, not analyzable, good, fair, and poor. We excluded the not done and not analyzable groups for these analyses (Supplemental Figure 6).
Outcomes
The outcome measures for this study included (1) cardiovascular events (heart failure, myocardial infarction, stroke, and peripheral vascular disease), (2) renal outcomes (50% decrease in eGFR or ESRD), and (3) all-cause mortality. These events were adjudicated from study entry to March of 2012. Participants’ follow-up was censored at the end of the follow-up time period, loss to follow-up, or death, whichever occurred first. Two independent reviewers evaluated the clinical data on the basis of predefined criteria to adjudicate each outcome. Detailed outcome ascertainment details are reported in Supplemental Material.
Covariates
Demographics and clinical information were obtained at the baseline and follow-up study visits by questionnaires, interviews, and physical examination. Data obtained at the year 1 visit for the CRIC Study were used for this analysis to coincide with the echocardiogram. History of any cardiovascular disease included prior myocardial infarction, revascularization, heart failure, stroke, or peripheral arterial disease. Current smoking was defined as self-report of current use of cigarettes and at least 100 cigarettes smoked. At each study visit, participants were queried about any medication usage in the prior 30 days. All antihypertensive medications were categorized into drug classes, and the total number of antihypertensive drug classes was calculated. Serum creatinine and 24-hour urine total protein were measured at the CRIC Central Laboratory using standard methods. Diabetes mellitus was defined as a fasting glucose >126 mg/dl, a nonfasting glucose >200 mg/dl, or use of insulin or other antidiabetic medication. Except for high-sensitivity C–reactive protein, serum phosphorus, and uric acid (for which baseline visit data were used), all other laboratory data obtained at the year 1 visit were used in this analysis.
Statistical Analyses
We calculated the overall prevalence rates of PH on the basis of age, sex, race, and stage of kidney disease. Descriptive statistics for baseline variables were compared among participants with and without PH using chi-squared and ANOVA models for categorical and continuous variables, respectively. A two–sided type I error probability of 0.05 was used throughout the analyses. Multivariate regression analyses were conducted to examine the predictors of PH. This model was adjusted for potential confounding variables, such as age, sex, race, eGFR, proteinuria, diabetes, hypertension, smoking, COPD, body mass index, LVEF, LVH, and hemoglobin levels. We also conducted a multivariate regression analysis, in which diastolic relaxation was used instead of LVH as a marker of diastolic relaxation.
To evaluate whether PH was associated with the individual primary outcome measures among this CKD population, Kaplan-Meier plots and log-rank tests were used. The associations of PH with individual outcomes of interest were examined using the Cox proportional hazards model while adjusting for age; sex; race; clinical center; diabetes; hypertension; smoking; body mass index; previous cardiovascular disease; chronic obstructive pulmonary disease; angiotensin–converting enzyme inhibitors/angiotensin receptor blockers use; use of diuretics, statins, and β-blockers; serum albumin; hemoglobin; LDL cholesterol; eGFR; urine albumin to creatinine ratio; LVEF; and LVH. We performed sequential modeling by first adjusting for demographics and then including comorbid conditions followed by adjustment for cardiac and kidney function as described above. A separate analysis was conducted by considering TRV as a continuous measure (each SD increase). Prespecified interactions between PH and age, sex, race, hypertension, and eGFR were tested, because these variables might modify the associations of PH with outcomes in CKD.
Sensitivity Analyses
In addition to examining TRV in a continuous analysis, we also compared the different categories of TRV and its relationship with outcomes (TRV=2.6–2.9, 3.0–3.4, and >3.4 versus <2.5 m/s). Similarly, we examined the associations between PASP and outcomes as continuous (each SD increase) and categorical variables (PASP=36–45, 46–55, and >55 versus <35 mmHg). We also conducted separate analysis to examine the associations of PH, PASP, and TRV with incident cardiovascular events (among those without previous cardiovascular disease at the year 1 CRIC Study visit) and the ESRD/death composite in the CRIC Study.
Disclosures
G.S. received research grants (principal investigator for multicenter studies) from Actelion and Gilead; was part of the Lectures/Speaker Bureau for Gilead, United Therapeutics, and Bayer; and also, reports travel arrangements to attend investigator/research meetings by Actelion. All other authors report no competing financial interests relevant to this manuscript.
Supplementary Material
Acknowledgments
Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under National Institute of Diabetes and Digestive and Kidney (NIDDK) Diseases Cooperative Agreements U01-DK060990, U01-DK060984, U01-DK061022, U01-DK061021, U01-DK061028, U01-DK060980, U01-DK060963, and U01-DK060902. In addition, this work was supported, in part, by Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) Grant UL1-TR000003, Johns Hopkins University Grant UL1-TR000424, University of Maryland General Clinical Research Center Grant M01RR16500, the Clinical and Translational Science Collaborative of Cleveland, NCATS Component of the NIH and NIH Roadmap for Medical Research Grant UL1-TR000439, Michigan Institute for Clinical and Health Research Grant UL1-TR000433, University of Illinois at Chicago Clinical and Translational Science Awards Grant UL1-RR029879, Tulane University Translational Research in Hypertension and Renal Biology Grant P30-GM103337, and Kaiser Permanente NIH/NCRR National Center for Research Resources Grant UCSF-CTSI UL1-RR024131. S.D.N. is supported by NIH Grant R01-DK101500.
Parts of the results were presented as a poster at the American Society of Nephrology annual abstract sessions held on November 14, 2014 in Philadelphia, PA.
The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Funding agencies did not have any role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
The CRIC Study investigators also include Drs. Harold I. Feldman (University of Pennsylvania), Alan S. Go (Kaiser Permanente of Northern California), James P. Lash (University of Illinois at Chicago), Lawrence J. Appel (Johns Hopkins University), Jiang He (Tulane University), and John W. Kusek (NIDDK/NIH).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Pulmonary Hypertension in CKD: Some Answers, Yet More Questions,” on pages 661–663.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111111/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt-Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen-Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R, SHARP Investigators : The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 377: 2181–2192, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jardine MJ, Kang A, Zoungas S, Navaneethan SD, Ninomiya T, Nigwekar SU, Gallagher MP, Cass A, Strippoli G, Perkovic V: The effect of folic acid based homocysteine lowering on cardiovascular events in people with kidney disease: Systematic review and meta-analysis. BMJ 344: e3533, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris K, Bourgoigne J, Gassman J, Hebert L, Middleton J, Phillips RA, Randall O, Rostand S, Sherer S, Toto RD, Wright JT, Jr., Wang X, Greene T, Appel LJ, Lewis J, AASK Study Group : Cardiovascular outcomes in the african american study of kidney disease and hypertension (AASK) trial. Am J Kidney Dis 48: 739–751, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, Wright JT, Jr., Barzilay JI, Brown CD, Colon PJS, Sr., Fine LJ, Grimm RH, Jr., Gupta AK, Baimbridge C, Haywood LJ, Henriquez MA, Ilamaythi E, Oparil S, Preston R, ALLHAT Collaborative Research Group : Long-term renal and cardiovascular outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) participants by baseline estimated GFR. Clin J Am Soc Nephrol 7: 989–1002, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolignano D, Rastelli S, Agarwal R, Fliser D, Massy Z, Ortiz A, Wiecek A, Martinez-Castelao A, Covic A, Goldsmith D, Suleymanlar G, Lindholm B, Parati G, Sicari R, Gargani L, Mallamaci F, London G, Zoccali C: Pulmonary hypertension in CKD. Am J Kidney Dis 61: 612–622, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Sise ME, Courtwright AM, Channick RN: Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int 84: 682–692, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Kawar B, Ellam T, Jackson C, Kiely DG: Pulmonary hypertension in renal disease: Epidemiology, potential mechanisms and implications. Am J Nephrol 37: 281–290, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Navaneethan SD, Wehbe E, Heresi GA, Gaur V, Minai OA, Arrigain S, Nally JV, Jr., Schold JD, Rahman M, Dweik RA: Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol 9: 855–863, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal R: Prevalence, determinants and prognosis of pulmonary hypertension among hemodialysis patients. Nephrol Dial Transplant 27: 3908–3914, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yigla M, Nakhoul F, Sabag A, Tov N, Gorevich B, Abassi Z, Reisner SA: Pulmonary hypertension in patients with end-stage renal disease. Chest 123: 1577–1582, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Shah SJ: Pulmonary hypertension. JAMA 308: 1366–1374, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Park M, Hsu CY, Li Y, Mishra RK, Keane M, Rosas SE, Dries D, Xie D, Chen J, He J, Anderson A, Go AS, Shlipak MG, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Associations between kidney function and subclinical cardiac abnormalities in CKD. J Am Soc Nephrol 23: 1725–1734, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Havlucu Y, Kursat S, Ekmekci C, Celik P, Serter S, Bayturan O, Dinc G: Pulmonary hypertension in patients with chronic renal failure. Respiration 74: 503–510, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Yigla M, Fruchter O, Aharonson D, Yanay N, Reisner SA, Lewin M, Nakhoul F: Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int 75: 969–975, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Abdelwhab S, Elshinnawy S: Pulmonary hypertension in chronic renal failure patients. Am J Nephrol 28: 990–997, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Mielniczuk LM, Chandy G, Stewart D, Contreras-Dominguez V, Haddad H, Pugliese C, Davies RA: Worsening renal function and prognosis in pulmonary hypertension patients hospitalized for right heart failure. Congest Heart Fail 18: 151–157, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP: Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. N Engl J Med 350: 886–895, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Janda S, Shahidi N, Gin K, Swiston J: Diagnostic accuracy of echocardiography for pulmonary hypertension: A systematic review and meta-analysis. Heart 97: 612–622, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Parent F, Bachir D, Inamo J, Lionnet F, Driss F, Loko G, Habibi A, Bennani S, Savale L, Adnot S, Maitre B, Yaïci A, Hajji L, O’Callaghan DS, Clerson P, Girot R, Galacteros F, Simonneau G: A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med 365: 44–53, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM: Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation 119: 2663–2670, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI, Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akmal M, Barndt RR, Ansari AN, Mohler JG, Massry SG: Excess PTH in CRF induces pulmonary calcification, pulmonary hypertension and right ventricular hypertrophy. Kidney Int 47: 158–163, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Klinger JR, Abman SH, Gladwin MT: Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 188: 639–646, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Nerpin E, Ingelsson E, Risérus U, Helmersson-Karlqvist J, Sundström J, Jobs E, Larsson A, Lind L, Ärnlöv J: Association between glomerular filtration rate and endothelial function in an elderly community cohort. Atherosclerosis 224: 242–246, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Tonelli AR, Haserodt S, Aytekin M, Dweik RA: Nitric oxide deficiency in pulmonary hypertension: Pathobiology and implications for therapy. Pulm Circ 3: 20–30, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeger W, Adir Y, Barberà JA, Champion H, Coghlan JG, Cottin V, De Marco T, Galiè N, Ghio S, Gibbs S, Martinez FJ, Semigran MJ, Simonneau G, Wells AU, Vachiéry JL: Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 62[Suppl]: D109–D116, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Amin M, Fawzy A, Hamid MA, Elhendy A: Pulmonary hypertension in patients with chronic renal failure: Role of parathyroid hormone and pulmonary artery calcifications. Chest 124: 2093–2097, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, Pochettino A, Kotloff RM: Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 167: 735–740, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS, Gomez-Sanchez MA, Jondeau G, Klepetko W, Opitz C, Peacock A, Rubin L, Zellweger M, Simonneau G, ESC Committee for Practice Guidelines (CPG) : ESC Committee for Practice Guidelines (CPG): Guidelines for the diagnosis and treatment of pulmonary hypertension: The task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30: 2493–2537, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Fishman AP: Clinical classification of pulmonary hypertension. Clin Chest Med 22: 385–391, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT, Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A: Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22: 107–133, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.