Abstract
Hyponatremia is the most common clinical electrolyte disorder. Once thought to be asymptomatic in response to adaptation by the brain, recent evidence suggests that chronic hyponatremia may be linked to attention deficits, gait disturbances, risk of falls, and cognitive impairments. Such neurologic defects are associated with a reduction in quality of life and may be a significant cause of mortality. However, because underlying diseases such as adrenal insufficiency, heart failure, liver cirrhosis, and cancer may also affect brain function, the contribution of hyponatremia alone to neurologic manifestations and the underlying mechanisms remain unclear. Using a syndrome of inappropriate secretion of antidiuretic hormone rat model, we show here that sustained reduction of serum sodium ion concentration induced gait disturbances; facilitated the extinction of a contextual fear memory; caused cognitive impairment in a novel object recognition test; and impaired long-term potentiation at hippocampal CA3–CA1 synapses. In vivo microdialysis revealed an elevated extracellular glutamate concentration in the hippocampus of chronically hyponatremic rats. A sustained low extracellular sodium ion concentration also decreased glutamate uptake by primary astrocyte cultures, suggesting an underlying mechanism of impaired long-term potentiation. Furthermore, gait and memory performances of corrected hyponatremic rats were equivalent to those of control rats. Thus, these results suggest chronic hyponatremia in humans may cause gait disturbance and cognitive impairment, but these abnormalities are reversible and careful correction of this condition may improve quality of life and reduce mortality.
Keywords: hyponatremia, dementia, electrolytes, vasopressin, water-electrolyte balance, osmolality
Hyponatremia is the most common clinical electrolyte disorder.1,2 Symptoms of hyponatremia depend chiefly on its magnitude and rapidity of onset.3 Acute hyponatremia can cause neurologic complications and death as a result of osmotically induced cerebral edema.4 On the other hand, chronic hyponatremia has been considered asymptomatic because the brain can successfully adapt to hyponatremia that is associated with hypo-osmolarity.5 However, recent evidence suggests that chronic hyponatremia may be linked to attention deficits,6 gait disturbances,6 a risk of fracture associated with falls,7,8 and cognitive impairments.9 Furthermore, in Study of Ascending Levels of Tolvaptan in Hyponatremia (SALT)-1 and SALT-2 trials, correcting chronic hyponatremia improved self-assessed mental health status.10 These neurologic impairments are associated with a reduction of quality of life and may be a significant cause of mortality. However, because underlying diseases such as adrenal insufficiency, heart failure, liver cirrhosis, and cancer may affect brain function, the contribution of hyponatremia itself to neurologic manifestations remains unknown.
Brain cells can adapt to hyponatremia. After an acute decrease in external osmolality, cells will initially swell, as a result of water movement into the cells along an osmotic gradient. Very soon thereafter, a process known as volume regulatory decrease begins, in which intracellular solutes (electrolytes and organic osmolytes) are extruded together with osmotically obligated water.5 Glutamate, a known major excitatory neurotransmitter, is one such organic osmolyte that is extruded into the extracellular space during cellular adaptation to hyponatremia.5 In fact, under acute hypo-osmotic conditions, the brain’s extracellular glutamate concentration is increased.11
In a chronically hyponatremic state, as a result of adaptation to hyponatremia, brain volume normalizes completely.12 However, the brain content of glutamate reportedly decreases by 38.6% after 14 days’ sustained hyponatremia in rats,12 which suggests that synaptic excitatory neurotransmissions are affected by chronic hyponatremia.5 The effect of chronic hyponatremia on neurotransmission, however, remains unknown. Furthermore, the extracellular glutamate concentration in a chronically hyponatremic state remains unquantified. The extracellular glutamate concentration must be kept low to maintain a high signal-to-noise ratio in synaptic and extrasynaptic transmissions and to prevent glutamate neurotoxicity resulting from excessive activation of glutamate receptors.13 The mechanism responsible for the long-term maintenance of a low extracellular glutamate concentration is astrocytic glutamate uptake, in which the sodium-dependent glial glutamate transporters, GLT-1 and GLAST, play a critical role.13 Considering that chronic hyponatremia reduces the brain’s glutamate content by about 40%, it is possible that chronic hyponatremia affects glial glutamate uptake and glutamate metabolism.
In this study, we developed a syndrome of inappropriate antidiuretic hormone secretion rat model with different serum sodium ion concentrations ([Na+]). Then, we showed that a reduction in serum [Na+] in chronic hyponatremia induces gait disturbances, memory impairment and decreased long-term potentiation (LTP) at hippocampal CA3–CA1 synapses as an underlying mechanism of memory impairment. Furthermore, the extracellular glutamate concentration was elevated in the chronically hyponatremic rat brain through decreased astrocytic glutamate uptake, which seems to be the cause of decreased LTP.
Results
Induction of Chronic Hyponatremia
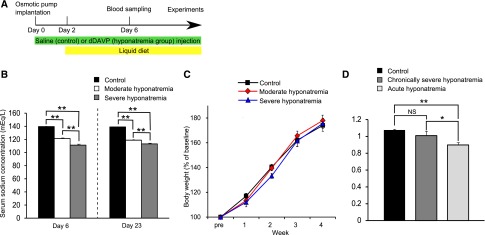
Continuous injections of the vasopressin V2 receptor agonist, 1-deamino-8-d-arginine vasopressin (dDAVP) and liquid diet feeding were used to induce hyponatremia in rats. A decreased serum [Na+] of 121.33±0.93 mEq/l in the moderate hyponatremia group (dDAVP was administered at a rate of 0.3 ng/h) and 111.33±1.54 mEq/l in the severe hyponatremia group (dDAVP was administered at a rate of 0.7 ng/h), as measured on day 6 after the start of dDAVP injections, was noted (Figure 1, A and B). Body weight was comparable among the three groups (Figure 1C). We measured the apparent diffusion coefficient (ADC) using magnetic resonance imaging (MRI) to rule out brain cell swelling in chronically hyponatremic rats. MRI analysis revealed no significant difference in ADC between control and chronically, severely hyponatremic rats, whereas the ADC of acutely hyponatremic rats (a serum [Na+] of 113.25±1.21 mEq/l) was significantly decreased as compared with the former two groups (Figure 1D). This meant that the brain water content of chronically hyponatremic animals could not be distinguished from that of controls with the methods used.
Figure 1.
Hyponatremia without brain cell swelling was induced in rats. (A) Experimental protocol. Injection of saline (control group) or 1-deamino-8-d-arginine vasopressin (dDAVP) (hyponatremia group) was started on day 0 and rats were fed a liquid diet from day 2. Experiments were performed after day 6. (B) Serum sodium concentrations in control (n=11), moderately hyponatremic (n=9) and severely hyponatremic (n=9) rats. One-way ANOVA followed by Fisher’s projected least significant difference test; **P<0.01. (C) Body weights in control (n=9), moderately hyponatremic (n=8) and severely hyponatremic (n=6) rats over time. Two-way ANOVA. (D) Ratio of brain apparent diffusion coefficient values before and after induction of hyponatremia (control n=6, chronically severe hyponatremia n=6, acute hyponatremia n=8). One-way ANOVA followed by Fisher’s projected least significant difference test; *P<0.05, **P<0.01. NS, not significant.
Chronic Hyponatremia Induces Gait Disturbances
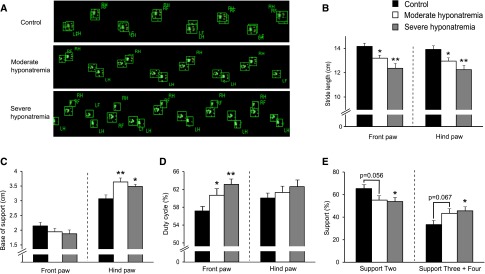
To investigate the influence of chronic hyponatremia on gait in detail, we performed CatWalk automated gait analysis.14 As seen in the movie (Supplemental data), moderately or severely hyponatremic rats with chronic disease seemed to walk with larger alternating, lateral trunk movements. Footprint images suggested moderately and severely hyponatremic rats had a smaller stride, wider-based stance, and separated front and hind paw prints as compared with control rats (Figure 2A). Quantitative analysis of footprint patterns showed moderately and severely hyponatremic rats displayed a significantly shorter stride length (Figure 2B) and a significantly wider base of support of hind paws as compared with control rats (Figure 2C). In addition, hyponatremic rats demonstrated a significantly larger duty cycle for front paws (Figure 2D) reflecting a significantly smaller support two gait phase (the relative duration of simultaneous contact with the glass plate of two paws) and a significantly larger support three plus four gait phase (that of three or four paws) (Figure 2E). These findings suggest that chronic hyponatremia causes ataxic gait.
Figure 2.
CatWalk automated gait analysis revealed chronic hyponatremia-induced gait disturbances. (A) Representative footprint images. (B–E) Quantitative analysis of footprint patterns in control (n=9), moderately hyponatremic (n=8) and severely hyponatremic (n=6) rats. (B) Stride length; (C) base of support; (D) duty cycle; and (E) support. One-way ANOVA followed by Fisher’s projected least significant difference test; *P<0.05, **P<0.01 versus control.
Chronic Hyponatremia Causes Memory Impairment
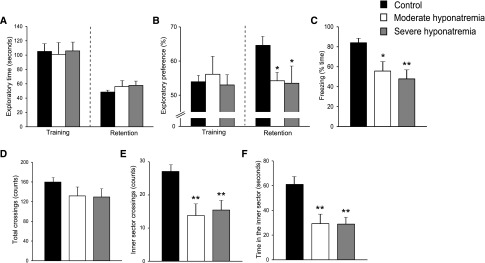
We evaluated the effect of chronic hyponatremia on recognition memory using a novel-object recognition test (NORT). There were no significant differences in exploratory times among the three groups (Figure 3A). During training sessions, all three groups spent approximately equal time with each object (Figure 3B). However, during retention sessions, the preference index of moderately and severely hyponatremic rats was significantly decreased as compared with control rats, indicating that chronic hyponatremia impairs recognition memory (Figure 3B).
Figure 3.
Chronic hyponatremia impairs memory without affecting locomotor activity. (A and B) Performance of control (n=11), moderately hyponatremic (n=8) and severely hyponatremic (n=7) rats in novel-object recognition tests (NORTs); (A) exploratory times and (B) exploratory preferences. (C) The freezing performance of control (n=9), moderately hyponatremic (n=8) and severely hyponatremic (n=6) rats during contextual fear conditioning tests. (D–F) Performance of control (n=11), moderately hyponatremic (n=9) and severely hyponatremic (n=9) rats in open field tests. The number of (D) total and (E) inner sector crossings. (F) Time in the inner sector. One-way ANOVA followed by Fisher’s projected least significant difference test; *P<0.05, **P<0.01 versus control.
We subsequently measured associative memory using a contextual fear conditioning test. Moderately and severely hyponatremic rats showed fewer freezing responses when they were placed in the conditioning cage, a day after delivery of foot shocks, indicating that chronic hyponatremia impairs associative memory (Figure 3C).
To rule out the difference in locomotor activity among the three groups, we performed an open field test. There was no significant difference in the number of total crossings (the sum of inner and outer sector crossings; Figure 3D) among the three groups indicating that chronic hyponatremia does not affect locomotor activity. However, unexpectedly, both moderately and severely hyponatremic rats showed a significantly reduced number of inner sector crossings (Figure 3E) and time in the inner sector (Figure 3F), suggesting that chronic hyponatremia increases anxiety levels.
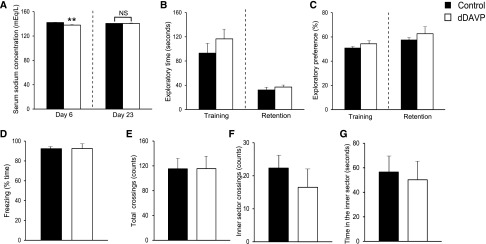
Many studies indicate that vasopressin per se influences memory and anxiety.15–19 Although most of the central actions of vasopressin are mediated via the V1b receptor, some clinical studies have suggested a V2 receptor mediated effect on memory.20 Therefore, to exclude any possible direct effect of subcutaneously injected dDAVP on behavior, we induced dDAVP-infused normonatremia in rats by injecting dDAVP at 0.7 ng/h and feeding them a high salt, liquid diet formula. Such rats’ serum [Na+] on day 6 was significantly lower than that of control rats, but the difference was extremely small (Figure 4A); by day 23, however, serum [Na+] was similar between the two groups (Figure 4A). dDAVP-infused normonatremic rats showed no significant changes when compared with control rats in the NORT, contextual fear conditioning and open field tests (Figure 4, B–G). These results indicate that subcutaneously injected dDAVP did not affect the behaviors tested in the present study and that it is therefore chronic hyponatremia itself that induces memory impairment.
Figure 4.
Continuous 1-deamino-8-d-arginine vasopressin (dDAVP) administration does not directly affect memory and anxiety. (A) Serum sodium concentration of dDAVP injected (n=6) and control (n=6) rats on days 6 and 23. (B and C) The performance of dDAVP injected (n=6) and control (n=6) rats in novel-object recognition tests (NORTs): (B) exploratory time and (C) exploratory preferences. (D) The freezing performance of dDAVP injected (n=6) and control (n=6) rats in contextual fear conditioning tests. (E–G) The performance of dDAVP injected (n=6) and control (n=6) rats in the open field test: number of (E) total and (F) inner sector crossings, and (G) time in the inner sector. Student’s t test; **P<0.01 versus control. NS, not significant.
Chronic Hyponatremia Impairs Hippocampal CA3–CA1 Synaptic Plasticity
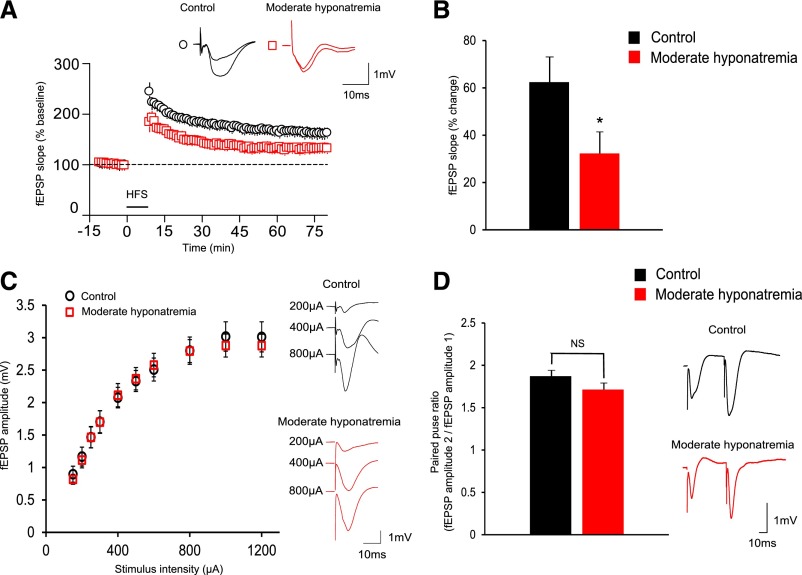
Then, we focused on chronic hyponatremia-induced memory impairment to elucidate the mechanisms underlying the behavioral abnormalities. It has been shown by others that LTP in the hippocampal CA1 region is involved in the formation of certain types of memory.21 We therefore studied high frequency stimulation (HFS)-induced LTP at hippocampal CA3–CA1 synapses in chronic, moderately hyponatremic rats in vivo.
At 60–69 minutes after HFS, the initial falling slope of field excitatory postsynaptic potential (fEPSP) of hyponatremic rats was significantly smaller than that observed in control rats (Figure 5, A and B). The input-output curve, used as a measure of basal synaptic transmission, was not significantly altered in hyponatremic as compared with control rats (Figure 5C). In addition, paired-pulse facilitation was indistinguishable between the two groups, suggesting that the presynaptic release probability is not altered in hyponatremic rats (Figure 5D). These results indicate that chronic hyponatremia decreases the magnitude of LTP at CA3–CA1 synapses without affecting basal synaptic transmission.
Figure 5.
Chronic hyponatremia impairs long-term potentiation (LTP) in hippocampal CA3–CA1 synapses. (A and B) The magnitude of LTP induced in moderately hyponatremic rats (n=12) is significantly smaller as compared with control (n=10) rats. (A) Plots of normalized field excitatory postsynaptic potential (fEPSP) slopes. (B) The average percentage change of fEPSP slopes before and 60–69 minutes after high frequency stimulation (HFS). Student’s t test; *P<0.05 versus control. (C) Input-output curve (input is given by the amplitude of stimulus intensity applied in the Schaffer collateral/commissural pathway and output is given by the amplitude of fEPSP) of moderately hyponatremic (n=12) and control (n=10) rats. Two-way ANOVA. (D) Paired-pulse ratio of moderately hyponatremic (n=12) and control (n=10) rats. The graph shows the averaged ratio of consecutive fEPSP amplitudes (second fEPSP amplitude/first fEPSP amplitude). Student’s t test; NS, not significant.
Chronic Hyponatremia Increases Extracellular Glutamate Concentration by Decreasing Astrocytic Glutamate Uptake
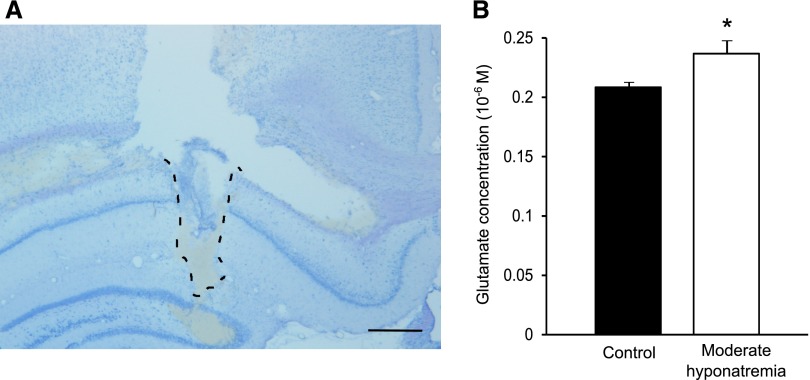
An elevated extracellular glutamate concentration has been shown to impair LTP induction.22,23 Acute hypo-osmotic conditions stimulate glutamate release from neurons and astrocytes24 and increase the extracellular glutamate concentration of the cerebral cortex as demonstrated by microdialysis.11 However, the extracellular glutamate concentration of the chronically hyponatremic brain remains unknown. We therefore investigated, by microdialysis, whether chronic hyponatremia increases the extracellular glutamate concentration of the hippocampal CA1 region (Figure 6A). As shown in Figure 6B, the steady state extracellular glutamate concentration of the hippocampal CA1 region in chronically hyponatremic rats was significantly increased compared with that of control rats.
Figure 6.
Chronic hyponatremia increases the extracellular glutamate concentration of the hippocampus CA1 region in vivo. (A) The area delineated by a dashed line shows the prior location of an intra-CA1 microdialysis probe. Scale bar, 500 µm. (B) The extracellular glutamate concentration of control (n=7) and moderately hyponatremic (n=7) rats as measured by microdialysis. For each rat, the average of six successive fractions is shown after the collection of baseline fractions for 2 hours. Student’s t test; *P<0.05 versus control.
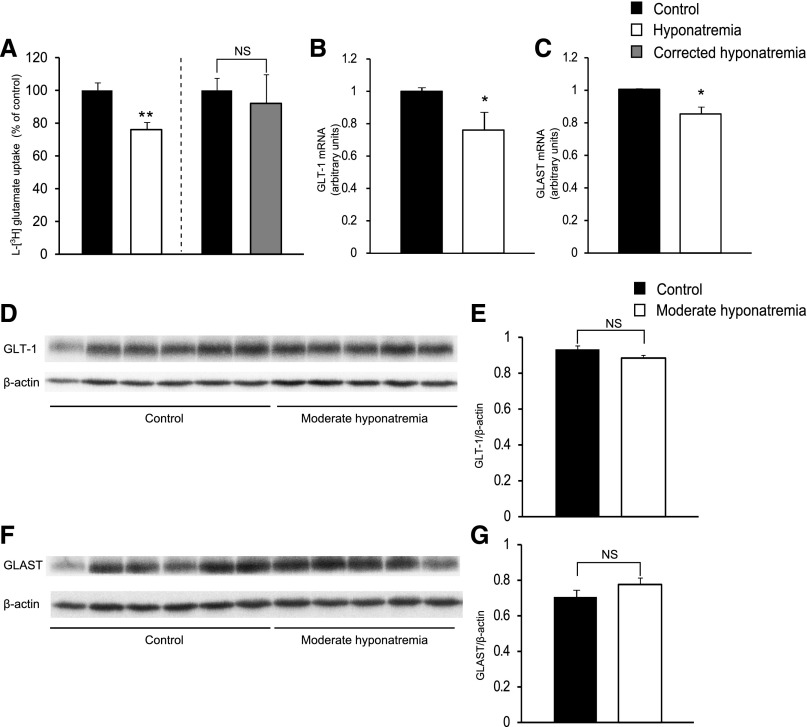
We subsequently hypothesized that decreased astrocytic glutamate uptake may cause an increase in extracellular glutamate concentration because astrocytic glutamate uptake is the mechanism responsible for the long-term maintenance of low extracellular concentrations of glutamate.13 We therefore evaluated what effect a chronic reduction in extracellular [Na+] has on astrocytic glutamate uptake by measuring l-[3H] glutamate uptake by primary mouse astrocytes cultured in medium in which [Na+] was gradually decreased to 117 mEq/l over 6 days, and which was then maintained hyponatremic for at least a further 2 days. As shown in Figure 7A, l-[3H] glutamate uptake by astrocytes was significantly decreased after long-term culturing in hyponatremic medium. In addition, after gradual correction of hyponatremia, l-[3H] glutamate uptake was comparable to that of controls (Figure 7A).
Figure 7.
Chronic hyponatremia decreased astrocytic glutamate uptake. (A) (Left) l-[3H] glutamate uptake by primary mouse astrocytes cultured in control (n=6) or hyponatremic medium (n=6). (Right) l-[3H] glutamate uptake by primary mouse astrocytes cultured in control (n=6) or corrected hyponatremic medium (n=6). (B and C) Expression of GLT-1 (B) and GLAST (C) mRNA in primary astrocytes cultured in control (n=7) or hyponatremic (n=7) medium was determined by quantitative PCR. (D and F) Western blot analysis of GLT-1 (D) and GLAST (F) in hippocampal membrane fractions from control (n=6) and moderately hyponatremic (n=5) rats. (E and G) Quantification of the data presented in D (E) and F (G). Student’s t test; *P<0.05, **P<0.01 versus control. NS, not significant.
The glutamate transporters GLT-1 and GLAST are reported to play a crucial role in removing glutamate from the synaptic cleft.25 We therefore measured the GLT-1 and GLAST mRNA expression levels of astrocytes cultured in hyponatremic medium and found these to be significantly decreased compared with those of astrocytes cultured in control medium (Figure 7, B and C). The results obtained in vitro using cultured mouse astrocytes may not translate into the in vivo situation as these cells would differ from in vivo astrocytes. Therefore, we evaluated GLT-1 and GLAST protein levels in membrane fractions and total protein lysates (data from total protein lysates were not shown) derived from the hippocampi and found that the expression of GLT-1 and GLAST proteins in moderately hyponatremic rats was comparable to that of control rats (Figure 7, D–G).
Chronic Hyponatremia Impairs Neuronal Mitochondrial Distribution and Decreases ATP Production
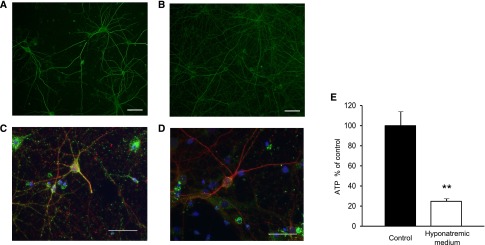
We next evaluated the direct effects of a chronic reduction in extracellular [Na+] on cultured primary neurons. Neuritic beading formation, thought to be an early pathologic sign of neuronal dysfunction,26 was indistinguishable between primary neurons cultured in control medium and those cultured in a low sodium concentration (117 mEq/l) (Figure 8, A and B). In primary neurons cultured in control medium, mitochondria, which were stained by anti-manganese superoxide dismutase (green), were distributed throughout neurites and the cell body (Figure 8C); however, in primary neurons cultured in hyponatremic medium, mitochondria disappeared from neurites, suggesting that chronic hyponatremia impaired neuritic transport and mitochondrial function (Figure 8D), as previously described.26 In addition, the ATP content of primary neurons cultured in hyponatremic medium was significantly decreased compared with that of neurons cultured in control medium (Figure 8E). These results suggest that chronic hyponatremia directly induces neuronal dysfunction by energy loss.
Figure 8.
Chronic hyponatremia influences neurons directly. (A and B) Neurons cultured in control (A) or hyponatremic (B) medium were stained with anti-microtubule-associated protein 2 (MAP2). Scale bar, 50 µm. (C and D) Neurons were stained with anti-neuron-specific tubulin βIII isoform (βIII-tubulin) (red), anti-manganese superoxide dismutase (MnSOD; a mitochondrial marker, green) and Hoechst (blue). Neurons were cultured in control (C) or hyponatremic (D) medium. Samples were visualized by immunofluorescence microscopy and representative micrographs shown. Note that neurons cultured in control medium displayed mitochondria throughout their cell body and neurite. On the other hand, neurons cultured in hyponatremic medium lost mitochondria in their neurites. Scale bar, 50 µm. (E) Intracellular ATP levels (control n=4; hyponatremic medium n=4) were measured by luminometric assay. Student’s t test; **P<0.01 versus control.
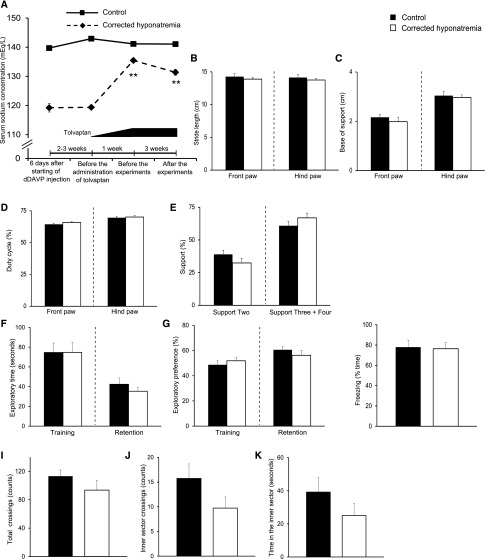
Gait and Memory Performances of Corrected Hyponatremic Rats are Comparable to those of Control Rats
Finally, we assessed whether correcting chronic hyponatremia restores gait disturbances and memory impairment. We corrected moderately chronic hyponatremia with increasing doses of tolvaptan, a vasopressin V2 receptor antagonist, after hyponatremia was maintained for 3–4 weeks. It has been shown that this method of administration of tolvaptan gradually increased serum [Na+] and did not cause osmotic demyelination syndrome in chronically hyponatremic rats.27 In fact, the serum [Na+] of our tolvaptan-administered rats gradually but significantly increased from 119.38±0.74 mEq/l to 135.46±0.73 mEq/l (Figure 9A, Supplemental Table 1). Gait performances, locomotor activities, recognition and associative memory of these corrected hyponatremic rats were equivalent to those of control rats (Figure 9, B–K) suggesting that chronic hyponatremia-induced behavioral abnormalities are reversible. In this experiment, we used a separate set of corrected animals because repeated determinations of locomotor and neurocognitive tests may be impacted by the rats having previously taken this test.
Figure 9.
Gait and memory performances of corrected hyponatremic rats were equivalent to those of control rats suggesting that chronic hyponatremia-induced behavioral abnormalities are reversible. (A) Serum sodium concentration of control (n=14) and corrected hyponatremic (n=13) rats. Two-way ANOVA followed by Tukey’s test; **P<0.01 versus corrected hyponatremia on day 6. (B–E) Quantitative analysis of footprint patterns in control (n=8) and corrected hyponatremic (n=8) rats; (B) stride length; (C) base of support; (D) duty cycle and (E) support. (F and G) Performance of novel-object recognition tests (NORTs) in control (n=11) and corrected hyponatremic (n=11) rats; (F) exploratory time; (G) exploratory preference. (H) The freezing performance of control (n=11) and corrected hyponatremic (n=11) rats in contextual fear conditioning tests. (I–K) Performance of control (n=11) and corrected hyponatremic (n=11) rats in open field tests; number of (I) total and (J) inner sector crossings, and (K) time in the inner sector. Student’s t test.
Discussion
Several clinical studies have identified a relationship between chronic hyponatremia and gait disturbances.6,7 However, a past animal experiment using the rotarod test failed to demonstrate an effect of chronic hyponatremia on gait.27 In the present study, we used CatWalk because this is more sensitive in measuring gait deficits in some animals than the rotarod test.28 Using CatWalk, we found that chronically hyponatremic rats displayed findings similar to those seen in Purkinje cell-specific vesicular γ-aminobutyric acid transporter knockout29 or atm-deficient mice,30 which exhibit cerebellar ataxia. The mechanisms underlying hyponatremia-induced gait abnormalities are currently unknown. However, a mutation in the glutamate transporter GLAST, which is mainly expressed in the cerebellum, has been reported to decrease glutamate uptake and cause episodic ataxia in human subjects.31,32 In addition, GLAST mutant mice exhibited mild motor discoordination.33 In light of such findings, the elevated extracellular glutamate concentrations observed in our hyponatremic rat model may therefore play an important role in hyponatremia-induced gait disturbances.
In relation to the detrimental effects of hyponatremia on memory, our results concur with those of a previous study in which a passive avoidance test was used to evaluate memory in a chronic hyponatremia rat model.27 In our study, we used two behavioral tests of memory, a contextual fear conditioning test and NORT, to evaluate the effect of chronic hyponatremia on memory more definitively. The contextual fear conditioning test evaluates associative memory and NORT evaluates recognition memory, and they are mainly dependent on the hippocampus34 and perirhinal cortex,35–37 respectively. Therefore, it is suggested that chronic hyponatremia affects several kinds of memory and a broad range of brain areas related to memory processing.
The extracellular glutamate concentration of the chronically hyponatremic rat hippocampus was shown to be elevated compared with that of control rats. An excessive extracellular glutamate concentration has been demonstrated to impair LTP without affecting basal glutamatergic transmission in GLT-1,22,38 aquaporin-423 and glia-specific tuberous sclerosis complex-1 knockout mice,39 in which the expression of glutamate transporters is decreased. One postulated mechanism of LTP impairment may be excessive activation of N-methyl-d-aspartate (NMDA) receptors during HFS because impaired LTP is restored by the presence of a low concentration of NMDA antagonist.22 Another possible mechanism may be the desensitization of NMDA receptors by ambient extracellular glutamate. A substantial portion of all ionotropic glutamate receptor subtypes (especially NMDA receptors) are tonically desensitized by ambient extracellular glutamate.40
In an acute hyponatremic state, glutamate is extruded as one of several organic osmolytes that reduce cell volume. However, this hypo-osmolarity-elicited glutamate efflux is rapidly activated and then inactivated.41 Our results indicate that a chronic reduction of extracellular [Na+] decreases astrocytic glutamate uptake. Glutamate uptake can be regulated by both changes in transporter expression and activity.42 In the light of the comparable protein expression levels of GLT-1 and GLAST in control and chronic hyponatremic rats, the impairment of glutamate uptake seen in chronic hyponatremia is thought to be caused by a decrease in transporter activity. One possible reason for the decrease in transporter activity is a decrease in extracellular [Na+] itself because these transporters utilize the ion gradient of sodium as an energy source for transport activity.13 Another possible reason is hyponatremia-induced oxidative stress.43 GLT-1 and GLAST both contain functional cysteine residues that are sensitive to the oxidative formation of cysteine bridges and cause the inhibition of glutamate flux through the transporters.44
GLT-1 (+/−) mice, a possible model of mild glutamatergic hyperactivity, show impaired contextual fear conditioning test performances in accordance with our results; however, they also show reduced levels of anxiety contrary to those seen in our hyponatremic rat.45 This suggests that the increased anxiety levels induced by chronic hyponatremia are not caused by increased extracellular glutamate. Therefore, further research is required to elucidate the exact mechanisms involved in elevated anxiety levels.
Several reports indicated that total brain water content, measured by weighing before and after desiccation, was not significantly different between hyponatremic and control rats.46,47 In contrast, Verbalis and Gullans reported that hyponatremic rats developed an element of brain edema.12 Such a contrast in findings is likely to be due to differences in the periods of chronic hyponatremia. In order to evaluate whether our hyponatremic rats show brain cell swelling, we measured ADC. A decrease in ADC is believed to be the result of water moving into the intracellular compartment in acute cerebral infarction.48 It has been reported that the plots of ADC versus total brain water showed a statistically significant, inverse linear relationship between ADC and increasing brain water.49 Several lines of evidence also showed that an approximately 10% decrease in ADC values is observed in the acute hyponatremic rat brain.49,50 ADC of the brains of severely hyponatremic rats was comparable to that of controls, indicating that our chronically hyponatremic rat model did not develop brain cell swelling.
In addition to its effect on astrocytes, our study suggests chronic hyponatremia also directly impairs mitochondrial distribution and decreases the ATP content of neurons, findings that are known to be induced by excessive glutamate.26 Therefore, the direct effects of a reduction in extracellular [Na+] on neurons is thought to reinforce neurologic symptoms in conjunction with elevated extracellular glutamate levels in the chronically hyponatremic rat.
Some points remain as matters to be evaluated further. Firstly, the threshold of serum [Na+] that induces gait and memory impairments remains uncertain. Secondly, differences in effects on the central nervous system by various methods of correction of chronic hyponatremia are unknown. In our study, we corrected chronic hyponatremia with tolvaptan because this method is reported to correct hyponatremia stably without causing osmotic demyelination syndrome in rats.27 However, in clinical settings, other methods (i.e., water restriction, high salt diet, urea, etc.) may be more suitable as appropriate treatment. Thirdly, reversibility should be evaluated by more rigorous methods. It would be preferable to compare behaviors before and after the induction of chronic hyponatremia, and after the correction of chronic hyponatremia, in the same animals. However, it is probable that some tests such as those of contextual fear conditioning, which includes electric shock, may affect the next behavior analysis, and the body weight gain during a long-term experiment for reversibility would modify the results of gait or memory tests. In addition, we did not directly compare animals with corrected hyponatremia to animals with uncorrected hyponatremia, and without such a comparison cannot say definitively that chronic hyponatremia-induced behavioral abnormalities are reversible.
Our results will encourage further research on the effect of chronic hyponatremia on human subjects and treatment for chronic hyponatremia, the importance of which has been constantly overlooked.
Concise Methods
Animal Experiments
All of the procedures were performed in accordance with the institutional guidelines for animal care at Nagoya University, Japan, which, in turn, conform to the National Institutes of Health animal care guidelines.
Male Sprague–Dawley rats (body wt, 160–230 g; Chubu Science Materials, Nagoya, Japan) were housed in a standard animal facility kept at constant temperature (23°C) and on a 12/12-hour light/dark cycle. Rats had ad libitum access to standard chow and tap water until the induction of hyponatremia according to previously described methods.51–53 Briefly, osmotic minipumps (Alzet model 2002 or 2004; DURECT Corporation, Cupertino, CA) containing dDAVP (10 µg/ml; Kyowa Hakko Kogyo Co. Ltd., Tokyo, Japan) were implanted subcutaneously into rats under ether anesthesia. For the moderately or severely hyponatremic groups, dDAVP was injected continuously at rates of 0.3 or 0.7 ng/h, respectively. After 2 days of dDAVP administration, rats were water loaded by substituting their daily feed with a liquid formula (Isocal plus; Mead-Johnson, Evansville, IN) and 10% glucose mixed at a 1:1 ratio. Control rats were implanted with osmotic minipumps containing saline and fed a liquid formula. More than 6 days after starting either dDAVP or saline injections, rats were used for experiments as described in the following sections.
To correct hyponatremia, tolvaptan mixed in a powder diet (delivered by Otsuka Pharmaceutical Co. Ltd., Tokushima, Japan) was administered orally by dose titrations after moderate hyponatremia was maintained by injecting dDAVP at a rate of 0.3 ng/h and feeding them a liquid diet formula for 3–4 weeks. Administered doses of tolvaptan were as follows (day 1 was defined as the first day of the administration of tolvaptan): 0.25 (day 1), 0.5 (day 2), 1.0 (day 3), 2.0 (day 4), 4.0 (day 5), and 8.0 (days 6 onwards) mg/kg.27 Behavioral tests were performed after day 12.
Normonatremic dDAVP-infused rats were induced by injecting them with dDAVP at a rate of 0.7 ng/h and feeding them a high salt liquid diet formula (Isocal plus, 10% glucose and 3% saline solution mixed at a 1:1 ratio). The final [Na+] of this high salt liquid diet formula was estimated to be 314 mEq/l.
Acute hyponatremia was induced in rats by a 15 ml dextrose solution (140 mmol/l) intraperitoneally and 1 µg dDAVP subcutaneously, followed by an additional dose of 10 ml dextrose intraperitoneally and 1 µg dDAVP subcutaneously after 40 minutes.54
Blood samples for the determination of serum [Na+] were obtained from the tail vein under light ether anesthesia. Serum [Na+] was measured at LSI Medience Corporation Co. Ltd., Tokyo, Japan.
Behavioral Analysis
CatWalk
CatWalk is a video-based analysis system to assess gait in voluntarily walking mice or rats (Noldus Information Technology, Wageningen, The Netherlands).14 CatWalk was performed 11 days after starting dDAVP injections. Animals were placed in a corridor and allowed to move freely across a glass plate walkway. When rat paws made contact on the glass plate, light became reflected downward. The illuminated contact areas were recorded with a CCD camera underneath the glass plate to visualize the different paw contacts. From these data, multiple parameters were calculated. For instance, in this study, we focused on stride length, base of support, duty cycle and support. A base of support was defined as the average width between either the front paws or the hind paws. A duty cycle was defined as the stance duration as a percentage of the step cycle duration, i.e., stance phase/(stance+swing phases)×100, where the stance phase is measured as the duration, in seconds, of contact of a paw with the glass plate, and the swing phase is measured as the duration, in seconds, of no contact of a paw with the glass plate. Support two was defined as the relative duration of simultaneous contact with the glass plate of two paws, and support three plus four as of that three or four paws. The animals were allowed to traverse the walkway as many times as needed to obtain at least three smooth crossings (without stops or hesitations).55
Open field test
The open field test was carried out as described previously, with minor modifications56 on day 12. The open field used in this study consisted of a square area with gray walls (100 cm width, 45 cm height) and was set in a dark, sound-attenuated room. The floor of the field was divided into 25 identical areas so that the animal’s ambulation could be precisely measured. The field was divided into inner (50 cm square) and outer sectors. An LED light was positioned 190 cm above the center of the floor of the apparatus. Each rat was placed in the center of the open field. The rats were allowed to explore the environment freely for 10 minutes. During this time, the ambulation of the rats was measured by counting the number of times that the animals crossed from one area to another. We also measured the time spent visiting the inner sector.
NORT
NORT was carried out as described previously56 with minor modifications between day 18 and day 22. We used the open field described above as the experimental apparatus. The apparatus was located in a sound-attenuated room and was illuminated with an LED light.
In a standard procedure, NORT consisted of three sessions: habituation, training, and retention. Each rat was individually habituated to the apparatus, with 10 minutes of exploration in the absence of objects for three consecutive days (habituation session, days 1–3). During the training session, two novel objects were symmetrically fixed to the floor of the apparatus, 25 cm from the walls, and each animal was allowed to explore the apparatus for 10 minutes (day 4). The objects were constructed from a plastic cup, a plastic ink bottle, and a sake bottle, which were different in shape and color but similar in size. An animal was considered to be exploring the object when its head was facing the object and/or it was touching or sniffing the object. The time spent exploring each object was recorded, and the rats were immediately returned to their home cages after training. The animals were placed back into the same apparatus 24 hours after each training session (retention session). During the retention session, one of the two familiar objects used during the training session was replaced with a novel object. The animals were then allowed to explore freely for 5 minutes and the time spent exploring each object was recorded. Throughout the experiments, the objects were used in a counterbalanced manner in terms of their physical complexity and emotional neutrality. During each retention session, a preference index, which is a ratio of the amount of time spent exploring the novel object to the total time spent exploring both objects, was used to measure cognitive function. In the training session, the preference index was calculated as the ratio of the time spent exploring the object that was replaced by a novel object in the retention session to the total exploring time.
Contextual fear conditioning test
A contextual fear conditioning test was performed in accordance with previous reports56 with minor modifications on days 27–28. Freezing behavior, as indicated by a rat’s head, arms and legs not moving, was measured by stop-watch. For measuring basal levels of a freezing response (preconditioning phase), rats were individually placed in a conditioning cage (a transparent Plexiglas box, 30×30×45 cm, W×L×H) for 2 minutes. For training (conditioning phase), rats were placed in the conditioning cage, and a foot shock of 0.6 mA was delivered by a shock generator (Brain Science Idea Co. Ltd., Osaka, Japan). This procedure was repeated four times at 15-second intervals. Contextual tests were carried out one day after fear conditioning; rats were placed in the conditioning cage and the freezing response was measured for 2 minutes.
Electrophysiological analysis
Electrophysiological analysis was performed as described previously57 with minor modifications. Rats were anesthetized with urethane (ethyl carbamate, 1.5 mg/kg, intraperitoneally) and then placed in a stereotaxic frame. A local anesthetic, lidocaine, was administered at pressure points caused by the stereotaxic frame and around surgical incisions. Body temperature was maintained at about 37°C. fEPSPs were recorded from the CA1 stratum radiatum of either hippocampal hemisphere using a tungsten electrode (300–500 KΩ; Frederick Haer & Co., Bowdoinham, ME) in response to stimulation of the Schaffer collateral/commissural pathway using a pair of bipolar stimulating tungsten electrodes (diameter, 100 μm; interpolar distance, about 200 μm). Electrode implantation sites were determined using stereotaxic coordinates relative to the bregma, with the recording site located 3 mm posterior and 2 mm lateral of the midline, and the stimulating electrode 4 mm posterior to the bregma and 3 mm lateral of the midline. The stimulating electrodes and the recording electrodes were slowly lowered through the cortex and the upper layers of the hippocampus into the CA1 region to a depth of 2.2 mm below the cortex surface and were positioned to record maximal fEPSPs.
In all experiments test EPSPs were evoked at a frequency of 0.1 Hz, and an input-output curve (stimulus intensity versus EPSP amplitude) was plotted for each experiment at this test frequency. For the test EPSPs, the stimulation intensity was adjusted to give an EPSP amplitude of 30–40% of maximum. The intensity was increased to give an EPSP of 60–70% maximum amplitude during stimulation to induce LTP.58 LTP was induced by applying HFS (five trains of 100 pulses at 100 Hz delivered at 120-second intervals) to the Schaffer collateral/commissural pathway.58 LTP was measured as a percentage of the baseline EPSP slope recorded over the 15-minute period before HFS. Paired-pulse facilitation was measured at an interstimulus interval of 50 ms.
Microdialysis
In vivo microdialysis was performed as described previously59,60 with minor modifications. Rats were anesthetized with sodium pentobarbital before stereotaxic implantation of a guide cannula into the hippocampus CA1 region (AP –3.8, ML +2.0 from the bregma, DV –2.1 from the skull).61 After 2 days, a dialysis probe (A-I-4–01; 1 mm membrane length; Eicom, Kyoto, Japan) was inserted through the guide cannula. The next day, the dialysis probe was perfused with artificial cerebrospinal fluid (147 mM sodium chloride, 4 mM potassium chloride, 2.3 mM calcium chloride) at a flow rate of 1.0 µl/min. Outflow fractions were collected every 10 minutes. After the collection of baseline fractions for 2 hours, we measured the levels of glutamate in six successive fractions with a HPLC system (Eicom).
Magnetic Resonance Imaging
MRI data were acquired by an MRmini SA device equipped with a 1.5-Tesla permanent magnet made of Nd-Fe-B material (DS Pharma Biomedical Co. Ltd., Osaka, Japan). Anesthesia was induced and maintained with 1.0% isoflurane. Sets of diffusion weighted spin-echo images were acquired using a repetition time of 2000 ms, echo time of 69 ms and b-values of 0, 45, 170, 380, 660 and 1000 s/mm2. The field of view was 30 mm × 60 mm and the data matrix was 128 × 256 for ten slices 2.0 mm thick throughout the cortex. An ADC map was calculated by MRI Analysis Calculator v. 1.0, and the ADC value was measured in two slices comprising the hippocampus and cerebral cortex.
Cell Culture
Astrocytes were prepared from the primary mixed glial cell cultures of newborn C57BL/6 mice (Chubu Science Materials), which contained both astrocytes and microglia, as previously described with minor modifications.62 Astrocytes and microglia made up the primary mixed glial cell cultures of newborn C57BL/6 mice from which microglia were isolated on the 14th day using the shaking off method. The remaining attached layer of the culture contained astrocytes, which were subsequently purified by repeated trypsinization and replating three to four times. Such cultures were more than 95% pure when examined by indirect immunofluorescence staining with an anti-glial fibrillary acidic protein (rabbit monoclonal; EMD Millipore, Billerica, MA). Cultures were maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% fetal bovine serum, 5 μg/ml bovine insulin, and 0.2% glucose.
Neuronal cultures were prepared from C57BL/6 mice at embryonic day 17 using a nerve-cell culture system (Sumitomo Bakelite Co. Ltd., Akita, Japan) as described previously.26 Briefly, cortices were dissected and freed of meninges. Cortical fragments were dissociated into single cells using dissociation solution, and were resuspended in nerve-cell culture medium (serum-free conditioned medium from 48 hour rat astrocyte confluent cultures based on Dulbecco’s modified Eagle’s minimum essential medium/F-12 with N2 supplement, Sumitomo Bakelite). Primary neuronal cells were plated on 12-mm polyethyleneimine-coated coverslips (Asahi Techno Glass Corp., Chiba, Japan) in 24-well multidishes at a density of 5 × 104 cells/well. The purity of the cultures was more than 95% as determined by NeuN-specific immunostaining.
In order to induce hyponatremia gradually, after confluent cell growth, half of the medium was replaced every 2 days with normal DMEM (control group) or hyponatremic ([Na+]=117 mEq/l) DMEM (hyponatremia group). After four medium changes, cells were cultured in DMEM (control group) or hyponatremic DMEM (hyponatremia group) for at least 2 days and used for subsequent experiments. For neuronal cultures, F-12 with N2 supplement was added to both normal and hyponatremic DMEM. To correct hyponatremia, after induction of hyponatremia described above, half of the medium was replaced every 2 days with normal DMEM for four times and cells were cultured in normal DMEM for 2 days.
Glutamate Uptake Assay
Glutamate uptake assays were performed as described previously63 with minor modifications. Briefly, control and hyponatremic astrocytes grown in 12-well mutidishes were washed twice and incubated with either prewarmed Krebs–Ringer solution (125 mM sodium chloride, 2.5 mM potassium chloride, 1.25 mM monosodium phosphate, 2 mM calcium chloride, 1 mM magnesium chloride, 25 mM sodium bicarbonate, 25 mM glucose) or prewarmed hyponatremic Krebs–Ringer solution (91 mM sodium chloride, 2.5 mM potassium chloride, 1.25 mM monosodium phosphate, 2 mM calcium chloride, 1 mM magnesium chloride, 25 mM sodium bicarbonate, 25 mM glucose), respectively. The glutamate uptake assay was initiated by adding a mixture of nonradioactive glutamate and 0.25 mCi/ml l-[3H] glutamate (specific activity, 51.1 Ci/mmol; PerkinElmer, Waltham, MA) to the reaction media to provide a final concentration of 100 nM glutamate. The reaction was continued for 10 minutes at 37°C and terminated by washing three times with ice-cold PBS, immediately followed by cell lysis in 1 ml of 1 N sodium hydroxide. An aliquot of 750 µl was taken out into scintillation vials and neutralized with 75 µl of 10 N hydrochloric acid. After adding 3 ml of liquid scintillation fluid, the radioactivity was measured using a liquid scintillation counter (LS 6500; Beckman Coulter, Brea, CA). Sample protein concentrations were determined by BCA assay (Thermo Fisher Scientific, Waltham, MA). Glutamate uptake activity was calculated as pmol glutamate/milligram protein/minute after correcting for protein levels.
Quantitative PCR
Total RNA was extracted from cultured astrocytes using an RNeasy Mini Kit (Qiagen, Venlo, The Netherlands), following the manufacturer’s instructions. cDNA was generated by SuperScript II (Invitrogen, Carlsbad, CA). Quantitative PCR was performed on reverse transcription products using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a Rotor-Gene Q Real-Time PCR cycler (Qiagen). Each sample’s mRNA level was normalized relative to glyceraldehyde-3-phosphate dehydrogenase mRNA (Table 1).
Table 1.
Primer sequences (5′ to 3′)
| GLT-1 | Forward | TGACCTTCATCATGGCCTAAACA |
| Reverseward | TCAGGCTCACCTGCATGACTACTAA | |
| GLAST | Forward | AGAGTGAGGCTCCCAAATGGTC |
| Reverseward | CTGATGTTCCAGAAGTTTGGGCTA | |
| GAPDH | Forward | TGTGTCCGTCGTGGATCTGA |
| Reverseward | TTGCTGTTGAAGTCGCAGGAG |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Western Blotting
Membrane protein fractions were prepared using ProteoExtract Transmembrane Protein Extraction Kit (EMD Millipore) following manufacturer’s instructions. Protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and probed by immunoblotting with the following primary antibodies: GLT-1 guinea pig polyclonal (1:2000; EMD Millipore), GLAST rabbit polyclonal (1:200; Santa Cruz Biotechnology, Dallas, TX), β-actin mouse monoclonal (1:2000; Sigma-Aldrich, St Louis, MO). Bound antibodies were detected by using appropriate horseradish peroxidase-conjugated secondary antibodies (1:20,000; Jackson ImmunoResearch, West Grove, PA) and SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). Western blots were quantified using ImageJ software. Optical density values were normalized to β-actin signal.
Immunocytochemistry
Neurons were fixed with 4% paraformaldehyde for 30 minutes and permeabilized with 0.05% Triton X-100 for 10 minutes at room temperature. Cells were stained with primary antibody at 4°C overnight as follows: mouse monoclonal anti–βIII-tubulin (1:2000; Chemicon International, Temecula, CA), mouse monoclonal anti-microtubule-associated protein 2 (1:500; Chemicon International), rabbit polyclonal anti- manganese superoxide dismutase (1:2000; Stressgen Biotechnologies Corp., Victoria, BC, Canada). They were subsequently stained with secondary antibody-conjugated Alexa-488 or 568 (1:1000; Molecular Probes-Life Technologies) at room temperature for 90 minutes. Cells were then counterstained with 1 µg/ml Hoechst 33342 (Molecular Probes) at room temperature for 10 minutes, and mounted in antifade reagent. Cells were analyzed under a deconvolution fluorescence microscope system (BZ-8000; Keyence, Osaka, Japan).
Assessment of Intracellular ATP Levels
To measure intracellular ATP levels, we used a luminometric assay, the ApoSENSOR Cell Viability Assay Kit (BioVision, Mountain View, CA) according to the manufacturer’s protocol. The ATP concentration was calculated as a percentage of control.
Statistical Analysis
Results are expressed as the mean±SEM. Statistical analyses were performed using Student’s t test (two-tailed), one-way ANOVA followed by Fisher’s projected least significant difference test or two-way ANOVA followed by Tukey's test as indicated in the figure legends. P values less than 0.05 were considered to be significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Japanese Society for the Promotion of Science 24591360 (to Y.S.) and a grant-in-aid for scientific research (Research on Hypothalamo-hypophyseal Disorders) (to Y.S. and Y.O.) from the Ministry of Health, Labor and Welfare, Japan. The authors thank Otsuka Pharmaceutical Co. Ltd., for the gift of tolvaptan.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Modeling the Neurologic and Cognitive Effects of Hyponatremia,” on pages 659–661.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014121196/-/DCSupplemental.
References
- 1.Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE: Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170: 294–302, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ: Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med 126: 256–263, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Decaux G: Is asymptomatic hyponatremia really asymptomatic? Am J Med 119[Suppl 1]: S79–S82, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Adrogué HJ, Madias NE: Hyponatremia. N Engl J Med 342: 1581–1589, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Verbalis JG: Brain volume regulation in response to changes in osmolality. Neuroscience 168: 862–870, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G: Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med 119: e1–e8, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G: Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101: 583–588, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Sandhu HS, Gilles E, DeVita MV, Panagopoulos G, Michelis MF: Hyponatremia associated with large-bone fracture in elderly patients. Int Urol Nephrol 41: 733–737, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Gunathilake R, Oldmeadow C, McEvoy M, Kelly B, Inder K, Schofield P, Attia J: Mild hyponatremia is associated with impaired cognition and falls in community-dwelling older persons. J Am Geriatr Soc 61: 1838–1839, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C, SALT Investigators : Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med 355: 2099–2112, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Haskew-Layton RE, Rudkouskaya A, Jin Y, Feustel PJ, Kimelberg HK, Mongin AA: Two distinct modes of hypoosmotic medium-induced release of excitatory amino acids and taurine in the rat brain in vivo. PLoS ONE 3: e3543, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verbalis JG, Gullans SR: Hyponatremia causes large sustained reductions in brain content of multiple organic osmolytes in rats. Brain Res 567: 274–282, 1991 [DOI] [PubMed] [Google Scholar]
- 13.Danbolt NC: Glutamate uptake. Prog Neurobiol 65: 1–105, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB, Gispen WH: Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma 18: 187–201, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Landgraf R, Gerstberger R, Montkowski A, Probst JC, Wotjak CT, Holsboer F, Engelmann M: V1 vasopressin receptor antisense oligodeoxynucleotide into septum reduces vasopressin binding, social discrimination abilities, and anxiety-related behavior in rats. J Neurosci 15: 4250–4258, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Londen L, Goekoop JG, Zwinderman AH, Lanser JB, Wiegant VM, De Wied D: Neuropsychological performance and plasma cortisol, arginine vasopressin and oxytocin in patients with major depression. Psychol Med 28: 275–284, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Alescio-Lautier B, Paban V, Soumireu-Mourat B: Neuromodulation of memory in the hippocampus by vasopressin. Eur J Pharmacol 405: 63–72, 2000 [DOI] [PubMed] [Google Scholar]
- 18.Frank E, Landgraf R: The vasopressin system—from antidiuresis to psychopathology. Eur J Pharmacol 583: 226–242, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Neumann ID, Landgraf R: Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35: 649–659, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Juul KV, Bichet DG, Nielsen S, Nørgaard JP: The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. Am J Physiol Renal Physiol 306: F931–F940, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Malenka RC, Bear MF: LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Katagiri H, Tanaka K, Manabe T: Requirement of appropriate glutamate concentrations in the synaptic cleft for hippocampal LTP induction. Eur J Neurosci 14: 547–553, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Li MX, Luo Y, Chen T, Liu J, Fang P, Jiang B, Hu ZL, Jin Y, Chen JG, Wang F: Chronic ceftriaxone treatment rescues hippocampal memory deficit in AQP4 knockout mice via activation of GLT-1. Neuropharmacology 75: 213–222, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Pasantes-Morales H, Alavez S, Sánchez Olea R, Morán J: Contribution of organic and inorganic osmolytes to volume regulation in rat brain cells in culture. Neurochem Res 18: 445–452, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Kanai Y, Clémençon B, Simonin A, Leuenberger M, Lochner M, Weisstanner M, Hediger MA: The SLC1 high-affinity glutamate and neutral amino acid transporter family. Mol Aspects Med 34: 108–120, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi H, Mizuno T, Zhang G, Wang J, Kawanokuchi J, Kuno R, Suzumura A: Neuritic beading induced by activated microglia is an early feature of neuronal dysfunction toward neuronal death by inhibition of mitochondrial respiration and axonal transport. J Biol Chem 280: 10444–10454, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki T, Ohmoto K, Hirose T, Fujiki H: Chronic hyponatremia impairs memory in rats: effects of vasopressin antagonist tolvaptan. J Endocrinol 206: 105–111, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Vandeputte C, Taymans JM, Casteels C, Coun F, Ni Y, Van Laere K, Baekelandt V: Automated quantitative gait analysis in animal models of movement disorders. BMC Neurosci 11: 92, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kayakabe M, Kakizaki T, Kaneko R, Sasaki A, Nakazato Y, Shibasaki K, Ishizaki Y, Saito H, Suzuki N, Furuya N, Yanagawa Y: Motor dysfunction in cerebellar Purkinje cell-specific vesicular GABA transporter knockout mice. Front Cell Neurosci 7: 286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus M, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A: Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86: 159–171, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Jen JC, Wan J, Palos TP, Howard BD, Baloh RW: Mutation in the glutamate transporter EAAT1 causes episodic ataxia, hemiplegia, and seizures. Neurology 65: 529–534, 2005 [DOI] [PubMed] [Google Scholar]
- 32.de Vries B, Mamsa H, Stam AH, Wan J, Bakker SL, Vanmolkot KR, Haan J, Terwindt GM, Boon EM, Howard BD, Frants RR, Baloh RW, Ferrari MD, Jen JC, van den Maagdenberg AM: Episodic ataxia associated with EAAT1 mutation C186S affecting glutamate reuptake. Arch Neurol 66: 97–101, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K: Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci 10: 976–988, 1998 [DOI] [PubMed] [Google Scholar]
- 34.Holland PC, Bouton ME: Hippocampus and context in classical conditioning. Curr Opin Neurobiol 9: 195–202, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Winters BD, Saksida LM, Bussey TJ: Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev 32: 1055–1070, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Gilbert PE, Kesner RP: Recognition memory for complex visual discriminations is influenced by stimulus interference in rodents with perirhinal cortex damage. Learn Mem 10: 525–530, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray EA, Bussey TJ, Hampton RR, Saksida LM: The parahippocampal region and object identification. Ann N Y Acad Sci 911: 166–174, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K: Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276: 1699–1702, 1997 [DOI] [PubMed] [Google Scholar]
- 39.Zeng L-H, Ouyang Y, Gazit V, Cirrito JR, Jansen LA, Ess KC, Yamada KA, Wozniak DF, Holtzman DM, Gutmann DH, Wong M: Abnormal glutamate homeostasis and impaired synaptic plasticity and learning in a mouse model of tuberous sclerosis complex. Neurobiol Dis 28: 184–196, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Featherstone DE, Shippy SA: Regulation of synaptic transmission by ambient extracellular glutamate. Neuroscientist 14: 171–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco R, Torres-Márquez ME, Pasantes-Morales H: Evidence for two mechanisms of amino acid osmolyte release from hippocampal slices. Pflugers Arch 442: 791–800, 2001 [DOI] [PubMed] [Google Scholar]
- 42.Anderson CM, Swanson RA: Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32: 1–14, 2000 [PubMed] [Google Scholar]
- 43.Barsony J, Sugimura Y, Verbalis JG: Osteoclast response to low extracellular sodium and the mechanism of hyponatremia-induced bone loss. J Biol Chem 286: 10864–10875, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trotti D, Danbolt NC, Volterra A: Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci 19: 328–334, 1998 [DOI] [PubMed] [Google Scholar]
- 45.Kiryk A, Aida T, Tanaka K, Banerjee P, Wilczynski GM, Meyza K, Knapska E, Filipkowski RK, Kaczmarek L, Danysz W: Behavioral characterization of GLT1 (+/–) mice as a model of mild glutamatergic hyperfunction. Neurotox Res 13: 19–30, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Verbalis JG, Drutarosky MD: Adaptation to chronic hypoosmolality in rats. Kidney Int 34: 351–360, 1988 [DOI] [PubMed] [Google Scholar]
- 47.Lien YH, Shapiro JI, Chan L: Study of brain electrolytes and organic osmolytes during correction of chronic hyponatremia. Implications for the pathogenesis of central pontine myelinolysis. J Clin Invest 88: 303–309, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer PW, Grant PE, Gonzalez RG: Diffusion-weighted MR imaging of the brain. Radiology 217: 331–345, 2000 [DOI] [PubMed] [Google Scholar]
- 49.Sevick RJ, Kanda F, Mintorovitch J, Arieff AI, Kucharczyk J, Tsuruda JS, Norman D, Moseley ME: Cytotoxic brain edema: assessment with diffusion-weighted MR imaging. Radiology 185: 687–690, 1992 [DOI] [PubMed] [Google Scholar]
- 50.Steier R, Aradi M, Pál J, Bukovics P, Perlaki G, Orsi G, Janszky J, Schwarcz A, Sulyok E, Dóczi T: The influence of benzamil hydrochloride on the evolution of hyponatremic brain edema as assessed by in vivo MRI study in rats. Acta Neurochir (Wien) 153: 2091–2097, discussion 2097, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Sugimura Y, Murase T, Takefuji S, Hayasaka S, Takagishi Y, Oiso Y, Murata Y: Protective effect of dexamethasone on osmotic-induced demyelination in rats. Exp Neurol 192: 178–183, 2005 [DOI] [PubMed] [Google Scholar]
- 52.Suzuki H, Sugimura Y, Iwama S, Suzuki H, Nobuaki O, Nagasaki H, Arima H, Sawada M, Oiso Y: Minocycline prevents osmotic demyelination syndrome by inhibiting the activation of microglia. J Am Soc Nephrol 21: 2090–2098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takagi H, Sugimura Y, Suzuki H, Iwama S, Izumida H, Fujisawa H, Ogawa K, Nakashima K, Ochiai H, Takeuchi S, Kiyota A, Suga H, Goto M, Banno R, Arima H, Oiso Y: Minocycline prevents osmotic demyelination associated with aquaresis. Kidney Int 86: 954–964, 2014 [DOI] [PubMed] [Google Scholar]
- 54.Vajda Z, Promeneur D, Dóczi T, Sulyok E, Frøkiaer J, Ottersen OP, Nielsen S: Increased aquaporin-4 immunoreactivity in rat brain in response to systemic hyponatremia. Biochem Biophys Res Commun 270: 495–503, 2000 [DOI] [PubMed] [Google Scholar]
- 55.Ferdinandusse S, Zomer AW, Komen JC, van den Brink CE, Thanos M, Hamers FP, Wanders RJ, van der Saag PT, Poll-The BT, Brites P: Ataxia with loss of Purkinje cells in a mouse model for Refsum disease. Proc Natl Acad Sci U S A 105: 17712–17717, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mizoguchi H, Ibi D, Takuma K, Toth E, Sato J, Itohara S, Nabeshima T, Yamada K: Alterations of emotional and cognitive behaviors in matrix metalloproteinase-2 and -9-deficient mice. The Open Behavioral Science Journal 4: 19–25, 2010 [Google Scholar]
- 57.Hölscher C, Anwyl R, Rowan MJ: Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J Neurosci 17: 6470–6477, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yilmaz-Rastoder E, Miyamae T, Braun AE, Thiels E: LTP- and LTD-inducing stimulations cause opposite changes in arc/arg3.1 mRNA level in hippocampal area CA1 in vivo. Hippocampus 21: 1290–1301, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mizoguchi H, Yamada K, Niwa M, Mouri A, Mizuno T, Noda Y, Nitta A, Itohara S, Banno Y, Nabeshima T: Reduction of methamphetamine-induced sensitization and reward in matrix metalloproteinase-2 and -9-deficient mice. J Neurochem 100: 1579–1588, 2007 [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi H, Mizoguchi H, Doi Y, Jin S, Noda M, Liang J, Li H, Zhou Y, Mori R, Yasuoka S, Li E, Parajuli B, Kawanokuchi J, Sonobe Y, Sato J, Yamanaka K, Sobue G, Mizuno T, Suzumura A: Blockade of gap junction hemichannel suppresses disease progression in mouse models of amyotrophic lateral sclerosis and Alzheimer’s disease. PLoS ONE 6: e21108, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paxinos G, Watson C: The Rat Brain in Stereotaxic Coordinates, San Diego, CA, Academic Press, 1998 [Google Scholar]
- 62.Liang J, Takeuchi H, Doi Y, Kawanokuchi J, Sonobe Y, Jin S, Yawata I, Li H, Yasuoka S, Mizuno T, Suzumura A: Excitatory amino acid transporter expression by astrocytes is neuroprotective against microglial excitotoxicity. Brain Res 1210: 11–19, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Karki P, Webb A, Zerguine A, Choi J, Son DS, Lee E: Mechanism of raloxifene-induced upregulation of glutamate transporters in rat primary astrocytes. Glia 62: 1270–1283, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.