Abstract
Endoplasmic reticulum (ER) stress is involved in the pathophysiology of kidney disease and aging, but the molecular bases underlying the biologic outcomes on the evolution of renal disease remain mostly unknown. Angiogenin (ANG) is a ribonuclease that promotes cellular adaptation under stress but its contribution to ER stress signaling remains elusive. In this study, we investigated the ANG-mediated contribution to the signaling and biologic outcomes of ER stress in kidney injury. ANG expression was significantly higher in samples from injured human kidneys than in samples from normal human kidneys, and in mouse and rat kidneys, ANG expression was specifically induced under ER stress. In human renal epithelial cells, ER stress induced ANG expression in a manner dependent on the activity of transcription factor XBP1, and ANG promoted cellular adaptation to ER stress through induction of stress granules and inhibition of translation. Moreover, the severity of renal lesions induced by ER stress was dramatically greater in ANG knockout mice (Ang−/−) mice than in wild-type mice. These results indicate that ANG is a critical mediator of tissue adaptation to kidney injury and reveal a physiologically relevant ER stress-mediated adaptive translational control mechanism.
Keywords: apoptosis, acute renal failure, cell biology and structure, chronic allograft nephropathy, renal cell biology, renal epithelial cell
ESRD is a medical condition resulting from CKD, which affects millions of persons worldwide and constitutes a major public health problem and economic challenge. AKI results in the destruction of the renal parenchyma and is a major risk factor for the development and progression of CKD and incident ESRD.1 Notably, the prognosis of injured kidneys depends on the extent of the damage rather than the underlying disease.2 AKIs lead to profound adaptive cellular reprogramming to maintain cellular homeostasis, promote cell survival, and eliminate stressors. Although primarily protective, these events also actively participate in tissue remodeling through the promotion of inflammation and fibrosis.3–5 Therefore, characterizing the molecular mechanisms underlying the cellular response to acute stress and their structural and functional consequences at the tissue level is crucial for the development of preventive and therapeutic strategies in renal medicine.
Many disturbances related to AKI, and including redox regulation, aberrant calcium fluxes, glucose deprivation, viral infections, altered glycosylation, or folding enzyme inhibition, interfere with the endoplasmic reticulum (ER) protein-folding machinery and subsequently lead to the accumulation of misfolded proteins in the ER lumen, a condition referred to as ER stress.6 In the kidney, ER stress often occurs in podocytes and tubular cells under an injured microenvironment and has thereby been implicated in the pathophysiology of various renal diseases.7–12 Upon ER stress, the unfolded protein response (UPR) is activated, engaging transcriptional, post-transcriptional and translational programs to reduce the misfolding burden in the ER by reducing the amount of proteins entering this compartment, increasing its folding capacity and ameliorating the clearance of accumulated proteins. The UPR is signaled through three ER stress sensors, including activated transcription factor 6 (ATF6), inositol-requiring enzyme 1α (IRE1α) and protein kinase RNA-like ER kinase (PERK). In addition, the UPR not only promotes the elimination of misfolded proteins but also engages cell nonautonomous responses (angiogenesis, inflammation) to promote tissue-level adaptation and serve as a signal for the surrounding healthy tissue (i.e., the cell nonautonomous adaptive response).
The secreted ribonuclease angiogenin (ANG) was first identified as an angiogenic factor in tumor cell–conditioned medium13 and is a component of stress response programs in yeast and mammalian cells.14,15 In mammalian cells, ANG promotes ribosomal RNA transcription and cell growth.16 Under stressful conditions, such as heat shock or oxidative stress, cytoplasmic ANG may contribute to stress-induced translational repression through the promotion of tRNA cleavage.15,17–19 Therefore, ANG may act both intracellularly and extracellularly to promote cell and tissue adaptation, respectively. Whether ANG signaling contributes to the UPR is currently unknown; however, this information would provide key insights into the regulatory and biologic functions of the UPR.
In the present study, we explored the molecular basis underlying the regulation of ANG by the UPR and characterized how this regulation promotes cellular adaptation during ER stress. Our results indicate that ANG, as a critical regulator of the stress response regulated by the UPR, plays a critical role in tissue adaptation in response to kidney injury.
RESULTS
ANG Expression is Induced in the Injured Kidney Epithelium
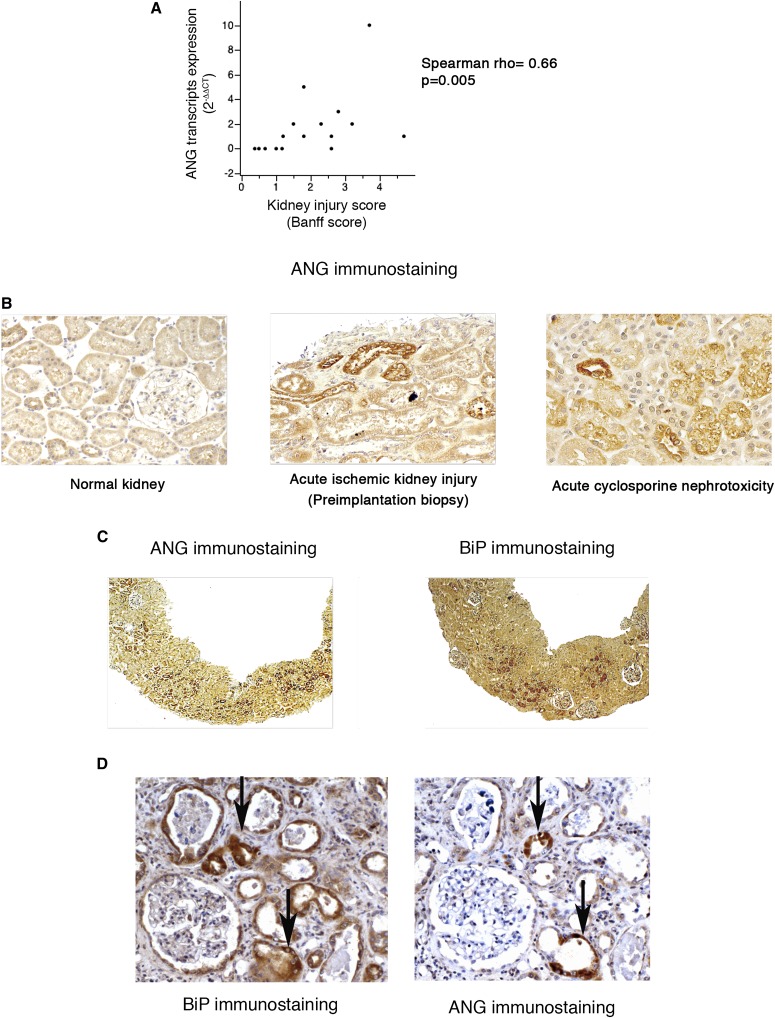
We first determined whether ANG is expressed in injured human kidneys. To this end, we analyzed the relative expression of ANG transcripts in a series of 16 consecutive kidney allograft biopsies, which are integrators of numerous injuries (toxic, immunologic and/or ischemic),20 systematically performed 3 months after transplantation. The results showed that the expression of ANG transcripts was significantly higher in injured tissue samples assessed using acute Banff scores, in which higher numbers indicate extended injured areas21 (Figure 1A). To further document the link between ANG expression and kidney injury, we performed an immunohistochemical analysis of kidney allografts. Whereas ANG was not expressed in the normal kidneys, we observed the expression of ANG in the tubular epithelium of allografts from injured kidneys (Figure 1B). Together, these data indicate that ANG is expressed in the acutely injured human kidney epithelium.
Figure 1.
ANG is expressed in the kidney epithelium in association with ER stress. (A) Regression plots between ANG transcripts expression and acute Banff scores in 16 kidney transplant biopsies using quantitative RT-PCR. The ANG/RPL13A ratios were calculated compared with a normal kidney cDNA. Rho=Spearman’s rank correlation coefficient. (B) Representative photomicrographs of ANG expression evaluated by immunohistochemistry using kidneys allografts from individuals with acute cyclosporine nephrotoxicity, acute ischemic damage (preimplantation biopsy), or without kidney injury (n=3 for each condition). Original magnification, ×200. (C, D) Representative photomicrographs of ANG and BiP coexpression evaluated by immunohistochemistry in kidneys allografts from individuals with AKI. Original magnification, ×100 (C); ×200 (D).
ER Stress Induces ANG Expression in Renal Epithelial Cells
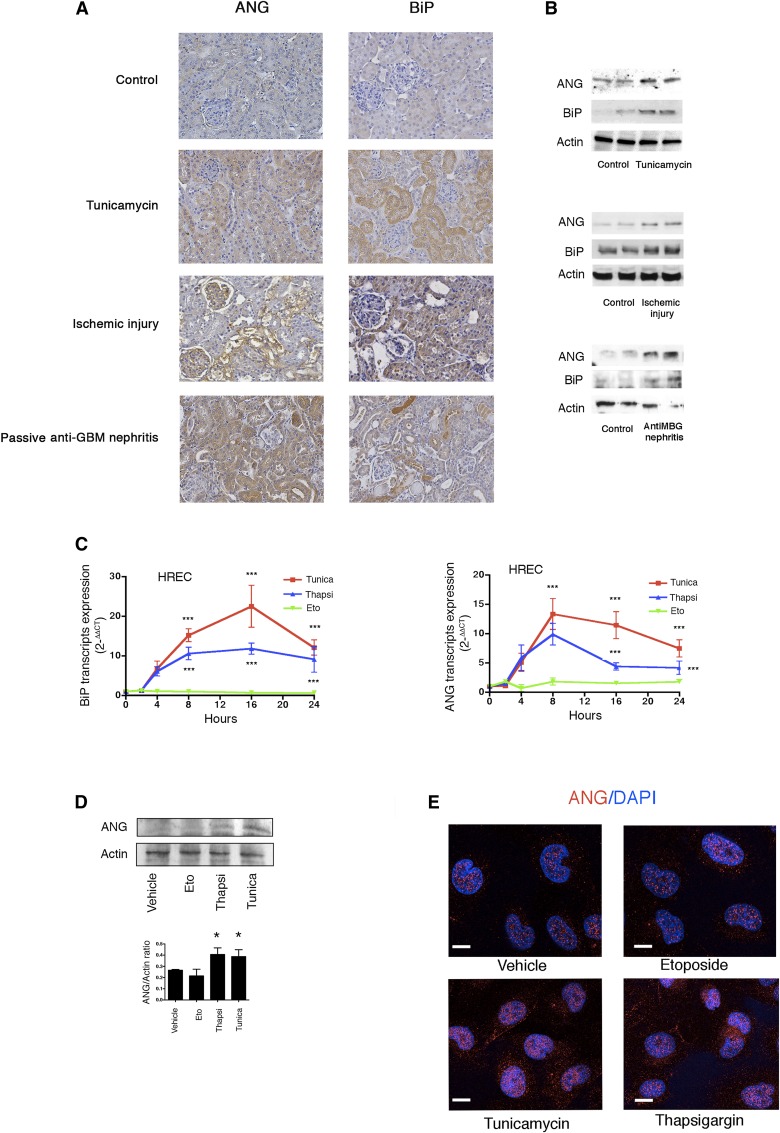
Accumulating evidence indicates that ER stress contributes to kidney disease, and constitutes a new progression factor.7,9 In addition, human renal epithelial cells express ANG during ER stress induced by tissue ischemia.22 Therefore, we investigated the biologic significance of ANG expression in injured kidney epithelium, and we examined whether ER stress promotes the epithelial expression of ANG. Expression levels of binding immunoglobulin protein (BiP) (a surrogate marker of ER stress) and ANG transcripts were significantly higher in injured tissue samples assessed using Banff scores (Supplemental Figure 1, A and B). Consistently, immunohistochemistry revealed that ANG and BiP were coexpressed in the renal epithelium of injured kidney allografts (Figure 1, C and D). In the kidney tissues of mice treated with tunicamycin, a molecule that inhibits N-linked glycosylation and promotes ER stress and AKI,23 ANG and BiP expression was induced in the renal epithelium (Figure 2, A and B, and Supplemental Figure 1C), with BiP and ANG being sometimes coexpressed in the same tubules (Supplemental Figure 1D). Similar findings were observed in rat kidneys subjected to cold ischemia. ANG and BiP were also expressed during AKI associated with passive anti-glomerular basement membrane (GBM) nephritis (Figure 2, A and B) a condition associated with significant proteinuria,24 which is known to promote ER stress.25 Finally, BiP and ANG expression was induced in rat kidneys with acute cyclosporin nephrotoxicity (Supplemental Figure 1C).
Figure 2.
ER stress induces ANG expression in renal epithelial cells. (A) Representative photomicrographs of ANG and BiP expression in kidneys of five mice treated with 1 mg/kg tunicamycin for 24 hours; or anti-GBM nephrotoxic serum; or five rats subjected to 24 hours of cold ischemic injury; all being evaluated using immunohistochemistry. Original magnification, ×200. (B) Immunoblots representing ANG, BiP, and actin protein expression in kidneys of five mice treated with 1 mg/kg tunicamycin for 24 hours, or five rats subjected to 24 hours of cold ischemic injury, or five mice injected with anti-GBM nephrotoxic serum for 14 days. (C) Graph representing the relative expression (means±SEM) of BiP and ANG transcripts measured through quantitative RT-PCR during a time course experiment using HREC incubated with 2 μg/ml tunicamycin, 0.25 μm thapsigargin, 100 μm etoposide or vehicle. The data were obtained from four independent experiments. One-way ANOVA with Dunnett’s post test. ***P<0.001. (D) Immunoblot representing ANG and actin protein expression in HREC incubated with 2 μg/ml tunicamycin, 0.25 μm thapsigargin, 100 μm etoposide, or vehicle for 24 hours. The immunoblot shown represents three independent experiments. Histogram shows densitometric analysis of three independent experiments. Mann–Whitney U test: *P<0.05 compared with control, with Dunnett’s post test for multiple comparisons to a single control. (E) Immunofluorescence analysis by confocal microscopy of ANG expression in HREC incubated 24 hours with 2 μg/ml tunicamycin, 0.25 μm thapsigargin, 100 μm etoposide or vehicle. The bar represents 10 μm.
To confirm that ER stress controls ANG expression in the human renal epithelium, human renal epithelial cells (HREC) were exposed to thapsigargin (which inhibits Sarco/endoplasmic reticulum Ca2+-ATPase pumps and disturbs calcium homeostasis) and tunicamycin. The topoisomerase inhibitor Etoposide, which promotes apoptosis without ER stress, was used as a control. ANG mRNA expression was induced after 4 hours and peaked after 8 hours of treatment with the ER stressors but not Etoposide, and progressively declined thereafter (Figure 2C). The upregulation of ANG upon ER stress was specific, as among the various RNases typically expressed in human renal epithelial cells, only RNase4, which shares the same promoter as ANG, was expressed (Supplemental Figure 2). ER stress weakly induced ANG protein expression in HREC (Figure 2, D and E). Notably, ER stress also induced ANG expression in primary cultured renal epithelial cells recovered from a human nephrectomy specimen26 (Supplemental Figure 3).
ANG Expression is Under the IRE1α/sX-Box Binding Protein 1 Axis in Response to ER Stress
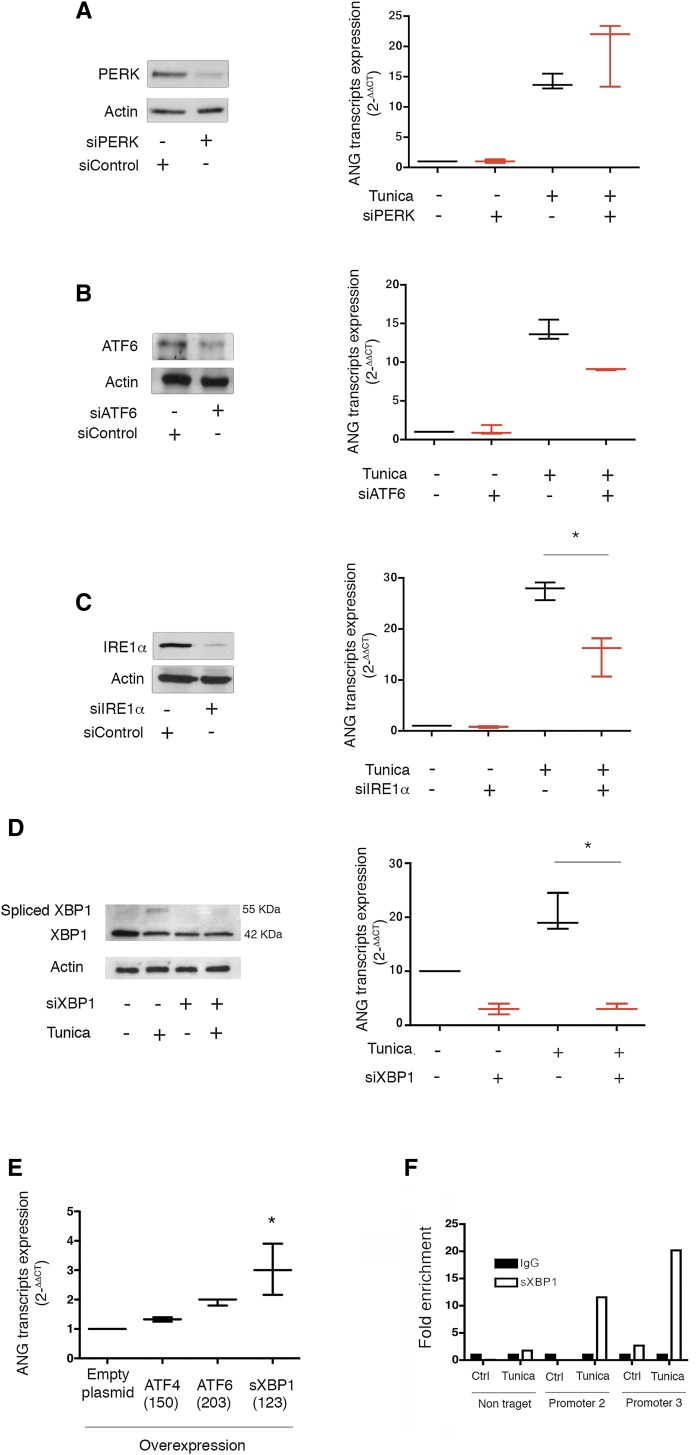
Evidence suggests that ANG is induced in HREC subjected to ER stress, so we determined whether and how the UPR regulates ANG expression using small interfering RNA (siRNA)-mediated silencing of PERK, ATF6 and IRE1α, respectively. Although PERK inhibition did not alter ANG expression (Figure 3A), ATF6 and IRE1α expression was required for ANG transcript expression upon ER stress (Figure 3, B and C), with only IRE1α inhibition reaching a statistically significant difference compared with controls. In line with these findings, the inhibition of the ribonuclease activity of IRE1α by the chemical compound 4μ8C27 reduced the expression levels of ANG under ER stress (Supplemental Figure 4A). To understand the mechanism by which IRE1α regulates ANG expression, we then silenced the expression of X-box binding protein 1 (XBP1), the transcription factor activated by the ribonuclease IRE1α, and which regulates the IRE1α-related transcriptional program.28 The siRNA-mediated XBP1 silencing (Figure 3D, Supplemental Figure 4B) resulted in reduced expression of ANG following ER stress (Figure 3D), consistent with the overexpression of sXBP1, the active form of XBP1, which induced the expression of ANG transcripts (Figure 3E). Notably, overexpression of ATF6 also led to an induction of ANG expression, albeit not statistically significant. The sXBP1-mediated chromatin immunoprecipitation assay showed a significant enrichment of the ANG promoter region (Figure 3F), consistent with the predicted presence of sXBP1 binding sites in the ANG promoter (Supplemental Figure 4C, Supplemental Table 1). These results indicate that the IRE1α/sXBP1 arm of the UPR controls ANG expression during ER stress.
Figure 3.
ANG synthesis during ER stress is mediated by IRE1α and sXBP1. (A) (Left) Immunoblot representing PERK and actin protein expression in HREC at 24 hours after transfection with siRNA targeting PERK mRNA. The immunoblot shown is representative of three independent experiments. (Right) Box and whiskers plots representing the relative expression levels of ANG transcripts in HREC transfected with siRNA, targeting PERK, or scrambled siRNA and incubated with 2 μg/ml tunicamycin or vehicle for 16 hours. The data were obtained from three independent experiments. (B) (Left) Immunoblot representing ATF6 and actin protein expression in HREC at 24 hours after transfection with siRNA targeting ATF6 mRNA. The immunoblot shown represents three independent experiments. (Right) Box and whiskers plots representing the relative expression levels of ANG transcripts in HREC transfected with a siRNA targeting ATF6, or a scrambled siRNA, and incubated with 2 μg/ml tunicamycin or vehicle for 16 hours. The data were obtained from three independent experiments. (C) (Left) Immunoblot representing IRE1α and actin protein expression in HREC at 24 hours after transfection of a siRNA targeting IRE1α mRNA. The immunoblot shown is representative of three independent experiments. (Right) Box and whiskers plots representing the relative expression levels of ANG transcripts in HREC transfected with an siRNA targeting IRE1α, or a scrambled siRNA, and incubated with 2 μg/ml tunicamycin or vehicle for 16 hours. The data were obtained from three independent experiments. Mann–Whitney U test: *P<0.05. (D) (Left) Immunoblot representing unspliced XBP1, spliced BXP1 and actin protein expression in HREC at 24 hours after transfection with siRNA targeting XBP1 mRNA, or a scrambled siRNA, and after incubation with 2 μg/ml tunicamycin or vehicle for 16 hours. The immunoblot shown is representative of three independent experiments. (Right) Box and whiskers plots representing ANG transcripts expression in HREC transfected with an siRNA targeting XBP1, or a scrambled siRNA, and incubated with 2 μg/ml tunicamycin or vehicle for 16 hours, analyzed using quantitative RT-PCR. The data were obtained from three independent experiments. Mann–Whitney U test: *P<0.05. (E) Box and whiskers plots representing ANG transcripts relative expression in HREC transfected with an empty plasmid or a plasmid expressing ATF4, ATF6, or sXBP1 for 36 hours analyzed using quantitative RT-PCR. *P<0.05 compared with control cells. In brackets are shown the expression levels of the transcripts of the respective transcription factors measured by quantitative PCR (F) Histograms representing the results of a chromatin immunoprecipitation assay of sXBP1, followed by amplification through the quantitative RT-PCR of ANG promoter using non-target primers or primers targeting two different regions of the promoter. HREC were incubated with 2 μg/ml tunicamycin or vehicle for 2 hours. The graph is representative of two experiments.
ANG Participates in Translation Inhibition During ER Stress
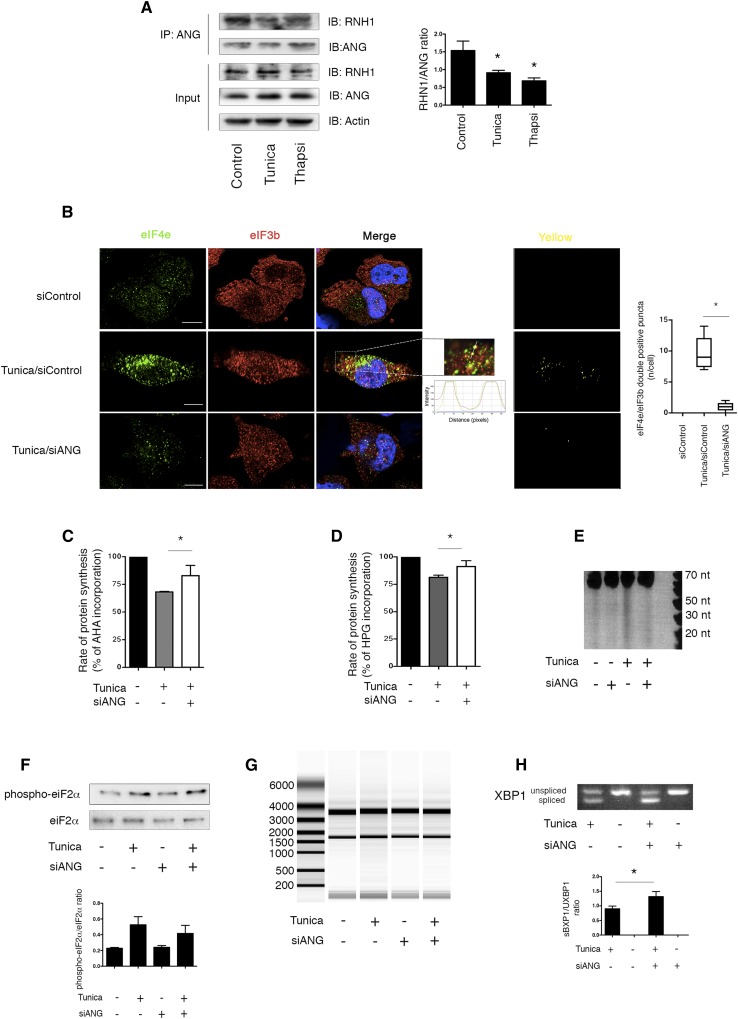
Provided that ANG participates in cell stress responses through intracellular pathways,18 we proposed that under ER stress, ANG could have intracellular properties. To examine the cell autonomous properties of ANG during ER stress, we focused on the early period after ER stress induction (<4 h) to rule out any autocrine effect of secreted ANG, which is not yet secreted at this time (Supplemental Figure 5). We first analyzed the interaction of ANG and the ribonuclease inhibitor ribonuclease/angiogenin inhibitor 1 (RNH1), an important regulator of ANG activity, whose dissociation under stress is required to allow ANG cellular activity.29 Co-immunoprecipitation analysis revealed that the association of ANG and RNH1 was disrupted following 2 hours of exposure to tunicamycin (Figure 4A), thus indicating that ER stress promotes the release of ANG from its inhibitor. Recent findings also indicate that during stress, ANG participates in the production of stress granules (SG).30 SG are dense cytoplasmic aggregates (Supplemental Figure 6) containing messenger RNA stalled in the initiation complex of translation, eventually promoting the adaptation and survival of stressed cells.31,32 Using elongation Initiation Factor 4E (eIF4E) and eIF3B as markers of SG,32 immunofluorescence analysis showed that siRNA-mediated ANG silencing (Supplemental Figure 7), was associated with the decreased abundance of SG in the cytoplasm of HRECs early after the induction of ER stress (Figure 4B), so suggesting that ANG participates in SG formation. To examine whether ANG is involved in translation control upon ER stress, we monitored protein synthesis using Click chemistry.33,34 Using the l-azidohomoalanine (AHA) + Alexa Fluor 488 alkyne reaction, or the inverse reaction, l-homopropargylglycine (HPG) and Alexa Fluor 488 azide, we observed that siRNA-mediated ANG silencing led to increased protein synthesis (by approximately 20%) after 2 hours of ER stress (Figure 4, C and D), compared with control cells. This indicated that ANG contributes to the reduction of protein synthesis during ER stress. Moreover, the analysis of small RNA in tunicamycin-treated HREC extracts resolved on denaturing gel and visualized using SYBR gold, revealed bands corresponding to RNAs centered at approximately 30–50 nucleotides (Supplemental Figure 8A). Northern blotting using cDNA probes complementary to the 3′ end of tRNAPro confirmed that these stress-induced RNAs were tRNA fragments (tiRNA) (Supplemental Figure 8B).15,18 To determine the role of ANG in the production of these tiRNA, we monitored production of small RNAs under ER stress in HREC extracts from cells silenced or not for ANG. These fragments were significantly reduced in cells transfected with siANG, indicating that their production might depend on ANG expression (Figure 4E). Notably, the activity of ANG on protein translation and SG formation was likely independent of eIF2α phosphorylation, as previously described during oxidative stress,15 because the inhibition of ANG expression did not alter ER stress-induced eIF2α phosphorylation (Figure 4F). The inhibition of ANG expression was not associated with degradation of total RNA, which could impact protein synthesis rate35,36 (Figure 4G). In addition, ANG silencing was associated with the increased production of the spliced product of X-box binding protein 1 (XBP1) mRNA, sXBP1, likely reflecting the exacerbation of misfolding burden on ER due to deficient inhibition of protein synthesis (Figure 4H). In line with an increased ER stress response, the expression of transcripts of UPR, including the proapoptotic factor CHOP, and the ER association degradation pathway component HERP, were found to be significantly increased when ANG expression was inhibited (Supplemental Figure 8C). These results indicate that the cellular effects of ANG during ER stress are, at least in part, mediated through a reduction in protein synthesis, potentially mediated through tiRNA production.
Figure 4.
ANG participates in translation inhibition during ER stress. (A) Blots representing a coimmunprecipitation assay of ANG, followed by RNH1 immunoblotting in HREC incubated with 2 μg/ml tunicamycin or vehicle for 2 hours. The immunoblot represents three independent experiments. Histogram shows densitometric analysis of three independent experiments. Mann–Whitney U test: *P<0.05 compared with control, with Dunnett’s post-test for multiple comparisons to a single control. (B) Immunofluorescence analysis by confocal microscopy of eIF4E and eIF3b colocalization in HREC transfected with an siRNA targeting ANG mRNA or a scrambled siRNA and incubated 4 hours with 2 μg/ml tunicamycin or vehicle. The bar represents 10 μm. Mann–Whitney U test: *P<0.05. (C, D) Histograms showing the incorporation rates of l- AHA and l- HPG in HREC transfected with siRNA targeting ANG mRNA or a scrambled siRNA after 2 hours of incubation with 2 μg/ml tunicamycin. The data were obtained from three independent experiments. Mann–Whitney U test: *P<0.05. (E) PAGE of small RNA extracted from HREC transfected with siRNA targeting ANG mRNA or scrambled siRNA after 2 hours of incubation with 2 μg/ml tunicamycin, followed by staining with SYBR gold. The data were obtained from three independent experiments. (F) Immunoblot representing phospho-eIF2α and eIF2α expression in HRECs transfected with an siRNA targeting ANG mRNA and incubated with 2 μg/ml tunicamycin for 2 hours. Immunoblot is representative of three independent experiments. Histogram shows densitometric analysis of three independent experiments. (G) Experion-based automated gel-based electrophoresis of total RNA extracted from HREC transfected with an siRNA targeting ANG mRNA and incubated with 2 μg/ml tunicamycin. (H) Agarose gel electrophoresis of PCR products of XBP1 cDNA from HREC transfected with siRNA targeting ANG mRNA or scrambled siRNA and incubated 4 hours with 2 μg/ml tunicamycin, or vehicle. Histogram shows densitometric analysis of three independent experiments. Mann–Whitney U test: *P<0.05.
ANG Deficiency Increases Susceptibility to ER Stress-Induced AKI
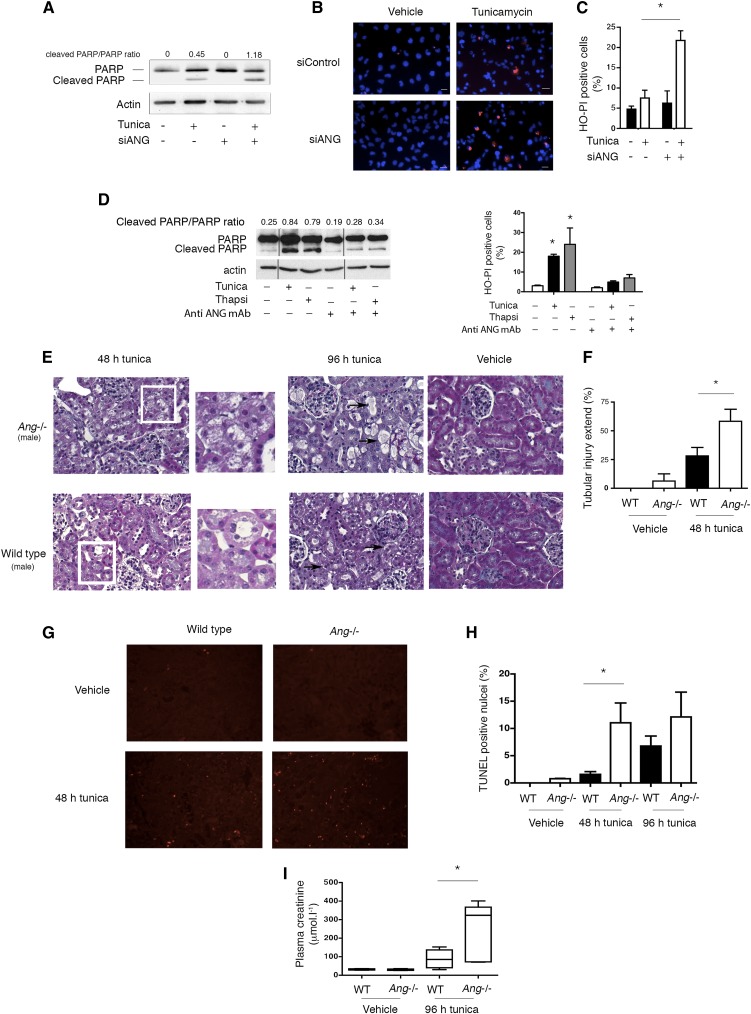
We next determined whether and how ANG affords protection under ER stress. To this end, we inhibited ANG expression using siRNA-mediated RNA interference in tunicamycin-treated cells (Supplemental Figure 7), which results in the inhibition of both expression and secretion of ANG, and monitored cell death. ANG silencing increased signs of HREC ER stress-induced cell death, possibly mediated by apoptosis and exemplified by increased PARP cleavage (Figure 5A). This indicated that ANG is cytoprotective upon ER stress. Accordingly, staining with the vital dyes Hoechst 33342 and propidium iodide,37 showed an increase in the number of dying cells with fragmented and pycnotic nuclei and permeabilized plasma membrane, compared with control cells (Figure 5, B and C). The incubation of HREC with monoclonal antibodies that block extracellular ANG reduced PARP cleavage under ER stress, as well as an increased number of apoptotic cells (Figure 5D), suggesting that secreted ANG may have deleterious autocrine effects on HREC viability under ER stress. Together, these results indicate that the net effect of the activation of ANG during ER stress in vitro (both at the intracellular and extracellular levels) results in increased cell viability, an effect likely supported by the intracellular adaptive properties of ANG.
Figure 5.
ANG deficiency increases susceptibility to ER stress-induced kidney injury. (A) Immunoblot representing PARP and actin protein expression in HREC transfected with siRNA targeting ANG mRNA or scrambled siRNA and incubated 24 hours with 2 μg/ml tunicamycin or vehicle. Immunoblot is representative of three independent experiments. (B, C) Representative epifluorescence photomicrograph of HREC transfected with a siRNA targeting ANG mRNA or a scrambled siRNA and incubated 24 hours with 2 μg/ml tunicamycin or vehicle and stained with 1 μg/ml Hoechst 33342 (HO) and 5 μg/ml propidium iodide (PI), and quantification of the number of HOPI-positive cells per high-powered field. The bar represents 50 μm. The data represent three independent experiments. (D) (Left) Immunoblot representing PARP and actin in HREC incubated with 2 μg/ml tunicamycin or 0.25 μm thapsigargin for 24 hours and with 30 μg/ml monoclonal anti-ANG antibody (26-2F; Sigma-Aldrich) or mouse IgG. Immunoblot is representative of three independent experiments. (Right) Quantification of the number of HOPI-positive cells per high-powered field in HREC incubated with 2 μg/ml tunicamycin, 0.25 μm thapsigargin or vehicle for 24 hours and with 30 μg/ml monoclonal anti-ANG antibody (26-2F; Sigma-Aldrich) or mouse IgG. Mann–Whitney U test: *P<0.05. (E) Photomicrographs (×400) of Ang−/− or wild-type mouse kidneys stained with Masson’s trichrome 48 and 96 hours (black arrows) after injection with 1 mg/kg tunicamycin, or vehicle. (F) Quantification of the extent of tubular damage at 48 hours in tunicamycin- or vehicle-treated wild-type (n=6) and Ang−/− mice (n=6), using a modified Shih scale.51 Mann–Whitney U test: *P<0.05. (G) Photomicrographs (×400) of Ang−/− or wild-type mouse kidneys stained for terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) 48 hours after injection with 1 mg/kg tunicamycin, or vehicle. (H) Quantification of the percentage of TUNEL-positive cells per high-powered field in kidneys from six wild-type and six Ang−/− mice 48 hours after tunicamycin injection. The number of TUNEL-positive nuclei per high-power field was reported to the number of DAPI positive nuclei (not shown) to calculate the percentage of TUNEL-positive nuclei. Mann–Whitney U test: *P<0.05. (I) Plasma creatinine concentration from six wild-type and six Ang−/− mice at 96 hours after tunicamycin injection. The median and 95% CI are represented as box and whisker plots. Mann–Whitney U test: *P<0.05.
We next examined a role for ANG in response to ER stress-induced kidney injury in vivo. To this end, we challenged mice that do not express ANG (Ang−/− mice) with 1 mg/kg tunicamycin. Ang−/− mice were born at the expected Mendelian ratio and exhibited no apparent renal phenotype under physiologic conditions (Supplemental Figures 9 and 10). Tunicamycin-treated wild-type mice developed severe renal lesions, including cell vacuolization, indicative of acute tubular necrosis (Figure 5E) and the frequency and severity of these lesions were significantly increased in Ang−/− mice (Figure 5F). Of note, 96 hours after tunicamycin injection, wild-type and Ang−/− mice displayed a similar frequency of tubular lesions, but the lesions were far more severe in Ang−/− mice (Figure 5E). The number of apoptotic cells, evaluated using terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining, was higher in Ang−/− mice challenged with tunicamycin (Figure 5, G and H). These findings were confirmed by an increased cleavage of caspase 3 in the idneys of Ang−/− mice, which also displayed more severe ultrastructural tubular lesions (Supplemental Figure 11). Moreover, tunicamycin prompted acute renal failure, characterized by an increase in plasma creatinine concentration, and Ang−/− mouse kidneys experienced more severe dysfunction compared with wild-type littermates (Figure 5I). These results indicate that ANG affords protection against AKI during ER stress.
DISCUSSION
Understanding UPR signaling in injured tissues is of paramount importance in medicine, and expanding the understanding of how cells respond to ER stress will fuel the discovery of novel therapeutic targets and potential biomarkers relevant to tissue injury. In the present study, based on the characterization of the cellular responses to kidney injuries, we provide the first evidence that ANG is directly and specifically regulated by the UPR through the IRE1α-activated transcription factor sXBP1. The discovery of a UPR-regulated original biologic pathway depending on ANG is of potential considerable importance, as the biologic properties of ANG have been associated with a wide range of pathophysiological processes, including tumorigenesis, tissue regeneration, inflammatory bowel disease, and inflammation.16,38–40
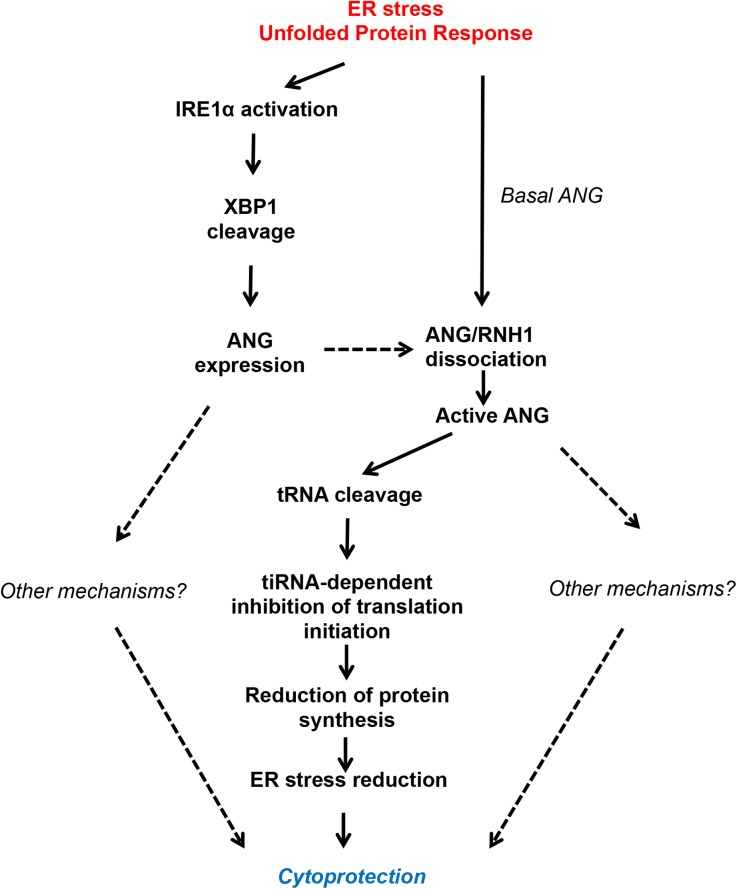
Based on our findings, we propose a working model in Figure 6. In the first few hours after ER stress arises, a basal pool of ANG dissociates from its inhibitor RNH1 and becomes active, thereby cleaving tRNA, promoting SG formation and translation reduction. The mechanism by which ER stress promotes ANG–RNH1 dissociation is not known, but might involve RNH1 nuclear translocation.29 In parallel, during ER stress ANG mRNA expression increases under the control of the IRE1α–XBP1 axis, as well as protein level, albeit to a lesser extent, maybe because the protein is in part secreted. Newly produced ANG is also involved in cytoprotection, and we cannot exclude that ANG has cytoprotective properties independent of tiRNA production. Therefore, the cytoprotective effects of ANG that we observed in vitro are, at least in part, mediated by ANG-mediated translation reduction. Our findings are consistent with those recently published indicating that, in various models of tissue damage (ischemic reperfusion, toxic injury and irradiation), the levels of circulating tRNA derivatives, which correspond to the conformational change in tRNA structure that occurs before fragmentation, increased rapidly and may serve as a marker of early tissue injury.41 Together, these and our results suggest that tRNA derivates are likely involved in renal cell adaptation under stress and highlight the biologic relevance of tRNA catabolism in kidney injury.
Figure 6.
Regulation of ANG activation and expression under ER stress. Under ER stress, ANG expression and activation are induced. ANG expression is induced by the transcription factor sXBP1, following IRE1α activation. Part of ANG is secreted by HREC under ER stress. In addition, early ER stress promotes residual ANG activation by freeing it from its inhibitor RNH1, the exact mechanism being unknown, but which could involve post-translational modifications. One of the mechanisms by which ANG promotes cell adaptation during ER stress implicates its ribonuclease activity, which cleaves tRNA to produce tiRNA that interfere with translation initiation. Consequently, nascent protein synthesis and ER protein load are reduced, ER stress is mitigated, and cell survival is prolonged. Importantly, this model does not exclude other mechanisms leading to cell adaptation mediated by ANG under ER stress. Dashed lines represent hypothetical process.
We demonstrate herein that, under ER stress, ANG engage a cell autonomous adaptive response, which implicates eIF2α-independent translation inhibition and SG formation. These findings are consistent with the fact that ANG contributes to translation repression and SG formation in response to oxidative stress.42 Mechanistically, ANG generates stress-induced tiRNAs that contribute to the displacement of eIF4G/A from capped and uncapped mRNA and eIF4E/G/A (eIF4F) from the m7G cap, thereby inhibiting translation and inducing SG assembly.18 In addition, ANG cleaves the conserved single-stranded 3′-CCA termini of all tRNAs, thereby promoting the deactivation of the aminoacyl-ends of tRNA and subsequently inhibiting translation.43 Therefore, ANG participates in translation attenuation in ER-stressed cells through an original process of RNA interference which therefore expands UPR-induced mechanisms for the reduction of protein flux into the ER and comes in addition to the previously described phosphorylation of eIF2α,44 regulated IRE1α-dependent decay of RNAs,36 and selective mRNA release from the ER.45
Our data also indicate that in the first hours after ER stress induction, ANG-mediated translation repression correlates with ANG dissociation from the ANG-inhibitor RNH1. It is likely that during early ER stress, the existing pool ANG is mobilized and activated, since adaptation occurs before ANG mRNA expression is induced by the IRE1α/XBP1 arm of the UPR. The mechanisms by which RNH1 dissociates from its inhibitor are not clear. A model for RNH1–ANG dissociation has been proposed whereby the RNH1–ANG complex is sensitive to oxidation, attributable to RNH1 cysteine residues. Indeed RNH1 contains 32 cysteines and loses activity in the absence of reducing agents.46 Treatment of RNH1–ANG complexes with p-hydroxymercuribenzoate rapidly dissociates the complex, releasing fully active RNase. Oxidation or derivatization of cysteine residues alters the structure of RNH1,47 which may lead to dissociation of ANG from the RNH1–ANG complex. Whether this mechanism occurs under ER stress, which can be associated with oxidative stress, remains to be established.
In parallel with this early ANG-mediated stress response, ER stress also promotes the induction of ANG expression. The fact that we observed a weak accumulation of ANG in whole cell lysate may be related to a secretion process activated upon ER stress, which modalities remain to be determined. In addition to cell autonomous responses mediated by ANG to eliminate the stress and promote cellular adaptation at the individual cellular level, one might propose that secreted ANG might as well be involved in cell non-autonomous responses. Those would likely involve paracrine communication with neighboring cells not yet exposed to stress, and consequently, would act as warning signals.48 These cellular responses afford tissue level adaptations to a challenge, facilitating the preservation of tissue structure and function.
In conclusion, our results provide new insights into how ANG modulates adaptation during kidney injury, and we describe an additional mechanism by which the UPR might control protein synthesis. We showed ANG as a key regulator of tissue homeostasis during AKI associated with ER stress. Over time, regulators of ANG activity could be developed to increase tissue adaptation to stress.
CONCISE METHODS
Human Studies
RNA Isolation from Kidney Transplant Biopsies
Sixteen surplus kidney allograft protocol biopsies, performed 3 months after transplantation, were retrospectively analyzed for BiP and ANG mRNA expression. Detailed methods are available in the Supplemental Material.
Immunohistochemistry of Human Kidney Biopsies with Acute Injury
Nine kidney allograft biopsies: three normal, three with acute cyclosporine nephrotoxicity lesions, and three with ischemic damage (preimplantation biopsy) were retrospectively analyzed for ANG immunohistochemistry.
Approvals
Kidney biopsies were not performed for the purpose of this noninterventional study, but only for patient care. Patients wrote informed consent for the eventual use of their surplus biologic samples. Analyses were performed anonymously. Paris Descartes University ethics committee approved this observational study. The authors adhere to the Declaration of Helsinki.
Experimental Animal Models
Ischemic Injury
Adult male Sprague-Dawley rats (Charles River laboratories, L’Arbresle, France) weighing 325–350 g were allowed free access to tap water. The abdomen was then opened through a midline incision, and the aorta was retrogradely cannulated below the renal arteries with an 18-gauge needle. With the aorta occluded by ligation above the renal arteries, and the renal vein opened by a small incision for outflow, the kidneys were perfused with 20 mL of cold heparinized saline. Kidneys were then washed with 10 mL IGL1 followed by incubation in IGL1 during 24 hours at 4°C.
Passive Anti-GBM Nephritis
Passive anti-GBM nephritis protocol has been induced as described previously.49 Anti-GBM nephrotoxic serum was injected to C57Bl6/J mice through the retro-orbital venous sinus at 6μl/g body wt for 3 days continuously. On day 14 animals were euthanized. Albuminuria was significantly increased in the animals with anti-GBM nephritis.24
Ang knockout (Ang−/−) mice.
Ang−/− mice were generated by crossing Ang1 gene floxed mice with EIIa-Cre mice and were backcrossed eight generations to obtain Ang−/− mice in pure C57BL/6 background. The Ang knockout mice used in this study were constitutive knockout. Eliia-Cre was used to delete floxed Ang gene in the germline. All experiments were performed on 12-week-old wild-type males and Ang−/− littermates from Ang+/− heterozygous crossings. Animals were fed ad libitum and housed at 25°C in a 12-hour light cycle. Tunicamycin (1 mg/kg) or vehicle (DMSO) was intraperitoneally injected at day 0, and mice were sacrificed 2 and 4 days post-injection. Kidneys were next processed for protein, RNA extraction, and Masson’s trichrome staining. Detailed methods are available in the Supplemental Material. Five to seven mice per group were analyzed.
Approvals
Experiments were conducted according to the French veterinary guidelines and those formulated by the European Community for experimental animal use (L358–86/609EEC), and were approved by the Institut National de la Santé et de la Recherche Médicale (INSERM). Animal procedures on Ang−/− mice were approved by the Institutional Animal Care and Use Committee of Tufts University (Protocol N°B2013–59).
Cell Culture
Normal HREC of proximal origin (HK-2) were purchased from ATCC/LGC Standards (lot number 60352186), and cultured according to previously published methods.26 Detailed methods are available in the Supplemental Material.
Small Interfering RNA Transfections
The transient inactivation of PERK, IRE1α, ATF6, ANG, and XBP1 was achieved using synthetic siRNAs designed and obtained from Qiagen and transfected using HiPerFect (Qiagen) according to the manufacturer’s protocol. The control siRNA we used is a scrambled siRNA called AllStars Negative Control siRNA (Ref. 1027281; Qiagen). AllStars Negative Control siRNA has no homology to any known mammalian gene. Validation has been performed using Affymetrix GeneChip arrays and a variety of cell-based assays to ensure minimal nonspecific effects on gene expression and phenotype.
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation (ChIP) was performed according to the manufacturer’s protocol (EZ-Magna CHIP G; EMD Millipore). Briefly, HREC were incubated with tunicamycin for 1 h. Subsequently, the cells were washed with PBS and crosslinked with 1% formaldehyde at 37°C. After terminating the crosslinking reaction, the cells were collected, washed, and resuspended in the SDS lysis buffer containing protease inhibitor cocktail (Roche Diagnostics). The lysates were sonicated five times followed by cooling on ice. The cell debris was cleared, and the supernatant was diluted in a ChIP dilution buffer. After a brief centrifugation, 1% of the total supernatant was put aside and one-tenth of this material was used as input control. Half of the remaining supernatant was incubated with an anti-sXBP1 antibody (made from hybridoma, provided by Dr. Eric Chevet,50 and the other half of the supernatant was incubated with a nonimmune rabbit immunoglobulin G and protein G magnetic beads at 4°C overnight with rotation. The beads were washed and pelleted using a magnetic separator. After elution, DNA fragments were purified using a spin column. For PCR, 10% of the immunoprecipitated materials were used as the DNA template in 40 cycles of amplification using the following primer sets. The primers sequences are listed in the Supplemental Table 2A. The results of the ChIP analysis were calculated and recorded as fold-enrichment values. The default Input fraction was 1%, which represents a dilution factor of 100 or 6.644 cycles (i.e., log2 of 100).
Coimmunoprecipitation Assay
The samples were immunoprecipitated using an anti-ANG antibody, followed by immunoblotting for RHN1 and ANG. Detailed methods are available in the Supplemental Material
Small RNAs Migration and Staining with SYBR Gold
Total RNA was extracted using the miRNeasy Mini Kit (Qiagen) RNA (10µg per well) was analyzed using TBE-urea gels and stained with SYBR gold to visualize stress-induced small RNAs. Detailed methods are available in the Supplemental Material.
Click-It Chemistry
Nascent protein synthesis analysis was performed using the Click-iT HPG/AHA Alexa Fluor 488 Protein Synthesis Assay Kits (Invitrogen) according to the manufacturer’s instructions, with minor modifications. HREC cultured on 96-well plates in l-methionine-free medium supplemented with 200 μm l-cystine, 2 mm l-glutamine, and 10 mm HEPES were fed with 50 μm l-HPG or l-AHA for 30 min. Subsequently, tunicamyin was added, and the cells were fixed after 2 hours of incubation with 3.7% formaldehyde in PBS, followed by a permeabilization step using 0.5% Triton X-10. After incubation with Click-iT reaction buffer containing Alexa Fluor 488 azide/alkyne, the fluorescence was visualized using an imaging platform with filters appropriate for Alexa Fluor 488.
Statistical Analysis
Distribution of variables is represented using box-and-whiskers plots: the bottom and top of the box are the first and third quartiles, the band inside the box is the median, and the ends of the whiskers represent the minimum and maximum of all the data. The proportions are represented using histograms. We used the Mann–Whitney U test for nonparametric data comparisons between two groups, and Student’s t test for the comparison of parametric data.
One-way ANOVA with Dunnett’s post-test correction for multiple comparisons to a single control was performed when necessary. Statistical analyses were performed using JMP.10 (SAS software) and graphs were produced using Prism-GraphPad software. P values <0.05 were considered significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was funded by grants from the INSERM, la Fédération Nationale pour l’Aide aux Insuffisants Rénaux (to N.P.), grants from INSERM (to E.C.) and National Institutes of Health grants R01-NS065237 and R01-CA105241 (to G.-F.H.). The authors thank the HisIM facility (Cochin Institute), the Laboratoire de Biochimie (Claude Bernard Institute), and Alain Schmitt (Electron Microscopy facility, Cochin Institute).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015020196/-/DCSupplemental.
References
- 1.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leung KC, Tonelli M, James MT: Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 9: 77–85, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Mariño G, Levine B: Autophagy and the integrated stress response. Mol Cell 40: 280–293, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Majmundar AJ, Wong WJ, Simon MC: Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell 40: 294–309, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sengupta S, Peterson TR, Sabatini DM: Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40: 310–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter P, Ron D: The unfolded protein response: from stress pathway to homeostatic regulation. Science 334: 1081–1086, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Cybulsky AV: The intersecting roles of endoplasmic reticulum stress, ubiquitin- proteasome system, and autophagy in the pathogenesis of proteinuric kidney disease. Kidney Int 84: 25–33, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Inagi R: Endoplasmic reticulum stress as a progression factor for kidney injury. Curr Opin Pharmacol 10: 156–165, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Inagi R, Ishimoto Y, Nangaku M: Proteostasis in endoplasmic reticulum—new mechanisms in kidney disease. Nat Rev Nephrol 10: 369–378, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Pallet N, Fougeray S, Beaune P, Legendre C, Thervet E, Anglicheau D: Endoplasmic reticulum stress: an unrecognized actor in solid organ transplantation. Transplantation 88: 605–613, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Kawakami T, Gomez IG, Ren S, Hudkins K, Roach A, Alpers CE, Shankland SJ, D’Agati VD, Duffield JS: Deficient Autophagy Results in Mitochondrial Dysfunction and FSGS. J Am Soc Nephrol 26: 1040–1052, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogo AB: The targeted podocyte. J Clin Invest 121: 2142–2145, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fett JW, Strydom DJ, Lobb RR, Alderman EM, Bethune JL, Riordan JF, Vallee BL: Isolation and characterization of angiogenin, an angiogenic protein from human carcinoma cells. Biochemistry 24: 5480–5486, 1985 [DOI] [PubMed] [Google Scholar]
- 14.Hartmann A, Kunz M, Köstlin S, Gillitzer R, Toksoy A, Bröcker EB, Klein CE: Hypoxia-induced up-regulation of angiogenin in human malignant melanoma. Cancer Res 59: 1578–1583, 1999 [PubMed] [Google Scholar]
- 15.Yamasaki S, Ivanov P, Hu GF, Anderson P: Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185: 35–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuji T, Sun Y, Kishimoto K, Olson KA, Liu S, Hirukawa S, Hu GF: Angiogenin is translocated to the nucleus of HeLa cells and is involved in ribosomal RNA transcription and cell proliferation. Cancer Res 65: 1352–1360, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fu H, Feng J, Liu Q, Sun F, Tie Y, Zhu J, Xing R, Sun Z, Zheng X: Stress induces tRNA cleavage by angiogenin in mammalian cells. FEBS Lett 583: 437–442, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Ivanov P, Emara MM, Villen J, Gygi SP, Anderson P: Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov P, O’Day E, Emara MM, Wagner G, Lieberman J, Anderson P: G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci U S A 111: 18201–18206, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Famulski KS, de Freitas DG, Kreepala C, Chang J, Sellares J, Sis B, Einecke G, Mengel M, Reeve J, Halloran PF: Molecular phenotypes of acute kidney injury in kidney transplants. J Am Soc Nephrol 23: 948–958, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7: 518–526, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Bouvier N, Fougeray S, Beaune P, Thervet E, Pallet N: The unfolded protein response regulates an angiogenic response by the kidney epithelium during ischemic stress. J Biol Chem 287: 14557–14568, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D: CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang J, Filipe A, Rahuel C, Bonnin P, Mesnard L, Guérin C, Wang Y, Le Van Kim C, Colin Y, Tharaux PL: Lutheran/basal cell adhesion molecule accelerates progression of crescentic glomerulonephritis in mice. Kidney Int 85: 1123–1136, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, Schlöndorff D: Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J Am Soc Nephrol 19: 2225–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pallet N, Thervet E, Le Corre D, Knebelmann B, Nusbaum P, Tomkiewicz C, Meria P, Flinois JP, Beaune P, Legendre C, Anglicheau D: Rapamycin inhibits human renal epithelial cell proliferation: effect on cyclin D3 mRNA expression and stability. Kidney Int 67: 2422–2433, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Hetz C, Chevet E, Harding HP: Targeting the unfolded protein response in disease. Nat Rev Drug Discov 12: 703–719, 2013 [DOI] [PubMed] [Google Scholar]
- 28.Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD: XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27: 53–66, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Pizzo E, Sarcinelli C, Sheng J, Fusco S, Formiggini F, Netti P, Yu W, D’Alessio G, Hu GF: Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival. J Cell Sci 126: 4308–4319, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Emara MM, Ivanov P, Hickman T, Dawra N, Tisdale S, Kedersha N, Hu GF, Anderson P: Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem 285: 10959–10968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Decker CJ, Parker R: P-bodies and stress granules: possible roles in the control of translation and mRNA degradation. Cold Spring Harb Perspect Biol 4: a012286, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson P, Kedersha N: Stress granules. Curr Biol 19: R397–R398, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Clark PM, Dweck JF, Mason DE, Hart CR, Buck SB, Peters EC, Agnew BJ, Hsieh-Wilson LC: Direct in-gel fluorescence detection and cellular imaging of O-GlcNAc-modified proteins. J Am Chem Soc 130: 11576–11577, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG: Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J Am Chem Soc 125: 3192–3193, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS: Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186: 323–331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maurel M, Chevet E, Tavernier J, Gerlo S: Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem Sci 39: 245–254, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Kepp O, Galluzzi L, Lipinski M, Yuan J, Kroemer G: Cell death assays for drug discovery. Nat Rev Drug Discov 10: 221–237, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Lee HS, Lee IS, Kang TC, Jeong GB, Chang SI: Angiogenin is involved in morphological changes and angiogenesis in the ovary. Biochem Biophys Res Commun 257: 182–186, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Koutroubakis IE, Xidakis C, Karmiris K, Sfiridaki A, Kandidaki E, Kouroumalis EA: Serum angiogenin in inflammatory bowel disease. Dig Dis Sci 49: 1758–1762, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Moenner M, Gusse M, Hatzi E, Badet J: The widespread expression of angiogenin in different human cells suggests a biological function not only related to angiogenesis. Eur J Biochem 226: 483–490, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Mishima E, Inoue C, Saigusa D, Inoue R, Ito K, Suzuki Y, Jinno D, Tsukui Y, Akamatsu Y, Araki M, Araki K, Shimizu R, Shinke H, Suzuki T, Takeuchi Y, Shima H, Akiyama Y, Toyohara T, Suzuki C, Saiki Y, Tominaga T, Miyagi S, Kawagisihi N, Soga T, Ohkubo T, Yamamura K, Imai Y, Masuda S, Sabbisetti V, Ichimura T, Mount DB, Bonventre JV, Ito S, Tomioka Y, Itoh K, Abe T: Conformational change in transfer RNA is an early indicator of acute cellular damage. J Am Soc Nephrol 25: 2316–2326, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiyagarajan N, Ferguson R, Subramanian V, Acharya KR: Structural and molecular insights into the mechanism of action of human angiogenin-ALS variants in neurons. Nat Commun 3: 1121, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Czech A, Wende S, Mörl M, Pan T, Ignatova Z: Reversible and rapid transfer-RNA deactivation as a mechanism of translational repression in stress. PLoS Genet 9: e1003767, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ron D: Translational control in the endoplasmic reticulum stress response. J Clin Invest 110: 1383–1388, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reid DW, Chen Q, Tay AS, Shenolikar S, Nicchitta CV: The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell 158: 1362–1374, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blackburn P, Wilson G, Moore S: Ribonuclease inhibitor from human placenta. Purification and properties. J Biol Chem 252: 5904–5910, 1977 [PubMed] [Google Scholar]
- 47.Fominaya JM, Hofsteenge J: Inactivation of ribonuclease inhibitor by thiol-disulfide exchange. J Biol Chem 267: 24655–24660, 1992 [PubMed] [Google Scholar]
- 48.Chovatiya R, Medzhitov R: Stress, inflammation, and defense of homeostasis. Mol Cell 54: 281–288, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, Gutierrez-Ramos JC: RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med 185: 1371–1380, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pluquet O, Dejeans N, Bouchecareilh M, Lhomond S, Pineau R, Higa A, Delugin M, Combe C, Loriot S, Cubel G, Dugot-Senant N, Vital A, Loiseau H, Gosline SJ, Taouji S, Hallett M, Sarkaria JN, Anderson K, Wu W, Rodriguez FJ, Rosenbaum J, Saltel F, Fernandez-Zapico ME, Chevet E: Posttranscriptional regulation of PER1 underlies the oncogenic function of IREα. Cancer Res 73: 4732–4743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shih W, Hines WH, Neilson EG: Effects of cyclosporin A on the development of immune-mediated interstitial nephritis. Kidney Int 33: 1113–1118, 1988 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.