Abstract
Podocytes are specialized, highly differentiated epithelial cells in the kidney glomerulus that are exposed to glomerular capillary pressure and possible increases in mechanical load. The proteins sensing mechanical forces in podocytes are unconfirmed, but the classic transient receptor potential channel 6 (TRPC6) interacting with the MEC-2 homolog podocin may form a mechanosensitive ion channel complex in podocytes. Here, we observed that podocytes respond to mechanical stimulation with increased intracellular calcium concentrations and increased inward cation currents. However, TRPC6-deficient podocytes responded in a manner similar to that of control podocytes, and mechanically induced currents were unaffected by genetic inactivation of TRPC1/3/6 or administration of the broad-range TRPC blocker SKF-96365. Instead, mechanically induced currents were significantly decreased by the specific P2X purinoceptor 4 (P2X4) blocker 5-BDBD. Moreover, mechanical P2X4 channel activation depended on cholesterol and podocin and was inhibited by stabilization of the actin cytoskeleton. Because P2X4 channels are not intrinsically mechanosensitive, we investigated whether podocytes release ATP upon mechanical stimulation using a fluorometric approach. Indeed, mechanically induced ATP release from podocytes was observed. Furthermore, 5-BDBD attenuated mechanically induced reorganization of the actin cytoskeleton. Altogether, our findings reveal a TRPC channel-independent role of P2X4 channels as mechanotransducers in podocytes.
Keywords: cell signaling, cell and transport physiology, glomerulosclerosis
Podocytes are unique visceral epithelial cells lining the inner part of the Bowman capsule, where they are important regulators of the kidney filtration barrier. Podocytes contain a cell body and so-called foot processes, which allow for protein-protein interactions with adjacent interdigitated foot processes, thereby forming the slit membrane.1,2 Slit-membrane proteins, such as nephrin or Neph1–3, as well as slit-membrane–associated proteins, such as podocin, synaptopodin, CD2-associated protein (CD2AP), and the transient receptor potential channel 6 (TRPC6), are tightly regulated to ensure proper function of the podocyte filtration barrier. Hypertension and the resulting increase in glomerular pressure are believed to result in podocyte damage, leading to proteinuria.3 Glomerular hypertension–provoked hypertrophy, foot process effacement, and detachment of podocytes are well characterized in animal models.4–6 There is also evidence for a damaging effect of hypertension in humans.7
Until now, it has been completely unknown how podocytes sense the changes in glomerular pressure. It was postulated that TRPC6 channels involved in the pathogenesis of familial FSGS8,9 may serve as mechanosensors in podocytes.2,10,11 However, at least in overexpression systems, TRPC6 itself was not inherently mechanosensitive.12–14 TRPC6 was shown to be expressed at the slit membrane,8,9 where it interacts with the podocyte-specific protein podocin.10 Because the podocin homolog MEC-2 is part of a mechanosensitive multiprotein ion channel complex in Caenorhabditis elegans, it was suggested that TRPC6 in complex with other slit-membrane proteins (including podocin) could form a mechanosensitive ion channel complex.2 Of note, in our previous studies, Gq/11-protein–coupled receptors (Gq/11PCRs) and not TRPC6 channels shaped up as mechanosensors in vascular smooth muscle cells13,15 contributing to myogenic vasoconstriction. There is still growing evidence for an inherent mechanosensitivity of G-protein–coupled receptors (GPCRs) in other tissues and organs (summarized by Storch et al.16). Furthermore, many other proteins, including structural proteins and various ion channels, have been discussed as potential mechanosensors.17 Because the mechanosensory and -transducing elements in podocytes are still largely elusive, we sought to analyze the role of TRPC6 and GPCRs as potential mechanosensors in podocytes and at uncovering novel candidates for mechanosensors or -transducers in podocytes. Deeper insight into the mechanisms leading to mechanically induced podocyte damage may be instrumental in devising targeted treatments for podocyte injuries, as seen in hypertensive nephropathy.18
RESULTS
Mechanical Membrane Stretch Induces Inwardly Rectifying Currents and Intracellular Calcium Increases in Podocytes
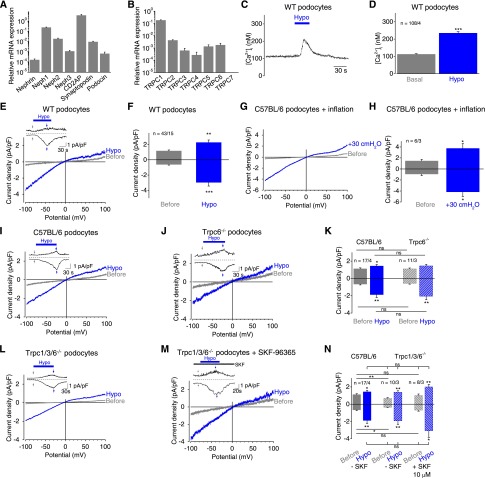
To analyze the mechanosensory and -transduction properties of podocytes, we used isolated murine podocytes, which express several podocyte-specific proteins, such as nephrin and podocin (Supplemental Figure 1). Quantitative RT-PCR was used to determine the expression of the podocyte markers nephrin, podocin, Neph1–3, synaptopodin, and CD2AP (Figure 1A) and of all TRPC channels exception TRPC7 (Figure 1B). Next, fluorometric calcium measurements with fura-2–loaded podocytes were performed. As a mechanical stimulus, cell swelling with hypoosmotic bath solution was deployed, resulting in transient calcium increases (Figure 1, C and D). Whole-cell measurements showed that podocytes responded to cell swelling with hypoosmotic bath solution with significantly increased inwardly rectifying cation currents (Figure 1, E and F); this finding indicates that podocytes are mechanosensitive. Because hypotonic cell swelling might trigger additional unspecific effects apart from exerting membrane stretch by increasing the cell volume, we next applied a positive pipette pressure of 30 cmH2O; this led to transient cation current increases with a similar current density voltage relationship, as observed upon hypoosmotic cell swelling (Figure 1, G and H). Similar results were obtained by analyzing conditionally immortalized murine podocytes19 that responded to hypoosmotic cell swelling and positive pipette pressure in a similar manner (Supplemental Figure 2, A–D).
Figure 1.
Mechanical membrane stretch induces inwardly rectifying currents and intracellular calcium increases in podocytes. (A and B) Relative mRNA expression levels of selected podocyte marker proteins (A) and TRPC channels (B) in primary podocytes determined by quantitative PCR analysis from three independent experiments. (C) Exemplary time courses of the intracellular calcium concentration [Ca2+]i of fura-2–loaded podocytes. Application of hypoosmotic bath solution “Hypo” is indicated. (D) Summary of [Ca2+]i before (gray bar) and during (blue bar) application of hypoosmotic bath solution. Numbers indicate the numbers of measured cells and of independent experiments. (E–N) Electrophysiologic whole-cell measurements of wild-type (WT) (E), C57BL/6 (G and I), TRPC6 gene-deficient (Trpc6−/−) (J), and TRPC1/3/6 gene-deficient (Trpc1/3/6−/−) (L and M) podocytes in the presence (M) or absence (L) of the nonselective TRPC blocker SKF-96365 with exemplary current density voltage (CDV) relationships. CDVs are displayed before (gray) and during (blue) application of hypoosmotic bath solution “Hypo” and of positive pipette pressure of 30 cmH2O “inflation” (G). Insets show current density time courses at holding potentials of ±100 mV. Stippled lines represent zero current. Blue bars indicate application of hypoosmotic solution. Arrows represent the time points of depicted CDV traces. (F, H, K, and N) Summary of current densities before (gray and gray hatched bars) and during application of hypoosmotic bath solution or of positive pipette pressure (blue and blue hatched bars) of respective control (solid bars) and gene-deficient podocytes (hatched bars) at ±100 mV. Numbers display the numbers of measured cells and of independent experiments. Nonsignificant (ns) differences are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.
Because it is hypothesized that in podocytes TRPC6 in complex with slit-membrane proteins (SMPs) is mechanosensitive, we analyzed isolated podocytes from TRPC6 gene-deficient (Trpc6−/−) mice.20 Interestingly, Trpc6−/− podocytes showed similar inwardly rectifying currents as observed in control podocytes (Figure 1, I–K), suggesting that TRPC6 is not essential for mechanosensation or -transduction. Moreover, the reconstitution of a potentially mechanosensitive ion channel complex by coexpressing TRPC6 together with the SMPs podocin, CD2AP, and nephrin in human embryonic kidney 293 (HEK293) cells was unsuccessful because TRPC6 current did not increase upon mechanical stimulation (Supplemental Figure 2, E and F), while subsequent stimulation with the TRPC6 activator oleoyl-2-acetyl-glycerol, a membrane-permeable diacylglycerol analogue, augmented TRPC6 currents. Taken together, these data argue against TRPC6 as a mechanosensor in podocytes. To rule out the involvement of other TRPC channels, C57BL/6 podocytes were incubated with the broad-range TRPC channel blocker SKF-96365 (10 µM), which had no effect on mechanically induced cation current increases (Supplemental Figure 2, G and H). Moreover, podocytes isolated from TRPC1/3/6 triple gene–deficient (Trpc1/3/6−/−) mice were analyzed in the presence and absence of 10 µM SKF-96365. Mechanical stimulation caused similar cation currents as monitored in control podocytes (Figure 1, L–N), indicating that TRPC channels are not essentially involved in mechanosensation or mechanotransduction in podocytes.
Gq/11-PCRs Are Not Mechanosensors in Podocytes
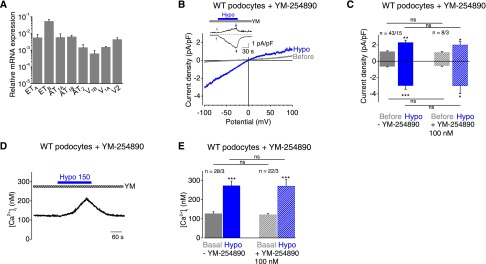
In cardiac and vascular smooth muscle, Gq/11PCRs and particularly angiotensin II (AT1) receptors (AT1Rs) were identified as direct mechanosensors.13,15,21 Moreover, there is evidence that overexpression of AT1Rs causes proteinuria,22 and AT1R antagonists and angiotensin-converting enzyme inhibitors are protective for podocytes and can improve proteinuria.23,24 Therefore, we analyzed the role of Gq/11PCRs as potential mechanosensors in podocytes. Performing quantitative RT-PCR, we documented the expression of several Gq/11PCRs known to play a role in the kidney, including endothelin type A and type B, AT1A, AT1B, and AT2 and vasopressin (V)1A, V1B, and V2 receptors (Figure 2A). Inhibition of all Gq/11 proteins by the selective Gq/11 protein inhibitor YM-254890, preventing the exchange of guanosine diphosphate for guanosine triphosphate during Gαq/11 activation,25 did not suppress mechanically induced currents (Figure 2, B and C) or intracellular calcium increases (Figure 2, D–F). This finding indicates that Gq/11PCRs are not involved in mechanosensation in podocytes.
Figure 2.
Gq/11-PCRs are not mechanosensors in podocytes. (A) Relative mRNA expression levels of selected GPCRs in primary podocytes determined by quantitative PCR analysis from three independent experiments. (B) Exemplary CDV relationships of podocytes preincubated with the Gq/11-inhibitor YM-254890 (100 nM) for 30 minutes before (gray) and during (blue) cell swelling. Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution is indicated. Arrows represent the time points of depicted CDV traces. (C) Summary of current densities before (gray and gray hatched bars) and during (blue and blue hatched bars) the application of hypoosmotic solution of control podocytes (solid bars) and podocytes preincubated with YM-254890 (hatched bars) determined at holding potentials of ±100 mV. Numbers display the number of measured cells and of independent experiments. (D) Exemplary time course of the [Ca2+]i of fura-2–loaded podocytes in the presence of YM-25489. Application of hypoosmotic bath solution “Hypo” is indicated. (E) Summary of [Ca2+]i before (gray and gray hatched bar) and during (blue and blue hatched bar) application of hypoosmotic bath solution in the presence or absence of YM-25489. Numbers indicate the numbers of measured cells and of independent experiments. Nonsignificant differences (ns) are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.
ATP Application and Mechanical Membrane Stretch Cause Similar Purinergic Currents in Podocytes
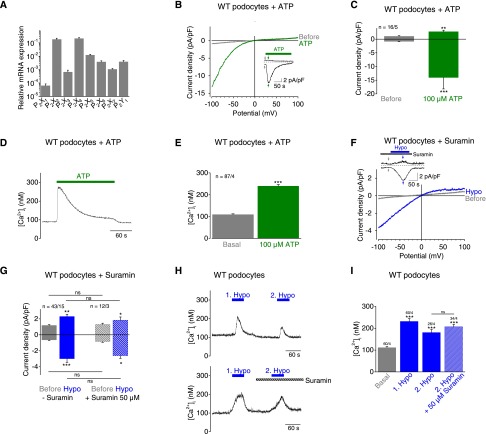
Interestingly, mechanically induced cation currents in podocytes share properties with purinergic (P2X) channels, such as nonselectivity and inward rectification.26,27 Performing quantitative RT-PCR, we observed expression of all seven P2X channel subunits (P2X1–P2X7) in podocytes. P2X2 and P2X4 showed highest mRNA expression levels, which were 15- to 17-fold higher compared with P2X5, exhibiting the third highest mRNA expression level (Figure 3A). Expression of the Gq/11-coupled P2Y1 receptor was also detected. Surprisingly, 100 µM ATP gave rise to inward currents with marked inward rectification (Figure 3, B and C), similar to mechanically induced currents. Further, ATP application provoked transient calcium increases in fura-2–loaded podocytes (Figure 3, D and E). ATP-induced currents slowly inactivated in the presence of ATP (Figure 3, B and D) with an inactivation constant of τ=40.8±7.2 seconds calculated at a holding potential of −100 mV. Homomeric P2X channels consisting of three P2X subunits can be distinguished by their current inactivation time within milliseconds (P2X1 and P2X3), within several seconds (P2X2 and P2X4), and by little and no inactivation (P2X5 and P2X7, respectively).27 Therefore, our estimated inactivation constant in podocytes points to P2X2 and P2X4 channels.
Figure 3.
ATP application and mechanical membrane stretch cause similar P2X currents in podocytes. (A) Relative mRNA expression levels of selected GPCRs of primary podocytes determined by quantitative PCR analysis from three independent experiments. (B, C, F, G) Electrophysiologic whole-cell measurements of podocytes. (B and F) Exemplary CDV relationships before (gray) and during (green) application of 100 µM ATP and of hypoosmotic solution “Hypo” in the presence of 50 µM suramin (blue) are displayed. Insets show current density time course at ±100 mV. Stippled lines represent zero current. Application of ATP is indicated. Arrows represent the time points of depicted CDV traces. (C and G) Summary of current densities before (gray bars) and during (green bars) application of ATP and of hypoosmotic solution in the presence (blue hatched bars) and absence (blue bars) of suramin at ±100 mV. (D and E) Fluorimetric calcium measurements with fura-2–loaded podocytes. (D) Exemplary time course of intracellular calcium concentration [Ca2+]i with application of 100 µM ATP is displayed. (E) Summary of [Ca2+]i before (gray bar) and during (green bar) application of ATP. Numbers indicate the numbers of measured cells and of independent experiments. (F and G) Electrophysiologic whole-cell measurements of podocytes. (H and I) Fluorimetric calcium measurements with fura-2–loaded podocytes. (H) Exemplary time courses of [Ca2+]i in the presence (lower panel) and absence (upper panel) of 50 µM suramin during the second hypoosmotic stimulation. Applications of hypoosmotic solution and of suramin are indicated. (I) Summary of [Ca2+]i before (gray bars) and during application of hypoosmotic solution in the presence (blue hatched bar) and absence (blue bars) of suramin. Numbers indicate the numbers of measured cells and of independent experiments. Nonsignificant differences (ns) are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.
To test whether mechanical stimulation as well as ATP activates P2X channels, we first applied 50 µM suramin blocking P2X1, P2X2, P2X3 P2X5, and P2X6 channels.28 Suramin did not significantly reduce mechanically induced currents in podocytes (Figure 3, F and G). Furthermore, suramin did not suppress subsequent hypoosmotically induced calcium transients in fura-2–loaded podocytes (Figure 3, H and I) compared with untreated cells; this finding indicates that P2X4 or P2X7 channels might be involved. Of note, the first hypoosmotic stimulus served as a control, illustrating the comparability of the cells. In light of the slow inactivation kinetics characteristic of P2X2 and P2X4 channels and the lack of any suramin effect, these findings suggest that the currents evoked by mechanical stimulation are mainly mediated by P2X4 channels. Moreover, 50 µM suramin uncouples G proteins from receptors,29 additionally indicating that GPCRs including P2Y receptors are not involved in mechanical responses of podocytes.
P2X4 Channels in Podocytes Are Activated upon Mechanical Stimulation
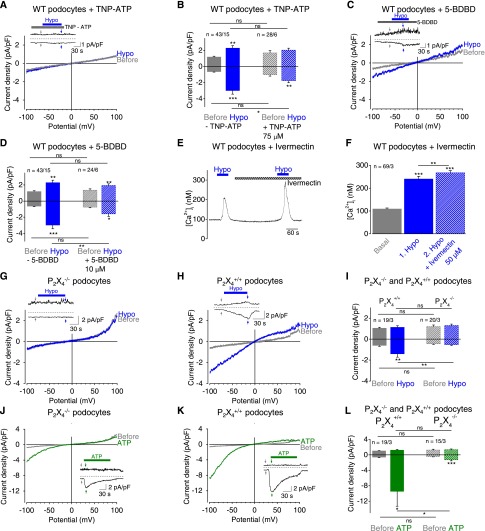
To ascertain mechanically induced P2X4 channel activation in podocytes, we applied the nonselective P2X channel blocker trinitrophenyl (TNP)-ATP (75 µM) affecting all homomeric P2X channels except P2X727 (Figure 4, A and B) and the selective P2X4 blocker 5-BDBD30 (10 µM) (Figure 4, C and D). Both blockers significantly reduced mechanically induced currents, indicating that P2X4 is crucially involved in the mechanical response of podocytes. This notion was further supported by analyzing conditionally immortalized podocytes, which likewise showed significantly suppressed currents in the presence of 10 µM 5-BDBD (Supplemental Figure 3, A and B). However, mechanically induced currents and intracellular calcium increases (Supplemental Figure 3, C and D) were not fully suppressed, which may be caused by the specific assembly of endogenous heteromeric P2X channels with distinct properties.
Figure 4.
P2X4 channels in podocytes are activated upon mechanical stimulation. (A–D) Electrophysiologic whole-cell measurements of podocytes. (A and C) Exemplary CDV relationships before (gray) and during (blue) application of hypoosmotic solution in the presence of 75 µM TNP-ATP (A) and of 10 µM 5-BDBD (C). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Applications of hypoosmotic solution and of TNP-ATP (A) or of 5-BDBD (C) are indicated. Arrows represent the time points of depicted CDV traces. (B and D) Summaries of current densities before (gray and gray hatched bars) and during application of hypoosmotic solution (blue and blue hatched bars) in the presence and absence of TNP-ATP (B) or of 5-BDBD (D) determined at holding potentials of ±100 mV. (E and F) Fluorimetric calcium measurements with fura-2–loaded podocytes. (E) Exemplary time courses of [Ca2+]i and applications of hypoosmotic solution and of 50 µM ivermectin are indicated. (F) Summary of [Ca2+]i before (gray bar) and during application of hypoosmotic solution in the presence (blue hatched bar) and absence (blue bar) of ivermectin. Numbers indicate the numbers of measured cells and of independent experiments. (G–L) Electrophysiologic whole-cell measurements of P2X4−/− and of P2X4+/+ control podocytes. (G, H, J, K) Exemplary CDV relationships of P2X4−/− (G and J) and of P2X4+/+ control podocytes (H and K) before (gray) and during application of hypoosmotic solution (blue) or of 100 µM ATP (green). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution and of ATP is indicated. Arrows represent the time points of depicted CDV traces. (I and L) Summaries of current densities before (gray and gray hatched bars) and during application of hypoosmotic solution (blue and blue hatched bars) (I) or of 100 µM ATP (green and green hatched bars) (L) of P2X4−/− and P2X4+/+ control podocytes determined at ±100 mV. Numbers indicate the numbers of measured cells and of independent experiments. Nonsignificant differences (ns) are indicated. *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.
To verify the prominent role of P2X4 channels, the selective P2X4 potentiator ivermectin was applied to fura-2–loaded podocytes. In the presence of 50 µM ivermectin mechanically induced calcium transients were significantly higher compared with the respective initial calcium response (Figure 4, E and F), whereas in the absence of ivermectin the second calcium response was always smaller (Figure 3, H [upper panel] and I). Altogether, these pharmacologic interventions identified P2X4 as a key component of the mechanotransduction process in podocytes. As a crucial proof-of-principle, isolated podocytes from P2X4 gene-deficient (P2X4−/−) mice31 were investigated. Indeed, P2X4−/− podocytes were devoid of mechanically induced current increases (Figure 4G) compared with their respective wild-type controls (Figure 4, H and I), confirming the cardinal role of P2X4−/−. Of note, ATP-induced currents were also significantly reduced in P2X4−/− compared with wild-type podocytes (Figure 4, J–L). The current inactivation constant was 40.4±5.0 seconds in P2X4−/− similar to that seen in P2X4+/+ control cells; this finding points to slow inactivating P2X2 currents activated by ATP in P2X4−/− podocytes. Altogether, these findings provide strong evidence for an essential involvement of P2X4 channels in the mechanical responsiveness of podocytes.
Mechanical Stimulation of Podocytes Causes ATP Release Leading to Subsequent P2X4 Channel Activation
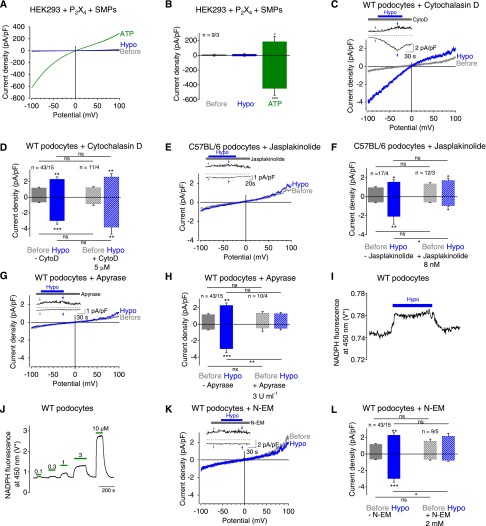
To analyze whether P2X4 channels in podocytes are mechanosensors or mechanotransducers, we performed whole-cell measurements with HEK293 cells overexpressing P2X2, P2X4, or both channel subunits together. Hypoosmotic cell swelling did not activate P2X channels (Supplemental Figure 4, A–C) in cells overexpressing P2X4, P2X2, or combinations thereof in the presence or absence of the SMPs podocin, CD2AP, and nephrin (Figure 5, A and B, and Supplemental Figure 4, D and E), suggesting that P2X channels are not inherently mechanosensitive. The involvement of the actin cytoskeleton in mechanosensation of podocytes was analyzed by incubation of podocytes with 5 µM cytochalasin D for 20 minutes at 37°C to disrupt actin fibers. This measure had no effect on hypoosmotically induced current responses in podocytes (Figure 5, C and D), indicating that the actin cytoskeleton per se is not crucial for mechanical activation in podocytes. Next, 8 nM jasplakinolide32 was administered for 20 minutes at 37°C to induce actin polymerization, resulting in significant reduction of mechanically induced currents (Figure 5, E and F). This finding suggests that stabilization of the actin cytoskeleton might prevent ATP release. This is in congruence with previous findings in PC12 cells showing impaired vesicle release by jasplakinolide.33,34 Of note, 200 nM jasplakinolide, a frequently used concentration in other cells, did not significantly suppress mechanically induced currents (Supplemental Figure 5, A and B), indicating that this concentration results in disruption rather than stabilization of the actin cytoskeleton, as seen in other cells.35,36 Moreover, applying 10 µM thiocolchicine for 2 hours at 37°C to prevent microtubule assembly did not affect mechanically induced current increases (Supplemental Figure 5, C and D). Altogether, these findings are compatible with the notion that mechanically induced release of prestored ATP-filled vesicles is impaired by actin cytoskeleton stabilization, but not by microtubule disassembly or actin cytoskeleton disruption.
Figure 5.
Mechanical stimulation of podocytes causes ATP release leading to subsequent P2X4 channel activation. (A–H, K, and L) Electrophysiologic whole-cell measurements of HEK293 cells (A and B) and podocytes (C–H, K, and L). (A) Exemplary CDV relationships of HEK293 cells overexpressing P2X4 and the SMPs podocin, nephrin, and CD2AP before (gray) and during (blue) application of hypoosmotic solution and of 100 µM ATP (green). (B) Summary of current densities before (gray bars) and during (blue bar) application of hypoosmotic bath solution and of ATP (green bar) at ±100 mV. Numbers display the number of measured cells and of independent experiments. (C, E, G, K) Exemplary CDV relationships of podocytes before (gray) and during (blue) application of hypoosmotic solution in the presence of 5 µM cytochalasin D (C), 8 nM jasplakinolide (E), 3 U/ml apyrase (G), and 2 mM N-ethylmaleimide (N-EM) (K). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution and of cytochalasin D (C), jasplakinolide (E), of apyrase (G) and of N-EM (K) is indicated. Arrows represent the time points of depicted CDV traces. (D, F, H, L) Summaries of current densities before (gray and gray hatched bars) and during (blue and blue hatched bars) application of hypoosmotic solution in the presence or absence of cytochalasin D, jasplakinolide, apyrase and N-EM determined at ±100 mV. Numbers indicate the numbers of measured cells and of independent experiments. (I and J) Photometric determination of ATP release from podocytes during hypoosmotic stimulation by measuring ATP-dependent conversion of NADP to NADPH. *Voltage of the transimpedance amplifier from the photodiode. (I) Exemplary trace of NADPH fluorescence at 450 nm with application of hypoosmotic solution (blue bar) is displayed. (J) Subsequent calibration curve with application of increasing ATP concentrations (green bars). *P<0.05; **P<0.01; ***P<0.001; ns P>0.05.
To analyze whether P2X channels are indeed activated by ATP released from podocytes upon mechanical stimulation, podocytes were treated with the ATP-converting enzyme apyrase, which rapidly converts ATP to AMP. Preincubation for 30 minutes at 37°C and addition of apyrase to all bath solutions completely abolished mechanically induced current increases (Figure 5, G and H). To measure ATP release from podocytes, we performed fluorescence photometry. Perfusion of cells with hypoosmotic bath solution (Figure 5I) and subsequent application of increasing ATP concentrations (Figure 5J) used for calibration purposes resulted in fluorescence increases at 450 nm, corresponding to 419±133 nM ATP (n=9). To further analyze the mechanism of ATP release in podocytes, we applied N-ethylmaleimide, an unspecific SNARE protein blocker that disables vesicular fusion, because podocytes express SNARE proteins.37 Incubation of podocytes with 2 mM N-ethylmaleimide for 2 minutes was sufficient to completely prevent hypoosmotically induced current increases (Figure 5, K and L), compatible with the notion that podocytes release ATP upon mechanical stimulation via fusion of ATP-loaded vesicles to the plasma membrane. Altogether, these findings strongly point to mechanically induced ATP release in podocytes.
Mechanically Induced P2X4 Channel Activation Is Podocin and Cholesterol Dependent and Is Crucial for Reorganization of the Actin Cytoskeleton
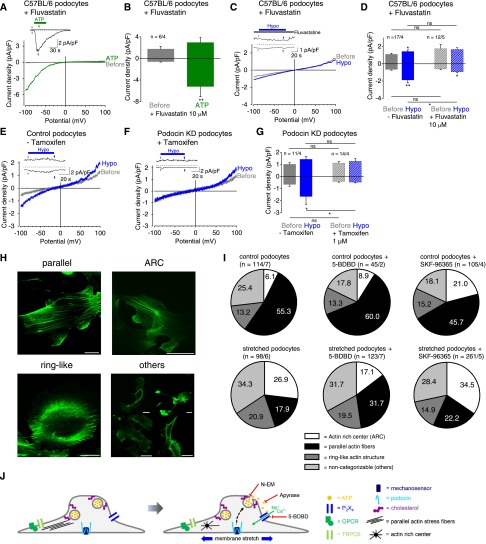
Exocytosis is inhibited by depletion of cholesterol38 distributed in lipid rafts in the podocyte cell membrane39 and is thought to play a role in the pathomechanism of podocyte injuries.40 To analyze whether cholesterol is involved in mechanically induced P2X4 activation, cells were preincubated with fluvastatin (10 µM)41 for 24 hours at 37°C to prevent cholesterol production and with the cholesterol-binding agent methyl-β-cyclodextrin (10 mM) for 10 minutes at 37°C. Interestingly, ATP-induced currents in cholesterol-depleted podocytes were still observed in fluvastatin and methyl-β-cyclodextrin–treated podocytes, although they were suppressed by a factor of 2.9 and 3.4, respectively (Figure 6, A and B, and Supplemental Figure 6, A and B). However, mechanically induced inward currents were nearly completely suppressed and typical inwardly rectifying P2X currents were abolished (Figure 6, C and D, and Supplemental Figure 6, A and B), suggesting that cholesterol is essential for mechanical P2X4 activation. Of note, slight current increases were still seen in methyl-β-cyclodextrin–treated podocytes evoked by membrane stretch, probably reflecting the presence of other cation channels (Supplemental Figure 6, C and D).
Figure 6.
Mechanically induced P2X4 channel activation is podocin and cholesterol dependent and is crucial for reorganization of the actin cytoskeleton. (A– G) Electrophysiologic whole-cell measurements of podocytes treated with fluvastatin (A–D) and of podocin knockdown (KD) and respective control podocytes (E–G). (A and C) Exemplary CDV relationships before (gray) and during (green) application of 100 µM ATP and of hypoosmotic solution (blue) in the presence of 10 µM fluvastatin. Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution, ATP, and fluvastatin is indicated. Arrows represent the time points of depicted CDV traces. (B and D) Summaries of current densities before (gray bar) and during (green bar) application of ATP (B) or of hypoosmotic solution (blue bar) (D) in the presence of fluvastatin at ±100 mV. Numbers indicate the numbers of measured cells and of independent experiments. (E and F) Exemplary CDV relationships before (gray) and during (blue) application of hypoosmotic solution of Nphs2flox/flox,Cre+/+ podocytes not treated with tamoxifen (control podocytes, E) and treated with 1 µM tamoxifen to induce podocin knockdown (podocin KD podocytes, F). Insets show current density time courses at ±100 mV. Stippled lines represent zero current. Application of hypoosmotic solution is indicated. Arrows represent the time points of depicted CDV traces. (G) Summary of current densities before (gray and gray hatched bars) and during (blue and blue hatched bars) application of hypoosmotic solution at ±100 mV. Numbers indicate the numbers of measured cells and of independent experiments. (H) Exemplary fluorescence pictures of actin fiber distribution of podocytes after subjection to radial tensile strain for 2 hours representing four categories: parallel stress fibers “parallel,” presence of ARCs, ring-like actin structure, and nondefinable actin structure (“others”). Actin fibers were stained with Alexa Fluor-488 phalloidin. Scale bar is 20 µm. (I) Frequency distribution of the four categories of stretched and nonstretched podocytes in the presence and absence of 10 µM 5-BDBD and of 10 µM SKF-96365. Numbers indicate percentage. Numbers in parentheses show numbers of podocytes and numbers of independent experiments. (J) Schematic model of mechanically induced P2X4 activation in podocytes. TRPC6 and GPCRs do not appear to be involved in the induction of ionic currents by mechanical membrane stretch. Podocin may participate in the function of a hitherto unidentified mechanosensor. Mechanical membrane stretch causes cholesterol-dependent ATP release by vesicle fusion via the SNARE complex. ATP subsequently activates P2X4 channels, resulting in disorganization of the actin cytoskeleton, which might contribute to the pathomechanism leading to hypertension-induced podocyte injury. Pharmacologic interventions with N-EM, which prevents vesicle fusion, with apyrase causing ATP degradation and of the selective P2X4 channel blocker 5-BDBD, are displayed. *P<0.05; **P<0.01; ns P>0.05.
Furthermore, podocin is known to bind to cholesterol and to potentiate diacylglycerol induced TRPC6 currents in an ion channel complex.42 Therefore, we next analyzed the involvement of podocin in mechanical P2X4 activation. To this end, podocin knockdown podocytes isolated from Nphs2flox/flox, Cre+/+ mice31,43 were used and tested for successful in vitro podocin downregulation according to Western blot analysis (Supplemental Figure 7). Interestingly, mechanically induced currents were completely abolished in podocin knockdown podocytes compared with respective control podocytes (Figure 6, E–G), whereas ATP-induced currents were unchanged (Supplemental Figure 8). Altogether, these findings indicate that podocin and cholesterol together determine mechanically induced P2X4 channel activation in podocytes.
Disorganization of the actin cytoskeleton, which is important for podocyte structure and function, damages podocytes.44–46 To investigate whether mechanically induced P2X activation affects the actin cytoskeleton, podocytes were seeded onto silicone membranes and subjected to cyclic tensile strain for 2 hours. After F-actin staining, the actin fiber organization in stretched and nonstretched podocytes was analyzed by categorizing the observed actin structures into four groups (Figure 6H): presence of well defined parallel actin stress fibers representing the physiologic state, actin structure formations displaying the disorganized state (characterized by the presence of actin-rich centers [ARCs] known to be evoked by membrane stretch47), ring-like actin fiber structures, and nondefinable actin structures (termed “others”, which do not fit in one of the categories but also reflects actin dysregulation). Nonstretched podocytes showed 55.3% parallel stress fibers; in stretched podocytes the percentage of podocytes with ARCs was increased 4-fold, ring-like structures 1.6-fold, and noncategorizable actin fiber formation 1.4-fold, whereas the percentage of podocytes with parallel stress fibers was decreased by a factor of 3 (Figure 6I). These findings illustrate a highly significant (P<0.001) reorganization of the actin cytoskeleton. Incubation of nonstretched podocytes with the P2X4 blocker 5-BDBD had no significant effect on actin fiber organization (P>0.6). Interestingly, actin disorganization by membrane stretch could be partially rescued by 5-BDBD, which significantly reduced the percentage of cells showing ARCs to 64% and increased the number of cells with parallel actin stress fibers to 177% compared with untreated stretched podocytes (P<0.01). This result points to a protective effect of 5-BDBD in mechanically stressed podocytes. Furthermore, in the presence of 5-BDBD, nonstretched and stretched podocytes showed significant differences (P<0.01) with regard to increased actin disorganization and loss of parallel actin stress fibers. Interestingly, the nonselective TRPC channel blocker SKF-96365 had no protective effect on actin cytoskeleton reorganization in mechanically stressed podocytes, suggesting that TRPC channels are not involved. Altogether, these findings strongly indicate that P2X4 channels play a crucial role for mechanically induced actin skeleton reorganization contributing to podocyte damage.
DISCUSSION
Proper podocyte function is of utmost importance for the maintenance of the filtration barrier. Several mutations (e.g., in podocin, CD2AP, nephrin, PLCε, and TRPC6, summarized by Machuca et al.48) are known causes for podocyte damage. However, very little is known about mechanically induced podocyte injury. A mechanosensitive TRPC6/podocin channel complex was assumed in podocytes,10 similar to the mechanosensitive degenerin and epithelial Na+ channel complex in Caenorhabditis elegans.
However, as shown here, reconstitution of the postulated channel complex by coexpressing TRPC6 and the SMPs podocin, CD2AP, and nephrin failed to give rise to mechanically induced TRPC6 currents. Moreover, Trpc6−/− podocytes were still mechanosensitive, demonstrating that TRPC6 is neither a mechanosensor nor mechanotransducer in podocytes, contrasting with observations by Wilson et al.49 One reason for the discrepancy observed might be the use of Trpc6−/− podocytes in our study compared with the use of small interfering RNA against TRPC6. Moreover, different bath solutions were used in the latter study; these solutions were devoid of the chloride channel blockers 5-nitro-2-(3-phenylpropylamino)benzoic acid (NPPB) and 4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (DIDS). Thus, volume-regulated chloride channels may have contributed to the currents measured by Wilson et al.49 However, in our study we did not obtain experimental evidence supporting a role of other TRPC channels for mechanosensation or mechanotransduction after analyzing Trpc1/3/6−/− podocytes and by applying the nonselective TRPC channel blocker SKF-96365 on wild-type and Trpc1/3/6−/− podocytes. Genetic inactivation of TRPC channels and pharmacologic blockade had no effect on mechanically induced inwardly rectifying currents. Furthermore, we could exclude Gq/11PCRs as mechanosensors in podocytes, although several GPCRs were expressed in these cells. Instead, our findings point to a key role of P2X4 channels for the mechanical responsiveness of podocytes.
ATP evoked similar inwardly rectifying currents in podocytes, as induced by membrane stretch with slow inactivation kinetics pointing to P2X2 and P2X4 channels, which were found to be highly expressed on the mRNA level. These findings are at odds with observations by Roshanravan and Dryer,50 who described ATP-induced TRPC6 channel activations. This discrepancy might also be explained by the use of different bath solutions without addition of chloride channel blockers, which were always present throughout our experiments in order to isolate cation currents. However, the inactivation constant of about 41 seconds we determined in podocytes was markedly higher than that observed in the analysis of homomeric P2X channels.27 This may be explained by the formation of endogenous heteromeric channel complexes with adapter proteins. This could also explain the incomplete suppression of mechanically induced currents of about 65%–75% by 5-BDBD and TNP-ATP. However, using a pharmacologic approach, we could verify a key role of P2X4 channels for the mechanical response of podocytes. As a crucial test, P2X4−/− podocytes were analyzed; this showed a complete suppression of mechanically induced currents, clearly demonstrating the essential role of P2X4 channels as mechanotransducers mediating mechanically induced cation influx in podocytes.
Interestingly, two studies show that P2X4 channels are sensitive to shear stress in endothelial cells and in oocytes without mechanistic explanation.51,52 However, we demonstrate for the first time that P2X4 channels are mechanotransducers in podocytes activated by ATP released upon mechanical stimulation. Using a fluorescence approach to monitor cell swelling induced ATP accumulation in the bath solution, we determined a concentration of about 400 nM, which should be sufficient for P2X activation considering dilution in the bath solution and the occurrence of high local ATP concentrations near the cell membrane. Moreover, apyrase abolished stretch-induced currents and using N-ethylmaleimide (N-EM) we obtained initial evidence that ATP might be released by vesicle fusion via the SNARE complex. Furthermore, heterologously expressed P2X channels were not directly mechanosensitive even in the presence of SMPs. Altogether, our findings strongly argue for an indirect mechanosensitivity of P2X channels.
Interestingly, in our study disruption of the actin cytoskeleton did not affect mechanosensation of podocytes, in contrast to the mechanosensitive degenerin and epithelial Na+ channel protein complex in C. elegans, because treatment with cytochalasin D was without effect. Instead, mechanosensation of podocytes was inhibited by stabilization of the actin cytoskeleton, possibly caused by impairment of mechanically induced ATP release. Disassembly of microtubules had no effect on ATP release. Moreover, we found cholesterol to be critically involved in mechanical responsiveness of podocytes because cholesterol depletion by fluvastatin and methyl-β-cyclodextrins completely suppressed mechanically induced P2X4 currents. Interestingly, cholesterol depletion only abolished mechanically but not ATP-induced P2X4 currents. Thus, cholesterol, which is important for exocytosis and for formation of lipid rafts in the slit diaphragm, is essential for mechanical responsiveness of podocytes probably as a regulator of ATP release. Moreover, our findings with podocin knockdown podocytes show that podocin also regulates mechanical P2X4 channel activation. In podocin knockdown podocytes, mechanically induced P2X4 currents were completely suppressed, indicating that podocin is primarily involved in an upstream mechanosensing process independent of the formation and function of TRPC6/podocin complexes in the plasma membrane.10 Altogether, we can demonstrate that cholesterol and podocin play dominant roles for mechanical responsiveness of podocytes.
There is evidence for an ATP-induced reorganization of the cytoskeleton in macrophages and mammary tumor cells.53,54 Beyond that, our findings demonstrate that mechanically induced P2X4 activation via ATP release plays a key role for the reorganization of the actin cytoskeleton in podocytes, indicating that mechanical P2X4 activation might be crucial for the pathomechanism leading to hypertension-induced podocyte damage. We found that nonstretched podocytes exhibited a 6% frequency of podocytes with ARCs; this is similar to Endlich and colleagues' study,47 which reported a frequency of about 15%. Interestingly, we observed a significant increase in podocytes with ARCs of about 27% after 2 hours compared with an increase of about 50% monitored after 24 hours, as shown by others.47 This finding indicates that reorganization of the actin cytoskeleton is a fast process. Remarkably, cyclic membrane stretch caused significant reorganization of the actin cytoskeleton, which could be rescued by the selective P2X4 blocker 5-BDBD while the nonselective TRPC channel blocker SKF-96365 had no effect. Altogether, we postulate a novel role of P2X4 channels as mechanotransducers in podocytes regulated by podocin and cholesterol and activated by membrane stretch-induced ATP release via the SNARE complex, finally resulting in reorganization of the actin cytoskeleton (summarized in Figure 6H). This signaling pathway may contribute to mechanically induced podocyte injury and open up novel strategies for nephroprotection in conditions such as hypertension.
CONCISE METHODS
Mice Used in the Study
If not stated otherwise, we used wild-type mice with the BKS.Cg-Dock7m+/+ background and with the C57BL/6 background obtained from Jackson Laboratory. P2X4−/− mice in C57BL/6 background were provided from GlaxoSmithKline and from Manfred Frick (Ulm, Germany). To induce podocin knockdown in cultured podocytes, inducible podocin knockdown (Nphs2flox/flox,Cre+/+) mice were used in C57BL/6 background developed in the laboratory of Corinne Antignac (Paris, France).31,43 Trpc6−/− and Trpc1/3/6−/− mice were in C57BL/6 background. All experiments and procedures were approved by the governmental oversight authority, district government of Upper Bavaria (Germany).
Materials
We used 1-Oleoyl-2-acetyl-sn-glycerol (Calbiochem), DIDS (Invitrogen), 5-BDBD (Tocris), Suramin (Tocris), TNP-ATP (Tocris), Normacin (Invivogen), and YM-254890 (Taiho Pharmaceutical). Unless otherwise stated, all other materials were obtained from Sigma-Aldrich.
Electrophysiologic Whole-Cell Recordings
Conventional whole-cell patch-clamp recordings were performed to analyze isolated podocytes and HEK293 cells transfected with cDNA coding for TRPC6 (NM_004621), nephrin (AAF91087.1), podocin (NM_130456.3) and CD2-associated protein (CD2AP, NM_009847.3) and P2X4 (AF089751) using Genejuice (EMD Millipore) reagent. All measurements were carried out at room temperature. Bath solutions contained (in mM): 110 NaCl, 0.1 CaCl2, 1 MgCl2, 10 HEPES (adjusted to pH 7.40 with NaOH), and 10 glucose supplemented with mannitol to 300 mOsm/kg. Hypoosmotic solution contained no mannitol, which resulted in an osmolality of 240 mOsm/kg. For podocyte measurements, all bath solutions additionally contained 100 µM NPPB and 300 µM DIDS to block chloride channels. Patch pipettes were made of borosilicate glass (Science Products, Hofheim, Germany) and had resistances of 1.9–3.6 MΩ when filled with the standard intracellular solution containing (in mM): 9.4 NaCl, 120 CsCl, 3.949 CaCl2, 0.2 Na3-GTP, 10 HEPES (pH 7.2 with CsOH), and 10 BAPTA, resulting in 100 nM free Ca2+. The liquid junction potential was 4.0 mV and corrected by the PatchMaster software. Data were collected with an EPC10 patch-clamp amplifier (HEKA, Lambrecht, Germany) using PatchMaster v2×52 software. Current voltage relationships were obtained from voltage ramps from −100 to 100 mV with a slope of 0.5 V/s at a frequency of 2 Hz. Data were acquired at a frequency of 5 kHz after filtering at 1.67 kHz. Measurements with basal inward current densities at −100 mV higher than −2.00 pA/pF for wild-type podocytes and −1.55 pA/pF for C57BL/6 podocytes were regarded as leak currents and were not further analyzed.
Podocytes Used in the Study
Primary podocytes were isolated from the kidneys of 3- to 4-week-old mice using a conventional sieving approach55,56 with some modifications. Kidneys were mechanically disrupted in 200 ml Ham's F12 medium containing 10% fetal calf serum (FCS, Invitrogen), 100 U/ml penicillin, 100 mg/ml streptomycin, and 2 mM glutamine using a set of steel sieves with a pore size of 100, 75, 50, and 36 µm (Retsch, Haan, Germany) coated by Ham's F12 medium containing 50% FCS (Invitrogen) at room temperature. Freshly isolated cells from the final fraction were centrifuged for 5 minutes at 200 g and cultured in Ham's F12 medium supplemented with 10% FCS, 2 mM glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 100 µg/ml normocin, 5 µg/ml transferrin, 5 ng/ml natriumselenite, 100 nM hydrocortisone, and 5 µg/ml recombinant human insulin (Sigma-Aldrich, Taufkirchen, Germany) in a humidified atmosphere with 5% CO2. Enrichment of podocytes was >95% as assessed by cell morphology, staining with an antipodocin antibody (Sigma-Aldrich) and by expression of nephrin, synapotopodin, and Wilms tumor protein (WT1), which is described in detail elsewhere.57 Cell membrane capacity was used as another selection criterion for podocytes. Cell membrane capacity was estimated to be 71.2±2.3 pF (n=362) for primary podocytes; thus, there was no significant difference to conditionally immortalized murine podocytes, which had cell membrane capacities of 63.4±3.3 pF (n=52). Primary podocytes were analyzed after the first cell passage approximately 10 days after isolation. Conditionally immortalized murine podocytes were cultured as described in detail elsewhere.19
Determination of Intracellular Calcium Concentrations
Intracellular free calcium concentration was determined in isolated podocytes using 5 µM fura-2 acetoxymethyl ester (Sigma-Aldrich) as described in detail elsewhere.58 In brief, cells were mounted on the stage of a monochromator-equipped (Polychrome V; TILL-Photonics, Martinsried, Germany) inverted microscope (Olympus IX 71 with an UPlanSApo 20×/0.85 oil immersion objective). Fluorescence was recorded with a 14-bit EMCCD camera (iXON 885; Andor, Belfast, UK). Fura-2 fluorescence was excited at 340 and 380 nm. Intracellular free calcium concentrations were calculated as described previously.59 During imaging, cells were continuously superfused at room temperature with an isotonic bath solution containing (in mM) the following: 55 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES (pH 7.40), and 10 glucose, supplemented with mannitol to 300 mOsm/kg. The hypoosmotic bath solution had the same salt concentration without added mannitol, resulting in an osmolality of 149–152 mOsm/kg.
Quantitative RT-PCR Analysis
Total RNA was isolated using the Tri Reagent (Sigma-Aldrich, Munich, Germany). First-strand synthesis was carried out with random hexamer primers, using REVERTAID reverse transcription (Fermentas, Sankt Leon-Roth, Germany). For detailed information and primers, see the Supplemental Material. The Wilcoxon rank-sum test was used for analysis of mRNA expression levels. All experiments were performed in quadruplets, and experiments were repeated at least three times.
Determination of Extracellular ATP Concentrations Using NADP Conversion
To measure ATP release from podocytes, photometry was performed by turning to account ATP-dependent conversion of NADP to NADPH in the presence of the enzymes hexokinase and glucose-6-phosphate dehydrogenase and of the substrates glucose and NADP, which resulted in an increased fluorescence emission at 450 nm. Quantification of ATP was essentially done as previously described.60 Podocytes were seeded onto a glass coverslip 24 hours before analysis. For analysis of ATP, cells were superfused with (1) isotonic bath solution (in mM: 110 NaCl, 0.1 CaCl2, 1 MgCl2, 10 HEPES [pH 7.40], and 10 glucose supplemented with mannitol to 300 mOsm/kg) additionally containing 2 mM NADP, 2 U/ml hexokinase, and 2 U/ml glucose-6-phosphate-dehydrogenase or (2) hypoosmotic solution without the supplementation of mannitol, resulting in about 240 mOsm/kg. NAPDH was excited with Polychrome V (TillPhotonics) at 340 nm, and emission was measured as voltage of the transimpedance amplifier from the photodiode at 450 nm. Data were collected by EPC10 amplifier (HEKA Lambrecht, Germany) with the Patchmaster software (HEKA). Hypoosmotic solution was applied, resulting in fluorescence increases, which were quantified by subsequently measuring different increasing ATP concentrations in isotonic bath solution for calibration.
Mechanical Membrane Stretch Using FlexCell Tension System
Cyclic radial tensile strains were applied to podocytes using the Flexcell Tension System (FX-5000T; Flexcell International Corp.). A total of 200,000 podocytes were seeded onto collagene-coated silicon membranes (BioFlex Culture Plate; Flexcell International Corp.) 24 hours before cells were subjected to cyclic 10% radial tensile strain with a frequency of 0.5 Hz for 2 hours. Thereafter, cells were fixated with 4% paraformaldehyde for 30 minutes at 4°C. Actin was labeled by 20-minute incubation of cells with Alexa Fluor 488–tagged phalloidin (Invitrogen). Subsequently, fluorescence pictures were taken using confocal microscopy with a Leica TCS SP5 using an HCX PL APO CS 63.0×/1.40 ultraviolet oil immersion objective. Probes were excited with 488 nm while emission was measured at 515±10 nm. Reorganization of the cytoskeleton was quantified using the chi-squared test for analysis of sample distribution.
Statistical Analyses
Data are presented as mean±SEM. Unless stated otherwise, data were compared by a paired or unpaired t test if a Gaussian distribution was confirmed by applying a Shapiro-Wilk (normality) test. Calcium measurements were analyzed using the one‐way ANOVA with Bonferroni post hoc means comparison. Significance was accepted at P<0.05. For statistical analysis the software Origin 7.5 and 8.0 (OriginLab Corporation, Northampton, MA) was used. Chi-squared analysis was done with Excel 2013 (Microsoft Corporation, Redmond, WA).
Disclosures
J.R. is cofounder and president of TRISAQ, a biopharmaceutical company aimed at developing kidney protective therapeutics. He stands to gain royalties from issued and pending patents relevant to treating kidney disease. None of the other authors report any conflict of interests.
Supplementary Material
Acknowledgments
We thank Joanna Zaißerer and Laura Danner for excellent technical assistance. We are grateful to GlaxoSmithKline and Manfred Frick (Ulm, Germany) for providing P2X4−/− mice, Lutz Birnbaumer for providing Trpc3−/− mice, and for critically reading the manuscript, Maike Fahlbusch and Jana Demleitner for providing Trpc6−/− podocytes, and Natsuko Kayakiri (Taiho Pharmaceutical) for providing YM-254890. This study was supported by Deutsche Forschungsgemeinschaft SFB/TRR152.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014111144/-/DCSupplemental.
References
- 1.Huber TB, Benzing T: The slit diaphragm: A signaling platform to regulate podocyte function. Curr Opin Nephrol Hypertens 14: 211–216, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Huber TB, Schermer B, Benzing T: Podocin organizes ion channel-lipid supercomplexes: Implications for mechanosensation at the slit diaphragm. Nephron, Exp Nephrol 106: e27–e31, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Endlich N, Endlich K: The challenge and response of podocytes to glomerular hypertension. Semin Nephrol 32: 327–341, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Kreisberg JI, Karnovsky MJ: Focal glomerular sclerosis in the fawn-hooded rat. Am J Pathol 92: 637–652, 1978 [PMC free article] [PubMed] [Google Scholar]
- 5.Kretzler M, Koeppen-Hagemann I, Kriz W: Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Archiv 425: 181–193, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Kriz W, Hosser H, Hähnel B, Simons JL, Provoost AP: Development of vascular pole-associated glomerulosclerosis in the Fawn-hooded rat. J Am Soc Nephrol 9: 381–396, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Bidani AK, Griffin KA: Long-term renal consequences of hypertension for normal and diseased kidneys. Curr Opin Nephrol Hypertens 11: 73–80, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Reiser J, Polu KR, Möller CC, Kenlan P, Altintas MM, Wei C, Faul C, Herbert S, Villegas I, Avila-Casado C, McGee M, Sugimoto H, Brown D, Kalluri R, Mundel P, Smith PL, Clapham DE, Pollak MR: TRPC6 is a glomerular slit diaphragm-associated channel required for normal renal function. Nat Genet 37: 739–744, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winn MP, Conlon PJ, Lynn KL, Farrington MK, Creazzo T, Hawkins AF, Daskalakis N, Kwan SY, Ebersviller S, Burchette JL, Pericak-Vance MA, Howell DN, Vance JM, Rosenberg PB: A mutation in the TRPC6 cation channel causes familial focal segmental glomerulosclerosis. Science 308: 1801–1804, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Huber TB, Schermer B, Müller RU, Höhne M, Bartram M, Calixto A, Hagmann H, Reinhardt C, Koos F, Kunzelmann K, Shirokova E, Krautwurst D, Harteneck C, Simons M, Pavenstädt H, Kerjaschki D, Thiele C, Walz G, Chalfie M, Benzing T: Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci U S A 103: 17079–17086, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Hindi S, Reiser J: TRPC channel modulation in podocytes-inching toward novel treatments for glomerular disease. Pediatr Nephrol 26: 1057–1064, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gottlieb P, Folgering J, Maroto R, Raso A, Wood TG, Kurosky A, Bowman C, Bichet D, Patel A, Sachs F, Martinac B, Hamill OP, Honore E: Revisiting TRPC1 and TRPC6 mechanosensitivity. Pflugers Archiv 455: 1097–1103, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T: Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27: 3092–3103, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharif-Naeini R, Folgering JH, Bichet D, Duprat F, Delmas P, Patel A, Honoré E: Sensing pressure in the cardiovascular system: Gq-coupled mechanoreceptors and TRP channels. J Mol Cell Cardiol 48: 83–89, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Blodow S, Schneider H, Storch U, Wizemann R, Forst AL, Gudermann T, Mederos y Schnitzler M: Novel role of mechanosensitive AT1B receptors in myogenic vasoconstriction. Pflugers Archiv 466: 1343–1353, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Storch U, Mederos y Schnitzler M, Gudermann T: G protein-mediated stretch reception. Am J Physiol Heart Circ Physiol 302: H1241–H1249, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Mederos y Schnitzler M, Storch U, Gudermann T: AT1 receptors as mechanosensors. Curr Opin Pharmacol 11: 112–116, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Mennuni S, Rubattu S, Pierelli G, Tocci G, Fofi C, Volpe M: Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J Hum Hypertens 28: 74–79, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R: Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Dietrich A, Mederos Y Schnitzler M, Gollasch M, Gross V, Storch U, Dubrovska G, Obst M, Yildirim E, Salanova B, Kalwa H, Essin K, Pinkenburg O, Luft FC, Gudermann T, Birnbaumer L: Increased vascular smooth muscle contractility in TRPC6-/- mice. Mol Cell Biol 25: 6980–6989, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I: Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol 6: 499–506, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann S, Podlich D, Hähnel B, Kriz W, Gretz N: Angiotensin II type 1 receptor overexpression in podocytes induces glomerulosclerosis in transgenic rats. J Am Soc Nephrol 15: 1475–1487, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Delles C, Jacobi J, John S, Fleischmann I, Schmieder RE: Effects of enalapril and eprosartan on the renal vascular nitric oxide system in human essential hypertension. Kidney Int 61: 1462–1468, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Ziai F, Ots M, Provoost AP, Troy JL, Rennke HG, Brenner BM, Mackenzie HS: The angiotensin receptor antagonist, irbesartan, reduces renal injury in experimental chronic renal failure. Kidney Int Suppl 57: S132–S136, 1996 [PubMed] [Google Scholar]
- 25.Takasaki J, Saito T, Taniguchi M, Kawasaki T, Moritani Y, Hayashi K, Kobori M: A novel Galphaq/11-selective inhibitor. J Biol Chem 279: 47438–47445, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS: Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev 63: 641–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North RA: Molecular physiology of P2X receptors. Physiol Rev 82: 1013–1067, 2002 [DOI] [PubMed] [Google Scholar]
- 28.North RA, Surprenant A: Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563–580, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Chung WC, Kermode JC: Suramin disrupts receptor-G protein coupling by blocking association of G protein alpha and betagamma subunits. J Pharmacol Exp Ther 313: 191–198, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Balazs B, Danko T, Kovacs G, Koles L, Hediger MA, Zsembery A: Investigation of the inhibitory effects of the benzodiazepine derivative, 5-BDBD on P2X4 purinergic receptors by two complementary methods. Cell Physiol Biochem 32: 11–24, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Mollet G, Ratelade J, Boyer O, Muda AO, Morisset L, Lavin TA, Kitzis D, Dallman MJ, Bugeon L, Hubner N, Gubler MC, Antignac C, Esquivel EL: Podocin inactivation in mature kidneys causes focal segmental glomerulosclerosis and nephrotic syndrome. J Am Soc Nephrol 20: 2181–2189, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernstein BW, Bamburg JR: Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci 23: 1–6, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ng YK, Lu X, Levitan ES: Physical mobilization of secretory vesicles facilitates neuropeptide release by nerve growth factor-differentiated PC12 cells. J Physiol 542: 395–402, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Richards DA: Spatial regulation of exocytic site and vesicle mobilization by the actin cytoskeleton. PLoS ONE 6: e29162, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bubb MR, Spector I, Beyer BB, Fosen KM: Effects of jasplakinolide on the kinetics of actin polymerization. An explanation for certain in vivo observations. J Biol Chem 275: 5163–5170, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Ou GS, Chen ZL, Yuan M: Jasplakinolide reversibly disrupts actin filaments in suspension-cultured tobacco BY-2 cells. Protoplasma 219: 168–175, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavaré JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Lang T: SNARE proteins and ‘membrane rafts’. J Physiol 585: 693–698, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orci L, Singh A, Amherdt M, Brown D, Perrelet A: Microheterogeneity of protein and sterol content in kidney podocyte membrane. Nature 293: 646–647, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Fornoni A, Merscher S, Kopp JB: Lipid biology of the podocyte—new perspectives offer new opportunities. Nat Rev Nephrol 10: 379–388, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Fountain SJ: Fluvastatin suppresses native and recombinant human P2X4 receptor function. Purinergic Signal 8: 311–316, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schermer B, Benzing T: Lipid-protein interactions along the slit diaphragm of podocytes. J Am Soc Nephrol 20: 473–478, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Bugeon L, Danou A, Carpentier D, Langridge P, Syed N, Dallman MJ: Inducible gene silencing in podocytes: A new tool for studying glomerular function. J Am Soc Nephrol 14: 786–791, 2003 [DOI] [PubMed] [Google Scholar]
- 44.Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P: Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Mathieson PW: Podocyte actin in health, disease and treatment. Nephrol Dial Transplant 25: 1772–1773, 2010 [DOI] [PubMed] [Google Scholar]
- 46.Endlich N, Endlich K: Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol 85: 229–234, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Endlich N, Kress KR, Reiser J, Uttenweiler D, Kriz W, Mundel P, Endlich K: Podocytes respond to mechanical stress in vitro. J Am Soc Nephrol 12: 413–422, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Machuca E, Benoit G, Antignac C: Genetics of nephrotic syndrome: Connecting molecular genetics to podocyte physiology. Hum Mol Genet 18[R2]: R185–R194, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Wilson C, Dryer SE: A mutation in TRPC6 channels abolishes their activation by hypoosmotic stretch but does not affect activation by diacylglycerol or G protein signaling cascades. Am J Physiol Renal Physiol 306: F1018–F1025, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Roshanravan H, Dryer SE: ATP acting through P2Y receptors causes activation of podocyte TRPC6 channels: Role of podocin and reactive oxygen species. Am J Physiol Renal Physiol 306: F1088–F1097, 2014 [DOI] [PubMed] [Google Scholar]
- 51.Kessler S, Clauss WG, Fronius M: Laminar shear stress modulates the activity of heterologously expressed P2X(4) receptors. Biochim Biophys Acta 1808: 2488–2495, 2011 [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto K, Korenaga R, Kamiya A, Ando J: Fluid shear stress activates Ca(2+) influx into human endothelial cells via P2X4 purinoceptors. Circ Res 87: 385–391, 2000 [DOI] [PubMed] [Google Scholar]
- 53.Pfeiffer ZA, Aga M, Prabhu U, Watters JJ, Hall DJ, Bertics PJ: The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J Leukoc Biol 75: 1173–1182, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Pubill D, Dayanithi G, Siatka C, Andrés M, Dufour MN, Guillon G, Mendre C: ATP induces intracellular calcium increases and actin cytoskeleton disaggregation via P2x receptors. Cell Calcium 29: 299–309, 2001 [DOI] [PubMed] [Google Scholar]
- 55.Mundel P, Reiser J, Kriz W: Induction of differentiation in cultured rat and human podocytes. J Am Soc Nephrol 8: 697–705, 1997 [DOI] [PubMed] [Google Scholar]
- 56.Schlondorff D: Preparation and study of isolated glomeruli. Methods Enzymol 191: 130–140, 1990 [DOI] [PubMed] [Google Scholar]
- 57.Kalwa H, Storch U, Demleitner J, Fiedler S, Mayer T, Kannler M, Fahlbusch M, Barth H, Smrcka A, Hildebrandt F, Gudermann T, Dietrich A: Phospholipase C epsilon (PLCε) induced TRPC6 activation: a common but redundant mechanism in primary podocytes. J Cell Physiol 230: 1389–1399, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Storch U, Forst AL, Philipp M, Gudermann T, Mederos y Schnitzler M: Transient receptor potential channel 1 (TRPC1) reduces calcium permeability in heteromeric channel complexes. J Biol Chem 287: 3530–3540, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grynkiewicz G, Poenie M, Tsien RY: A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985 [PubMed] [Google Scholar]
- 60.Corriden R, Insel PA, Junger WG: A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol 293: C1420–C1425, 2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.