Abstract
The transient receptor potential melastatin type 6 (TRPM6) epithelial Mg2+ channels participate in transcellular Mg2+ transport in the kidney and intestine. Previous reports suggested a hormonal cAMP-dependent regulation of Mg2+ reabsorption in the kidney. The molecular details of this process are, however, unknown. Adenylate cyclase 3 (Adcy3) has been shown to colocalize with the Na+/Cl− cotransporter, a marker of the distal convoluted segment of the kidney, the principal site of TRPM6 expression. Given the critical role of TRPM6 in Mg2+ reabsorption, an inducible kidney-specific Adcy3 deletion mouse model was characterized for blood and urinary electrolyte disturbances under a normal—and low—Mg2+ diet. Increased urinary Mg2+ wasting and Trpm6 mRNA levels were observed in the urine and kidney of Adcy3-deleted animals compared with wild-type controls. Serum Mg2+ concentration was significantly lower in Adcy3-deleted animals at day 7 on the low Mg2+ diet. Using patch clamp electrophysiology, cell surface biotinylation, and total internal reflection fluorescence live cell imaging of transfected HEK293 cells, we demonstrated that cAMP signaling rapidly potentiates TRPM6 activity by promoting TRPM6 accumulation at the plasma membrane and increasing its single-channel conductance. Comparison of electrophysiological data from cells expressing the phosphorylation-deficient S1252A or phosphomimetic S1252D TRPM6 mutants suggests that phosphorylation at this intracellular residue participates in the observed stimulation of channel activity. Altogether, these data support a physiologically relevant magnesiotropic role of cAMP signaling in the kidney by a direct stimulatory action of protein kinase A on the plasma membrane trafficking and function of TRPM6 ion channels.
Keywords: cell and transport physiology, cell signaling, cAMP, electrophysiology, magnesium, ion channel
Magnesium (Mg2+) participates in the regulation of numerous physiologic processes, including acting as a cofactor in multiple enzymatic reactions, participating in maintaining cardiac rhythm, ion homeostasis, neurologic function, and neuromuscular activity.1,2 Mg2+ homeostasis is achieved by the concerted action of intestinal absorption, bone storage, and kidney extrusion.1,3 In the kidney, the majority of Mg2+ is passively reabsorbed along the thick ascending limb (TAL) of the nephron. The last step of Mg2+ reabsorption occurs in the distal convoluted tubule (DCT) of the nephron,1,3 in which the apically expressed Mg2+-permeant transient receptor potential melastatin type 6 (TRPM6) channels mediate the initial rate-limiting step in transcellular transport of Mg2+.4, 5 Loss-of-function mutations in TRPM6 are the primary cause of inherited hypomagnesemia with secondary hypocalcemia.6–8 Inactivation of TRPM6 in mice is lethal, whereas heterozygous deletion of Trpm6 or chemical damage to the DCT is associated with hypomagnesemia.9–11 In addition to TRPM6, various transporters and channels are proposed to either directly mediate Mg2+ reabsorption or indirectly stimulate it by maintaining a favorable electrochemical gradient.1,3,12,13
Micromolar levels of extracellular divalents inhibit TRPM6 currents.4,14,15 Intracellular Mg2+ reduces TRPM6 activity, with reported affinities between 0.02 and 1 mM.4,16,17 In addition, TRPM6 is modulated by intracellular and extracellular pH,15,18 halides,19 oxidation,20 phosphatidylinositol 4,5-bisphosphate (PIP2),21 flavaglines,22 and sphingolipids.23 TRPM6 channels comprise a C-terminal serine/threonine α-kinase domain, the function of which and its link to the channel activity is incompletely understood.4,6,16,17,19,24 TRPM6 undergoes autophosphorylation,24,25 which leads to a shift in the inhibition by intracellular Mg2+/Mg2+ nucleotides to higher concentrations.17
In the kidneys, TRPM6 is regulated by the EGF,26–28 insulin,29 estrogens,30,31 purinergic signaling,32 dietary Mg2+ intake,30 and acid-base status.33 Previous studies have suggested increased DCT Mg2+ reabsorption in response to hormones such as parathyroid hormone (PTH), glucagon, calcitonin, and arginine vasopressin.34 From these, PTH has been shown to increase Ca2+ reabsorption in the DCT and connecting tubule by directly stimulating the open probability of the Ca2+ channel TRPV5 in a cAMP/protein kinase A (PKA)–dependent manner.35 These hormones bind Gαs-coupled receptors, which activate adenylate cyclases (ACs), leading to an increase in intracellular cAMP, which then activates PKA. Mammalian organisms express nine different membrane-bound ACs and one soluble adenylate cyclase (sAC).36 Forskolin (FSK) activates all membrane-bound ACs but does not affect sAC. Within the nephron, seven of nine ACs are expressed (AC2–AC7 and AC9) with different patterns of expression in distinct tubular segments.36 In the DCT, AC2–AC4, AC6, and AC9 expression has been demonstrated at the mRNA and protein levels.36 There, AC3 colocalizes with the Na+/Cl− cotransporter (NCC) at the apical membrane, suggesting proper subcellular localization for putative modulation of TRPM6, with NCC being a marker of DCT, the principal site of TRPM6 expression.4,36,37
Here, the effects of cAMP signaling on the function of TRPM6 are investigated using in vivo inducible nephron-specific genetic deletion of Adcy3 (AC3−/−), patch clamp electrophysiology, cell surface biotinylation, and total internal reflection fluorescence (TIRF) imaging. It was demonstrated that cAMP signaling potentiates the activity and plasma membrane expression of TRPM6 and that AC3−/− mice waste Mg2+ in urine, an effect that is partially compensated by an increase in renal TRPM6 expression.
Results
Urinary Mg2+ Wasting in an Inducible Nephron-Specific Adcy3 Knockout Mice Model
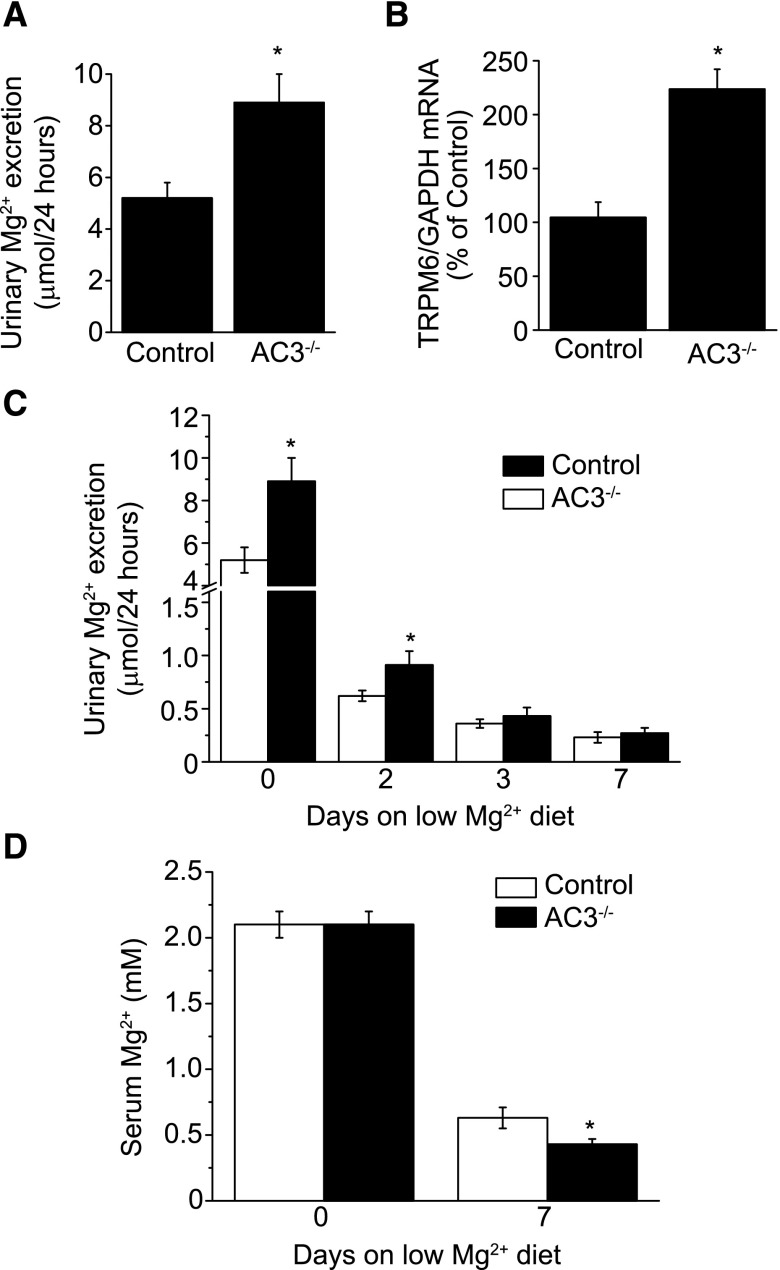
A doxycycline-inducible nephron-specific AC3 knockout mice (AC3−/−) model38,39 was assessed for electrolyte disturbances (Supplemental Figure 1, Supplemental Methods). Nephron-specific Adcy3 recombination was achieved in all tubular segments by the expression of the reverse tetracycline repressor (rTetR) under the promoter of Pax8,38,40,41 a procedure that does not affect the normal histology of the kidney, Na+ and water handling, and BP.38 AC3−/− mice were housed in metabolic cages and urine was collected every 24 hours for 3 days. Food and fluid intakes were similar between controls and AC3−/− mice (Table 1). Urine volume and urinary excretion of Na+, K+, and Ca2+ were comparable between the two groups. By contrast, urinary Mg2+ excretion was significantly increased by approximately 1.7-fold in AC3−/− mice compared with controls (Figure 1A, Table 1). Plasma concentrations of Mg2+ were not different between controls and AC3−/− mice (Table 1). Renal levels of TRPM6 mRNA were significantly increased by approximately 2-fold in AC3−/− mice compared with controls (Figure 1B).
Table 1.
Effect of nephron-specific AC3 knockout on food and water intake, renal fluid, and electrolyte excretion
| Parameters | Control | Nephron-Specific AC3−/− |
|---|---|---|
| Food intake, g/d | 5.0±0.2 | 4.8±0.6 |
| Water intake, ml/d | 3.8±0.5 | 3.7±0.3 |
| Urine volume, ml/d | 1.3±0.2 | 1.4±0.2 |
| UMgV, µmol/d | 5.2±0.6 | 8.9±1.1a |
| UCaV, µmol/d | 1.4±0.3 | 1.9±0.3 |
| UNaV, µmol/d | 286±26 | 258±20 |
| UKV, µmol/d | 262±20 | 289±25 |
| Plasma [Mg2+], mM | 2.1±0.1 | 2.1±0.1 |
Ux is the fractional excretion of the electrolyte X, and plasma Mg2+ is the concentration in control and nephron-specific AC3−/− mice. n=16–17 per data point.
P<0.05 versus control.
Figure 1.
Characterization of the doxycycline-inducible whole nephron Adcy3 (AC3) knockout mice. (A) Twenty-four–hour urine collection reveals a significant increase in urinary Mg2+ excretion in AC3−/− mice (n=16–17). (B) TRPM6 mRNA expression is increased in total cortex preparation from AC3−/− mice compared with controls (n=5–6). (C) Mg2+ excretion is significantly increased in AC3−/− mice at days 0 and 2 compared with the wild type (n=10–22). (D) Serum Mg2+ concentration is lower in AC3−/− at day 7 (n=6). *P<0.05 versus control. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
To assess the specific renal contribution in Mg2+ wasting, mice were fed a low Mg2+ diet for 7 days. Increased Mg2+ excretion persisted for 2 days from the start of the low Mg2+ diet in AC3−/− mice (Figure 1C). Although no differences in urinary Mg2+ excretion were observed at days 3 and 7, AC3−/− had significantly lower serum Mg2+ values at day 7 (Figure 1D). Altogether these data suggest that AC3−/− presents a renal Mg2+ leak that is at least in part compensated by an increase in renal TRPM6 expression.
cAMP Signaling Stimulates the Activity of TRPM6
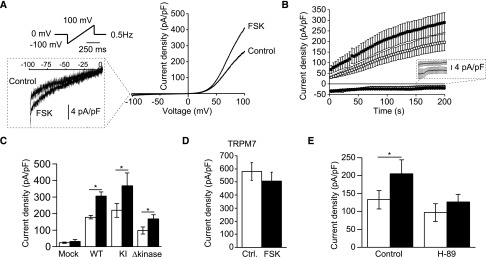
Human embryonic kidney 293 (HEK293) cells were transfected with a human TRPM6 construct and subjected to whole-cell patch clamp, as previously described.4 Currents were elicited by a series of voltage ramps applied every 2 seconds from a holding voltage of 0 mV (Figure 2A). Large slowly developing outwardly rectifying currents were observed in response to this ramp protocol (Figure 2, A and B). To assess the putative modulation of TRPM6 by cAMP signaling, cells were preincubated with the AC activator FSK (10 μM, 15 minutes) or vehicle (EtOH, 0.01% vol/vol, 15 minutes). This treatment significantly increased the maximal current density (measured at +80 mV, 200 seconds after the establishment of the whole-cell configuration) by approximately 1.6-fold without affecting the characteristic shape of the IV curve or the time-dependent current development (Figure 2, A–C). HEK293 cells express low levels of endogenous TRPM7-mediated Mg2+-nucleotide–inhibited (MagNuM/MIC) currents.4 Here, these currents were small (<30 pA/pF) and not sensitive to FSK (mock; Figure 2C). To assess the involvement of the intrinsic α-kinase domain of TRPM6 in the FSK-evoked potentiation, cells were transfected with the previously described kinase-truncated (L1749X, Δkinase) or kinase-inactive (K1804R) TRPM6 constructs.16 Although the first construct lacks the complete kinase domain, the kinase-inactive construct is mutated at a single amino acid residue, leading to a loss in phosphotransferase activity. Here, a similar increase in current density was observed for the kinase-inactive and Δkinase mutants upon treatment with FSK (Figure 2C). In agreement with the lack of FSK sensitivity of the endogenous currents, cells transfected with TRPM7 yielded currents that were insensitive to FSK (Figure 2D). To assess the specific contribution of PKA to the FSK-mediated potentiation of TRPM6 currents, cells were pretreated with the PKA inhibitor H-89 (10 μM, 20 minutes) or vehicle (DMSO, 0.1% vol/vol, 20 minutes) before the treatment with FSK (10 μM, 15 minutes) or vehicle (EtOH, 0.01% vol/vol, 15 minutes). This significantly prevented the potentiation of TRPM6 by FSK (Figure 2E).
Figure 2.
Activation of cAMP signaling specifically potentiates the activity of TRPM6 in a PKA-dependent manner. (A) TRPM6 currents are evoked by a series of 500-millisecond voltage ramp from −100 to +100 mV applied every 2 seconds from a holding potential of 0 mV (top left inset). Typical current-voltage curves measured 200 seconds after break-in are shown for a cell treated with FSK (10 μM, 15 minutes) or vehicle (control, 0.1% vol/vol EtOH, 15 minutes). (B) The average time course of TRPM6 current development is shown for current values measured at +80 mV (n=23, circles) and −80 mV (n=23, squares) in vehicle-treated (open symbols) and FSK-treated (filled symbols) conditions. (C) Incubation of cells with FSK (filled bars) significantly increases current densities at +80 mV 200 seconds after break-in in wild-type TRPM6 (n≥84), kinase-inactive (K1804R, n≥17), and kinase-truncated mutant (Δkinase, n≥14) cells, but not in mock-transfected cells (n=6). (D) TRPM7 currents are not sensitive to the treatment with FSK (filled bar, n=12). (E) Preincubation with the PKA inhibitor H-89 (10 μM, 20 minutes) abolishes the effects of FSK (filled bars) on wild-type TRPM6 (n≥16). *P<0.05 versus control. Ctrl, control; KI, kinase-inactive; WT, wild type.
PKA Rapidly Increases the Plasma Membrane Expression of TRPM6
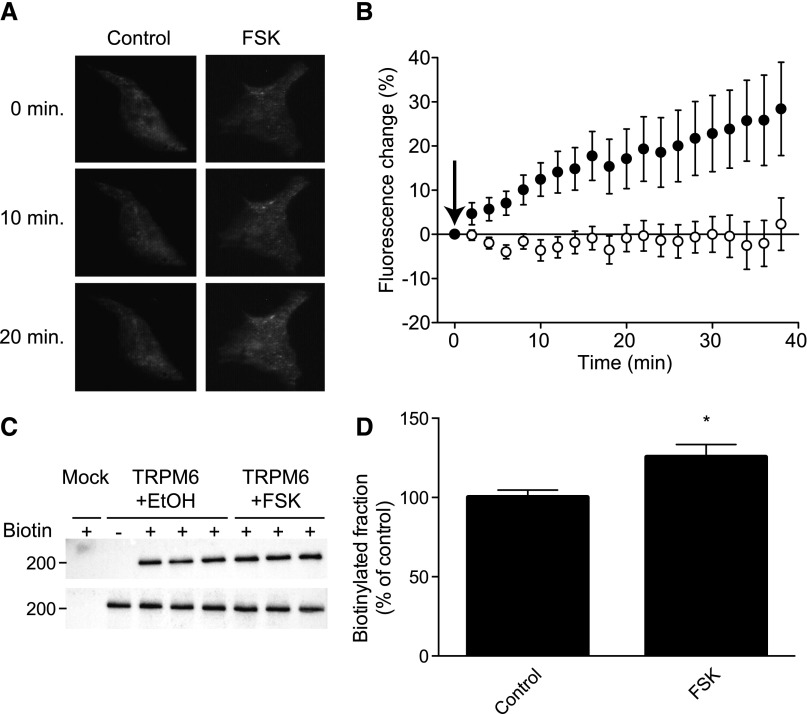
The membrane insertion of green fluorescent protein (GFP)-TRPM6 fusion channels was monitored over time by TIRF microscopy. After the establishment of a 15-minute stable fluorescence baseline, cells were perfused with FSK (10 μM) or vehicle (EtOH, 0.01% vol/vol). Perfusion of FSK induced a time-dependent increase in punctate GFP fluorescence at the cell surface, whereas vehicle did not induce such response (Figure 3, A and B).
Figure 3.
cAMP signaling induces an increase in TRPM6 surface expression. (A) Typical TIRF live cell microscopy frames from cells expressing a TRPM6-GFP fusion construct at 0, 10, and 20 minutes after the establishment of a stable baseline. Left and right panels show typical change in GFP fluorescence observed in a cell perfused with vehicle (EtOH, 0.1% vol/vol) or FSK (10 μM), respectively. (B) The average TIRF fluorescence is plotted over time for cells treated with vehicle (EtOH, 0.1% vol/vol, n=10, open symbols) or FSK (10 μM, n=9, filled symbols). The arrow indicates the start of compound perfusion. (C and D) Cell surface biotinylation of HEK293 cells expressing TRPM6 and treated with vehicle (EtOH, 0.1% vol/vol, 15 minutes) or FSK (10 µM, 15 minutes). (C) A representative immunoblot showing increased TRPM6 expression at the cell surface (upper blot) and an expression control (lower blot) are shown. (D) Quantification by blot intensities is shown corrected for total TRPM6 expression (n=17). *P<0.05 versus vehicle-treated cells.
To further validate the observed increase in cell surface expression of TRPM6, cell surface biotinylation was used to assess the changes in the number of channels on the plasma membrane. Using this technique, an approximately 25% increase in surface TRPM6 was observed upon FSK treatment (10 μM, 15 minutes) compared with control (EtOH, 0.01% vol/vol; Figure 3, C and D). Together, these results suggest that FSK induces a rapid (<15 minutes) increase in cell surface expression of TRPM6.
cAMP Increases the Conductance of TRPM6 Channels in Cell-Attached Mode
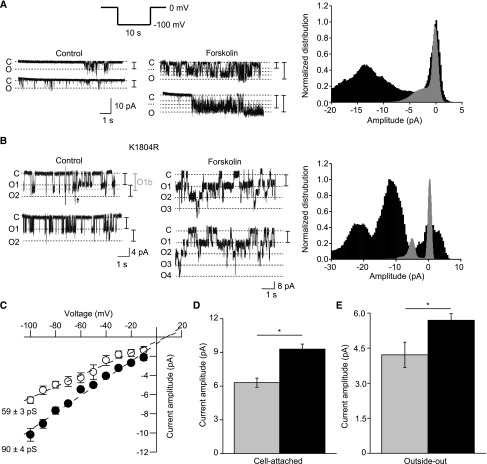
Cells expressing TRPM6 were subjected to cell-attached patch clamp recordings. Inward Na+ currents were recorded in response to a series of 5- to 10-second −100-mV voltage pulses applied from a holding voltage of 0 mV (Figure 4A). Using this protocol, measurable step-like and flickering single-channel activity was frequently detected in TRPM6-transfected but not in mock-transfected cells (data not shown). In control conditions (EtOH, 0.1% vol/vol, 15 minutes) openings were of short duration and had maximal amplitudes of approximately 4–5 pA (Figure 4A). Preincubation with FSK (10 μM, 15 minutes) induced the appearance of channel openings going through what appears like subconductance states (Figure 4A). The transient and complex nature of these events, occurring as bursts of channel activity, did not allow for the accurate quantification of the channel open probability. The incubation with FSK increased the frequency of rare and short-lived small endogenous currents (approximately 2 pA at −100 mV, not shown).
Figure 4.
cAMP signaling increases the maximal single-channel current amplitude of wild-type and kinase inactive mutant in cell-attached mode and in outside-out excised patches. (A) Cell-attached recordings are performed using the same divalent-free pipette solution used in whole-cell recordings. Inward Na+ currents are evoked by a series of 100-mV pulses from a holding potential of 0 mV (top left inset). Typical recording from cells expressing wild-type TRPM6 and treated with vehicle (control, EtOH, 0.1% vol/vol, 15 minutes) or with FSK (10 μM, 15 minutes). C indicates the closed state and O indicates the open state. Intermediate conductance levels are illustrated with a dashed line. The normalized amplitude histograms of the full recordings in vehicle-treated (gray histogram) and FSK-treated (black histogram) conditions are shown in the right panel. (B) Cells expressing the kinase inactive (K1804R) TRPM6 mutant are subjected to the same protocol. A recording from a patch of membrane containing two channels is shown on the left panel. A transition to a different conductance state is observed in the depicted recording (O1b to O1 transition, indicated by an arrow). Ox indicates the different channels in this recording. FSK significantly increases the single-channel current amplitude at −100 mV. The normalized amplitude histograms of the full recordings in control (gray histogram) and FSK-treated (black histogram) conditions are shown in the right panel. (C) FSK (filled symbols) significantly increases the single-channel conductance of the kinase inactive TRPM6 mutant in cell-attached recordings (n=3–7 per data point). Conductances are obtained by fitting a linear equation to the averaged data points. (D) Average cell-attached single-channel amplitude in vehicle-treated (gray bar) or FSK-treated (black bar) conditions (n≥19). (E) Average outside-out single-channel amplitude in vehicle-treated (gray bar) or FSK-treated (black bar) conditions (n=5). *P<0.05.
Next, cells expressing the kinase-inactive TRPM6 mutant were subjected to the same protocol. Single-channel cell-attached patches with stable activity were more frequently obtained from cells expressing this mutant. Single-channel activity with transitions to apparent subconductance states openings were observed (Figure 4B, left panel), although less frequently, in FSK-treated cells (Figure 4B). Like wild-type TRPM6, treatment of the kinase-inactive TRPM6 mutant with FSK increased the amplitude of channel openings (Figure 4, B–D). The single-channel conductance of FSK-treated cells was significantly higher than that of vehicle-treated cells (Figure 4C). The reversal potential, obtained by linear regression of the current-voltage curve, was similar between the various conditions. In a subset of cells, the patch was excised and the channel activity investigated in the outside-out configuration. The difference between control- and FSK-treated cells was preserved but reduced (IFSK/Icontrol=1.35±0.17, n=5; P<0.05) for vehicle- and FSK-treated cells (Figure 4E).
PKA Directly Phosphorylates TRPM6 in its C-Terminal Part
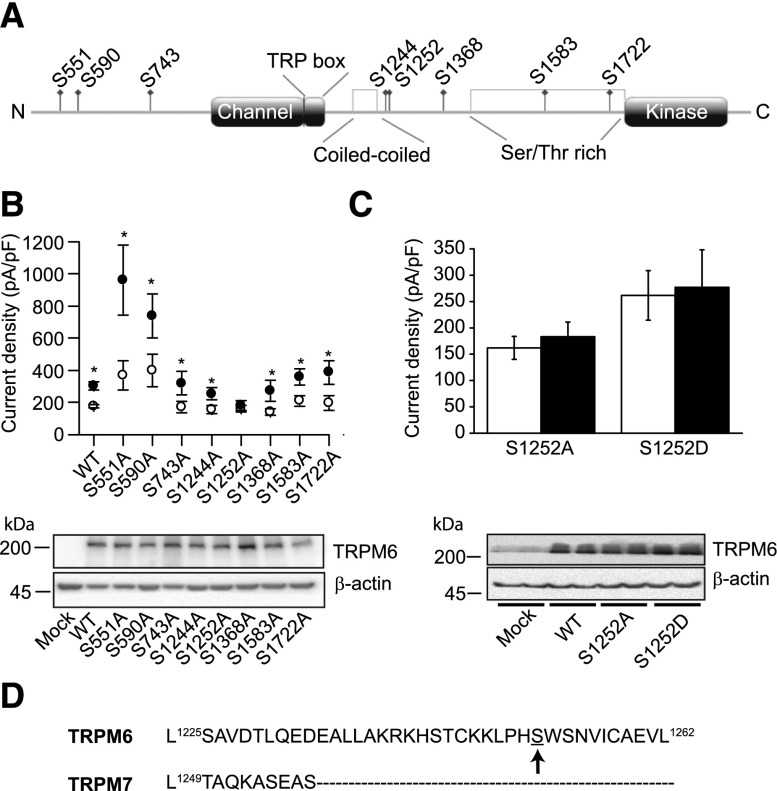
Putative PKA phosphorylation sites were predicted using bioinformatics servers (Supplemental Methods), an approach previously successfully applied to identify a PKA-dependent regulatory site of TRPV5.35 Eight potential intracellular PKA phosphorylation sites were identified (Figure 5A). These sites were individually mutated to alanine in order to produce phosphorylation deficient residues. Seven of the eight tested mutants responded to FSK (10 μM, 15 minutes) in a manner comparable to the wild type (Figure 5B). By contrast, S1252A failed to react to FSK stimulation (Figure 5, B and C). The phosphomimetic aspartate (D) mutant of S1252 was generated and assessed for FSK responsiveness using an identical protocol. This mutant was not stimulated by FSK (Figure 5C). All mutants showed comparable expression levels (Figure 5, B and C). Interestingly, alignment of TRPM7 sequence on TRPM6 revealed that S1252 is located in a unique region that has no equivalent in TRPM7 (Figure 5D).
Figure 5.
The potentiation of TRPM6 by cAMP signaling depends on a serine residue in the C-terminal tail. (A) Localization of the predicted PKA phosphorylation sites along TRPM6. (B) The predicted PKA phosphorylation sites are individually mutated to alanine mutants and tested for their sensitivity to FSK. All mutants except S1252A responded to the treatment with FSK (10 μM, 15 minutes, full symbols, n≥10 per mutants and conditions). The bottom panel illustrates the expression of the tested mutants. (C) Average whole-cell S1252A and S1252D current amplitudes with FSK (10 μM, 15 minutes, n≥11, full bars) or vehicle (EtOH, 0.1% vol/vol, 15 minutes, n≥11, open bars). Both mutants exhibited normal expression (bottom panel). (D) The TRPM6 segment containing serine 1252 does not align with TRPM7. The TRPM6 residue S1252 is indicated by an arrow. *P<0.05. WT, wild type.
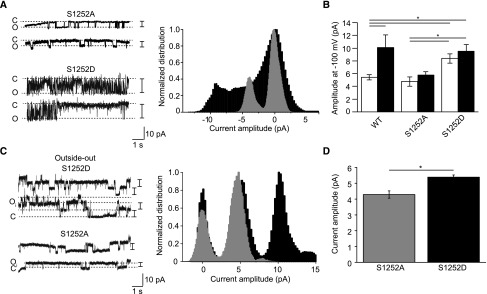
Single-channel recordings of the S1252A and S1252D mutants were obtained using cell-attached patch clamp. The average amplitude of openings of unstimulated S1252D mutant channels was approximately 1.6-fold higher than that of S1252A and wild-type TRPM6 channels (Figure 6, A and B). Importantly, the S1252A and S1252D single-channel current amplitudes were not affected by the FSK treatment (Figure 6B). The average opening amplitudes of the S1252D mutant in control and FSK-treated conditions were not statistically different from wild-type TRPM6 treated with FSK (P>0.20; Figure 6B). The difference in current amplitudes between S1252A and S1252D were smaller in outside-out single-channel recording mode (IS1252D/IS1252A=1.25±0.07; Figure 6, C and D).
Figure 6.
Microscopic behavior of the S1252A and S1252D mutants. (A) Typical single-channel cell-attached recordings obtained from S1252A- and S1252D-expressing cells. The normalized amplitude histograms of the full recordings in vehicle-treated (EtOH, 0.1% vol/vol, 15 minutes, gray histogram) and FSK-treated (10 μM, 15 minutes, black histogram) conditions are shown in the right panel. (B) The maximal single-channel current amplitude of S1252D is significantly higher than that of S1252A and wild-type TRPM6 channels. Single-channel current amplitude of both S1252A and S1252D were insensitive to the treatment with FSK (10 μM, 15 minutes, black bars, n≥5 per condition). (C) Outside-out excised single-channel recordings were obtained from cells expressing the S1252A or S1252D mutants. The normalized amplitude histograms of the full recordings in vehicle-treated (gray histogram) and FSK-treated (black histogram) conditions are shown in the right panel. (D) The single-channel amplitude of S1252D (black bar) is significantly higher than that of S1252A (gray bar, n≥10). *P<0.05.
Discussion
Our study demonstrated that inducible nephron-specific deletion of AC3 leads to urinary Mg2+ wasting and TRPM6 mRNA upregulation in the kidney. Using in vitro functional approaches, it was shown that cAMP signaling potentiates the activity of TRPM6 in a PKA-dependent manner. This potentiation is explained by a rapid increase in cell surface expression and conductance of the channels. The intracellular TRPM6 amino acid residue S1252 was identified as a likely target of PKA phosphorylation.
Using TIRF microscopy and cell surface biotinylation, an approximately 1.3-fold enrichment in cell surface expression of channels was detected within 15 minutes of FSK application. In parallel, outside-out excised plasma membrane patches revealed an approximately 1.3-fold stimulation in single-channel amplitude upon FSK treatment. Together, these observations quantitatively account for the approximately 1.6-fold FSK-evoked increase in whole-cell current density observed for wild-type and kinase-inactive TRPM6 mutants. These data suggest that the activation of PKA stimulates the activity of TRPM6 in a dual manner by increasing channel expression on the plasma membrane and the intrinsic channel conductance.
By assessing the FSK responsiveness of a series of phosphorylation-deficient serine to alanine point mutants, S1252 was identified as the target of PKA-dependent phosphorylation. S1252 is located in the cytoplasmic region between the membrane-embedded channel domain and the α-kinase moiety of TRPM6 (Figure 5A). Sequence alignment revealed that S1252 is not conserved in TRPM7 (Figure 5D), explaining the lack of FSK responsiveness of endogenous and overexpressed TRPM7 channels (Figure 2, C and D). Excluding channel trafficking effects, two scenarios can be considered to explain the PKA-mediated potentiation: (1) direct phosphorylation of S1252 by PKA induces a conformational change that affects the intrinsic properties of the channel, and (2) phosphorylation of S1252 prevents or facilitates the binding of intracellular factor(s) to TRPM6. Here, the modulation of wild-type TRPM6 by FSK was reduced upon excision of the patches from the plasma membrane, suggesting that the binding of an intracellular factor stabilizes the channel in a more conductive state. The regulation of TRPM6 activity by intracellular factors other than Mg2+ or Mg nucleotides has been demonstrated for RACK1 (GNB2L1),42 REA (PHB2),31 PIP2,21 and sphingolipids.23 RACK1 and REA require a functional α-kinase domain to mediate their inhibitory effects and are, therefore, excluded as candidates.31,42 Although the sphingolipid inhibitory regulatory site is unknown,23 PIP2 binds to the signature transient receptor potential (TRP) box (1085–1098) region of TRPM6 and potentiates its function.21 Modulation of gating by the interplay between PIP2 and the TRP box has been observed for other TRP channels.43 Based on the crystal structure of a C-terminal assembly domain of TRPM7, it was suggested that the 1174–1226 region of TRPM6 participates in a 4-fold coiled-coiled binding assembly.44 At the moment, the hypothesis that the phosphorylation status of S1252 influences the binding of PIP2 to the TRP box or disrupt the structure of the coiled-coiled interaction cannot be excluded. Alternatively, phosphorylation of S1252 could interfere with the binding of a cellular Ca2+/Mg2+ sensor such as calmodulin45 or an intracellular carrier-like calbindin protein.46 Evidence demonstrating direct regulation of TRPM6 by calmodulin or carrier-like calbindin proteins is, however, lacking and therefore remains highly hypothetical. Further experiments are needed to identify the link between the phosphorylation status of S1252 and channel function. Gαs-coupled receptor–dependent stimulation of Mg2+ transport has been previously observed in vivo and in immortalized renal cells.34 In addition to transcellular transport in the DCT, a substantial fraction (approximately 70%) of filtered Mg2+ is reabsorbed in the TAL by passive paracellular transport.3 One could hypothesize that the deletion of AC3 in the TAL affects paracellular Mg2+ reabsorption and leads to urinary Mg2+ wasting. AC3 is, however, not expressed in the TAL.36,47 Other AC isoforms (AC2, AC6, and AC9) are expressed in the DCT and might, therefore, compensate for AC3 loss.47 In that respect, the fact that AC3 is the only Ca2+-stimulated isoform in the distal part of the tubule36,48 might impart significant differences in its function there. Using micropuncture, microperfusion, and Mg2+ uptake in a DCT cell line, increased Mg2+ reabsorption has been observed in response to hormones such as PTH, glucagon, calcitonin, and arginine vasopressin.34 In addition, insulin has been reported to stimulate Mg2+ uptake in a DCT cell line.49 EGF and insulin increase the cell surface expression of TRPM6 via a PI3K-Akt-Rac1 signaling cascade.26,29 The effects of EGF and insulin are most likely mediated by a rapid insertion of a cytoplasmic pool of TRPM6-containing vesicles into the plasma membrane, a phenomenon previously described for the TRPC5 and TRPV1 channels.50,51 Here, it is observed that cAMP signaling potentiates the activity of the S1583A TRPM6 mutant, a channel that is not inserted in the membrane upon insulin stimulation.29 This suggests that PKA does not act along the previously identified stimulatory pathway of TPRM6 (PI3K-Akt-Rac1). In agreement with the current observations, other investigators have reported additive effects of PTH and insulin on Mg2+ transport.34 Additional work will be needed to identify the hormone(s) and receptors responsible for the cAMP-dependent stimulation of TRPM6 activity. In addition, further genetic work will be required to delineate the function of each AC in the context of transcellular transport.
Current in vivo and in vitro data suggest an AC3-dependent stimulation of TRPM6-mediated Mg2+ reabsorption in the DCT by direct PKA-dependent stimulation of channel activity and membrane expression. Phosphorylation of TRPM6 at residue S1252 was not necessary for channel activity, as evidenced by the functional S1252A mutant. AC3−/− mice fed a normal diet did not show differences in plasma Mg2+ values but had increased urinary Mg2+ wasting and higher TRPM6 mRNA levels in the kidney, suggesting an adaptive increase in intestinal Mg2+ absorption in kidney-specific AC3−/−. Given the low level of recombination of Adcy3 in the intestine with the current mouse model,39 an upregulation of putative native intestinal AC3 might be responsible for this effect. The involvement of putative AC3 and other ACs on intestinal Mg2+ absorption will, however, need further investigation to explain this observation. When fed a low Mg2+ diet, AC3−/− mice presented a urinary Mg2+ leak and persistent hypomagnesemia. Taken together, our data suggest a model in which AC3−/− mice are not able to stimulate Mg2+ reabsorption via a cAMP-dependent phosphorylation of TRPM6 residue S1252, leading to a reduction in DCT Mg2+ reabsorption. This potentially harmful Mg2+ wasting is, however, compensated by an increase in TRPM6 channel mRNA expression. Our results suggest that targeting cAMP signaling in the DCT might prove to be a treatment of drug-induced or inherited hypomagnesemia.
Concise Methods
Generation of Inducible Whole Nephron-Specific AC3 Knockout Mice
Mice transgenic for Pax8-rtTA and LC-1 transgenes were bred with mice containing loxP-flanked exons 4–6 of the Adcy3 gene.39 One-month-old male mice homozygous for the floxed Adcy3 gene and hemizygous for Pax8-rtTA and for LC-1 transgenes were given doxycycline (2 mg/ml, in 2% wt/vol sucrose drinking water) for 14 days. The complete procedure is described in the Supplemental Methods.
Metabolic Cage Studies and Electrolyte Measurement
Nephron AC3 knockout and control mice fed a normal Mg2+ diet (3 μmol/ml, LD101; LabDiet, Richmond, IN) were placed in metabolic cages for 3 days. Twenty-four–hour urine was collected each day. The urine of day 3 was analyzed for volume, Na+, K+, Mg2+, and Ca2+ concentration. Mice fed a low Mg2+ diet (negligible Mg2+ concentration, TD.93106; Harlan Laboratories, Madison, WI) were placed in metabolic cages for 7 days. Blood was collected at days 0 and 7 and analyzed for Mg2+ content. Urine collected at days 0, 2, 3, and 7 were similarly analyzed for Mg2+ content. Blood was taken from the heart and was used to analyze plasma Mg2+ concentration. Electrolyte concentrations were determined in the clinical laboratory at Associated Regional University Pathologists.
Cell Culture and Transfection
HEK293 cells were transfected with HA-tagged TRPM64 or a TRPM6-GFP fusion construct29 (1 μg/well in 12-well plates). Mutations were introduced in the wild-type HA-tagged TRPM6 construct using the QuikChange site-directed mutagenesis kit (Agilent, Amstelveen, The Netherlands). Approximately 36 hours after transfection, cells were seeded on glass coverslips coated with 50 μg/ml fibronectin (Roche, Mannheim, Germany).
Immunoblotting and Cell Surface Biotinylation
For cell surface biotinylation experiments, HEK293 cells were transfected and cell surfaces were biotinylated for 30 minutes at 4°C 48 hours later by adding sulfo-NHS-LC-LC-biotin (Pierce, Rockford, IL). Subsequently, protein lysates were incubated overnight with neutravidin-agarose beads (Pierce) to isolate cell surface biotinylated proteins. Western blot membranes were incubated overnight with either mouse anti–HA-tag antibody (6E2, 1:5000; Bioké, Leiden, The Netherlands) or mouse anti–β-actin antibody (1:5000; Sigma-Aldrich). The next day, membranes were incubated with a mouse peroxidase-coupled secondary antibody (1:10,000; Jackson ImmunoResearch, Suffolk, UK). Proteins were revealed using the SuperSignal West Pico and Femto chemiluminescence kits (Thermo Fisher Scientific, Waltham, MA).
Electrophysiology
Whole-cell recordings were acquired with a low-pass filter set at 2.9 kHz, whereas single-channel recordings had a low-pass filter set at 5 kHz. Whole-cell patch clamp pipettes were pulled from thin wall borosilicate glass (Harvard Apparatus, March-Hugstetten, Germany) and had resistance between 1 and 3 MΩ when filled with the pipette solution. Single-channel patch clamp pipettes were made of thick wall borosilicate glass (Harvard Apparatus) and had resistance between 8 and 11 MΩ when filled with the pipette solution. Current densities were obtained by normalizing the current amplitude (obtained at +80 mV 200 seconds after break-in) to the cell capacitance.
Solutions and Compound Application
The extracellular solution contained 150 mM NaCl, 1 mM CaCl2, and 10 mM HEPES/NaOH, pH 7.4.4 The pipette solution was made of 150 mM NaCl, 10 mM Na2EDTA, and 10 mM HEPES/NaOH, pH 7.2.4 Unless otherwise mentioned, cells were preincubated 15 minutes at 37°C in bath solution containing the compound of interest or vehicle (0.1% vol/vol DMSO and/or 0.1% vol/vol EtOH).
TIRF Microscopy
A microscope with 63×1.45 oil immersion objective (Olympus) was used to image GFP-labeled TRPM6 channels. Baseline fluorescence intensity from the cells was established for 15 minutes before switching to a solution-containing vehicle (EtOH, 0.1% vol/vol) or FSK (10 μM). Detailed methods can be obtained from a previous published research article.29
Statistical Analyses
All results are depicted as the mean±SEM. Direct comparisons of means between conditions were performed using the t test. When multiple groups were compared, one-way ANOVA and Fisher’s post hoc tests were used to assess statistical significance. Difference with P values <0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Michael Thomassen for technical support and Drs. Sjoerd Verkaart and Jenny van der Wijst for comments on this manuscript and for valuable discussions.
This work was supported by grants from the Netherlands Organization for Scientific Research (Grant ALW 818.02.001 and Vici Grant 016.130.668 to J.G.J.H.) and EURenOmics funding from the European Union Seventh Framework Program (FP7/2007–2013, Agreement Number 305608).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2014121228/-/DCSupplemental.
References
- 1.de Baaij JH, Hoenderop JG, Bindels RJ: Magnesium in man: Implications for health and disease. Physiol Rev 95: 1–46, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Jahnen-Dechent W, Ketteler M: Magnesium basics. Clinical Kidney Journal 5: i3–i14, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Baaij JHF, Hoenderop JGJ, Bindels RJM: Regulation of magnesium balance: Lessons learned from human genetic disease. Clinical Kidney Journal 5[Suppl 1]: i15–i24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG: TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem 279: 19–25, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Dimke H, Hoenderop JG, Bindels RJ: Molecular basis of epithelial Ca2+ and Mg2+ transport: Insights from the TRP channel family. J Physiol 589: 1535–1542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlingmann KP, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, Klingel K, Kratz M, Haddad E, Ristoff E, Dinour D, Syrrou M, Nielsen S, Sassen M, Waldegger S, Seyberth HW, Konrad M: Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet 31: 166–170, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, Borochowitz Z, Boettger MB, Beck GE, Englehardt RK, Carmi R, Sheffield VC: Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet 31: 171–174, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Lainez S, Schlingmann KP, van der Wijst J, Dworniczak B, van Zeeland F, Konrad M, Bindels RJ, Hoenderop JG: New TRPM6 missense mutations linked to hypomagnesemia with secondary hypocalcemia. Eur J Hum Genet 22: 497–504, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walder RY, Yang B, Stokes JB, Kirby PA, Cao X, Shi P, Searby CC, Husted RF, Sheffield VC: Mice defective in Trpm6 show embryonic mortality and neural tube defects. Hum Mol Genet 18: 4367–4375, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woudenberg-Vrenken TE, Sukinta A, van der Kemp AW, Bindels RJ, Hoenderop JG: Transient receptor potential melastatin 6 knockout mice are lethal whereas heterozygous deletion results in mild hypomagnesemia. Nephron, Physiol 117: 11–19, 2011 [DOI] [PubMed] [Google Scholar]
- 11.van Angelen AA, Glaudemans B, van der Kemp AW, Hoenderop JG, Bindels RJ: Cisplatin-induced injury of the renal distal convoluted tubule is associated with hypomagnesaemia in mice. Nephrol Dial Transplant 28: 879–889, 2013 [DOI] [PubMed] [Google Scholar]
- 12.de Baaij JH, Groot Koerkamp MJ, Lavrijsen M, van Zeeland F, Meijer H, Holstege FC, Bindels RJ, Hoenderop JG: Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg(2+) handling. Am J Physiol Renal Physiol 305: F1563–F1573, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Ferrè S, Hoenderop JG, Bindels RJ: Insight into renal Mg2+ transporters. Curr Opin Nephrol Hypertens 20: 169–176, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Topala CN, Groenestege WT, Thébault S, van den Berg D, Nilius B, Hoenderop JG, Bindels RJ: Molecular determinants of permeation through the cation channel TRPM6. Cell Calcium 41: 513–523, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Li M, Du J, Jiang J, Ratzan W, Su LT, Runnels LW, Yue L: Molecular determinants of Mg2+ and Ca2+ permeability and pH sensitivity in TRPM6 and TRPM7. J Biol Chem 282: 25817–25830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thébault S, Cao G, Venselaar H, Xi Q, Bindels RJ, Hoenderop JG: Role of the alpha-kinase domain in transient receptor potential melastatin 6 channel and regulation by intracellular ATP. J Biol Chem 283: 19999–20007, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Yu H, Huang J, Faouzi M, Schmitz C, Penner R, Fleig A: The TRPM6 kinase domain determines the Mg·ATP sensitivity of TRPM7/M6 heteromeric ion channels. J Biol Chem 289: 5217–5227, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Jiang J, Yue L: Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol 127: 525–537, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Zhang Z, Lis A, Penner R, Fleig A: TRPM7 is regulated by halides through its kinase domain. Cell Mol Life Sci 70: 2757–2771, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao G, Lee KP, van der Wijst J, de Graaf M, van der Kemp A, Bindels RJ, Hoenderop JG: Methionine sulfoxide reductase B1 (MsrB1) recovers TRPM6 channel activity during oxidative stress. J Biol Chem 285: 26081–26087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie J, Sun B, Du J, Yang W, Chen HC, Overton JD, Runnels LW, Yue L: Phosphatidylinositol 4,5-bisphosphate (PIP(2)) controls magnesium gatekeeper TRPM6 activity. Sci Rep 1: 146, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blanchard MG, de Baaij JH, Verkaart SA, Lameris AL, Basmadjian C, Zhao Q, Désaubry L, Bindels RJ, Hoenderop JG: Flavaglines stimulate transient receptor potential melastatin type 6 (TRPM6) channel activity. PLoS One 10: e0119028, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin X, Yue Z, Sun B, Yang W, Xie J, Ni E, Feng Y, Mahmood R, Zhang Y, Yue L: Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels. Br J Pharmacol 168: 1294–1312, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Wijst J, Blanchard MG, Woodroof HI, Macartney TJ, Gourlay R, Hoenderop JG, Bindels RJ, Alessi DR: Kinase and channel activity of TRPM6 are co-ordinated by a dimerization motif and pocket interaction. Biochem J 460: 165–175, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E, van Leeuwen FN: Massive autophosphorylation of the Ser/Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS One 3: e1876, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thebault S, Alexander RT, Tiel Groenestege WM, Hoenderop JG, Bindels RJ: EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol 20: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groenestege WM, Thébault S, van der Wijst J, van den Berg D, Janssen R, Tejpar S, van den Heuvel LP, van Cutsem E, Hoenderop JG, Knoers NV, Bindels RJ: Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest 117: 2260–2267, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dimke H, van der Wijst J, Alexander TR, Meijer IM, Mulder GM, van Goor H, Tejpar S, Hoenderop JG, Bindels RJ: Effects of the EGFR inhibitor erlotinib on magnesium handling. J Am Soc Nephrol 21: 1309–1316, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair AV, Hocher B, Verkaart S, van Zeeland F, Pfab T, Slowinski T, Chen YP, Schlingmann KP, Schaller A, Gallati S, Bindels RJ, Konrad M, Hoenderop JG: Loss of insulin-induced activation of TRPM6 magnesium channels results in impaired glucose tolerance during pregnancy. Proc Natl Acad Sci U S A 109: 11324–11329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groenestege WM, Hoenderop JG, van den Heuvel L, Knoers N, Bindels RJ: The epithelial Mg2+ channel transient receptor potential melastatin 6 is regulated by dietary Mg2+ content and estrogens. J Am Soc Nephrol 17: 1035–1043, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Cao G, van der Wijst J, van der Kemp A, van Zeeland F, Bindels RJ, Hoenderop JG: Regulation of the epithelial Mg2+ channel TRPM6 by estrogen and the associated repressor protein of estrogen receptor activity (REA). J Biol Chem 284: 14788–14795, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Baaij JH, Blanchard MG, Lavrijsen M, Leipziger J, Bindels RJ, Hoenderop JG: P2X4 receptor regulation of transient receptor potential melastatin type 6 (TRPM6) Mg2+ channels. Pflugers Arch 466: 1941–1952, 2014 [DOI] [PubMed] [Google Scholar]
- 33.Nijenhuis T, Renkema KY, Hoenderop JG, Bindels RJ: Acid-base status determines the renal expression of Ca2+ and Mg2+ transport proteins. J Am Soc Nephrol 17: 617–626, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Dai LJ, Ritchie G, Kerstan D, Kang HS, Cole DE, Quamme GA: Magnesium transport in the renal distal convoluted tubule. Physiol Rev 81: 51–84, 2001 [DOI] [PubMed] [Google Scholar]
- 35.de Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJ, Hoenderop JG: Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693–1704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rieg T, Kohan DE: Regulation of nephron water and electrolyte transport by adenylyl cyclases. Am J Physiol Renal Physiol 306: F701–F709, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ: Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci U S A 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kittikulsuth W, Stuart D, Van Hoek AN, Kohan DE: Lack of an effect of nephron-specific deletion of adenylyl cyclase 3 on renal sodium and water excretion or arterial pressure. Physiol Rep 3: e12316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kittikulsuth W, Stuart D, Van Hoek AN, Stockand JD, Bugaj V, Mironova E, Blount MA, Kohan DE: Lack of an effect of collecting duct-specific deletion of adenylyl cyclase 3 on renal Na+ and water excretion or arterial pressure. Am J Physiol Renal Physiol 306: F597–F607, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stuart D, Rees S, Woodward SK, Koesters R, Strait KA, Kohan DE: Disruption of the endothelin A receptor in the nephron causes mild fluid volume expansion. BMC Nephrol 13: 166, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Traykova-Brauch M, Schönig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Gröne HJ, Koesters R: An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med 14: 979–984, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao G, Thébault S, van der Wijst J, van der Kemp A, Lasonder E, Bindels RJ, Hoenderop JG: RACK1 inhibits TRPM6 activity via phosphorylation of the fused alpha-kinase domain. Curr Biol 18: 168–176, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Nilius B, Mahieu F, Karashima Y, Voets T: Regulation of TRP channels: A voltage-lipid connection. Biochem Soc Trans 35: 105–108, 2007 [DOI] [PubMed] [Google Scholar]
- 44.Fujiwara Y, Minor DL, Jr: X-ray crystal structure of a TRPM assembly domain reveals an antiparallel four-stranded coiled-coil. J Mol Biol 383: 854–870, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Groot T, Kovalevskaya NV, Verkaart S, Schilderink N, Felici M, van der Hagen EA, Bindels RJ, Vuister GW, Hoenderop JG: Molecular mechanisms of calmodulin action on TRPV5 and modulation by parathyroid hormone. Mol Cell Biol 31: 2845–2853, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lambers TT, Mahieu F, Oancea E, Hoofd L, de Lange F, Mensenkamp AR, Voets T, Nilius B, Clapham DE, Hoenderop JG, Bindels RJ: Calbindin-D28K dynamically controls TRPV5-mediated Ca2+ transport. EMBO J 25: 2978–2988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bek MJ, Zheng S, Xu J, Yamaguchi I, Asico LD, Sun XG, Jose PA: Differential expression of adenylyl cyclases in the rat nephron. Kidney Int 60: 890–899, 2001 [DOI] [PubMed] [Google Scholar]
- 48.Sadana R, Dessauer CW: Physiological roles for G protein-regulated adenylyl cyclase isoforms: Insights from knockout and overexpression studies. Neurosignals 17: 5–22, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai LJ, Ritchie G, Bapty BW, Kerstan D, Quamme GA: Insulin stimulates Mg2+ uptake in mouse distal convoluted tubule cells. Am J Physiol 277: F907–F913, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE: Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol 6: 709–720, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Stein AT, Ufret-Vincenty CA, Hua L, Santana LF, Gordon SE: Phosphoinositide 3-kinase binds to TRPV1 and mediates NGF-stimulated TRPV1 trafficking to the plasma membrane. J Gen Physiol 128: 509–522, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.