Abstract
Traditionally, arterial hypertension and subsequent end-organ damage have been attributed to hemodynamic factors, but increasing evidence indicates that inflammation also contributes to the deleterious consequences of this disease. The immune system has evolved to prevent invasion of foreign organisms and to promote tissue healing after injury. However, this beneficial activity comes at a cost of collateral damage when the immune system overreacts to internal injury, such as prehypertension. Renal inflammation results in injury and impaired urinary sodium excretion, and vascular inflammation leads to endothelial dysfunction, increased vascular resistance, and arterial remodeling and stiffening. Notably, modulation of the immune response can reduce the severity of BP elevation and hypertensive end-organ damage in several animal models. Indeed, recent studies have improved our understanding of how the immune response affects the pathogenesis of arterial hypertension, but the remarkable advances in basic immunology made during the last few years still await translation to the field of hypertension. This review briefly summarizes recent advances in immunity and hypertension as well as hypertensive end-organ damage.
Keywords: hypertension, innate immunity, adaptive immunity, dendritic cells
Hypertension is the most common modifiable risk factor for cardiovascular disease and death. Worldwide, it is estimated that >1 billion adults have hypertension, and this figure is projected to climb to 1.5 billion by the year 2025. An improved understanding of the mechanisms underlying arterial hypertension and its subsequent damage to the kidneys, heart, and vascular system is clearly needed. For many years, it was believed that hypertensive end-organ damage is merely the result of an increased hydrostatic pressure on the vessel walls. Indeed, in 1977 Beilin stated that pressure, if it is great enough, will eventually disrupt any structure.1 In intact vessels, pressure mediates cellular stretch, which can in turn lead to cell death and initiate a tissue response aimed at removing dead cells and replacing them to restore homeostasis. These repair processes are a substantial component of the immune response, and it is therefore self-evident that activation of both the innate and adaptive arm of the immune system occurs in arterial hypertension.
A hint for a role of the immune system in hypertension was the observation that pressor doses of angiotensin II (Ang II) administered for a short time period resulted in subsequent salt-sensitive hypertension and renal infiltration and activation of immune cells. Both of these sequelae were reduced by immunosuppression.2 The concept that inflammation is not only a consequence of the physically induced injury but also a cause of hypertension, was further supported in a 2007 study.3 That study found that the increase in BP caused by Ang II infusion was significantly blunted in mice lacking the recombinase-activating gene 1 (RAG-1−/− mice), a strain that lacks T and B cells. In these experiments, adoptive transfer of T cells, but not B cells, restored the hypertensive response,3 indicating that T cells play an important role in generation of arterial hypertension. These results have been confirmed in severe combined immunodeficiency mice and in Dahl salt-sensitive rats in which the RAG-1 or the essential T cell receptor component CD3 have been deleted using zinc finger nuclease technology.4–6 This seminal work has caused great interest in the immunologic aspects of hypertensive disease.7–10
This review will give a brief overview of the components of the innate and adaptive arm of the immune system in hypertension and summarize recent advances in immunologic research, with a special focus on their potential relevance for hypertension and hypertensive end-organ damage. Very recent findings on the role of dendritic cells as well as CD8+ and TH17 T cells in hypertension will be discussed.
Innate Immunity
Innate immunity is considered to be the immune system’s first line of defense against invading pathogens. The term “innate” refers to the inherent capacity of this arm of immune defense to be rapidly activated by external stimuli without need for time-consuming, antigen-specific education or interaction with other cells. The essential cellular components of innate immunity are granulocytes, macrophages, mast cells, and certain subsets of lymphocytes, whereas the most important humoral component beside the defensin system is the complement system.
Complement
The complement system is one of the oldest components of immunity and is central to the detection and destruction of invading pathogens.11 It is a system of fluid-phase and cell membrane–bound proteins. Complement activation produces anaphylatoxins, such as C3a and C5a, which are chemotactic stimuli and can activate a variety of immune effector cell types. Both C3a and C5a serum levels are significantly increased after induction of hypertension by Ang II infusion. Interestingly, hearts of C5a knockout mice are protected from hypertensive cardiac injury.12 Moreover, treatment with a C5aR antagonist also prevented cardiac remodeling in Ang II–induced hypertension.13
Reactive Oxygen Species
While there are several reactive oxygen species (ROS)-producing enzymes in mammalian cells, a major source are the nicotinamide adenine dinucleotide phosphate-(NADPH) oxidases. Professional phagocytes use the NADPH oxidase to produce superoxide and kill invading organisms. Superoxide serves as a progenitor for numerous other ROS, including hydrogen peroxide, hydroxyl radical, and peroxynitrite. The NADPH oxidases have evolved to have numerous roles in other cells, including modulation of sympathetic outflow, vascular tone, and renal sodium reabsorption, all of which contribute to hypertension.14,15 The sympathetic nervous system and hypothalamic pituitary axis are two major pathways in which the NADPH oxidases modulate neuroimmune communication. The sympathetic nervous system innervates both primary (thymus, bone marrow) and secondary tissues and lymphoid tissues (spleen, lymph nodes), and most immune cells express receptors for neurohormones and catecholamines.16 Inhibition of the NADPH oxidase subunit p22phox in the subfornical organ eliminated the Ang II–induced hypertensive response and vascular inflammation.17 In addition, a peripheral neuroimmune response in the spleen, mediated by celiac ganglion efferent nerve fibers, is essential for the development of hypertension.18 The NADPH oxidase in antigen-presenting cells plays a role in their activation in hypertension, and inhibition of this enzyme blunts the vascular inflammation present in hypertension.19,20
Innate Sensing of Injury
Signature molecules of microbial origin (pathogen-associated molecular patterns) or molecules that are released by host cells in response to stress or damage (damage-associated molecular patterns [DAMPs]) can activate resident and innate immune cells via action on many germline-encoded pattern recognition receptors (PRRs). The inflammatory response to cell stress or injury has been called “sterile inflammation.”21 The best-characterized pattern recognition receptors of the immune system are toll-like receptors (TLRs). These are expressed on a variety of immune and somatic cells and are activated by a variety of pathogen-associated molecular patterns and DAMPs to initiate inflammatory responses. TLR4 was elevated in spontaneously hypertensive rats, and treatment with an anti-TLR4 antibody lowered BP in this model.22 TLR4 is also increased in cardiomyocytes of the spontaneously hypertensive rat and angiotensin-converting enzyme inhibition lowers its expression.23 G-nitro-l-arginine-methyl ester (l-NAME)–induced hypertension is blunted in TLR4-deficient mice.24 TLR9 is expressed in aortic vascular smooth muscle cells, and its activation augments contractile responses in conduit arteries.25 Activation of nicotinic acetylcholine receptors in splenocytes inhibits TLR-mediated inflammation in Wistar-Kyoto rats but paradoxically increases inflammation in the SHR.26 TLRs likely represent a link between host-derived “danger” molecules (DAMPs), low-grade inflammation, vascular remodeling, and hypertension. This topic has recently been reviewed in detail.25,27
Inflammasome
Some pattern recognition receptors trigger a distinct proinflammatory mechanism that involves assembly of cytosolic protein complexes called inflammasome. Once assembled, inflammasomes activate caspase-1, which in turn processes the proinflammatory IL-1 family cytokines IL-1β and IL-18 from their inactive to their active forms. A well described stimulus for inflammasome activation involves the action of extracellular ATP on the P2×7 receptor. Cellular damage increases extracellular ATP, which in turn serves as a danger signal that ultimately stimulates IL-1 release.28 Interestingly, P2×7-deficient mice develop blunted hypertension in response to deoxycorticosterone acetate (DOCA) salt challenge, suggesting that the inflammasome is involved in the pathogenesis of hypertension.29 Other stimuli for inflammasome activation include cholesterol and uric acid crystals. Current studies of mice lacking components of the inflammasome promise to provide additional insight into the role of inflammation in hypertension.
Monocytes/Macrophages and Other Bone Marrow–Derived Cells
Cells of myeloid lineage, including monocytes, macrophages, granulocytes, and dendritic cells, are major effectors of the innate immune system. Selective ablation of lysozyme M-positive myelomonocytic cells markedly blunts Ang II–induced infiltration of these cells into the vascular wall and attenuates Ang II–induced hypertension and vascular dysfunction.30 Osteopetrotic (Op/Op) mice, in which macrophages are functionally deficient, exhibit reduced hypertension and vascular remodeling in response to Ang II or DOCA salt.31,32 As in the case of other immune cells, the bone marrow is the source of circulating monocytes, monocyte-derived dendritic cells, and most inflammatory macrophages. Of interest, bone marrow transplant studies have emphasized the role of the bone marrow in hypertension. Santisteban et al. have recently shown that transplant of marrow from spontaneously hypertensive rats to Wistar-Kyoto rats increases BP in the latter.33 Likewise, Saleh et al. showed that transplant of marrow from LNK−/− mice, which are prone to inflammation and hypertension, markedly enhances the response to low-dose angiotensin II infusion in wild-type recipient mice.34
Lymphocytes of the Innate Immune System
Natural killer (NK) cells are the classic effector lymphocytes of the innate immune system.35 Only in the last few years has another population of lymphocytes that belongs to the innate arm of the immune system been identified and studied extensively. Because of their antigen-independent activation and lymphoid morphology, these cells have been called “innate lymphoid cells" (ILCs). NK cells share characteristics of innate lymphoid cells and, according to the most recent nomenclature of these cells, are considered to be “killer” ILCs.36
Innate Lymphoid Cells
NK cells (or “killer” ILCs) recognize stressed and damaged cells by a repertoire of activating and inhibitory NK cell receptors. Upon activation, NK cells exert cytotoxic effects on target cells. In Ang II–induced hypertension, NK cells migrate into the aortic wall and become a local source for interferon-γ. Depletion of NK cells reduces Ang II–induced vascular dysfunction.37 The family of “nonkiller” ILCs, which are also called “helper-like” ILCs, can be further divided into three groups that differ in their cytokine production, transcription factor usage, tissue localization, and functional characteristic.36,38,39,40 The role of these cells in hypertension remains undefined.
Innate-Like T Cell Subsets
It has become evident that certain T cell subsets that bear T cell receptors with a restricted repertoire can be activated independently from antigen directly by cytokine stimuli. Therefore they are regarded as being on the border between innate and adaptive immunity. These “innate-like” T cells include γδ T cells, NK T cells, and mucosa-associated invariant T cells.41 The γδ T cells are a population of cells that express the T cell receptor γδ chains. γδ T cells are a major source of IL-17 in hypertension, as discussed below. The other cells have not been examined in arterial hypertension.
Adaptive Immunity
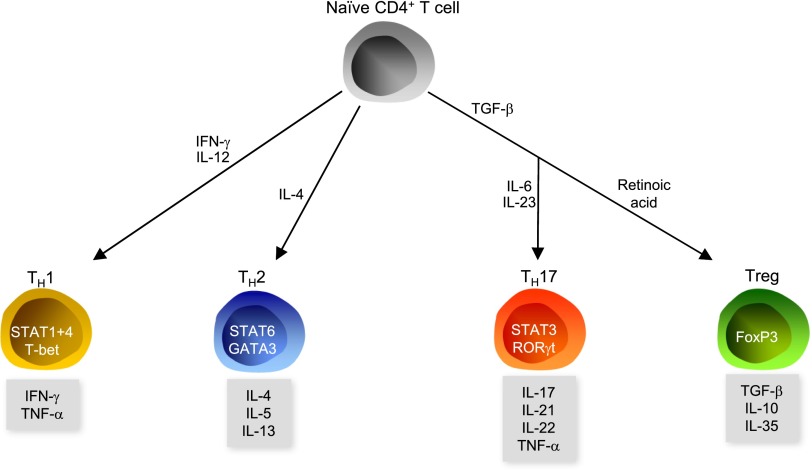
B cells, CD4+ cells, and CD8+ T cells are the major effectors of the adaptive immune system. As noted above, studies of RAG-1–deficient mice demonstrated that T cells contribute to the development of hypertension. On the basis of their cytokine production profile and expression of specific transcription factors, CD4+ T cells are classified into four major subsets: TH1, TH2, TH17, and regulatory T cells (Tregs) (Figure 1). The evidence for a role of the four subtypes in hypertension is summarized in Table 1.
Figure 1.
Polarization of CD4+ cells. Naive CD4+ T cells polarize into TH1, TH2, or TH17 cells or Tregs.
Table 1.
Evidence for a role of the four subtypes in hypertension
| Variable | TH1 | TH2 | TH17 | Treg |
|---|---|---|---|---|
| Function | Intracellular bacteria, cell mediated autoimmunity | Helminth infections, allergic disease | Extracellular bacteria, fungi, cell-mediated autoimmunity | Downregulation of the immune response in autoimmunity and infection |
| Observations in experimental arterial hypertension | Increased IFN-γ production in Ang II–induced hypertension42 | Decreased IL-4 production in Ang II–induced hypertension42 | Increased IL-17a production in Ang II–induced hypertension (plasma, aorta, heart)47–49 | Number of Tregs in the tissue follows the amount of injury |
| Increased number of TH17 cells in kidney of hypertensive mice55 | ||||
| Effect of knockout | IFN-γ deficiency has no effect on BP or albuminuria in Ang II–infused mice44 | No data | IL-17a deficiency lowers BP and reduces hypertensive injury in response to Ang II infusion47–49 | Not done because Treg knockout is lethal |
| IFN-γ receptor deficiency has no effect on BP leads to but less cardiac injury and mixed results in the kidney in response to Ang II infusion45 | IL-17a and IL-23p19 deficiency enhances renal injury in response to DOCA+Ang II infusion55 | |||
| IFN-γ deficiency lowers BP in response to low-dose Ang II43 | IL-6 deficiency lowers BP and endothelial dysfunction in response to Ang II infusion52,53 | |||
| T-bet- and IFN-γ deficiency have no effect on BP but less vascular dysfunction in response to Ang II infusion37 | ||||
| Effect of neutralizing antibodies | IFN-γ antibody has no effect on BP in hypertensive NZB mice46 | IL-4 antibody reduces BP in hypertensive NZB mice46 | No effect of IL-17 and IL-23 antibody in response to Ang II infusion45 | No data |
| Effect of overexpression or administration | Overexpression of IFN-γ induces vascular dysfunction37 | No data | Overexpression of IL-17a in the skin induces hypertension51 | Administration of Tregs lowers BP and ameliorates hypertensive injury in different models of hypertension65–68 |
| Administration of IL-17 increases BP50 |
T-bet, T-box expressed in T cells.
TH1 and TH2
Substantial evidence suggests that hypertension skews T cells toward a proinflammatory TH1 phenotype, as indicated by increased production of the TH1 cytokine IFN-γ and a decrease in TH2-mediated responses.42 The TH1 response in hypertension has been examined with different gene deletion approaches, and these studies suggest that IFN-γ and TH1 polarization contributes to the hypertension and end-organ damage caused by angiotensin II or DOCA salt hypertension challenge.37,43–46 Data on TH2 in hypertension are lacking. In one study, treatment with IL-4 antibodies lowered BP in NZB mice.46
TH17
IL-17 is the defining cytokine of TH17 cells. Several isoforms of IL-17 exist, with IL-17A and -F being the most abundant. Ang II infusion increases IL-17A production by T cells and IL-17 protein in the aortic media and the heart.47 The initial hypertensive response to Ang II infusion is similar in IL-17A−/− mice and wild-type mice. However, hypertension is not sustained in IL-17A−/− mice, reaching levels 30 mmHg lower than in wild-type mice by 4 weeks of Ang II infusion.48 In addition, mice lacking IL-17A are also protected against aortic stiffening and cardiac fibrosis after infusion of Ang II.47,49 Obviously, γδ T cell–derived IL-17 mediates the adverse effects of IL-17 in hypertension.47 In addition, IL-17 treatment increased systolic BP in mice.50 TH17 cells need IL-23 for expansion and survival. IL-23 receptor antibody treatment or IL-17A antibody treatment of Ang II–infused mice showed no major effect on cardiac or renal injury.45 It has to be kept in mind that antibody treatment never has an efficiency of 100%, whereas a genetic approach knocks down the gene and protein of interest to 0%. Dermal overexpression of IL-17A induces not only psoriasis-like lesions but also endothelial dysfunction, vascular oxidative stress, and arterial hypertension.51 The importance of IL-17 in hypertension is supported by studies of IL-6–deficient mice. IL-6 is needed for polarization of TH17 cells, and mice lacking this cytokine have lower BP and less endothelial dysfunction in response to Ang II infusion.52,53 IL-6 may also increase BP by stimulating epithelial sodium channels in collecting duct cells.54 Interestingly, in response to a combination of high-level Ang II infusion and DOCA salt administration, IL-17 deficiency can have adverse effects. Significantly higher renal injury was also found in IL-23p19–deficient mice with DOCA+Ang II–induced hypertension.55 In contrast, neither IL-17A nor IL-23p19 deficiency changed the degree of cardiac injury. Collectively, these findings show that IL-17 plays a major role in the pathophysiology of hypertension and hypertensive end-organ damage. A different role is possible in models with severe hypertensive end-organ damage, such as the combination of DOCA salt and Ang II.
T helper cells were originally considered to have stable phenotypes without changing of cytokine expression. However, experiments with TH17 cells in particular displayed dramatic instability of IL-17 secretion in vitro56,57 and in an animal model of multiple sclerosis (experimental autoimmune encephalomyelitis).58,59 Plasticity of TH17 cells in cardiovascular diseases and the potential impact on BP and tissue damage has not been investigated so far.
A fascinating relation exists between salt intake and IL-17. Two independent studies showed that sodium chloride can exaggerate TH17 production by CD4+ T cells due to activation of the serum and glucocorticoid-regulated kinase 1, which stabilizes the IL-23 receptor and reinforces the TH17 phenotype.60,61 Thus, a decreased TH17 response may significantly contribute to the beneficial effects of salt restriction in patients with hypertension. Serum and glucocorticoid-regulated kinase 1 is also the link between salt intake and accelerated allograft rejection.62 Activation of renal sodium transporters is blunted in INF-γ and IL-17A–deficient mice in response to Ang II, suggesting that sodium transporters are an important link between IFN-γ and IL-17 and hypertension.43 Titze and coworkers identified another important link between sodium and the immune system. In a series of elegant experiments, these authors found that high salt intake promotes skin Na+ accumulation and that this in turn causes macrophages to produce factors including VEGF-C, which modulates skin interstitial lymphatic density.63 In addition, Na+ accumulates at the site of bacterial skin infections in humans and mice and boosts classic macrophage activation, thus helping to ward off skin infection. Thus, salt deposition might serve as an ancient mechanism to aid in immune-mediated pathogen removal.64
Tregs and IL-10
The main function of Tregs is maintenance of immunologic tolerance. Several groups have shown that adoptive transfer of Tregs lowers BP and ameliorates cardiac and renal injury in different models of hypertension.65–68 Mice deficient in IL-10, an important product of Tregs, develop similar degrees of hypertension in response to angiotensin II, but have severe endothelial dysfunction and vessels from these mice show an increased superoxide production when incubated with angiotensin II.69 These and other data suggest that Treg-derived IL-10 mediates the beneficial effects of Tregs in hypertension.70 IL-2/anti–IL-2 immune complex treatment of hypertensive mice expands Tregs and reduces aortic stiffening.71 Administration of IL-10 lowers BP in preeclampsia.72
CD8+ T Cells
Convincing evidence suggests that T cells contribute to BP elevation. A major question, however, is what T cells are doing to cause these effects. A recent study has suggested that CD8+ T cells are a major source of IFN-γ and that these accumulate in the kidney in hypertension. Mice lacking CD8+ T cells developed blunted hypertensive responses to Ang II, while mice lacking CD4+ cells are not similarly protected. Vascular rarefaction in the kidney was noted in wild-type and CD4−/− mice, but not in CD8−/− mice. This in turn could promote sodium and volume retention.73 It should be recognized that mice lacking CD4+ cells also lack Tregs and this perhaps predisposes these animals to worsened hypertension.
Antigens and Dendritic Cells
The canonical function of dendritic cells is activation of T cells.74 After acquiring antigens, dendritic cells process these to peptides, migrate to the secondary lymphoid organ, and present these antigenic peptides in the context of MHCs to T cells. Antigen-activated T cells proliferate and produce clones specific for the antigen. The kidney harbors a network of dendritic cells. “Neoantigens” might be produced in response to early hypertensive mechanical trauma injury by induction of cell death and release of intracellular antigens that normally are immune privileged. Interestingly, various hypertensive stimuli increase ROS production in dendritic cells by activation of the NADPH oxidase. This leads to lipid oxidation and formation of γ ketoaldehydes or isoketals. Isoketals rapidly ligate to protein lysines in the dendritic cells, forming proteins that are interpreted as nonself as shown in Figure 2. Peptides from these proteins are presented to T cells, leading to their proliferation. In addition, isoketal formation also promotes production of cytokines, including IL-1β, IL-6, and IL-23, which polarize T cells to produce effector cytokines. Transfer of isoketal-activated dendritic cells raises BP in wild-type mice but not in RAG-1−/− mice that lack T cells. Moreover, treatment with isoketal scavengers blunts hypertension and its associated renal injury in mice.19 A role of dendritic cells in hypertension was also suggested by prior work using abatacept, which blocks CD80 and CD86 on dendritic cells. CD28 binds to these co-stimulatory molecules on dendritic cells and acts as a co-stimulatory receptor in the activation and maturation of T lymphocytes. Abatacept treatment reversed Ang II and DOCA salt–induced hypertension, confirming a role of T cell priming by dendritic cells in hypertension.75
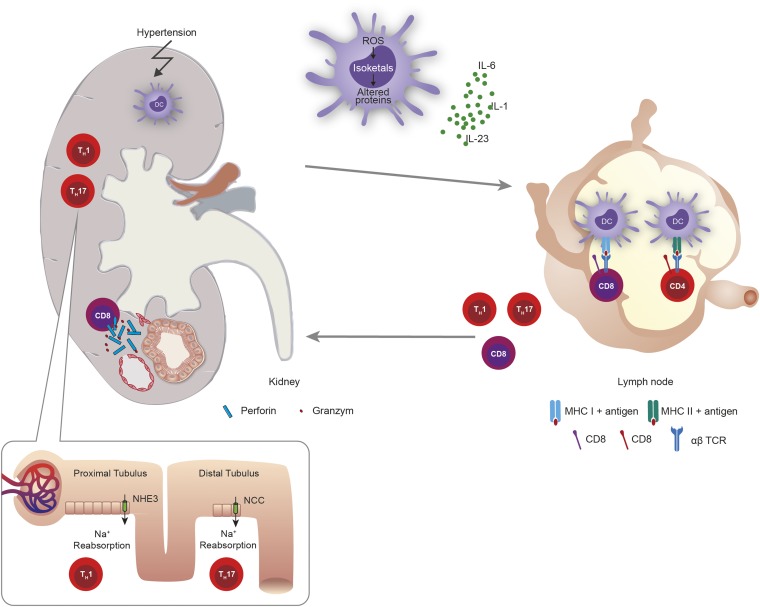
Figure 2.
Role of dendritic cells as well as CD4+ and CD8+ cells in hypertension. Hypertension causes lipid oxidation in dendritic cells (DC) resulting in formation of isoketal adducts of various self-proteins. These isoketal-modified proteins behave like DAMPs and activate dendritic cells. The dendritic cells secrete cytokines such as IL-1, IL-6, and IL-23. In the renal lymph node or other tissues dendritic cells may activate CD8+ and CD4+ T cells that migrate into the kidney. CD8+ T cells release cytotoxins such as perforin and granzyme leading to apoptosis or cell death of peritubular capillaries causing vascular rarefaction. IL-17A and IFN-γ released from TH17 and TH1 activate the renin-angiotensin system and may stimulate or upregulate transport channels in the proximal and distal convoluted tubules, including the sodium hydrogen exchanger 3 (NHE3) and the sodium chloride cotransporter (NCC). Both in turn causes sodium and volume retention. Modified from reference 10.
Microbiome
The human gut is the natural niche for >1014 bacteria of >1000 different species. The microbiota seem to modulate immune responses, and increasing data show that alterations in the microbiota contribute to immune disorders and cardiovascular diseases.76,77 Interestingly, recent data have shown that short-chain fatty acids produced by gut microbiota modulate renin release by binding to olfactory receptors expressed in the renal juxtaglomerular apparatus.78 A link between microbial content and BP regulation was also found in Dahl S and R rats.79 The cross-talk between the gut microbiota and the renal and cardiovascular systems could be relevant to the pathogenesis and treatment of hypertension. An interesting and unanswered question is whether salt intake influences the microbiome and the gut-derived IL-17 response enhancing the development of arterial hypertension.
Mechanisms of Experimental Models of Hypertension
How does the immune system cause hypertension? Convincing data demonstrate that inflammatory cytokines such as IL-17, IFN-γ, or IL-6 may modulate sodium reabsorption, as shown in Figure 2. In addition, infiltration of cytotoxic CD8 T cells into the renal interstitium may also lead to capillary rarefication, as shown in Figure 2. The inflammation associated with hypertension also causes deposition of collagen in the aortic adventitia and media with a loss of Windkessel or capacitance function.49
Most data on hypertension and inflammation in mice have been obtained through Ang II or norepinephrine infusion with osmotic mini-pumps or DOCA salt treatment. These experimental interventions reproduce several features of human hypertension. Ang II infusion resembles the condition in hypertensive patients with an inappropriately activated renin-angiotensin system, whereas norepinephrine infusion mimics the state of patients with an increased sympathetic drive. DOCA salt treatment models use enhanced mineralocorticoid activity, which is increasingly recognized in patients with resistant hypertension and so-called low-renin hypertension, based on the efficacy of mineralocorticoid receptor blockade in these patients. Furthermore, new mouse models are necessary to investigate the influence of genetic variants conferring an increased susceptibility to hypertensive renal injury associated with hypertension, as demonstrated for the gene that encodes the molecular motor protein nonmuscle myosin 2a (MYH9).80
Summary
Why should evolution favor immunity as a regulator of BP? Control of BP and host defense are essential mechanisms of homeostasis. Infection can cause hypotension via fluid loss in fever, tachypnea, and diarrhea. Septicemia induces inflammation-related vascular fluid losses. Thus, as suggested recently, the risk of hypotension related to inflammation might have favored selection of mechanisms that link immune activation to BP increases for short-term survival benefits. Such an evolutionary force may be responsible for the fact that important antimicrobial effectors such as IL-17 or ROS have direct hypertensive effects by vasoconstriction or sodium retention.81
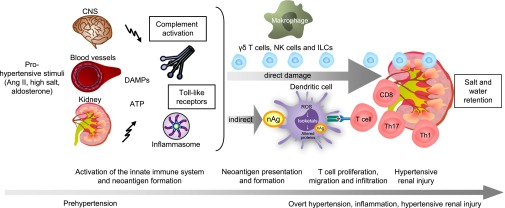
Synthesis of all of the available evidence has led us to propose the following hypothesis as a mechanism for inflammation and hypertension (Figure 3). Small increases in prohypertensive factors, such as Ang II, salt retention, or aldosterone, activate the innate immune system that triggers or aggravates hypertension. This process includes complement activation and release of DAMPs that activate TLRs. These mechanisms can damage the kidney directly. Hypertensive stimuli also induce NADPH oxidase–dependent ROS production in dendritic cells, which leads to formation of neoantigens that can activate adaptive immune cells. Activation of innate-like lymphocytes, such as γδ T cells and NK cells, as well as the adaptive immune system, consequently aggravates renal injury. Our hypothesis will almost certainly require refinement as new information becomes available. Are there clinical consequences? The major reason to treat hypertension is to prevent end-organ damage. While BP-lowering is clearly important to reach this goal, prevention of local inflammation that accompanies hypertensive end-organ damage should also to be addressed. Currently, no drugs are available to accomplish this latter goal.10 In addition, vaccines could be a therapeutic option for prevention or treatment of arterial hypertension.
Figure 3.
Working hypothesis describing the role of immunity in hypertension. Hypertensive factors such as Ang II, salt, or aldosterone lead to a cascade of events: They directly activate the innate immune system. This process includes complement activation and release of DAMPs, which can activate toll-like receptors and also the inflammasome. On the one hand these mechanisms damage the kidney directly. On the other hand, they promote the release of cryptic antigens and ROS production, isoketal formation, and alteration of proteins in dendritic cells. These nonself recognized proteins are presented to T cells. Polarized CD4+ and CD8+ T cells migrate into the heart, vessels, and kidney and mediate the hypertensive end-organ damage or aggravate this damage, as in the case of kidney arterial hypertension due to salt and volume retention. In addition activation of cells, such as monocytes/macrophages, γδ T cells, NK cells and ILCs may mediate or aggravate hypertensive renal injury. Modified from reference 7. CNS, central nervous system, nAg, neoantigen.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Beilin LJ, Goldby FS, Mohring J: High arterial pressure versus humoral factors in the pathogenesis of the vascular lesions of malignant hypertension. Clin Sci Mol Med 52: 111–117, 1977 [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez-Iturbe B, Pons H, Quiroz Y, Gordon K, Rincón J, Chávez M, Parra G, Herrera-Acosta J, Gómez-Garre D, Largo R, Egido J, Johnson RJ: Mycophenolate mofetil prevents salt-sensitive hypertension resulting from angiotensin II exposure. Kidney Int 59: 2222–2232, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG: Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med 204: 2449–2460, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowley SD, Song YS, Lin EE, Griffiths R, Kim HS, Ruiz P: Lymphocyte responses exacerbate angiotensin II-dependent hypertension. Am J Physiol Regul Integr Comp Physiol 298: R1089–R1097, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H: Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304: R407–R414, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL, PhysGen Knockout Program : CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension 63: 559–564, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM: Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Crowley SD: Role of T lymphocytes in hypertension. Curr Opin Pharmacol 21: 14–19, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Idris-Khodja N, Mian MO, Paradis P, Schiffrin EL: Dual opposing roles of adaptive immunity in hypertension. Eur Heart J 35: 1238–1244, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMaster WG, Kirabo A, Madhur MS, Harrison DG: Inflammation, immunity, and hypertensive end-organ damage. Circ Res 116: 1022–1033, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolev M, Le Friec G, Kemper C: Complement—tapping into new sites and effector systems. Nat Rev Immunol 14: 811–820, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Zhang C, Li Y, Wang C, Wu Y, Cui W, Miwa T, Sato S, Li H, Song WC, Du J: Complement 5a receptor mediates angiotensin II-induced cardiac inflammation and remodeling. Arterioscler Thromb Vasc Biol 34: 1240–1248, 2014 [DOI] [PubMed] [Google Scholar]
- 13.Zhang C, Li Y, Wang C, Wu Y, Du J: Antagonist of C5aR prevents cardiac remodeling in angiotensin II-induced hypertension. Am J Hypertens 27: 857–864, 2014 [DOI] [PubMed] [Google Scholar]
- 14.Harrison DG: Basic science: Pathophysiology: Oxidative stress. J Am Soc Hypertens 8: 601–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montezano AC, Touyz RM: Reactive oxygen species, vascular Noxs, and hypertension: Focus on translational and clinical research. Antioxid Redox Signal 20: 164–182, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, Gordon FJ, Harrison DG: Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res 107: 263–270, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lob HE, Schultz D, Marvar PJ, Davisson RL, Harrison DG: Role of the NADPH oxidases in the subfornical organ in angiotensin II-induced hypertension. Hypertension 61: 382–387, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carnevale D, Pallante F, Fardella V, Fardella S, Iacobucci R, Federici M, Cifelli G, De Lucia M, Lembo G: The angiogenic factor PlGF mediates a neuroimmune interaction in the spleen to allow the onset of hypertension. Immunity 41: 737–752, 2014 [DOI] [PubMed] [Google Scholar]
- 19.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, Bikineyeva AT, Dikalov S, Xiao L, Chen W, Saleh MA, Trott DW, Itani HA, Vinh A, Amarnath V, Amarnath K, Guzik TJ, Bernstein KE, Shen XZ, Shyr Y, Chen SC, Mernaugh RL, Laffer CL, Elijovich F, Davies SS, Moreno H, Madhur MS, Roberts J, 2nd, Harrison DG: DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest 124: 4642–4656, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ: NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol 23: 776–782, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Epelman S, Liu PP, Mann DL: Role of innate and adaptive immune mechanisms in cardiac injury and repair. Nat Rev Immunol 15: 117–129, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bomfim GF, Echem C, Martins CB, Costa TJ, Sartoretto SM, Dos Santos RA, Oliveira MA, Akamine EH, Fortes ZB, Tostes RC, Webb RC, Carvalho MH: Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci 122: 1–7, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eissler R, Schmaderer C, Rusai K, Kühne L, Sollinger D, Lahmer T, Witzke O, Lutz J, Heemann U, Baumann M: Hypertension augments cardiac Toll-like receptor 4 expression and activity. Hypertens Res 34: 551–558, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Sollinger D, Eißler R, Lorenz S, Strand S, Chmielewski S, Aoqui C, Schmaderer C, Bluyssen H, Zicha J, Witzke O, Scherer E, Lutz J, Heemann U, Baumann M: Damage-associated molecular pattern activated Toll-like receptor 4 signalling modulates blood pressure in L-NAME-induced hypertension. Cardiovasc Res 101: 464–472, 2014 [DOI] [PubMed] [Google Scholar]
- 25.McCarthy CG, Goulopoulou S, Wenceslau CF, Spitler K, Matsumoto T, Webb RC: Toll-like receptors and damage-associated molecular patterns: Novel links between inflammation and hypertension. Am J Physiol Heart Circ Physiol 306: H184–H196, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harwani SC, Chapleau MW, Legge KL, Ballas ZK, Abboud FM: Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res 111: 1190–1197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh MV, Abboud FM: Toll-like receptors and hypertension. Am J Physiol Regul Integr Comp Physiol 307: R501–R504, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan SM, Sobey CG, Latz E, Mansell A, Drummond GR: IL-1β and IL-18: Inflammatory markers or mediators of hypertension? Br J Pharmacol 171: 5589–5602, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji X, Naito Y, Weng H, Endo K, Ma X, Iwai N: P2X7 deficiency attenuates hypertension and renal injury in deoxycorticosterone acetate-salt hypertension. Am J Physiol Renal Physiol 303: F1207–F1215, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Wenzel P, Knorr M, Kossmann S, Stratmann J, Hausding M, Schuhmacher S, Karbach SH, Schwenk M, Yogev N, Schulz E, Oelze M, Grabbe S, Jonuleit H, Becker C, Daiber A, Waisman A, Münzel T: Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation 124: 1370–1381, 2011 [DOI] [PubMed] [Google Scholar]
- 31.De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL: Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: Evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 25: 2106–2113, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Ko EA, Amiri F, Pandey NR, Javeshghani D, Leibovitz E, Touyz RM, Schiffrin EL: Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: Evidence from m-CSF-deficient mice. Am J Physiol Heart Circ Physiol 292: H1789–H1795, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, Raizada MK, Zubcevic J: Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res 117: 178–191, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, Michell D, Huan T, Zhang Y, Takaki S, Titze J, Levy D, Harrison DG, Madhur MS: Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest 125: 1189–1202, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S: Innate or adaptive immunity? The example of natural killer cells. Science 331: 44–49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eberl G, Di Santo JP, Vivier E: The brave new world of innate lymphoid cells. Nat Immunol 16: 1–5, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Kossmann S, Schwenk M, Hausding M, Karbach SH, Schmidgen MI, Brandt M, Knorr M, Hu H, Kröller-Schön S, Schönfelder T, Grabbe S, Oelze M, Daiber A, Münzel T, Becker C, Wenzel P: Angiotensin II-induced vascular dysfunction depends on interferon-γ-driven immune cell recruitment and mutual activation of monocytes and NK-cells. Arterioscler Thromb Vasc Biol 33: 1313–1319, 2013 [DOI] [PubMed] [Google Scholar]
- 38.McKenzie AN, Spits H, Eberl G: Innate lymphoid cells in inflammation and immunity. Immunity 41: 366–374, 2014 [DOI] [PubMed] [Google Scholar]
- 39.Zhou L: Striking similarity: GATA-3 regulates ILC2 and Th2 cells. Immunity 37: 589–591, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Pietro C, Falcone M: The role of invariant NKT cells in organ-specific autoimmunity. Front Biosci (Landmark Ed) 19: 1240–1250, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Ussher JE, Klenerman P, Willberg CB: Mucosal-associated invariant T-cells: New players in anti-bacterial immunity. Front Immunol 5: 450, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shao J, Nangaku M, Miyata T, Inagi R, Yamada K, Kurokawa K, Fujita T: Imbalance of T-cell subsets in angiotensin II-infused hypertensive rats with kidney injury. Hypertension 42: 31–38, 2003 [DOI] [PubMed] [Google Scholar]
- 43.Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA: Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ-/- and interleukin-17A-/- mice. Hypertension 65: 569–576, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, Crowley SD: Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension 64: 1275–1281, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markó L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Müller DN: Interferon-γ signaling inhibition ameliorates angiotensin II-induced cardiac damage. Hypertension 60: 1430–1436, 2012 [DOI] [PubMed] [Google Scholar]
- 46.van Heuven-Nolsen D, De Kimpe SJ, Muis T, van Ark I, Savelkoul H, Beems RB, van Oosterhout AJ, Nijkamp FP: Opposite role of interferon-gamma and interleukin-4 on the regulation of blood pressure in mice. Biochem Biophys Res Commun 254: 816–820, 1999 [DOI] [PubMed] [Google Scholar]
- 47.Li Y, Wu Y, Zhang C, Li P, Cui W, Hao J, Ma X, Yin Z, Du J: γδT Cell-derived interleukin-17A via an interleukin-1β-dependent mechanism mediates cardiac injury and fibrosis in hypertension. Hypertension 64: 305–314, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ, Harrison DG: Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension 55: 500–507, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG: Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 114: 616–625, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen H, Chiasson VL, Chatterjee P, Kopriva SE, Young KJ, Mitchell BM: Interleukin-17 causes Rho-kinase-mediated endothelial dysfunction and hypertension. Cardiovasc Res 97: 696–704, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Karbach S, Croxford AL, Oelze M, Schüler R, Minwegen D, Wegner J, Koukes L, Yogev N, Nikolaev A, Reißig S, Ullmann A, Knorr M, Waldner M, Neurath MF, Li H, Wu Z, Brochhausen C, Scheller J, Rose-John S, Piotrowski C, Bechmann I, Radsak M, Wild P, Daiber A, von Stebut E, Wenzel P, Waisman A, Münzel T: Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler Thromb Vasc Biol 34: 2658–2668, 2014 [DOI] [PubMed] [Google Scholar]
- 52.Schrader LI, Kinzenbaw DA, Johnson AW, Faraci FM, Didion SP: IL-6 deficiency protects against angiotensin II induced endothelial dysfunction and hypertrophy. Arterioscler Thromb Vasc Biol 27: 2576–2581, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Wang W, Yu H, Zhang Y, Dai Y, Ning C, Tao L, Sun H, Kellems RE, Blackburn MR, Xia Y: Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59: 136–144, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li K, Guo D, Zhu H, Hering-Smith KS, Hamm LL, Ouyang J, Dong Y: Interleukin-6 stimulates epithelial sodium channels in mouse cortical collecting duct cells. Am J Physiol Regul Integr Comp Physiol 299: R590–R595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krebs CF, Lange S, Niemann G, Rosendahl A, Lehners A, Meyer-Schwesinger C, Stahl RA, Benndorf RA, Velden J, Paust HJ, Panzer U, Ehmke H, Wenzel UO: Deficiency of the interleukin 17/23 axis accelerates renal injury in mice with deoxycorticosterone acetate+angiotensin ii-induced hypertension. Hypertension 63: 565–571, 2014 [DOI] [PubMed] [Google Scholar]
- 56.Mathur AN, Chang HC, Zisoulis DG, Kapur R, Belladonna ML, Kansas GS, Kaplan MH: T-bet is a critical determinant in the instability of the IL-17-secreting T-helper phenotype. Blood 108: 1595–1601, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT: Late developmental plasticity in the T helper 17 lineage. Immunity 30: 92–107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kurschus FC, Croxford AL, Heinen AP, Wörtge S, Ielo D, Waisman A: Genetic proof for the transient nature of the Th17 phenotype. Eur J Immunol 40: 3336–3346, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B: Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12: 255–263, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA: Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu C, Yosef N, Thalhamer T, Zhu C, Xiao S, Kishi Y, Regev A, Kuchroo VK: Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496: 513–517, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Safa K, Ohori S, Borges TJ, Uehara M, Batal I, Shimizu T, Magee CN, Belizaire R, Abdi R, Wu C, Chandraker A, Riella LV: Salt accelerates allograft rejection through serum- and glucocorticoid-regulated kinase-1-dependent inhibition of regulatory T cells [published online ahead of print April 1, 2015]. J Am Soc Nephrol 10.1681/ASN.2014090914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Titze J, Machnik A: Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens 19: 385–392, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Jantsch J, Schatz V, Friedrich D, Schröder A, Kopp C, Siegert I, Maronna A, Wendelborn D, Linz P, Binger KJ, Gebhardt M, Heinig M, Neubert P, Fischer F, Teufel S, David JP, Neufert C, Cavallaro A, Rakova N, Küper C, Beck FX, Neuhofer W, Muller DN, Schuler G, Uder M, Bogdan C, Luft FC, Titze J: Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 21: 493–501, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matrougui K, Abd Elmageed Z, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M: Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol 178: 434–441, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kvakan H, Kleinewietfeld M, Qadri F, Park JK, Fischer R, Schwarz I, Rahn HP, Plehm R, Wellner M, Elitok S, Gratze P, Dechend R, Luft FC, Muller DN: Regulatory T cells ameliorate angiotensin II-induced cardiac damage. Circulation 119: 2904–2912, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL: T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 57: 469–476, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL: T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 59: 324–330, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Didion SP, Kinzenbaw DA, Schrader LI, Chu Y, Faraci FM: Endogenous interleukin-10 inhibits angiotensin II-induced vascular dysfunction. Hypertension 54: 619–624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K: Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol 31: 2534–2542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Majeed B, Tawinwung S, Eberson LS, Secomb TW, Larmonier N, Larson DF: Interleukin-2/anti-interleukin-2 immune complex expands regulatory T cells and reduces angiotensin II-induced aortic stiffening. Int J Hypertens 2014: 126365, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chatterjee P, Chiasson VL, Seerangan G, Tobin RP, Kopriva SE, Newell-Rogers MK, Mitchell BM: Cotreatment with interleukin 4 and interleukin 10 modulates immune cells and prevents hypertension in pregnant mice. Am J Hypertens 28: 135–142, 2015 [DOI] [PubMed] [Google Scholar]
- 73.Trott DW, Thabet SR, Kirabo A, Saleh MA, Itani H, Norlander AE, Wu J, Goldstein A, Arendshorst WJ, Madhur MS, Chen W, Li CI, Shyr Y, Harrison DG: Oligoclonal CD8+ T cells play a critical role in the development of hypertension. Hypertension 64: 1108–1115, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weisheit CK, Engel DR, Kurts C: Dendritic cells and macrophages: Sentinels in the kidney [published online ahead of print January 7, 2015]. Clin J Am Soc Nephrol 10.2215/CJN.07100714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vinh A, Chen W, Blinder Y, Weiss D, Taylor WR, Goronzy JJ, Weyand CM, Harrison DG, Guzik TJ: Inhibition and genetic ablation of the B7/CD28 T-cell costimulation axis prevents experimental hypertension. Circulation 122: 2529–2537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surana NK, Kasper DL: Deciphering the tête-à-tête between the microbiota and the immune system. J Clin Invest 124: 4197–4203, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tang WH, Hazen SL: The contributory role of gut microbiota in cardiovascular disease. J Clin Invest 124: 4204–4211, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ: Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B: Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics 47: 187–197, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Freedman BI, Sedor JR: Hypertension-associated kidney disease: Perhaps no more. J Am Soc Nephrol 19: 2047–2051, 2008 [DOI] [PubMed] [Google Scholar]
- 81.Anders HJ, Baumann M, Tripepi G, Mallamaci F: Immunity in arterial hypertension: Associations or causalities? [Published online ahead of print March 11, 2015] Nephrol Dial Transplant 10.1093/ndt/gfv057 [DOI] [PMC free article] [PubMed] [Google Scholar]