Abstract
Preeclampsia is a devastating complication of pregnancy. Soluble Fms-like tyrosine kinase-1 (sFlt-1) is an antiangiogenic protein believed to mediate the signs and symptoms of preeclampsia. We conducted an open pilot study to evaluate the safety and potential efficacy of therapeutic apheresis with a plasma-specific dextran sulfate column to remove circulating sFlt-1 in 11 pregnant women (20–38 years of age) with very preterm preeclampsia (23–32 weeks of gestation, systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg, new onset protein/creatinine ratio >0.30 g/g, and sFlt-1/placental growth factor ratio >85). We evaluated the extent of sFlt-1 removal, proteinuria reduction, pregnancy continuation, and neonatal and fetal safety of apheresis after one (n=6), two (n=4), or three (n=1) apheresis treatments. Mean sFlt-1 levels were reduced by 18% (range 7%–28%) with concomitant reductions of 44% in protein/creatinine ratios. Pregnancy continued for 8 days (range 2–11) and 15 days (range 11–21) in women treated once and multiple times, respectively, compared with 3 days (range 0–14) in untreated contemporaneous preeclampsia controls (n=22). Transient maternal BP reduction during apheresis was managed by withholding pre-apheresis antihypertensive therapy, saline prehydration, and reducing blood flow through the apheresis column. Compared with infants born prematurely to untreated women with and without preeclampsia (n=22 per group), no adverse effects of apheresis were observed. In conclusion, therapeutic apheresis reduced circulating sFlt-1 and proteinuria in women with very preterm preeclampsia and appeared to prolong pregnancy without major adverse maternal or fetal consequences. A controlled trial is warranted to confirm these findings.
Keywords: clinical trial, preeclampsia, pregnancy, vascular endothelial growth factor, sFlt-1, therapeutic apheresis
Preeclampsia remains a potentially devastating medical complication of pregnancy, with adverse consequences to both mother and fetus, most notably when the condition occurs very preterm.1–3 Preeclampsia affects 3%–8% of pregnancies worldwide and is among the hypertensive disorders contributing to maternal mortality.1–4 Preventive strategies for preeclampsia have been largely unsuccessful,2,5,6 in part, because our understanding of the pathogenesis has been limited.
Although several putative factors have been suggested, mounting evidence suggests that soluble Fms-like tyrosine kinase-1 (sFlt-1) plays a role in the maternal signs and symptoms of preeclampsia.7,8 Possible approaches to antagonize sFlt-1 and its physiologic effects include saturating the system with its natural ligands (i.e., vascular endothelial growth factor [VEGF] or placental growth factor [PlGF]), administering anti-sFlt-1 antibodies or small molecules to reduce sFlt-1 production (e.g., small interfering [si]RNA), and removing circulating sFlt-1 with an extracorporeal device.9
Although we and others have performed experimental studies for each strategy,10–13 therapeutic apheresis exploits the fact that sFlt-1 circulates in blood with a strongly positive charge, and complementary negatively charged columns employed in apheresis devices have already been approved worldwide and are safely used for the treatment of familial hypercholesterolemia, including in pregnant women.14–16 Moreover, selective removal of circulating sFlt-1 avoids the removal of placental sFlt-1, which may be necessary for maintaining placental health.17
We previously demonstrated that whole-blood apheresis using a negatively charged dextran sulfate column reduces circulating sFlt-1 and proteinuria in women with very preterm preeclampsia.13 To improve the efficiency of sFlt-1 removal, minimize the need for therapeutic anticoagulation, reduce potential activation of immune cells, and obtain detailed outcome information regarding fetuses and neonates, we conducted this single-arm pilot study using an apheresis device that first separates plasma from whole blood before passing through a plasma-specific dextran sulfate (PSDS) column. These columns are widely available16 and we suspected that their side-effect profiles might be superior to whole-blood column apheresis when used in women with preterm preeclampsia. We aimed to treat 10–12 patients to provide evidence of safety sufficient to inform design of a subsequent randomized trial; the first several patients were to be treated once, and the rest more than once. Twenty-two women with preterm preeclampsia not treated with apheresis and 22 women who delivered preterm for reasons other than preeclampsia were selected as contemporaneous controls so that the outcomes of infants born to untreated versus treated women could be compared.
RESULTS
Maternal Effects of Apheresis
Eleven pregnant women with very preterm preeclampsia18 (gestational ages ranging from 25+0/7 to 30+4/7 weeks) were enrolled. More than half (6 of 11 women) had BP measurements at enrollment that were consistent with existing definitions of severe disease.19 See Supplemental Table 1 for their individual demographic and baseline clinical features.
The 11 women received a total of 17 apheresis treatments (six were treated once, four were treated twice and one was treated three times); all experienced a reduction of sFlt-1 (Table 1). The mean pre-apheresis sFlt-1 concentration was 17,394 (range 7916–35,301) pg/ml and fell post-apheresis to 14,265 (6446–26,259) pg/ml. The mean percentage reduction in sFlt-1 per treatment (measured within 4 hours following apheresis) was 18% (7%–28%). Thirteen treatments were associated with a decrease in protein/creatinine (P/C) ratios following apheresis. The mean starting P/C ratio in ten patients with both pretreatment and post-treatment values was 6.5 g/g (0.4–16.9 g/g), which fell to a mean of 3.2 (0.2–8.6) g/g. The mean percentage reduction in P/C ratio per treatment was 44% (range: 88% reduction to a 0% change). Changes in systolic and diastolic BP, hematocrit, and platelet counts pre- and post-apheresis averaged among all treated women are summarized in Table 2.
Table 1.
Characteristics of women undergoing apheresis treatment(s)
| Patient | Gestational Age (GA) at Admission (weeks + days) | sFlt-1/PlGF | sFlt-1 (pg/ml) | P/C ratio (g/g) | Apheresis volume (ml) | GA at delivery (weeks + days) | Continuation of pregnancy (days) | Reason for Delivery | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | Pre | Post | Change | |||||||
| One Treatment | ||||||||||||
| A | 30+3 | 143 | 8243 | 7628 | −7% | 15.8 | 6.7 | −58% | 1000 | 32+0 | +11 | Abnormal fetal heart variability |
| B | 30+0 | 1194 | 21,847 | 19,997 | −8% | 1.7 | 0.2 | −88% | 1000 | 30+2 | +2 | Abdominal pain; Worsening hypertension |
| C | 27+2 | 567 | 17,358 | 14,004 | −19% | 7.9 | 2.5 | −68% | 900 | 28+3 | +8 | Worsening hypertension |
| D | 27+3 | 1753 | 18,929 | 14,848 | −22% | 1.3 | 1.2 | −8% | 1000 | 28+5 | +9 | Headache; Visual symptoms; Worsening hypertension |
| E | 29+4 | 1012 | 15,397 | 11,740 | −24% | 0.4 | 0.4 | 0% | 400 | 30+4 | +7 | Worsening thrombocytopenia |
| Fa | 30+4 | 325 | 35,301 | 26,259 | −26% | 3.0 | 1.6 | −47% | 1500 | 32+0 | +10 | Headache |
| Two Treatments | ||||||||||||
| G | 28+0 | 150 | 11,276 | 9800 | −13% | 1.7 | — | — | 700 | 30+0 | +14 | Worsening thrombocytopenia |
| 12,245 | 10,836 | −12% | 3.0 | — | — | 1000 | ||||||
| H | 25+0 | 1150 | 19,550 | 17,320 | −11% | 19.3 | 7.7 | −60% | 500 | 26+4 | +11 | Abnormal fetal heart variability |
| 23,569 | 23,162 | −2% | 14.5 | 9.4 | −35% | 500 | ||||||
| I | 28+6 | 674 | 19,019 | 14,955 | −21% | 9.9 | 11.8 | +19% | 600 | 30+3 | +11 | Worsening hypertension |
| 25,024 | 20,063 | −20% | 11.1 | 2.4 | −78% | 600 | ||||||
| J | 30+0 | 95 | 7069 | 5161 | −27% | 3.8 | 2.2 | −42% | 700 | 32+4 | +18 | HELLP syndrome |
| 8763 | 7731 | −12% | 2.7 | 2.4 | −11% | 1000 | ||||||
| Three Treatments | ||||||||||||
| K | 28+6 | 245 | 7546 | 5222 | −31% | 1.4 | 0.5 | −64% | 1000 | 31+6 | +21 | Oligo-hydramnios |
| 12,928 | 8814 | −32% | 5.2 | 1.9 | −63% | 1000 | ||||||
| 12,525 | 9750 | −22% | 6.2 | 1.8 | −71% | 1000 | ||||||
—, missing data.
Patient F gave birth to twins.
Table 2.
Mean blood pressure, hematocrit, and platelet counts in women treated with apheresis (n=11)
| Mean ±SD | |||
|---|---|---|---|
| Baseline | Pretreatmenta | Post-treatmenta | |
| Systolic BP (mmHg) | 161±11 | 165±18 | 149±14 |
| Diastolic BP (mmHg) | 101±9 | 105±10 | 96±10 |
| Hematocrit (%) | 36±3 | 36±3 | 34±3 |
| Platelets (×103/µl) | 198±47 | 188±61 | 182±55 |
Systolic BP, diastolic BP, hematocrit, and platelet values for patients treated more than once were averaged over all treatments.
In six women treated once (A through F [Table 1]), mean pre-apheresis blood sFlt-1 concentrations and urine P/C ratios were 19,513 (range 8243–35,301) pg/ml and 5.0 (0.4–15.8) g/g, respectively. Following single treatments, mean sFlt-1 concentrations decreased to 15,746 pg/ml and P/C ratios fell to 2.1 g/g. On average, pregnancy continued in these women for an additional 8 days (2–11 days) from day of admission.
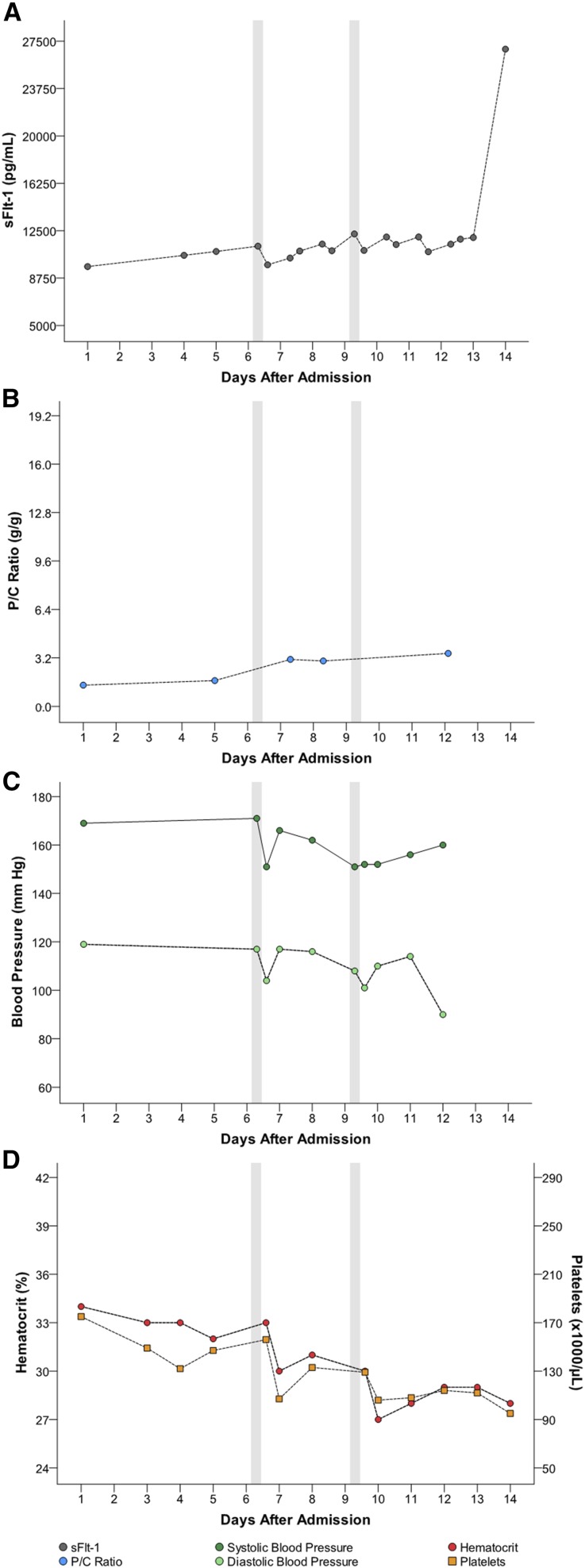
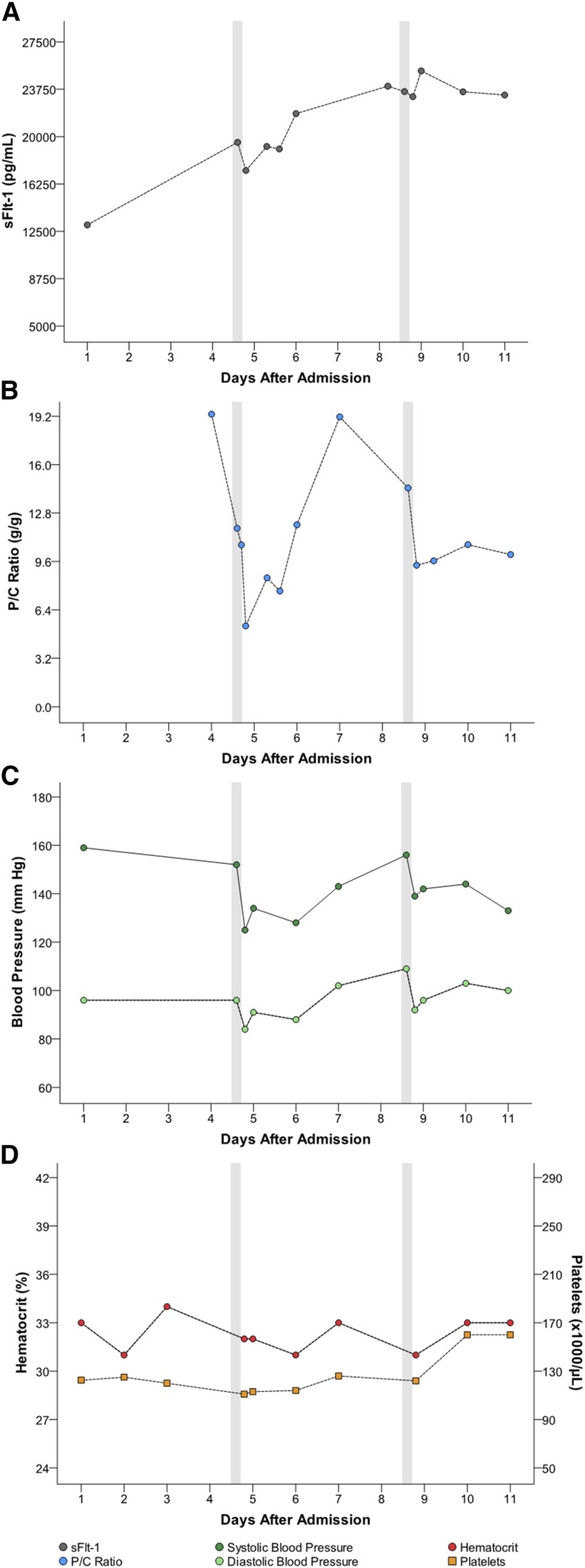
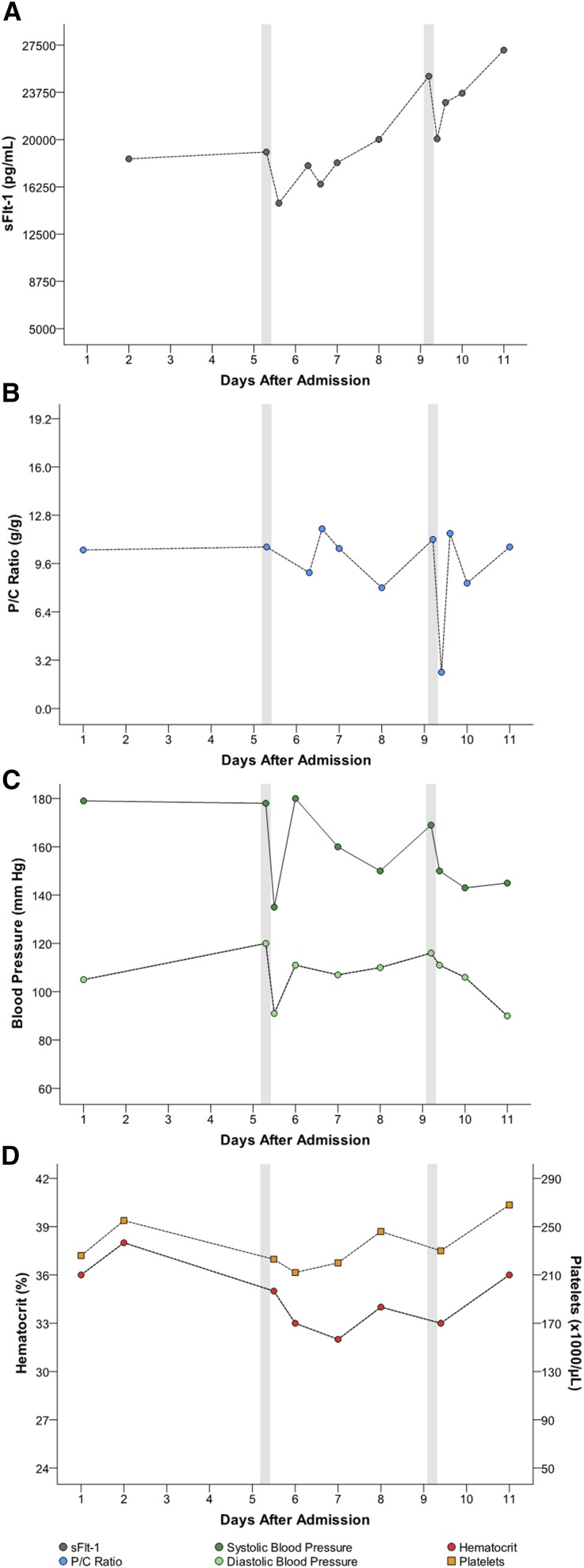
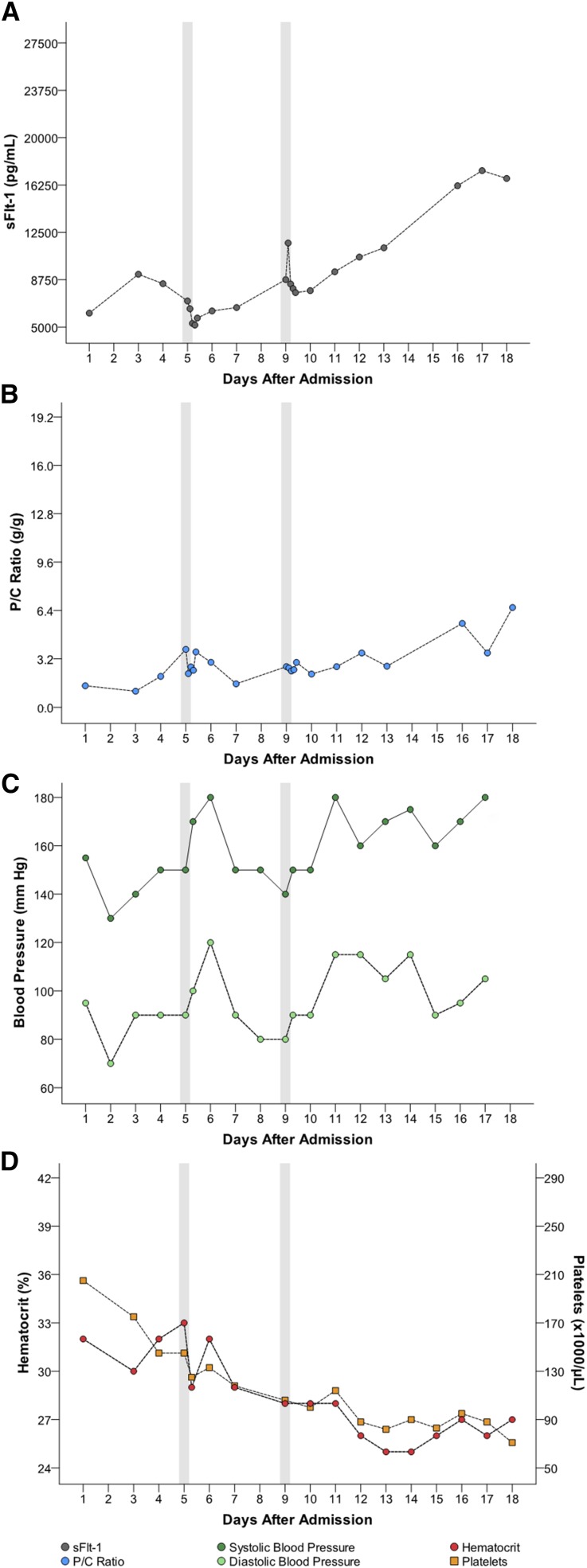
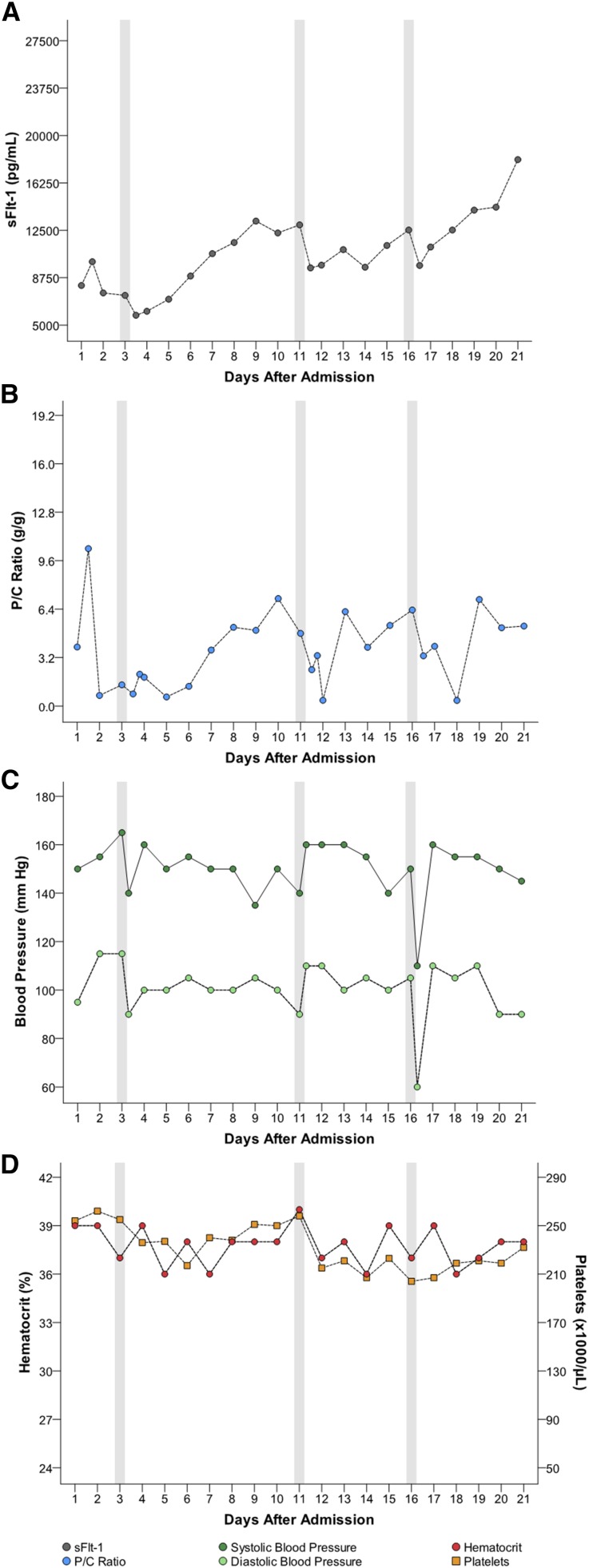
Five women had multiple apheresis treatments (G through K [Table 1]). Figures 1, 2, 3, 4, and 5 detail changes in maternal sFlt-1 concentrations and P/C ratios pre- and post-apheresis in each of these women. Excursions of systolic and diastolic BP, hematocrit, and platelet counts measured during the course of their hospitalization and apheresis treatments are also displayed, showing transient fluctuations in BP but no clinically significant changes in hematocrit or platelet counts. Among women treated multiple times, pregnancy continued on average for 15 days (range 11–21). Narrative summaries of each patient that underwent multiple apheresis treatments are provided in the figure legends.
Figure 1.
Patient G: changes in circulating sFlt-1 levels and corresponding parameters after two apheresis treatments. Patient G was a 31-year-old gravida 0 para 1 who, prior to treatment, was 28+4/7 weeks of gestation with BP 171/117 mmHg, P/C ratio of 1.7 g/g, and sFlt-1 of 11,276 pg/ml. Her sFlt-1/PlGF ratio was 150. She underwent two apheresis treatments without complications (see Table 1). Following the first treatment, sFlt-1 fell to 9800 pg/ml (13% reduction). Her second pretreatment sFlt-1 level had climbed to 12,245 pg/ml and fell to 10,836 pg/ml post-treatment (12% reduction). The P/C ratios post-treatments (within about 12 hours post-treatment) were not available; however her overall P/C ratios did not appear to markedly change during hospitalization. Estimated fetal birthweight before the first treatment was 1048 g. The mother’s pregnancy continued for an additional 14 days (counting from day of admission). At birth (30+0/7 weeks), actual neonatal weight was 1310 g. At 41 days post-delivery (neonate discharge date), the infant weighed 1905 g. At the most recent follow-up conducted 2 months post-discharge, the baby’s growth percentiles were normal. There were no persistent complications associated with treatment, only transient reductions in BP which were managed as detailed in the manuscript.
Figure 2.
Patient H: changes in circulating sFlt-1 levels and corresponding parameters after two apheresis treatments. Patient H was a 26-year-old gravida 2 para 1 who, prior to treatment, was 25+4/7 weeks of gestation with a BP of 152/96 mmHg, P/C ratio of 19.3 g/g, sFlt-1 of 19,550 pg/ml and sFlt-1/PlGF ratio of 1150. She underwent two treatments of apheresis without complications (see Table 1). Following the first treatment, sFlt-1 fell to 17,320 pg/ml (11% reduction). Her second pretreatment sFlt-1 had risen to 23,569 pg/ml and following the treatment was 23,162 pg/ml (2% reduction). The corresponding P/C ratio after the first treatment was 7.7 g/g (60% reduction). P/C ratios before and after the second treatment were 14.5 g/g and 9.4 g/g, respectively, a 35% reduction. Estimated fetal birthweight before the first treatment was 571 g. Her pregnancy continued for an additional 11 days (counting from day of admission). At birth (26+4/7 weeks), actual neonatal weight was 580 g. The infant’s weight was 1640 g at the time of hospital discharge (day 90). There were no persistent complications with treatment.
Figure 3.
Patient I: changes in circulating sFlt-1 levels and corresponding parameters after two apheresis treatments. Patient I was a 23-year-old gravida 0 para 1 who, prior to treatment, was 29+3/7 weeks of gestation with a BP of 178/120 mmHg, P/C ratio of 9.9 g/g, sFlt-1 of 19,019 pg/ml and sFlt-1/PlGF ratio of 674. She underwent two apheresis treatments without complications (see Table 1). Following the first treatment, sFlt-1 fell to 14,955 pg/ml (21% reduction). Her second pretreatment sFlt-1 had risen to 25,024 pg/ml and following the treatment was 20,063 pg/ml (20% reduction). P/C ratio immediately after the first treatment was 11.8 g/g (19% increase). With the second treatment, before and after P/C ratios were 11.1 g/g and 2.4 g/g (78% reduction). Estimated fetal birthweight before the first treatment was 1206 g. The mother’s pregnancy continued for an additional 11 days (counting from day of admission). At birth (30+3/7 weeks), actual neonatal weight was 1280 g. At discharge from the hospital (39 days after delivery) the infant’s weight was 1860 g. There were no persistent complications with treatment.
Figure 4.
Patient J: changes in circulating sFlt-1 levels corresponding parameters after two apheresis treatments. Patient J was a 26-year-old gravida 0 para 2 who, prior to treatment, was 30+5/7 weeks of gestation with a BP of 150/90 mmHg, P/C ratio of 3.8 g/g, sFlt-1 of 7069 pg/ml and sFlt-1/PlGF ratio of 95. She underwent two apheresis treatments without complications (see Table 1). Following the first treatment, sFlt-1 fell to 5161 pg/ml (27% reduction). Her second pretreatment sFlt-1 had risen to 8763 pg/ml and following the treatment was 7731 pg/ml (12% reduction). Before and after treatment P/C ratios for the first treatment were 3.8 and 2.2 g/g (42% reduction) and for the second treatment were 2.7 and 2.4 g/g (11% reduction). Estimated fetal birthweight before the first treatment was 1307 g. The mother’s pregnancy continued for an additional 18 days (counting from day of admission). At birth (32+4/7 weeks), actual neonatal weight was 1490 g. At discharge from the hospital (38 days after delivery) the infant’s weight was 2030 g. There were no persistent complications with treatment.
Figure 5.
Patient K: changes in circulating sFlt-1 levels and corresponding parameters after three apheresis treatments. Patient K was a 20-year-old gravida 0 para 1 who, prior to treatment, was 29+1/7 weeks of gestation with BP 165/115 mmHg, P/C ratio of 1.4 g/g, sFlt-1 level of 7546 pg/ml and sFlt-1/PlGF ratio of 245. She underwent three apheresis treatments without complications (see Table 1). Following the first treatment, sFlt-1 fell to 5222 pg/ml (31% reduction). Her second pretreatment sFlt-1 level had risen to 12,928 pg/ml and following the treatment was 8814 pg/ml (32% reduction). Her third pretreatment sFlt-1 rose again to 12,525 pg/ml and following the third and final treatment was 9750 pg/ml (22% reduction). Corresponding P/C ratios following each treatment were as follows: Treatment 1, 1.4 g/g decreased to 0.5 g/g (64% reduction); Treatment 2, 5.2 g/g decreased to 1.9 g/g (63% reduction); Treatment 3, 6.2 g/g decreased to 1.8 g/g (71% reduction). Estimated fetal birthweight before the first treatment was 1076 g. The mother’s pregnancy continued for an additional 21 days (counting from day of admission). At birth (31+6/7 weeks), actual neonatal weight was 1256 g. At discharge from the hospital (44 days after delivery), the infant’s weight was 2100 g. At 1 month after delivery, the mother had normal BP 110/70 mmHg, no proteinuria, and her sFlt-1 level was 86 pg/ml. At 4, 8, and 12 months after delivery, the child’s growth centiles were normal. There were no persistent complications with treatment.
Maternal Safety of Apheresis
Despite withholding antihypertensive therapy on the morning of treatment (see Supplemental Table 2 for apheresis protocol), the primary side-effect experienced by all treated women was a transient drop in BP within 30 minutes of initiating apheresis. These episodes were successfully managed by saline infusions and by temporarily reducing blood flow rates through the device. No women experienced hypotension severe enough to necessitate termination of apheresis.
Maternal Controls
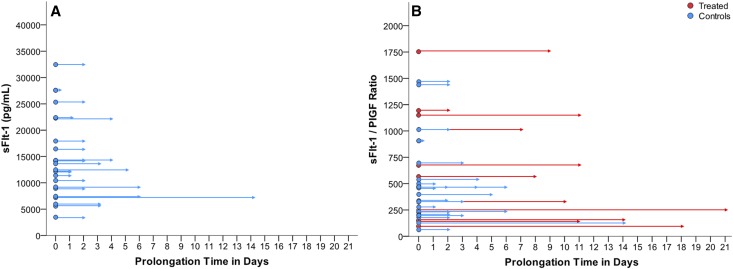
Baseline and demographic characteristics of women selected as contemporaneous controls with preeclampsia (n=22) or without preeclampsia (n=22) are summarized in Supplemental Table 3 along with summary data from treated women. Controls were matched for a number of factors, most notably for gestational age at delivery (Supplemental Methods) to determine if neonatal and fetal parameters were adversely affected by apheresis treatments. Figure 6A shows data on baseline sFlt-1 measurements and pregnancy prolongation in the 22 untreated contemporaneous preeclamptic controls. Mean prolongation of pregnancy was 3 days (range 0–14) in this untreated group. Figure 6B shows sFlt-1/PlGF ratios and pregnancy prolongation for both treated and untreated women with preeclampsia.
Figure 6.
Prolongation time of pregnancy in women with preeclampsia. (A) Pregnancy prolongation in contemporaneous untreated women with preeclampsia (n=22 controls) by sFlt-1 levels (pg/ml) at baseline. (B) Pregnancy prolongation in women with preeclampsia treated with apheresis (n=11) and untreated contemporaneous controls (n=22) by pretreatment sFlt-1/PlGF ratios.
Fetal and Neonatal Effects of Apheresis
All fetuses were evaluated in utero using fetal heart rate monitoring and ultrasounds. Notably, there were no significant changes in fetal cardiotocography during the transient BP reductions we observed in mothers during apheresis treatments.
Estimated fetal weights were obtained weekly. Actual fetal weights at birth were consistently similar to or higher than estimated in utero birthweights (Supplemental Figure 1). No antepartum, intrapartum, or neonatal deaths occurred. Birthweight was highly correlated with gestational age (P<0.001).
Neonates born to women in the apheresis group (n=12 in 11 treated women) required fewer days of supplemental oxygen compared with neonates born to women in the preeclampsia control group 1 (Table 3). Neonatal adaptation after birth (Apgar scores, umbilical cord blood pH) and the estimation of biologic risks (Clinical Risk Index for Babies [CRIB] score) did not differ between groups. None of the index infants and no infant in either control group exhibited any complications including intraventricular hemorrhage >stage 1, periventricular leukomalacia, bronchopulmonary dysplasia, necrotizing enterocolitis, focal-intestinal perforation, or retinopathy of prematurity >stage 2. Duration of neonatal intensive care unit stay and overall length of hospital stay between the treated and control groups were similar.
Table 3.
Neonatal characteristics of infants born to treated and untreated mothers
| Apheresis Group (n=12)a | Control Group 1 (n=22) | Control Group 2 (n=22) | ||||
|---|---|---|---|---|---|---|
| Mean±SD | (Min–Max) | Mean±SD | (Min–Max) | Mean±SD | (Min–Max) | |
| Data collected at birth | ||||||
| Gestational age (weeks) | 30±2 | (26–32) | 30±2 | (25–32) | 30±2 | (25–33) |
| Birthweight (g) | 1183±306 | (580–1630) | 1204±328 | (600–1800) | 1300±381 | (530–1970) |
| Length (cm) | 39±3 | (31–44) | 39±3 | (31–43) | 39±5 | (25–46) |
| Head circumference (cm) | 27±2 | (24–29) | 28±3 | (22–34) | 28±3 | (22–32) |
| Cord blood pH | 7.28±0.08 | (7.12–7.41) | 7.29±0.06 | (7.15–7.40) | 7.33±0.08 | (7.16–7.47) |
| Apgar (5-min) | 8±1 | (7–9) | 8±1 | (5–9) | 8±1 | (5–9) |
| Apgar (10-min) | 9±1 | (7–9) | 9±1 | (7–9) | 8±1 | (8–9) |
| CRIB score | 3±2 | (1–8) | 2±2 | (1–7) | 2±3 | (0–10) |
| Data collected during follow-up | ||||||
| NICU stay (days) | 16±14 | (2–45) | 12±11 | (1–42) | 15±21 | (1–94) |
| LOS (days) | 45±17 | (27–90) | 45±15 | (21–77) | 50±23 | (14–118) |
| Ventilation (days) | 1±1 | (1–2) | 5±5 | (1–14) | 2±1 | (1–4) |
| Treated (%) | 3 (25) | 6 (27) | 6 (27) | |||
| nCPAP (days) | 6±5 | (2–17) | 13±13 | (1–56) | 9±11 | (1–48) |
| Treated (%) | 12 (100) | 22 (100) | 22 (100) | |||
| O2 (days)b | 2±2 | (1–7) | 11±15 | (1–58) | 7±13 | (1–49) |
| Treated (%) | 9 (75) | 15 (68) | 12 (55) | |||
| Surfactant (doses) | 1±0 | (1–1) | 1±1 | (1–2) | 1±1 | (1–2) |
| Treated (%) | 6 (50) | 9 (41) | 10 (45) | |||
Apheresis Group: Preterm infants of mothers with preeclampsia treated with apheresis; Control Group 1: Preterm infants of mothers with preeclampsia but not treated with apheresis; Control Group 2: Infants of mothers with preterm delivery due to other causes.
NICU, neonatal intensive care unit; LOS, total length of hospital stay; Ventilation, duration of mechanical ventilation; nCPAP, duration of nasal continuous positive airway pressure; O2, duration of oxygen supplementation.
One mother delivered twins, hence, 12 infants born to 11 treated mothers.
P<0.05 for comparison between Apheresis Group and Control Group 1; all other comparisons were not significant (P>0.05).
DISCUSSION
Given the many clinical and management challenges that arise when caring for both mother and fetus, interventions in pregnancy are few. Historically, agents that traverse the placenta and potentially adversely affect the fetus represent one of the most formidable barriers to introducing a novel agent into maternal circulation.20 For this reason we chose to remove the putative factor, sFlt-1. In our previous study with a whole-blood dextran sulfate apheresis device,13 the challenges we faced were BP reductions during treatments and the efficiency of sFlt-1 removal given various substances (i.e., cellular elements, clotting factors) in blood that could interfere with this process. Here we demonstrate that extracorporeal removal of excess circulating sFlt-1 with a PSDS-device is safe and potentially effective for reducing proteinuria and potentially prolonging pregnancy in women with very preterm, severe preeclampsia. Among women treated once, pregnancy continued for an average of 8 days and, among women treated multiple times, pregnancy continued on average for 15 days. Although we did not perform a parallel randomized trial, this compares favorably to the 3-day prolongation observed for untreated contemporaneous preeclamptic controls in the present study and the approximately 4 days in historical controls we previously observed under similar conditions.13
This study represents a proof-of-concept strategy based on a unique pathway that is altered in most women with preeclampsia.7–9 We targeted the antiangiogenic protein sFlt-1 given the mounting evidence of its involvement in the pathogenesis of human preeclampsia.5,7,8,21,22 Importantly, we required women to demonstrate significantly altered blood levels of sFlt-1 and PlGF at enrollment (sFlt-1/PlGF ratio >85), which we and others have linked to adverse outcomes including almost immediate need for delivery.8,23 These observations have been replicated in the present study (Figure 6B). We cannot immediately refute the possibility that removal of other factors including LDL-cholesterol24 may confer benefit, and studies using ligand-specific apheresis columns (e.g., configured with anti-sFlt-1 antibody or VEGF) are needed to determine the relative contribution of sFlt-1 depletion versus depletion of other potential mediators of preeclampsia. We acknowledge that the negatively charged column employed in the device removes other critical positively charged proteins from maternal circulation, e.g., fibrinogen, but as we demonstrated previously, these alterations are transient,13 and resolve even more rapidly than the reduction in sFlt-1. Given the extensive safety record of such devices used in pregnancy (e.g., dialysis, plasma exchange14–16) amounting to several thousand person-years of experience, we felt that this was a reasonably safe strategy to pursue for a devastating condition in which no alternative effective therapy other than delivery is available.
Reductions in P/C ratios observed in parallel with reductions in sFlt-1 in treated women were not unexpected based on observations in animal models in which VEGF pathways are antagonized10,12,25,26 and consistent with our previous data.13 We considered whether this was due to mild reductions in BP during apheresis treatments; however, reductions were transient (minutes) and we even observed reductions in P/C ratios among women without treatment-related BP reductions. Based on current thinking about the pathology underlying proteinuria in preeclamptic women (i.e., glomerular endotheliosis),27 such acute resolution of proteinuria may be related to improvement of glomerular hemodynamics proposed by Smithies.28 Furthermore, we know that the abundance of proteinuria immediately improves following delivery of the placenta in women with severe preeclampsia,10,29 again a finding unlikely to be due to acute structural changes. Our observations following apheresis treatments warrant closer experimental study of mechanisms related to human proteinuria.
In our previous study using a whole-blood apheresis device, the primary side-effect encountered was transient reduction in systolic BP during the procedure in the range of approximately 20–30 mmHg, but in some women was more profound, albeit for brief periods.13 Rapid BP reduction should be avoided because uteroplacental perfusion is adapted to the hypertensive situation (a pathologic feature of preeclampsia) and the high placental resistance likely requires an elevated perfusion pressure to maintain the fetal supply. In contrast to whole-blood devices, reductions in systolic BP using the PSDS-device were less profound (approximately 10–20 mmHg in most cases) and immediately improved with saline administration and reducing blood flow rates. Whether this attenuation can be attributed to differential effects on cytokine/chemokine activation by different apheresis columns30 will require further study.
We acknowledge that the careful control of BP with antihypertensive agents (or by apheresis) might increase the latency period for woman with severe preeclampsia. Antihypertensives are used routinely to prevent malignant hypertension and seizures in pregnant women.31 A meta-analysis (23 randomized trials) concluded that antihypertensive therapies in pregnant women do not lower the incidence of preeclampsia; however, they may reduce the risk of uncontrolled hypertension, which itself may be an indication for preterm delivery.32 Although women with preeclampsia are intravascular volume depleted, the use of diuretics in preeclampsia may be beneficial, especially in the setting of cerebral or pulmonary edema.33 Management of BP and volume in a randomized control trial should follow standard-of-care guidelines, and the therapy introduced (e.g., apheresis) must be shown to safely prolong pregnancy over and above these standard-of-care measures with minimal impact on resource use.
In addition to changes in BP, we reported serial hemoglobin and platelet counts in women treated more than once. Changes in hematologic parameters are commonly reported in pregnancy and especially in severe preeclampsia,34 and our treatments did not appear to alter (worsen or improve) otherwise observed trends in these parameters during the hospitalization course.
The natural history of rising sFlt-1 levels over time among women with preeclampsia has been the subject of several manuscripts.5,13,23,35–37 In some women there appeared to be a rebound following apheresis (see Figures 2, 4, and 5). Given that the volume of distribution of sFlt-1 is large, with significant quantities bound to the interstitial matrix and <20% of total body sFlt-1 circulating,38 rebound following equilibration with the interstitial matrix compartment is not surprising. Although our ability to compare maternal changes in sFlt-1 levels over time in untreated contemporaneous preeclamptic controls to sFlt-1 levels in treated women should be considered exploratory, our study does emphasize, importantly, that any comparison of maternal and fetal outcomes between treated and untreated women should include women of similar preeclampsia severity. This may be facilitated by ensuring similar baseline sFlt-1/PlGF ratios, which have robust diagnostic and prognostic performances.35–37
Short-term neonatal outcomes of preterm infants born to treated women compared with untreated women with preeclampsia and to infants born preterm for reasons other than preeclampsia were similar, except that neonates born to treated women required fewer days on supplementary oxygen; no infants died. The similar Apgar scores, umbilical cord blood pHs, and estimated biologic risks suggest that apheresis did not influence fetal circulation or mechanisms of neonatal adaptation. No unexpected respiratory complications occurred and there was no excess need for surfactant treatment, respiratory support, or oxygen supplementation was observed. The potential improvement in lung function in neonates of treated women as reflected in significantly fewer days on supplementary oxygen compared with control group 1 must be addressed in a randomized trial, but is not entirely surprising given that preeclampsia-associated toxins, particularly sFlt-1, have been directly implicated in the etiology of respiratory distress syndrome and bronchopulmonary dysplasia in preterm infants.39–41
Cerebral and intestinal morbidity were negligible in all groups, and, given the relationship of VEGF to the underlying pathogenesis of retinopathy of prematurity,42,43 it is particularly reassuring that no infant developed this condition. We acknowledge that the small sample size in this study may have precluded observation of uncommon adverse events.
The most important challenge when developing therapies for pregnant women is to ensure the safety of any intervention in both mother and fetus. By monitoring each treatment for maternal blood pressure, oxygen saturation, and fetal wellbeing as well as short-term neonatal outcomes, we are encouraged that these data suggest therapeutic apheresis using a PSDS-column is well-tolerated. A randomized clinical trial will be needed to rigorously test whether removal of sFlt-1 by apheresis is safe, effective, and favors efficient use of resources in women with very preterm severe preeclampsia.
CONCISE METHODS
We first carried out in vitro experiments comparing the efficiency of sFlt-1 removal using negatively charged dextran sulfate adsorption columns configured to process either whole blood or plasma (Supplemental Figure 2, Supplemental Methods, Supplemental Table 4).
The primary end point of this single-arm pilot study (Clinicaltrials.gov NCT01404910) was percentage reduction of sFlt-1 levels in maternal blood measured immediately before and after apheresis therapy ([pre-sFlt–1 – post-sFlt–1]/pre-sFlt–1) as described previously.13 Fetal assessments included: gestational age at delivery, body weight, body length and head circumference at birth, Apgar score (5 and 10 minutes), Doppler ultrasound, cardiotocography, CRIB score, cord blood pH, total neonatal intensive care unit and hospital stay, duration of mechanical ventilation, administration of nasal continuous airway pressure, supplemental oxygen, and the doses of surfactant administered for early therapeutic treatment of respiratory distress syndrome. See also Supplemental Methods and Schema (Supplemental Figure 3). We conducted the study at two sites in Germany (Cologne and Leipzig) where testing for blood concentrations of sFlt-1 and PlGF is available for routine prenatal care measures.36 The study conduct adhered to the Declaration of Helsinki, with ethics approval of the Massachusetts General Hospital and the University of Cologne/University of Leipzig. Each patient signed written informed consent before any study procedures.
After consent was obtained, blood levels of sFlt-1 and PlGF (Roche Diagnostics, Germany23) were measured as previously described7 to confirm eligibility (see Supplemental Methods for complete inclusion/exclusion criteria). Briefly, we enrolled women with preterm preeclampsia defined as ≥23 and ≤32 weeks of gestation at diagnosis; systolic or diastolic BP ≥140 mmHg or ≥90 mmHg, respectively; proteinuria defined as P/C ratio ≥0.30 g/g, and sFlt-1/PlGF concentration ratio in blood >85 (which has been shown to herald adverse outcomes including the need for delivery within 3–5 days).37
We modified a standard LDL-apheresis protocol44 to accommodate pregnant women, and altered other procedures based on our previous experience using a related device (Supplemental Table 2).13 Maternal and fetal follow-up continued after delivery.
Supplemental Methods provide a description of statistical analyses and study conduct information.
DISCLOSURES
R.T. received grant support from Kaneka Corporation, is a consultant to Thermo Fisher Scientific, and has financial interest in Aggamin LLC and patents on diagnostics for preeclampsia. H.H. received grant support from Kaneka Corporation. S.A.K. is a co-inventor on patents on preeclampsia markers and therapies, received grant support from Thermo Fisher Scientific, has financial interest in Aggamin LLC and is a consultant to Siemens and Roche Diagnostics. T.B. was in receipt of a grant from the German Research Foundation (DFG BE 2212). All other authors have nothing to disclose.
Supplementary Material
Acknowledgments
The authors wish to thank the women who participated in this study, and members of the Data Safety Monitoring Board: Dr. Christoph Wanner, Chair, Dr. Jeffrey Ecker, and Dr. Stefan Verlohren.
Funding for the study was provided by Kaneka Corporation. Kaneka was not involved in the design, analysis, and interpretation of results, or the preparation of the manuscript.
This work was also partly supported by Forschungspool Klinische Studien, Faculty of Medicine, University of Cologne.
R.T. and T.B. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Apheresis to Treat Preeclampsia: Insights, Opportunities and Challenges,” on pages 663–665.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020157/-/DCSupplemental.
REFERENCES
- 1.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ: Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J 156: 918–930, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Sibai BM: Prevention of preeclampsia: a big disappointment. Am J Obstet Gynecol 179: 1275–1278, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Walker JJ: Pre-eclampsia. Lancet 356: 1260–1265, 2000 [DOI] [PubMed] [Google Scholar]
- 4.Kassebaum NJ, Bertozzi-Villa A, Coggeshall MS, Shackelford KA, Steiner C, Heuton KR, Gonzalez-Medina D, Barber R, Huynh C, Dicker D, et al. Global, regional, and national levels and causes of maternal mortality during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 980–1004, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA: Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 350: 672–683, 2004 [DOI] [PubMed] [Google Scholar]
- 6.McCoy S, Baldwin K: Pharmacotherapeutic options for the treatment of preeclampsia. Am J Health Syst Pharm 66: 337–344, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA: Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 111: 649–658, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA: Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 125: 911–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thadhani R: Inching towards a targeted therapy for preeclampsia. Hypertension 55: 238–240, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilbert JS, Verzwyvelt J, Colson D, Arany M, Karumanchi SA, Granger JP: Recombinant vascular endothelial growth factor 121 infusion lowers blood pressure and improves renal function in rats with placentalischemia-induced hypertension. Hypertension 55: 380–385, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui AH, Irani RA, Zhang Y, Dai Y, Blackwell SC, Ramin SM, Kellems RE, Xia Y: Recombinant vascular endothelial growth factor 121 attenuates autoantibody-induced features of pre-eclampsia in pregnant mice. Am J Hypertens 24: 606–612, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergmann A, Ahmad S, Cudmore M, Gruber AD, Wittschen P, Lindenmaier W, Christofori G, Gross V, Gonzalves AC, Gröne HJ, Ahmed A, Weich HA: Reduction of circulating soluble Flt-1 alleviates preeclampsia-like symptoms in a mouse model. J Cell Mol Med 14[6B]: 1857–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thadhani R, Kisner T, Hagmann H, Bossung V, Noack S, Schaarschmidt W, Jank A, Kribs A, Cornely OA, Kreyssig C, Hemphill L, Rigby AC, Khedkar S, Lindner TH, Mallmann P, Stepan H, Karumanchi SA, Benzing T: Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation 124: 940–950, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Teruel JL, Lasunción MA, Navarro JF, Carrero P, Ortuño J: Pregnancy in a patient with homozygous familial hypercholesterolemia undergoing low-density lipoprotein apheresis by dextran sulfate adsorption. Metabolism 44: 929–933, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Kroon AA, Swinkels DW, van Dongen PW, Stalenhoef AF: Pregnancy in a patient with homozygous familial hypercholesterolemia treated with long-term low-density lipoprotein apheresis. Metabolism 43: 1164–1170, 1994 [DOI] [PubMed] [Google Scholar]
- 16.Klingel R, Göhlen B, Schwarting A, Himmelsbach F, Straube R: Differential indication of lipoprotein apheresis during pregnancy. Ther Apher Dial 7: 359–364, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR: Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest 124: 4941–4952, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization: Preterm Birth. Fact Sheet No. 363. 2014 (November). http://www.who.int/mediacentre/factsheets/fs363/en/ Accessed April, 2015
- 19.Sibai BM, Mercer BM, Schiff E, Friedman SA: Aggressive versus expectant management of severe preeclampsia at 28 to 32 weeks’ gestation: a randomized controlled trial. Am J Obstet Gynecol 171: 818–822, 1994 [DOI] [PubMed] [Google Scholar]
- 20.Ward RM: Maternal-placental-fetal unit: unique problems of pharmacologic study. Pediatr Clin North Am 36: 1075–1088, 1989 [DOI] [PubMed] [Google Scholar]
- 21.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, Thadhani R, Wolf M, Harger G, Markovic N: Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta 26: 563–573, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, Ecker J, Karumanchi SA: First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin Endocrinol Metab 89: 770–775, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H: An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 202: 161.e1–161.e111, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Winkler K, Hoffmann MM, Putz G. Letter by Winkler et al regarding article, “Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia”. Circulation 125: e522; author reply e523–524, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto H, Hamano Y, Charytan D, Cosgrove D, Kieran M, Sudhakar A, Kalluri R: Neutralization of circulating vascular endothelial growth factor (VEGF) by anti-VEGF antibodies and soluble VEGF receptor 1 (sFlt-1) induces proteinuria. J Biol Chem 278: 12605–12608, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Zhang Y, Ying Ma J, Kapoun AM, Shao Q, Kerr I, Lam A, O’Young G, Sannajust F, Stathis P, Schreiner G, Karumanchi SA, Protter AA, Pollitt NS: Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension 50: 686–692, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Wagner SJ, Craici IM, Grande JP, Garovic VD: From placenta to podocyte: vascular and podocyte pathophysiology in preeclampsia. Clin Nephrol 78: 241–249, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Smithies O: Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci U S A 100: 4108–4113, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berks D, Steegers EA, Molas M, Visser W: Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol 114: 1307–1314, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Hovland A, Hardersen R, Sexton J, Mollnes TE, Lappegård KT: Different inflammatory responses induced by three LDL-lowering apheresis columns. J Clin Apher 24: 247–253, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Gillon TE, Pels A, von Dadelszen P, MacDonell K, Magee LA: Hypertensive disorders of pregnancy: a systematic review of international clinical practice guidelines. PLoS ONE 9: e113715, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abalos E, Duley L, Steyn DW, Henderson-Smart DJ: Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev (1): CD002252, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Podymow T, August P: Antihypertensive drugs in pregnancy. Semin Nephrol 31: 70–85, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Townsley DM: Hematologic complications of pregnancy. Semin Hematol 50: 222–231, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sunderji S, Gaziano E, Wothe D, Romero R, Kusanovic JP, Rogers L, Hodges-Savola C, Roberts S, Wassenberg J: Automated assays for sVEGF R1 and PlGF as an aid in the diagnosis of preterm preeclampsia: a prospective clinical study. Am J Obstet Gynecol 202: 40.e41–40.e47, 2010. [DOI] [PubMed] [Google Scholar]
- 36.Schaarschmidt W, Rana S, Stepan H: The course of angiogenic factors in early- vs. late-onset preeclampsia and HELLP syndrome. J Perinat Med 41: 511–516, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohkuchi A, Hirashima C, Matsubara S, Takahashi K, Matsuda Y, Suzuki M: Threshold of soluble fms-like tyrosine kinase 1/placental growth factor ratio for the imminent onset of preeclampsia. Hypertension 58: 859–866, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Wu FT, Stefanini MO, Mac Gabhann F, Popel AS: A compartment model of VEGF distribution in humans in the presence of soluble VEGF receptor-1 acting as a ligand trap. PLoS ONE 4: e5108, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansen AR, Barnés CM, Folkman J, McElrath TF: Maternal preeclampsia predicts the development of bronchopulmonary dysplasia. J Pediatr 156: 532–536, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Tang JR, Karumanchi SA, Seedorf G, Markham N, Abman SH: Excess soluble vascular endothelial growth factor receptor-1 in amniotic fluid impairs lung growth in rats: linking preeclampsia with bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 302: L36–L46, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang A, Holston AM, Yu KF, Zhang J, Toporsian M, Karumanchi SA, Levine RJ: Circulating anti-angiogenic factors during hypertensive pregnancy and increased risk of respiratory distress syndrome in preterm neonates. J Matern Fetal Neonatal Med 25: 1447–1452, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feng F, Cheng Y, Liu QH: Bevacizumab treatment reduces retinal neovascularization in a mouse model of retinopathy of prematurity. Int J Ophthalmol 7: 608–613, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yenice O, Cerman E, Ashour A, Firat R, Haklar G, Sirikci O, Akman I, Kazokoglu H: Serum erythropoietin, insulin-like growth factor 1, and vascular endothelial growth factor in etiopathogenesis of retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina 44: 549–554, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Gordon BR, Kelsey SF, Bilheimer DW, Brown DC, Dau PC, Gotto AM, Jr, Illingworth DR, Jones PH, Leitman SF, Prihoda JS, et al. The Liposorber Study Group : Treatment of refractory familial hypercholesterolemia by low-density lipoprotein apheresis using an automated dextran sulfate cellulose adsorption system. Am J Cardiol 70: 1010–1016, 1992 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.