Abstract
Monocytes have a crucial role in both proinflammatory and anti-inflammatory phenomena occurring during sepsis. Monocyte recruitment and activation are orchestrated by the chemokine receptors CX3CR1 and CCR2 and their cognate ligands. However, little is known about the roles of these cells and chemokines during the acute phase of inflammation in sepsis. Using intravital microscopy in a murine model of polymicrobial sepsis, we showed that inflammatory Ly6Chigh monocytes infiltrated kidneys, exhibited altered motility, and adhered strongly to the renal vascular wall in a chemokine receptor CX3CR1-dependent manner. Adoptive transfer of Cx3cr1-proficient monocyte-enriched bone marrow cells into septic Cx3cr1-depleted mice prevented kidney damage and promoted mouse survival. Modulation of CX3CR1 activation in septic mice controlled monocyte adhesion, regulated proinflammatory and anti-inflammatory cytokine expression, and was associated with the extent of kidney lesions such that the number of lesions decreased when CX3CR1 activity increased. Consistent with these results, the pro-adhesive I249 CX3CR1 allele in humans was associated with a lower incidence of AKI in patients with sepsis. These data show that inflammatory monocytes have a protective effect during sepsis via a CX3CR1-dependent adhesion mechanism. This receptor might be a new therapeutic target for kidney injury during sepsis.
Keywords: immunology, chemokine receptor, kidney dysfunction
Sepsis is defined as widespread inflammation secondary to infection.1 It is the major cause of admission and death in intensive care units.2,3 Its pathophysiology involves numerous components of innate immunity, especially mononuclear phagocytes.4,5
Monocytes are believed to generate the cytokine storm that triggers a chain reaction leading to tissue damage and death.5 They also perform regulatory functions during inflammatory processes.6–9 They are divided into two subsets: inflammatory monocytes, which are recruited early during inflammation,6,10 and resident monocytes, which patrol the steady-state endothelium.11 Recent studies have called attention to the dual role of inflammatory monocytes in acute inflammation and especially infection. They protect tissues during infectious processes, such as pneumonia or gastrointestinal toxoplasmosis, notably via their secretion of IL-1 receptor antagonist9,12,13 (IL-1ra) or prostaglandin E2.8 Essential crosstalk among monocytes, neutrophils, and tissue (especially epithelial cells) controls the equilibrium between inflammatory and anti-inflammatory processes.
CX3CR1, the receptor of CX3CL1, may be involved in the pathophysiology of sepsis. Kidneys are subject to insult during septic episodes; AKI is a common feature of sepsis and is associated with increased mortality. AKI results, in part, from leukocyte infiltration of kidney tissue and the generation of proinflammatory and proapoptotic mediators.14,15 Studies have shown that sepsis is associated with monocyte infiltration of the kidneys14 and with increased levels of CX3CL1 in situ.16
Here, we show that during polymicrobial sepsis, inflammatory monocytes emigrated from the bone marrow and induced monocytosis accompanied within a few hours by enhanced CX3CR1-dependent adhesion to the renal cortex endothelium. CX3CR1 deficiency increased renal damage and mouse mortality and was correlated with reduced monocyte margination. We further confirmed that the CX3CR1 conferred protective functions linked to inflammatory monocyte adhesiveness and reduced production of IL-1ra in Ly6Chigh monocytes. The involvement of CX3CR1 in the physiopathology of sepsis was confirmed in humans, through a CX3CR1 gene polymorphism study that showed that the I249 CX3CR1 allele is associated with both increased monocyte adhesiveness and reduced kidney damage. Our work describes the protection conferred by inflammatory monocytes against the distant kidney damage caused by septic inflammation.
RESULTS
Ly6Chigh Monocytes Exhibit Increased Adhesion to the Renal Endothelium during Sepsis
To determine the role of monocytes in kidney damage during sepsis, we used a standard murine model of abdominal sepsis induced by cecal ligation and puncture (CLP).17 In our experimental conditions, 50% of the mice died of sepsis in 4 days (Supplemental Figure 1A). Six hours after sepsis induction, the number of CD11b+NK1.1negF4/80+Ly6Chigh cells defined as Ly6Chigh monocytes increased in the blood, concomitant to their release from the bone marrow, while the number of CD11b+NK1.1negF4/80+Ly6Clow cells defined as Ly6Clow monocytes remained constant. Few Ly6Chighmonocytes accumulated in the kidneys, while the number of Ly6Clow monocytes and of CD11b+NK1.1negF4/80highCD11chigh cells defined as renal dendritic cells (renal DCs18,19) number did not change (Supplemental Figure 1B). Twenty-four hours after CLP, the number of Ly6Chigh monocytes reverted to that in the sham (control) group. The kinetics of mobilization in the blood and infiltration into the kidneys was the same for neutrophils as for Ly6Chigh monocytes (Supplemental Figure 1B).
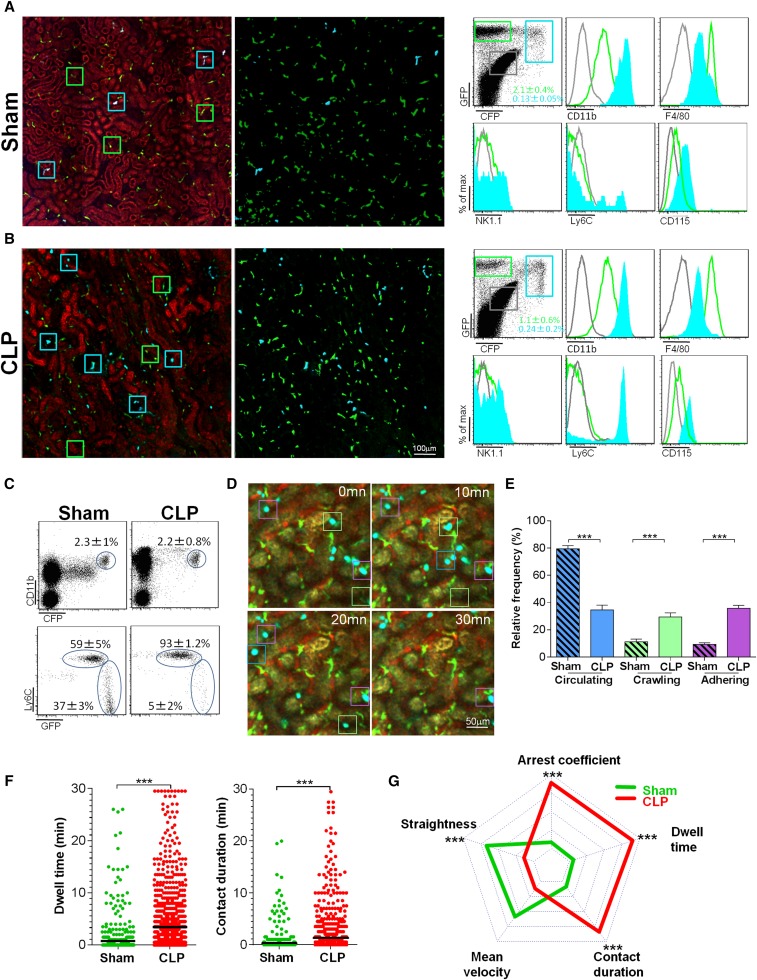
To examine the functional role of monocytes, we performed multiphoton intravital imaging on kidneys from sham- and CLP-operated MacBlue×Cx3cr1gfp/+ mice. In the MacBlue mouse,20 all blood monocytes are strongly positive for enhanced cyan fluorescent protein (ECFP), whereas most tissue macrophages do not express the reporter protein.21 In contrast, renal DCs are CX3CR1+ and strongly express the green fluorescent protein (GFP) reporter.22 In the sham-operated group, two particular subsets of myeloid cells were distinguished in the renal cortex, according to their expression of GFP and/or ECFP: A dominant population of stellate GFP+ECFPneg cells was evenly distributed throughout the tissue, while a few sparse GFP+ECFP+ cells were found (Figure 1A). GFP+ECFPneg cells expressed high levels of F4/80 and low levels of CD11b, Ly6C, and CD115 and may correspond to renal interstitial DCs.18,19 The GFP+ECFP+ cell phenotypes were stellate or round in shape, were CD11b+CD115+F4/80low, and expressed high or low levels of Ly6C (Figure 1A). Six hours after CLP, the number of GFP+ECFPneg cells remained constant, and small, round intravascular GFP+ ECFP+ cells (likely monocytes) accumulated in large numbers (Figure 1B). Of the blood ECFP+ cells in the sham-operated mice, 60% were CX3CR1low/dimLy6Chigh, corresponding to the phenotype of inflammatory monocytes, and 40% were CX3CR1highLy6Clow, corresponding to the phenotype of patrolling monocytes. At 6 hours after CLP, >90% of the ECFP+ cells were Ly6Chigh monocytes (Figure 1C). Time-lapse imaging of the kidneys of the sham-operated mice showed that numerous ECFP+ cells traveled through the cortex in the bloodstream, interacting very little with the endothelium, while the activity of renal DCs was strongly protrusive (Supplemental Movie 1). At 6 hours after CLP, renal DC behavior did not change, but the number of ECFP+ cells adhering to the luminal side of the vessel increased (Supplemental Movie 2). We defined three motility patterns of ECFP+ cells: circulating, crawling, and adhering (Figure 1D). Six hours after CLP, the proportion of circulating ECFP+ cells had fallen by >50%, while the proportion of crawling and adhering ECFP+ cells had increased above the levels in the sham-operated mice (Figure 1E). Mean dwell time and mean contact duration quadrupled (Figure 1F). Contact duration increased for ECFP+, but they were mainly released without any evidence of extravasation toward the kidney tissue.
Figure 1.
Ly6Chigh monocytes exhibit increased adhesion to the renal endothelium during sepsis. Two-photon laser scanning microscopic images (left) with volume rendering (right) of ECFP+ (cyan squares) and GFP+ (green squares) cells in kidneys of MacBlue×Cx3cr1gfp/+ mice 6 hours after (A) sham or (B) CLP operations. CFP signals are in blue, GFP signals are in green, and autofluorescent renal tubules are in red. Overlay of flow cytometric surface marker expression gated on GFP+ (green histograms) and ECFP+ cells (cyan histograms) in kidneys are shown for each condition. Mean percentages±SD of gated cells are indicated (n=6 mice per group out of three independent experiments). Background staining (gray histograms) gated on nonfluorescent cells is represented. (C) Dot plots represent Ly6C and CX3CR1-GFP expression, gated on blood CD11b+ECFP+ cells 6 hours after sham or CLP operations. Mean percentages±SD of gated cells are indicated (n=6 mice per group out of three independent experiments). (D) Time series two-photon laser scanning microscopic images of kidney cortex of MacBlue×Cx3cr1gfp/+ mice 6 hours after CLP. Examples of circulating (blue squares), crawling (green squares), and adhering monocytes (purple squares) are presented. ECFP signals are in cyan, GFP signals are in green, renal tubules are autofluorescent, and blood vessels are visualized by 2 MDa rhodamine-dextran. (E) Relative frequency of the three behaviors. Bars represent mean±SEM (n=4 sham and n=3 control from independent experiments; ANOVA with Bonferroni adjustment for multiple comparisons were used; ***P<0.001). (F) Dwell time and contact duration with renal endothelium of ECFP+ monocytes. Black bars indicate means. (n=4 sham and n=4 control from independent experiments; Mann-Whitney test were used; ***P<0.001). (G) Radar chart representation shows ECFP+ cell dynamic signatures in sham-operated (green) and CLP-operated (red) mice. Mean values are presented within the 95% confidence interval of the measured value scale for each parameter. Data represent a pool of cells from (n=4 sham and n=4 control from independent experiments; Mann-Whitney test were used; *** P<0.001). (See also Supplemental Figure 1 and Supplemental Movies 1–4).
Intravital imaging on CLP-operated MacBlue×Cx3cr1gfp/+×Ccr2−/− mice, defective for circulating Ly6Chigh monocytes,23 showed a near-complete abolition of the accumulation of ECFP+ adherent cells on the renal endothelium strongly suggests that they were Ly6Chigh monocytes (Supplemental Movie 3). This phenotype was further confirmed by the intravital imaging of the combined Cx3cr1gfp/+Ccr2rfp/+ mice, which showed that the adhering cells coexpressed red fluorescent protein (RFP) and GFP (Supplemental Movie 4). In Ccr2−/− mice, Ly6Chigh monocytes did not accumulate after CLP in blood or in the kidney. After CLP, Ly6Clow monocytes were more numerous in the blood of Ccr2−/− mice than in wild-type (WT) mice, and their numbers were similar in the kidney for both groups (Supplemental Figure 2A). However, no ECFP+ cells adhering to the renal vascular wall were imaged in Ccr2−/− mice, in contrast to WT mice (Supplemental Movie 3). Altogether these results indicate that Ly6Chigh monocytes are the main cells imaged by intravital imaging. Radar chart representation of the different cell dynamic measures provides a signature of the ECFP+ cell dynamic behavior (Figure 1G). Overall, CLP strongly modified the signature of ECFP+ cells compared with that of sham-operated mice. Adhesion of the ECFP+ cells increased after CLP, and their track straightness declined, as did their mean velocity. At the same time, their arrest coefficient, dwell time, and contact duration with the endothelium all increased (Figure 1G).
These findings show that within a few hours after CLP, the number of Ly6Chigh monocytes in the blood increased and they interacted with the renal endothelium.
CX3CR1 Promotes Ly6Chigh Monocyte Adhesion and Prevents Renal Damage during Sepsis
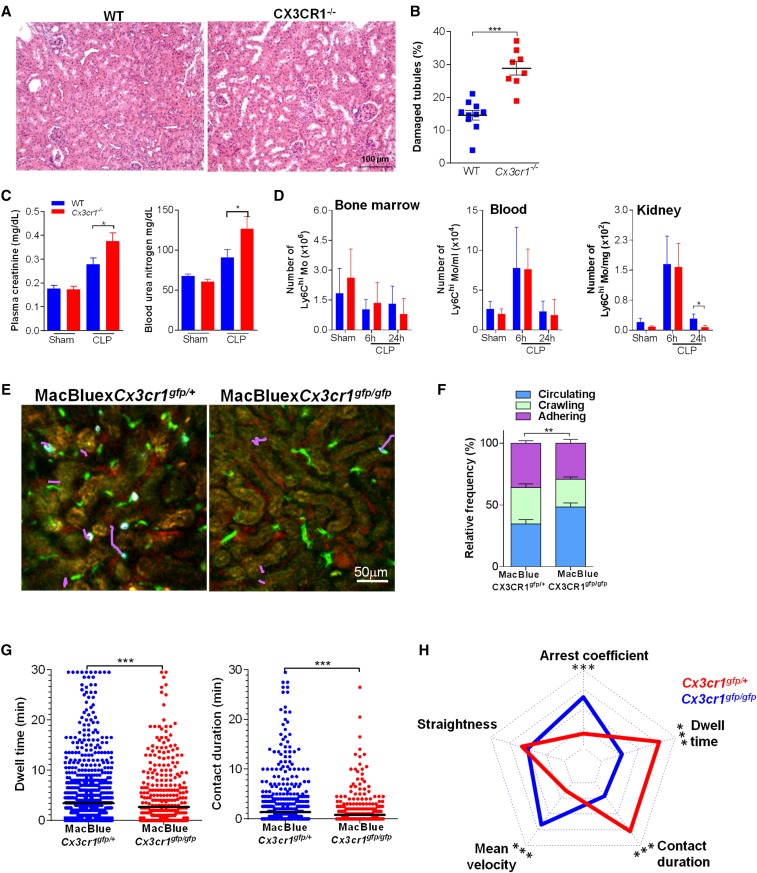
Previous reports have shown that CX3CR1 deficiency is associated with increased mortality after CLP,24 but the precise mechanisms involved have not been adequately defined. We sought to determine the role of CX3CR1 in the pathogenesis of organ damage during sepsis. Cx3cr1−/− mice had conspicuously more kidney histologic lesions than WT mice (Figure 2A). The proportion of damaged tubules during CLP was two times higher in the Cx3cr1−/− mice (Figure 2B), which also had substantially higher levels of markers of renal failure, such as creatinemia and uremia (Figure 2C). The kidney was not the only affected organ, but renal damages were associated with increased mortality after CLP (Supplemental Figure 3A). To determine whether this phenotype could be due to impaired bactericidal activity, as suggested,25 we compared mice survival in a sterile inflammation model, by injecting mice with a lethal dose of LPS. The strong reduction in survival of Cx3cr1−/− mice suggests that the phenotype observed was independent of control of the bacterial burden and emphasizes the relationship with organ damage (Supplemental Figure 3B). On the other hand, and as others have recently reported,26 these two types of mice did not differ in the number of Ly6Chigh monocytes (Figure 2D) or neutrophils (not shown) in bone marrow, kidneys, or blood. This prompted us to perform functional imaging to compare dynamic behavior of Ly6Chigh monocytes from CX3CR1-deficient and WT mice during sepsis.
Figure 2.
CX3CR1 promotes Ly6Chigh monocyte adhesion and prevents renal damage during sepsis. (A) Photomicrographs and (B) quantification of kidney histologic lesions 24 hours after CLP in WT (blue) and Cx3cr1−/− mice (red). Bars represent mean±SD (n=10 WT, n=8 Cx3cr1−/−, from at least two repeated experiments; ANOVA with Bonferroni adjustment was used; ***P<0.001). (C) Urea and creatinine measurements in plasma of CLP-operated WT and Cx3cr1−/− mice bars represent mean±SEM (n=10 WT sham, 6 Cx3cr1−/− sham, 15 WT, and 15 Cx3cr1−/− CLP, from at least two repeated experiments; ANOVA with Bonferroni adjustment was used; *P<0.05). (D) Number of Ly6Chigh monocytes in bone marrow, blood, and kidney of WT (blue) and Cx3cr1−/− (red) mice. Bars represent mean±SD (n=10 WT sham, 5 WT CLP at 6 hours, 12 WT CLP at 24 hours, and 6 Cx3cr1−/− sham, CLP at 6 hours, and CLP at 24 hours, from at least two repeated experiments; ANOVA with Bonferroni adjustment was used; *P<0.05). (E) Two-photon laser scanning microscopic images with overlay of monocyte migratory tracks (pink) in kidney cortex of MacBlue×Cx3cr1gfp/+ and MacBlue×Cx3cr1gfp/gfp mice, 6 hours after CLP. ECFP signals are in cyan, GFP signals are in green, renal tubules are autofluorescent, and blood vessels are visualized by 2 MDa rhodamine-dextran. (F) Relative frequency of the three monocyte behaviors in MacBlue×Cx3cr1gfp/+ and MacBluexC×3cr1gfp/gfp mice. Bars represent mean±SEM (n=3 MacBlue×Cx3cr1gfp/+; n=4 MacBluexC×3cr1gfp/gfp from independent experiments; ANOVA with Bonferroni adjustment was used; **P<0.01). (G) Dwell time and contact duration with the renal endothelium of ECFP+Cx3cr1gfp/+ and ECFP+Cx3cr1gfp/gfp monocytes. Black bars indicate means. (H) Radar chart representation shows ECFP+ cell dynamic signatures in CLP-operated MacBlue×Cx3cr1gfp/+ (red) and MacBlue×Cx3cr1gfp/gfp (blue) mice. Mean values are presented within the 95% confidence interval of the measured value scale for each parameter (for all two-photon experiments, n=3 MacBlue×Cx3cr1gfp/+; n=4 MacBluexC×3cr1gfp/gfp from independent experiments; Mann-Whitney test for multiple comparisons was used; ***P<0.001). (See also Supplemental Figure 2 and Supplemental Movie 5).
Thus, we used MacBlue×Cx3cr1gfp/gfp mice 6 hours after CLP (Figure 2E). In the absence of CX3CR1, the proportion of adhering and crawling ECFP+ cells fell (Figure 2F), as did ECFP+ cell dwell time and contact duration (Figure 2G). The radar chart representation of the motility pattern showed distinct cell dynamic signature between septic MacBlue×Cx3cr1gfp/+ and septic MacBlue×Cx3cr1gfp/gfp mice (Figure 2H). This loss of adhesion was specific to ECFP+ cells: Neutrophil behavior did not differ between the two strains (Supplemental Figure 3, C–E). To further address that the reduced adherence is intrinsic to the CX3CR1 deficiency, Cx3cr1−/− and Cx3cr1+/+ ECFP+ cells were transferred into septic WT mice and the proportion that adhered to the renal vascular wall was determined (Supplemental Figure 3F). Accordingly, Cx3cr1−/−ECFP+ cells adherence was much weaker than that of Cx3cr1+/+ ECFP+ cells. We conclude that the CX3CR1 receptor is functionally important during sepsis; its absence leads to reduced inflammatory monocyte adhesion to the renal vascular wall, more numerous kidney lesions, and increased mortality. This observation suggests that inflammatory monocytes have a potent unexpected protective effect during sepsis.
Bone Marrow–Derived Monocytes Protect against Damage to Kidney Tissue during Sepsis
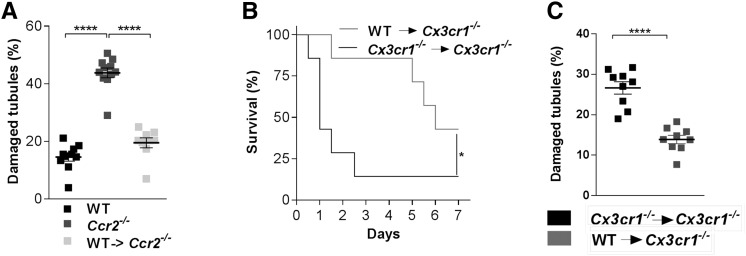
To examine the protective role circulating Ly6Chigh monocytes may play, we first performed CLP in Ccr2−/− mice that display a Ly6Chigh monocytopenia (Supplemental Figure 2A). Consistent with our hypothesis, the number of kidney lesions was dramatically higher in Ccr2−/− than in WT mice (Figure 3A). It has also been reported that phagocyte are renoprotective during sepsis and that Cx3cr1−/− mice have a defect in renal DC number.26,27 The number of renal DCs in Ccr2−/− mice was not impaired compared with the number in WT mice, confirming that the presence of renal DCs was not sufficient to protect from renal lesion (Supplemental Figure 2B).
Figure 3.
Bone marrow–derived monocytes protect against damage to kidney tissue during sepsis. (A) Quantification of kidney histologic lesions 24 hours after CLP in WT, Ccr2−/− and Ccr2−/− mice with adoptive transfer of WT bone marrow monocytes before surgery. Bars represent mean±SD (n=10 WT, 11 Ccr2−/−, 9 WT in Ccr2−/−; data from at least two repeated experiments; ANOVA with Bonferroni adjustment was used; ****P<0.0001). (B) Survival of CLP-operated Cx3cr1−/− mice after adoptive transfer of WT (gray line) or Cx3cr1−/− bone marrow monocytes (black line) (n=7 per group out of three independent experiments; survival curves were compared with a log-rank test; *P<0.05). (C) Quantification of kidney histologic lesions 24 hours after CLP in Cx3cr1−/− mice with adoptive transfer of WT (gray) or Cx3cr1−/− (black) bone marrow monocytes before surgery (n=9 per group from at least two repeated experiments; ANOVA with Bonferroni adjustment was used; **** P<0.0001).
Adoptive transfers of CCR2-proficient monocyte-enriched bone marrow cells (MBMs) into Ccr2−/− mice drastically reduced kidney lesions during sepsis and thus further confirmed their protective effect (Figure 3A). Finally, adoptive transfer of Cx3cr1-proficient MBM into septic Cx3cr1−/− mice provided significant protection against mortality (Figure 3B) and resulted in a diminution in kidney lesions to a level similar to that of WT mice (Figure 3C), compared with the transfer of Cx3cr1-deficient MBMs. These observations confirm that bone marrow–derived monocytes have a protective role in organ damage during sepsis via a CX3CR1-dependent mechanism.
CX3CR1 Activation Controls Ly6Chigh Monocyte Adherence and Outcome of CLP-Mediated Sepsis
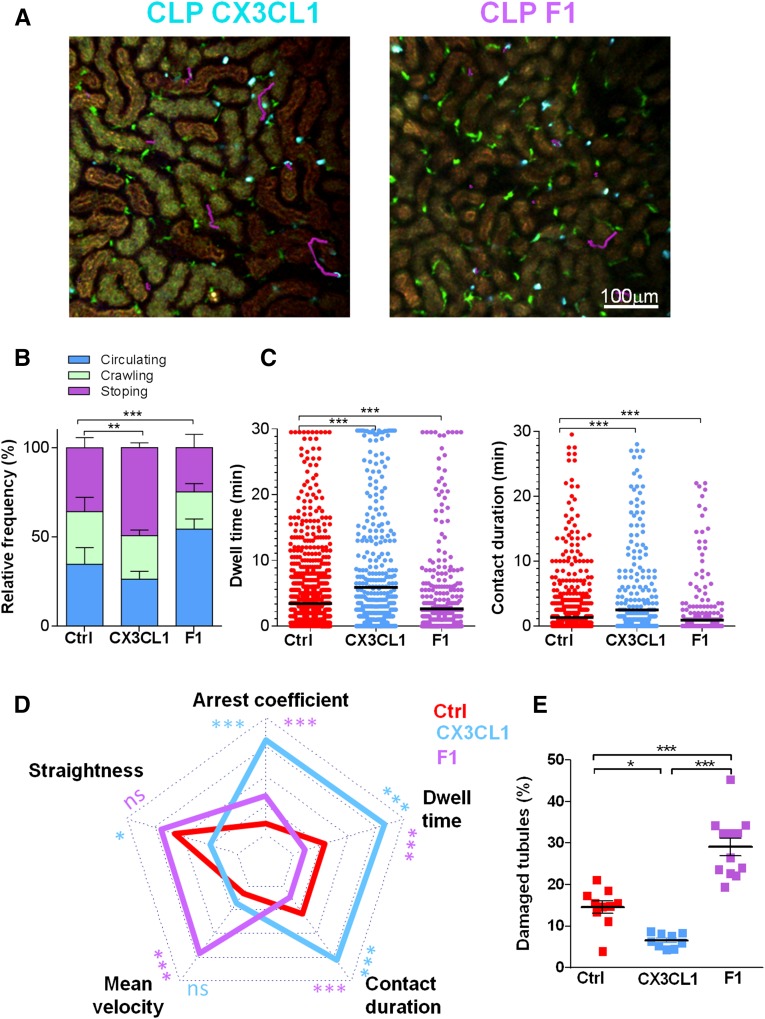
In further considering the role of CX3CR1-dependent adhesion of Ly6Chigh monocytes during sepsis, we treated septic mice with the CX3CR1 ligand (CX3CL1) or the antagonist (F1) of CX3CR1 that we have previously shown to inhibit monocyte adhesion to CX3CL1.28 The proportion of adherent monocytes increased in the presence of the agonist and diminished in the presence of the antagonist (Figure 4, A and B). In addition, dwell time and contact duration of monocytes increased after treatment with CX3CL1 and decreased after treatment with F1 (Figure 4C). The cell dynamic signature was inversely affected by treatment with CX3CL1 and F1 compared with control CLP-operated mice (Figure 4D). These different signatures were associated with a different level of organ failure. In particular, injection of the antagonist, F1, led to increased lesions (Figure 4E) and mortality (data not shown) without altering renal DC numbers (Supplemental Figure 2B). In contrast, CX3CL1 injection diminished the number of kidney lesions (Figure 4E). These findings show that pharmacologic modulation of CX3CR1 activation strongly correlates with Ly6Chigh monocyte margination and kidney damage, thereby demonstrating CX3CR1 as a potential therapeutic target.
Figure 4.
CX3CR1 activation controls Ly6Chigh monocyte adherence and the outcome of CLP-mediated sepsis. (A) Two-photon laser scanning microscopic images with overlay of monocyte migratory tracks (pink) in kidney cortex of MacBlue×Cx3cr1gfp/+ mice treated with CX3CL1 or F1, 6 hours after CLP. ECFP signals are in cyan, GFP signals are in green, and renal tubules are autofluorescent. (B) Relative frequency of the three behaviors and (C) dwell time and contact duration in MacBlue×Cx3cr1gfp/+ untreated (red), treated with CX3CL1 (blue), or treated with F1 (purple). Bars represent mean±SEM (n=3 mice per group from independent experiments; ANOVA with Bonferroni adjustment was used; **P<0.01; ***P<0.001). (D) Radar chart representation shows ECFP+ cell dynamic signatures in the different experimental conditions. Mean values are presented within the 95% confidence interval of the measured value scale for each parameter. For all two-photon experiments (n=3 mice per group from independent experiments; Mann-Whitney test was used; *P<0.05; ***P<0.001). (E) Quantification of kidney histologic lesions 24 hours after CLP in control, CX3CL1, and F1-treated mice. Bars represent mean±SD (n=10 control, 12 F1, and 9 CX3CL1, from at least two independent experiments; ANOVA with Bonferroni adjustment was used; *P<0.05; **P<0.01; ***P<0.001). (See also Supplemental Movies 6 and 7.)
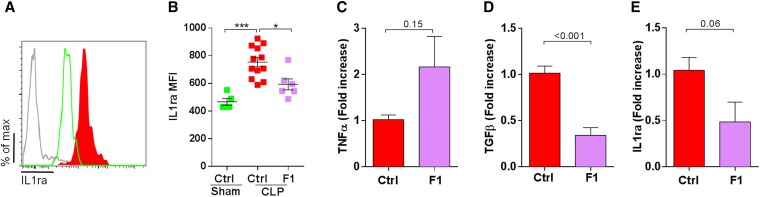
CX3CR1 Blockade Increases Kidney Inflammation and Reduces IL-1ra Production by Ly6Chigh Monocyte during Sepsis
To further investigate the molecular mechanism involved in protective effect by Ly6Chigh monocytes, we measured by flow cytometry the intracellular production of IL-1ra that was previously shown to attenuate lung injury after LPS treatment9 (Figure 5A). IL-1ra mean fluorescence intensities were nearly doubled in Ly6Chigh monocytes 6 hours after CLP compared with sham-operated mice. F1 treatment efficiently reduced IL-1ra mean fluorescence intensities in Ly6Chigh monocytes (Figure 5B) and leads to increased TNF-α (Figure 5C), decreased TGF-β (Figure 5D), and IL1ra (Figure 5E) transcripts in the whole kidney 6 hours after CLP. In conclusion, CX3CR1 blockade is associated with reduced production of IL-1ra by monocytes and with a higher proinflammatory environment in the kidney.
Figure 5.
CX3CR1 blockade increases kidney inflammation and reduces IL-1ra production by Ly6Chigh monocyte during sepsis. (A) Intracellular production of IL-1ra was evaluated by intracellular cytokine staining gated on CD11b+ Ly6Gneg NK1.1neg Ly6Chigh cells from sham-operated (green) and CLP-operated mice (red) 6 hours after surgery. Gray histogram represents isotype staining. (B) Mean fluorescence intensity of IL-1ra intracellular staining was compared between sham-operated (green) and CLP-operated mice (red) treated or not treated with F1 (purple), 6 hours after surgery (n=6 sham, F1, and 12 WT CLP from two independent experiments; ANOVA t test was used; *P<0.05; ***P<0.001). Kidneys from CLP-operated mice treated with PBS (control) or F1 were extracted 6 hours after CLP and were evaluated by quantitative PCR for (C) TNF-α production, (D) TGF-β production, and (E) IL1ra production. Results are represented as fold increase of CLP-operated mice (n=6 in each groups from two independent experiments; t test was performed; P value are indicated).
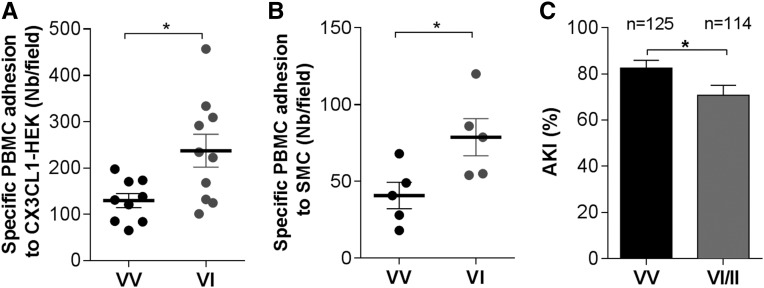
The Proadhesive CX3CR1 I249 Allele Is Associated with a Reduced Incidence of AKI in Septic Patients
To evaluate whether the mechanism we described in this murine model might be relevant to the pathophysiology of human disease, we studied the effect of a frequent CX3CR1 polymorphism, I249. This polymorphism was shown to increase monocyte adhesion to CX3CL1 and could modulate the course of coronary artery disease, atherosclerosis, age-related macular degeneration, glioblastoma, or obesity.29–33 We tested the effects of this polymorphism in vitro in cell adhesion assays and in a cohort of patient admitted to intensive care for sepsis. PBMCs from individuals with CX3CR1 VV genotype or VI genotype were assayed for adhesion (Figure 6, A and B). PBMCs from donors heterozygous for the I249 allele adhere significantly more than those homozygous for V249 allele, confirming our previous results.34 We then studied the effect of the I249 allele on the occurrence of AKI in a cohort of patients with sepsis. This study included 239 patients without chronic renal failure. In most cases, sepsis origins were in primary or secondary peritonitis and respiratory tract infections, with no difference between groups. At least one I249 allele was present in nearly 50% of the patients, with a distribution of the VV, VI, and II genotype of 52%, 41%, and 6%, respectively. The I249 allele (VI or II genotypes) was associated with a lower incidence of AKI (Figure 6C), with no differences between the groups in terms of age, disease severity, or comorbidities. Patients carrying the I249 allele had fewer coagulation disorders but the same rate of circulatory or respiratory failures and the same 28-day mortality (Supplemental Table 1). In the multivariate analysis, CX3CR1 was still significantly associated with a reduced occurrence of AKI after adjustment for age, Simplified Acute Physiology Score II, IL-6 levels, and number of comorbidities. The odds ratio for AKI in a patient with the I249 allele was 0.43 (95% confidence interval, 0.27 to 0.93) (Table 1). Thus, we showed that the I249 allele of CX3CR1 is associated with reduced AKI incidence in septic patients.
Figure 6.
The proadhesive CX3CR1 I249 allele is associated with a reduced incidence of AKI in septic patients. PBMCs from individuals with CX3CR1 VV (black) or VI (gray) genotypes were assayed for adhesion with (A) CX3CL1-expressing HEK (n=9–10 individuals) or (B) with adherent smooth muscle cells (SMCs) treated with TNF-α and IFN-γ (n=5 individuals in each group). Bars indicate mean±SEM. A t test was used; *P<0.05. (C) V249I polymorphism distribution and incidence of AKI in patients with VV− (black bar) or VI/II− (gray bar) polymorphisms (n=239; chi-squared test was used; and 95% confidence intervals of the estimate are presented).
Table 1.
Univariate and multivariate analyses of measures associated with AKI
| Variable | Univariate/Multivariate | OR (95% CI) |
|---|---|---|
| CX3CR1VI/II | Univariate | 0.50 (0.27 to 0.93) |
| CX3CR1VI/II | Multivariate | 0.43 (0.21 to 0.89) |
| SAPS II | Multivariate | 1.05 (1.01 to 1.08) |
| Age | Multivariate | 0.97 (0.94 to 0.99) |
| Log10 IL-6 | Multivariate | 1.69 (1.12 to 2.55) |
| Comorbidities | Multivariate | 1.76 (1.25 to 2.49) |
Data are presented as mean±SEM. Comparison of incidence of AKI was made with a two-tailed t test. See also Supplemental Table 1. SAPS, Simplified Acute Physiology Score; OR, odds ratio; 95% CI, 95% confidence interval.
DISCUSSION
Inflammatory monocytes play crucial roles during sepsis through cytokine secretion and direct cell contact.5,35–37 Recent studies have identified a regulatory protective role for inflammatory monocytes in infectious diseases as they synthetize and secrete anti-inflammatory mediators, such as IL-1ra and prostaglandin E2.8,9 However, the specific contribution of inflammatory monocytes to the regulation of inflammation in early phases of sepsis remains unclear. Our results have unveiled a key role these monocytes play in renal tissue protection via a CX3CR1-dependent adhesion of inflammatory monocytes to the renal vascular endothelium.
Previous studies have shown that inflammatory monocytes are involved in controlling inflammation in gram-negative pneumonia and abdominal infections.8,9,38–42 A lower number of inflammatory monocytes has been associated with increased lesions in the lung and in the intestinal lamina propria.8,9,38,41 Other studies have shown that the CX3CR1/CX3CL1 axis is involved in the pathogenesis of sepsis. Genetic disruption of the Cx3cr1 gene has been associated with increased mortality without any effect on neutrophil or monocyte recruitment.24 In that study, the indirect interaction of monocytes with neutrophils reduced the ability of Cx3cr1−/− mice to eradicate bacteria. Recent findings by Hochheiser et al.26 showed that CX3CR1 deficiency was associated with a reduced entry of DC precursors in the inflamed kidney. Additionally, this work showed that in a model of infectious GN, the absence of CX3CR1 was not associated with an increased bacterial burden in the kidney.26 Antibacterial role associated with Cx3cr1 was unlikely to be the main role for this gene during sepsis. Indeed, we showed that mortality increased in Cx3cr1−/− mice subjected to sterile lethal inflammation induced by LPS injection. Regarding our result during CLP, the reduced number of renal DCs in Cx3cr1−/− mice is unlikely the only cause of the adverse phenotype because in both bone marrow–derived cell-adoptive transfer that rescued CX3CR1-deficient mice from renal lesions and treatment of the antagonist F1 that mimicked the knockout phenotype in WT mice, renal DC numbers were unchanged.
Carlin et al.43 showed that Ly6Clow monocytes were specifically retained by inflamed capillaries in a G αi- and Toll-like receptor (TLR)-7–dependent manner and cleared dead cells. In accordance with our results, they observed that TLR4 activation by LPS painting did not induce any recruitment of Ly6Clow monocytes. Thus, these data could delineate a specific role of Ly6Clow monocytes during viral infections (while Ly6Chigh would be more recruited in bacterial infections) or a specific biphasic response with a first wave of Ly6Chigh monocytes recruited in a TLR4-dependent manner followed by a second wave of Ly6Clow monocytes that will clear the vessels from dead cells.
Both Cx3cr1 gene deletion and pharmacologic inhibition of CX3CR1 led to enhanced renal damage and reduced survival, whereas treatment by the chemokine agonist CX3CL1 prevented kidney damage. In addition, adoptive transfer of Cx3cr1−/− monocytes confirmed that reduced adherence is due to CX3CR1 expression by monocytes. The CX3CR1 receptor is involved in monocyte adhesion to the vascular wall via various mechanisms; the first is direct binding of CX3CR1 to the membrane-anchored form of CX3CL144 and the other is an integrin-mediated adhesion. Treatments with Inflammatory agents such as LPS, TNF-α or IL-1 induce CX3CL1 overexpression by endothelial cells.45 During sepsis, levels of soluble CX3CL1 also increase within 6 hours after sepsis onset and peak by 24 hours.24,46 The activation of CX3CR1 promotes the integrin-dependent adhesion mechanisms that are responsible for cell adhesion.47,48 Indeed, during sepsis the integrins cd11a and cd11b are involved in leukocyte adhesion to the pulmonary vascular wall.49 We showed here that CX3CR1 is a major determinant of this phenomenon and that CX3CR1 deficiency reduces the adhesive properties of Ly6Chigh monocytes.
The result that CX3CR1 play a protective role during sepsis is consistent with previous work24,46 showing that the administration of CX3CL1 can improve inflammatory response during sepsis and even reduce mortality.24,46 We did not observe improved survival, but we did observe reduced kidney lesions. Some of the anti-inflammatory functions of monocytes are closely related to their endothelial adhesion. The anti-inflammatory properties that IL-10 can exert on monocytes appear to occur only when these cells are adhesive.50 Furthermore, integrin signaling is also responsible for increased expression of the IL-1ra gene.51 Consistently, we have shown that CX3CR1 blockade led to reduced intracellular IL-1ra content in Ly6Chigh monocytes. This may explain how a deficiency in the CX3CR1 receptor could increase mortality and/or kidney lesions in mice and how the CX3CR1 I249 allele might reduce the incidence of AKI in septic patients. The I249 allele was also associated with a lower incidence of coagulation disorders during sepsis. This is consistent with a more pronounced endothelium protective effect of the monocytes carrying the rare allele. This observation could also participate in the reduction of AKI by diminishing the rate of microvascular dysfunction and clots that could account for AKI.52 We observed no difference in patients’ mortality at day 28. This could be due to an insufficient number of patients to observe an effect or to possible other effects of CX3CR1 polymorphism that we could not investigate in this study. CX3CL1 might therefore be proposed as a new therapeutic agent to prevent or to cure AKI in patients with sepsis.
In summary, our data delineated a CX3CR1-dependent renoprotective role played by Ly6Chigh monocytes during the early phase of sepsis.
CONCISE METHODS
Experimental details are provided in the Supplemental Material.
Mice
Male C57BL/6 mice were purchased from Elevage Janvier (Saint Isle, France). Cx3cr1gfp/+-Ccr2rfp/+ mice were kindly provided by Israel Charo (Gladstone Institute, San Francisco).53 Ccr2−/− and Cx3cr1−/− C57BL/6 mice, Cx3crR1gfp/+54 and Cx3cr1gfp/gfp, Csf1r-Gal4VP16/UAS-ECFP (MacBlue),20 MacBlue×Cx3cr1gfp/+, MacBlue×Cx3cr1gfp/gfp, and MacBlue×Cx3cr1gfp/+-Ccr2rfp/- mice were bred in our animal facility. All experiments and protocols were approved by the local animal experimentation ethics committee.
Human Polymorphism Study
We retrospectively studied DNA from patients included in previous studies.55 Patients meeting the criteria for severe sepsis/septic shock and who had at least two organ failures defined by the Sepsis-related Organ Failure Assessment25 were included. Patients with chronic renal failure were excluded. The Cx3cr1 V249I polymorphism (rs3732379) was detected as previously described.32 The AP-HP Cochin Hospital Ethics Committee approved the study. The patient or the patient's next-of-kin provided written informed consent.
Polymicrobial Sepsis Induction
Polymicrobial sepsis was generated after a CLP procedure as described using a 21-gauge needle.24 In the control animals, the cecum was exteriorized and reinserted in the abdomen. For some experiments, 3 µg of fractalkine (full-length fractalkine/CX3CL1; R&D Systems) was injected intraperitoneally 30 minutes before surgery. For F1 (kindly provided by A. Proudfoot), 50 µg was injected intraperitoneally 30 minutes before and 6 hours after surgery.
LPS Injection
LPS (Escherichia coli O111:B4) was injected at a dose of 15 mg/kg intraperitoneally.
Histologic Analysis
Quantification was performed on periodic acid-Schiff–stained, 3- to 5-μm kidney sections and on at least three mice per condition. For each mouse, the percentage of damaged tubules was calculated on three to five different randomly chosen fields comprising 200–300 tubules.
Flow Cytometry
Cell staining for flow cytometry and antibodies used are described in the Supplemental Material. Flow cytometry was performed with the FACScanto (BD Biosciences) flow cytometer. Analysis was performed with FlowJo software (TreeStar, Inc.).
Adoptive Transfer Experiments
Bone marrow cells were isolated from MacBlue-Cx3cr1gfp/+ and MacBlue-Cx3cr1gfp/gfp mice; cells were co-transferred at a 1:1 ratio 30 minutes before the CLP procedure. Analyses were done 6 hours after CLP. Bone marrow monocytes were extracted after negative selection removal of other cell types (see Supplemental Material). Before sorting, Ly6Chi monocytes represented nearly 16% of myeloid cells and were enriched to nearly 60% after sorting, while the neutrophils population was drastically reduced. In all experimental conditions, mice were injected with 4–5×106 monocytes just before the CLP procedure.
Multiphoton Imaging
Mice were anesthetized with isoflurane. Their temperatures were maintained at 37°C. An incision was made in the flank, and the kidney was exposed. In some experiments, 2×106 molecular weight tetramethylrhodamine-dextran (Invitrogen) was injected to stain the vasculature. Cell motility was measured every 30 seconds. Cells were tracked for 30 minutes with three-dimensional automatic tracking and manual correction with Imaris software. Definition of cells behavior and additional details are provided in the Supplemental Material.
Statistical Analyses
Data are reported as mean±SD or mean±SEM +/− SD/SEM as indicated. Groups were compared using a two-tailed unpaired t test and adjusted for multiple comparison analysis. Nonparametric Mann-Whitney test or ANOVA with adjustments was performed according to Gaussian distribution of each sample. Survival curves were compared using a log-rank test. Multivariate analysis of the human cohort was performed with JMP (SAS Institute, Inc., Cary, NC).
Disclosures
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale (INSERM), the Université Pierre et Marie Curie (UPMC), the European Community's Seventh Framework Project FP7-Health-2009 ENDOSTEM and the ANR Programme Emergence 2012 (ANR-EMMA-050). A.L. is a recipient of École de l'Inserm Bettencourt. B.G.C. is a recipient of a contract Inserm Poste d'accueil and was supported by Fondation pour la Recherche Médicale.
Supplementary Material
Acknowledgments
The authors thank Drs. Medhi Daoudi, Ludovic Arnold, and Valérie Faivre for their advice and technical assistance; Jo Ann Cahn for manuscript editing; the Plateforme Imagerie Pitié-Salpétrière for assistance with the two-photon microscope; and the animal facility NAC for assistance with mice breeding.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010009/-/DCSupplemental.
References
- 1.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644–1655, 1992 [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR: Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall JR, Payen D, Sepsis Occurrence in Acutely Ill Patients Investigators : Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med 34: 344–353, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, van der Poll T: Severe sepsis and septic shock. N Engl J Med 369: 840–851, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Cohen J: The immunopathogenesis of sepsis. Nature 420: 885–891, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Arnold L, Henry A, Poron F, Baba-Amer Y, van Rooijen N, Plonquet A, Gherardi RK, Chazaud B: Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frantz S, Hofmann U, Fraccarollo D, Schäfer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, Pachel C, Schön MP, Kneitz S, Bobinger T, Weidemann F, Ertl G, Bauersachs J: Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J 27: 871–881, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, Legrand F, Koo LY, Brenchley JM, Fraser ID, Belkaid Y: Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 19: 713–721, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Herold S, Tabar TS, Janssen H, Hoegner K, Cabanski M, Lewe-Schlosser P, Albrecht J, Driever F, Vadasz I, Seeger W, Steinmueller M, Lohmeyer J: Exudate macrophages attenuate lung injury by the release of IL-1 receptor antagonist in gram-negative pneumonia. Am J Respir Crit Care Med 183: 1380–1390, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ: The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 204: 3037–3047, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F: Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Arend WP, Malyak M, Guthridge CJ, Gabay C: Interleukin-1 receptor antagonist: Role in biology. Annu Rev Immunol 16: 27–55, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Remick DG, Call DR, Ebong SJ, Newcomb DE, Nybom P, Nemzek JA, Bolgos GE: Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit Care Med 29: 473–481, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Lerolle N, Nochy D, Guérot E, Bruneval P, Fagon JY, Diehl JL, Hill G: Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med 36: 471–478, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Schrier RW, Wang W: Acute renal failure and sepsis. N Engl J Med 351: 159–169, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Raspé C, Höcherl K, Rath S, Sauvant C, Bucher M: NF-κB-mediated inverse regulation of fractalkine and CX3CR1 during CLP-induced sepsis. Cytokine 61: 97–103, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Rittirsch D, Huber-Lang MS, Flierl MA, Ward PA: Immunodesign of experimental sepsis by cecal ligation and puncture. Nat Protoc 4: 31–36, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.John R, Nelson PJ: Dendritic cells in the kidney. J Am Soc Nephrol 18: 2628–2635, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Schraml BU, van Blijswijk J, Zelenay S, Whitney PG, Filby A, Acton SE, Rogers NC, Moncaut N, Carvajal JJ, Reis e Sousa C: Genetic tracing via DNGR-1 expression history defines dendritic cells as a hematopoietic lineage. Cell 154: 843–858, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Ovchinnikov DA, van Zuylen WJ, DeBats CE, Alexander KA, Kellie S, Hume DA: Expression of Gal4-dependent transgenes in cells of the mononuclear phagocyte system labeled with enhanced cyan fluorescent protein using Csf1r-Gal4VP16/UAS-ECFP double-transgenic mice. J Leukoc Biol 83: 430–433, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Sauter KA, Pridans C, Sehgal A, Bain CC, Scott C, Moffat L, Rojo R, Stutchfield BM, Davies CL, Donaldson DS, Renault K, McColl BW, Mowat AM, Serrels A, Frame MC, Mabbott NA, Hume DA: The MacBlue binary transgene (csf1r-gal4VP16/UAS-ECFP) provides a novel marker for visualisation of subsets of monocytes, macrophages and dendritic cells and responsiveness to CSF1 administration. PLoS ONE 9: e105429, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soos TJ, Sims TN, Barisoni L, Lin K, Littman DR, Dustin ML, Nelson PJ: CX3CR1+ interstitial dendritic cells form a contiguous network throughout the entire kidney. Kidney Int 70: 591–596, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Serbina NV, Pamer EG: Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Ishida Y, Hayashi T, Goto T, Kimura A, Akimoto S, Mukaida N, Kondo T: Essential involvement of CX3CR1-mediated signals in the bactericidal host defense during septic peritonitis. J Immunol 181: 4208–4218, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Hochheiser K, Heuser C, Krause TA, Teteris S, Ilias A, Weisheit C, Hoss F, Tittel AP, Knolle PA, Panzer U, Engel DR, Tharaux PL, Kurts C: Exclusive CX3CR1 dependence of kidney DCs impacts glomerulonephritis progression. J Clin Invest 123: 4242–4254, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lionakis MS, Swamydas M, Fischer BG, Plantinga TS, Johnson MD, Jaeger M, Green NM, Masedunskas A, Weigert R, Mikelis C, Wan W, Lee CC, Lim JK, Rivollier A, Yang JC, Laird GM, Wheeler RT, Alexander BD, Perfect JR, Gao JL, Kullberg BJ, Netea MG, Murphy PM: CX3CR1-dependent renal macrophage survival promotes Candida control and host survival. J Clin Invest 123: 5035–5051, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorgham K, Ghadiri A, Hermand P, Rodero M, Poupel L, Iga M, Hartley O, Gorochov G, Combadière C, Deterre P: An engineered CX3CR1 antagonist endowed with anti-inflammatory activity. J Leukoc Biol 86: 903–911, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Lavergne E, Labreuche J, Daoudi M, Debré P, Cambien F, Deterre P, Amarenco P, Combadière C, GENIC Investigators : Adverse associations between CX3CR1 polymorphisms and risk of cardiovascular or cerebrovascular disease. Arterioscler Thromb Vasc Biol 25: 847–853, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Moatti D, Faure S, Fumeron F, Amara M-W, Seknadji P, McDermott DH, Debré P, Aumont MC, Murphy PM, de Prost D, Combadière C: Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 97: 1925–1928, 2001 [DOI] [PubMed] [Google Scholar]
- 31.McDermott DH, Halcox JP, Schenke WH, Waclawiw MA, Merrell MN, Epstein N, Quyyumi AA, Murphy PM: Association between polymorphism in the chemokine receptor CX3CR1 and coronary vascular endothelial dysfunction and atherosclerosis. Circ Res 89: 401–407, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Rodero M, Marie Y, Coudert M, Blondet E, Mokhtari K, Rousseau A, Raoul W, Carpentier C, Sennlaub F, Deterre P, Delattre JY, Debré P, Sanson M, Combadière C: Polymorphism in the microglial cell-mobilizing CX3CR1 gene is associated with survival in patients with glioblastoma. J Clin Oncol 26: 5957–5964, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Sirois-Gagnon D, Chamberland A, Perron S, Brisson D, Gaudet D, Laprise C: Association of common polymorphisms in the fractalkine receptor (CX3CR1) with obesity. Obesity (Silver Spring) 19: 222–227, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Daoudi M, Lavergne E, Garin A, Tarantino N, Debré P, Pincet F, Combadière C, Deterre P: Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J Biol Chem 279: 19649–19657, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Aird WC: The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood 101: 3765–3777, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Cavaillon JM, Adib-Conquy M: Monocytes/macrophages and sepsis. Crit Care Med 33[Suppl]: S506–S509, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Serbina NV, Jia T, Hohl TM, Pamer EG: Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol 26: 421–452, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winter C, Taut K, Srivastava M, Länger F, Mack M, Briles DE, Paton JC, Maus R, Welte T, Gunn MD, Maus UA: Lung-specific overexpression of CC chemokine ligand (CCL) 2 enhances the host defense to Streptococcus pneumoniae infection in mice: Role of the CCL2-CCR2 axis. J Immunol 178: 5828–5838, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Chen L, Zhang Z, Barletta KE, Burdick MD, Mehrad B: Heterogeneity of lung mononuclear phagocytes during pneumonia: Contribution of chemokine receptors. Am J Physiol Lung Cell Mol Physiol 305: L702–L711, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seeley EJ, Barry SS, Narala S, Matthay MA, Wolters PJ: Noradrenergic neurons regulate monocyte trafficking and mortality during gram-negative peritonitis in mice. J Immunol 190: 4717–4724, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winter C, Herbold W, Maus R, Länger F, Briles DE, Paton JC, Welte T, Maus UA: Important role for CC chemokine ligand 2-dependent lung mononuclear phagocyte recruitment to inhibit sepsis in mice infected with Streptococcus pneumoniae. J Immunol 182: 4931–4937, 2009 [DOI] [PubMed] [Google Scholar]
- 42.Dunay IR, Damatta RA, Fux B, Presti R, Greco S, Colonna M, Sibley LD: Gr1(+) inflammatory monocytes are required for mucosal resistance to the pathogen Toxoplasma gondii. Immunity 29: 306–317, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F: Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal. Cell 153: 362–375, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX3C motif. Nature 385: 640–644, 1997 [DOI] [PubMed] [Google Scholar]
- 45.Harrison JK, Jiang Y, Wees EA, Salafranca MN, Liang HX, Feng L, Belardinelli L: Inflammatory agents regulate in vivo expression of fractalkine in endothelial cells of the rat heart. J Leukoc Biol 66: 937–944, 1999 [DOI] [PubMed] [Google Scholar]
- 46.He M, Moochhala SM, Adhikari S, Bhatia M: Administration of exogenous fractalkine, a CX3C chemokine, is capable of modulating inflammatory response in cecal ligation and puncture-induced sepsis. Shock 31: 33–39, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H: CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol 164: 4313–4320, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Kerfoot SM, Lord SE, Bell RB, Gill V, Robbins SM, Kubes P: Human fractalkine mediates leukocyte adhesion but not capture under physiological shear conditions; a mechanism for selective monocyte recruitment. Eur J Immunol 33: 729–739, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Roller J, Menger MD, Thorlacius H: Sepsis-induced leukocyte adhesion in the pulmonary microvasculature in vivo is mediated by CD11a and CD11b. Eur J Pharmacol 702: 135–141, 2013 [DOI] [PubMed] [Google Scholar]
- 50.Petit-Bertron AF, Fitting C, Cavaillon JM, Adib-Conquy M: Adherence influences monocyte responsiveness to interleukin-10. J Leukoc Biol 73: 145–154, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Yurochko AD, Liu DY, Eierman D, Haskill S: Integrins as a primary signal transduction molecule regulating monocyte immediate-early gene induction. Proc Natl Acad Sci U S A 89: 9034–9038, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarbock A, Gomez H, Kellum JA: Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care 20: 588–595, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saederup N, Cardona AE, Croft K, Mizutani M, Cotleur AC, Tsou CL, Ransohoff RM, Charo IF: Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5: e13693, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Combadière C, Potteaux S, Gao JL, Esposito B, Casanova S, Lee EJ, Debré P, Tedgui A, Murphy PM, Mallat Z: Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 107: 1009–1016, 2003 [DOI] [PubMed] [Google Scholar]
- 55.Payen D, Lukaszewicz AC, Legrand M, Gayat E, Faivre V, Megarbane B, Azoulay E, Fieux F, Charron D, Loiseau P, Busson M: A multicentre study of acute kidney injury in severe sepsis and septic shock: Association with inflammatory phenotype and HLA genotype. PLoS ONE 7: e35838, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.