Abstract
The course of autosomal dominant polycystic kidney disease (ADPKD) varies among individuals, with some reaching ESRD before 40 years of age and others never requiring RRT. In this study, we developed a prognostic model to predict renal outcomes in patients with ADPKD on the basis of genetic and clinical data. We conducted a cross-sectional study of 1341 patients from the Genkyst cohort and evaluated the influence of clinical and genetic factors on renal survival. Multivariate survival analysis identified four variables that were significantly associated with age at ESRD onset, and a scoring system from 0 to 9 was developed as follows: being male: 1 point; hypertension before 35 years of age: 2 points; first urologic event before 35 years of age: 2 points; PKD2 mutation: 0 points; nontruncating PKD1 mutation: 2 points; and truncating PKD1 mutation: 4 points. Three risk categories were subsequently defined as low risk (0–3 points), intermediate risk (4–6 points), and high risk (7–9 points) of progression to ESRD, with corresponding median ages for ESRD onset of 70.6, 56.9, and 49 years, respectively. Whereas a score ≤3 eliminates evolution to ESRD before 60 years of age with a negative predictive value of 81.4%, a score >6 forecasts ESRD onset before 60 years of age with a positive predictive value of 90.9%. This new prognostic score accurately predicts renal outcomes in patients with ADPKD and may enable the personalization of therapeutic management of ADPKD.
Keywords: end-stage renal disease, ADPKD, risk factors, genetic renal disease, progression of renal failure
Autosomal dominant polycystic kidney disease (ADPKD) is the most common Mendelian kidney disorder, and it represents 5%–10% of the ESRD causes in incident patients needing RRT.1,2 ADPKD is genetically heterogeneous with two causative genes identified: PKD1, located at 16p13.3, and PKD2, located at 4q21, with >1400 pathogenic mutations reported so far.3 ADPKD is characterized by fluid-filled cyst development that causes progressive and irreversible deterioration of kidney function. Approximately 50% of the patients experience ESRD onset in the sixth decade,4 but a high level of variability exists in relation to renal outcomes, ranging from rare and very severe neonatal forms5,6 to only moderate GFR reductions after 75 years of age.7
Substantial progress in understanding the cellular mechanisms underlying ADPKD8 has triggered the development of targeted therapies that interfere with cyst formation and proliferation. However, despite the impressive effects of candidate drugs in murine models, which include mammalian target of rapamycin inhibitors,9,10 somatostatin analogues,11 and vasopressin-2 receptor antagonists,12 the results from most of the clinical trials have been disappointing. For instance, two clinical trials involving mammalian target of rapamycin inhibitors have shown no benefit.9,10 Furthermore, octreotide administration over a 3-year period did not significantly reduce the increase in total kidney volume (TKV) or kidney function deterioration.11 Additionally, a large trial evaluating tolvaptan, a vasopressin-2 receptor antagonist, demonstrated a moderate but significant reduction in TKV and kidney function deterioration during a 3-year period.12 One explanation for these mixed results may be the heterozygous nature of the cohorts of patients selected in some of these trials. Indeed, demonstrating the significant effects of candidate molecules is difficult when slow and rapidly progressing patients are enrolled.
Undoubtedly, one or more of the new drugs will eventually gain marketing authorization, leading to questions about who should be treated and for how long, and when to start treatment. The benefit-to-risk ratio will be the key issue for physicians to consider, while they also take into account the risk of evolution to ESRD, efficacy, the adverse effects of the molecules, and the treatment costs. As targeted therapies emerge, there is a particular need for accurate prognostic tools that can provide early predictions of renal outcomes in patients with ADPKD. Additionally, a prediction tool would be useful to inform and possibly reassure patients about the likely course of their disease.
In this study, we aimed to assess the predictive value of genotypic and clinical factors and to develop a prognostic algorithm to forecast the progression to ESRD in patients with ADPKD.
RESULTS
Description of the Genkyst Cohort and Overall Renal Survival
Initially, we assessed a cohort of 1341 individuals from 913 pedigrees who participated between late 2009 and January 2015 and already had a molecular analysis of PKD1 and PKD2 genes completed. Table 1 summarizes the cohort’s main characteristics, including age, sex, CKD stage at inclusion based on the eGFR, and genetic status. The median age at ESRD onset was 61.7 years (interquartile range [IQR], 51.8–74.4 years), and 539 patients had reached ESRD. Table 1 presents the causative mutations identified in 1271 patients (850 pedigrees).
Table 1.
Characteristics of the patients
| Characteristic | Value |
|---|---|
| Patients (pedigrees), n (n) | 1341 (913) |
| Median age (IQR) (yr) | 54.7 (43.3–64.3) |
| Male sex, n (%) | 609 (45.4) |
| CKD stage according to eGFR (MDRD formula), n (%)a | |
| I | 175 (13) |
| II | 214 (16) |
| IIIa | 110 (8.2) |
| IIIb | 113 (8.4) |
| IV | 132 (9.8) |
| V Total | 597 (44.6) |
| Requiring RRT (dialysis or transplantation) (n) | 539 |
| Molecular analysis: n pedigrees (%), n patients | |
| PKD1 mutation | 678 (74.3), 1271 |
| PKD2 mutation | 172 (18.8), 248 |
| No mutation identified | 63 (6.9), 70 |
MDRD, Modification of Diet in Renal Disease.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int 3(Suppl): 1–150, 2013.
Results of Univariate Analysis
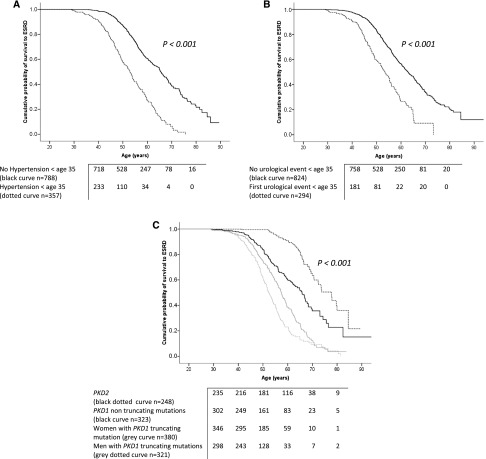
In the univariate analysis, we initially investigated the influence of clinical factors on renal outcomes. Patients who had required treatment for hypertension before age 35 years had significantly worse renal prognoses than patients who had not (Figure 1A, Table 2). The occurrence of any of the three main urologic manifestations of ADPKD (hemorrhagic events involving gross hematuria or cyst hemorrhages, cyst infections, or flank pain related to cysts) before 35 years of age was strongly associated with renal survival (Table 2). Patients presenting with at least one of these urologic manifestations before 35 years of age had more severe renal outcomes than patients with no urologic manifestations before that age (Figure 1B). Smoking status did not influence renal survival. Among women, a higher number of births did not compromise the renal prognosis (Table 2).
Figure 1.
Age at hypertension onset, age at first urologic complication and the causative mutation all influence renal survival. (A) Significant difference in renal survival between patients treated for hypertension before 35 years of age (dotted curve, n=357) and patients who did not receive treatment for hypertension before 35 years of age (black curve, n=788). (B) Significant difference in renal survival between patients presenting with their first urologic event (gross hematuria, flank pain, or cyst infection) before 35 years of age (n=294, dotted curve) and patients without any urologic events before that age (n=824, black curve). (C) Significant differences in renal survival between the PKD2 mutation carriers (genetic group 1, n=248, black dotted curve), PKD1 nontruncating mutation carriers (genetic group 2, n=322, black curve), women with truncating PKD1 mutations (genetic group 3, n=380, gray curve), and men with truncating PKD1 mutations (genetic group 4, n=321, gray dotted curve).
Table 2.
Univariate Cox analysis
| Variable | Patients (n) | Univariate HR (95% CI) | P Value |
|---|---|---|---|
| Sex | |||
| Female | 732 | ||
| Male | 609 | 1.3 (1.0 to 1.4) | 0.017 |
| Hypertension before age 35 yr | |||
| No | 788 | ||
| Yes | 357 | 3.1 (2.6 to 3.8) | <0.001 |
| Macroscopic hematuria before age 35 yr | |||
| No | 964 | ||
| Yes | 150 | 2.9 (2.2 to 3.7) | <0.001 |
| Cyst infection before age 35 yr | |||
| No | 1012 | ||
| Yes | 84 | 2.1 (1.5 to 3.0) | <0.001 |
| Flank pain related to cysts before age 35 yr | |||
| No | 938 | ||
| Yes | 170 | 2.6 (1.9 to 3.4) | <0.001 |
| ≥1 urologic complication before age 35 yr (hematuria, pain, or cyst infection) | |||
| No | 824 | ||
| Yes | 294 | 2.4 (2.0 to 3.0) | <0.001 |
| Smoking status | |||
| Never | 742 | ||
| Active or weaned, <20 pack-years | 389 | 11 (0.8 to 1.2) | 0.92 |
| Active or weaned, >20 pack-years | 118 | 1.2 (0.9 to 1.5) | 0.27 |
| Number of births | |||
| 0 | 112 | ||
| 1 or 2 | 403 | 1.2 (0.8 to 1.8) | 0.389 |
| >2 | 217 | 1.3 (0.9 to 1.6) | 0.085 |
| Mutation | |||
| PKD2 | 248 | ||
| PKD1 nontruncating | 322 | 2.6 (1.9 to 3.7) | <0.001 |
| PKD1 truncating | 701 | 6.2 (4.6 to 8.4) | <0.001 |
95% CI, 95% confidence interval.
We identified the influence of genetic factors on renal survival. Patients harboring truncating PKD1 mutations were more likely to develop ESRD earlier than patients with nontruncating PKD1 mutations and patients with PKD2 mutations, with corresponding median ages for ESRD onset of 55.1 [IQR, 48.5–62.1], 65.8 [IQR, 53–76.5], and 77.8 [IQR, 66.3–84.5] years, respectively. Renal outcomes were significantly worse in men with truncating PKD1 mutations (Figure 1C). Sex was not identified as an influence in patients with nontruncating PKD1 mutations or in patients with PKD2 mutations.
Multivariate Analysis and Development of a Prognostic Model to Predict Survival to ESRD: PRO-PKD Score
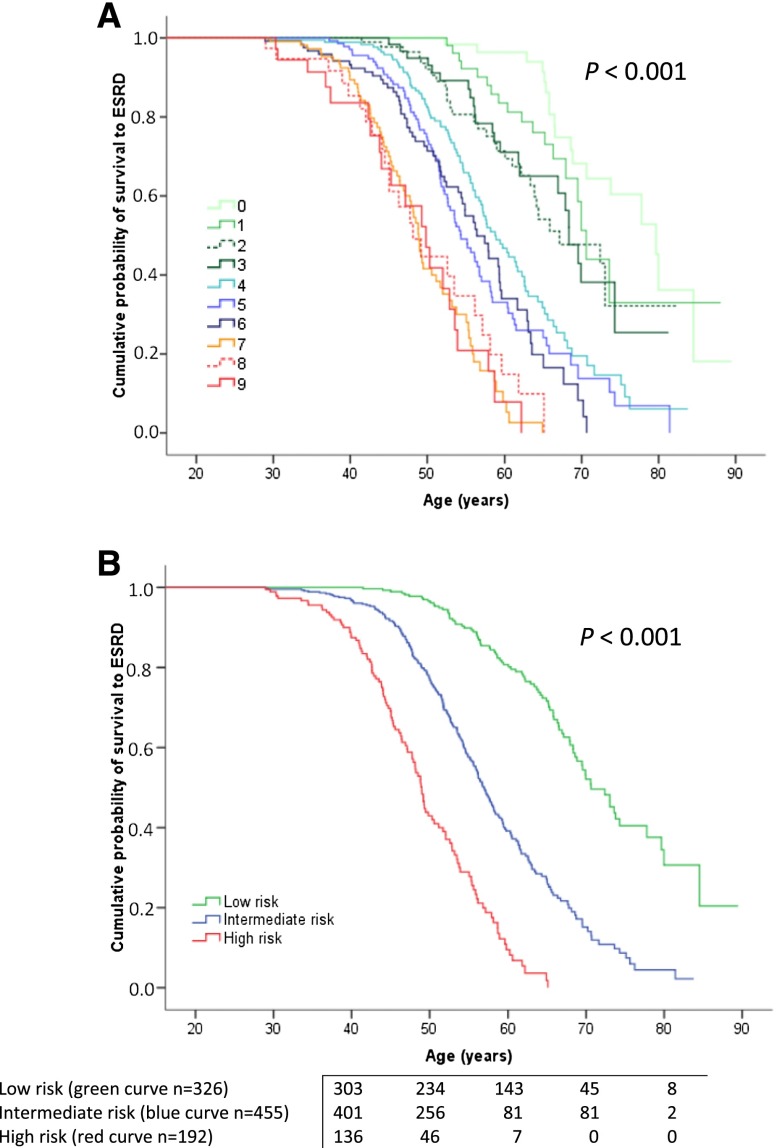
In the multivariate Cox regression model that involved 973 patients (Supplemental Figure 1), four variables remained as independent predictors of the progression to ESRD, namely, sex, need for antihypertensive therapy before 35 years of age (referred hereinafter as age at hypertension onset), occurrence of the first urologic event before 35 years of age, and genetic status (Table 3). These results were confirmed by cross-validation (Supplemental Table 1) and bootstrap resampling analysis (Table 3). No violation of proportionality assumption was found for any variable. A prognostic weighting was derived for each variable based on the hazard ratio (HR) obtained. The highest prognostic weighting was associated with the genotype, with 4 points for truncating PKD1 mutations, 2 points for nontruncating PKD1 mutations, and 0 points for PKD2 mutations. Hypertension onset before 35 years of age and urologic complications before 35 years of age both had weightings of 2 points, and being a man had a weighting of 1 point. Finally, the predicting renal outcomes in ADPKD (PROPKD) score was calculated as the sum of these weightings for the four variables, with results ranging from 0 to 9 points (Table 3). The accuracy of the score at predicting renal survival in the 973 patients was high, with a mean (±SEM) time-dependent area under the receiver-operating characteristic curve (AUC) of 0.84±0.02 at 65 years of age. Three risk categories were defined on the basis of the prognostic scoring, with patients at low, intermediate, and high risks of progression to ESRD scoring 0–3, 4–6, and 7–9 points, respectively. Median ages at ESRD significantly decreased from the low-risk to the intermediate-risk group and from the intermediate-risk to the high-risk group (Figure 2, Table 4). This result was confirmed by the cross-validation strategy (Supplemental Figure 2). At age 60 years, the probabilities of ESRD obtained by Kaplan-Meier analysis were 19.3% in the low-risk group, 60.8% in the intermediate-risk group, and 91.9% in the high-risk group (Table 4). Table 5 presents the performance characteristics of the scores at the two cutoff points of 3 and 6 for the prediction of renal survival at ages 40–65 years. Detailed performances of the score at all the cutoff points are presented in Supplemental Table 2. A PROPKD score ≤3 points allows the possibility of ESRD onset before 60 years of age to be eliminated with a negative predictive value (NPV) of 81.4%. Conversely, a PROPKD score >6 points forecasts ESRD onset before 60 years of age with a positive predictive value (PPV) of 90.9%.
Table 3.
Multivariate Cox analysis
| Variable | Patients (n) | HR (95% CI) | 95% CI from Bootstrap Analysis | P Value | Points for PROPKD Score |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 541 | 0 | |||
| Male | 432 | 1.55 (1.29 to 1.88) | 1.27 to 1.89 | <0.001 | 1 |
| Hypertension before age 35 yr | |||||
| No | 679 | 0 | |||
| Yes | 294 | 2.11 (1.71 to 2.61) | 1.71 to 2.62 | <0.001 | 2 |
| ≥1 urologic event before age 35 yr | |||||
| No | 734 | 0 | |||
| Yes | 239 | 1.73 (1.38 to 2.18) | 1.35 to 2.24 | <0.001 | 2 |
| Mutation | |||||
| PKD2 | 186 | 0 | |||
| PKD1 nontruncating | 239 | 2.27 (1.57 to 3.28) | 1.61 to 3.18 | 0.002 | 2 |
| PKD1 truncating | 548 | 4.75 (3.41 to 6.60) | 3.63 to 6.60 | <0.001 | 4 |
95% CI, 95% confidence interval.
Figure 2.
The PROPKD score enables stratification of risk of progression to ESRD in patients with ADPKD. (A) Renal survival based on PROPKD, with scores ranging from 0 to 9 points. (B) Significant differences in renal survival in patients from the three prognostic categories, as follows: low risk (0–3 points), intermediate risk (4–6 points), and high risk (7–9 points) of early progression to ESRD.
Table 4.
Comparisons of survival without ESRD in patients from the low-, intermediate-, and high-risk categories, based on Kaplan-Meier and Cox analyses
| Prognostic Group | Patients (n) | Kaplan-Meier Analysis | Cox Analysis | ||||
|---|---|---|---|---|---|---|---|
| Median Age at ESRD (IQR) (yr) | Age at ESRD (Minimum–Maximum) (yr) | P Value | Risk of ESRD at Age 60 yr±SEM (%) | HR (95% CI) | P Value | ||
| Low risk | 326 | 70.6 (63.6–84.5) | 41.5–84.6 | 19.3±2.7 | |||
| Intermediate risk | 455 | 56.9 (50.7–65.4) | 28.9–81.5 | <0.001 | 60.8±3.0 | 3.72 (2.89 to 4.78) | <0.001 |
| High risk | 192 | 49 (43.8–55.6) | 29–65.5 | <0.001 | 91.9±3.2 | 10.89 (8.06 to 14.7) | <0.001 |
Table 5.
Performance characteristics of the PROPKD score for the cutoff points (or thresholds) of 3 and 6 (delineating the low-, intermediate-, and high-risk categories) and for different censored ages
| Cutoff Point and Censored Age | Time-Dependent Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 3 (inferior or equal to versus superior) | ||||
| 40 yr | 100±±0 | 36.2±1.7 | 5.8±1.0 | 100±0 |
| 45 yr | 96.2±2.2 | 38.2±1.8 | 14.5±1.5 | 98.9 (0.6) |
| 50 yr | 94.5±1.8 | 43.6±2.1 | 31.9±2.1 | 96.6±1.1 |
| 55 yr | 90.0±1.9 | 50.8 (2.6) | 50.5±2.4 | 90.1±1.9 |
| 60 yr | 86.2±2.0 | 61.9±3.2 | 69.9±2.5 | 81.4±2.7 |
| 65 yr | 82.8±2.4 | 70.1±3.9 | 81.1±2.5 | 72.5±3.3 |
| 6 (inferior or equal to versus superior) | ||||
| 40 yr | 60.0±8.3 | 83.8±1.3 | 12.8±2.6 | 98.1±0.5 |
| 45 yr | 55.7±5.4 | 87.0±1.3 | 31.8±3.9 | 94.7±0.9 |
| 50 yr | 44.4±3.8 | 91.4±1.2 | 59.1±4.5 | 85.5±1.4 |
| 55 yr | 33.3±2.9 | 92.9±1.3 | 72.3±4.4 | 71.4±1.9 |
| 60 yr | 29.5±2.5 | 97.0±1.1 | 90.9±3.2 | 57.3±2.2 |
| 65 yr | 26.5±2.2 | 99.3±0.7 | 98.3±1.7 | 46.6±2.4 |
Percentages are expressed with SEMs. The test result is positive when the score is greater than the cutoff point and negative when the score is less than or equal to the cutoff point (n=973 patients).
Annual Rates of eGFR Decline in the Three Prognostic Groups Defined by the PROPKD Score
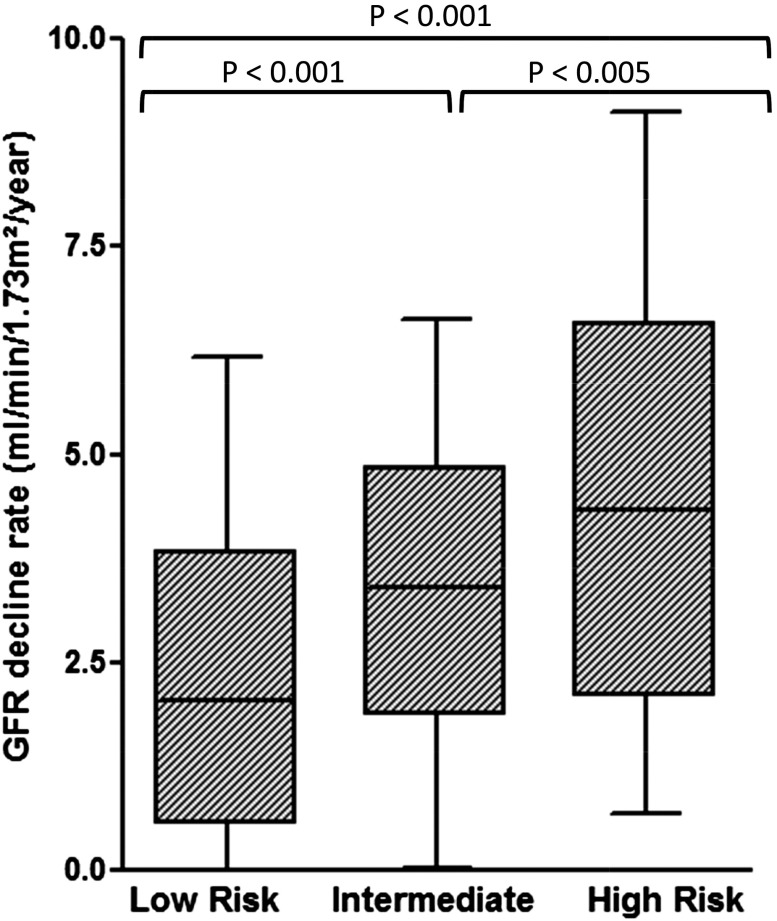
To test whether our prognostic model was suitable for differentiating patients who may progress more rapidly to ESRD, we compared the eGFR decline rates within the different prognostic groups. Data were available for 460 patients. Median annual eGFR decline rate was 3.4 (IQR, 1.3–4.9) ml/min per 1.73 m2 per year. Annual rates of eGFR decline increased across the three prognostic groups: 2 (IQR, 0.6–3.4) ml/min per 1.73 m2 per year in the low-risk group (n=180), 3.4 (IQR, 1.9–4.8) ml/min per 1.73 m2 per year in the intermediate-risk group (n=202), and 4.4 (IQR, 2.1–6.6) ml/min per 1.73 m2 per year (n=78) in the high-risk group (P<0.001) (Figure 3).
Figure 3.
Annual loss of kidney function is statistically different in the three prognostic groups defined by the PROPKD score. Median rates of eGFR decline (ml/min per 1.73 m2 per year) are presented with their 10% confidence interval (whiskers) in patients at inclusion who had at least two eGFR values (minimum, 2 eGFR values; maximum, 13 eGFR values) of 15–90 separated by at least 1 year. Significant differences are apparent between the low-risk group (n=180; median eGFR decline, 2 [IQR, 0.6–3.4] ml/min per 1.73 m2 per year), the intermediate-risk group (n=202; median eGFR decline, 3.4 [IQR, 1.9–4.8] ml/min per 1.73 m2 per year), and the high-risk group (n=78; median eGFR decline, 4.4 [IQR, 2.1–6.6] ml/min per 1.73 m2 per year).
Alternative for Patients with Missing Clinical Data: The Genetic Score
To address the issue of patients aged <35 years and patients with missing clinical data for whom the PROPKD score is not applicable, we evaluated the predictive ability of a genetic score that comprised the genetic data and the sex only. According to the Cox and Kaplan-Meier analyses, four categories of patients differed significantly from one another in terms of renal survival, and four prognostics groups were defined as follows: patients with PKD2 mutations: 1 point; patients with nontruncating PKD1 mutations: 2 points; women with truncating PKD1 mutations: 3 points; and men with truncating PKD1 mutations: 4 points (Figure 1D). A genetic score ≥2 points, which incorporates the presence of a truncating PKD1 mutation, predicted ESRD onset before age 65 years with a sensitivity of 73.8%, a specificity of 74.3%, a PPV of 80.4%, and an NPV of 66.6%. Table 6 presents the performance characteristics of the genetic score.
Table 6.
Performance characteristics of the genetic score for the cutoff points (or thresholds) of 1, 2, and 3 and for different censored ages
| Cutoff Point and Censored Age | Time-Dependent Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|
| 1 (inferior or equal to versus superior) | ||||
| 40 yr | 100±0 | 21.5±1.3 | 4.5±0.7 | 100±0 |
| 45 yr | 99.0±1.0 | 23.7±1.5 | 12.2±1.2 | 99.5±0.5 |
| 50 yr | 99.5±0.5 | 27.6±1.7 | 26.7±1.7 | 99.5±0.5 |
| 55 yr | 97.4±1.0 | 32.5±2.26 | 43.6±2.0 | 95.9±1.5 |
| 60 yr | 95.9±1.1 | 39.9±2.9 | 60.2±2.1 | 91.1±2.3 |
| 65 yr | 93.1±1.4 | 45.8±3.7 | 71.0±2.2 | 82.4±3.4 |
| 2 (inferior or equal to versus superior) | ||||
| 40 yr | 84.8±5.7 | 46.4±1.6 | 5.5±0.9 | 98.8±0.5 |
| 45 yr | 79.6±4.1 | 48.1±1.7 | 14.1±1.5 | 95.6±1.0 |
| 50 yr | 81.3±2.8 | 52.2±2.0 | 31.1±2.1 | 91.3±1.4 |
| 55 yr | 77.9±2.4 | 57.9±2.3 | 49.7±2.4 | 83.0±1.9 |
| 60 yr | 76.7±2.2 | 68.4±2.7 | 69.7±2.5 | 75.6±2.3 |
| 65 yr | 73.8±2.2 | 74.3±3.3 | 80.4±2.4 | 66.6±2.8 |
| 3 (inferior or equal to versus superior) | ||||
| 40 yr | 46.6±8.0 | 75.8±1.4 | 6.6±1.5 | 97.5±0.6 |
| 45 yr | 46.5±5.0 | 77.3±1.4 | 18.1±2.4 | 93.1±0.9 |
| 50 yr | 45.5±3.6 | 80.5±1.6 | 38.2±3.3 | 84.8±1.4 |
| 55 yr | 43.0±2.9 | 84.0±1.7 | 58.9±3.5 | 73.4±1.8 |
| 60 yr | 40.0±2.5 | 88.7±1.9 | 77.0±3.4 | 60.9±2.1 |
| 65 yr | 37.8±2.3 | 91.6±2.1 | 86.5±3.1 | 50.8±2.3 |
Percentages are expressed with SEMs. The test result is positive when the score is greater than the cutoff point and negative when the score is less than or equal to the cutoff point (n=946 patients).
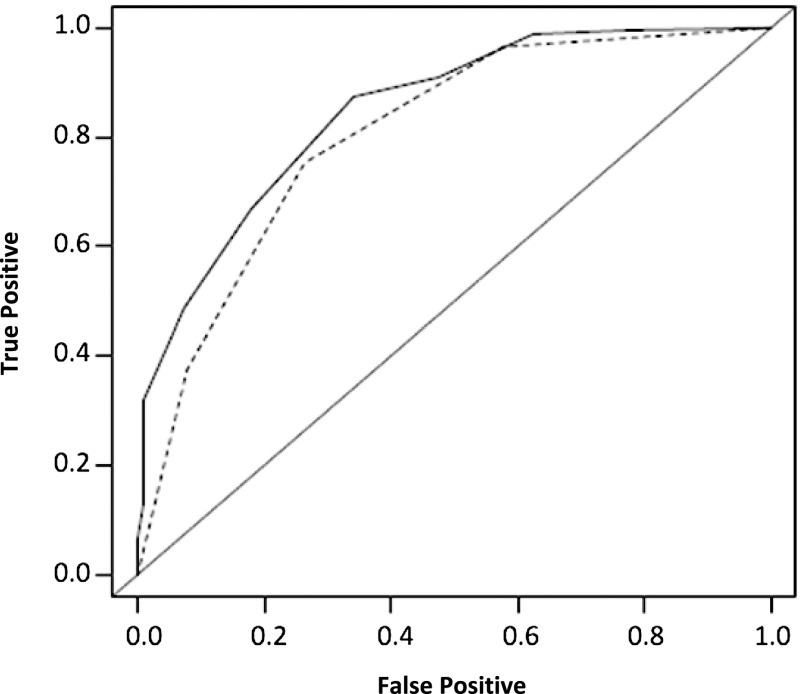
We then compared the performances of the PROPKD and the genetic scores. The PROPKD time-dependent AUC was significantly higher (P<0.002) for all ages censored between 40 years and 65 years (Figure 4).
Figure 4.
The genetic scoring offers a good prediction of ESRD but is less accurate than the PROPKD score. A significant difference was seen between the AUC of the genetic scoring (mean AUC [±SEM], 0.79±0.02; dotted line) and of the PROPKD score (AUC, 0.84±0.02; black line) at 65 years of age (P<0.001).
DISCUSSION
While we are moving to the era of targeted therapies in ADPKD, prognostic tools are necessary for the optimal selection of patients in clinical trials and, thereafter, the choice of treated patients when the treatments will be available. In addition, an increasing number of patients express a demand of information on their individual risk of progression to ESRD. Herein, we have proposed a new algorithm that integrates molecular genetic and clinical data to predict renal survival in patients with ADPKD and enables a personal approach to clinical decision making.
TKV has been proven highly predictive of eGFR decline in different studies.13–15 In particular, height-adjusted TKV >600 ml/m predicted the development of stage 3 CKD within 8 years.14 More recently, Irazabal and colleagues developed an imagery-based prognostic model15 to predict GFR decline in patients with ADPKD. The authors demonstrated that ellipsoid TKV at a given age enabled to predict GFR decline slope in patients with ADPKD and proposed to forecast GFR value at any time, entering TKV, age, and creatinine at baseline. Although the overall prediction value of the model is very good, its performance is less accurate in patients with normal kidney function. Additionally, TKV should be reevaluated in patients in whom the model predicts a good renal prognosis, as more than one out of five of them will later move to another prognostic category. Last, the model could not be applied in 8.8% of the patients of the initial cohort with atypical imagery presentation. For all these reasons, the imagery-based model, which performs well in predicting eGFR decline in a short-term perspective, seems more appropriate to select patients in the clinical trials than for long-term prediction of renal outcome in patients with ADPKD. The prognostic model proposed by Irazabal and ours should therefore not be considered as mutually exclusive but as two different and complementary tools.
To demonstrate significant effects of candidate molecules, future trials should focus on patients with poor renal prognoses, and we believe that the PROPKD score may be a valuable tool that could identify these patients. Indeed, patients from the high-risk group, (19.7% of the patients in our cohort), appear to meet this definition, as they have a 90.9% risk of evolution to ESRD before age 60 years. In sharp contrast, patients from the low-risk group (33.5% of the patients in our cohort), should not be exposed to the potential adverse effects of candidate molecules, because they have good renal prognoses, and this is supported by the NPV of 81.4% for a PROPKD score ≤3 points at 60 years of age.
The same rationale may help to customize therapeutic decision making when the treatments become available. Indeed, while the decision to treat or not to treat will be relatively straightforward for patients in the high- and low-risk categories, different factors may influence the decision to treat patients with an intermediate risk of ESRD onset, which will include the patient’s tolerance of the molecule, the quality of the patient’s life, and health reimbursement policies in the different countries. It is worth underlining that risk assessment provided by our score does not preclude classic follow-up, especially monitoring of kidney function. The observation of a faster eGFR decline than expected in a patient from the low-risk category, for instance, shall lead to a reconsideration of the risk assessment and subsequent therapeutic decisions.
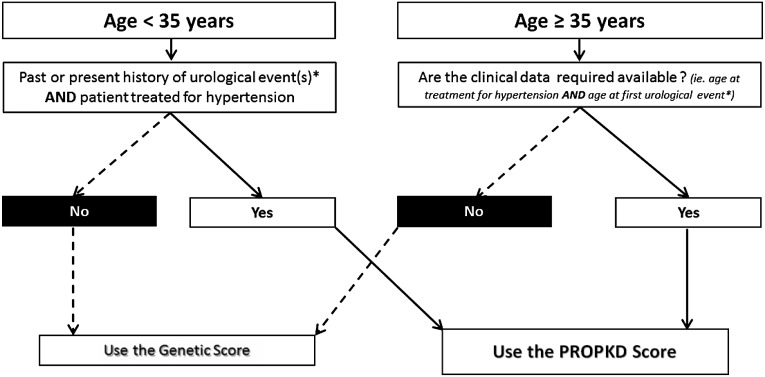
The PROPKD score can be used from 35 years of age or earlier in patients with both hypertension and urologic complications before this age. For patients requiring earlier evaluations of their prognoses or for those whose clinical data are missing, including the age at first antihypertensive therapy or when the first urologic complication occurred, the genetic score, although less accurate, provides a good estimate of renal prognosis (Figure 5). In the absence of other large existing cohorts with patients recruited at all the CKD stages, including ESRD, and with both precise retrospective clinical data and molecular genetic results available, external validation has not been possible. However, model robustness was internally evaluated using a conjunction of bootstrap and cross-validation testing, which led to highly consistent findings. A longitudinal replication analysis would be an ideal method to confirm the prognostic value of our scoring system; however, since ADPKD is a slowly progressive disease, this will not be feasible in the near future.
Figure 5.
Decision-support algorithm to assist in the selection of a prognostic model to predict renal outcomes in patients with ADPKD. For patients younger than 35 years of age who have already experienced urologic events, including gross hematuria, flank pain, or cyst infections, and are already receiving treatment for hypertension and for patients older than 35 years of age with clinical data available, the PROPKD score is applicable. For patients with ADPKD under 35 years of age and/or for whom clinical data are lacking, the genetic score, although less accurate, can be used to predict renal survival.
Although mutation detection rates have increased significantly in the last 10 years, molecular analysis of the PKD1 and PKD2 mutations remains negative in 7%–10% of the pedigrees16–18 (6.3% in our cohort), rendering the genetic and the PROPKD scores inapplicable to this patient subset. In addition, we are conscious that the cost of molecular analysis remains an important barrier to its widespread use, but this cost should decline steadily in the forthcoming years, because of the automation of reaction preparations and the development of next-generation sequencing technologies.17 Furthermore, not all patients with ADPKD require comprehensive analyses of both genes because confirmation of a previously identified familial mutation will be sufficient for some patients, and it is much less expensive.
Predicting renal outcomes is, more than ever before, a key question for the patient and the physician in ADPKD. As targeted therapies approach, nephrologists should expect to more patients with milder forms of ADPKD referred to them. They will have the responsibility of determining which patients should be treated, who can benefit from regular monitoring, and who should be reassured. We believe that the PROPKD score will be valuable in assisting with this difficult task. As illustrated in this study, combining clinical and genetic data to predict a disease’s evolution aligns perfectly with the entry in the era of personalized medicine.
CONCISE METHODS
Participants
Genkyst is a cross-sectional study involving >70 nephrologist investigators working in Brittany, France. Patients with ADPKD from the Genkyst cohort were recruited from dialysis centers and through nephrology and transplant consultations between 2009 and January 2015. ADPKD diagnoses were made using previously described criteria.19 The patients’ clinical data were obtained during medical interviews at the time of their inclusion and from medical records and were entered in standardized clinical research forms. The data evaluated included kidney function, calculated using the Modification of Diet in Renal Disease formula, or the age at ESRD onset, which was defined according to the requirement for dialysis or transplantation, and factors that have been reported to influence renal survival,20 such as BP, age at hypertension (defined as the age when the first antihypertensive therapy was administered), the previous occurrence of urologic events (gross hematuria, symptomatic cyst hemorrhage, cyst infections, and flank pain related to cysts), the number of births in women, and smoking status. According to the literature, the threshold of 35 years was chosen for all complications to categorize patients.21 All participants provided informed consent, and the local ethics committee approved the study.
Molecular Analysis
A comprehensive molecular analysis of the PKD1 and PKD2 genes was conducted for all the patients. Mutation detection and pathogenicity predictions for missense mutations were performed as previously described.16,18 Supplemental Table 3 shows all of the mutations identified. PKD1 mutations were divided into two categories: truncating mutations, including nonsense, frameshift, splicing mutations, and large rearrangements, and nontruncating mutations, including missense mutations and in-frame short deletions and insertions.
Statistical Analyses
All statistical analyses were performed using SPSS software, version 19 (IBM Corp., Armonk, NY), and R software, version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria). Renal survival was analyzed using the Kaplan-Meier method. The differences between the survival curves were assessed using a log-rank test with a 0.05 significance level. Univariate Cox proportional-hazards regression analysis was used to identify variables of possible prognostic significance. Variables were entered into the Cox multivariate analysis when they were associated with ESRD onset in the univariate analysis at a conservative threshold of 20%. The final model was chosen through backward selection based on an estimation of the maximum likelihood. We verified the proportional hazard assumption by testing covariate-by-time interactions for each variable in the final model.22
The validities of the HRs and the confidence intervals were confirmed using two different internal validation strategies. First, we used a cross-validation algorithm that used 90% of the cohort’s data for training and 10% for validation; this was repeated 10 times. The second validation strategy used a nonparametric bootstrapping resampling analysis with replacements. We then constructed a scoring system by entering all of the significant variables obtained using multivariate analysis. The number of points for each prognostic factor was obtained by dividing each HR by the smallest HR considered and rounding the resultant ratio to the higher integer. To assess the validity of this scoring system, 10 scores were built from the HR obtained in each of the 10 training datasets (90% of the patients for each) and applied in the patients omitted in each training dataset (i.e., the 10% validation subsets).
Time-dependent receiver-operating characteristic (ROC) curves and the AUCs for censored survival data were used to quantify the discriminative abilities of each score in the prediction of renal survival up to 65 years and to compare the developed scores23; therefore, the Kaplan-Meier estimator of the censored distribution was used. The time-dependent ROC AUC represented the probability that the score of a case (i.e., a patient who undergoes the event at time T before time t) was greater than the score of a control (i.e., an event-free patient at the same time t). Thereby, the time-dependent ROC AUC quantifies how well our scores can distinguish patients who reach ESRD by a given time t from patients who reach ESRD after this time (or who do not reach ESRD).
To assess the predictive performance of the scoring system, time-dependent sensitivities, specificities, PPVs, and NPVs were estimated with their SEMs at several given cutoff points, that is, for different score threshold values. An R package, timeROC version 0.2,24 was used for the time-dependent ROC curve analyses. Additionally, retrospective annual rates of eGFR decline (ml/min per 1.73 m2 per year) were calculated for all the patients who had at least two eGFR values of 15–90 ml/min per 1.73 m2 noted in their standardized case report forms, separated by at least 1 year. Median rates of eGFR decline across the three prognostic groups that were defined by our prognostic scoring system were compared using the Kruskal-Wallis test and Mann-Whitney test for pairwise comparisons after Bonferroni correction of risk.
Disclosures
The authors have no conflicts of interests relevant to this study. E.C.L.G. and Y.L.M. received speaking fees from Otsuka.
Supplementary Material
Acknowledgments
The authors would like to thank all the patients and the Genkyst Group investigators: Dr. Grall, Dr. Tanquerel, Dr. Hanrotel, Dr. Treguer, Dr. Segalen, Dr. Mesguen, Dr. Gosselin, Dr. Kersale, Dr. Hemon, Dr. Lanfranco (Centre Hospitalier Régional Universitaire, Brest); Professor Giral, Dr. Meurette, Dr. Lino, Dr. Garandeau, Dr. Touzot, Dr. Hodemon-Corne, Dr. Allain-Launay, Dr. Cantarovich, Professor Blancho, Dr. Hristea, Dr. Couvrat, Professor Fakhouri, Dr. Lavainne, Dr. Vercel, Dr. Chapal, Dr. Dufay (Centre Hospitalier Universitaire, Nantes); Professor Le Pogamp, Dr. Gie, Dr. Rivalan, Dr. Laruelle, Dr. Richer, Dr. Lorcy (Centre Hospitalier Universitaire, Rennes); Dr. Gatault, Dr. Merieau, Dr. Barbet, Professor Buchler, Dr. Golea, Dr. Ghouti, Dr. Gautard, Professor Halimi, Dr. Sautenet, Dr. François, Dr. Fournier, Dr. Baron, Dr. Prat, Dr. Salmon, Dr. Von Ey, Dr. Rabot, Dr. D’Halluin, Dr. Chevallier, Dr. Moles (Centre Hospitalier Régional Universitaire, Tours); Dr. Stanescu, Dr. Le Cacheux, Dr. Ang, Dr. Coulibaly, Dr. Baluta, Dr. Leonetti, Dr. Boulahrouz (Centre Hospitalier Yves le Foll, Saint Brieuc); Dr. Michez, Dr. Mandart, Dr. Menoyo, Dr. Pincon (Centre Hospitalier de Bretagne Atlantique, Vannes); Dr. Metes (Centre Hospitalier de Cornouaille, Quimper); Dr. Dolley-Hitze (Centre Hospitalier Broussais, Saint Malo); Dr. Desport, Dr. Thierry, Dr. Ecotiere, Professor Touchard, Professor Bridoux, Dr. Belmouaz, Dr. Javaugue, Dr. Bauwens, Dr. Fride Leroy, Dr. Diolez, Dr. Colombo, Dr. Galinier (Centre Hospitalier Universitaire, Poitiers); Dr. Latif, Dr. Massad (Centre Hospitalier du Centre Bretagne, Pontivy); Dr. Depraetre, Dr. Strullu, Dr. Chaffara, Dr. Le Mee (Association des Urémiques de Bretagne, Brest-Morlaix); Dr. Goulesque (Centre de Néphrologie et de Dialyse d’Armorique, Brest); Dr. Regnier-Le Coz, Dr. Blanpain, Dr. Durault (Centre Hospitalier Georges Charpak, Saint-Nazaire); Dr. Chamontin, Dr. Georgescu (Centre Hospitalier de Bretagne Sud, Lorient); Dr. Rifaat; Dr. Dimulescu (Association des Urémiques de Bretagne, Quimper); Dr. Querard, Dr. Target, Dr. Jaulin, Dr. Ottavioli (Centre Hospitalier Départementale de Vendée, La Roche Sur Yon); Dr. Ferrier, Dr. Durand (ECHO, Vannes); Dr. Savoiu, Dr. Lefrançois (ECHO, Saint Herblain-Nantes); Dr. Bertheleme (Centre Héliomarin, Roscoff).
The authors would also like to acknowledge the clinical research team (Christelle Ratajczak, Christelle Guillerm-Regost, and Stéphanie Bouvier) and the Molecular Genetic Laboratory of Brest (]oëlle Creff, Isabelle Quéré, Sandrine Maestri, Caroline Benech, and Sylvia Redon).
This study was conducted with the support of the National Plan for Clinical Research (PHRC regional 2010), Groupement Inter-Régional de Recherche Clinique et d’Innovation (GIRGI grand-ouest), the Société Française de Néphrologie, and the Institut National de la Santé et de la Recherche Médicale.
Part of this work was presented as an oral communication at the annual meeting of the American Society of Nephrology, November 2013, Atlanta, Georgia.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015010016/-/DCSupplemental.
References
- 1.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Shaw C, Simms RJ, Pitcher D, Sandford R: Epidemiology of patients in England and Wales with autosomal dominant polycystic kidney disease and end-stage renal failure. Nephrol Dial Transplant 29: 1910–1918, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Cornec-Le Gall E, Audrézet M-P, Meur YL, Chen J-M, Férec C: Genetics and pathogenesis of autosomal dominant polycystic kidney disease: 20 years on. Hum Mutat 35: 1393–1406, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Cornec-Le Gall E, Audrézet M-P, Chen J-M, Hourmant M, Morin M-P, Perrichot R, Charasse C, Whebe B, Renaudineau E, Jousset P, Guillodo M-P, Grall-Jezequel A, Saliou P, Férec C, Le Meur Y: Type of PKD1 mutation influences renal outcome in ADPKD. J Am Soc Nephrol 24: 1006–1013, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zerres K, Rudnik-Schöneborn S, Deget F: Childhood onset autosomal dominant polycystic kidney disease in sibs: Clinical picture and recurrence risk. German Working Group on Paediatric Nephrology (Arbeitsgemeinschaft für Pädiatrische Nephrologie. J Med Genet 30: 583–588, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fick GM, Johnson AM, Strain JD, Kimberling WJ, Kumar S, Manco-Johnson ML, Duley IT, Gabow PA: Characteristics of very early onset autosomal dominant polycystic kidney disease. J Am Soc Nephrol 3: 1863–1870, 1993 [DOI] [PubMed] [Google Scholar]
- 7.Torra R, Badenas C, Pérez-Oller L, Luis J, Millán S, Nicolau C, Oppenheimer F, Milà M, Darnell A: Increased prevalence of polycystic kidney disease type 2 among elderly polycystic patients. Am J Kidney Dis 36: 728–734, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Harris PC, Torres VE: Genetic mechanisms and signaling pathways in autosomal dominant polycystic kidney disease. J Clin Invest 124: 2315–2324, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walz G, Budde K, Mannaa M, Nürnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Hörl WH, Obermüller N, Arns W, Pavenstädt H, Gaedeke J, Büchert M, May C, Gschaidmeier H, Kramer S, Eckardt K-U: Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363: 830–840, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, Visciano B, Imbriaco M, Messa P, Cerutti R, Dugo M, Cancian L, Buongiorno E, De Pascalis A, Gaspari F, Carrara F, Rubis N, Prandini S, Remuzzi A, Remuzzi G, Ruggenenti P, ALADIN study group : Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo-controlled, multicentre trial. Lancet 382: 1485–1495, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS, TEMPO 3:4 Trial Investigators : Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367: 2407–2418, 2012. 23121377 [Google Scholar]
- 13.Grantham JJ, Torres VE, Chapman AB, Guay-Woodford LM, Bae KT, King BF, Jr, Wetzel LH, Baumgarten DA, Kenney PJ, Harris PC, Klahr S, Bennett WM, Hirschman GN, Meyers CM, Zhang X, Zhu F, Miller JP, CRISP Investigators : Volume progression in polycystic kidney disease. N Engl J Med 354: 2122–2130, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Chapman AB, Bost JE, Torres VE, Guay-Woodford L, Bae KT, Landsittel D, Li J, King BF, Martin D, Wetzel LH, Lockhart ME, Harris PC, Moxey-Mims M, Flessner M, Bennett WM, Grantham JJ: Kidney volume and functional outcomes in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 7: 479–486, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE, the CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audrézet M-P, Cornec-Le Gall E, Chen J-M, Redon S, Quéré I, Creff J, Bénech C, Maestri S, Le Meur Y, Férec C: Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat 33: 1239–1250, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Rossetti S, Hopp K, Sikkink RA, Sundsbak JL, Lee YK, Kubly V, Eckloff BW, Ward CJ, Winearls CG, Torres VE, Harris PC: Identification of gene mutations in autosomal dominant polycystic kidney disease through targeted resequencing. J Am Soc Nephrol 23: 915–933, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossetti S, Consugar MB, Chapman AB, Torres VE, Guay-Woodford LM, Grantham JJ, Bennett WM, Meyers CM, Walker DL, Bae K, Zhang QJ, Thompson PA, Miller JP, Harris PC, CRISP Consortium : Comprehensive molecular diagnostics in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 18: 2143–2160, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D: Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20: 205–212, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrier RW, Brosnahan G, Cadnapaphornchai MA, Chonchol M, Friend K, Gitomer B, Rossetti S: Predictors of autosomal dominant polycystic kidney disease progression. J Am Soc Nephrol 25: 2399–2418, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AM, Gabow PA: Identification of patients with autosomal dominant polycystic kidney disease at highest risk for end-stage renal disease. J Am Soc Nephrol 8: 1560–1567, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Harrell FE, Jr, Lee KL, Mark DB: Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361–387, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Heagerty PJ, Zheng Y: Survival model predictive accuracy and ROC curves. Biometrics 61: 92–105, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Blanche P, Dartigues J-F, Jacqmin-Gadda H: Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med 32: 5381–5397, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.