Abstract
Obesity is considered detrimental to women's reproductive health. Although most of the attention has been focused on the effects of obesity on hypothalamic function, studies suggest a multifactorial impact. In fact, obesity is associated with reduced fecundity even in women with regular cycles, indicating that there may be local ovarian effects modulating fertility. Here we describe a novel mechanism for leptin actions directly in the ovary that may account for some of the negative effects of obesity on ovarian function. We find that normal cycling, obese, hyperleptinemic mice fed with a high-fat diet are subfertile and ovulate fewer oocytes compared with animals fed with a normal diet. Importantly, we show that leptin induces expression of the neuropeptide cocaine- and amphetamine-regulated transcript (CART) in the granulosa cells (GCs) of ovarian follicles both in vitro and in vivo. CART then negatively affects intracellular cAMP levels, MAPK signaling, and aromatase mRNA expression, which leads to lower estradiol synthesis in GCs and altered ovarian folliculogenesis. Finally, in human samples from patients undergoing in vitro fertilization, we show a significant positive correlation between patient body mass index, CART mRNA expression in GCs, and CART peptide levels in follicular fluid. These observations suggest that, under obese conditions, CART acts as a local mediator of leptin in the ovary to cause ovarian dysfunction and reduced fertility.
Obesity is an epidemic and contributes to a large number of diseases, including impaired fertility (1). Although the negative impacts of obesity on female fecundity are well documented (2, 3), the mechanism(s) that links obesity to reduced fertility are yet to be fully elucidated. The etiologies of obesity related infertility are myriad; however, one common underlying feature associated with obesity is hyperleptinemia. Although a critical amount of leptin is essential for normal fertility (2, 3), obesity-induced hyperleptinemia is associated with both anovulatory infertility and ovulatory subfertility. Anovulatory infertility is generally linked with central obesity and perturbations to the hypothalamic-pituitary-ovarian axis that result in menstrual cycle disturbances and oligo-/anovulation (4–6). However, normal cycling obese women with no obvious reasons for fertility problems also have a propensity of lower conception rates, a poor response to infertility treatment, and an increased risk of early pregnancy loss (7). Little is known about the basis of this ovulatory subfertility (8, 9); however, local leptin signaling in the ovary is thought to be one potential underlying cause, because leptin has been shown to inhibit ovarian steroidogenesis through yet unknown mechanisms. In summation, it is now clear that the adverse effects of obesity on female reproductive health cannot be exclusively accounted for by central leptin actions in the brain (10–14) but are more likely due to the cumulative effects of leptin in the brain and ovary.
In this study we show that the adverse actions of elevated leptin levels associated with obesity are mediated, at least in part, through a neuro-peptide called cocaine- and amphetamine-regulated transcript (CART) in the ovary. CART possesses well-characterized biological activity in the hypothalamus and in other regions of the brain, along with the pituitary (15). Numerous pleiotropic actions of the CART peptide, including anorexigenic (16–19), neuroendocrine (20–24), and antipsychostimulant (25, 26) effects have been described in the brain across species. The gastrointestinal tract (27) and pancreatic cells (28, 29) are the prominent nonneural sites of CART actions. Ovarian CART expression was first reported in the bovine ovary (30), and intraovarian CART expression was shown to be associated with poor health status of follicles. Moreover, studies in the bovine ovary demonstrated CART to be a potent negative regulator of FSH and IGF-1 actions in vitro (31), and an inhibitor of follicular estradiol production in vivo (32).
Here we show that CART is significantly expressed in the ovarian granulosa cells (GCs) of obese mice fed with high-fat diet (HFD) relative to mice fed with normal diet (ND). Leptin promotes CART expression in GCs, both in vivo and in vitro, and CART subsequently inhibits GC aromatase mRNA expression and steroidogenesis. Furthermore, exogenous administration of CART has many of the same adverse effects on fertility as obesity or leptin treatment. Finally, we report a significant positive correlation between CART mRNA expression in GCs and CART protein levels in follicular fluids with body mass index (BMI) in women undergoing in vitro fertilization (IVF). Thus, our data suggest that under obese conditions, leptin induces CART expression in the ovary, which then inflicts adverse effects on ovarian functions that lead to fertility problems.
Materials and Methods
Animals and cell culture
Mouse studies were performed in accordance with the guidelines for the care and use of Laboratory animals and were approved by the University Committee on Animal Resources at the University of Rochester. Five-week-old C57BL/6J mice (The Jackson Laboratory) were fed with ND or HFD (60% kcal fat diet, Takelad custom research diet; Harlan Laboratories) for 12 weeks, and their weights were measured every other week (n = 35 animals/diet group). Estrous cycle was determined by vaginal smears as described previously (33) for the last 2 weeks of feeding. Only 19 animals were cycling normally after the HFD regime, and these were selected for further studies. Nineteen normally cycling mice fed with ND were used as corresponding controls. Collection and culture of mouse GCs were performed as previously described (34). For both in vitro and in vivo studies, CART treatment involved rat CART peptide 55–102 (Fisher Scientific), and inactive CART peptide 55–76 was used as control.
Assays
Leptin assay
Serum leptin levels (n = 10 animals/diet group) were measured by Leptin mouse ELISA kit (Abcam) with a detectable range of 4.1–1000 pg/mL as per the manufacturer's instruction. The intraassay coefficient of variation was 7.8%.
cAMP assay
Intracellular cAMP levels were measured by cAMP competitive ELISA (Thermo Scientific) as per the manufacturer's instructions. The intraassay coefficient of variation was 8.6%.
Estradiol assay
Estradiol levels were measured by a 17-β estradiol ELISA kit (Abcam) with a detectable range of 20–2000 pg/mL as per the manufacturer's instructions. The intraassay coefficient of variation was 5.5%.
CART peptide assay
CART peptide levels in follicular fluids isolated during oocyte retrieval from women undergoing IVF and CART peptide levels in mouse primary GC culture media were measured by a CART EIA (RayBio) as per the manufacturer's instructions. The intraassay coefficients of variations were 8.3% and 6.8% for human follicular fluid and mouse primary GC culture media, respectively.
FSH levels
Serum FSH levels were measured by the Center for Research in Reproduction, Ligand Assay and Analysis Core at University of Virginia School of Medicine. The intraassay coefficient of variation was 2.7%.
Fertility test, oocyte count, and follicle count
To evaluate the reproductive performance of these ND- and HFD-fed animals (n = 9 animals/diet group), a continuous mating study was performed for 12 weeks as described previously (33). Throughout the fertility test, animals were kept on ND or HFD. Weights were measured at the start and end of the fertility test. For oocyte counts, estrous cycling was again determined by vaginal smears for 2 weeks after the mating experiment, and thereafter the number of ovulated oocytes was determined at estrus. Oocyte/cumulus masses were surgically isolated from the oviduct and counted as reported previously (34). For follicle counting, ovaries (n = 3 ovaries from 3 different animals/diet group) were paraffin embedded, processed for sectioning (5-μm sections taken at 30-μm intervals), and stained with hematoxylin and eosin for morphological analysis using previously published criteria (33, 34). Briefly, follicle numbers were evaluated as: 1) primordial follicles, identified by an oocyte partially or completely encapsulated by flattened squamous cells; 2) primary follicles, single layer of cuboidal GCs around the oocyte; 3) secondary-tertiary follicles (preantral), 2 layers or more of cuboidal GCs (no antrum); 4) antral follicles, presence of an antrum; and 5) corpus luteum. Follicles were considered atretic based on any of the 2 criteria: 3 or more pyknotic nuclei or atretic bodies (depending on the follicle type) in GC layers or follicular antrum, GCs pulling away from basement membrane, broken basement membrane, uneven GC layer, and nonintact oocyte or nucleus. The data are represented as percentage of follicles.
For experiments involving CART in vivo injections, estrous cycling by daily vaginal smears was determined for 1 week before treatments as well as during one week of CART treatment and number of ovulated oocytes was determined at estrus.
CART knockdown experiments
Small interfering RNA-mediated CART knockdown experiments were performed as described (35) using nontargeting siRNA pool or ON-TARGET plus SMARTpool mouse CARTPT siRNA (catalog number L055671–00) according to manufacturer's instructions (Thermo Fisher Scientific).
Western blot analysis
Western blottings were performed as described previously (36). Samples were separated on 12.5% sodium dodecyl sulphate-polyacrylamide gel, and primary antibodies used were rabbit polyclonal p44/42-MAPK3/1 (catalog number 9102L) and phospho-p44/42-MAPK3/1 (T202/Y204, catalog number 9101) at 1:1000 dilution (Cell Signaling Technology) (see Table 1). Western blotting data was quantified by densitometric analysis as described previously (37). Briefly, phosphorylated and total MAPK3/1 levels were determined using computer-aided densitometry, and MAPK3/1 activation expressed as relative increase in phosphorylated/total MAPK3/1 vs media (M).
Table 1.
Antibody Table
| Name of Antibody | Manufacturer, Catalog Number | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|
| p44–42 MAPK (ERK1/2) | Cell Signaling, catalog number 9102 | Rabbit; polyclonal | 1:1000 WB |
| Phospho-p44/42 MAPK | Cell Signaling Technology | Rabbit | 1:1000 |
RNA extraction and real-time PCR
RNA from GCs was isolated by using RNeasy mini kit (QIAGEN) according to the manufacturer's instructions and levels of Cart, aromatase (Cyp19a1) and GAPDH mRNA expression were analyzed by Δ/Δ curve threshold (Ct) method using TaqMan gene expression assay primers (assay ID Mm00484049_m1-Cyp19a1, Mm00489086_m1-CARTPT and Mm03302249_g1-GAPDH; Hs04187545_g1-CARTPT, Hs00899423_g1-CARTPT and Hs03929097_g1-GAPDH; Applied Biosystems) and ABI StepOnePlus Real-Time PCR machine; 1 μg of RNA was used for all the RT-PCR reactions.
Collection of human GC and follicular fluid samples
Follicular fluids were obtained from oocyte aspiration during routine IVF procedure, and clumps of GCs were isolated from the follicular fluid after oocyte retrieval under dissecting scope using a Pasteur pipette. Thereafter, GCs were washed and subjected to 60%-percoll gradient to remove red blood cells and frozen in liquid nitrogen for RNA isolation. Moreover, follicular fluid was centrifuged to remove tissue/cell debris and blood clots and frozen for further analysis. In total, n = 45 patient samples across different BMIs (normal BMI, 16; overweight, 12; and obese, 17 samples) were used for measuring CART mRNA levels. For the CART peptide levels in follicular fluid the total number of patient samples used was 11 (normal BMI, 3; overweight, 3; and obese, 5).
Statistical analysis
Each in vitro experiment was repeated at least 3 times or more, and data are displayed as mean ± SEM. Statistical analysis was performed using Prism version 6 (GraphPad). ANOVA was used to detect differences between treatments. P ≤ .05 was considered significant.
Results
Diet-induced obesity causes subfertility in normally cycling mice
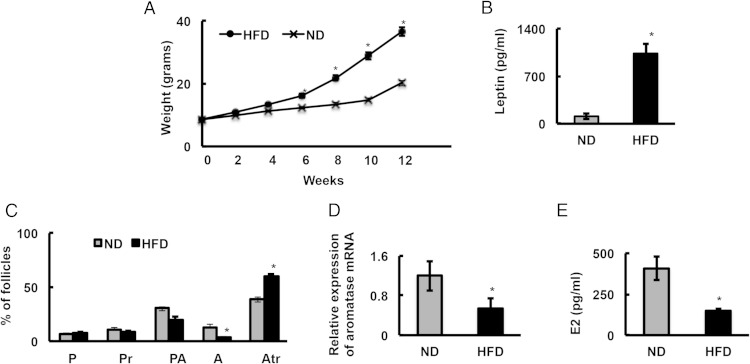
To begin to separate central from peripheral effects of obesity on fertility, we placed mice on a HFD and selected obese mice that were still cycling normally, as determined by examination of vaginal smears. Figure 1 shows the weights (Figure 1A) and final serum leptin levels (Figure 1B) of 5-week-old female mice fed with HFD or ND for 12 weeks. After 12 weeks, mice on HFD not only weighed significantly more, but also had higher serum leptin levels than mice on ND. To determine whether normally cycling obese mice still had reduced fertility, we subjected these ND- and HFD-fed animals to a 12-week-long fertility test. At the start of the fertility test, the ND and HFD mice on average weighed 26.52 ± 3.2 and 41.8 ± 6.3 g, respectively. After the 12-week fertility test, the average weights of the mice were 34.2 ± 4.7 and 65.2 ± 8.3 g, respectively. Our results show that the obese animals were subfertile, as indicated by smaller litter size (Table 2), and ovulated fewer oocytes (Table 3). In contrast, the number of litters per animal (n = 9) and FSH levels were unchanged between ND and HFD mice (6.2 ± 1 vs 9 ± 1.8 ng/mL). These observations further suggest that the observed subfertility was due to ovarian and not hypothalamic effects of obesity.
Figure 1.
Granulosa cells from ovaries of obese mice have inherent defects with respect to follicular physiology. A, Five-week-old mice (n = 18 animals/diet group) were fed with ND or HFD (60% kcal fat diet) for 12 weeks, and their weights were measured every other week. B, Serum leptin levels (n = 10 animals/diet group) were determined after the 12-week diet; *, P ≤ .05. C, Histological analysis of ovarian morphology of HFD and ND mice (n = 3 ovaries from 3 different mice/diet). P, primordial; Pr, primary; PA, preantral; A, antral; Atr, atretic. D, Aromatase mRNA levels in GCs isolated from ND and HFD mouse ovaries determined by real-time PCR (n = 10 animals/diet group). E, Estradiol levels extracted from ovaries of ND and HFD mice (n = 10 animals/diet group); *, P ≤ .05.
Table 2.
Fertility Test
| n | Pups | Litter | Pups/Litter | Litter/Female | |
|---|---|---|---|---|---|
| ND | 9 | 247 | 31 | 8 ± 0.5 | 3.4 ± 0.2 |
| HFD | 9 | 141 | 31 | 4.6 ± 0.6a | 3.4 ± 0.2 |
Normally cycling obese mice are subfertile. To evaluate the reproductive performance of the normal diet (ND) and high fat diet (HFD)-fed female mice, a continuous mating study for 12 weeks was performed. Two females were housed with 1 6- to 10-week-old fertile wild-type male mouse. The male was replaced every 2 weeks with other fertile male. Cages were monitored daily, and the number of pups and litters was recorded.
P ≤ .05.
Table 3.
Number of Ovulated Oocytes
| n | Ovulated Oocytes | |
|---|---|---|
| ND | 7 | 9.3 ± 0.7 |
| HFD | 7 | 5 ± 0.4a |
Estrous cycle of normal diet (ND) and high fat diet (HFD) mice was determined by daily vaginal smears for 2 weeks, and thereafter, normally cycling animals were killed at estrus. Oocyte/cumulus masses were surgically isolated from the oviduct and counted. Notably, the observed smaller litter size in the fertility test is reflective of the fewer ovulated oocytes.
P ≤ .05.
To gain further insight into the reduced fertility in normal cycling obese mice, ovarian morphology was examined. Although there was no difference in the percentage of primary, primordial, and preantral follicles between HFD and ND mice, HFD mice had significantly fewer antral and more atretic follicles compared with ND mice (Figure 1C). Moreover, aromatase mRNA levels (Figure 1D) in GCs and serum estradiol levels (Figure 1E) from HFD animals were significantly lower compared with ND control animals. These results demonstrate that GCs from ovaries of obese animals may have inherent defects with respect to follicular physiology.
Obesity-induced hyperleptinemia promotes CART expression in mouse GCs
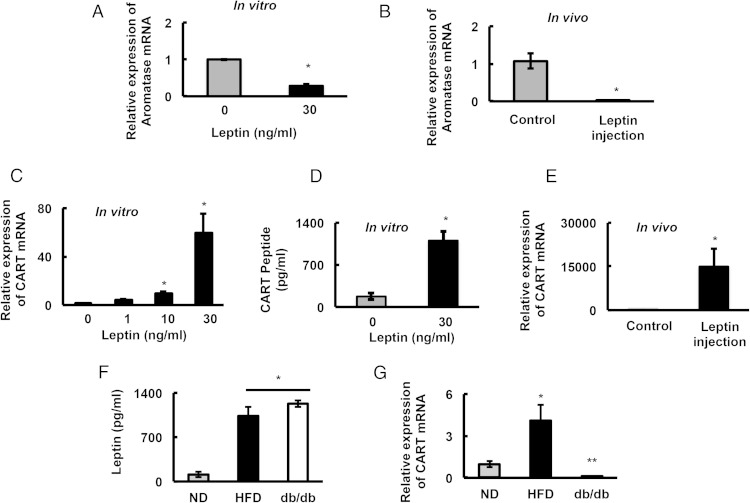
Given that the HFD-fed obese animals had high leptin levels, and based on previous studies showing negative leptin effects on ovarian physiology (38–42), we hypothesized that the observed subfertility in cycling obese mice was due to direct leptin effects on follicular development. In fact, similar to these previous studies, we found that leptin significantly inhibited aromatase (Cyp19a1) mRNA expression both in isolated primary GCs from normal mice (in vitro) (Figure 2A) and in ovaries from normal mice injected with leptin in vivo (Figure 2B).
Figure 2.
Leptin inhibits aromatase mRNA levels and induces CART expression in the ovaries. Aromatase expression in (A) primary culture of mouse GCs (n = 3) isolated from ND mice treated with leptin for 18 hours and in (B) GCs isolated from ovaries of ND mice (n = 5 animals/treatment) injected (ip) with vehicle or 30 mg of leptin/d for 1 week. CART mRNA levels in primary culture of mouse GCs (n = 3) isolated from ND mice treated with different concentrations of leptin for 18 hours (C) and CART (55–102) peptide levels in the media of mouse primary culture GCs (n = 3) treated with/without leptin (30 ng/mL) (D). E, CART mRNA levels in GCs isolated from ovaries of ND mice (n = 5 animals/treatment) injected (ip) with vehicle or 30 mg of leptin/d for 1 week. Serum leptin levels (n = 10 animals/diet group) (F) and CART mRNA expression in GCs (n = 14 animals/diet group) (G) of ND and HFD mice compared with leptin receptor-deficient mice (db/db mice, n = 3 animals). Data are normalized to GAPDH; *, P ≤ .05.
Interestingly, in the brain, leptin is known to induce the expression of the neuropeptide CART (16). Furthermore, CART is reported to suppress aromatase expression and negatively regulate follicle development in bovine GCs (32). Thus, we postulated that leptin might be inducing the expression of CART in mouse GCs, which in turn suppresses aromatase expression and thereby alters normal follicle development. Indeed, CART expression in GCs from ND mice was virtually undetectable. However, leptin induced a marked increase in Cart mRNA expression (Figure 2C) and CART peptide levels (measured in culture media) (Figure 2D) in GCs, in vitro. In addition, in vivo leptin administration promoted GC Cart mRNA expression in ND mice (Figure 2E).
To further demonstrate that leptin receptor signaling directly induces CART expression in the ovary, we determined Cart mRNA expression in GCs of db/db mice lacking the leptin receptor. Notably, serum leptin levels in the db/db and wild-type mice on a HFD were similar but were significantly higher compared with mice on a ND (Figure 2F). In contrast, although Cart mRNA levels were markedly elevated in GCs from obese mice on HFD, they were virtually undetectable in ND mice or db/db mice (Figure 2G). These observations establish a direct relationship between obesity induced hyperleptinemia, leptin receptor expression, and GC CART expression.
CART suppresses aromatase expression in GCs and attenuates ovulation
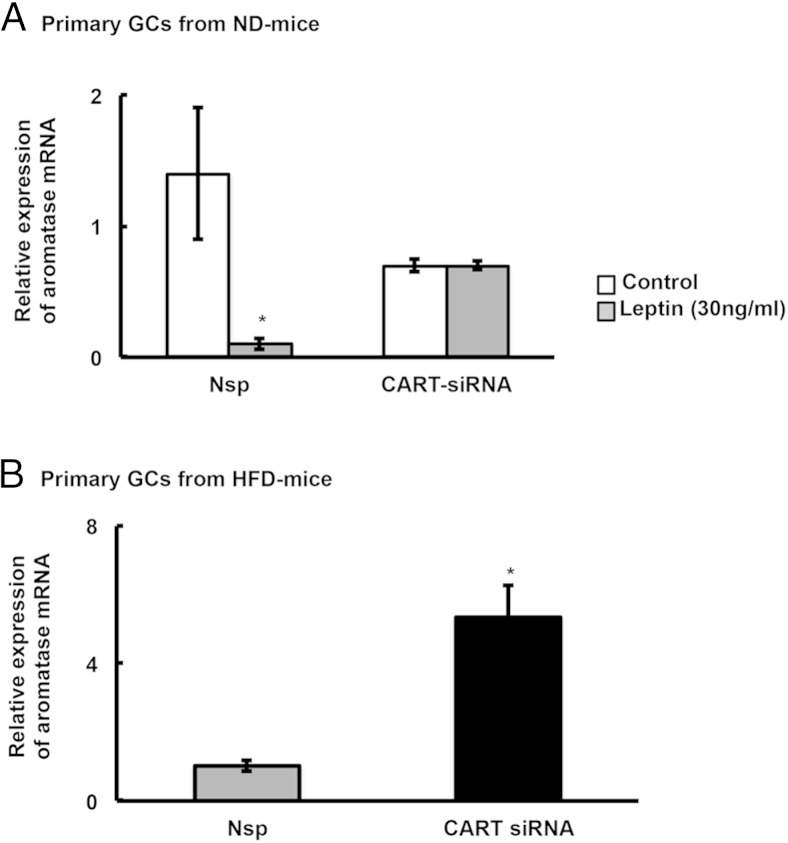
Because leptin promotes CART expression in mouse GCs, and because CART suppresses aromatase expression in bovine GCs (32), we examined whether leptin effects on aromatase expression in primary mouse GCs might be mediated via CART. Therefore, we treated primary GCs from ND-fed mice with nonspecific (Nsp) or CART-specific siRNAs, stimulated the cells with leptin, and then measured expression of aromatase mRNA. As expected, leptin stimulated Cart mRNA expression (Supplemental Figure 1A) and suppressed aromatase mRNA expression in GCs from ND mice treated with Nsp siRNAs. In contrast, in GCs treated with CART-specific siRNA, Cart mRNA expression remained low after leptin stimulation (Supplemental Figure 1A), and leptin was no longer able to suppress aromatase mRNA expression (Figure 3A). This suggests that CART is a key mediator of the negative actions of leptin in the ovary. Accordingly, siRNA-mediated knockdown of CART expression in GCs isolated from ovaries of HFD mice (Supplemental Figure 1B) exhibited increased aromatase expression compared with Nsp siRNA treated GCs from the same ovaries (Figure 3B). Together, these results suggest that the inherent defects in follicular physiology observed in obese animals (Figure 1, C–E) is likely due to obesity-induced hyperleptinemia regulating CART expression.
Figure 3.
Leptin- or HFD-induced inhibition of aromatase expression is mediated through CART. A, Primary GCs from normal mice were cultured in vitro and transfected with CART or non-specific (Nsp) siRNA and knockdown of CART mRNA was confirmed by real-time PCR as shown in Supplemental Figure 1. GC cultures were then treated for 18 hours with/without leptin (30 ng/mL), and aromatase expression was measured by real-time PCR. B, siRNA-mediated knockdown of CART expression rescues aromatase expression in GCs isolated from HFD-treated obese mice. Data are normalized to GAPDH and represented as mean of 3 separate experiments; *, P ≤ .05 vs Nsp-control.
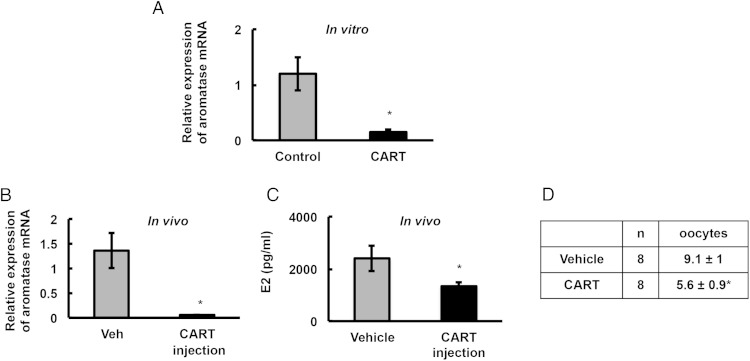
To further prove that CART is detrimental to follicular physiology, we treated ND animals with CART (55–102) peptide both in vitro and in vivo. In vitro treatment of GCs from ND animals with CART peptide significantly decreased aromatase mRNA expression (Figure 4A). Similarly, CART peptide injected into ND animals in vivo significantly reduced aromatase mRNA expression (Figure 4B) as well as estradiol levels (Figure 4C). Furthermore, similar to cycling animals on HFD, ND animals injected with CART ovulated significantly fewer oocytes compared with vehicle treated animals (Figure 4D). Because CART expression is low in the normal mouse ovary, our data suggest a direct relationship between hyperleptinemia, ovarian CART expression, dysfunctional follicular development, and subfertility associated with obesity.
Figure 4.
In vitro and in vivo CART treatment disrupts follicular physiology. A, Primary mouse GC cultures from ND mice were treated with 0.1μM CART (55–102) peptide for 18 hours, and aromatase mRNA level was determined by real-time PCR. Data are normalized to GAPDH and represented as mean of 3 separate experiments; *, P ≤ .05. CART (55–102) peptide (50 ng/mouse) or vehicle (0.2 mL/mouse) was injected (ip) for 1 week in ND-fed mice (n = 8 animals); and GC aromatase mRNA expression (B), estradiol levels (C), and number of ovulated oocytes (D) were determined; *, P ≤ .05.
CART inhibits cAMP and MAPK3/1 signaling in GCs
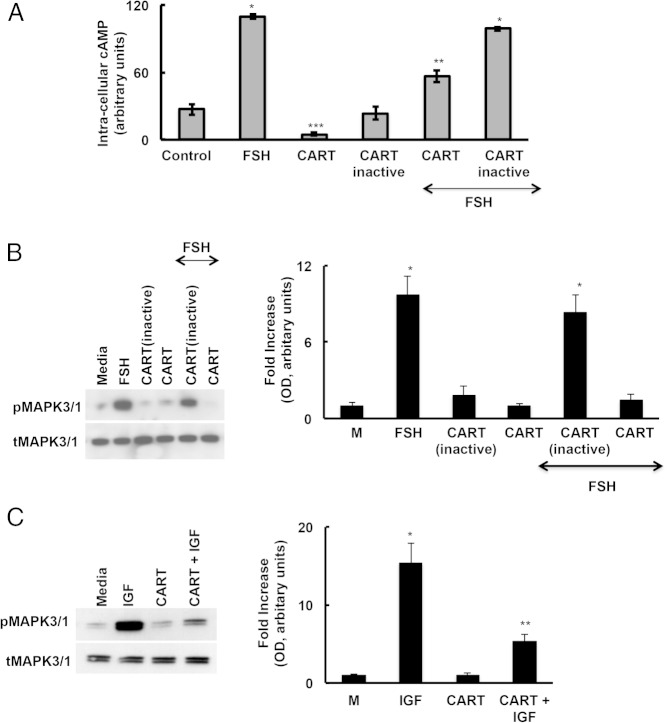
Because CART suppresses aromatase expression in GCs, and aromatase expression in vitro and in vivo is FSH regulated, we hypothesized that CART might be suppressing FSH-induced signaling. Therefore, we examined CART effects on FSH-induced intracellular cAMP levels and MAPK3/1 activation in primary GCs isolated from ND animals (Figure 5). We found that CART partially reduced FSH-induced cAMP production relative to an inactive CART peptide (Figure 5A). However, CART also reduced basal intracellular cAMP levels relative to GCs treated with inactive CART peptide (Figure 5A), suggesting that the negative effects of CART may be due to general suppression of cAMP production, rather than specifically effecting FSH-induced cAMP production.
Figure 5.
CART inhibits cAMP and pMAPK3/1 signaling in GCs. Primary GCs isolated from ND mice were pretreated with CART (55–102) or an inactive CART (negative control) peptide for 15 minutes followed by FSH or IGF stimulation (30 min). Intracellular cAMP (A) and total and phosphorylated (B and C) MAPK3/1 levels were determined by ELISA and Western blot analysis, respectively. Western blottings were quantified by densitometric analyses where phospho-MAPK3/1 levels were normalized to corresponding total-MAPK3/1 levels, and data are displayed as mean ± SEM of fold increase relative to media (M). Data are represented as mean of 3 separate experiments; P ≤ .05.
In contrast, CART treatment significantly decreased FSH-induced MAPK3/1 activation with no effect on basal levels (Figure 5B). To determine whether this mechanism was specific to FSH-induced signaling, we investigated CART effects on IGF-induced MAPK3/1 activation. Similar to FSH, CART inhibited IGF-induced MAPK3/1 phosphorylation (Figure 5C), again suggesting that CART might be a universal inhibitor of MAPK3/1 signaling rather than a specific inhibitor of FSH-induced signaling.
Correlation between BMI and CART levels in human samples
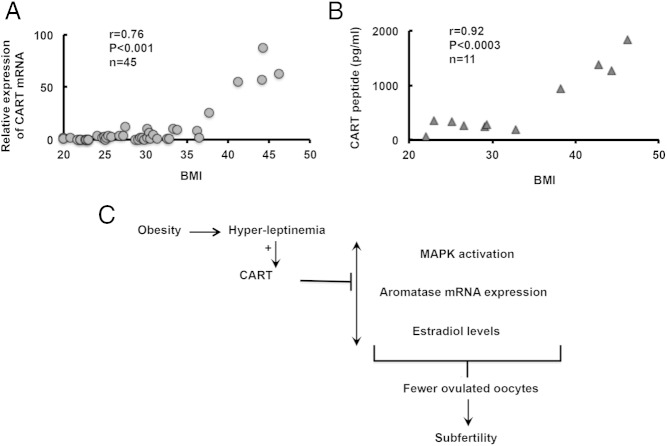
To determine whether there is a relationship between GC CART expression and BMI in humans, we extended our investigation to human GCs isolated during oocyte retrieval from patients undergoing IVF. Results (Figure 6A) show that although there were detectable levels of CART mRNA (average Ct value 27.5) in GCs from women with normal BMI (BMI, 19–24.9), CART mRNA expression was significantly higher in overweight (BMI, 25–29.9; average Ct value, 20.9) and obese (BMI, ≥30; average Ct value, 18.8) women. Similarly, CART peptide levels (Figure 6B) in follicular fluid from women with normal and overweight BMI were in the range of 250.5 ± 95.2 and 261 ± 14.2 pg/mL, respectively. In contrast, in obese women, the follicular fluid CART peptide levels were significantly elevated, in the range of 1125.2 ± 274.9 pg/mL and directly proportional with CART mRNA levels. Notably, we found a significant positive correlation between CART levels and BMI, especially in obese women with more than or equal to 30 BMI.
Figure 6.
Correlation between body mass index (BMI) and follicular CART levels in humans. GCs and follicular fluids were isolated during oocyte retrieval in IVF patients and (A) CART mRNA in GCs and (B) CART peptide in follicular fluids were determined by real-time PCR and ELISA, respectively; and correlated with corresponding patient BMI. Each circle or triangle represents 1 patient. C, Proposed model of CART-mediated obesity-related infertility.
Discussion
This study shows that elevated leptin levels linked with obesity induce the expression of the neuropeptide CART in the GCs of ovarian follicles. CART then inflicts adverse effects on intracellular cAMP levels, MAPK signaling and aromatase gene expression. These responses then lead to lower estradiol synthesis and fewer ovulated oocytes, resulting in subfertility (Figure 6C). Results thus raise the possibility that leptin-induced CART expression in the ovary may account in part for adverse effects of obesity on female reproductive health.
Although the central effects of leptin have been examined extensively (10–14), the contribution of direct leptin actions in the ovary in obesity-related fertility/subfertility remains controversial. However, several studies have reported direct local leptin effects on ovarian functions. Leptin receptors are expressed in all the follicular cell types (43–46), and their expression patterns fluctuate with estrous cycle (47, 48). Moreover, in vitro (49) and in vivo (50), leptin treatment has a negative effect on ovarian steroidogenesis (38–42) in different species (51), and leptin is an important regulator of follicle development (52). Importantly, in patients undergoing IVF, low serum and follicular fluid leptin levels have been associated with a high number of “good-quality” embryos, normal peak estradiol levels, and high implantation and pregnancy rates (53, 54). Despite all of these observations, the underlying mechanism(s) in the ovary by which obesity-induced hyperleptinemia exacerbates subfertility/infertility remains unclear. Our results here partly provide a mechanistic insight into the previously reported observations of direct leptin effects in the ovary and establish CART as a mediator of leptin-induced adverse actions in the ovary.
The intraovarian expression of CART was first discovered while generating a bovine oocyte cDNA microarray (30). Although intraovarian CART expression was initially documented in the oocyte, the GC layer is the prominent source of CART expression in the bovine ovary (55). CART is expressed in antral, but not preantral follicles in the bovine ovary, suggesting that local CART action is restricted to follicles that have undergone advanced stages of differentiation (55, 56). Interestingly, studies by others (56) and us (data not shown) find that CART expression is either undetectable or expressed in very low levels in ovaries of polytocous farm animals and rodents. In contrast, CART mRNA is expressed in bovine and human GCs. Intriguingly, studies in the bovine GC show that CART may play a critical role in the selection of a single dominant follicle during folliculogenesis (56); thus, the physiological function of ovarian CART expression may be to regulate the species-specific ovulatory quota in monotocous species such as cattle and humans. In contrast, polyovulatory species such as rodents may require a less stringent selection mechanism to ovulate multiple follicles, which may provide an evolutionary explanation for the absence or low expression of CART in the ovary of polytocous species (56). In accordance with this hypothesis, our results show that, although ND-fed mice have low levels of CART expression, obesity-associated or exogenous leptin can induce CART expression in the mouse ovary. Moreover, our in vitro studies in mouse GCs, as well as the correlation study between BMI and CART expression in IVF patients, suggest that there may be a threshold for leptin-induced ovarian CART expression. Under normal conditions, leptin concentrations may be below this threshold, with obesity-induced hyperleptinemia shifting the threshold towards elevated CART expression. This induction of CART expression then lowers the number of ovulated oocytes, as observed in obese mice, or disrupts the follicular selection process in obese women, thereby contributing to the subfertility associated with obesity.
Studies in the bovine reported CART as a potent negative regulator of FSH and IGF-1 actions in GCs in vitro (32, 37), and a potent inhibitor of follicular estradiol production in vivo (31). The inhibitory actions of CART on FSH signaling and estradiol production may be dependent on the Go/i-subclass of inhibitory G proteins and have been linked to multiple components of the FSH signal transduction pathway that ultimately result in reduced aromatase (Cyp19a1) mRNA expression and subsequent estradiol production (37). Moreover, in the bovine model, CART expression was found to be greater in atretic vs healthy follicles (55). Although we found similar effects of CART in our HFD-induced obese mouse model, our results show that CART appears to be a general inhibitor of cAMP production and MAPK signaling, regardless of the stimulant. In fact, in the bovine studies, CART effects on basal cAMP levels and/or CART actions on other agonists were not investigated. Regardless of the species, however, increased CART expression in the ovary is detrimental to follicular development and female fertility. Notably, the receptor for CART is still not known, which is a major limitation towards developing strategies to inhibit CART effects.
The positive correlation between CART mRNA and CART peptide levels in GCs and follicular fluid, respectively, relative to patient BMI, demonstrates that our observation in the mouse model holds true in humans. However, in contrast to mice, mono-ovulatory humans have higher basal ovarian CART levels even in normal weight women. These CART levels are then significantly higher in overweight and obese patients. Therefore we propose that in women, although CART likely acts as a gatekeeper for follicle selection, too much of CART (in case of obesity) prevents follicles from selection, preovulatory development, and ovulation.
In summary, here, we report a novel intracellular mechanism of action for leptin in the ovary that accounts for most of known negative effects of obesity on ovarian function. Our results in mouse and human samples show that obesity-associated elevated leptin levels induce the expression of CART in follicular GCs that in turn negatively alters GC function affecting follicular development, leading to subfertility. Thus, ovarian CART expression may be a potential biomarker for obesity-related fertility problems and may possess predictive value for IVF outcomes in obese patients.
Acknowledgments
This work was supported by the Foundation for Human Reproduction (A.S.) and the National Institutes of Health Grant R01 GM101709 (to S.R.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CART
- cocaine- and amphetamine-regulated transcript
- Ct
- curve threshold
- GC
- granulosa cell
- HFD
- high-fat diet
- IVF
- in vitro fertilization
- ND
- normal diet
- Nsp
- nonspecific
- siRNA
- small interfering RNA.
References
- 1. Zain MM, Norman RJ. Impact of obesity on female fertility and fertility treatment. Womens Health (Lond Engl). 2008;4:183–194. [DOI] [PubMed] [Google Scholar]
- 2. Pandey S, Pandey S, Maheshwari A, Bhattacharya S. The impact of female obesity on the outcome of fertility treatment. J Hum Reprod Sci. 2010;3:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kulie T, Slattengren A, Redmer J, Counts H, Eglash A, Schrager S. Obesity and women's health: an evidence-based review. J Am Board Fam Med. 2011;24:75–85. [DOI] [PubMed] [Google Scholar]
- 4. Grodstein F, Goldman MB, Cramer DW. Body mass index and ovulatory infertility. Epidemiology. 1994;5:247–250. [DOI] [PubMed] [Google Scholar]
- 5. Bolúmar F, Olsen J, Rebagliato M, Sáez-Lloret I, Bisanti L. Body mass index and delayed conception: a European Multicenter Study on Infertility and Subfecundity. Am J Epidemiol. 2000;151:1072–1079. [DOI] [PubMed] [Google Scholar]
- 6. van der Steeg JW, Steures P, Eijkemans MJ, et al. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum Reprod. 2008;23:324–328. [DOI] [PubMed] [Google Scholar]
- 7. Pandey S, Bhattacharya S. Impact of obesity on gynecology. Womens Health (Lond Endl). 2010;6:107–117. [DOI] [PubMed] [Google Scholar]
- 8. Tortoriello DV, McMinn J, Chua SC. Dietary-induced obesity and hypothalamic infertility in female DBA/2J mice. Endocrinology. 2004;145:1238–1247. [DOI] [PubMed] [Google Scholar]
- 9. Kelesidis T, Kelesidis I, Chou S, Mantzoros CS. Narrative review: the role of leptin in human physiology: emerging clinical applications. Ann Intern Med. 2010;152:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Swerdloff RS, Peterson M, Vera A, Batt RA, Heber D, Bray GA. The hypothalamic-pituitary axis in genetically obese (ob/ob) mice: response to luteinizing hormone-releasing hormone. Endocrinology. 1978;103:542–547. [DOI] [PubMed] [Google Scholar]
- 11. Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. [DOI] [PubMed] [Google Scholar]
- 12. Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol. 2003;15:61–68. [DOI] [PubMed] [Google Scholar]
- 13. Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. [DOI] [PubMed] [Google Scholar]
- 14. Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. [DOI] [PubMed] [Google Scholar]
- 15. Rogge G, Jones D, Hubert GW, Lin Y, Kuhar MJ. CART peptides: regulators of body weight, reward and other functions. Nat Rev Neurosci. 2008;9:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kristensen P, Judge ME, Thim L, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. [DOI] [PubMed] [Google Scholar]
- 17. Lambert PD, Couceyro PR, McGirr KM, Dall Vechia SE, Smith Y, Kuhar MJ. CART peptides in the central control of feeding and interactions with neuropeptide Y. Synapse. 1998;29:293–298. [DOI] [PubMed] [Google Scholar]
- 18. Vrang N, Tang-Christensen M, Larsen PJ, Kristensen P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain Res. 1999;818:499–509. [DOI] [PubMed] [Google Scholar]
- 19. Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ. CART in feeding and obesity. Trends Endocrinol Metab. 2004;15:454–459. [DOI] [PubMed] [Google Scholar]
- 20. Vrang N, Larsen PJ, Kristensen P, Tang-Christensen M. Central administration of cocaine-amphetamine-regulated transcript activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 2000;141:794–801. [DOI] [PubMed] [Google Scholar]
- 21. Raptis S, Fekete C, Sarkar S, et al. Cocaine- and amphetamine-regulated transcript co-contained in thyrotropin-releasing hormone (TRH) neurons of the hypothalamic paraventricular nucleus modulates TRH-induced prolactin secretion. Endocrinology. 2004;145:1695–1699. [DOI] [PubMed] [Google Scholar]
- 22. Kuriyama G, Takekoshi S, Tojo K, Nakai Y, Kuhar MJ, Osamura RY. Cocaine- and amphetamine-regulated transcript peptide in the rat anterior pituitary gland is localized in gonadotrophs and suppresses prolactin secretion. Endocrinology. 2004;145:2542–2550. [DOI] [PubMed] [Google Scholar]
- 23. Smith SM, Vaughan JM, Donaldson CJ, et al. Cocaine- and amphetamine-regulated transcript activates the hypothalamic-pituitary-adrenal axis through a corticotropin-releasing factor receptor-dependent mechanism. Endocrinology. 2004;145:5202–5209. [DOI] [PubMed] [Google Scholar]
- 24. Baranowska B, Woliñska-Witort E, Chmielowska M, Martyñska L, Baranowska-Bik A. Direct effects of cocaine-amphetamine-regulated transcript (CART) on pituitary hormone release in pituitary cell culture. Neuroendocrinol Lett. 2003;24:224–226. [PubMed] [Google Scholar]
- 25. Dominguez G, Vicentic A, Del Giudice EM, Jaworski J, Hunter RG, Kuhar MJ. CART peptides: modulators of mesolimbic dopamine, feeding, and stress. Ann NY Acad Sci. 2004;1025:363–369. [DOI] [PubMed] [Google Scholar]
- 26. Kimmel HL, Gong W, Vechia SD, Hunter RG, Kuhar MJ. Intra-ventral tegmental area injection of rat cocaine and amphetamine-regulated transcript peptide 55–102 induces locomotor activity and promotes conditioned place preference. J Pharmacol Exp Ther. 2000;294:784–792. [PubMed] [Google Scholar]
- 27. Ekblad E, Kuhar M, Wierup N, Sundler F. Cocaine- and amphetamine-regulated transcript: distribution and function in rat gastrointestinal tract. Neurogastroenterol Motil. 2003;15:545–557. [DOI] [PubMed] [Google Scholar]
- 28. Jensen PB, Kristensen P, Clausen JT, et al. The hypothalamic satiety peptide CART is expressed in anorectic and non-anorectic pancreatic islet tumors and in the normal islet of Langerhans. FEBS Lett. 1999;447:139–143. [DOI] [PubMed] [Google Scholar]
- 29. Wierup N, Kuhar M, Nilsson BO, Mulder H, Ekblad E, Sundler F. Cocaine- and amphetamine-regulated transcript (CART) is expressed in several islet cell types during rat development. J Histochem Cytochem. 2004;52:169–177. [DOI] [PubMed] [Google Scholar]
- 30. Yao J, Ren X, Ireland JJ, Coussens PM, Smith TP, Smith GW. Generation of a bovine oocyte cDNA library and microarray: resources for identification of genes important for follicular development and early embryogenesis. Physiol Genomics. 2004;19:84–92. [DOI] [PubMed] [Google Scholar]
- 31. Lv L, Jimenez-Krassel F, Sen A, et al. Evidence supporting a role for cocaine- and amphetamine-regulated transcript (CARTPT) in control of granulosa cell estradiol production associated with dominant follicle selection in cattle. Biol Reprod. 2009;81:580–586. [DOI] [PubMed] [Google Scholar]
- 32. Sen A, Bettegowda A, Jimenez-Krassel F, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript regulation of follicle-stimulating hormone signal transduction in bovine granulosa cells. Endocrinology. 2007;148:4400–4410. [DOI] [PubMed] [Google Scholar]
- 33. Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24:1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sen A, Prizant H, Light A, et al. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111:3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sen A, De Castro I, Defranco DB, et al. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122:2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Evaul K, Hammes SR. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem. 2008;283:27525–27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sen A, Lv L, Bello N, Ireland JJ, Smith GW. Cocaine- and amphetamine-regulated transcript accelerates termination of follicle-stimulating hormone-induced extracellularly regulated kinase 1/2 and Akt activation by regulating the expression and degradation of specific mitogen-activated protein kinase phosphatases in bovine granulosa cells. Mol Endocrinol. 2008;22:2655–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zachow RJ, Weitsman SR, Magoffin DA. Leptin impairs the synergistic stimulation by transforming growth factor-β of follicle-stimulating hormone-dependent aromatase activity and messenger ribonucleic acid expression in rat ovarian granulosa cells. Biol Reprod. 1999;61:1104–1109. [DOI] [PubMed] [Google Scholar]
- 39. Agarwal SK, Vogel K, Weitsman SR, Magoffin DA. Leptin antagonizes the insulin-like growth factor-I augmentation of steroidogenesis in granulosa and theca cells of the human ovary. J Clin Endocrinol Metab. 1999;84:1072–1076. [DOI] [PubMed] [Google Scholar]
- 40. Zachow RJ, Magoffin DA. Direct intraovarian effects of leptin: impairment of the synergistic action of insulin-like growth factor-I on follicle-stimulating hormone-dependent estradiol-17 β production by rat ovarian granulosa cells. Endocrinology. 1997;138:847–850. [DOI] [PubMed] [Google Scholar]
- 41. Spicer LJ, Francisco CC. Adipose obese gene product, leptin, inhibits bovine ovarian thecal cell steroidogenesis. Biol Reprod. 1998;58:207–212. [DOI] [PubMed] [Google Scholar]
- 42. Spicer LJ, Francisco CC. The adipose obese gene product, leptin: evidence of a direct inhibitory role in ovarian function. Endocrinology. 1997;138:3374–3379. [DOI] [PubMed] [Google Scholar]
- 43. Cioffi JA, Shafer AW, Zupancic TJ, et al. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat Med. 1996;2:585–589. [DOI] [PubMed] [Google Scholar]
- 44. Karlsson C, Lindell K, Svensson E, et al. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab. 1997;82:4144–4148. [DOI] [PubMed] [Google Scholar]
- 45. Ryan NK, Van der Hoek KH, Robertson SA, Norman RJ. Leptin and leptin receptor expression in the rat ovary. Endocrinology. 2003;144:5006–5013. [DOI] [PubMed] [Google Scholar]
- 46. Ryan NK, Woodhouse CM, Van der Hoek KH, Gilchrist RB, Armstrong DT, Norman RJ. Expression of leptin and its receptor in the murine ovary: possible role in the regulation of oocyte maturation. Biol Reprod. 2002;66:1548–1554. [DOI] [PubMed] [Google Scholar]
- 47. Fungfuang W, Nakada T, Nakao N, et al. Serum leptin concentrations, leptin mRNA expression, and food intake during the estrous cycle in rats. Lab Anim Res. 2013;29:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Löffler S, Aust G, Köhler U, Spanel-Borowski K. Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod. 2001;7:1143–1149. [DOI] [PubMed] [Google Scholar]
- 49. Ghizzoni L, Barreca A, Mastorakos G, et al. Leptin inhibits steroid biosynthesis by human granulosa-lutein cells. Horm Metab Res. 2001;33:323–328. [DOI] [PubMed] [Google Scholar]
- 50. Kendall NR, Gutierrez CG, Scaramuzzi RJ, Baird DT, Webb R, Campbell BK. Direct in vivo effects of leptin on ovarian steroidogenesis in sheep. Reproduction. 2004;128:757–765. [DOI] [PubMed] [Google Scholar]
- 51. Lei MM, Wu SQ, Li XW, Wang CL, Chen Z, Shi ZD. Leptin receptor signaling inhibits ovarian follicle development and egg laying in chicken hens. Reprod Biol Endocrinol. 2014;12:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kikuchi N, Andoh K, Abe Y, Yamada K, Mizunuma H, Ibuki Y. Inhibitory action of leptin on early follicular growth differs in immature and adult female mice. Biol Reprod. 2001;65:66–71. [DOI] [PubMed] [Google Scholar]
- 53. Anifandis G, Koutselini E, Stefanidis I, et al. Serum and follicular fluid leptin levels are correlated with human embryo quality. Reproduction. 2005;130:917–921. [DOI] [PubMed] [Google Scholar]
- 54. Anifandis G, Koutselini E, Louridas K, et al. Estradiol and leptin as conditional prognostic IVF markers. Reproduction. 2005;129:531–534. [DOI] [PubMed] [Google Scholar]
- 55. Kobayashi Y, Jimenez-Krassel F, Li Q, et al. Evidence that cocaine- and amphetamine-regulated transcript is a novel intraovarian regulator of follicular atresia. Endocrinology. 2004;145:5373–5383. [DOI] [PubMed] [Google Scholar]
- 56. Smith GW, Sen A, Folger JK, Ireland JJ. Putative role of cocaine- and amphetamine-regulated transcript (CARTPT) in dominant follicle selection in cattle. Soc Reprod Fertil Suppl. 2010;67:105–117. [PubMed] [Google Scholar]