Abstract
Hepatitis C virus (HCV) transmission between spouses remains poorly characterized, largely due to the limited availability of samples from the early stage of infection, as well as methodological constraints. A fifty-eight year-old male developed acute hepatitis C infection and his 53-year old spouse has been HCV-positive for over 10 years. Serum samples were collected from both at the time of acute hepatitis C diagnosis in male (baseline) and then at 9 and 13 months. Hypervariable region 1 (HVR1) and 5’ untranslated region (5’UTR) sequences were amplified and subjected to next generation sequencing (NGS) using a pyrosequencing platform. Genetic variants were inferred by Shorah reconstruction method and compared by phylogenetic and sequence diversity analysis. As the sequencing error of the procedure was previously determined to be ≤ 1.5%, the analysis was conducted with and without the 1.5% cut-off with regard to the frequency of variants. No identical HVR1 variants were identified in spouses at baseline and follow-up samples regardless whether the cut-off was applied or not. However, there was high similarity (98.3%) between a minor baseline donor variant (1.7% frequency) and the most abundant baseline recipient variant (62.5% frequency). Furthermore, donor and recipient strains clustered together when compared to 10 control subjects from the same area and infected with the same HCV subtype. There was an increase in HVR1 complexity (number of genetic variants) over time in both spouses. In contrast, the 5'UTR region was stable and of low complexity throughout the study. In conclusion, intrafamilial HCV transmission may be established by a very minor variant and investigation of this phenomenon requires high-sensitivity assays, such as NGS.
Introduction
Characterization of hepatitis C virus (HCV) transmission patterns remains challenging due to the long incubation periods, asymptomatic course of infection and the scarcity of samples from donor and recipient around the time of transmission [1, 2]. In up to 40% of HCV infections, no clear risk factor can be identified [3].
Horizontal transmission of HCV from spouse to spouse has been shown in a number of publications [4–8]. Such studies require HCV sequence comparison followed by phylogenetic analysis to verify the common ancestry of HCV strains [4]. However, HCV similarity assessment has usually been done by analyzing the consensus sequence only, and such an approach does not take into account high viral diversity [9]. Analysis of the entire spectrum of genetic variants (quasispecies) could be more informative particularly in the light of the high turnover rate of the virus, which may result in rapid changes in the spectrum of circulating viral variants. Furthermore, infection of the recipient is often initiated by a single donor variant [10].
Next-generation sequencing (NGS) techniques, which allow for the evaluation of a wide spectrum of quasispecies including minor variants, is uniquely suited for the investigation of transmission [11]. This approach has been successfully applied in a wide range of viral analyses including human papilloma virus (HPV) genotyping, characterization of HCV and human immunodeficiency virus (HIV) quasispecies, and detection of minor drug-resistant HIV, HCV, and hepatitis B virus (HBV) variants [12–15].
Recently, several studies approached in-depth analysis of hepatitis C virus transmission patterns, using single-genome or next-generation sequencing [16, 17]. However, these studies were focused on molecular identification of putative transmitter/founder variants and not on comparing viral populations of donor and recipient [18, 19].
The aim of the current study was to use NGS to analyze transmission and selection of HCV variants from chronically infected female spouse (donor) to her male spouse (recipient) who subsequently developed acute infection and chronic hepatitis C. We studied two different HCV genomic regions: HVR1 (hypervariable region 1) and 5’UTR (5’ untranslated region). HVR1 is a highly exposed fragment of envelope 2 glycoprotein and a major target for specific antiviral response and its variability facilitates immune evasion and reflects the immune pressure of the host [20]. In contrast, the highly conserved 5’UTR is a non-coding region harboring internal ribosomal entry site (IRES) and its variability impacts the efficiency of translation [21, 22]. Our study provides evidence for the selective transmission of a minor HCV variant and its subsequent rapid molecular evolution in the recipient.
Materials and Methods
The study involved serum samples from a 53-year old female (donor) who had documented chronic hepatitis C for 10 years (most likely due to iatrogenic infection) and her 58-year old male spouse who developed an acute infection evolving to chronic hepatitis (recipient). Both were infected by the same subtype 1b and samples were collected at baseline (October 2012), which was the time of acute infection in the male (month 0) and after 9 and 13 months. Some clinical and virological data on both spouses are presented in Table 1.
Table 1. Clinical and Virological Characteristics of Female and Male Spouses Infected with Hepatitis C Virus.
| Female (chronic hepatitis C) | Male (acute hepatitis C evolving to chronic hepatitis C)c | |||||
|---|---|---|---|---|---|---|
| Time | baseline | 9 months | 13 months | baseline | 9 months | 13 months |
| Age (years) | 53 | 58 | ||||
| Alanine aminotransferase levels [U L-1]; ref. values: 10–40 U L-1 | 50 | 58 | 69 | 1447 | >2000 | >1000 |
| Liver histologya staging | F0/F1 | F1 | ||||
| Viral load [IU mL-1]b | 0.23×106 | 1.43×106 | 5.24×106 | 0.21×106 | 0.27×106 | 4.13×106 |
aMETAVIR Histologic Scoring System [23].
bRoche Cobas Taqman HCV assay (Roche, Indianapolis, IN, USA).
cPatient was treated for acute HCV infection with interferon alfa-2b (Intron A, Schering Plough Corporation, Kenilworth, NJ, USA) for 6 months right after baseline sample collection.
Following recommendations the male spouse was treated for 6 months with interferon alfa-2b (Intron A, Schering Plough Corporation, Kenilworth, NJ, USA) 3 mln IU given daily for the first 4 weeks and three times per week thereafter for 20 weeks, but without success (Table 1). Except for the exposure to infected spouse, no other risk factors were identified.
Sera from 10 randomly selected, chronic hepatitis C patients were used as controls for phylogenetic and genetic distance analysis. These patients were of similar age, were infected with same HCV subtype, and were recruited at the same time as the study subjects. The study was approved by the Bioethical Committee of the Medical University of Warsaw (Approval Number KB/17/2013) and all patients provided written informed consent.
HVR1 and 5’UTR amplification
HVR1 and 5’UTR amplifications were performed as described previously [24, 25]. In brief, total RNA was extracted from 250 μl of serum by a modified guanidinium thiocyanate-phenol/chlorophorm method using Trizol (Life Technologies, Carlsbad, CA, USA). RNA was subjected to reverse transcription at 37°C for 30 minutes using AccuScript High Fidelity Reverse Transcriptase (Agilent Technologies, Santa Clara, CA, USA). A region of 175 nt encompassing HVR1 and 250 nt covering 5’UTR were amplified in two-step PCR using FastStart High Fidelity Taq DNA Polymerase (Roche, Indianapolis, IN, USA). Primers used for reverse transcription and first round HCV HVR1 amplification were as follows: 5′-CATTGCAGTTCAGGGCCGTGCTA-3′ (nt 1632–1610) and 5′-GGTGCTCACTGGGGAGTCCT-3′ (nt 1389–1408). Primers used for reverse transcription and first round HCV 5’UTR amplification were as follows: 5’-TGRTGCACGGTCTACGAGACCTC-3’ (nt 342–320) and 5’-RAYCACTCCCCTGTGAGGAAC-3’ (nt 33–55). Primers employed in the second round PCR contained tags recognized by GS Junior sequencing platform, standard 10-nucleotide multiplex identifiers and target-complementary sequence [24]. Target-complementary sequences of primers for the second round PCR for HVR1 amplification were as follows: 5′- TCCATGGTGGGGAACTGGGC-3′ (positions 1428–1447) and 5′-TGCCAACTGCCA TTGGTGTT-3′ (nt 1603–1584). Target-complementary sequences of primers for the second round PCR for 5’UTR amplification were as follows: 5’-ACTGTCTTCACGCAGAAAGCGTC-3’ (nt 57–79) and 5’-CAAGCACCCTATCAGGCAGTACC-3’ (nt 307–285).
Pyrosequencing
The amount of DNA equivalent to 3×107 amplicons was subjected to emulsion PCR using GS Junior Titanium emPCR Lib-A Kit (454 Life Sciences, Branford, CT, USA). Pyrosequencing was carried out according to the manufacturer’s protocol for amplicons using GS Junior System (454 Life Sciences).
Data analysis
Sequencing errors (mismatches, insertions and deletions) were corrected and haplotypes inferred using the program diri_sampler from the Shorah software (https://www1.ethz.ch/bsse/cbg/software/shorah) [26]. Haplotypes that had posterior probability > 95% and were represented by at least 10 reads were extracted with LStructure (https://github.com/ozagordi/LocalVariants/blob/master/src/LStructure.py).
Additionally, although error correction allows for a reliable estimation of variants at a lower frequency, we applied a 1.5% frequency cut-off to improve the specificity of our analysis [27]. As demonstrated in our earlier study based on sequencing of cloned HVR1 sequences, this particular cut-off value corresponds to the aggregate error of amplification and sequencing with the GS Junior platform [27]. Subsequently, 5’UTR and HVR1 haplotypes were aligned to the 1b HCV reference sequences (GenBank accession number AJ242654 and AJ406073, respectively) and the latter was translated into amino acid sequences by MEGA (Molecular Evolutionary Genetics Analysis), version 5.0 (http://www.megasoftware.net/) [28]. Phylogenetic trees of both regions were constructed according to the Maximum Likelihood method based on the Tamura-Nei model [29] using MEGA 5.0. Molecular clock analysis was performed in MEGA 5.0. Genetic diversity and distance parameters were assessed by DNA SP version 5 (http://www.ub.edu/dnasp/) and MEGA 5.0. Sequence similarity was compared using Clustal 2.1 Percent Identity Matrix (http://www.clustal.org/omega/) [30]. Amino acid sequence logos were generated by Web Logo (http://weblogo.berkeley.edu/) [31].
Results
Altogether, 71,923 reads were obtained from 6 analyzed samples, 13,410 for HVR1 and 58,513 for 5’UTR region. After reconstruction, 7 to 49 variants were inferred per sample for HVR1 and from 6 to 10 for 5’UTR. Application of the cut-off lowered the number of inferred variants as there were now 4 to 20 variants per sample for the HVR1 and from 1 to 2 for the 5’UTR. For the unrelated 10 chronic hepatitis C patients, 33,810 HVR1 reads were obtained (4 to 16 variants per sample).
HVR1 sequence variability
Similarity between spouses
No identical HVR1 variants were identified to be present in both spouses (either above or below the 1.5% cut-off value), but a minor baseline donor variant (1.7% frequency) was found to be closely related to all recipient variants present at baseline (97.1%–98.3% sequence similarity). Furthermore, recipient consensus sequence at baseline differed only by two nucleotide substitutions and one insertion when compared to the putative infecting variant of 1.7% frequency. Only one amino acid difference was present between the infecting minor variant and the recipient major variant (substitution S to A within the epitope for neutralizing antibodies, described below as Epitope 1 [32]).
Recipient male spouse
When employing the 1.5% cut-off, the number of variants in the male recipient increased from four (baseline) to five at month 9 and to seven at month 13. At baseline, the HVR1 population was composed of one predominant variant (62.5%), one variant of 24.7% frequency and two minor (defined as <10% frequency) variants. Sequence similarity of the predominant variant to other baseline variants was from 97.7%, to 98.9%. None of the variants present in the initial sample were found in the two follow-up samples, and only one variant was present both at 9 and 13 months (constituting 76.0% and 2.8% of the total, respectively). At 9 months, the frequency of predominant variant was 76.0% but at month 13 the population became more dispersed, with two most abundant variants constituting 52.8% and 23.0% of the population, and five minor variants. Nucleotide diversities per site within HVR1 populations are shown in Table 2. Genetic distances between HVR1 populations (intrahost) were 0.020 (baseline and month 9), 0.028 (month 9 and month 13) and 0.021 (baseline and month 13).
Table 2. Nucleotide Diversity Parameters of 5’UTR and HVR1 HCV Variants in Analyzed Spouses.
| 5’UTR | HVR1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Baseline (month 0) | Month 9 | Month 13 | Baseline (month 0) | Month 9 | Month 13 | ||||||
| Analysis of variants | Cut-off 1.5% | No cut-off | Cut-off 1.5% | No cut-off | Cut-off 1.5% | No cut-off | Cut-off 1.5% | No cut-off | Cut-off 1.5% | No cut-off | Cut-off 1.5% | No cut-off |
| Number of variants in female (donor) | 2 | 9 | 2 | 9 | 2 | 8 | 14 | 41 | 19 | 38 | 20 | 49 |
| Number of variants in male (recipient) | 1 | 10 | 1 | 6 | 1 | 7 | 4 | 7 | 5 | 11 | 7 | 17 |
| Number of nucleotide substitutionsa in female (donor) | 1 | 2 | 1 | 5 | 1 | 1 | 50 | 55 | 56 | 52 | 55 | 50 |
| Number of nucleotide substitutionsa in male (recipient) | 1 | 3 | 1 | 1 | 1 | 3 | 35 | 35 | 37 | 45 | 38 | 39 |
| Nucleotide diversity per sitea in female (donor) | 0.003 | 0.002 | 0.003 | 0.006 | 0.003 | 0.002 | 0.066 | 0.048 | 0.067 | 0.047 | 0.061 | 0.044 |
| Nucleotide diversity per sitea in male (recipient) | 0.004 | 0.002 | 0.004 | 0.001 | 0.004 | 0.004 | 0.082 | 0.055 | 0.080 | 0.056 | 0.062 | 0.038 |
Without the cut-off, the number of variants in the recipient increased from seven (baseline) to 11 at month 9 and 17 at month 13. At baseline, HVR1 population was composed of one predominant variant (62.5%), one variant of 24.7% frequency and five minor variants (defined as <10% frequency). Sequence similarity of the predominant variant to other baseline variants ranged from 97.1%, to 99.4%. None of the variants present in the initial sample was found in the follow-up samples, and only one variant was present both at month 9 and 13 (constituting 76.0% and 2.8%, respectively). At 9 months the frequency of predominant variant was 76.0% but at month 13 the population became more dispersed, with the two most abundant variants constituting 52.8% and 23.0% of the population, and 15 minor variants. These populations gave rise to a steep curve on the cumulative distribution plots (Fig 1). Nucleotide diversities per site within HVR1 populations are presented in Table 2. Genetic distances between HVR1 populations (intrahost) were 0.017 (baseline and month 9), 0.029 (month 9 and month 13) and 0.019 (baseline and month 13).
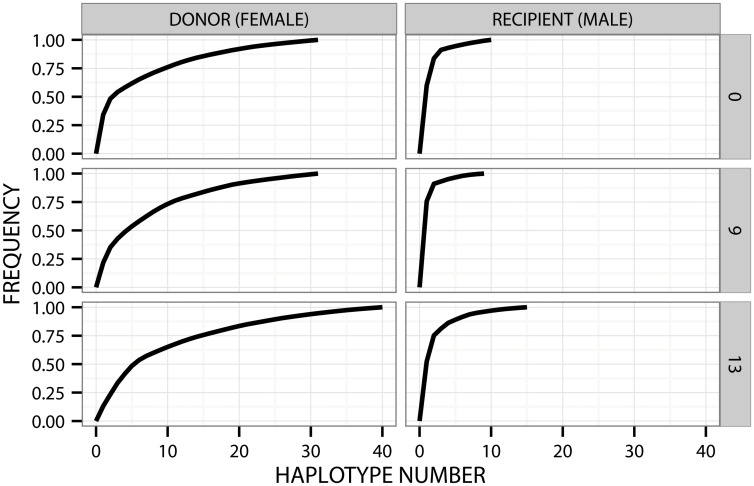
Fig 1. HVR1 quasispecies profile in both spouses at three different time points (0, 9 and 13 months).
On the X axis are the haplotypes ordered by decreasing frequency, while cumulative distribution is shown on Y axis. The flatter the curve, the more complex the quasispecies, with more haplotypes within the population. The male spouse was treated for 6 months with interferon starting immediately after the baseline serum was drawn, but the therapy was ultimately unsuccessful. Presented are haplotypes that had posterior probability > 95% and represent at least 10 reads.
Donor female spouse
The number of variants above the cut-off value of 1.5% increased in the donor during the follow-up from 14 (baseline) to 19 (month 9) and 20 (month 13). The baseline HCV population consisted of one predominant variant (33.2%), followed by a variant representing 13.6% and 12 minor variants. At 9 months, the contribution of minor variants increased to 17, while the frequency of the major variant declined to 20.9%. At 13 month, the population was even more dispersed with the most abundant variant constituting only 12.8% of the population and 18 different variants of lower frequency. Seven of the baseline variants were also present at month 9 (36.8% of population) and six baseline variants were still detectable at month 13 sample (30.0% of variants). The frequency of variants found in at least two donor samples ranged from 1.5% to 33.2%. Nucleotide diversity per site within HVR1 in the donor are presented in Table 2. Intrahost genetic distances between HVR1 populations were 0.051 (baseline vs month 9), 0.048 (month 9 vs month 13) and 0.050 (baseline vs month 13).
Genetic distance between donor’s and recipient’s baseline populations (interhost) was 0.054, remained the same at month 9 and increased to 0.057 at month 13.
When conducting the analysis without the cut-off, the number of variants in the donor fluctuated during the follow-up from 41 (baseline) to 38 (month 9) and 49 (month 13). The baseline HCV population consisted of one predominant variant (33.2%), followed by a variant representing 13.6% and 39 minor variants (< 10% each). At 9 months, the number of minor variants was 37, while the proportion of the major variant decreased to 20.9%. At 13 months the population was even more dispersed with the most abundant variant constituting only 12.8% of the population and the presence of 48 different minor variants. These populations resulted in a flatter curves on the cumulative distribution plots when compared to the recipient spouse (Fig 1). Eighteen of the baseline variants were also present at month 9 (47.4% of population) and 13 were still detectable at month 13 (26.5% of all variants). Variants found in at least two donor samples had frequencies of 0.3% to 33.2%. Nucleotide diversities per site in the donor are presented in Table 2. Intrahost genetic distances between HVR1 populations were 0.038 (baseline vs month 9), 0.041 (month 9 vs month 13) and 0.040 (baseline vs month 13).
Genetic distance between donor’s and recipient’s populations (interhost) were 0.037 at baseline, 0.041 at 9 months, and increased to 0.053 at 13 months.
5’UTR sequence variability
Recipient male spouse
When conducting the analysis with 1.5% cut-off, one predominant HCV variant, which was identical to one of donor baseline variants, was present in all samples (92.3%, 95.7% and 95.9% frequency).
Conducting the analysis without the cut-off did not change the frequency of the predominant variant, but there were now nine minor variants at baseline, five at 9 months and six at 13 months. Their frequency ranged from 0.1% to 1.4% of the total population.
Donor female spouse
Two variants of similar frequency (46.5% and 51.0%) were present in the donor spouse serum at baseline and during the follow-up period (49.4% and 47.2% at month 9); (54.4% and 41.7% at month 13). Interhost genetic distance between baseline populations was 0.002, and remained the same at month 9 and 13.
When conducting the analysis without the cut-off, the above two dominant variants were present at identical frequency at baseline (46.5% and 51.0%), at 9 months (49.4% and 47.2%), and at 13 months (54.4% and 41.7%). However, there were now also minority variants present: seven at baseline, seven at 9 months, and six at 13 months, ranging in frequence from 0.1% to 1.1%. Genetic distance between baseline populations (interhost) was 0.007, 0.006 at month 9 and 0.004 at month 13.
Minor variants in both spouses mostly differed by deletions and/or insertions in the homopolymeric cytosine region within 5’UTR (positions 127–129 of the AJ242654 reference genome) and at positions 66–71 (AJ242654 reference genome).
Phylogenetic analysis of HVR1 variants
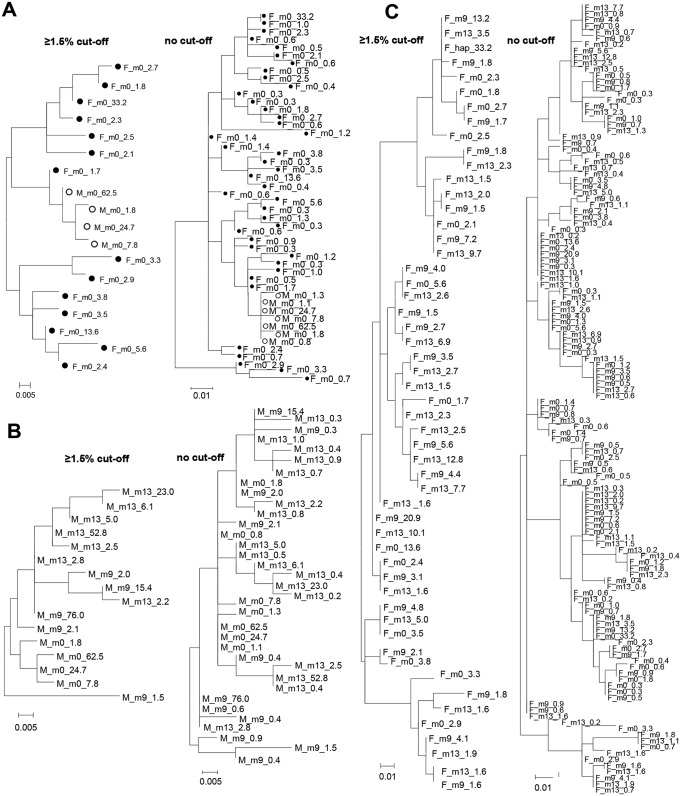
Phylogenetic analysis revealed that HVR1 recipient variants were highly similar to one minor donor variant of 1.7% frequency (Fig 2). Based on the estimated mutation rate of HVR1 sequence (8.6 × 10−2 substitutions per site per year, [33]), molecular clock analysis suggested that the major recipient (62.5%) variant had a common ancestor with the putative infecting variant of 1.7% frequency approximately 1.5 months prior to baseline sample. Furthermore, there was no clustering of variants from any particular time point.
Fig 2. Phylogenetic analysis of HVR1 variants.
(A) Variants present in serum at baseline (m0) in the female donor (F, black dot) and recipient male (M, open circle); (B) Variants present in serum over time of observation (m0, month 0; m9, month 9; m13, month 13) in the recipient (M); ultimately unsuccessful interferon monotherapy was given for six months starting immediately after drawing the baseline (m0) sample (C) variants present in the female donor (F) serum at baseline (m0), 9 months (m9) and 13 months (m13). Left panels show variants ≥ 1.5% cut-off, whereas the right panels show all reconstructed variants. Variant frequencies are expressed as percent values and follow time point of sample collection. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [29]. Evolutionary analyses were conducted using MEGA 5.0 [28].
Phylogenetic comparison of HVR1 with non-related chronic hepatitis C patients
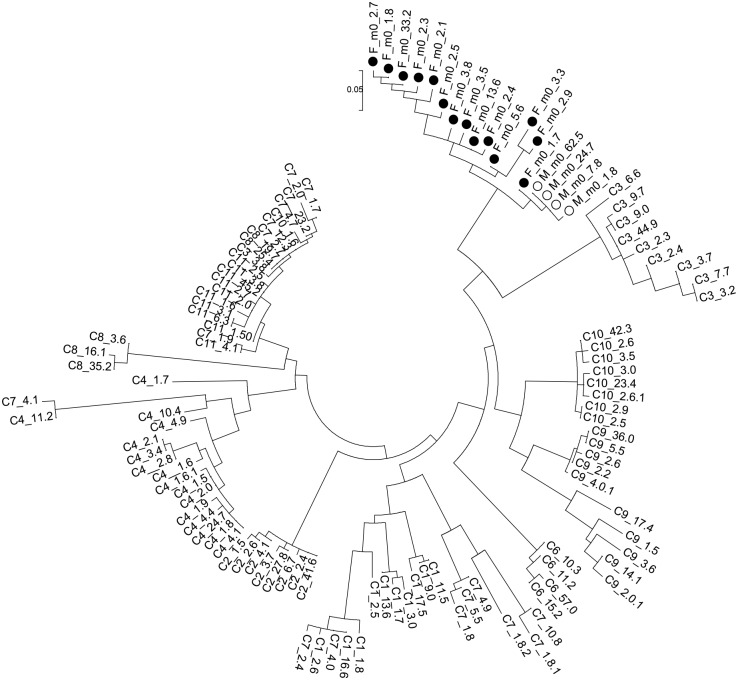
Baseline viral variants from both spouses were compared to sequences from 10 non-related chronic hepatitis C subjects (Fig 3). As seen, donor and recipient variants clustered together and were divergent from HCV variants in all 10 unrelated subjects. Mean distances between these control populations and those of the recipient were 0.280, 0.274, 0.294, 0.292, 0.341, 0.276, 0.316, 0.322, 0.242 and 0.211.
Fig 3. Phylogenetic analysis of HVR1 variants present in both spouses at baseline (m0) and 10 unrelated patients.
F, black dot denotes female spouse (F), while the open circle denotes the male spouse (M). Unrelated patients come from the same area and time and were infected with the same HCV subtype 1b. Variant frequencies are expressed as percent values and follow time point of sample collection. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [29]. Evolutionary analyses were conducted using MEGA 5.0 [28].
Evolution of HVR1 epitopes
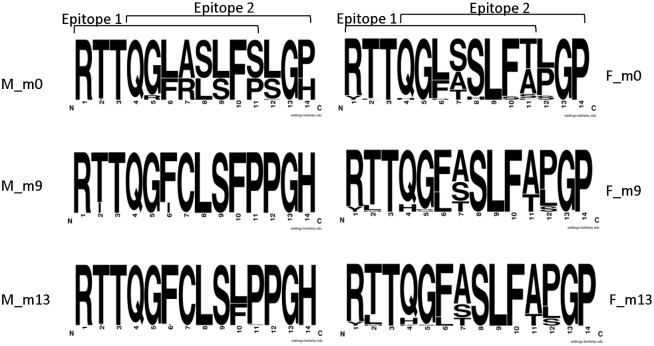
Close to the C-terminus of HVR1 amino acid sequence, two overlapping epitopes for neutralizing antibodies were described, encompassing amino acid positions 394–404 (Epitope 1) and 397–407 (Epitope 2) [32, 34]. In the recipient, the majority of these positions (57.1%) were homogeneous, i.e. composed of only one amino acid across the population. The remaining positions were heterogeneous: positions 2, 5, 6, 7, 8, 9, 11, 12, 14 at month 0, positions 2, 6, 7, 10 and 14 at month 9, and positions 2, 6, 10, 11 at month 13. Over time, nine positions were found to be unstable (change in amino acid composition: positions 5, 6, 7, 8, 9, 10, 11, 12 and 14 (Fig 4).
Fig 4. Amino acid sequence logos of HVR1 epitopes generated from populations of HVR1 sequence variants circulating in the male (M) and female (F) spouse.
Serum samples were collected at baseline (m0), and at 9 (m9) and 13 months (m13). The male spouse was treated for 6 months with interferon starting immediately after the baseline serum was drawn, but the therapy was ultimately unsuccessful. Epitope 1 comprises positions 1–11 (codon positions 394–404) and epitope 2 comprises positions 4–14 (397–407) [32, 34]. Height of letters within the stack indicates the relative frequency of each amino acid at this position. The analysis was conducted on variants ≥ 1.5% cut-off.
In the donor, 40.5% of positions were homogenous. Nine positions were heterogeneous at baseline (positions 1, 2, 4, 6, 7, 8, 10, 11, 12), eight at month 9 (positions 1, 2, 4, 5, 6, 7, 11, 12) and eight at month 13 (positions 1, 2, 4, 5, 6, 7, 11 and 12). Over the time of follow up, seven positions were unstable (positions 2, 4, 5, 6, 8, 10, 11).
Discussion
The increased risk of transmission from HCV-positive patients to household members, including siblings, parents, offspring as well as homo- and heterosexual partners, has been well documented [35–40]. It is of note, that the intrafamilial exposure to infection is high, as over 50% of the seronegative sexual partners of hepatitis C patients develop a specific cellular immune response against the virus without seroconversion or HCV-RNA presence in serum [41].
The actual prevalence of HCV among family members of infected patients was found to be very diverse ranging from 1.3% to 36.4% and depends on the studied population [38, 42]. In the majority of epidemiological studies, no analysis was done to confirm that partners were indeed infected with the same virus [36, 42–46], and even genotyping was done only occasionally [37, 39, 47]. However, genotyping is largely unreliable as it is likely to be similar for a given population in a certain area. Confirmation that a horizontal transmission event has occurred could be demonstrated by a high homology between the respective HCV genomes. Nevertheless, few of the previous studies included phylogenetic analysis [48–51] and sequence comparison was done only on the consensus sequence level [5, 51–53].
In our study phylogenetic analysis has demonstrated much more similarity between HCV strains in spouses than between unrelated subjects, supporting the occurence of intrafamilial transmission [8]. To the best of our knowledge, this study is the first using NGS to demonstrate intrafamilial spouse-to–spouse HCV transmission by a minor frequency variant. Already at baseline (acute infection in the male recipient), no identical HVR1 variants were present in spouses. However, high similarity (98.3%) of one out of the minor donor variants (1.7% frequency) to the most abundant recipient variant, as well as phylogenetic linkage and low interhost distance of baseline HVR1 populations when compared with 10 unrelated patients imply a common ancestry of donor and recipient variants. Indeed, the molecular clock analysis suggested a divergence from a common ancestor 1.5 months prior to baseline, which is compatible with the timing of infection.
Importantly, this minor donor variant would have been overlooked with the use of classical Sanger sequencing approach as it would require sequencing of 175 clones in order to detect a variant of 1.7% frequency with 95% probability. This observation is of special importance for transmission studies, because relationship traits between viral populations may be rapidly lost, especially when using Sanger-based techniques with the lowest detectable variants typically having ≥ 10–20% of frequency [18, 54, 55].
It must be noted that the frequency of the variant putatively transmitted to a new host (1.7%) was close to the applied 1.5% cut-off. This particular cut-off value corresponds to the error rate of the amplification and sequencing procedures determined previously by analysing of cloned HVR1 [27]. Of note, HVR1 and 5’UTR contain several homopolymeric regions (consecutive repeats of identical bases) and pyrosequencing chemistry is highly susceptible to errors at these regions [27, 56]. Importantly, despite repeating the analysis without application of the cut-off, no common HVR1 variants were detectable in both spouses.
The baseline HVR1 population found in the recipient was very narrow which is compatible with the bottleneck phenomenon and is consistent with some other studies in which only a single variant established infection [10, 18, 19]. In our study, the putative infecting variant constituted a small minority of the donor variants (1.7% of frequency) which suggests it may have had some major advantage in the new host. Interestingly, it contained high basic amino acid residues content (29.6%, data not shown) and the presence of basic residues in HVR1 have been previously shown to aid viral entry [57].
Despite the high sensitivity of NGS, it is impossible to determine the exact transmission route, but both sexual and household transmissions seem plausible. We collected cervical-vaginal lavage (CVL) to verify the presence of viral RNA, but neither 5’UTR nor HVR1 could be amplified, which is in line with some previous finding of low prevalence of HCV RNA in genital tract of HCV-monoinfected women [58]. Interestingly, among couples in long-term monogamous heterosexual relationships, the risk of sexual transmission of HCV has been assessed to be very low (0–0.6% per year) [59, 60] and has been even found to be null in a recent metaanalysis of more than 80 studies [61].
Molecular evolution of HCV HVR1 was strikingly different in the chronically infected donor and acutely infected recipient. Recipient's population showed increased complexity (number of variants) over time as well as change in variants composition, with only a few variants dominating the population. This change, called selective sweep, could be the result of selection pressures and is common for such pathogens as influenza [62], HCV [63, 64] and HIV [65]. Furthermore, there was a marked turnover of amino acids within the analyzed epitopes for neutralizing antibodies over time. This observation is compatible with immune selection pressure in acute infection.
After an unsuccessful antiviral treatment was attempted, the composition of variants was entirely changed (baseline vs 9 months). While interferon activates cellular rather than humoral response and thus would be expected to have a limited impact on the variability of the HVR1, rapid evolution of this region during therapy is common and predictive with respect to outcome [66–69]. However, a similar change occurred also between month 9 and month 13 when the patient was not receiving any antiviral treatment.
In contrast, the majority of female spouse variants were present in subsequent samples, most of them at a higher frequency. This could be due to virus adaptation and/or limited immune pressure possibly due to immune exhaustion, which is common in chronic HCV infection [70]. Similarly, amino acid composition within two HVR1 epitopes was largely conserved over time.
The 5’UTR sequence remained relatively stable, probably due to lack of selective pressures.
In conclusion, it seems that intrafamilial HCV transmission may be established by a very minor variant and thus the investigation of this phenomenon requires high-sensitivity assays, such as NGS.
Data Availability
All NGS files are available from the Zenodo database: https://zenodo.org/record/44605. DOI: 10.5281/zenodo.44605.
Funding Statement
This work was supported by grant 1M24/PM11/14 from the Medical University of Warsaw.
References
- 1.Cochrane A, Searle B, Hardie A, Robertson R, Delahooke T, Cameron S, et al. A genetic analysis of hepatitis C virus transmission between injection drug users. J Infect Dis. 2002;186(9):1212–21. Epub 2002/10/29. doi: JID020314 [pii] 10.1086/344314 . [DOI] [PubMed] [Google Scholar]
- 2.Gotz HM, van Doornum G, Niesters HG, den Hollander JG, Thio HB, de Zwart O. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS. 2005;19(9):969–74. Epub 2005/05/21. doi: 00002030-200506100-00015 [pii]. . [DOI] [PubMed] [Google Scholar]
- 3.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. The Lancet Infectious diseases. 2005;5(9):558–67. 10.1016/S1473-3099(05)70216-4 . [DOI] [PubMed] [Google Scholar]
- 4.Indolfi G, Nesi A, Resti M. Intrafamilial transmission of hepatitis C virus. J Med Virol. 2013;85(4):608–14. 10.1002/jmv.23522 . [DOI] [PubMed] [Google Scholar]
- 5.de Carvalho-Mello IM, Filho JE, Gomes-Gouvea MS, de Mello Malta F, de Queiroz AT, Pinho JR, et al. Molecular evidence of horizontal transmission of hepatitis C virus within couples. J Gen Virol. 2010;91(Pt 3):691–6. 10.1099/vir.0.015594-0 . [DOI] [PubMed] [Google Scholar]
- 6.Matthews GV, Pham ST, Hellard M, Grebely J, Zhang L, Oon A, et al. Patterns and characteristics of hepatitis C transmission clusters among HIV-positive and HIV-negative individuals in the Australian trial in acute hepatitis C. Clin Infect Dis. 2011;52(6):803–11. Epub 2011/02/02. 10.1093/cid/ciq200ciq200 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Indolfi G, Bartolini E, Azzari C, Becciolini L, Moriondo M, de Martino M, et al. Intrafamilial transmission of hepatitis C virus: infection of the father predicts the risk of perinatal transmission. J Med Virol. 2008;80(11):1907–11. Epub 2008/09/25. 10.1002/jmv.21316 . [DOI] [PubMed] [Google Scholar]
- 8.Plancoulaine S, Mohamed MK, Arafa N, Bakr I, Rekacewicz C, Tregouet DA, et al. Dissection of familial correlations in hepatitis C virus (HCV) seroprevalence suggests intrafamilial viral transmission and genetic predisposition to infection. Gut. 2008;57(9):1268–74. 10.1136/gut.2007.140681 . [DOI] [PubMed] [Google Scholar]
- 9.Moradpour D, Penin F, Rice CM. Replication of hepatitis C virus. Nature reviews Microbiology. 2007;5(6):453–63. 10.1038/nrmicro1645 . [DOI] [PubMed] [Google Scholar]
- 10.D'Arienzo V, Moreau A, D'Alteroche L, Gissot V, Blanchard E, Gaudy-Graffin C, et al. Sequence and functional analysis of the envelope glycoproteins of hepatitis C virus variants selectively transmitted to a new host. J Virol. 2013;87(24):13609–18. 10.1128/JVI.02119-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzon L, Lavezzo E, Costanzi G, Franchin E, Toppo S, Palu G. Next-generation sequencing technologies in diagnostic virology. J Clin Virol. 2013;58(2):346–50. 10.1016/j.jcv.2013.03.003 . [DOI] [PubMed] [Google Scholar]
- 12.Barzon L, Militello V, Lavezzo E, Franchin E, Peta E, Squarzon L, et al. Human papillomavirus genotyping by 454 next generation sequencing technology. J Clin Virol. 2011;52(2):93–7. 10.1016/j.jcv.2011.07.006 . [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann C, Minkah N, Leipzig J, Wang G, Arens MQ, Tebas P, et al. DNA bar coding and pyrosequencing to identify rare HIV drug resistance mutations. Nucleic acids research. 2007;35(13):e91 10.1093/nar/gkm435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verbinnen T, Van Marck H, Vandenbroucke I, Vijgen L, Claes M, Lin TI, et al. Tracking the evolution of multiple in vitro hepatitis C virus replicon variants under protease inhibitor selection pressure by 454 deep sequencing. J Virol. 2010;84(21):11124–33. 10.1128/JVI.01217-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solmone M, Vincenti D, Prosperi MC, Bruselles A, Ippolito G, Capobianchi MR. Use of massively parallel ultradeep pyrosequencing to characterize the genetic diversity of hepatitis B virus in drug-resistant and drug-naive patients and to detect minor variants in reverse transcriptase and hepatitis B S antigen. J Virol. 2009;83(4):1718–26. 10.1128/JVI.02011-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prosperi MC, De Luca A, Di Giambenedetto S, Bracciale L, Fabbiani M, Cauda R, et al. The threshold bootstrap clustering: a new approach to find families or transmission clusters within molecular quasispecies. PLoS One. 2010;5(10):e13619 Epub 2010/11/05. 10.1371/journal.pone.0013619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Stoddard MB, Wang S, Blair LM, Giorgi EE, Parrish EH, et al. Elucidation of hepatitis C virus transmission and early diversification by single genome sequencing. PLoS pathogens. 2012;8(8):e1002880 10.1371/journal.ppat.1002880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bull RA, Luciani F, McElroy K, Gaudieri S, Pham ST, Chopra A, et al. Sequential bottlenecks drive viral evolution in early acute hepatitis C virus infection. PLoS pathogens. 2011;7(9):e1002243 10.1371/journal.ppat.1002243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang GP, Sherrill-Mix SA, Chang KM, Quince C, Bushman FD. Hepatitis C virus transmission bottlenecks analyzed by deep sequencing. J Virol. 2010;84(12):6218–28. 10.1128/JVI.02271-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke KP, Cox AL. Hepatitis C virus evasion of adaptive immune responses: a model for viral persistence. Immunol Res. 2010;47(1–3):216–27. Epub 2010/01/13. 10.1007/s12026-009-8152-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhat P, Shwetha S, Sharma DK, Joseph AP, Srinivasan N, Das S. The beta hairpin structure within ribosomal protein S5 mediates interplay between domains II and IV and regulates HCV IRES function. Nucleic acids research. 2015. 10.1093/nar/gkv110 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, et al. Hepatitis-C-virus-like internal ribosome entry sites displace eIF3 to gain access to the 40S subunit. Nature. 2013;503(7477):539–43. 10.1038/nature12658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–32. . [DOI] [PubMed] [Google Scholar]
- 24.Caraballo Cortes K, Zagordi O, Laskus T, Ploski R, Bukowska-Osko I, Pawelczyk A, et al. Ultradeep pyrosequencing of hepatitis C virus hypervariable region 1 in quasispecies analysis. Biomed Res Int. 2013;2013:626083 Epub 2013/05/28. 10.1155/2013/626083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laskus T, Radkowski M, Wang LF, Nowicki M, Rakela J. Uneven distribution of hepatitis C virus quasispecies in tissues from subjects with end-stage liver disease: confounding effect of viral adsorption and mounting evidence for the presence of low-level extrahepatic replication. J Virol. 2000;74(2):1014–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zagordi O, Bhattacharya A, Eriksson N, Beerenwinkel N. ShoRAH: estimating the genetic diversity of a mixed sample from next-generation sequencing data. BMC Bioinformatics. 2011;12:119 Epub 2011/04/28. 10.1186/1471-2105-12-119 1471-2105-12-119 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cortes KC, Zagordi O, Perlejewski K, Laskus T, Maroszek K, Bukowska-Osko I, et al. Deep sequencing of hepatitis C virus hypervariable region 1 reveals no correlation between genetic heterogeneity and antiviral treatment outcome. BMC Infect Dis. 2014;14:389 10.1186/1471-2334-14-389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular biology and evolution. 2011;28(10):2731–9. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Molecular biology and evolution. 1993;10(3):512–26. . [DOI] [PubMed] [Google Scholar]
- 30.McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N, et al. Analysis Tool Web Services from the EMBL-EBI. Nucleic acids research. 2013;41(Web Server issue):W597–600. 10.1093/nar/gkt376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome research. 2004;14(6):1188–90. 10.1101/gr.849004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato N, Nakazawa T, Ootsuyama Y, Sugiyama K, Ohkoshi S, Shimotohno K. Virus isolate-specific antibodies against hypervariable region 1 of the hepatitis C virus second envelope protein, gp70. Japanese journal of cancer research: Gann. 1994;85(10):987–91. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herring BL, Tsui R, Peddada L, Busch M, Delwart EL. Wide range of quasispecies diversity during primary hepatitis C virus infection. J Virol. 2005;79(7):4340–6. 10.1128/JVI.79.7.4340-4346.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahid A, Dubuisson J. Virus-neutralizing antibodies to hepatitis C virus. J Viral Hepat. 2013;20(6):369–76. 10.1111/jvh.12094 . [DOI] [PubMed] [Google Scholar]
- 35.Kouyos RD, Rauch A, Boni J, Yerly S, Shah C, Aubert V, et al. Clustering of HCV coinfections on HIV phylogeny indicates domestic and sexual transmission of HCV. International journal of epidemiology. 2014;43(3):887–96. 10.1093/ije/dyt276 . [DOI] [PubMed] [Google Scholar]
- 36.Chang TT, Liou TC, Young KC, Lin XZ, Lin CY, Shin JS, et al. Intrafamilial transmission of hepatitis C virus: the important role of inapparent transmission. J Med Virol. 1994;42(1):91–6. . [DOI] [PubMed] [Google Scholar]
- 37.Nakashima K, Ikematsu H, Hayashi J, Kishihara Y, Mutsutake A, Kashiwagi S. Intrafamilial transmission of hepatitis-C virus among the population of an endemic area of Japan. Jama. 1995;274(18):1459–61. . [PubMed] [Google Scholar]
- 38.Noguchi S, Sata M, Suzuki H, Mizokami M, Tanikawa K. Routes of transmission of hepatitis C virus in an endemic rural area of Japan. Molecular epidemiologic study of hepatitis C virus infection. Scandinavian journal of infectious diseases. 1997;29(1):23–8. . [DOI] [PubMed] [Google Scholar]
- 39.Akhtar S, Moatter T, Azam SI, Rahbar MH, Adil S. Prevalence and risk factors for intrafamilial transmission of hepatitis C virus in Karachi, Pakistan. J Viral Hepat. 2002;9(4):309–14. . [DOI] [PubMed] [Google Scholar]
- 40.Paez Jimenez A, Sharaf Eldin N, Rimlinger F, El-Daly M, El-Hariri H, El-Hoseiny M, et al. HCV iatrogenic and intrafamilial transmission in Greater Cairo, Egypt. Gut. 2010;59(11):1554–60. 10.1136/gut.2009.194266 . [DOI] [PubMed] [Google Scholar]
- 41.Kamal SM, Amin A, Madwar M, Graham CS, He Q, Al Tawil A, et al. Cellular immune responses in seronegative sexual contacts of acute hepatitis C patients. J Virol. 2004;78(22):12252–8. 10.1128/JVI.78.22.12252-12258.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hajiani E, Masjedizadeh R, Hashemi J, Azmi M, Rajabi T. Hepatis C virus transmission and its risk factors within families of patients infected with hepatitis C virus in southern Iran: Khuzestan. World journal of gastroenterology: WJG. 2006;12(43):7025–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim YS, Chi HS, Ahn YO, Lee HS, Klag MJ. Lack of familial clustering of hepatitis C virus infection. International journal of epidemiology. 1998;27(3):525–9. . [DOI] [PubMed] [Google Scholar]
- 44.La Torre G, Miele L, Mannocci A, Chiaradia G, Berloco F, Gabrieli ML, et al. Correlates of HCV seropositivity among familial contacts of HCV positive patients. BMC public health. 2006;6:237 10.1186/1471-2458-6-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed MK, Abdel-Hamid M, Mikhail NN, Abdel-Aziz F, Medhat A, Magder LS, et al. Intrafamilial transmission of hepatitis C in Egypt. Hepatology. 2005;42(3):683–7. 10.1002/hep.20811 . [DOI] [PubMed] [Google Scholar]
- 46.Tahan V, Karaca C, Yildirim B, Bozbas A, Ozaras R, Demir K, et al. Sexual transmission of HCV between spouses. The American journal of gastroenterology. 2005;100(4):821–4. 10.1111/j.1572-0241.2005.40879.x . [DOI] [PubMed] [Google Scholar]
- 47.Minola E, Baldo V, Baldovin T, Trivello R, Floreani A. Intrafamilial transmission of hepatitis C virus infection. European journal of epidemiology. 2006;21(4):293–7. 10.1007/s10654-006-0016-8 . [DOI] [PubMed] [Google Scholar]
- 48.Rice PS, Smith DB, Simmonds P, Holmes E. Heterosexual transmission of hepatitis C virus. Lancet. 1993;342(8878):1052–3. . [DOI] [PubMed] [Google Scholar]
- 49.Skidmore SJ, Collingham KE, Drake SM. Brief report: sexual transmission of hepatitis C. J Med Virol. 1994;42(3):247–8. . [DOI] [PubMed] [Google Scholar]
- 50.Orlando R, Lirussi F. Hepatitis C virus infection: sexual or non-sexual transmission between spouses? A case report and review of the literature. Infection. 2007;35(6):465–8. 10.1007/s15010-007-6188-7 . [DOI] [PubMed] [Google Scholar]
- 51.Nguyen O, Sheppeard V, Douglas MW, Tu E, Rawlinson W. Acute hepatitis C infection with evidence of heterosexual transmission. J Clin Virol. 2010;49(1):65–8. 10.1016/j.jcv.2010.06.008 . [DOI] [PubMed] [Google Scholar]
- 52.Nakamura I, Tanaka Y, Ochiai K, Moriyasu F, Mizokami M, Imawari M. Clarification of interspousal hepatitis C virus infection in acute hepatitis C patients by molecular evolutionary analyses: Consideration on sexual and non-sexual transmission between spouses. Hepatology research: the official journal of the Japan Society of Hepatology. 2011;41(9):838–45. 10.1111/j.1872-034X.2011.00843.x . [DOI] [PubMed] [Google Scholar]
- 53.Cavalheiro Nde P, La Rosa A, Elagin S, Tengan FM, Barone AA. Hepatitis C virus: molecular and epidemiological evidence of male-to-female transmission. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2010;14(5):427–32. . [DOI] [PubMed] [Google Scholar]
- 54.Cruz-Rivera M, Carpio-Pedroza JC, Escobar-Gutierrez A, Lozano D, Vergara-Castaneda A, Rivera-Osorio P, et al. Rapid hepatitis C virus divergence among chronically infected individuals. Journal of clinical microbiology. 2013;51(2):629–32. 10.1128/JCM.03042-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A. 2008;105(21):7552–7. 10.1073/pnas.0802203105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilles A, Meglecz E, Pech N, Ferreira S, Malausa T, Martin JF. Accuracy and quality assessment of 454 GS-FLX Titanium pyrosequencing. BMC genomics. 2011;12:245 10.1186/1471-2164-12-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Callens N, Ciczora Y, Bartosch B, Vu-Dac N, Cosset FL, Pawlotsky JM, et al. Basic residues in hypervariable region 1 of hepatitis C virus envelope glycoprotein e2 contribute to virus entry. J Virol. 2005;79(24):15331–41. 10.1128/JVI.79.24.15331-15341.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nowicki MJ, Laskus T, Nikolopoulou G, Radkowski M, Wilkinson J, Du WB, et al. Presence of hepatitis C virus (HCV) RNA in the genital tracts of HCV/HIV-1-coinfected women. J Infect Dis. 2005;192(9):1557–65. 10.1086/491742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Terrault NA. Sexual activity as a risk factor for hepatitis C. Hepatology. 2002;36(5 Suppl 1):S99–105. 10.1053/jhep.2002.36797 . [DOI] [PubMed] [Google Scholar]
- 60.Vandelli C, Renzo F, Romano L, Tisminetzky S, De Palma M, Stroffolini T, et al. Lack of evidence of sexual transmission of hepatitis C among monogamous couples: results of a 10-year prospective follow-up study. The American journal of gastroenterology. 2004;99(5):855–9. 10.1111/j.1572-0241.2004.04150.x . [DOI] [PubMed] [Google Scholar]
- 61.Tohme RA, Holmberg SD. Is sexual contact a major mode of hepatitis C virus transmission? Hepatology. 2010;52(4):1497–505. 10.1002/hep.23808 . [DOI] [PubMed] [Google Scholar]
- 62.Koelle K, Cobey S, Grenfell B, Pascual M. Epochal evolution shapes the phylodynamics of interpandemic influenza A (H3N2) in humans. Science. 2006;314(5807):1898–903. 10.1126/science.1132745 . [DOI] [PubMed] [Google Scholar]
- 63.Sheridan I, Pybus OG, Holmes EC, Klenerman P. High-resolution phylogenetic analysis of hepatitis C virus adaptation and its relationship to disease progression. J Virol. 2004;78(7):3447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farci P, Shimoda A, Coiana A, Diaz G, Peddis G, Melpolder JC, et al. The outcome of acute hepatitis C predicted by the evolution of the viral quasispecies. Science. 2000;288(5464):339–44. . [DOI] [PubMed] [Google Scholar]
- 65.Shankarappa R, Margolick JB, Gange SJ, Rodrigo AG, Upchurch D, Farzadegan H, et al. Consistent viral evolutionary changes associated with the progression of human immunodeficiency virus type 1 infection. J Virol. 1999;73(12):10489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Wagner M, Lee JH, Ruster B, Kronenberger B, Sarrazin C, Roth WK, et al. Dynamics of hepatitis C virus quasispecies turnover during interferon-alpha treatment. J Viral Hepat. 2003;10(6):413–22. . [DOI] [PubMed] [Google Scholar]
- 67.Polyak SJ, McArdle S, Liu SL, Sullivan DG, Chung M, Hofgartner WT, et al. Evolution of hepatitis C virus quasispecies in hypervariable region 1 and the putative interferon sensitivity-determining region during interferon therapy and natural infection. J Virol. 1998;72(5):4288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pawlotsky JM, Germanidis G, Frainais PO, Bouvier M, Soulier A, Pellerin M, et al. Evolution of the hepatitis C virus second envelope protein hypervariable region in chronically infected patients receiving alpha interferon therapy. J Virol. 1999;73(8):6490–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Caraballo Cortes K, Laskus T, Bukowska-Osko I, Pawelczyk A, Berak H, Horban A, et al. Variability of hepatitis C virus hypervariable region 1 (HVR-1) during the early phase of pegylated interferon and ribavirin therapy. Advances in medical sciences. 2012;57(2):370–4. 10.2478/v10039-012-0024-8 . [DOI] [PubMed] [Google Scholar]
- 70.Penna A, Pilli M, Zerbini A, Orlandini A, Mezzadri S, Sacchelli L, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45(3):588–601. Epub 2007/02/28. 10.1002/hep.21541 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All NGS files are available from the Zenodo database: https://zenodo.org/record/44605. DOI: 10.5281/zenodo.44605.