Abstract
Timely and appropriate levels of thyroid hormone (TH) signaling are necessary to ensure normal developmental outcomes in many tissues. Studies using pharmacological models of altered TH status have revealed an influence of these hormones on testis development and size, but little is known about the role of endogenous determinants of TH action in the developing male gonads. Using a genetic approach, we demonstrate that the type 3 deiodinase (D3), which inactivates TH and protects developing tissues from undue TH action, is a key factor. D3 is highly expressed in the developing testis, and D3-deficient (D3KO) mice exhibit thyrotoxicosis and cell proliferation arrest in the neonatal testis, resulting in an approximately 75% reduction in testis size. This is accompanied by larger seminiferous tubules, impaired spermatogenesis, and a hormonal profile indicative of primary hypogonadism. A deficiency in the TH receptor-α fully normalizes testis size and adult testis gene expression in D3KO mice, indicating that the effects of D3 deficiency are mediated through this type of receptor. Similarly, genetic deficiencies in the D2 or in the monocarboxylate transporter 8 partially rescue the abnormalities in testis size and gonadal axis gene expression featured in the D3KO mice. Our study highlights the testis as an important tissue in which determinants of TH action coordinately converge to ensure normal development and identifies D3 as a critical factor in testis development and in testicular protection from thyrotoxicosis.
Thyroid hormone (TH) is known primarily for their critical roles in brain development and the regulation of metabolism, but TH receptors are expressed in many tissues, suggesting that TH influence many aspects of development and physiology. These include the male gonads, where TH receptors are expressed during development and adulthood (1–3).
In rodent models, overt changes in altered TH levels during development exert a significant effect on testicular size. Hypothyroidism during neonatal life results in significantly enlarged testes in mice (4–10). In contrast, thyrotoxicosis during the developmental period in rodents, as achieved by the administration of pharmacological doses of T4 or T3 during neonatal life (“Neo-TH” models), results in reduced testis size (11–13). Published evidence indicates that TH influences Leydig cell proliferation, differentiation (14–17), and steroidogenesis (18, 19). However, TH effects on Sertoli cells exert a major influence on testis size (6). Thus, hypothyroidism extends the proliferation and delays the differentiation of Sertoli cells (6, 9, 20, 21), whereas hyperthyroidism prematurely arrests their proliferation and promotes their differentiation (13, 22–25). In mouse Neo-TH models, a reduction in testis size may be accompanied by hormonal abnormalities in hypothalamic-pituitary-gonadal (HPG) axis (13).
TH action in tissues is determined not only by serum levels of TH, but also by local factors such as transporters and deiodinases that modulate local TH availability, and by TH receptors and associated proteins that are responsible for TH signaling at the transcription level. Despite the effects of an altered TH status on testis size, the relative importance of those factors during testicular development remains largely unevaluated, and limited information is available about the extent to which these determinants influence TH action in the testis and influence gonadal and reproductive outcomes. In particular, the relative importance of the physiological mechanisms that ensure appropriate levels of TH during testicular development has not been sufficiently explored. In the testis, some factors may facilitate or enhance TH signaling. These include the TH receptor α (TRα1), which is one of the receptors responsible for the regulation of gene transcription in response to TH (26), is prominently expressed in the developing testis (1, 3), and its specific absence in Sertoli cells leads to significant gene expression changes in the postnatal testis (27); the type 2 deiodinase (D2), which catalyzes the conversion of T4 into the active hormone T3 (28), its activity in the testis increases during neonatal life (29), and it is present in spermatids in the adult rat testis (30); and the monocarboxylate transporter 8 (MCT8), the most efficient transporter of T3 into cells (31) and also present in testicular tissue (32). Other factors may limit TH availability. These include the D3, which is encoded by the imprinted gene Dio3 (33, 34), catalyzes the conversion of T4 and T3 to inactive metabolites, and is a major factor regulating TH availability in developing tissues (28, 35, 36). We and others have demonstrated its prominent role in the development of the hypothalamus, the retina, the cochlea, and the pancreatic β-cell. In these tissues, D3 deficiency leads to growth retardation, abnormal programming of the hypothalamus-pituitary-thyroid axis, sensory impairments, and deficits in insulin secretion and glucose homeostasis (37–40). We thus hypothesized that D3 also plays an important role in the developing testis by maintaining TH availability within levels that are locally and developmentally appropriate.
Here, we show that mice lacking a functional D3 manifest markedly reduced testis size, decreased cell proliferation in the neonatal testis, impaired fertility, and abnormal hormonal and gene expression profiles in tissues of the HPG axis. We demonstrate that this phenotype can be partially or completely rescued by alterations in other determinants of TH action. Our findings identify the D3 as a critical factor influencing TH action in the developing testis and, thus, as an important determinant of fertility and HPG axis function.
Materials and Methods
Experimental animals, genotyping, and tissue collection
D3-deficient (D3KO) mice were in a 129X1/Svj genetic background, and the mutation they carry has been previously described (37). Wild-type (WT) and D3KO experimental animals were littermates generated by breeders heterozygous for the Dio3 gene mutation. All heterozygous breeders used to generate experimental animals were themselves generated by crossing WT males with heterozygous females. Therefore, they were all systemically D3 sufficient, because the active Dio3 allele is that inherited from the father in most fetal tissues (33, 34). Mice carrying a mutation in the Mct8 gene were also in a 129X1/Svj genetic background and were generously provided by Dr Dumitrescu and Dr Refetoff (University of Chicago) (41). Mice carrying the inactivating deletion in the Dio2 gene or in the TRα1 (Thratm1 allele deleting TRα1) gene were in a mixed 129/Sv/C57Bl6 genetic background and have been previously described (42, 43). Mice were kept under a 12-hour light, 12-hour dark cycle and provided food and water ad libitum. Only male mice were used in these studies. To assess the effect of a T3 challenge, mice were treated with T3 (0.25 μg/mL) in the drinking water for 4 weeks. Experimental animals of weaning age or older were killed by asphyxiation with CO2, and blood was obtained postmortem from the ascending cava vein. Trunk blood was collected from young neonates, which were killed by decapitation. Blood samples were maintained at 4°C for 2 hours, and then serum was obtained by centrifugation and stored at −80°C until further analysis. Testes, pituitary, and hypothalamus were harvested and frozen on a dry ice-cold surface, then stored at −80°C until being processed. Genotyping of the Dio3, Mct8, Dio2, and Thra mutations was performed as previously described by PCR of genomic DNA extracted from tail snips (37, 41, 43). The sequences of the primers used for genotyping are listed in the Supplemental Table 1. All animal procedures were approved by Institutional Animal Care and Use Committees of Dartmouth College and Maine Medical Center Research Institute.
D3 enzymatic assay and serum determinations
D3 enzymatic activities were determined as previously described (37). In brief, tissues were homogenized in a 10mM Tris-HCl, 0.25M sucrose (pH 7.4) buffer. A suitable volume of tissue homogenate was used in the enzymatic reaction to ensure that deiodination did not exceed 40% and was proportional to the protein content. Tissue homogenates were incubated at 37°C for an hour with 2nM 125I-labeled T3 (PerkinElmer) in the presence of 25mM dithiothreitol. Deiodination was determined based on the percentage of 125I-3,3′-diiodothyronine produced. The latter was determined by measuring the amount of radioactivity associated with the reaction products after separation by paper chromatography, as described (44). Serum levels of testosterone, FSH, and LH were determined using commercial ELISA kits (Cayman Chemicals and Biotang, respectively), according to the manufacturer's instructions. Total serum TH levels were determined using RIA kits following the manufacturer's directions (TKT4 and TKT3 from Siemens).
Gene expression
Total RNA was isolated from mouse tissues using the RNeasy kit (QIAGEN). Total RNA (1 μg) was reverse transcribed for 1 hour at 42°C with 1 μL of Moloney Murine Leukemia Virus reverse transcriptase (Life Technologies). The mix was heated at 75°C for 15 minutes to inactivate the reverse transcriptase and diluted 1:20 with water. Aliquots of the mixes from a given experiment were pooled before dilution to establish the first point of an internal standard. Three consecutive 1:4 dilutions of this standard were done to generate 3 additional standard points; 10 μL of each of the diluted samples were mixed with 12.5 μL of SYBR Select Master Mix (Life Technologies) and 2.5 μL of the appropriate gene-specific primer mix (3.33 pmol/μL for each primer). The mixture was subjected to PCR cycling using a MyiQ Single Color Real-Time PCR Detection System from Bio-Rad. PCRs were performed in triplicate. Gapdh was used as a control gene, and its expression level did not vary significantly between experimental groups. Expression levels for each gene were read from the standard curve, corrected by Gapdh expression, and are reported in arbitrary units. The sequences of the primers used for gene expression are shown in the Supplemental Table 1.
Spermatogenesis
Daily sperm production (DSP) and efficiency of sperm production were determined as previously described (45). In brief, 1 testis per mouse was homogenized for 3 minutes in 25 mL of a physiological saline solution containing 0.05% Triton X-100 (Sigma) using a Waring blender. step 14–16 spermatids (stage II–VIII) survived this homogenization, and their nuclei were counted using a hematocytometer. For this, a 200-μL sample of homogenate was diluted with 300 μL of saline and 500 μL of 4% trypan blue, which stains spermatids. Sample aliquots of 5.5 μL were placed on the hematocytometer, and the spermatids were counted twice under the microscope using a ×100 magnification. The average of the values obtained was divided by 4.84, which is the number of days developing spermatids spend in steps 14–16 during spermatogenesis in the mouse, to obtain the DSP. This number was divided by the weight of the testis to obtain the efficiency of sperm production.
Immunohistochemistry
Testes were fixed in Bouin's solution for 2 hours (fetal and neonatal testes) or overnight (adult testes), briefly washed in 70% ethanol, cut transversally in 2 halves and paraffin embedded for sectioning at the cut area. Hematoxylin and eosin (H&E) staining, as well as immunohistochemistry staining for the antigen identified by monoclonal antibody KI67 (see antibody table, Table 1) was performed at the Histology Core facility at Maine Medical Center Research Institute following standard procedures. Seminiferous tubule size was measured using ImageJ software on H&E-stained testis sections cut at the center of the testis perpendicularly to its length. Fifteen sections per genotype and age were used, representing 3 mice per experimental group (5 sections per animal).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| MKI67 | KI67 | Thermo Scientific | Rabbit | 1:250 |
Statistical analysis
We used the GraphPad Prism software for statistical analysis. Unless stated otherwise, comparisons between more than 2 experimental groups were performed by ANOVA and Tukey's post hoc test, whereas statistical significance between 2 groups was determined by the Student's t test or, in the case of some fertility parameters, by Pearson's χ2 test. P < .05 was considered statistically significant.
Results
Testicular D3 ontogeny and neonatal D3KO testis gene expression
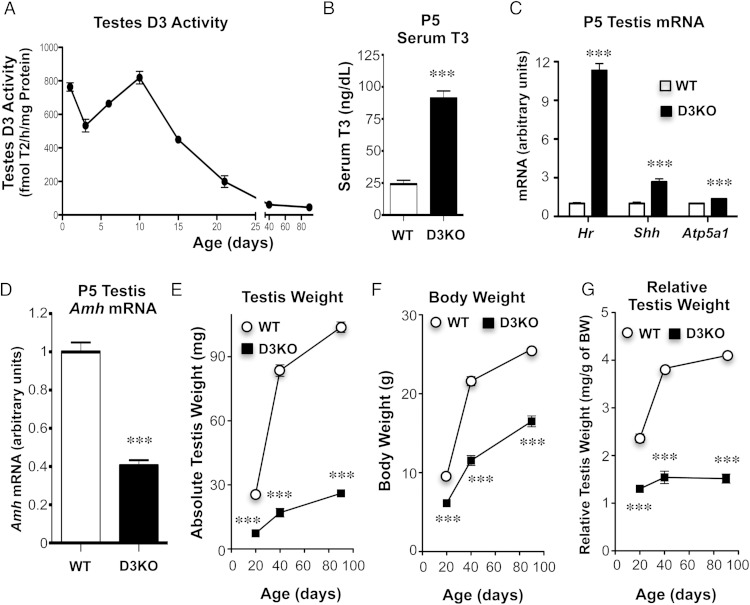
We determined D3 activity in the testis of WT mice from postnatal day (P)1 to adulthood. Testicular D3 activities were very high in the first 10 days of life, started to decline by P15, and stabilized at very low levels in the adult (Figure 1A). The difference in testis D3 activity between neonatal and adult life was approximately 2 orders of magnitude.
Figure 1.
D3 and testis size. A, Ontogeny of D3 activity in normal testis. Four to 6 animals were used per age. B, Serum T3 at P5. Four samples per experimental group were used, each representing pooled serum from 3 mice. C and D, Altered gene expression in the testes of P5 D3KO mice (n = 6, 5 for WT and D3KO, respectively). E–G, Testis weight, body weight, and relative testis weight, respectively, of WT and D3KO mice of different ages. The average weight of the 2 testes of each animal was used. Each data point represents 7–22 mice. All data in the figure represents the mean ± SEM; *, P < .05; **, P < .01; ***, P < .001, as determined by the Student's t test.
The absence of a functional D3 leads to a systemic impairment in T3 clearance and elevated serum T3 in neonatal mice (37), a finding that was confirmed in the present study (Figure 1B). Given the high D3 expression in the neonatal testis, we hypothesized that the developing testis are particularly sensitive to D3 deficiency and the resultant systemic and local T3 excess. To test this hypothesis, we measured the mRNA expression of Hr, Shh, and Atp5a1. These genes are significantly expressed in the testes and, especially the first one, are regulated by T3, as indicated by a recent publication compiling a list of T3-regulated genes that are expressed in the brain (46). We observed that P5 D3KO mice exhibited a 10-, 3-, and 1.4-fold increase, respectively, in the testicular expression of Hr, Shh, and Atp5a1 (Figure 1C), suggesting an elevated level of TH action. In addition, mRNA expression of Sertoli cell marker gene Amh, which encodes anti-Mullerian hormone, was 60% lower than in control animals (Figure 1D), suggesting that D3 deficiency also affects the expression of genes of importance to testis physiology and development.
Reduced testis size and impaired fertility in D3KO mice
Based on published reports from Neo-TH models indicating that administration of pharmacological doses of TH leads to reduced testis size (11–13), we compared the testis weight in WT and D3KO littermate mice at different ages. By weaning age, the weight of the D3KO testis is only about 30% of that of the WT testis, and by adult age, it is 25% of WT values (Figure 1E). We have previously shown that D3KO mice are growth retarded (37), a phenotype confirmed in the present studies by the weight data shown in Figure 1F. This raised the possibility that the reduced testis size in D3KO mice resulted from the overall stunted growth. However, after correcting by body weight, the relative testis weight of D3KO mice at weaning age is markedly lower than that in WT mice and this phenotype persists in adulthood (Figure 1G). The reduction in relative testis weight by weaning age suggests that D3 deficiency plays a specific role in the developing testis. Notably, D3KO mice appear to lack the testis growth spurt associated with puberty that is easily appreciated in WT mice between P20 and P40 (Figure 1G).
To assess whether male fertility is compromised in D3 deficiency, we compiled data from 64 WT and 44 D3KO adult male mice. These male mice were mated at the age of 7 weeks with WT females that were between 7 and 20 weeks of age. The experimental males were paired with these females for at least 4 months. Only 52% of D3KO males were capable of producing offspring, compared with 95% of WT males (Table 2). Furthermore, only 27% of D3KO males produced more than 1 pregnancy during the 4 months they remained caged with the female. The latency to induce pregnancy after pairing was significantly longer in D3KO mice, and the size of the litters generated was significantly reduced (Table 2). Preliminary observations indicate the fertility of the male D3KO mouse is also impaired, although slightly improved, in mixed genetic backgrounds or outbred strains, whereas the relative reduction in testis size is not changed. These findings demonstrate the importance of D3 expression for male reproductive function.
Table 2.
Fertility Data From WT and D3KO Male Mice
| Genotype | Number of Mice Analyzed | Number of Mice That Produced Offspring | Number of Mice That Produced More Than 1 Pregnancy | Latency (d) to Impregnate 1st Female | Litter Size |
|---|---|---|---|---|---|
| WT | 64 | 61 (95%) | 61 (95%) | 11.7 ± 2.1 (n = 61) | 7.10 ± 0.23 (n = 174) |
| D3KO | 44 | 23 (52%) | 12 (27%) | 22.7 ± 3.3 (n = 23) | 4.73 ± 0.63 (n = 59) |
| P < .01a | P < .001a | P < .01b | P < .01b |
Data were compiled from mice that were mated with WT females for at least 4 months since the age of 7 weeks.
Statistical significance determined by Pearson's χ2 test.
Statistical significance determined by Student's t test.
Histology of D3KO testis and spermatogenesis
Histological analysis of adult D3KO testis using H&E staining did not reveal any major abnormality in cytoarchitecture other than an enlargement of the average size of seminiferous tubules (Figure 2, A and B). The larger area of the seminiferous tubules in D3 deficiency was significant from birth to adulthood but more prominent at an early neonatal stage (Figure 2B). This occurs despite the fact that testis size is reduced, suggesting a reduction in the relative number of seminiferous tubules in D3KO mice. In addition, DSP was markedly reduced in D3KO mice (Figure 2C), and the efficiency of sperm production, calculated as DSP per milligram of testis, was also significantly lower in D3KO mice (Figure 2D). These results indicate that spermatogenesis is adversely affected by D3 deficiency.
Figure 2.
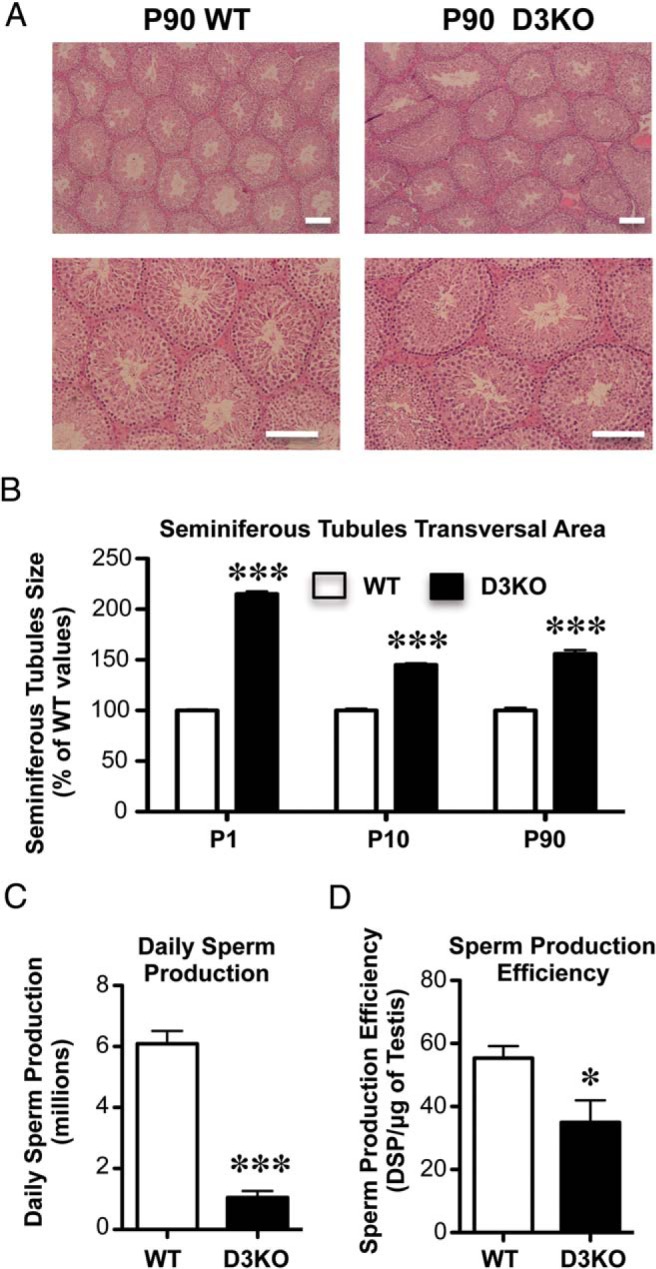
Testis histology and spermatogenesis. A, Representative testicular section of P90 WT and D3KO. Scale bars, 200 μm. B, Area of seminiferous tubules in WT and D3KO testis at different ages. C and D, Spermatogenesis in adult WT and D3KO mice (n = 6 per group). Data represent the mean ± SEM of determinations in 5–33 animals per group; *, P < .05 and ***, P < .001, WT vs D3KO as determined by the Student's t test.
The enlarged size of seminiferous tubules in D3KO mice is already apparent during late fetal life (Figure 3, A and B), at P1 (Figure 3, C and D) and at P10 (Figure 3, E and F). Published work has demonstrated that in Neo-TH models the administration of pharmacological doses of T3 leads to a premature arrest of Sertoli cell proliferation and premature lumen formation in seminiferous tubules (13, 22–24, 47, 48). We assessed cell proliferation in the testis of P10 WT and D3KO mice by immunostaining for the cell proliferation marker MKI67. Immunohistochemistry results reveal a marked decrease in MKI67 immunoreactivity in D3KO testis, affecting both staining intensity and the number of stained cells (Figure 3, G and H). These findings demonstrate an impact of D3 deficiency on cell proliferation in the neonatal testis. At this age, an enlarged lumen is clearly appreciated in the seminiferous tubules of D3KO mice (Figure 3, F and H vs E and G).
Figure 3.
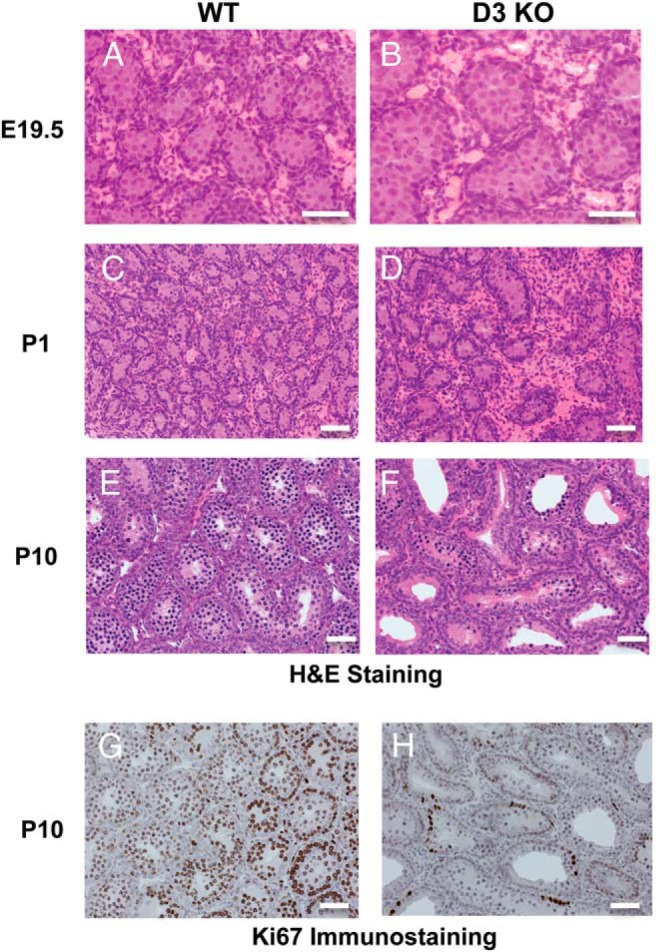
Neonatal testis histology and cell proliferation. A–F, Representative images of H&E-stained E19.5 (A and B), P1 (C and D), and P10 (E and F) WT and D3KO testis. G and H, Ki67 IHC of WT and D3KO testis at P10. Magnification, ×400 (A and B) and ×200 (C–H). Scale bars, 200 μm.
The reproductive axis of D3KO males
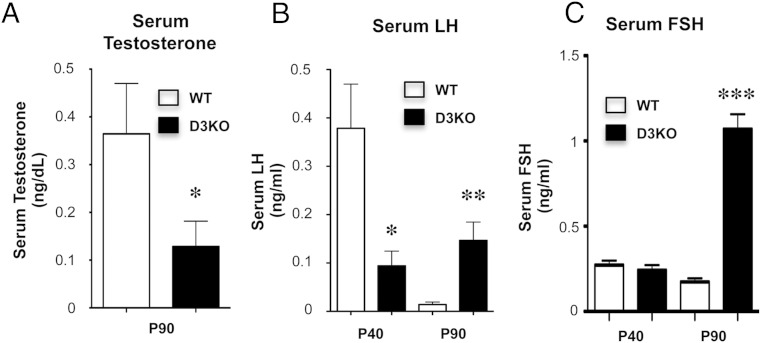
The neonatal overexposure to TH leads in adult D3KO mice to central abnormalities in the hypothalamic-pituitary-thyroid axis, which present as a moderate central hypothyroidism (49). Considering the reduced testis size in D3KO males, we hypothesized that D3 deficiency may also cause alterations in the HPG axis. We thus analyzed serum and tissue parameters related to the HPG axis. At P90, D3KO males exhibited a significant reduction in serum testosterone (Figure 4A). At P40, an age close to the end of puberty and the start of reproductive capacity, D3KO male mice exhibit lower serum levels of LH than those in WT mice (Figure 4B). However, later in adulthood, serum LH is significantly elevated in D3KO males, whereas the serum LH level in WT mice is low (Figure 4B). Adult D3KO mice exhibit markedly elevated levels of serum FSH (Figure 4C), but this parameter was not significantly different between WT and D3KO mice at P40.
Figure 4.
Testosterone and gonadotropin serum levels in P40 and P90 WT and D3KO mice. A, Serum testosterone. B, Serum LH. C, Serum FSH. Data represent the mean ± SEM of determinations in 6–12 mice per group. *, P < .05; **, P < .01; ***, P < .001, as determined by the Student's t test.
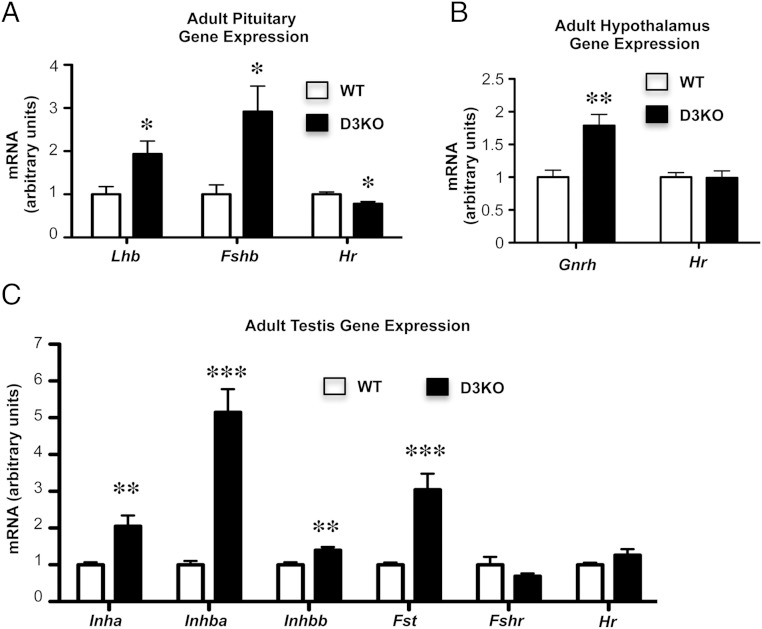
We observed that elevated levels of FSH and LH in the serum of P90 D3KO males were associated with increased mRNA expression of Lhb and Fshb genes in the pituitary (Figure 5A). In contrast, pituitary expression of Hr was slightly reduced in D3KO mice, consistent with their systemic hypothyroidism (Figure 5A). Furthermore, hypothalamic mRNA expression of gonadotropin-releasing hormone (Gnrh) was also significantly increased in D3KO male mice, whereas Hr mRNA was not changed in the hypothalamus of D3KO mice at this age (Figure 5B), suggesting that abnormal Gnrh expression is not a direct effect of an altered thyroid status in this tissue at this time.
Figure 5.
Gene expression in HPG axis tissues of adult WT and D3KO mice. A, Pituitary expression of Lhb, Fshb, and Hr mRNAs. B, Hypothalamic expression of Gnrh and Hr mRNAs. C, Adult testicular expression of Inha, Inhba, Inhbb, Fst, Fshr, and Hr mRNAs. Data represent the mean ± SEM of determinations in 5–8 mice per group; *, P < .05; **, P < .01; ***, P < .001, as determined by the Student's t test.
The expression of genes relevant to the HPG axis was altered in the testes of adult D3KO mice. Testicular mRNA levels of inhibin genes (Inha, Inhba, and Inhbb) and follistatin (Fst) were elevated in D3KO mice (Figure 5C). No significant differences were observed in the mRNA expression of FSH receptor (Fshr) or Hr, the latter suggesting a euthyroid state in the adult D3KO testis. Overall, these data reflect an abnormal regulation of the gonadal axis and a profile of primary hypogonadism in adult D3KO mice.
To determine whether the abnormal gene expression in the gonadal axis tissues of D3KO mice was due to increased susceptibility to TH action, we treated WT and D3KO mice with T3 (0.25 μg/mL) in the drinking water for 4 weeks and analyzed the response in gene expression. T3 treatment resulted in a 2- and 8-fold increase in serum T3, respectively, in WT and D3KO mice (data not shown), but no significant response in Fshb and Hr mRNA expression was observed in the pituitary of either group of mice (Supplemental Figure 1A). In the hypothalamus, T3 treatment significantly increased Hr mRNA expression in WT mice and, to a greater extent, in D3KO mice, but no changes were noticed in Gnrh mRNA expression (Supplemental Figure 1B). Similarly, testis gene expression revealed no response to T3 treatment in WT or D3KO mice, except for Hr expression, whose response to T3 treatment was much higher in D3KO mice than in WT mice (Supplemental Figure 1C). The lack of T3 responsiveness in genes relevant to the regulation of the HPG axis in adults suggests that the apparent primary hypogonadism of D3KO males is not a consequence of local TH excess in adult tissues of the HPG axis but likely due to the effects of excessive TH levels during development. (All the gene expression data concerning the adult HPG axis, in untreated and T3-treated mice of both genotypes, are shown together in Supplemental Figure 2.)
Rescue of the testis size phenotype of D3KO mice
The small testis size of D3KO mice is presumably the result, at least partially, of the excessive TH action occurring during development in this tissue. We thus hypothesized that deficiencies in factors that enhance or facilitate TH signaling would lead to a less severe D3KO phenotype. Such factors include TRα1, MCT8, and D2. To test that hypothesis, we crossed D3KO mice with mice lacking functional Trha, Dio2, or Mct8 genes.
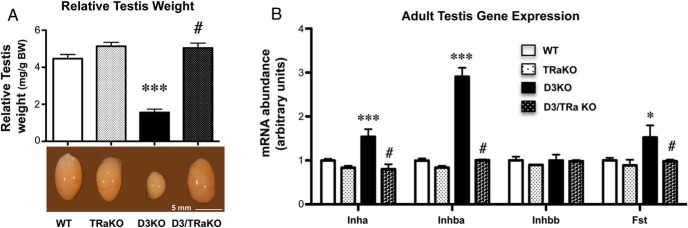
Our results showed that the absence of TRα1 normalizes the testis size of D3KO mice (Figure 6A). The normal adult testis size in adult D3/TRα1 double KO mice was accompanied by normal testicular expression levels of inhibin A and B and follistatin (Figure 6B). These data demonstrate that TRα1 is the critical mediator of TH signaling in the testis and further demonstrates that the reduced testis size of D3KO males is the consequence of enhanced TH signaling.
Figure 6.
Testis size and gene expression in D3 and TRα1 double deficiency. A, Relative testis weight in adult mice of the 4 genotypes. B, Testicular gene expression of Inha, Inhba, Inhbb, and Fst, of adult mice of the 4 genotypes. Data represent the mean ± SEM of determinations in 4 animals per group. Statistical significance was determined by the ANOVA and Tukey's post hoc test. *, P < .05 and ***, P < .001, when compared with the WT group; #, P < .05, when compared with the D3KO group.
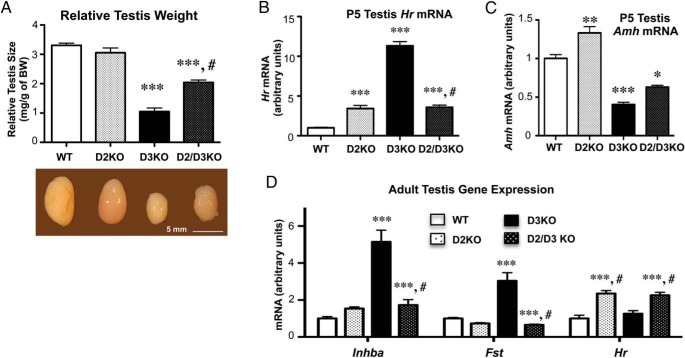
A deficiency in D2 resulted in a significant increase in the testis size of D3KO mice but did not fully rescue this phenotype (Figure 7A). In the P5 testis, the abnormal gene expression observed in D3KO mice is ameliorated by D2 deficiency, which significantly reduced the mRNA expression of Hr (Figure 7B) and completely normalized Amh expression (Figure 7C). Interestingly, D2 deficiency alone also altered testicular gene expression of Hr and Amh at this age. In the adult testis, D2 deficiency fully normalized the expression of Fst mRNA and, to a large extent, that of Inhba mRNA (Figure 7D). However, Hr mRNA expression in the testis of adult D2/D3 double KO mice was elevated above normal levels to an extent similar to that observed in mice with single D2 deficiency (Figure 7D).
Figure 7.
Testis size and gene expression in D3 and D2 double deficiency. A, Relative testis weight in adult mice of the 4 genotypes. B and C, Testicular expression of Hr and Amh mRNAs, respectively, in P5 animals. D, Testicular gene expression of Inhba, Fst, and Hr mRNAs in adult mice. Data represent the mean ± SEM of determinations in 4–9 animals per group. Statistical significance was determined by the ANOVA and Tukey's post hoc test; *, P < .05; **, P < .01; ***, P < .001, respectively, when compared with the WT group; #, P < .05, when compared with the D3KO group.
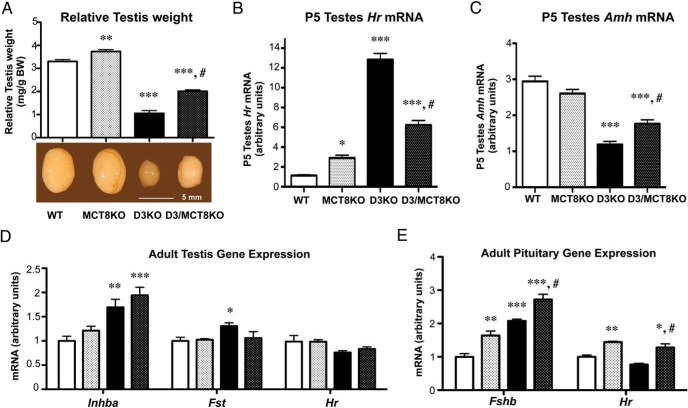
The mRNA encoded by the TH transporter gene Mct8 is significantly expressed in the testes and, just like Dio3 mRNA, displays a marked developmental pattern of expression, with much higher testicular expression during development than in adulthood (Supplemental Figure 3). A deficiency in MCT8 also resulted in a partial rescue of the testis size and gene expression phenotype of adult D3KO mice (Figure 8A). In addition, a modest but significant increase in testis size was noted in MCT8-deficient mice when compared with WT mice (Figure 8A). A phenotypic rescue of similar degree is observed in P5 testis gene expression, where MCT8 deficiency ameliorates but does not normalize the expression of Amh or Hr mRNAs (Figure 8, B and C). When compared with that of WT testis, an increase in Hr mRNA expression is observed in MCT8-deficient P5 testis, suggesting increased TH action. In adult testis, MCT8 deficiency exerted varied effects on the gene expression of D3KO mice. The abnormal increase in Fst mRNA expression observed in D3KO mice was corrected by MCT8 deficiency (Figure 8D). In contrast, the lack of MCT8 exacerbated the elevated expression of Inhba mRNA previously observed in D3KO mice (Figure 8D). No significant difference in the testicular expression of Hr was observed across the 4 genotypes, suggesting overall euthyroidism in this tissue (Figure 8D). Pituitary expression of Fshb mRNA was significantly elevated in MCT8KO mice, although not as much as in D3KO mice. Double D3/MCT8 deficiency resulted in further elevation of Fshb mRNA expression (Figure 8E). However, pituitary expression of Hr mRNA was elevated only in those animals with single MCT8 or double D3/MCT8 deficiencies, suggesting again that the abnormal Fshb expression does not correlate with an abnormal thyroid status in the pituitary.
Figure 8.
Testis size and gene expression in D3 and MCT8 double deficiency. A, Relative testis weight in adult mice of the 4 genotypes. B and C, Testicular expression of Hr and Amh mRNAs, respectively, in P5 animals. D, Testicular gene expression of Inha, Fst, and Hr mRNAs in adult mice. E, Pituitary expression of Fshb and Hr mRNAs in adult mice. Data represent the mean ± SEM of determinations in 4–9 animals per group. Statistical significance was determined by the ANOVA and Tukey's post hoc test; *, P < .05; **, P < .01; ***, P < .001, respectively, when compared with the WT group; #, P < .05, when compared with the D3KO group.
Parental-specific effects of D3 deficiency
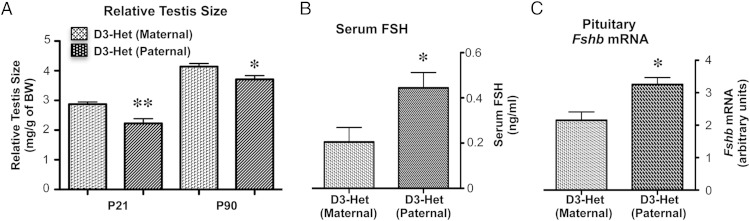
Considering that Dio3 is an imprinted gene that is preferentially expressed from the paternal allele in most developing tissues (33, 34), we examined testis size in heterozygous mice that inherited the Dio3 mutation from the mother or from the father. The results show a testis size reduction in heterozygous males with paternal inheritance of the Dio3 mutation when compared with heterozygous males with maternal inheritance of the mutation (Figure 9A). The testis size phenotype of heterozygous males with paternal inheritance of the mutation was milder than that previously observed in D3KO males. In addition, the smaller testis in heterozygous males with paternal inheritance of the Dio3 mutation was accompanied by a modest but significant elevation in serum FSH (Figure 9B) and pituitary mRNA expression of Fshb (Figure 9C). These results suggest that genetic or epigenetic abnormalities affecting the allelic expression of Dio3 could also significantly influence the HPG axis and the development of the testis.
Figure 9.
Effects of parental-specific inheritance of the D3 mutation on testis size and serum FSH. Relative testis size (A) and serum FSH (B) and pituitary Fshb mRNA (C) in heterozygous adult mice with paternal vs maternal inheritance of the D3-inactivating mutation. Data represent the mean ± SEM of determinations in 6–14 animals per group; *, P < .05 and **, P < .01, respectively, as determined by the Student's t test.
Discussion
Alterations in neonatal TH status have been shown to alter the development and ultimate weight of the testis, but it is unclear what determinants of TH action influence these effects in physiological conditions. An important role of D3 in the developing testis is suggested by its marked developmental pattern of expression in this tissue; testicular D3 activity is 2 orders of magnitude higher in the neonate than in the adult. Supporting this hypothesis is the dramatic reduction in testis size observed in mice with D3 deficiency. In this condition, insufficient clearance of T3 results in excessive TH action in neonatal tissues, including the testis, as manifest by the 10-fold increase in Hr mRNA expression in this organ. This observation is consistent with previous studies indicating that neonatal hyperthyroidism leads to reduced testis size (11, 12). Notably, the severity of this phenotype is significantly greater in D3 deficient animals when compared with Neo-TH models (11, 12), despite the fact that the latter models exhibit much higher level of neonatal serum T3 (50). The difference in severity might result from in utero TH excess in D3KO mice, or it may be that T3 activation of D3 in Neo-TH models provides significant protection against thyrotoxicosis, underscoring the important role of D3 action in protecting the developing testis from inappropriate degrees of TH action.
A significant arrest in the neonatal proliferation of Sertoli cells, in association with reduced testis weight, has been observed in a Neo-TH mouse model (13), a finding that is again in agreement with the marked reduction in cell proliferation that we observed in the testis of D3KO neonates. Not only is Sertoli cell proliferation a major determinant of testicular mass (6), but a reduction in the number of these cells and their premature differentiation would lead to an increase in the diameter of seminiferous tubules and premature lumen formation, an outcome we observed in D3KO mice and has also been reported in Neo-TH models (13). D3 deficiency also impacts the neonatal expression of Sertoli cell-specific Amh. This may indicate that Amh is regulated by TH (51), or it may reflect the lower proportion of these cells in the D3KO testis, a finding also described in Neo-TH mouse models (11).
The small testis size of D3KO mice is accompanied by reduced fertility and impaired spermatogenesis, as would be predicted based on both the important roles that Sertoli cells play in those processes and the influence of neonatal thyroid status on germ cell development (52). In addition, D3KO mice exhibit hormonal and gene expression abnormalities of the HPG axis. A low-serum LH at the time of puberty suggests an abnormality at this critical developmental phase. The pattern observed in the adult D3KO male mice, in which serum testosterone is decreased, whereas serum LH and FSH, pituitary Lhb and Fshb expression, hypothalamic Gnrh expression, and testicular inhibin B expression are all elevated, delineates a profile of primary hypogonadism. Notably, the abnormal gene expression observed in tissues of the HPG axis occurs in the absence of marked changes in Hr expression, suggesting that these tissues are euthyroid, and that the abnormalities observed are not secondary to an adult deficiency of D3 in those tissues but rather result from the consequences of D3 deficiency during development. However, this has to be interpreted with caution as the overall tissue expression of Hr in a given tissue does not necessarily reflect the thyroid status of particular cell types. The likely developmental origin of the abnormalities in the HPG axis is further supported by the observation that systemic T3 treatment did not significantly change the expression patterns of genes relevant to the HPG axis, but it did change, except in the pituitary, the expression of the T3-responsive gene Hr in a manner that was dependent on the presence of D3. Consistent with a developmental origin of the hormonal abnormalities of the D3KO mice is the observation of alterations in serum inhibin and FSH in a Neo-TH mouse model (13), in which animals are D3 sufficient and experience enhanced TH action only during neonatal life.
That the reduced testis size of D3KO mice is due to enhanced TH action during development is demonstrated by the phenotypic rescue observed by TRα1 deficiency. Indeed, adult testis gene expression and testis size is fully normalized in mice with both D3 and TRα1 deficiencies. This rescue occurs even though the serum levels of T3 that have been reported in D3/TRα1 double KO neonates remain abnormally elevated, as in D3KO mice (42). Similarly, TRα1 deficiency, but not TRβ, also largely rescues the testicular phenotype observed in a Neo-TH mouse model (11). These findings confirm the prominent role for the TRα1 in the developing testis, as previously reported (3).
Other determinants of cellular TH action have effects on the testis and HPG gene expression phenotype of D3KO mice. In a background of D2 deficiency, some of the abnormal characteristics of the D3KO mouse are ameliorated. These include adult testis size, adult testicular expression of Inhba and Fst, and expression levels of Hr and Amh in the neonatal testis. Preliminary results in our laboratory indicate that serum levels of T3 in the D3KO neonate are largely normalized by D2 deficiency. This suggests that a large proportion of the T3 excess that cannot be cleared by D3KO neonates originates from T4 to T3 conversion by D2. The contribution of D2 to the elevated serum T3 of D3KO neonates is probably enhanced by its increased activity in this animal model (37, 53). This contribution may explain the significant normalization of gene expression in the testis of D2/D3 double KO neonates. If we assume that the HPG axis gene expression phenotype of the adult D3KO mouse has a large developmental component, its rescue by D2 deficiency could also be due to the amelioration of T3 exposure during neonatal life. The modest, but significant, elevation of Hr expression observed in the P5 D2KO testis is consistent with the high levels of serum T3 and T4 that have been previously reported in D2KO neonates (54). The reason for these serum values in the D2KO neonate has not been further investigated, but they are measured at a time in which the set-point of the thyroid axis has not been fully established. It is possible that the elevated serum TH levels in D2KO neonates may be the result of elevated TSH and increased TH secretion from the thyroid gland, before central regulation is established. The elevated serum TH levels in D2KO neonates (54) are not accompanied by any reduction in Amh expression, as it occurs in D3KO neonates. This is possibly due to cell differences in the expression of Hr, Amh, and D2 in testicular tissue that could make certain cell types or genes differentially sensitive to systemic vs locally generated T3.
A deficiency in the principal T3 cell transporter, MCT8, results in the partial normalization of adult testis size and in the testicular expression of Amh and Hr in the D3KO mouse neonate. The phenotypic rescue occurs even though preliminary data suggest that neonatal T3 exposure is unchanged with respect to that in D3KO neonates. These findings suggest that the MCT8 transporter plays a significant role in facilitating TH action in Sertoli cells and that a deficiency in this protein mitigates the overall T3 excess in testicular cells. In contrast to D2 deficiency, MCT8 deficiency does not reduce the elevated expression of testicular Inhba or pituitary Fshb of the adult D3KO mice. In addition, single MCT8 deficiency results in an elevation of Hr expression in the P5 testis and a significant increase in testis size. The elevated Hr expression could be explained by the serum and tissue hyperthyroidism in MCT8KO newborn mice that has been recently described (55), whereas the slightly larger testis of MCT8KO mice could be the result of a mild hypothyroidism in Sertoli cells during late neonatal life as a result from decreased T3 transport, because this may prolong proliferation and delay differentiation of these cells. Taken together, our data suggest complex and important roles of MCT8 in modulating TH action in the developing male gonadal axis.
A milder testicular phenotype concerning testis size and serum FSH is observed in heterozygous mice with paternal inheritance of the D3 mutation but not in those heterozygous mice that inherited the mutation from their mother. This finding is not surprising given that the Dio3 gene is imprinted and preferentially expressed from the paternal allele. Although we have shown that Dio3 monoallelic expression is relaxed in certain mouse tissues, including the testis (56), the finding of increased serum levels of T3 in heterozygous neonates with paternal, but not maternal, inheritance of the D3 mutation (56) is likely to result in enhanced TH action in the neonatal testis. These results suggest that alterations in the epigenetic regulation of Dio3 can be of consequence to testis development and the function of the HPG axis.
In summary, our results demonstrate that, in the context of the regulation of TH action, D3 is a critically important factor that affects testis size, fertility and the function of the HPG axis. The developmental consequences of D3 deficiency are influenced by the action of TRα1, D2, and MCT8, illustrating the complexity of the control of TH signaling in testis development. Future experiments with these and available floxed-Dio3 mouse models will delineate more precisely at the cellular level the timely developmental roles of those factors in regulating TH availability in the testis, and their influences on reproductive function.
Acknowledgments
We thank Samuel Refetoff and Alexandra Dumitrescu for providing us with the MCT8KO mice and Bjorn Vennstrom for the TRα1 KO mice.
This work was partially supported by Institutes of Health Grants DK054716 (to D.S.G.), MH083220 (to A.H.), DK095908 (to A.H.), and MH096050 (to A.H.) and by the Intramural Research Program at NIDDK at the National Institutes of Health (D.F. and R.P.P.). Our studies used the Histopathology and the Molecular Phenotyping CORE facilities at Maine Medical Center Research Institute that are part of Centers of Biomedical Research Excellence Grants P30GM103392 and P30GM106391 (to Robert Friesel and Don Wojchowsky, PIs).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- D2
- type 2 deiodinase
- D3KO
- D3 deficient
- DSP
- daily sperm production
- Fst
- follistatin
- Gnrh
- gonadotropin-releasing hormone
- H&E
- hematoxylin and eosin
- HPG
- hypothalamic pituitary gonadal
- Inh
- inhibin
- MCT8
- monocarboxylate transporter 8
- P
- postnatal day
- TH
- thyroid hormone
- TRα1
- TH receptor α
- WT
- wild type.
References
- 1. Buzzard JJ, Morrison JR, O'Bryan MK, Song Q, Wreford NG. Developmental expression of thyroid hormone receptors in the rat testis. Biol Reprod. 2000;62:664–669. [DOI] [PubMed] [Google Scholar]
- 2. Canale D, Agostini M, Giorgilli G, et al. Thyroid hormone receptors in neonatal, prepubertal, and adult rat testis. J Androl. 2001;22:284–288. [PubMed] [Google Scholar]
- 3. Jannini EA, Dolci S, Ulisse S, Nikodem VM. Developmental regulation of the thyroid hormone receptor α 1 mRNA expression in the rat testis. Mol Endocrinol. 1994;8:89–96. [DOI] [PubMed] [Google Scholar]
- 4. Cooke PS, Porcelli J, Hess RA. Induction of increased testis growth and sperm production in adult rats by neonatal administration of the goitrogen propylthiouracil (PTU): the critical period. Biol Reprod. 1992;46:146–154. [DOI] [PubMed] [Google Scholar]
- 5. Cooke PS, Meisami E. Early hypothyroidism in rats causes increased adult testis and reproductive organ size but does not change testosterone levels. Endocrinology. 1991;129:237–243. [DOI] [PubMed] [Google Scholar]
- 6. De França LR, Hess RA, Cooke PS, Russell LD. Neonatal hypothyroidism causes delayed Sertoli cell maturation in rats treated with propylthiouracil: evidence that the Sertoli cell controls testis growth. Anat Rec. 1995;242:57–69. [DOI] [PubMed] [Google Scholar]
- 7. Lagu SK, Bhavsar NG, Sharma RK, Ramachandran AV. Neonatal hypothyroidism-induced changes in rat testis size, dependence on temperature. Neuro Endocrinol Lett. 2005;26:780–788. [PubMed] [Google Scholar]
- 8. Meisami E, Sendera TJ, Clay LB. Paradoxical hypertrophy and plasticity of the testis in rats recovering from early thyroid deficiency: a growth study including effects of age and duration of hypothyroidism. J Endocrinol. 1992;135:495–505. [DOI] [PubMed] [Google Scholar]
- 9. Hess RA, Cooke PS, Bunick D, Kirby JD. Adult testicular enlargement induced by neonatal hypothyroidism is accompanied by increased Sertoli and germ cell numbers. Endocrinology. 1993;132:2607–2613. [DOI] [PubMed] [Google Scholar]
- 10. Joyce KL, Porcelli J, Cooke PS. Neonatal goitrogen treatment increases adult testis size and sperm production in the mouse. J Androl. 1993;14:448–455. [PubMed] [Google Scholar]
- 11. Holsberger DR, Kiesewetter SE, Cooke PS. Regulation of neonatal Sertoli cell development by thyroid hormone receptor α1. Biol Reprod. 2005;73:396–403. [DOI] [PubMed] [Google Scholar]
- 12. Bakke JL, Lawrence N, Wilber JF. The late effects of neonatal hyperthyoridism upon the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology. 1974;95:406–411. [DOI] [PubMed] [Google Scholar]
- 13. van Haaster LH, de Jong FH, Docter R, de Rooij DG. High neonatal triiodothyronine levels reduce the period of Sertoli cell proliferation and accelerate tubular lumen formation in the rat testis, and increase serum inhibin levels. Endocrinology. 1993;133:755–760. [DOI] [PubMed] [Google Scholar]
- 14. Hardy MP, Kirby JD, Hess RA, Cooke PS. Leydig cells increase their numbers but decline in steroidogenic function in the adult rat after neonatal hypothyroidism. Endocrinology. 1993;132:2417–2420. [DOI] [PubMed] [Google Scholar]
- 15. Hardy MP, Sharma RS, Arambepola NK, et al. Increased proliferation of Leydig cells induced by neonatal hypothyroidism in the rat. J Androl. 1996;17:231–238. [PubMed] [Google Scholar]
- 16. Mendis-Handagama SM, Ariyaratne HB, Teunissen van Manen KR, Haupt RL. Differentiation of adult Leydig cells in the neonatal rat testis is arrested by hypothyroidism. Biol Reprod. 1998;59:351–357. [DOI] [PubMed] [Google Scholar]
- 17. Siril Ariyaratne HB, Ian Mason J, Mendis-Handagama SM. Effects of thyroid and luteinizing hormones on the onset of precursor cell differentiation into leydig progenitor cells in the prepubertal rat testis. Biol Reprod. 2000;63:898–904. [DOI] [PubMed] [Google Scholar]
- 18. Manna PR, Kero J, Tena-Sempere M, Pakarinen P, Stocco DM, Huhtaniemi IT. Assessment of mechanisms of thyroid hormone action in mouse Leydig cells: regulation of the steroidogenic acute regulatory protein, steroidogenesis, and luteinizing hormone receptor function. Endocrinology. 2001;142:319–331. [DOI] [PubMed] [Google Scholar]
- 19. Manna PR, Roy P, Clark BJ, Stocco DM, Huhtaniemi IT. Interaction of thyroid hormone and steroidogenic acute regulatory (StAR) protein in the regulation of murine Leydig cell steroidogenesis. J Steroid Biochem Mol Biol. 2001;76:167–177. [DOI] [PubMed] [Google Scholar]
- 20. Van Haaster LH, De Jong FH, Docter R, De Rooij DG. The effect of hypothyroidism on Sertoli cell proliferation and differentiation and hormone levels during testicular development in the rat. Endocrinology. 1992;131:1574–1576. [DOI] [PubMed] [Google Scholar]
- 21. Maran RR, Ravisankar B, Ravichandran K, Valli G, Arunakaran J, Aruldhas MM. Impact of neonatal onset hypothyroidism on Sertoli cell number, plasma and testicular interstitial fluid androgen binding protein concentration. Endocr Res. 1999;25:307–322. [DOI] [PubMed] [Google Scholar]
- 22. Cooke PS, Zhao YD, Bunick D. Triiodothyronine inhibits proliferation and stimulates differentiation of cultured neonatal Sertoli cells: possible mechanism for increased adult testis weight and sperm production induced by neonatal goitrogen treatment. Biol Reprod. 1994;51:1000–1005. [DOI] [PubMed] [Google Scholar]
- 23. Buzzard JJ, Wreford NG, Morrison JR. Thyroid hormone, retinoic acid, and testosterone suppress proliferation and induce markers of differentiation in cultured rat Sertoli cells. Endocrinology. 2003;144:3722–3731. [DOI] [PubMed] [Google Scholar]
- 24. Holsberger DR, Jirawatnotai S, Kiyokawa H, Cooke PS. Thyroid hormone regulates the cell cycle inhibitor p27Kip1 in postnatal murine Sertoli cells. Endocrinology. 2003;144:3732–3738. [DOI] [PubMed] [Google Scholar]
- 25. Holsberger DR, Cooke PS. Understanding the role of thyroid hormone in Sertoli cell development: a mechanistic hypothesis. Cell Tissue Res. 2005;322:133–140. [DOI] [PubMed] [Google Scholar]
- 26. Forrest D, Vennström B. Function of thyroid hormone receptors in mice. Thyroid. 2000;10:41–52. [DOI] [PubMed] [Google Scholar]
- 27. Chatonnet F, Livera G, Fumel B, FouchÉCourt S, Flamant F. Direct and indirect consequences on gene expression of a thyroid hormone receptor α 1 mutation restricted to Sertoli cells. Mol Reprod Dev. 2014;81:1159–1166. [DOI] [PubMed] [Google Scholar]
- 28. St Germain DL, Galton VA, Hernandez A. Defining the roles of iodohyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140:844–851. [DOI] [PubMed] [Google Scholar]
- 30. Wajner SM, dos Santos Wagner M, Melo RC, et al. Type 2 iodothyronine deiodinase is highly expressed in germ cells of adult rat testis. J Endocrinol. 2007;194:47–54. [DOI] [PubMed] [Google Scholar]
- 31. Heuer H, Visser TJ. The pathophysiological consequences of thyroid hormone transporter deficiencies: insights from mouse models. Biochim Biophys Acta. 2013;1830:3974–3978. [DOI] [PubMed] [Google Scholar]
- 32. Kinne A, Schulein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011;4(suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernandez A, Fiering S, Martinez E, Galton VA, St Germain D. The gene locus encoding iodothyronine deiodinase type 3 (Dio3) is imprinted in the fetus and expresses antisense transcripts. Endocrinology. 2002;143:4483–4486. [DOI] [PubMed] [Google Scholar]
- 34. Tsai CE, Lin SP, Ito M, Takagi N, Takada S, Ferguson-Smith AC. Genomic Imprinting contributes to thyroid hormone metabolism in the mouse embryo. Curr Biol. 2002;12:1221–1226. [DOI] [PubMed] [Google Scholar]
- 35. Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid. 2005;15:865–874. [DOI] [PubMed] [Google Scholar]
- 36. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. [DOI] [PubMed] [Google Scholar]
- 37. Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Medina MC, Molina J, Gadea Y, et al. The thyroid hormone-inactivating type III deiodinase is expressed in mouse and human β-cells and its targeted inactivation impairs insulin secretion. Endocrinology. 2011;152:3717–3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ng L, Lyubarsky A, Nikonov SS, et al. Type 3 deiodinase, a thyroid-hormone inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30:3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ng L, Hernandez A, He W, et al. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150:1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120:3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peeters RP, Hernandez A, Ng L, et al. Cerebellar abnormalities in mice lacking type 3 deiodinase and partial reversal of phenotype by deletion of thyroid hormone receptor α1. Endocrinology. 2013;154:550–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Galton VA, Wood ET, St Germain EA, et al. Thyroid hormone homeostasis and action in the type 2 deiodinase-deficient rodent brain during development. Endocrinology. 2007;148:3080–3088. [DOI] [PubMed] [Google Scholar]
- 44. Galton VA, Hiebert A. Hepatic iodothyronine 5-deiodinase activity in Rana catesbeiana tadpoles at different stages of the life cycle. Endocrinology. 1987;121:42–47. [DOI] [PubMed] [Google Scholar]
- 45. Thayer KA, Ruhlen RL, Howdeshell KL, et al. Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17α-ethinyl oestradiol. Hum Reprod. 2001;16:988–996. [DOI] [PubMed] [Google Scholar]
- 46. Chatonnet F, Flamant F, Morte B. A temporary compendium of thyroid hormone target genes in brain. Biochim Biophys Acta. 2015;1849:122–129. [DOI] [PubMed] [Google Scholar]
- 47. Gilleron J, Nebout M, Scarabelli L, et al. A potential novel mechanism involving connexin 43 gap junction for control of Sertoli cell proliferation by thyroid hormones. J Cell Physiol. 2006;209:153–161. [DOI] [PubMed] [Google Scholar]
- 48. Fumel B, Guerquin MJ, Livera G, et al. Thyroid hormone limits postnatal Sertoli cell proliferation in vivo by activation of its α1 isoform receptor (TRα1) present in these cells and by regulation of Cdk4/JunD/c-myc mRNA levels in mice. Biol Reprod. 2012;87:16, 11–19. [DOI] [PubMed] [Google Scholar]
- 49. Hernandez A, Martinez ME, Liao XH, et al. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148:5680–5687. [DOI] [PubMed] [Google Scholar]
- 50. Martin SM, Moberg GP. Effects of early neonatal thyroxine treatment on development of the thyroid and adrenal axes in rats. Life Sci. 1981;29:1683–1688. [DOI] [PubMed] [Google Scholar]
- 51. Arambepola NK, Bunick D, Cooke PS. Thyroid hormone and follicle-stimulating hormone regulate Mullerian-inhibiting substance messenger ribonucleic acid expression in cultured neonatal rat Sertoli cells. Endocrinology. 1998;139:4489–4495. [DOI] [PubMed] [Google Scholar]
- 52. Auharek SA, de França LR. Postnatal testis development, Sertoli cell proliferation and number of different spermatogonial types in C57BL/6J mice made transiently hypo- and hyperthyroidic during the neonatal period. J Anat. 2010;216:577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155:4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ng L, Goodyear RJ, Woods CA, et al. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101:3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ferrara AM, Liao XH, Gil-Ibáñez P, et al. Changes in thyroid status during perinatal development of MCT8-deficient male mice. Endocrinology. 2013;154:2533–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Martinez ME, Charalambous M, Saferali A, et al. Genomic imprinting variations in the mouse type 3 deiodinase gene between tissues and brain regions. Mol Endocrinol. 2014;28:1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]