Abstract
Because of the shortage of agalsidase-β supply between 2009 and 2012, patients with Fabry disease either were treated with reduced doses or were switched to agalsidase-α. In this observational study, we assessed end organ damage and clinical symptoms with special focus on renal outcome after 2 years of dose-reduction and/or switch to agalsidase-α. A total of 89 adult patients with Fabry disease who had received agalsidase-β (1.0 mg/kg body wt) for >1 year were nonrandomly assigned to continue this treatment regimen (regular-dose group, n=24), to receive a reduced dose of 0.3–0.5 mg/kg and a subsequent switch to 0.2 mg/kg agalsidase-α (dose-reduction-switch group, n=28), or to directly switch to 0.2 mg/kg agalsidase-α (switch group, n=37) and were followed-up for 2 years. We assessed clinical events (death, myocardial infarction, severe arrhythmia, stroke, progression to ESRD), changes in cardiac and renal function, Fabry-related symptoms (pain, hypohidrosis, diarrhea), and disease severity scores. Determination of renal function by creatinine and cystatin C–based eGFR revealed decreasing eGFRs in the dose-reduction-switch group and the switch group. The Mainz Severity Score Index increased significantly in these two groups (P=0.02 and P<0.001, respectively), and higher frequencies of gastrointestinal pain occurred during follow-up. In conclusion, after 2 years of observation, all groups showed a stable clinical disease course with respect to serious clinical events. However, patients under agalsidase-β dose-reduction and switch or a direct switch to agalsidase-α showed a decline of renal function independent of the eGFR formula used.
Keywords: chronic kidney disease, creatinine clearance, Fabry’s disease, enzyme, replacement therapy, outcomes, cystatin C clearance
Fabry disease (FD) is an X-linked rare progressive multisystemic disorder resulting from lysosomal enzyme α-galactosidase A (GLA) deficiency. Fabry-specific manifestations, such as early stroke, malignant arrhythmia, myocardial infarction, and progressive renal and cardiac failure, result from the differential systemic cellular accumulation of globotriaosylceramide (Gb3).1 Treatment with enzyme replacement therapy (ERT) with recombinant GLA, including agalsidase-α (Replagal, Shire) and agalsidase-β (Fabrazyme, Genzyme), results in subcellular Gb3 clearance, leading to stabilization or at least slowing of disease progression.2–7
The shortage in agalsidase-β supply (from June 2009 to January 2012) resulted in a change of treatment regimen in many patients. Patients who had formerly received agalsidase-β in a standard dose (1.0 mg/kg body wt every other week [e.o.w.]) were dose-reduced (0.3–0.5 mg/kg body wt e.o.w.) or switched to agalsidase-α (0.2 mg/kg body wt e.o.w.). Very limited clinical data on the basis of small potentially statistically underpowered8 patient cohorts demonstrated no relevant effects on clinical outcome.9–12 In our recent multicenter study comprising 105 patients with FD followed-up for 12 months, patients receiving a regular agalsidase-β dose had a stable disease course, but dose reduction was associated with worsening of renal function and symptoms.13 Switching to agalsidase-α was safe, but renal function declined and Fabry-related symptoms increased in some patients.13
Because the 1-year follow-up period was relatively short, a long-term follow-up study of patients who were dose reduced and/or switched is warranted.
In this study we assessed clinical stability and safety during agalsidase-β standard dose, ERT dose reduction (agalsidase-β, 0.3 or 0.5 mg/kg e.o.w.), and subsequent switch or a direct switch to agalsidase-α (0.2 mg/kg) after 2 years of observation, with a special focus on clinical events, renal end organ damage, disease-related clinical symptoms, and disease severity.
Results
Patients
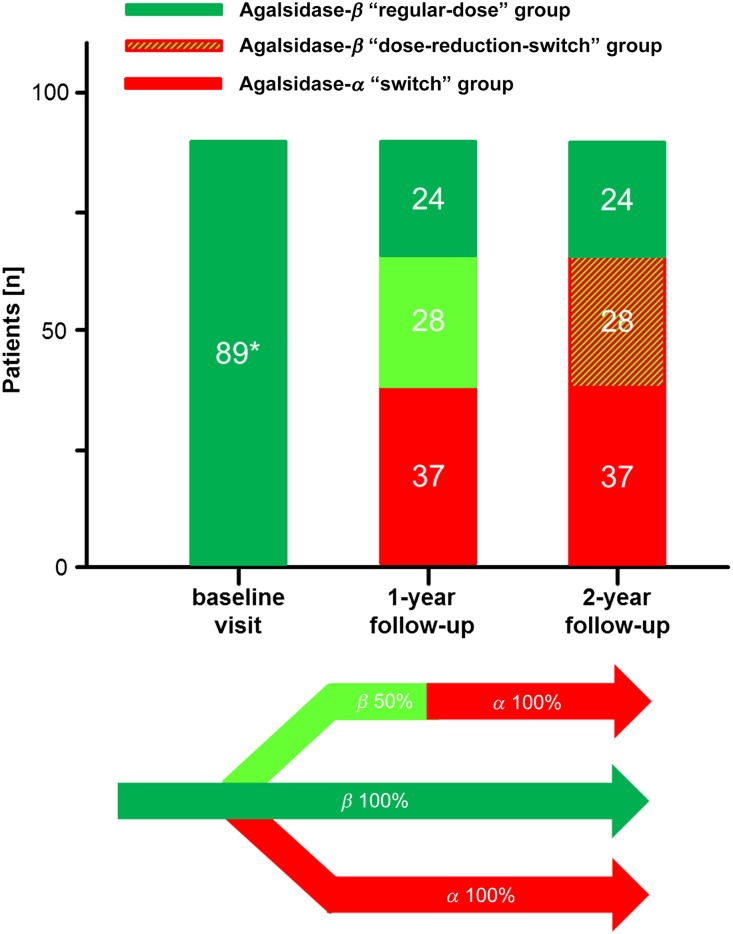
Figure 1 represents an overview of this retrospective observational study. A comprehensive diagnostic workup was performed as previously reported.13 The baseline characteristics of all patients are presented in Tables 1–3. In total, 89 patients with FD were included. The mean age was 45.5±12.5 years, and 37.1% were women. Patients received agalsidase-β, 1.0 mg/kg body wt e.o.w. for 44.2±26.3 months at baseline. Because of the ongoing supply shortage of agalsidase-β, patients receiving 0.5 mg/kg e.o.w. were switched to agalsidase-α (0.2 mg/kg e.o.w.) after 12 months of observation (Figure 1). Because of the observational design (real-world design), patients were not randomized. The long-term treatment strategy was decided by the interdisciplinary physicians of the Fabry centers. This strategy resulted in quite inhomogeneous treatment groups, with more severely affected patients with FD in the regular-dose group and suspected milder affected patients within the dose-reduction-switch and switch groups. In detail, compared with the switch-group and the dose-reduction-switch group, the regular-dose group comprised more male patients (each P<0.05) (Table 1), resulting in a lower residual enzymatic GLA activity (each P<0.05) (Table 1). In terms of FD-typical symptoms, patients in the regular-dose group more often showed edema compared with the dose-reduction-switch group (P<0.05) (Table 1). In addition, patients in the regular-dose group and dose-reduction-switch groups suffered more often from pain attacks compared with those in the switch group (each P<0.05) (Table 1).
Figure 1.
General overview of the study population. All patients were at least 12 months under a stable dose of agalsidase-β (1.0 mg/kg e.o.w.). After the baseline visit, the treatment strategy for the following years was chosen by a FD specialist physician team and the patients. Because of ongoing supply shortage, patients under agalsidase-β reduction (0.5 mg/kg e.o.w.) were switched after 12 months to agalsidase-α (0.2 mg/kg e.o.w.). *Patients were at least 12 months under a regular-dose of agalsidase-β.
Table 1.
Baseline characteristics of the patient groups
| Characteristic | Regular-Dose (n=24) | Dose-Reduction-Switch (n=28) | Switch (n=37) | Total (N=89) |
|---|---|---|---|---|
| Patients | ||||
| Women | 3 (12.5)a,b | 13 (46.4) | 17 (46.0) | 33 (37.1) |
| Age, y | 43.5±11.3 | 47.4±12.7 | 46.1±13.9 | 45.5±12.5 |
| Weight, kg | 74.0±15.1 | 74.5±13.1 | 72.8±13.4 | 73.9±13.6 |
| Height, cm | 177.0±7.9 | 172.3±10.2 | 171.5±10.2 | 173.4±13.6 |
| Heart rate, bpm | 62.5±20.3 | 67.4±7.8 | 65.3±11.8 | 65.2±13.5 |
| Systolic BP, mmHg | 118.2±13.9 | 118.6±12.2 | 120.0±10.1 | 118.9±11.8 |
| Diastolic BP, mmHg | 78.9±10.4 | 79.0±11.6 | 80.5±8.4 | 79.5±10.1 |
| GLA activity, nmol/min per mg of protein | 0.04±0.05a,b | 0.09±0.12 | 0.10±0.10 | 0.08±0.10 |
| GLA activity in men, nmol/min per mg of protein | 0.02±0.01 | 0.03±0.01 | 0.02±0.01 | 0.02±0.01 |
| Diuretics | 6 (40.0) | 6 (30.0) | 9 (47.4) | 21 (38.9) |
| RAAS blockers | 9 (60.0) | 14 (73.7) | 12 (60.0) | 35 (64.8) |
| Analgesics | 4 (30.8) | 6 (31.6) | 6 (31.6) | 16 (31.4) |
| Premedication | 2 (11.8) | 2 (8.7) | 1 (2.9) | 5 (6.8) |
| Duration of ERT, mo | 45.5±22.9 | 44.0±27.9 | 42.6±27.9 | 44.2±26.3 |
| Fabry-typical manifestations and symptoms | ||||
| Angiokeratoma | 13 (65.0) | 11 (45.8) | 14 (43.8) | 38 (50.7) |
| Edema | 11 (55.5)b | 2 (8.3) | 8 (24.4) | 21 (27.6) |
| Gastrointestinal pain | 5 (25.0) | 3 (12.5) | 3 (9.1) | 11 (14.5) |
| Diarrhea | 5 (25.0) | 7 (30.4) | 4 (11.8) | 16 (20.8) |
| Hypohidrosis | 15 (75.0) | 16 (66.7) | 16 (51.6) | 47 (62.2) |
| Cornea verticillata | 12 (60.0) | 8 (33.3) | 15 (46.9) | 35 (45.3) |
| Tinnitus | 8 (40.0) | 6 (25.0) | 7 (21.2) | 21 (26.3) |
| Hypoacusis | 5 (25.0) | 7 (29.2) | 5 (15.2) | 17 (22.4) |
| Paresthesia | 15 (75.0) | 18 (75.0) | 21 (63.6) | 54 (69.7) |
| Pain attacks | 5 (25.0)a | 6 (25.0)c | 1 (3.0) | 12 (15.8) |
| Permanent pain | 8 (40.0) | 13 (54.2) | 6 (18.2) | 27 (35.5) |
| Pain crisis | 4 (20.0) | 3 (12.5) | 7 (21.2) | 14 (18.4) |
| TIA/stroke | 2 (8.3) | 0 (0.0) | 1 (3.0) | 3 (4.0) |
| Fatigue | 4 (20.0) | 6 (25.0) | 4 (12.1) | 14 (17.1) |
| Stroke/TIA | 2 (10.0) | 0 (0.0) | 1 (3.0) | 3 (3.9) |
| MSSI score | 26.5±12.1 | 18.5±9.5 | 17.6±8.3 | 20.3±10.4 |
Values are given as mean±SD or n (%). Differences between groups have been tested via one-way ANOVA for continuous variables or Fisher’s exact test for categorical variables. Because of the multicenter approach, some parameters were not available for the entire study cohort. RAAS, renin-angiotensin-aldosterone system; TIA, transitory ischemic attack.
P<0.05 for regular-dose versus switch group.
P<0.05 for regular-dose versus dose-reduction-switch group.
P<0.05 for dose-reduction-switch versus switch group.
Table 3.
Baseline cardiac and neurologic measures of the patient groups
| Measures | Regular-Dose (n=24) | Dose-Reduction-Switch (n=28) | Switch (n=37) | Total (N=89) |
|---|---|---|---|---|
| Cardiac measures | ||||
| NYHA class | ||||
| 0 | 2 (10.5) | 3 (14.3) | 4 (12.9) | 9 (12.7) |
| I | 9 (47.4) | 9 (42.9) | 13 (41.9) | 31 (43.7) |
| II | 7 (36.8) | 6 (28.6) | 11 (35.4) | 24 (33.8) |
| III | 1 (5.3) | 3 (14.3) | 3 (9.7) | 7 (9.9) |
| IV | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| LV diastolic diameter, mm | 52.4±6.8a | 47.8±8.0 | 46.6±6.2 | 49.2±6.8 |
| LV systolic diameter, mm | 34.4±8.1 | 30.5±6.1 | 29.1±7.3 | 31.6±7.5 |
| LVSd, mm | 12.7±2.7 | 13.5±3.7 | 13.7±2.8 | 13.1±2.8 |
| LVH | 10 (55.6) | 10 (52.6) | 17 (70.8) | 37 (60.7) |
| Posterior wall diameter, mm | 12.2±2.4 | 12.3±3.4 | 12.8±2.8 | 12.3±2.7 |
| Ejection fraction, % | 60.1±8.9 | 60.3±5.1 | 61.8±6.9 | 60.8±7.2 |
| ECG abnormalities | 3 (15.8) | 5 (22.7) | 6 (24.0) | 14 (19.4) |
| Pacemaker | 3 (15.0) | 2 (9.1) | 2 (6.1) | 7 (9.3) |
| Neurologic measures | ||||
| CES-D score | 18.4±9.0 | 17.5±10.6 | 18.3±9.1 | 18.0±9.4 |
| GCPS 2 (maximum pain) | 4.6±2.9 | 5.3±2.3 | 3.8±3.8 | 4.6±3.0 |
| GCPS 5 (impairment) | 1.5±2.1 | 2.1±2.9 | 1.8±3.2 | 1.8±2.7 |
| NPSI sum score | 0.15±0.15 | 0.25±0.21 | 0.13±0.11 | 0.18±0.17 |
| Sural nerve | ||||
| SNAP, µV | 11.7±6.1 | 19.1±13.2 | 17.1±5.1 | 16.3±9.1 |
| NCV, m/s | 39.9±2.9 | 42.5±5.6 | 45.6±6.6 | 42.9±5.8 |
| CDT | −17.1±5.6 | −15.7±7.2 | −12.4±7.0 | −14.8±6.8 |
Values are given as mean±SD or n (%). Differences between groups have been tested via one-way ANOVA for continuous variables or Fisher’s exact test for categorical variables. Because of the multicenter approach, some parameters were not available for the entire study cohort. NYHA, New York Heart Association; LV, left ventricular; LVSd, left ventricular septum thickness in diastole; LVH, left ventricular hypertrophy (left ventricular septum thickness in diastole >12 mm); ECG, electrocardiogram; CES-D, Center for Epidemiologic Studies-Depression scale; GCPS, Graded Chronic Pain Scale; NPSI, Neuropathic Pain Symptom Inventory; SNAP, sensory nerve action potential; NCV, nerve conduction velocity; CDT, cold detection threshold.
P<0.05 for regular-dose versus switch group.
At baseline, all three groups did not significantly differ in eGFR on the basis of different formulae (Table 2). Cardiac measures revealed only a slightly increased left ventricular diastolic diameter in the regular-dose group in comparison with the switch group (P<0.05) (Table 3). Neurologic measures revealed no differences in baseline parameters between the groups (Table 3).
Table 2.
Baseline renal measures of the patient groups
| Measures | Regular-Dose (n=24) | Dose-Reduction-Switch (n=28) | Switch (n=37) | Total (N=89) |
|---|---|---|---|---|
| Creatinine, mg/dl | 2.05±2.49 | 1.49±1.58 | 1.89±2.63 | 1.82±2.31 |
| Creatinine, mg/dla | 1.19±0.48b | 0.82±0.18 | 1.01±0.33 | 0.99±0.35 |
| Cystatin C, mg/l | 1.53±1.43 | 1.39±1.29 | 1.07±0.54 | 1.32±1.10 |
| Cystatin C, mg/la | 1.02±0.51 | 0.76±0.14 | 0.98±0.39 | 0.93±0.39 |
| eGFR, ml/min per 1.73 m2 | ||||
| eGFRcreat | 69.9±36.6 | 74.2±34.5 | 72.8±35.9 | 72.5±35.2 |
| eGFRcreata | 81.9±28.0 | 86.5±24.4 | 81.9±21.0 | 83.3±23.1 |
| eGFRcys | 79.9±42.4 | 91.1±46.7 | 89.4±37.9 | 87.4±41.4 |
| eGFRcysa | 89.7±29.3 | 114.2±25.0 | 92.1±31.6 | 97.4±30.5 |
| eGFRcreat-cys | 73.7±39.9 | 83.3±41.2 | 83.7±34.1 | 80.2±38.9 |
| eGFRcreat-cysa | 87.1±31.5 | 95.3±28.6 | 86.7±25.8 | 89.2±27.4 |
| ACR, mg/g creatinine | 185±264 | 324±373 | 189±300 | 226.3±301 |
| Albuminuria | 9 (69.2) | 9 (75.0) | 12 (70.6) | 30 (71.4) |
| Hemoglobin, mg/dl | 13.7±1.1 | 13.8±1.2 | 13.5±1.6 | 13.6±1.4 |
| Kidney transplantation | 3 (13.0) | 4 (14.8) | 2 (5.9) | 9 (10.7) |
| Dialysis | 8 (34.8) | 4 (14.8) | 5 (14.7) | 17 (20.2) |
| Hyperfiltration | 0 (0.0) | 1 (3.6) | 1 (2.7) | 2 (2.2) |
Values are given as mean±SD or n (%). Differences between groups have been tested via one-way ANOVA for continuous variables or Fisher’s exact test for categorical variables. Because of the multicenter approach, some parameters were not available for the entire study cohort. eGFRcreat is calculated via the CKD-EPI formula according to Levey et al.14 eGFRcys and eGFRcreat-cys are calculated via the CKD-EPI formula according to Inker et al.15 Albuminuria, ACR>30 mg/g protein; hyperfiltration, eGFRcreat>120 ml/min per 1.73 m2.
Values without patients with kidney transplantation, dialysis, or hyperfiltration.
P<0.05 for regular-dose versus dose-reduction-switch group.
Outcome for Clinical Events
The outcome for serious clinical events, such as stroke/transient ischemic attack, dialysis, renal transplantation, or a pacemaker/implantable cardioverter defibrillator, did not significantly differ between baseline and 2-year follow-up in any of the three groups (Table 4).
Table 4.
Development of major events between baseline and 2-year follow-up
| Group/Event | Patients at Baseline | Patients at 2-y Follow-Up | P Value | Additional Patients |
|---|---|---|---|---|
| Regular-dose | ||||
| TIA/strokes | 2 (8.7) | 2 (8.7) | 0.99 | 0 |
| Dialysis | 8 (34.8) | 8 (34.8) | 0.99 | 0 |
| Kidney transplantation | 3 (13.0) | 4 (17.4) | 0.99 | 1 |
| Pacemaker/ICD | 3 (13.0) | 4 (17.4) | 0.99 | 1 |
| Dose-reduction-switch | ||||
| TIA/strokes | 0 (0.0) | 3 (15.8) | 0.23 | 3 |
| Dialysis | 4 (14.8) | 5 (18.5) | 0.99 | 1 |
| Kidney transplantation | 4 (14.8) | 4 (14.8) | 0.99 | 0 |
| Pacemaker/ICD | 2 (7.7) | 3 (11.5) | 0.99 | 1 |
| Switch | ||||
| TIA/strokes | 1 (3.6) | 2 (7.1) | 0.99 | 2 |
| Dialysis | 5 (14.7) | 5 (14.7) | 0.99 | 1a |
| Kidney transplantation | 2 (5.9) | 3 (8.8) | 0.99 | 1 |
| Pacemaker/ICD | 2 (6.3) | 3 (9.4) | 0.99 | 1 |
Values are given as n (%) or as otherwise indicated. TIA, transitory ischemic attack; ICD, implantable cardioverter defibrillator.
One patient stopped dialysis and received kidney transplantation.
Outcome for Change in Organ Function and Structure
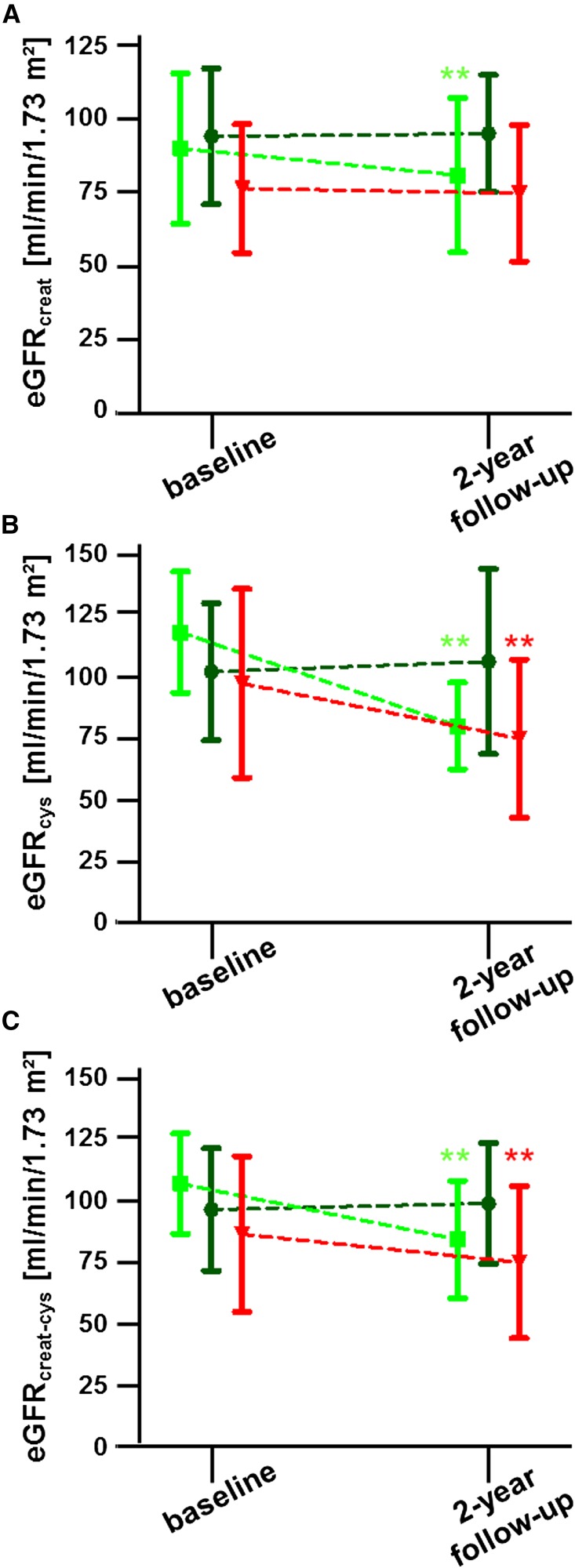
Because we recently reported a significant impairment of renal function after 12 months of agalsidase-β dose-reduction or switch to agalsidase-α,13 we now focused on renal parameters, such as eGFR and albumin-to-creatinine ratio (ACR), after further 12 months. Therefore, eGFRs were calculated by three different Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formulae on the basis of creatinine and cystatin C: (1) creatinine-based (eGFRcreat) according to Levey and colleagues,14 (2) cystatin C-based (eGFRcys), and (3) creatinine/cystatin C-based (eGFRcreat-cys), both according to Inker and colleagues,15 as recommended within the actual Kidney Disease Improving Global Outcomes (KDIGO) guidelines.16 Because patients within the three groups revealed different eGFR values at baseline, calculations of renal function (i.e., annual changes) have been adjusted for baseline values (Table 5). eGFR values of patients within the regular-dose group remained stable between baseline and 2-year follow-up, independent of the used eGFR formula for calculation (Figure 2, Table 5). Within the dose-reduction-switch group, eGFRcreat values decreased by 5.00% per year or –3.74 ml/min per 1.73 m2 per y (adjusted), respectively (Figure 2, Table 5). Similar results were obtained by cystatin C–based and creatinine/cystatin C–based eGFR calculations in that both calculations revealed significant decreases between baseline and 2-year follow-up (Figure 2, Table 5). Within the switch group, the annual percentage change for eGFRcreat remained stable, but the multivariate regression model revealed a slight decline of 2.45 ml/min per 1.73 m2 per y after adjustment (Table 5). In addition, cystatin C–based and creatinine/cystatin C–based eGFR values decreased significantly over time within this group (Table 5).
Table 5.
eGFRs determined by creatinine-, cystatin C- and creatinine/cystatin C–based CKD-EPI formulae and changes per year
| Group | eGFR, ml/min per 1.73 m2 | Baseline | 1-y Follow-Up | 2-y Follow-Up | % Change in eGFR per Year (95% CI) | Change in ml/min per 1.73 m2 per y (95% CI) |
|---|---|---|---|---|---|---|
| Regular-dose (n=10) | eGFRcreat | 93.7±23.0 | 92.8±19.5a | 94.6±19.8 | 1.58 (–4.28 to 7.43) | 0.13 (–3.75 to 4.00) |
| eGFRcys | 101.7±27.9 | 94.8±28.8a | 105.8±37.6 | 2.42 (–6.00 to 10.84) | −1.77 (–7.51 to 3.98) | |
| eGFRcreat-cys | 96.4±24.9 | 95.1±29.3a | 98.9±24.6 | 2.23 (–4.68 to 9.14) | −0.47 (–4.50 to 3.55) | |
| Dose-reduction-switch (n=15) | eGFRcreat | 90.0±25.5 | 87.4±27.9 | 80.9±26.2 | −5.00 (–7.67 to –2.33) | −3.74 (–6.63 to –0.85) |
| eGFRcys | 118.2±24.7 | 92.1±21.1 | 80.1±17.6 | −14.07 (–18.89 to –9.25)b | −14.02 (–19.43 to –8.60)b | |
| eGFRcreat-cys | 107.0±20.4 | 94.7±25.2 | 84.3±23.9 | −9.67 (–13.2 to –6.12)b | −9.33 (–13.32 to –5.34)b | |
| Switch (n=22) | eGFRcreat | 76.4±21.9 | 76.2±20.5 | 74.8±23.1 | −2.20 (–5.31 to 0.91) | −2.45 (–4.67 to –0.24) |
| eGFRcys | 97.4±38.4 | 82.0±24.6 | 75.0±32.2 | −7.14 (–10.93 to –3.35)c | −8.64 (–12.62 to –4.65) | |
| eGFRcreat-cys | 86.4±31.6 | 81.8±25.0 | 75.0±31.0 | −5.16 (–8.07 to –2.25)c | −4.97 (–7.73 to –2.20) |
eGFRcreat is calculated via the CKD-EPI formula according to Levey et al.14 eGFRcys and eGFRcreat-cys are calculated according to Inker et al.15 Patients under dialysis, with renal transplantation, or hyperfiltration (>120 ml/min per 1.73 m2 on the basis of eGFRcreat) have been excluded from calculations. Slopes of eGFR (annual percentage change) were calculated with the respective eGFR formula, and their 95% confidence intervals are reported. Differences between groups have been tested using one-way ANOVA with post hoc analysis corrected for multiple comparisons according to Bonferroni. Slopes of eGFR (annual change in ml/min per 1.73 m2) were adjusted for baseline eGFR values, age, sex, and premedication. 95% CI, 95% confidence interval.
Limited data are available.
P<0.05 for regular-dose versus dose-reduction-switch group.
P<0.05 for regular-dose versus switch group.
Figure 2.
Changes in renal function quantified by eGFR over time. eGFR was quantified using the CKD-EPI equation on the basis of (A) creatinine (eGFRcreat), (B) cystatin C (eGFRcys), and (C) the combination of both levels (eGFRcreat-cys). Patients under dialysis, with renal transplantation or hyperfiltration (>120 ml/min per 1.73 m2 on the basis of eGFRcreat) have been excluded. Dark green color represents the regular-dose group, bright green color represents the dose-reduction-switch group, and red represents the switch group. Values are given in mean±SD. **P<0.01.
The analyses of raw serum creatinine and cystatin C concentrations over time in all three groups showed that creatinine values remained stable within the regular-dose (P=0.39) and switch groups (P=0.43) but increased significantly within the dose-reduction-switch group (P<0.01) (Supplemental Figure 1). Raw cystatin C values remained also stable over time within the regular-dose group (P=0.83), but they increased within the dose-reduction-switch and the switch groups (both P<0.01) (Supplemental Figure 1).
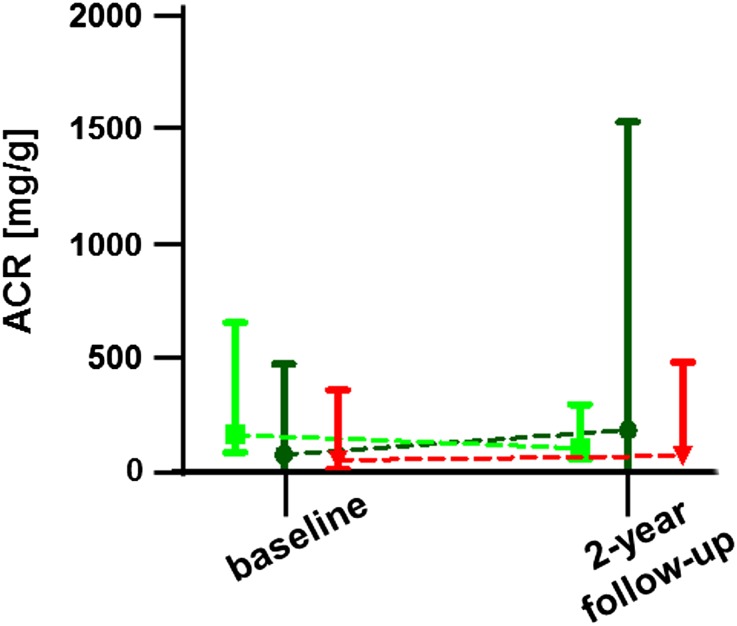
ACR values did not differ between baseline and 2-year follow-up in any of the three groups (Figure 3). In detail, median ACRs differed not significantly between the three groups at baseline (regular-dose group: 81 [range, 0–741] mg/g; dose-reduction-switch group: 162 [range, 0–1292] mg/g; switch group: 57 [range, 0–1426] mg/g; P=0.49). After 2 years, all three groups revealed stable ACRs (Figure 3). Additionally, ACRs at 2-year follow-up did not differ between the groups (regular-dose group: 188 [range, 8–2420] mg/g; dose-reduction-switch group: 103 [range, 5–552] mg/g; switch group: 76 [range, 0–1457] mg/g; P=0.71).
Figure 3.
Changes in ACR over time. Patients under dialysis, with renal transplantation, or hyperfiltration (>120 ml/min per 1.73 m2 on the basis of eGFRcreat) have been excluded. Dark green color represents the regular-dose group, bright green color represents the dose-reduction-switch group, and red represents the switch group. Values are given as medians (95% confidence interval).
Outcome for Changes in FD-Related Symptoms
Changes in FD-related symptoms are shown in Table 6. Frequencies of pain attacks, permanent pain, and diarrhea remained stable in all three groups between baseline and 2-year follow-up (Table 6). However, frequencies of gastrointestinal pain increased significantly in the dose-reduction-switch group (relative risk: 1.87; 95% confidence interval, 1.18 to 2.95) and the switch group (relative risk: 1.73; 95% confidence interval, 1.10 to 2.71) after 2 years of observation (Table 6). As a marker of general disease progression and severity, Mainz Severity Score Index (MSSI) scores were assessed (Table 7). Because the MSSI scores might be influenced by nonchangeable aspects, only values within the groups have been analyzed between baseline and 2-year follow-up. Although patients in the regular-dose group remained stable, patients in the dose-reduction-switch group and switch group showed increases of MSSI scores after 2 years (P<0.05 and P<0.001, respectively) (Table 7).
Table 6.
Development of symptoms between baseline and 2-year follow-up
| Group/Symptom | Baseline | 2-y Follow-Up | Relative Risk (95% CI) |
|---|---|---|---|
| Regular-dose | |||
| Pain attacks | 5 (25.0) | 7 (29.2) | 1.10 (0.62 to 1.96) |
| Permanent pain | 8 (40.0) | 9 (37.5) | 0.95 (0.54 to 1.67) |
| Gastrointestinal pain | 5 (25.0) | 8 (33.3) | 1.19 (0.69 to 2.06) |
| Diarrhea | 5 (25.0) | 8 (33.3) | 1.19 (0.69 to 2.06) |
| Dose-reduction-switch | |||
| Pain attacks | 6 (26.1) | 9 (33.3) | 1.17 (0.69 to 1.97) |
| Permanent pain | 13 (56.5) | 14 (51.9) | 0.92 (0.55 to 1.53) |
| Gastrointestinal pain | 3 (13.0) | 12 (44.4) | 1.87 (1.18 to 2.95) |
| Diarrhea | 7 (30.4) | 13 (48.1) | 1.44 (0.87 to 2.38) |
| Switch | |||
| Pain attacks | 1 (3.0) | 3 (9.7) | 1.61 (0.86 to 3.01) |
| Permanent pain | 6 (18.2) | 7 (21.9) | 1.12 (0.63 to 2.00) |
| Gastrointestinal pain | 3 (9.1) | 9 (28.1) | 1.73 (1.10 to 2.71) |
| Diarrhea | 4 (12.1) | 8 (25.0) | 1.47 (0.90 to 2.42) |
Values are given as n (%) or as otherwise indicated. Differences between baseline and 2-year follow-up have been tested using Fisher’s exact test. 95% CI, 95% confidence interval.
Table 7.
Differences in MSSI score, LVSd, and ejection fraction between baseline and 2-year follow-up
| Group | Baseline | 1-y Follow-Up | 2-y Follow-Up | P Value |
|---|---|---|---|---|
| Regular-dose | ||||
| MSSI | 26.1±12.7 | 26.2±13.2 | 24.9±14.4 | 0.61 |
| LVSd, mm | 12.7±2.2 | 12.4±2.4 | 12.8±2.6 | 0.44 |
| Ejection fraction, % | 58.5±8.4 | 55.8±9.5 | 56.4±6.0 | 0.75 |
| Dose-reduction-switch | ||||
| MSSI | 20.3±9.8 | 21.9±11.6 | 23.9±10.7a | 0.02 |
| LVSd, mm | 13.3±3.7 | 13.0±3.1 | 13.1±3.9 | 0.05 |
| Ejection fraction, % | 60.5±5.6 | 57.5±8.3 | 59.9±9.2 | 0.42 |
| Switch | ||||
| MSSI | 17.9±8.4 | 20.2±9.0 | 22.5±10.6b | 0.001 |
| LVSd, mm | 14.2±2.4 | 13.8±1.8 | 14.4±2.6 | 0.45 |
| Ejection fraction, % | 62.2±6.7 | 59.1±7.0 | 57.8±8.7 | 0.27 |
Values are given in mean±SD or as otherwise indicated. LVSd, left ventricular septum thickness in diastole.
P<0.05 for baseline versus 2-year follow-up.
P<0.001 for baseline versus 2-year follow-up.
In terms of cardiac involvement (i.e., left ventricular septum in diastole and ejection fraction), no differences were detected within the three groups during observation (Table 7).
Additional Medication
Information on additional medication (diuretics, renin-angiotensin-aldosterone system blockers, and analgesics) is presented in Table 1. The three groups did not differ significantly at baseline visit in any additional medication (Table 1). During 1-year and 2-year follow-up the number of patients receiving medication and drug doses was unchanged (data not shown). For all types of medication, no significant changes or differences among the groups and the visits were observed. Patients under 0.5 mg/kg agalsidase-β tolerated switch to agalsidase-α well. In general, three (13.0%) patients of the former dose-reduction group developed mild infusion reactions, such as mild fever and increased fatigue, after infusion. These patients were premedicated with steroids and histamine antagonists before infusions.
Discussion
The agalsidase-β supply shortage between 2009 and 2012 forced the practitioners to treat the patients with FD with a reduced dose of agalsidase-β or to switch them to a regular-dose of agalsidase-α. In our initial study, comprising 105 patients with FD followed-up for 12 months, patients receiving regular agalsidase-β doses had a stable disease course, but both dose-reduction and switch to agalsidase-α resulted in worsening of renal function and symptoms.13 Because the 1-year follow-up period was relatively short, a further 2-year follow-up study of patients who were dose reduced and/or switched was warranted.
In this study, we assessed clinical stability and safety during agalsidase-β dose-reduction and/or switch to agalsidase-α in a 2-year follow-up with a special focus on clinical events, renal end organ damage, disease-related clinical symptoms, and disease severity score.
Our main findings are as follows: (1) patients with FD irrespective of the ERT regime showed a stable clinical disease course with respect to serious clinical events; (2) patients with FD under a regular agalsidase-β dose showed stable renal function and FD-related symptoms; and (3) patients with FD on a reduced agalsidase-β dose for 12 months and subsequent switch to a regular agalsidase-α dose for another 12 months and those patients with FD after a direct switch to agalsidase-α showed a significant decline in renal function, an increase of MSSI score, and a higher frequency of gastrointestinal pain.
ERT in FD
Recent studies with agalsidase-α and agalsidase-β have shown that ERT is safe and efficient for treatment of patients with FD.2–4,17–20 Because adverse events and acute infusion reactions remained limited, initially ERT compounds and dosages usually remain unchanged over time. Because of a viral contamination in 2009, the worldwide agalsidase-β supply was limited for 2.5 years,12 necessitating a dose-reduction of agalsidase-β or a switch to agalsidase-α for many patients. Limited clinical data on the basis of potentially statistically underpowered8 patient cohorts suggested no effects on clinical outcome.9–12 However, a decrease in quality of life in women (SF-36 questionnaire), and increased lyso-Gb3 levels in patients who received a reduced dose of agalsidase-β or were switched to agalsidase-α, has been previous reported.12 In our first multicenter study, we observed a decline of renal function, higher frequencies of FD-related symptoms, and an increase of the MSSI after 1 year after dose-reduction or switch in a larger cohort of 105 patients with FD.13 These results were interpreted by Warnock and Mauer in a recent editorial, emphasizing that the dose of the drug matters in FD treatment and suggesting that the full dose of agalsidase-α could be too low to guarantee results as effective as those of agalsidase-β.8
Here we report our results after additional 12 months of observation, with a special focus on renal outcome. Nephropathy is a dominant organ manifestation in FD, and progressive GFR decline may lead to ESRD in the third to fifth decade of life.21 Kidney function is one of the critical determinants of the clinical outcome in patients with FD. The estimated GFR is the clinical standard for the assessment of kidney function, mostly on the basis of creatinine.16
The KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease recommend use of the 2009 CKD-EPI equation14 instead of the Modification of Diet in Renal Disease study equation22 to estimate eGFRcreat. Second, they suggest use of the 2012 CKD-EPI equations15 to estimate GFR (eGFRcys or eGFRcreat-cys) whenever cystatin C has been measured.16
The superior performance of cystatin C or the combination of creatinine and cystatin C versus creatinine alone to estimate GFR and predict CKD outcomes has been repeatedly demonstrated.15,23–25 Furthermore, reduced values for eGFRcys and eGFRcreat-cys have a consistent linear association with increased risk of death for all eGFR levels below approximately 85 ml/min per 1.73 m2, which is well above the threshold of 60 ml/min per 1.73 m2 for the detection of CKD with a creatinine-based eGFR.16,26,27 However, cystatin C–based eGFR has limitations for an association with CKD risk factors as such and outcomes similar to that of measured GFR.25 Furthermore, already Inker et al. mentioned that unmeasured and largely unknown non-GFR determinants of cystatin C are similar in magnitude to those of creatinine.15 In this respect, the equation that combines creatinine and cystatin C provides the most precise and accurate estimate of GFR across the range of GFRs and in subgroups on the basis of demographic and clinical characteristics.15
Similar to patients with diabetes mellitus, most patients with FD are at high risk for CKD. In that respect, Perkins and colleagues demonstrated the accuracy of the cystatin C–based GFR equation to detect eGFR decline over time, especially in patients with diabetes with normal or even high GFR values28; however, the use of cystatin C in patients with hyperfiltration is controversially discussed.25
Indeed, the primary goal of our study was to assess effects of the change or reduction of ERT on renal function between baseline and the 2-year follow-up visit and the discrimination of clinical outcomes. In this respect, we determined eGFR by the CKD-EPI formula on the basis of creatinine, cystatin C, and the combination of both. Patients on a regular agalsidase-β dose showed a stable renal function over 2 years irrespective of the CKD-EPI formula used. Because of ongoing agalsidase-β supply shortage we were forced to switch most patients formally receiving a reduced dose of agalsidase-β to a regular-dose of agalsidase-α. Patients under a reduced dose of agalsidase-β for 12 months and subsequent switch to a regular-dose of agalsidase-α for additional 12 months showed an eGFR decline irrespective of the three calculations used. In patients directly switched to agalsidase-α an adjusted annual eGFR decline with all three formulae was observed. This may indicate that directly switched patients suffer from a more pronounced renal impairment after 2-years follow-up. Besides the observed renal impairment, patients with FD presented with increasing frequencies of gastrointestinal pain and worsened MSSI scores in the dose-reduction-switch and switch groups. However, cardiac measurements remained stable in all three groups during the 2 years of follow-up.
In contrast with our findings after 1 year follow-up,13 ACR values did not significantly differ between baseline and 2-year follow-up in any of the three groups. These results have to be interpreted with caution because the ACR values might be influenced by several factors, such as glycemic and BP control, treatment with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, nondihydropyridine calcium channel blockers (diltiazem and verapamil), salt consumption, exercise in the 24 hours before the measurement, day time of the measurement, and muscle mass (overestimated albumin excretion in a cachectic patient in whom muscle mass and creatinine excretion are markedly reduced).29,30
Clinical Effect
As reported previously, the supply restriction for agalsidase-β caused distress and concern in both patients and physicians. Because of the observational study design, it cannot be concluded that the regular agalsidase-β dose regimen is the optimal treatment for all patients with FD. In addition, the observed eGFR decline in patients directly switched to agalsidase-α might not only be the result of a lower ERT dose, but it may also be the result of a more severe renal impairment in these patients when ERT was initiated. On the basis of our study, we conclude that whenever agalsidase-β is chosen, a dose-reduction and subsequent switch to agalsidase-α should be avoided. In addition, a direct switch from agalsidase-β to agalsidase-α is safe in general, but clinicians should be aware that eGFR may decline.
Limitations
Our study has some limitations, such as its observational nature and therefore an unavoidable selection of patients. Because of the observational design (real-world design), patients were not randomized. Therefore, outcomes of comparison between the three groups should be carefully interpreted. The total number of patients was slightly lower than the initial publication13 because (1) one inclusion criterion was the determination of a complete dataset at baseline and after 2 years and (2) patients switching the therapy regimen more than two times during the follow-up were excluded. Because of the multicenter approach, some parameters and especially lyso-Gb3 levels as a potential marker for disease progression were not available for the entire study cohort. The observed clinical response may be influenced by the development of inhibitory antibodies against ERT, which were not systematically assessed in our multicenter approach; however, a switch of product has no effect on their formation.31 The analysis of the longitudinal 2-year data revealed a renal impairment in patients after dose reduction and/or switch, even if kidney biopsies are missing to demonstrate a direct injury. As already mentioned by Pisani and colleagues in their recent editorial,32 a detected increase of gastrointestinal pain may be influenced by anxiety of patients or greater attention by physicians. Even though our study has intrinsic limits, we could conclude that both products show efficacy and safety concerning serious clinical events.
Conclusions
After 2 years of observation, patients receiving a regular-dose of agalsidase-β had a stable disease course, whereas those with dose reduction and switch to agalsidase-α had an impairment of renal function.
Concise Methods
Study Design
In this retrospective observational study, a cohort of 89 patients (56 men, 33 women) with genetically confirmed FD from three German Fabry Centers in Berlin, Münster, and Würzburg were consecutively recruited and followed-up for 2 years. Patients had been on a stable treatment with agalsidase-β 1.0 mg/kg body wt e.o.w. for at least 1 year and reported at the Fabry centers for their regular clinical follow-ups. The routine clinical assessment included cardiac, renal, and neurologic parameters. The documentation of assessments followed clinical practice of the German Fabry Expert Centers for a rare multisystemic disorder. Inclusion criteria were as follows: (1) adult patients (≥18 years of age) with genetically determined FD, (2) at least 12 months stable treatment with a regular-dose of agalsidase-β 1.0 mg/kg body wt e.o.w., (3) 1- and 2-year follow-up visits at the respective FD centers after dose reduction and/or switch to agalsidase-α 0.2 mg/kg body wt e.o.w., and (4) informed consent for examinations and participation in the study. All investigations were performed after approval of the ethics committees of the participating centers (project no.: 2011–347-f), and written informed consent of the patients for molecular analysis and publication was obtained.
Patients changing therapy more often than twice after at least 12 months of stable treatment with the regular-dose of agalsidase-β 1.0 mg/kg body wt e.o.w. (inclusion criterion 2) were excluded. Our study comprised 89 instead of the initial 105 patients with FD because of dropouts and incomplete datasets for some parameters that were not available for the entire study cohort. The baseline visit was defined as the last visit when patients were still treated with the regular-dose of agalsidase-β 1.0 mg/kg body wt e.o.w. After this visit the long-term therapy strategy was determined individually by the treating physicians in accordance with the patient. The following options were proposed (Figure 1): (1) remain on the regular-dose of agalsidase-β of 1.0 mg/kg body wt e.o.w. (regular-dose group); (2) change to a reduced dose of agalsidase-β of 0.3 or 0.5 mg/kg body wt for 12 months and subsequently switch (ongoing supply shortage) to agalsidase-α with a standard dose of 0.2 mg/kg body wt e.o.w. (dose-reduction-switch group) for another 12 months; or (3) compound switch to agalsidase-α with a standard dose of 0.2 mg/kg body wt e.o.w. (switch group). The regular-dose group should not be considered as a control group but serves for a better understanding of the results obtained in the 2 core groups (dose-reduction-switch group and switch group). The determined long-term treatment was initiated, and patients were invited for follow-up visits (1- and 2-year follow-up) after 1 year of stable treatment with the implemented therapy. Therefore, in the final dataset, all data from three consecutive visits were included (baseline-visit, 1-year follow-up-visit, and 2-year follow-up-visit).
The detailed clinical work-up of patients has been previously reported.13 In brief, renal function was quantified by the eGFR using the CKD-EPI–based equation on the basis of serum creatinine (eGFRcreat),14 cystatin C levels (eGFRcys),15 and a combination of creatinine and cystatin C (eGFRcreat-cys)15 and albuminuria (ACR) from spot urine. Microalbuminuria was defined as an ACR between 30 and 300 mg albumin per gram of creatinine. For the eGFR analyses, patients with hyperfiltration (>120 ml/min per 1.73 m2, eGFRcreat) or under dialysis were excluded. All patients underwent neurologic examination and a clinical interview focusing on a history of strokes/transient ischemic attacks and pain.
Outcome Parameters
To quantify the clinical outcome, three groups of Fabry-related progression parameters were analyzed.
Clinical Events
For clinical events, the following parameters were analyzed: (1) death; (2) symptomatic cardiac arrhythmia requiring an implantable cardioverter defibrillator or pacemaker, myocardial infarction, coronary artery bypass graft, or percutaneous transluminal coronary angioplasty; (3) progression of renal disease to CKD stage 5 (eGFR <15 ml/min per 1.73 m2 [with at least 30% decrease of eGFR], kidney transplantation or dialysis); and (4) stroke/transient ischemic attack.
Change in Organ Function and/or Structure
For cardiac changes, end-diastolic septal and posterior wall thickness and ejection fraction were analyzed. For renal changes, eGFR and ACR in spot urine were analyzed. For neurologic changes, clinical examination and interview on stroke and transitory ischemic attacks were performed.
Change of Fabry-related Symptoms
Appearrance of gastrointestinal pain (abdominal pain, tenesmus, or cramping more than once a week), diarrhea (more than three loose bowels or >250 g of stool weight per day), hypohidrosis or anhidrosis (impairment or loss of ability to sweat), tinnitus, Fabry-associated pain (i.e., pain attacks, permanent pain or pain crises) was assessed; additionally the MSSI score33 was applied.
Data Analyses
Data are presented as mean±SD, median (range), or number (%), where appropriate. Differences between groups and between baseline and follow-ups have been tested via one-way (Kruskal–Wallis) or repeated-measure (Friedman) ANOVA for continuous variables or Fisher’s exact test for categorical variables, including correction for multiple testing. Annual percentage changes in eGFRs were analyzed using one-sample t test. Annual changes (in ml/min per 1.73 m2) were adjusted for baseline eGFR values, age, sex, and premedication. The P values <0.05 were considered statistically significant. Data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC) and GraphPad PRISM V5.0 software (GraphPad Software, La Jolla, CA).
Disclosures
F.W., J.K., M.L., C.W., T.D., S.C.K., and E.B. have received speaker honoraria from Genzyme and Shire Corporation. F.W. and C.W. are members of the Fabry Registry European Board of Advisors and have received travel assistance and speaker honoraria. Research grants were given to the institutions (Würzburg and Münster) by Genzyme and Shire Corporations. NÜ has received travel assistance and speaker honoraria from Genzyme Corporation and Shire Corporation. C.S. and J.S. have received speaker honoraria from Genzyme Corporation. S.M.B. has received speaker honoraria from Shire Corporation. H.W.H., D.B., and S.R. report no disclosures.
Supplementary Material
Acknowledgments
We thank Barbara Broll, Irina Turkin, Anne Huster, Samira Schiwek, and Jutta Beilker for expert technical assistance.
This study was funded by Genzyme Europe B.V. Eva Brand was supported by a Heisenberg professorship from the Deutsche Forschungsgemeinschaft (Br1589/8-2).
The funding agency had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. The researchers were independent of the funding agency.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030337/-/DCSupplemental.
References
- 1.Zarate YA, Hopkin RJ: Fabry’s disease. Lancet 372: 1427–1435, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ, International Collaborative Fabry Disease Study Group : Safety and efficacy of recombinant human alpha-galactosidase A--replacement therapy in Fabry’s disease. N Engl J Med 345: 9–16, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Schiffmann R, Kopp JB, Austin HA, 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO: Enzyme replacement therapy in Fabry disease: A randomized controlled trial. JAMA 285: 2743–2749, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ, Fabry Disease Clinical Trial Study Group : Agalsidase-beta therapy for advanced Fabry disease: A randomized trial. Ann Intern Med 146: 77–86, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Hughes DA, Elliott PM, Shah J, Zuckerman J, Coghlan G, Brookes J, Mehta AB: Effects of enzyme replacement therapy on the cardiomyopathy of Anderson-Fabry disease: A randomised, double-blind, placebo-controlled clinical trial of agalsidase alfa. Heart 94: 153–158, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Mehta A, Beck M, Elliott P, Giugliani R, Linhart A, Sunder-Plassmann G, Schiffmann R, Barbey F, Ries M, Clarke JT, Fabry Outcome Survey investigators : Enzyme replacement therapy with agalsidase alfa in patients with Fabry’s disease: An analysis of registry data. Lancet 374: 1986–1996, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Wraith JE, Tylki-Szymanska A, Guffon N, Lien YH, Tsimaratos M, Vellodi A, Germain DP: Safety and efficacy of enzyme replacement therapy with agalsidase beta: An international, open-label study in pediatric patients with Fabry disease. J Pediatr 152: 563–570.e1, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Warnock DG, Mauer M: Fabry disease: Dose matters. J Am Soc Nephrol 25: 653–655, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linthorst GE, Germain DP, Hollak CE, Hughes D, Rolfs A, Wanner C, Mehta A, European Medicines Agency : Expert opinion on temporary treatment recommendations for Fabry disease during the shortage of enzyme replacement therapy (ERT). Mol Genet Metab 102: 99–102, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Tsuboi K, Yamamoto H: Clinical observation of patients with Fabry disease after switching from agalsidase beta (Fabrazyme) to agalsidase alfa (Replagal). Genet Med 14: 779–786, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisani A, Spinelli L, Visciano B, Capuano I, Sabbatini M, Riccio E, Messalli G, Imbriaco M: Effects of switching from agalsidase Beta to agalsidase alfa in 10 patients with anderson-fabry disease. JIMD Rep 9: 41–48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smid BE, Rombach SM, Aerts JM, Kuiper S, Mirzaian M, Overkleeft HS, Poorthuis BJ, Hollak CE, Groener JE, Linthorst GE: Consequences of a global enzyme shortage of agalsidase beta in adult Dutch Fabry patients. Orphanet J Rare Dis 6: 69, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weidemann F, Krämer J, Duning T, Lenders M, Canaan-Kühl S, Krebs A, Guerrero González H, Sommer C, Üçeyler N, Niemann M, Störk S, Schelleckes M, Reiermann S, Stypmann J, Brand SM, Wanner C, Brand E: Patients with Fabry disease after enzyme replacement therapy dose reduction versus treatment switch. J Am Soc Nephrol 25: 837–849, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS, CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 17.Weidemann F, Breunig F, Beer M, Sandstede J, Turschner O, Voelker W, Ertl G, Knoll A, Wanner C, Strotmann JM: Improvement of cardiac function during enzyme replacement therapy in patients with Fabry disease: A prospective strain rate imaging study. Circulation 108: 1299–1301, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Weidemann F, Niemann M, Breunig F, Herrmann S, Beer M, Störk S, Voelker W, Ertl G, Wanner C, Strotmann J: Long-term effects of enzyme replacement therapy on fabry cardiomyopathy: Evidence for a better outcome with early treatment. Circulation 119: 524–529, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Lenders M, Karabul N, Duning T, Schmitz B, Schelleckes M, Mesters R, Hense H-W, Beck M, Brand SM, Brand E: Thromboembolic events in Fabry disease and the impact of factor V Leiden. Neurology 84: 1009–1016, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Germain DP, Charrow J, Desnick RJ, Guffon N, Kempf J, Lachmann RH, Lemay R, Linthorst GE, Packman S, Scott CR, Waldek S, Warnock DG, Weinreb NJ, Wilcox WR: Ten-year outcome of enzyme replacement therapy with agalsidase beta in patients with Fabry disease. J Med Genet 52: 353–358, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branton M, Schiffmann R, Kopp JB: Natural history and treatment of renal involvement in Fabry disease. J Am Soc Nephrol 13[Suppl 2]: S139–S143, 2002 [PubMed] [Google Scholar]
- 22.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS: Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69: 399–405, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, Solbu MD, Toft I: The role of cystatin C in improving GFR estimation in the general population. Am J Kidney Dis 59: 32–40, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Rule AD, Glassock RJ: GFR estimating equations: Getting closer to the truth? Clin J Am Soc Nephrol 8: 1414–1420, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT, CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J: Chronic kidney disease. Lancet 379: 165–180, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Perkins BA, Nelson RG, Ostrander BE, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: Results of a 4-year follow-up study. J Am Soc Nephrol 16: 1404–1412, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mogensen CE, Vestbo E, Poulsen PL, Christiansen C, Damsgaard EM, Eiskjaer H, Frøland A, Hansen KW, Nielsen S, Pedersen MM: Microalbuminuria and potential confounders. A review and some observations on variability of urinary albumin excretion. Diabetes Care 18: 572–581, 1995 [DOI] [PubMed] [Google Scholar]
- 30.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJ, de Jong PE, Gansevoort R: First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lenders M, Stypmann J, Duning T, Schmitz B, Brand SM, Brand E: Serum-mediated inhibition of enzyme replacement therapy in Fabry disease [published online ahead of print April 30, 2015]. J Am Soc Nephrol 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pisani A, Riccio E, Sabbatini M: Agalsidase alfa and agalsidase beta in the treatment of Fabry disease: Does the dose really matter? Genet Med 17: 21–23, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whybra C, Kampmann C, Krummenauer F, Ries M, Mengel E, Miebach E, Baehner F, Kim K, Bajbouj M, Schwarting A, Gal A, Beck M: The Mainz Severity Score Index: A new instrument for quantifying the Anderson-Fabry disease phenotype, and the response of patients to enzyme replacement therapy. Clin Genet 65: 299–307, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.