Abstract
Among the different types of methionine-derived aliphatic glucosinolates (GS), sinigrin (2-propenyl), the final product in 3C GS biosynthetic pathway is considered very important as it has many pharmacological and therapeutic properties. In Brassica species, the candidate gene regulating synthesis of 3C GS remains ambiguous. Earlier reports of GSL-PRO, an ortholog of Arabidopsis thaliana gene At1g18500 as a probable candidate gene responsible for 3C GS biosynthesis in B. napus and B. oleracea could not be validated in B. juncea through genetic analysis. In this communication, we report the isolation and characterization of the gene CYP79F1, an ortholog of A. thaliana gene At1g16410 that is involved in the first step of core GS biosynthesis. The gene CYP79F1 in B. juncea showed presence-absence polymorphism between lines Varuna that synthesizes sinigrin and Heera virtually free from sinigrin. Using this presence-absence polymorphism, CYP79F1 was mapped to the previously mapped 3C GS QTL region (J16Gsl4) in the LG B4 of B. juncea. In Heera, the gene was observed to be truncated due to an insertion of a ~4.7 kb TE like element leading to the loss of function of the gene. Functional validation of the gene was carried out through both genetic and transgenic approaches. An F2 population segregating only for the gene CYP79F1 and the sinigrin phenotype showed perfect co-segregation. Finally, genetic transformation of a B. juncea line (QTL-NIL J16Gsl4) having high seed GS but lacking sinigrin with the wild type CYP79F1 showed the synthesis of sinigrin validating the role of CYP79F1 in regulating the synthesis of 3C GS in B. juncea.
Introduction
Glucosinolates (GS) are nitrogen- and sulfur-rich secondary metabolites characteristic of the order Capparales [1]. GS biosynthesis is highly complex. Brassica crops primarily contain methionine derived aliphatic GS (up to 95% of the total GS). Aliphatic GS can be broadly divided into propyls (three-carbon, 3C), butyls (four-carbon, 4C) and pentyls (five-carbon, 5C) on the basis of side chain length. Brassica species consist of various combinations of the above three types of aliphatic GS namely, 2-propenyl (commonly known as sinigrin) of 3C GS, 3-butenyl (known as gluconapin) and 2-hydroxy-3-butenyl (known as progoitrin) of 4C GS and 4-pentenyl (known as glucobrassicanapin) of 5C GS. The diploid B. nigra (BB, 2n = 16) contains 3C GS sinigrin, B. oleracea (CC, 2n = 18) contains either sinigrin or 4C GS, and B. rapa (AA, 2n = 20) contains both 4C and 5C GS. The GS profile of the three allotetraploid Brassica species, B. juncea (AABB, 2n = 36), B. napus (AACC, 2n = 38) and B. carinata (BBCC, 2n = 34) is a combination of the GS profiles of the progenitor diploid Brassica species [2]. In allotetraploid oilseed mustard (Brassica juncea; AABB), 3C and 4C constitute about 99% of the total aliphatic GS. Among the two gene pools in mustard—the east European and the Indian [3], cultivars belonging to the former primarily contain sinigrin of 3C GS whereas those belonging to the latter contain both sinigrin of 3C GS (~20%) and gluconapin of 4C GS (~80%) [4,5].
Among the different types of aliphatic GS, sinigrin, the final product in 3C GS pathway is considered to have great importance in human health as well as in plant defence processes. Its degradation product, allyl isothiocyanate has been shown to have bactericidal and antimicrobial activities [6] and anti-proliferative activities against liver [7] and bladder cancer [8]. Sinigrin and its degradation products suppress nitric oxide production in macrophages [9] and reduce plasma triglyceride level [10]. Due to its beneficial role, sinigrin is now even available as a nutrition supplement. In plants it is known to trigger stomatal closure by acting on K+ channels [11], prevent water loss and provide defence against fungal invasions [12] and bacterial pathogens [13].
Genetic analysis of 3C GS in Brassica species was initiated [14] in B. napus by QTL mapping. Since natural B. napus do not synthesize sinigrin of 3C GS, the experiment used a resynthesized B. napus line that contained the C genome from a wild form of B. oleracea which synthsizes sinigrin. The analysis led to the identification of a single locus controlling the genetic variation of sinigrin. A follow up work in B. oleracea [15] reported that alleles of a single locus GSL-PRO (Arabidopsis thaliana gene ID: At1g18500; B rapa gene code: Bra025899 belonging to MAM gene family) regulate the presence or absence of sinigrin. Further work in B. oleracea revealed the presence of two tightly linked duplicated genes, GSL-PROa (At1g18500) and GSL-PROb (At1g74040) by sequencing and were shown to map to the same position in the LG C5 [16,17]. However, the functional validity of GSL-PRO for the biosynthesis of sinigrin in Brassica species still remains to be demonstrated through genetic transformation experiments.
In B. juncea, the detailed genetic analysis of aliphatic GS was initiated [18] using an Indian type high seed GS line (Varuna) that contains sinigrin of 3C GS, gluconapin of 4C GS and traces of 5C GS (Table 1 of [18]) and identified four major QTL namely, J2Gsl1, J3Gsl2, J9Gsl3 and J16Gsl4 in LGs A2, A3, A9 and B4, respectively and one minor QTL, J17Gsl5 in LG B1. Subsequent fine mapping study by candidate gene markers of aliphatic GS pathway genes and their co-segregation with the phenotypes [19] revealed that J2Gsl1 controls the 4C GS as one homolog of ELONG gene mapped to this locus, J3Gsl2 controls both 5C GS and total aliphatic GS as another homolog of ELONG and a homolog of Myb28 mapped at a distance of 1.6 cM to the QTL region. J9Gsl3 and J17Gsl5 both control total aliphatic GS as one homolog each of Myb28 mapped to these two QTL regions. The QTL J16Gsl4 in the LG B4 was identified to be controlling the sinigrin fraction of 3C GS in B. juncea. Since the QTL J16Gsl4 mapped to a chromosome of B sub-genome of B. juncea, it indicated that the 3C GS pathway in B. juncea is inherited from the B-genome progenitor. Furthermore, an attempt to map the GSL-PRO to QTL J16Gsl4 region did not yield positive result as none of the two B genome specific paralogues for GSL-PRO identified in B. juncea could be mapped to QTL J16Gsl4 that was implicated in regulating sinigrin biosynthesis in B. napus and B. oleracea. The two B-genome paralogues of GSL-PRO instead mapped to LGs B6 and B7 of B. juncea [19]. A follow up exercise of QTL mapping in B. juncea using an east European high seed GS line (Donskaja IV) that primarily synthesizes sinigrin also identified a QTL corresponding to J16Gsl4 [20].
Since no candidate gene known to be responsible for the biosynthesis of sinigrin could be identified where the trait was shown to be mapping to the B genome of B. juncea, we concentrated on the identification of the candidate gene regulating sinigrin synthesis in this important oilseed and condiment Brassica species. In this communication we describe identification of the candidate gene responsible for biosynthesis of sinigrin in B. juncea. Analysis showed that CYP79F1, a homologue of the gene At1g16410 (named as BjuB.CYP79F1) mapped to the QTL J16Gsl4 in LG B4 of B. juncea. Further, we confirmed the role of BjuB.CYP79F1 in regulating the synthesis of sinigrin by validation through genetic and transgenic approaches in B. juncea.
Materials and Methods
Plant Materials used
Several genetic stocks consisting of different oilseed Brassica species and two mapping populations were used in the study. For gene isolation experiment, two B. juncea (AABB) lines—Varuna [a high GS Indian variety (>110 μmol g-1 seed with 18–20 μmol g-1 seed of sinigrin)] and Heera [a low GS east European line (~12 μmol g-1 seed and virtually free from sinigrin) (Table 1 of [18]) containing low alleles for Myb28, ELONGs and QTL J16Gsl4 [19] were used. The study also includes one line each from B. rapa (AA) cv. YSPB-24 and B. nigra (BB) cv. IC 257 with high seed GS content. For genetic analyses and mapping, two bi-parental populations–(i) A Varuna x Heera F1DH mapping population (VH) consisting of 123 lines (the population was previously used in the lab to construct a high-density comparative linkage map in B. juncea) [21] and QTL mapping of seed GS [18]. (ii) An F2 population of 95 segregants derived from a cross between Varuna x QTL-NIL J16Gsl4. The line QTL-NIL J16Gsl4 is a near isogenic line (NIL) which contains Heera allele of QTL J16Gsl4 in the genetic background of Varuna and is high in seed GS (>110 μmol g-1 seed) with trace amount of sinigrin. The NIL J16Gsl4 was developed by following a recurrent selection backcross (RSB) scheme [18] and was identified as NIL (near iso-genic line) for the QTL J16Gsl4 through genotyping for all GS QTL/genes from a population of 785 BC4DH lines segregating for aliphatic GS trait [19]. Finally for the transformation experiment, two lines namely, QTL-NIL J16Gsl4 (high seed GS) and EH-2 (an EMS induced early flowering mutant of low GS line Heera containing mutant alleles for the loci Myb28, ELONGs and QTL J16Gsl4 as in Heera and also having comparable aliphatic seed GS profile as that of Heera) were used. We used EH-2 in place of Heera because the lab has standardized transformation protocol for EH-2.
Cloning, sequencing and mapping of the gene
Genomic DNA was isolated from well-expanded leaves from the field grown plants following the protocol of Rogers and Bendich [22]. All PCR amplifications were carried out in a 20 μl reaction volume containing 50 ng of template DNA, 800 μM of dNTPs, 2 mM of MgCl2, 10 pmol each of forward and reverse primers and enzyme Taq polymerase (1 Unit, i-Taq DNA polymerase, Intron Biotechnology, Inc.). The PCR profile included initial denaturation at 94°C for 5 min followed by 36 cycles of 94°C for 30 sec, annealing at 62°C for 30 sec and 72°C for 1 min 30 sec and a final extension at 72°C for 12 min. For the purpose of sequencing, amplified fragments were cloned in the pGEM-T Easy TA cloning vector (Promega, Madison, USA). Sequencing was done by capillary electrophoresis on Applied Biosystems 3730 Genetic Analyzer. Sequencing of each sample was done in a minimum of 3 independent replicates to remove chances of any PCR related error. Sequence files were assembled and analysed using EditSeq, SeqMan and MegAlign software packages of DNAStar (DNAStar Inc.). For mapping, marker genotyping data of the mapping population were added to the existing map of B. juncea [21] using the program JoinMap version 4.0 [23]. Genome walk was performed using PCR genomic libraries based on the method described by GenomeWalker Universal Kit (Clontech Ltd.).
Screening of BAC libraries and verification
Development of BAC libraries used in the study has been described by Sharma et al. [24]. BAC screening, identification and contig construction was performed according to the protocol followed by Sharma et al. [24].
Sequencing of BAC and sequence analysis
Sizing of the BAC insert and Sanger sequencing of BAC was outsourced to Amplicon Express, USA. BAC sequencing by 454/Roche GS-FLX Titanium pyrosequencing and sequence assembly using GS De Novo Assembler (v 2.9) was performed at Macrogen Inc., South Korea. Annotation of the assembled contigs was performed using the Brassica BAC annotation pipeline (http://brassica.nbi.ac.uk/annotate.html). Transposable elements (TE) in the BAC were predicted and located by using the program RepeatMasker (http://www.repeatmasker.org/). Sequence of the BAC was aligned to the corresponding A. thaliana sequences obtained from TAIR (https://www.arabidopsis.org/) and B. rapa sequences obtained from BRAD (http://brassicadb.org/brad/) using NCBI BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE_TYPE=BlastSearch&PROG_DEF=blastn&BLAST_PROG_DEF=megaBlast&BLAST_SPEC=blast2seq) to analyze gene conservation.
Reverse transcriptase (RT)-PCR
Total RNA was extracted from frozen tissues using the RNeasy Plant Mini Kit (Qiagen), complying with manufacturer’s instructions. Total RNA isolated was treated with DNase using the RNase-Free DNase Set (Qiagen) and column purified. cDNA library was synthesized by reverse transcribing 2 μg of total RNA with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, USA) in a 20 μl reaction volume using mixture of random primers and oligo-dTs in 1:1 ratio according to manufacturer’s instructions. PCR amplification was carried out in a 20 μl reaction volume containing 1 μl of cDNA, 800 μM of dNTPs, 2 mM of MgCl2, 10 pmol each of forward and reverse primers and 1 Unit of Taq polymerase enzyme (i-Taq DNA polymerase, Intron Biotechnology, Inc.). The RT-PCR profile included initial denaturation at 94°C for 5 min followed by 30 cycles of 94°C for 30 sec, annealing at 60°C for 30 sec and 72°C for 30 sec and a final extension at 72°C for 5 min. The PCR products were analysed by agarose gel electrophoresis. Actin gene forward primer Actin-FP (5’–GGC TCC TCT TAA CCC AAA GG– 3’) and reverse primer Actin-RP (5’–TTC TCG ATG GAA GAG CTG GT– 3’) was used as endogenous control for equal template loading.
Genetic transformation of B. juncea and GS analysis
Agrobacterium tumefaciens mediated genetic transformation of B. juncea was performed using hypocotyl explants following the protocol described by Jagannath et al. [25]. Extraction and estimation of seed GS content and analysing the GS profiles was done by HPLC [20].
Data deposition
The sequences reported in this paper are deposited in the National Center for Biotechnology Information GenBank database with accession numbers KT254222 (BjuB.CYP79F1 from B. juncea cv. Varuna) and KT254223 (BniB.CYP79F1 from B. nigra cv. IC 257).
Results
Identification and amplification of aliphatic GS genes from QTL J16Gsl4 region of B. juncea
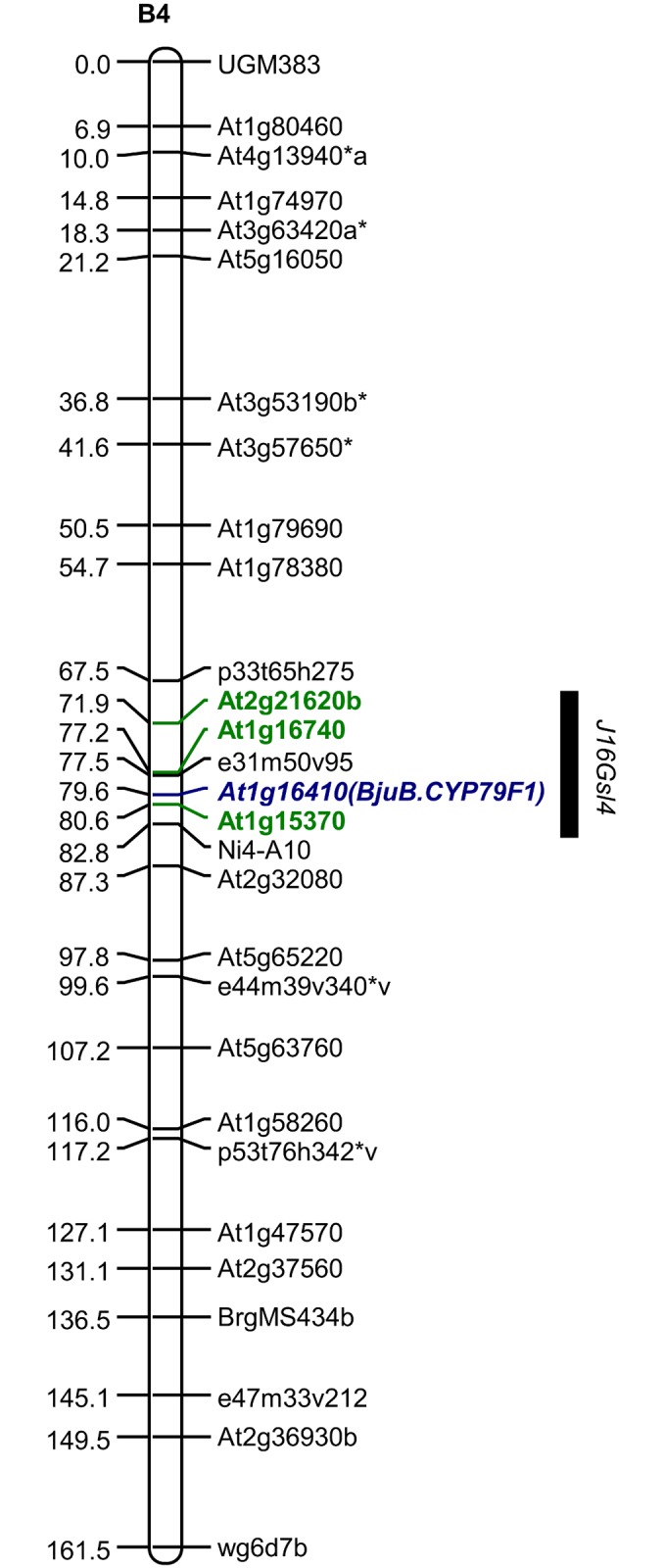
An in silico search of aliphatic GS genes from 1g and 2g chromosomes of Arabidopsis ([26] (sequences downloaded from http://www.arabidopsis.org/) encompassing the syntenous region of QTL J16Gsl4 (Fig 1) flanked by the markers At1g15370 (block A) and At2g21620 (block I) in B. juncea [21] and the corresponding regions in B. rapa ([27] (sequences downloaded from http://brassicadb.org/brad/) was undertaken. The search identified a total of 15 genes of aliphatic GS biosynthetic pathway (GSL-PRO not included). A total of 14 degenerate primers (wherever necessary) to accommodate the multiple copies of the genes were used (S1 Table) for amplification from two allotetraploid B. juncea (AABB) lines Varuna and Heera having contrasting phenotype for sinigrin and also from the diploid parental species B. rapa cv. YSPB-24 (AA) and B. nigra cv. IC257 (BB). None of these primer pairs showed clear-cut polymorphism between Heera and Varuna (S1 Fig). Hence, emphasis was laid for uncovering B genome specific allelic polymorphism of the genes as the QTL J16Gsl4 regulating the sinigrin synthesis was located in the LG B4 of B. juncea. The process was initiated with the genes CYP79F1 (At1g16410) and CYP79F2 (At1g16400) (tandemly duplicated in Arabidopsis) involved in the first step of synthesis of core GS structure. Priority was given to these two genes because of the fact that these gene IDs (At1g16400 and At1g16410) were assumed to be located nearer to the peak LOD value marker (At1g16740) of the QTL J16Gsl4 [18,19] (Fig 1) as revealed by the synteny information from a comparative map of B. juncea [21].
Fig 1. Map of LG B4 showing positions of marker At1g16410 (named as BjuB.CYP79F1) and QTL J16Gsl4 in B. juncea.
Isolation of gene BjuB.CYP79F1 from B. juncea and its mapping
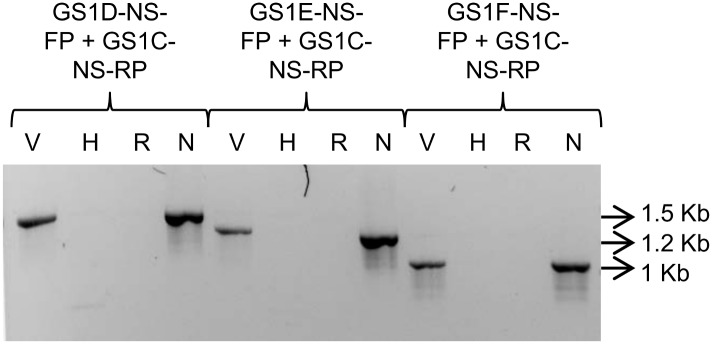
The initial non-polymorphic amplified products (S1 Fig: ~1700 bp from the primer pair GS1-FP and GS1-RP) from the two parental species B. rapa and B. nigra which were presumed to contain the sequences of both CYP79F1 and CYP79F2 were cloned into pGEM-T easy vector and sequenced. The sequences from both 5’ and 3’ ends of the cloned products revealed only one sequence variant each in B. rapa and B. nigra. Based on the sequence divergence from the deduced partial sequences of the two orthologs of CYP79F1/CYP79F2 from B. rapa and B. nigra, three sets of B. nigra-specific forward primers (GS1D-NS-FP, GS1E-NS-FP and GS1F-NS-FP) and one reverse primer (GS1C-NS-RP) were designed for amplification (for detailed primer sequences see S2 Table and for the positions of the primers see S2 Fig). PCR amplification using these three sets of B. nigra-specific nested primers led to the amplification of only one band of different molecular weights by each primer. B. nigra (B genome) specificity of these three primers was confirmed by the fact that none of the primer pairs amplified any bands from B. rapa (A genome), whereas all the three primers amplified one band each from B. juncea line Varuna with the same molecular weight as that from B. nigra (Fig 2). However, no band was amplified from Heera indicating that the gene is either truncated or lost in the B genome of B. juncea line Heera.
Fig 2. PCR amplification by three B genome specific primers (names of primers used have been given on top of the gel lanes) for CYP79F1 from B. juncea lines Varuna (V) and Heera (H); B. rapa (R); B. nigra (N).
Using one of these three primer pairs (GS1D-NS-FP and GS1C-NS-RP) showing dominant polymorphism between Varuna and Heera, the gene CYP79F1/CYP79F2 was mapped to the existing VH map of Panjabi et al. [21] (Fig 1). It mapped to the QTL interval of QTL J16Gsl4 indicating that the CYP79F1/CYP79F2 could be the probable candidate gene for biosynthesis of sinigrin.
Complete sequences of the B genome orthologs of the gene CYP79F1/ CYP79F2 from B. juncea cv. Varuna and from B. nigra cv. IC257 were derived by using a common set of nested primers and through 5’ and 3’ genome walking (See S2 Table for primer sequences). Complete genomic sequence of the gene in Varuna was observed to be 2163 bp long (S2 Fig). A data search of genome sequences of Brassica species revealed the presence of only CYP79F1 in B. rapa [27], B. oleracea [28] and B. napus [29]. Alignment by BLAST search of these two deduced sequences from B. nigra and B. juncea with the CYP79F1 from B. rapa, B. oleracea and B. napus and the two tandemly duplicated genes CYP79F1 and CYP79F2 from Arabidopsis revealed high similarity with CYP79F1 of Brassica species than the sequences of CYP79F1 and CYP79F2 of Arabidopsis. Hence, the isolated genes from the B genome of B. juncea and from B. nigra were named as BjuB.CYP79F1 and BniB.CYP79F1, respectively. The predicted coding region comprised three exons, as in CYP79F1 from A. thaliana (S3 Fig). The coding sequence of BjuB.CYP79F1 was compared with the orthologous sequences of the gene from A. thaliana (At1g16410 and At1g16400), B. nigra (BniB.CYP79F1, isolated in the present study) and other Brassica species. The gene was almost identical to BniB.CYP79F1 (99.8%) and also showed high sequence identity (>93%) with CYP79F1 of other Brassica species (S4 Fig).
The full length 1626 bp BjuB.CYP79F1 cDNA encodes a predicted 62 kDa polypeptide of 541 amino acids. Several motifs in the deduced amino acid sequences of BjuB.CYP79F1 exhibited similarities with the conserved motifs found in cytochrome P450 enzymes: a short N-terminal signal peptide for targeting to the endoplasmic reticulum (ER), a stretch of hydrophobic amino acids constituting the transmembrane helix, which anchors the protein in the ER; a positively charged domain; a proline-rich motif and a heme binding domain towards the C-terminal [30,31]. When queried using the PredictProtein software (https://www.predictprotein.org/; [32,33]), subcellular localization of BjuB.CYP79F1 and BniB.CYP79F1 was predicted to be in the ER membrane, and amino acids 16–34 in BjuB.CYP79F1 and amino acids 14–34 in BniB.CYP79F1 were predicted to constitute the transmembrane helix (S3 Fig).
Resolving the structural organization of BjuB.CYP79F1 in Heera
Our initial PCR experiment (previous section) could not succeed in amplifying the BjuB.CYP79F1 from the parent Heera. Hence, the gene organization of BjuB.CYP79F1 in Heera was resolved through BAC sequencing. Initially, a sequence of 1177 bp starting from -645 to +532 bp was established by 5’genome walking which showed that the 5’ end of the gene in Heera is intact. The sequence was identical to the corresponding sequence of the gene in Varuna. Subsequently, a primer pair that would amplify -428 to +532 bp was used for screening the two BAC libraries of the Heera genome [24]. Of the four positive BAC clones identified, the clone with the biggest insert was sequenced.
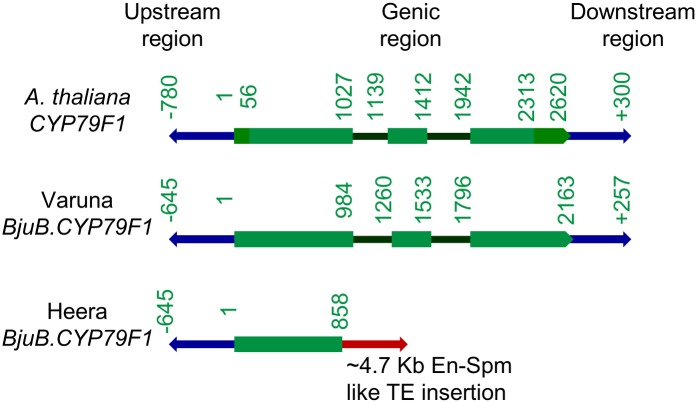
The BAC sequence data revealed the length of the insert as 94.989 kb and predicted a total of 30 genes (S3 Table). The sequence analysis also uncovered the presence of 24 transposable elements (TE) in the region. The gene BjuB.CYP79F1 was observed to be truncated in the first exon with next two exons missing. The truncated first exon was completely identical up to 855 bp from the 5’ end with B. rapa CYP79F1 (Bra026058) and up to 858 bp from the 5’ end with wild type BjuB.CYP79F1 gene of Varuna. Following the truncation point, presence of a ~4.7 Kb long En-Spm like DNA transposon downstream of BjuB.CYP79F1 was detected (Fig 3). It suggests that the insertion of the transposon might have led to exon loss, and probably caused the loss of function of BjuB.CYP79F1 in Heera.
Fig 3. Representative gene models depicting structural organization of CYP79F1 from Arabidopsis and two B. juncea lines Varuna and Heera.
Bases 1–55 and 2313–2620 in A. thaliana CYP79F1 constitute the 5’UTR and 3’UTR, respectively.
Functional validation of BjuB.CYP79F1 as the candidate gene responsible for 3C GS
Genetic approach
An F2 population of 95 individuals from a cross between Varuna and QTL-NIL J16Gsl4 segregating for the sinigrin was used for the validation through co-segregation of the genotype and the phenotype. The scheme for the development of F2 population has been shown in S5 Fig. The NIL status of QTL-NIL J16Gsl4 was initially established using 59 background markers distributed throughout the genome spanning over 18 LGs of B. juncea and two flanking foreground markers (At1g15370 and At2g21620b) (Fig 1). The genotyping data confirmed the line as QTL-NIL J16Gsl4 as all the background markers revealed the genotype of the recurrent parent Varuna except a ~13.9 cM introgression of the QTL region of J16Gsl4 from the donor parent Heera.
Analysis of 95 F2 segregants revealed a perfect co-segregation between the phenotype and the genotype wherein F3 seeds from 76 F2 segregants showed the synthesis of sinigrin ranging from 8.1–31.5 μmol g-1 (mean 13.7 μmol g-1) and the presence of ‘Varuna’ allele for BjuB.CYP79F1. The remaining 19 segregants showed no synthesis (or traces) of sinigrin and the presence of ‘Heera’ allele for BjuB.CYP79F1 (S6 Fig). The segregation data conformed to the 3:1 ratio showing a single locus inheritance (Table 1). Details of the aliphatic seed GS profiles of parents and F2 segregants have been shown in S4 Table.
Table 1. Phenotypic (for sinigrin) and genotypic (for BjuB.CYP79F1) co-segregation data of a F2 population from a cross between QTL-NIL J16Gsl4 line and Varuna.
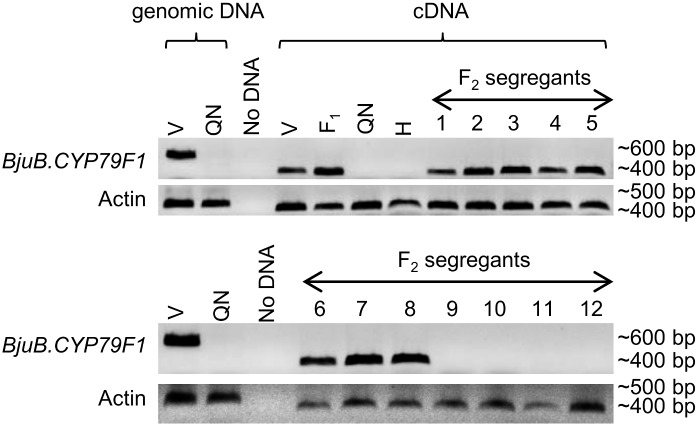
An expression analysis was done from the developing siliques (20 days after pollination) of the F2 segregants through reverse transcriptase (RT) PCR. Eight randomly selected F2 plants that showed the presence of gene BjuB.CYP79F1 (Varuna allele) and four F2 plants that did not (Heera allele) were chosen for the expression analysis. All the eight F2 segregants showing the presence of the gene BjuB.CYP79F1 also showed amplification from BjuB.CYP79F1 cDNA. On the other hand, the four lines that did not amplify the gene BjuB.CYP79F1 did not show amplification from cDNA as well (Fig 4). This lack of transcription from the parents Heera, QTL-NIL J16Gsl4 and four F2 segregants containing ‘Heera’ allele indicates that the truncated BjuB.CYP79F1 of Heera is non-functional.
Fig 4. RT-PCR analysis from developing siliques showing expression of BjuB.CYP79F1 in parents and F2 segregants derived from a cross between Varuna and QTL-NIL J16Gsl4.
RT-PCR reactions were performed using gene specific primers for BjuB.CYP79F1 (forward primer GS1G-NS-FP and reverse primer GS1B-NS-RP) and Actin (forward primer Actin-FP and reverse primer Actin-RP). Amplification from genomic DNA was done by primer pair GS1G-NS-FP and GS1B-NS-RP (S2 Table). No DNA lane was used as control. V—Varuna, QN—QTL-NIL J16Gsl4 line, F1 –F1 between Varuna x QTL-NIL J16Gsl4, H—Heera, cDNA—complementary DNA, 1–8 –F2 segregants where BjuB.CYP79F1 is present (Varuna allele), 9–12 –F2 segregants where BjuB.CYP79F1 is absent (Heera allele).
Transgenic approach
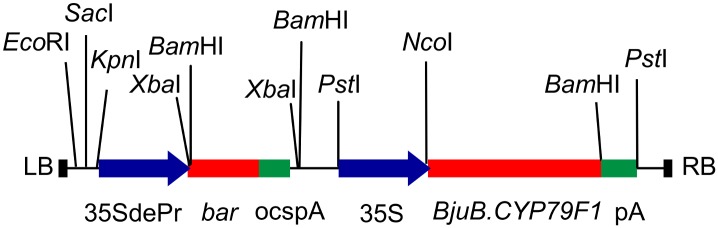
A gene construct for plant transformation was developed under the control of CaMV35S promoter and terminator that contained wild-type BjuB.CYP79F1 from Varuna in the binary vector pPZP200 (Fig 5). The bar gene, conferring phosphinothricin resistance and driven by double enhancer CaMV35S promoter was used as a selectable marker. The construct was introduced into electro-competent Agrobacterium tumefaciens strain GV3101. Two B. juncea lines, EH-2 (low seed GS content) and QTL-NIL J16Gsl4 (high seed GS content) both almost free from sinigrin and containing Heera allele of the gene, were used for transformation.
Fig 5. Map of T-DNA construct of BjuB.CYP79F1 used for genetic transformation of B. juncea.
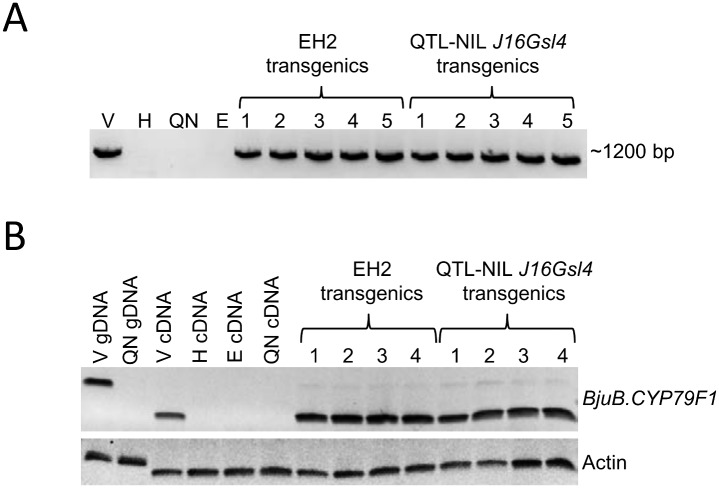
A total of 37 and 211 independent putative (T0) transgenic plants of EH-2 and QTL-NIL J16Gsl4, respectively, were transferred to a containment net house during the mustard growing season. Basta (200 mg l-1) was sprayed 30 days after transplantation. Basta resistant plants were grown to maturity and were allowed to set seeds through selfing as well as open pollination. The presence of the transgene and its expression were confirmed by PCR amplification of the genomic DNA (Fig 6A) and by RT-PCR of leaf mRNA (Fig 6B) respectively, from randomly selected T0 plants of QTL-NIL J16Gsl4 and EH-2. Control (untransformed) parental lines namely, Varuna, EH-2 and QTL-NIL J16Gsl4 were also grown in the containment net house for comparative seed GS profile analysis.
Fig 6.
(A) Gel picture showing amplification of BjuB.CYP79F1 (PCR using forward primer GS1B-NS-FP and reverse primer GS1B-NS-RP) in five T0 transgenics each of EH-2 and QTL-NIL J16Gsl4. (B) Expression analysis of BjuB.CYP79F1 from leaves of T0 transgenic lines by RT-PCR using the same primers as in Fig 4. V—Varuna, H—Heera, E—EH-2, QN—QTL-NIL J16Gsl4, gDNA—genomic DNA, cDNA—complementary DNA.
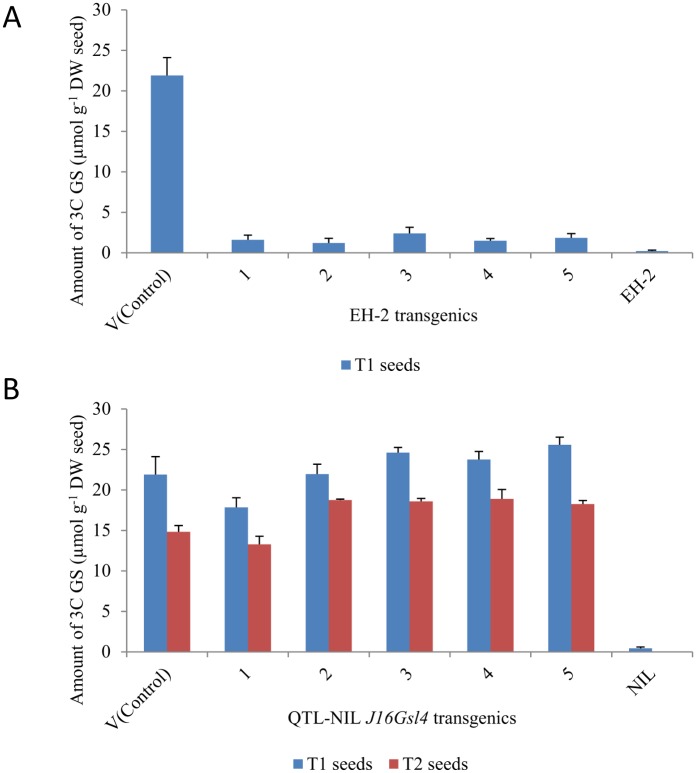
Open-pollinated T1 seeds from 20 randomly selected transgenic plants each from EH-2 and QTL-NIL J16Gsl4 along with the untransformed parental controls were analysed for total GS and profiles of aliphatic GS by HPLC. The aliphatic GS profiles of seeds from controls (with high GS) grown under containment net house showed an upward trend in the seed GS content (S5 Table) as compared to the plants grown in open field (S4 Table). We invariably, observe this trend between the field grown and net house grown plants as several environmental factors influence the GS production [2]. The EH-2 transgenic lines had low GS content conforming to the status of the wild type parent EH-2. It was also observed that none of these 20 transgenic lines showed significant increase in the sinigrin content as compared to the untransformed wild type. The range of sinigrin content varied from 0.2 to 2.4 μmol g-1 dry weight. It implies that even the presence of a functional copy(ies) of BjuB.CYP79F1 gene did not enhance synthesis of sinigrin in the low GS EH-2 transgenics. On the other hand, the range of sinigrin content in the 20 transgenic lines of QTL-NIL J16Gsl4 varied from 0.5 to 25.5 μmol g-1 dry weight wherein five transgenic lines showed sinigrin content varying from 17.8 to 25.5 μmol g-1 dry weight in the T1 seeds. Selfed T1 seeds of these five QTL-NIL J16Gsl4 transgenic lines showing high sinigrin content were grown in containment in the next growing season to obtain T2 seeds. After Basta (200 mg l-1) spraying, resistant segregants of each line were grown to maturity and selfed seeds from each line were harvested separately. Analysis of open-pollinated T2 seeds by HPLC revealed consistency in the sinigrin content and was comparable to the sinigrin content of the wild type B. juncea line Varuna (Fig 7, S5 Table). It implies that BjuB.CYP79F1 has effectively restored sinigrin biosynthesis in QTL-NIL J16Gsl4 line, which lacked only sinigrin in its otherwise high GS profile.
Fig 7. Graph showing seed 3C GS profile (T1 and T2 seeds) of five independent lines showing high sinigrin expression each from (A) EH-2 (T1 seeds only) and (B) QTL-NIL J16Gsl4 containing transgene 35S:BjuB.CYP79F1.
Discussion
Prior information on QTL mapping of the 3C aliphatic GS (J16Gsl4) to the LG B4 [18] and comparative mapping of B. juncea genome [21,34] along with the genome sequence data of both Arabidopsis and B. rapa helped in searching for the CYP79F1 gene in the QTL region. The genome search of B. rapa (AA) [27], B. oleracea (CC) [28] and B. napus (AACC) [29] revealed the presence of only CYP79F1 in all these Brassica species. The gene in the A genome of B. napus was observed to be tandemly duplicated. In the present study, we detected only CYP79F1 (BjuB.CYP79F1) from the B genome of B. juncea and also one each from B. nigra and B. rapa. It indicated that at least two copies of CYP79F1, one in each homoeolog, should be present in B. juncea.
The observed truncation of the gene BjuB.CYP79F1 in B. juncea line Heera (Fig 3) due to the insertion of a ~4.7 kb TE in the first exon led to the loss of function of the gene as no mRNA was detected either from the developing siliques or from the leaves (Figs 4 and 6B). It could be substantiated with the fact that Heera is a low GS line containing primarily 4C GS and only trace amount of sinigrin (3C GS). On the other hand, it was possible to isolate the full length gene of BjuB.CYP79F1 from the wild type B. juncea cv. Varuna that synthesizes sinigrin. The gene was almost identical to BniB.CYP79F1 (99.8%) of B. nigra and showed high sequence identity (>93%) with CYP79F1 of other Brassica species. The BjuB.CYP79F1 protein was predicted to be localized on the ER membrane inside the cell. It is known that methionine derivatives with carbon side chains are synthesized inside the chloroplast [35] and are translocated across the chloroplast membrane to CYP79F1 which is anchored on the ER [31]. High level of identity and similar domain structure deduced from the sequence of BjuB.CYP79F1 with the known and characterized CYP79F1 from A. thaliana suggests that these are orthologous genes and in all probability perform similar function.
Validation of BjuB.CYP79F1 (a B genome homoeolog of CYP79F1) as the gene responsible for regulating sinigrin biosynthesis in B. juncea was first undertaken by genotype-phenotype co-segregation analysis. All the Varuna x QTL-NIL J16Gsl4 F2 segregants containing the Varuna allele of the gene (wild type) showed synthesis of sinigrin while the segregants having Heera allele did not show synthesis of sinigrin (S6 Fig).
The final validation for the gene was obtained by genetic transformation of the line QTL-NIL J16Gsl4 with the gene construct containing wild type gene BjuB.CYP79F1. QTL-NIL J16Gsl4 is a high GS line having truncated BjuB.CYP79F1 allele from Heera and is devoid of sinigrin in the seed. The observation of the synthesis of sinigrin up to 25.5 μmol g-1 in the T1 transgenic seeds comparable to the sinigrin content of wild type line Varuna (21.9 μmol g-1 seed; Fig 7) is indicative of the fact that BjuB.CYP79F1 is the candidate gene regulating the synthesis of sinigrin in B. juncea. Similar trend of sinigrin synthesis in T2 transgenics indicated the stable inheritance of the trait. Moreover, the use of QTL-NIL J16Gsl4 that contains Heera allele for the locus J16Gsl4 and Varuna alleles for all other loci in GS pathway indicated that no other enzyme in the pathway produced by Varuna allele is compensating for the synthesis of sinigrin. We, therefore, propose that BjuB.CYP79F1 plays a major role in the conversion of homomethionine to 4-methylthiobutanaldoxime that leads to the synthesis of sinigrin according to the proposed GS pathway [1]. That some of the transgenic lines of QTL-NIL J16Gsl4 in the study did not show increase in the sinigrin content could be attributed to position effect causing low expression of the transgene in these lines. This type of gene expression variation is of general occurrence in transgenic research.
The EH-2 transgenics did not show any significant increase in sinigrin content over that of the wild type EH-2, although there is accumulation of transcript of the transgene (Fig 6B). EH-2 is a low GS line (~12 μmol g-1 DW of seed) having mutated MYB28, that controls the basal level synthesis of aliphatic GS [19,20]. Transgenic lines of EH-2, therefore, have limited availability of the substrates for GS core structure formation and modification and hence, remain low in sinigrin despite accumulation of BjuB.CYP79F1 transcripts. Such uncoupling of GS metabolite levels from the level of transcript accumulation for aliphatic GS biosynthetic genes has been shown in A. thaliana [36]. It has also been demonstrated that expression of most of the genes involved in aliphatic GS biosynthesis is repressed in Arabidopsis plants in which MYB28 was knocked out [37,38]. This fact has been put to practice in B. juncea by down regulating MYB28 through transgenic approach to develop low GS lines [39]. This corroborates with our observation on EH-2 transgenic lines.
Our results also lend credence to the earlier reports from Arabidopsis [31,40] regarding the involvement of the gene CYP79F1 (At1g16410) in modulating the level of short-chain methionine-derived aliphatic GS. Hansen et al. [40] reported that when CYP79F1 of Arabidopsis was heterologously expressed in E. coli, there was conversion of dihomo- and trihomomethionine to their corresponding aldoximes. In the same year, an Arabidopsis mutant bus1-1f that contained an En-1 insertion in the gene CYP79F1 revealed a complete lack of short-chain methionine-derived GS. Conversely, the overexpression of CYP79F1 in Arabidopsis led to an increased level of short-chain methionine-derived GS [31].
It was inferred from the present study on B. juncea and the sequence analyses of other Brassica species, that CYP79F2 is absent in all these cases. It has been demonstrated in A. thaliana that CYP79F1 catalyses synthesis of both short and long chain aliphatic GS, while CYP79F2 catalyses that of only long chain aliphatic GS [40,41]. Therefore the absence of CYP79F2 in Brassica species corresponds well with the fact that all profiles of aliphatic GS in brassicas are composed of short chain GS [27].
Some recent genomic studies provide indirect evidence in support of our findings. Comparative genome analysis using genome survey sequences identified non-synonymous SNPs in CYP79F1 between a sinigrin rich B. juncea var. tumida and a B. rapa line free of sinigrin [42]. The CYP79F1 expression was found to be upregulated in leaves of B. juncea as compared to B. rapa, suggesting difference in aliphatic GS content between B. juncea and B. rapa. However, it was not clear from the study whether the comparison of CYP79F1 of B. rapa was with CYP79F1 of the A or B genome of B. juncea. RNA-seq analysis of transcriptome and GS metabolism in a broccoli (B. oleracea var. italica) line rich in 4C GS could not detect orthologs for both CYP79F1 and CYP79F2 [43].
The present study resolved the genetic control of 3C GS and identified BjuB.CYP79F1 located in the LG B4 of B genome as the candidate gene responsible for the 3C GS biosynthesis in B. juncea. B. nigra (BB) is the provider of B genome in allopolyploid B. juncea (AABB) and contributes a functional 3C GS pathway to B. juncea because sinigrin is the predominant aliphatic GS fraction in B. nigra. On the other hand, the A genome contributor B. rapa (AA) does not synthesize sinigrin and hence does not contribute to sinigrin biosynthesis in B. juncea. The fact was earlier confirmed by QTL analysis by Ramchiary et al. [18] where 3C GS QTL was detected only in the B genome (J16Gsl4 in LG B4) and none in the A genome. Moreover, the observation of restoration of the phenotype in transgenic lines of NIL J16Gsl4 in the present study not only identified the CYP79F1 as the candidate gene for sinigrin biosynthesis in B. juncea but also revealed the fact that genes upstream and downstream to CYP79F1 in aliphatic GS biosynthesis are functional in NIL J16Gsl4. It could be one of the major reasons for not being able to map a B genome paralogue of GSL-PRO (a member of the MAM gene family involved in the side chain elongation of aliphatic GS and is an upstream gene to CYP79F1) to the NIL J16Gsl4 QTL region [19]. On the contrary, GSL-PRO has been proposed as the candidate gene controlling sinigrin biosynthesis in B. oleracea [15–17]. This points to the possible existence of two types of controls for 3C GS biosynthesis in Brassica species, one that operates at the chain elongation level in the C genome and the other that works at core GS structure formation level in the B genome.
The study also indicates that BjuB.CYP79F1 in all probability is involved in conversion of methionine substrate (homomethionine) to aldoxime in the 3C GS biosynthesis pathway. If so, it puts forth the obvious question—are there specific CYP79F1 homologs that are involved in the 4C and 5C pathways in B. juncea? A detailed molecular analysis of A genome specific CYP79F1 which has been shown to be functional from the transcriptome data of B. rapa [44] and B. juncea [34] might throw some light on the specificity of this homolog.
Natural germplasm of B. juncea can be classified into two chemo-types for aliphatic GS [5]. Depending on the end use of the product, different artificial selection pressures have been applied for the genetic improvement of this crop. In India, mustard is grown for edible oil purpose; hence yield components and oil content enhancement traits have been the focus for selection and no selection was done for GS types. Thereby all the Indian germplasm contain both 3C (sinigrin) and 4C (gluconapin) types of GS. On the other hand, in the western hemisphere, only east European type mustard is grown which is used as leafy vegetable and condiment and lines with beneficial sinigrin fraction was mainly selected. Hence from the breeding point of view, the candidate gene polymorphism in BjuB.CYP79F1 elaborated in the present study in B. juncea can be used as functional foreground selection marker for the efficient transfer of the trait (either high or low allele for sinigrin, as required) through marker-assisted breeding.
Supporting Information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We would like to thank Dr. Martin Trick from John Innes Centre for helping in annotation of BAC clones using Brassica BAC annotation pipeline. We also thank Dr. Naveen C. Bisht from National Institute of Plant Genome Research, New Delhi for critical scientific suggestions.
Abbreviations
- μmol g-1
Micromoles per gram
- 3C
Three-carbon
- 4C
Four-carbon
- 5C
Five-carbon
- cDNA
Complementary deoxyribonucleic acid
- cM
CentiMorgan
- cv
Cultivar
- CYP79F1
Cytochrome P450 monooxygenase 79F1
- CYP79F2
Cytochrome P450 monooxygenase 79F2
- DW
Dry weight
- ER
Endoplasmic reticulum
- GS
Glucosinolates
- LG
Linkage group
- mRNA
Messenger ribonucleic acid
- NIL
Near-isogenic line
- QTL
Quantitative trait locus
- RT-PCR
Reverse transcriptase polymerase chain reaction
- TE
Transposable element
Data Availability
All CYP79F1 sequence files from Brassica juncea cv. Varuna and Brassica nigra are available from the National Center for Biotechnology Information GenBank database (accession numbers KT254222 and KT254223). All other relevant data are within the paper and its Supporting Information files.
Funding Statement
The study was funded by the National Dairy Development Board (NDDB) (http://www.nddb.org/) (PF&A:DU:882) and also partly supported by the Department of Biotechnology (http://www.dbtindia.nic.in/), Government of India, through the award of a Centre of Excellence on Brassica breeding (BT/01/COE/08/06-II). MS acknowledges the receipt of research fellowship from the University Grants Commission (UGC) (http://www.ugc.ac.in/), India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Halkier BA, Gershenzon J. Biology and biochemistry of glucosinolates. Annu. Rev. Plant Biol. 2006; 57: 303–333. [DOI] [PubMed] [Google Scholar]
- 2.Ishida M, Hara M, Fukino N, Kakizaki T, Morimitsu Y. Glucosinolate metabolism, functionality and breeding for the improvement of Brassicaceae vegetables. Breed. Sci. 2014; 64: 48–59. 10.1270/jsbbs.64.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Srivastava A, Gupta V, Pental D, Pradhan AK. AFLP-based genetic diversity assessment amongst agronomically important natural and some newly synthesized lines of Brassica juncea. Theor. Appl. Genet. 2001; 102: 193–199. [Google Scholar]
- 4.Gland A, Röbbelen G, Thies W. Variation of alkenyl glucosinolates in seeds of Brassica species. Zeitschrift fuer Pflanzenzuechtung; 1981: 87. [Google Scholar]
- 5.Sodhi YS, Mukhopadhyay A, Arumugam N, Verma JK, Gupta V, Pental D, et al. Genetic analysis of total glucosinolate in crosses involving a high glucosinolate Indian variety and a low glucosinolate line of Brassica juncea. Plant breeding. 2002; 121: 508–511. [Google Scholar]
- 6.Wang T, Liang H, Yuan Q. Separation of sinigrin from Indian mustard (Brassica juncea L.) seed using macroporous ion-exchange resin. Korean J. Chem. Eng. 2012; 29: 396–403. [Google Scholar]
- 7.Jie M, Cheung WM, Yu V, Zhou Y, Tong PH, Ho JWS. Anti-proliferative activities of sinigrin on carcinogen-induced hepatotoxicity in rats. PLoS ONE. 2014; 9: e110145 10.1371/journal.pone.0110145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya A, Li Y, Wade KL, Paonessa JD, Fahey JW, Zhang Y. Allyl isothiocyanate-rich mustard seed powder inhibits bladder cancer growth and muscle invasion. Carcinogenesis. 2010; 31: 2105–2110. 10.1093/carcin/bgq202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel DK, Patel K, Gadewar M, Tahilyani V. A concise report on pharmacological and bioanalytical aspect of sinigrin. Asian Pac. J. Trop. Biomed. 2012; 2: S446–S448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okulicz M. Multidirectional time-dependent effect of sinigrin and allyl isothiocyanate on metabolic parameters in rats. Plant Foods Hum. Nutr. 2010; 65: 217–224. 10.1007/s11130-010-0183-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tungngoen K, Kongsawadworakul P, Viboonjun U, Katsuhara M, Brunel N, Sakr S, et al. Involvement of HbPIP2;1 and HbTIP1;1 aquaporins in ethylene stimulation of latex yield through regulation of water exchanges between inner liber and latex cells in Hevea brasiliensis. Plant Physiol. 2009; 151: 843–856. 10.1104/pp.109.140228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khokon MAR, Jahan MS, Rahman T, Hossain MA, Muroyama D, Minami I, et al. Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ. 2011; 34: 1900–1906. 10.1111/j.1365-3040.2011.02385.x [DOI] [PubMed] [Google Scholar]
- 13.Sotelo T, Lema M, Soengas P, Cartea ME, Velasco P. In vitro activity of glucosinolates and their degradation products against Brassica-pathogenic bacteria and fungi. Appl. Environ. Microbiol. 2015; 81: 432–440. 10.1128/AEM.03142-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magrath R, Bano F, Morgner M, Parkin I, Sharpe A, Lister C, et al. Genetics of aliphatic glucosinolates. I. Side chain elongation in Brassica napus and Arabidopsis thaliana. Heredity. 1994; 72: 290–299. [Google Scholar]
- 15.Li G, Gao M, Yang B, Quiros CF. Gene for gene alignment between the Brassica and Arabidopsis genomes by direct transcriptome mapping. Theor. Appl. Genet. 2003; 107: 168–180. [DOI] [PubMed] [Google Scholar]
- 16.Gao M, Li G, Potter D, McCombie WR, Quiros CF. Comparative analysis of methylthioalkylmalate synthase (MAM) gene family and flanking DNA sequences in Brassica oleracea and Arabidopsis thaliana. Plant Cell Rep. 2006; 25: 592–598. [DOI] [PubMed] [Google Scholar]
- 17.Gao M, Li G, Yang B, Qiu D, Farnham M, Quiros C. High-density Brassica oleracea linkage map: Identification of useful new linkages. Theor. Appl. Genet. 2007; 115: 277–287. [DOI] [PubMed] [Google Scholar]
- 18.Ramchiary N, Bisht NC, Gupta V, Mukhopadhyay A, Arumugam N, Sodhi YS, et al. QTL analysis reveals context-dependent loci for seed glucosinolate trait in the oilseed Brassica juncea: importance of recurrent selection backcross scheme for the identification of 'true' QTL. Theor. Appl. Genet. 2007; 116: 77–85. [DOI] [PubMed] [Google Scholar]
- 19.Bisht NC, Gupta V, Ramchiary N, Sodhi YS, Mukhopadhyay A, Arumugam N, et al. Fine mapping of loci involved with glucosinolate biosynthesis in oilseed mustard (Brassica juncea) using genomic information from allied species. Theor. Appl. Genet. 2009; 118: 413–421. 10.1007/s00122-008-0907-z [DOI] [PubMed] [Google Scholar]
- 20.Rout K, Sharma M, Gupta V, Mukhopadhyay A, Sodhi YS, Pental D, et al. Deciphering allelic variations for seed glucosinolate traits in oilseed mustard (Brassica juncea) using two bi-parental mapping populations. Theor. Appl. Genet. 2015; 128: 657–666. 10.1007/s00122-015-2461-9 [DOI] [PubMed] [Google Scholar]
- 21.Panjabi P, Jagannath A, Bisht NC, Lakshmi KP, Sharma S, Gupta V, et al. Comparative mapping of Brassica juncea and Arabidopsis thaliana using intron polymorphism (IP) markers: homoeologous relationships, diversification and evolution of the A, B and C Brassica genomes. BMC Genomics. 2008; 9: 113 10.1186/1471-2164-9-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi In Plant Mol. Biol. Manual. Gelvin SB and Schilperoot RA, Eds. Kluwer Academic Publishers, Springer; Netherlands: 1994; 183–190. [Google Scholar]
- 23.Van Ooijen JW. JoinMap 4 Software for the calculation of genetic linkage maps in experimental populations. Kyazma BV, Wageningen, Netherlands: 2006; 33. [Google Scholar]
- 24.Sharma S, Padmaja KL, Gupta V, Paritosh K, Pradhan AK, Pental D. Two plastid DNA lineages—Rapa/Oleracea and Nigra—within the tribe Brassicaceae can be best explained by reciprocal crosses at hexaploidy: Evidence from divergence times of the plastid genomes and R-block genes of the A and B genomes of Brassica juncea. PLoS ONE. 2014; 9: e93260 10.1371/journal.pone.0093260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jagannath A, Bandyopadhyay P, Mehra S, Arumugam N, Burma PK, Pental D. Agrobacterium-mediated genetic transformation of Brassica juncea In: Jaiwal PK and Singh RP (eds) Plant Genetic Engineering Volume 2: Improvement of Food Crops. Sci Tech Publishing LLC, U.S.A. 2003; 349–360. [Google Scholar]
- 26.Sønderby IE, Geu-Flores F, Halkier BA. Biosynthesis of glucosinolates—gene discovery and beyond. Trends Plant Sci. 2010; 15: 283–290. 10.1016/j.tplants.2010.02.005 [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Wu J, Sun S, Liu B, Cheng F, Sun R, et al. Glucosinolate biosynthetic genes in Brassica rapa. Gene. 2011; 487: 135–142. 10.1016/j.gene.2011.07.021 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IAP, et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nature Comm. 2014; 5: 3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalhoub B, Denoeud F, Liu S, Parkin IAP, Tang H, Wang X, et al. Early allopolyploid evolution in the post-neolithic Brassica napus oilseed genome. Science. 2014; 345: 950–953. 10.1126/science.1253435 [DOI] [PubMed] [Google Scholar]
- 30.Chapple C. Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998; 49: 311–343. [DOI] [PubMed] [Google Scholar]
- 31.Reintanz B, Lehnen M, Reichelt M, Gershenzon J, Kowalczyk M, Sandberg G, et al. bus, a bushy Arabidopsis CYP79F1 knockout mutant with abolished synthesis of short-chain aliphatic glucosinolates. Plant Cell. 2001; 13: 351–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldberg T, Hamp T, Rost B. LocTree2 predicts localization for all domains of life. Bioinformatics. 2012; 28: i458–i465. 10.1093/bioinformatics/bts390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rost B, Fariselli P, Casadio R. Topology prediction for helical transmembrane proteins at 86% accuracy. Prot. Sci. 1996; 5: 1704–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paritosh K, Gupta V, Yadava SK, Singh P, Pradhan AK, Pental D. RNA-seq based SNPs for mapping in Brassica juncea (AABB): synteny analysis between the two constituent genomes A (from B. rapa) and B (from B. nigra) shows highly divergent gene block arrangement and unique block fragmentation patterns. BMC Genomics. 2014; 15: 396 10.1186/1471-2164-15-396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroymann J, Textor S, Tokuhisa JG, Falk KL, Bartram S, Gershenzon J, et al. A gene controlling variation in Arabidopsis glucosinolate composition is part of the methionine chain elongation pathway. Plant Physiol. 2001; 127: 1077–1088. [PMC free article] [PubMed] [Google Scholar]
- 36.Sønderby IE, Burow M, Rowe HC, Kliebenstein DJ, Halkier BA. A complex interplay of three R2R3 MYB transcription factors determines the profile of aliphatic glucosinolates in Arabidopsis. Plant Physiol. 2010; 153: 348–363. 10.1104/pp.109.149286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gigolashvili T, Yatusevich R, Berger B, Müller C, Flügge UI. The R2R3-MYB transcription factor HAG1/MYB28 is a regulator of methionine-derived glucosinolate biosynthesis in Arabidopsis thaliana. Plant J. 2007; 51: 247–261. [DOI] [PubMed] [Google Scholar]
- 38.Hirai MY, Sugiyama K, Sawada Y, Tohge T, Obayashi T, Suzuki A, et al. Omics-based identification of Arabidopsis Myb transcription factors regulating aliphatic glucosinolate biosynthesis. Proc. Natl. Acad. Sci. USA. 2007; 104: 6478–6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Augustine R, Mukhopadhyay A, Bisht NC. Targeted silencing of BjMYB28 transcription factor gene directs development of low glucosinolate lines in oilseed Brassica juncea. Plant Biotechnol. J. 2013; 11: 855–866. 10.1111/pbi.12078 [DOI] [PubMed] [Google Scholar]
- 40.Hansen CH, Wittstock U, Olsen CE, Hick AJ, Pickett JA, Halkier BA. Cytochrome P450 CYP79F1 from Arabidopsis Catalyzes the Conversion of Dihomomethionine and Trihomomethionine to the Corresponding Aldoximes in the Biosynthesis of Aliphatic Glucosinolates. J. Biol. Chem. 2001; 276: 11078–11085. [DOI] [PubMed] [Google Scholar]
- 41.Chen S, Glawischnig E, Jørgensen K, Naur P, Jørgensen B, Olsen CE, et al. CYP79F1 and CYP79F2 have distinct functions in the biosynthesis of aliphatic glucosinolates in Arabidopsis. Plant J. 2003; 33: 923–937. [DOI] [PubMed] [Google Scholar]
- 42.Yang J, Song N, Zhao X, Qi X, Hu Z, Zhang M. (2014) Genome survey sequencing provides clues into glucosinolate biosynthesis and flowering pathway evolution in allotetrapolyploid Brassica juncea. BMC Genomics. 2014; 15: 107 10.1186/1471-2164-15-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao J, Yu X, Ma F, Li J. RNA-seq analysis of transcriptome and glucosinolate metabolism in seeds and sprouts of broccoli (Brassica oleracea var. italic). PLoS ONE. 2014; 9: e88804 10.1371/journal.pone.0088804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paritosh K, Yadava SK, Gupta V, Panjabi-Massand P, Sodhi YS, Pradhan AK, et al. RNA-seq based SNPs in some agronomically important oleiferous lines of Brassica rapa and their use for genome-wide linkage mapping and specific-region fine mapping. BMC Genomics. 2013; 14: 463 10.1186/1471-2164-14-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All CYP79F1 sequence files from Brassica juncea cv. Varuna and Brassica nigra are available from the National Center for Biotechnology Information GenBank database (accession numbers KT254222 and KT254223). All other relevant data are within the paper and its Supporting Information files.