Abstract
Environmental enrichment (EE), a housing condition providing complex physical, social, and cognitive stimulation, leads to improved metabolic health and resistance to diet-induced obesity and cancer. One underlying mechanism is the activation of the hypothalamic-sympathoneural-adipocyte axis with hypothalamic brain-derived neurotrophic factor (BDNF) as the key mediator. VGF, a peptide precursor particularly abundant in the hypothalamus, was up-regulated by EE. Overexpressing BDNF or acute injection of BDNF protein to the hypothalamus up-regulated VGF, whereas suppressing BDNF signaling down-regulated VGF expression. Moreover, hypothalamic VGF expression was regulated by leptin, melanocortin receptor agonist, and food deprivation mostly paralleled to BDNF expression. Recombinant adeno-associated virus-mediated gene transfer of Cre recombinase to floxed VGF mice specifically decreased VGF expression in the hypothalamus. In contrast to the lean and hypermetabolic phenotype of homozygous germline VGF knockout mice, specific knockdown of hypothalamic VGF in male adult mice led to increased adiposity, decreased core body temperature, reduced energy expenditure, and impaired glucose tolerance, as well as disturbance of molecular features of brown and white adipose tissues without effects on food intake. However, VGF knockdown failed to block the EE-induced BDNF up-regulation or decrease of adiposity indicating a minor role of VGF in the hypothalamic-sympathoneural-adipocyte axis. Taken together, our results suggest hypothalamic VGF responds to environmental demands and plays an important role in energy balance and glycemic control likely acting in the melanocortin pathway downstream of BDNF.
In 2014, 52% of adults worldwide were overweight or obese, thus this epidemic has undoubtedly become a global health, social, and economic problem (1, 2). Substantial obesity-related morbidity and mortality have prompted many investigations to explore genes involved in metabolic regulation. We previously manipulated the environment and demonstrated that environmental enrichment (EE), a housing condition providing complex stimuli, leads to robust improvements in metabolism including decreased adiposity, resistance to diet-induced obesity, and mitigation of obesity-associated liver steatosis and insulin resistance in addition to inhibition of peripheral cancers (3–5). Moreover, our mechanistic studies have elucidated a novel neuroendocrine axis, the hypothalamic-sympathoneural-adipocyte (HSA) axis, underlying the EE-induced resistance to obesity and cancer (3, 5, 6). The social, physical, and cognitive stimuli provided by the EE up-regulated brain-derived neurotrophic factor (BDNF) expression in the hypothalamus and subsequently increased sympathetic tone preferentially to the white fat leading to at least 2 consequences. The first was characterized by the induction of beige cells and the ensuing elevated energy expenditure (5), whereas the second was a sharp suppression of leptin expression and release contributing to the cancer inhibition (3, 7). The browning of white adipose tissue (WAT) and leptin suppression can be dissociated. For example, adipose vascular endothelial growth factor up-regulation was required for EE-induced browning but not the drop in leptin (8). Here, we attempted to identify other mediators of the HSA axis in the hypothalamus and found VGF to be closely associated with the HSA axis activation.
VGF (nonacronymic) was first identified as a nerve growth factor-inducible transcript in PC12 pheochromocytoma cells and is expressed widely throughout the nervous system, with the highest expression in the hypothalamus (9–13). VGF expression in hypothalamic neuroendocrine cells leads to its peripheral release and may function by reaching target tissues via circulation or paracrine signaling (14). Aside from nerve growth factor, VGF is also induced by other neurotrophins such as BDNF (15). Accumulating evidence suggests that VGF acts in the melanocortin pathway that regulates energy balance and glucose homeostasis through its sympathetic and parasympathetic innervation to the brown adipose tissue (BAT), WAT, and pancreas (16–21). Homozygous VGF germline knockout mice are small, lean, hypermetabolic, infertile, and have shortened life span (22). The metabolic phenotype is associated with decreased fat storage, increased fatty acid oxidation, increased uncoupling protein 1 (UCP1) expression in BAT, increased lipolysis, and decreased lipogenesis in WAT (23, 24). In addition, heterozygous VGF knockout mice as well as ablation of several bioactive C-terminal VGF peptides showed a decrease in body weight and adiposity, and increased energy expenditure. Germline VGF depletion is also resistant to diet-, lesion- and genetic-induced forms of obesity such as in Ay/a agouti and melanocortin 4 receptor (MC4R)-deficient mice (25, 26). Conversely, germline overexpression of VGF resulted in increased body weight, although had little effect on energy expenditure (27). Paradoxically, however, central and peripheral administration of TLQP-21, the most well-characterized active VGF peptide deriving from the VGF peptide precursor, resulted in increased energy expenditure, decreased adiposity, elevated lipolysis, enhanced glucose-stimulated insulin secretion, and improved glycemic control (28–30). Similarly, activating the HSA axis via environmental (by EE) or genetic (by overexpressing BDNF in the hypothalamus) methods lead to leanness, increased energy expenditure, and up-regulated VGF expression. Interestingly, these findings are the opposite of what the VGF germline knockout would have predicted. It has been hypothesized that these contrasting findings are due to bioactive peptides derived from VGF peptide precursor having antagonistic functions, eg, TLQP-21 is catabolic, whereas neuroendocrine regulatory peptide-2 is anabolic (31, 32). Notably, VGF peptides have also shown heterogeneous expression in several neuroendocrine tissues including the stomach and pancreas (30, 33). In addition, the compensatory responses during development may confound the germline knockout findings. This clearly highlights the complexity of VGF functions and the necessity for tissue specific manipulation studies. Here, we further investigated the hypothalamic VGF expression responding to environmental stimulations and its relationship with BDNF. To study the specific role of hypothalamic VGF in energy balance, we used the Cre-loxP system to knockdown VGF specifically in the hypothalamus of adult mice. Furthermore, the role of hypothalamic VGF in EE-induced metabolic adaptations was investigated.
Materials and Methods
Mice
C57BL/6 mice were purchased from Charles River. VGFflpflox/flpflox breeders (>98% C57BL/6J background; MAX-BAX; Charles River Laboratories) were provided by Dr S. Salton of Icahn School of Medicine at Mount Sinai. Vgf gene targeting in VGFflpflox/flpflox mice has recently been described (34); the entire Vgf coding sequence is flanked by 5′ and 3′ loxP sites, and the pgk-neo selection cassette in the Vgf 3′ untranslated region was excised in this line. All mice were housed in temperature (22°C–23°C) and humidity controlled rooms with a 12-hour light, 12-hour dark cycle, and maintained on a standard rodent diet (7912 rodent chow; Teklad) with food and water ad libitum. All use of animals was approved by and conducted in accordance with the Ohio State University Institutional Animal Care and Use Committees.
EE protocol
Male 3-week old C57BL/6 mice (from Charles River) were housed in large cages (63 × 49 × 44 cm, 5 mice per cage) supplemented with running wheels, tunnels, igloos, huts, retreats, wood toys, a maze, and nesting material in addition to standard lab chow and water. Control mice were housed under standard laboratory conditions (5 mice per cage).
Recombinant adeno-associated virus (rAAV) vector construction and packaging
The rAAV plasmid contains a vector expression cassette consisting of the cytomegalovirus enhancer and chicken β-actin promoter, woodchuck posttranscriptional regulatory element, and bovine GH poly-A flanked by AAV inverted terminal repeats. Transgenes: human TrkB isoform 1 (TrkB.T1) cDNA, human BDNF, destabilized yellow fluorescent protein (YFP), were inserted into the multiple cloning sites between the chicken β-actin promoter and woodchuck posttranscriptional regulatory element sequence. rAAV serotype 1 vectors carrying TrkB.T1, BDNF, or YFP were packaged and purified as described elsewhere (35). An engineered hybrid rAAV serotype Rec2 vector carrying Cre or YFP were packaged and purified as described previously (36).
AAV-mediated BDNF overexpression
Male C57BL/6 mice, 8 weeks of age, were randomly assigned to receive AAV1-BDNF or AAV1-YFP. Mice were anesthetized with a single dose of ketamine/xylazine (100 and 20 mg/kg; ip) and secured via ear bars and incisor bar on a Kopf stereotaxic frame. A midline incision was made through the scalp to reveal the skull and 2 small holes were drilled into the skull with a dental drill above the injection sites (−0.8 anteroposterior (AP), ±0.3 medial lateral (ML), −5.0 dorsal ventral (DV); mm from bregma). rAAV1 vectors (1 × 109 vg per site) were injected bilaterally into the hypothalamus at a rate of 0.1 μL/min using a 10-μL Hamilton syringe attached to Micro4 Micro Syringe Pump Controller (World Precision Instruments, Inc). At the end of infusion, the syringe was slowly removed from the brain and the scalp was sutured. Animals were placed back into a clean cage and carefully monitored postsurgery until fully recovered from anesthesia. Mice were maintained on normal chow diet until the end of the study (4 wk after surgery).
AAV-mediated overexpression of dominant-negative TrkB.T1
Male C57BL/6 mice, 8 weeks of age, were randomly assigned to receive AAV1-TrkB.T1 or AAV1-YFP. AAV vectors were injected bilaterally to the hypothalamus (−1.2AP, ±0.5ML, −6.2DV; mm from bregma, 5.0 × 109 vg per site). Body weight and food intake were monitored every 5–7 days. Mice were maintained on normal chow diet until the end of the study (4 wk after surgery).
AAV-microRNA experiment
Seven-week-old male C57BL/6 mice were randomly assigned to receive AAV-miR-Bdnf (n = 12) or AAV-miR-scr (n = 12). AAV vectors were injected bilaterally into the hypothalamus at the stereotaxic coordinates described above (1.4 × 1010 vg per site). Ten days after surgery, half of the miR-Bdnf mice and miR-scr mice were housed in EE (large cage, 1.5 × 1.5 × 1.0 m) with the other half of the groups were maintained in standard housing. After 4 weeks of enriched housing, mice were killed and fat pads were dissected and weighed. The hypothalamus was also microdissected.
BDNF heterozygous mice
BDNF+/− mice breeders were provided by Dr F. Lee of Weill Medical College of Cornell University. Male BDNF+/− mice (n = 4) and age matched C57BL/6 wild-type littermates (n = 4) were killed at the age of 2 months for analysis.
Melanotan-II (MT II) experiment
Male C57BL/6 mice, 6 weeks of age, were single housed and sham injected ip for 3 consecutive days. Mice were fasted overnight and randomly assigned to receive ip injection of MT II (1 μg/g body weight, catalog 2566; R&D Systems) or PBS. Food was offered 15 minutes after MT II injection. Body weight and food intake was recorded 3 hours after injection. Hypothalamus was block dissected, and a portion of cortex was used as control.
Leptin experiment
Male C57BL/6 mice, 6 weeks of age, were single housed and sham ip injected for 3 consecutive days. Mice were randomly assigned to receive ip injection of mouse leptin (2 μg/g body weight per day, catalog 498-OB-01M; R&D Systems) or PBS as control for 2 days. Body weight and food intake were monitored. Mice were killed 30 minutes after the third dose of leptin injection. Hypothalamus was block dissected, and a portion of cortex was used as control.
Acute BDNF experiment
Male C57BL/6 mice, 6 weeks of age, were single housed. Human BDNF (1.428 μg in 2-μL PBS, catalog 248-BD-025/CF; R&D Systems) was injected unilaterally to the hypothalamus (−1.3AP, 0.5ML, −5.7DV; mm from bregma). Body weight and food intake were monitored. Mice were killed 24 hours after BDNF injection. Hypothalamus was block dissected, and a portion of cortex was used as control.
AAV-Cre-mediated knockdown of hypothalamic VGF
VGFflpflox/flpflox mice, 6 weeks of age, were assigned randomly to receive Rec2-Cre or Rec2-YFP vector. AAV vectors were injected bilaterally into the hypothalamus at the stereotaxic coordinates described above (1 × 109 vg per site). Both male and female mice were used. Body weights were recorded before injections and each succeeding week for the duration of the 9-week study. Food consumption was recorded at the same time points of body weight as the total food consumption of each cage housing 4–5 mice and represented as the average of food consumption per mouse per day.
Body temperature
Body temperature was recorded 6 weeks after rAAV-Cre injection. Each mouse was anesthetized using isoflourane for 3 minutes, upon which time the rectal temperature was measured with a rectal thermometer.
Hypothalamic dissection
Brains were quickly isolated on ice. The hypothalamus was dissected from 2-mm-thick coronal sections (−0.7 to −2.7 mm from bregma, 1.5 mm dorsal to the bottom of the brain, 1 mm bilateral to the midline) under a dissection scope and stored at −80°C for further analysis.
Western blotting
Hypothalamus dissections were homogenized by 1.5-mL pestle with cordless motor (VWR) in ice-cold radioimmunoprecipitation assay buffer containing phosphatase inhibitors and protease inhibitors cocktail (Pierce). The protein content was assayed by Bicinchoninic acid assay kit (Pierce). A total of 20 μg of protein from each sample was loaded to a precast 4%–20% gradient SDS-PAGE polyacrylamide gel (Bio-Rad), then transferred to nitrocellulose membranes and blocked in PBS containing 5% nonfat dry milk for 1 hour at room temperature. Blots were incubated overnight at 4°C with either rabbit antibody against VGF (Thermo Scientific) or β-actin (13E5; Cell Signaling) diluted (1:1000) in PBS containing 5% nonfat dry milk and 0.1% Tween 20. Membranes were then washed in PBS containing 0.1% Tween 20 and incubated with secondary antirabbit horseradish peroxidase-conjugated antibody (Bio-Rad) for 1 hour at room temperature. Bound antibodies were detected via Super Signal West Pico Chemiluminescent Substrate (Thermo Scientific). Band densities were quantified with ImageJ software.
Quantitative RT-PCR
Fat pads and hypothalamus were dissected at killing. Total RNA was isolated using RNeasy Lipid kit plus ribonuclease-free deoxyribonuclease treatment (QIAGEN). First-strand cDNA was generated using TaqMan Reverse Transcription Reagent (Applied Biosystems). Real-time PCR was carried out using StepOnePlus System (Applied Biosystems) with the Power SYBR Green PCR Master Mix (Applied Biosystems). Primer sequences are available on request. Data were calibrated to endogenous control Actb or Hprt1, and the relative gene expression was quantified using the 2−ΔΔCT method (37).
Glucose tolerance test
Five weeks after rAAV-Cre injection, mice were ip injected with glucose solution (1-mg glucose/g body weight) after an overnight fast. Blood was drawn from the tail at various time points, and the blood glucose concentrations were measured with a portable glucose meter (ReliOn Ultima).
Metabolic studies
We measured energy expenditure and activity of mice using the Oxymax Lab Animal Monitoring System (Columbus Instruments). Individual mouse was habituated to the instrument overnight, and the physiological and behavioral parameters were monitored for 24 hours (activity, food and water consumption, metabolic performance, and temperature). Oxygen consumption, carbon dioxide production, and methane production were normalized to the body weight and corrected to an effective mass value according to the manufacturer's software.
VGF knockdown in EE
VGFflpflox/flpflox mice were randomly assigned to receive Rec2-Cre or Rec2-YFP. AAV vectors were injected bilaterally into the hypothalamus at the stereotaxic coordinates described above (1 × 109 vg per site). Ten days after surgery, half of the YFP mice and Cre mice were housed in EE with the other half of the groups were maintained in standard housing. After 4 weeks of EE, mice were killed and fat pads were dissected and weighed. Hypothalamus was microdissected for analysis.
Serum harvest and biomarker measurement
Blood was collected after decapitation. Serum was prepared by allowing the blood to clot for 30 minutes on ice followed by centrifugation. Serum was at least diluted 1:5 in serum assay diluent and assayed using the following DuoSet ELISA Development System (R&D Systems): mouse leptin, adiponectin/Acrp30. Insulin was measured using Mercodia ultrasensitive mouse insulin ELISA (ALPCO Diagnostic). Glucose was measured using QuantiChrom Glucose Assay (BioAssay Systems). Total cholesterol was measured using Cholesterol E test kit (Wako Diagnostics). Triglyceride was measured using L-Type test (Wako Dianostics).
Confocal image
Mice were intracardially perfused with 4% paraformaldehyde (Sigma); fixed brains were sectioned on a Leica cryostat at 30 μm. Sections were washed in PBS, blocked, and incubated with primary antibody (rabbit anti-neuropeptide Y (NPY), 1:2000, catalog ab30914 [Abcam]; rabbit anti-proopiomelanocortin (POMC), 1:50, catalog H-029-30 [Phoenix Pharmaceuticals]) overnight at 4°C followed by incubation with secondary antibody (Invitrogen Alexa Fluor) at room temperature for 1 hour. Sections were mounted and coverslipped with ProLong Diamond Antifade Mountant with 4',6-Diamidino-2-phenylindole (Thermo Fisher), and ×60 imaging was performed on a Zeiss FluoView FV1000 confocal microscope.
Adipose tissue immunohistochemistry
Paraffin-embedded sections (4 μm) of adipose tissues were subjected to citrate-based antigen retrieval following by incubations with antibody against UCP1 (1:500; Abcam). The sections were visualized with 3,3'-diaminobenzidine and counterstained with hematoxylin.
Statistical analysis
Data are expressed as mean ± SEM. We used JMP software to analyze the following: unpaired, 2-tailed Student's t test for comparison between 2 groups; one-way ANOVA with a post hoc test for comparisons between multiple groups. Differences were considered statistically significant at P < .05.
Results
VGF acts downstream of BDNF in the melanocortin pathway
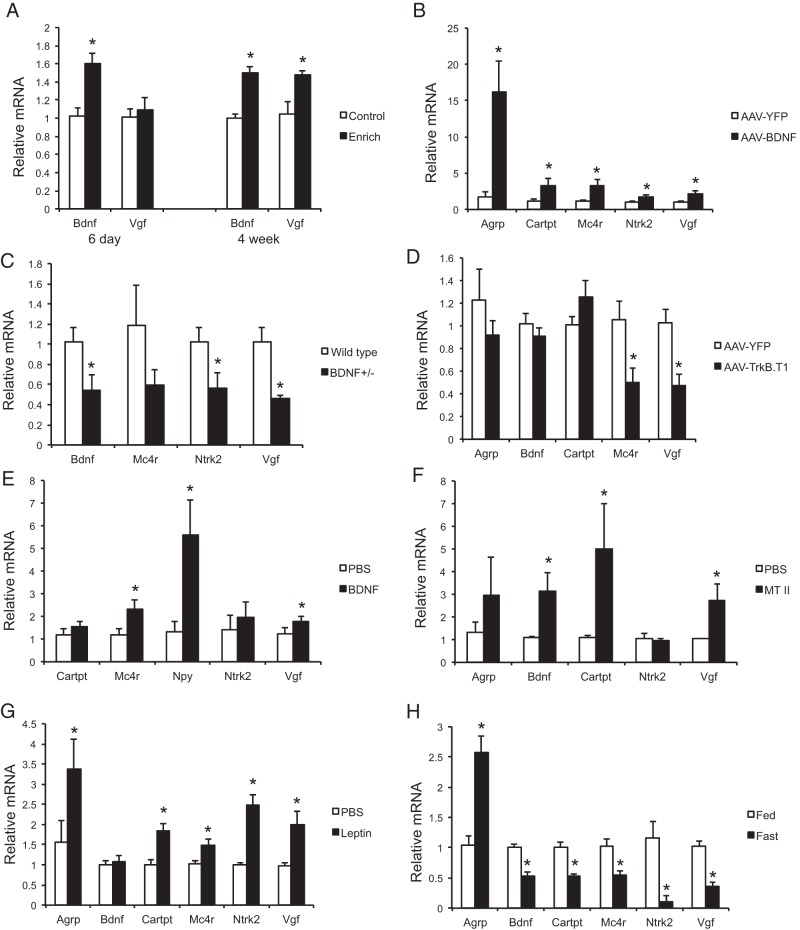
Male C57Bl/6 mice were housed in EE or control housing for 6 days or 4 weeks. Quantitative RT-PCR of the hypothalamus block dissection showed significant up-regulation of Bdnf mRNA levels as early as 6 days EE and the up-regulation sustained to 4 weeks. In contrast, Vgf expression was up-regulated only at 4-week time point (Figure 1A). rAAV-mediated overexpression of human BDNF in the hypothalamus of mice resulted in the up-regulation of Vgf, as well as Mc4r (upstream of BDNF) (38), BDNF receptor TrkB (encoded by Ntrk2), and neuropeptides involved in regulation of energy balance such as agouti-related peptide (encoded by Argp) and cocaine- and amphetamine-regulated transcript prepropeptide (encoded by Cartpt) compared with YFP control (Figure 1B). BDNF heterozygous mice, whose BDNF protein level is about 40% of wild type, develop adult onset obesity (39). Vgf expression was down-regulated together with Bdnf and Ntrk2 in the hypothalamus of BDNF heterozygous mice (Figure 1C). To inhibit BDNF signaling specifically in the hypothalamus, we generated a rAAV vector to deliver a dominant-negative truncated form of the high-affinity BDNF receptor (TrkB.T1) to the hypothalamus. TrkB.T1 mice showed hyperphagia and obesity (5). The functional blockade by TrkB.T1 significantly down-regulated both Mc4r and Vgf expression in the hypothalamus without affecting Bdnf expression (Figure 1D).
Figure 1.
Hypothalamic Vgf expression responding to the HSA axis activation and genetic or pharmacological use of molecules of the melanocortin pathway. Hypothalamic gene expression profile (A) after 6 days and 4 weeks of EE, (B) 4 weeks after rAAV-BDNF injection to the hypothalamus, (C) of heterozygous BDNF mice, (D) 6 weeks after rAAV-TrkB.T1 injection to the hypothalamus, (E) after acute injection of human BDNF protein to the hypothalamus, (F) after ip injection of MT II, (G) after ip injection of leptin, and (H) after food deprivation for 48 hours. Data are mean ± SEM, n = 5–6 per group; *, P < .05.
In addition to genetic manipulations, pharmacological approaches were used to investigate the acute effects of BDNF, leptin, and MC4R agonist on hypothalamic Vgf expression. Direct injection of BDNF protein to the hypothalamus decreased weight gain (PBS, −1.517 ± 0.323 g and BDNF, −2.767 ± 0.165 g; P = .02) with no significant effect on food intake (PBS, 1.517 ± 0.409 g and BDNF, 1.567 ± 0.308 g; P = .91) and significantly up-regulated the expression of Vgf, Mc4r, and Npy (encoding neuropeptide Y) (Figure 1E). Intraperitoneal administration of MT II, a synthetic analog of α-melanocyte-stimulating hormone and an MC4R agonist that suppresses food intake and body weight (weight gain: PBS, 1.002 ± 0.365 g and MT II, −1.010 ± 0.258 g; P = .002, and food intake: PBS, 1.333 ± 0.211 g and MT II, 0.500 ± 0.224; P = .022) (40) lead to up-regulation of Vgf together with Bdnf (Figure 1F). Leptin acts on the MC4R pathway in the hypothalamus to regulate energy balance (41). Intraperitoneal injection of leptin induced the expression of both Mc4r and Vgf (Figure 1G) with no significant effects on weight gain or food intake (weight gain: PBS, 0.267 ± 0.148 g and leptin, −0.217 ± 0.199 g; P = .082, and food intake: PBS, 3.717 ± 0.315 g and leptin, 3.983 ± 0.309; P = .56). The expression of Vgf in cortex was not changed by acute BDNF, MT II, or leptin (data not shown). Lastly, food deprivation has been shown to inhibit the melanocortin pathway (38). A 48-hour fast resulted in a collective down-regulation of Mc4r, Bdnf, Ntrk2, Vgf, and anorexic neuropeptide Cartpt, which resulted in the up-regulation of orexigenic Agrp (Figure 1H), consistent with the activation of hunger response. Taken together, these results indicated that hypothalamic Vgf responded to environmental challenges, energy demand, and was involved in the melanocortin pathway, likely acting downstream of BDNF.
Hypothalamic knockdown of VGF leads to metabolic disturbance in male mice
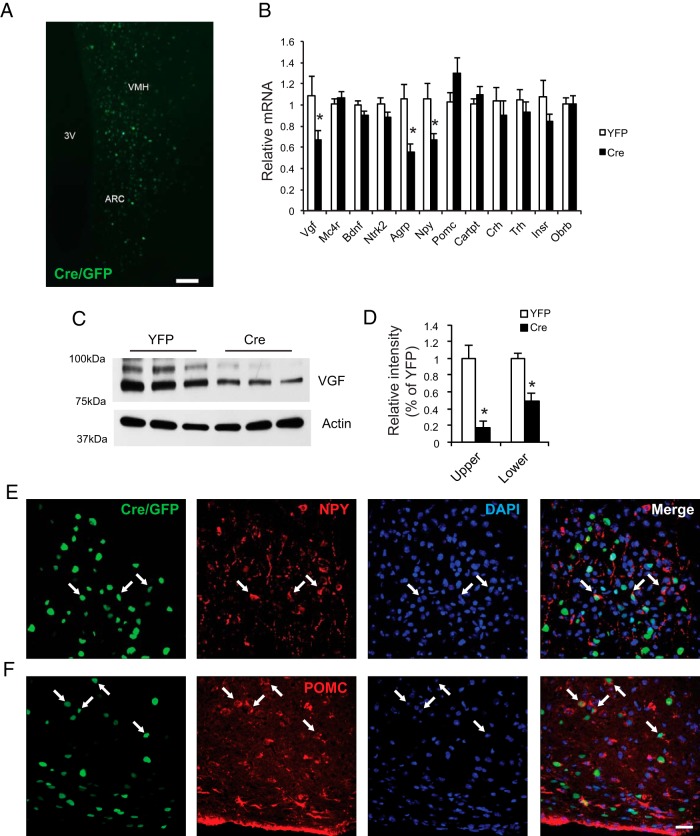
We reported that injection of a novel engineered rAAV serotype Rec2 carrying Cre recombinase fused with GFP (Cre/GFP) to the BAT and WAT of insulin receptorflox/flox mice efficiently knocked down insulin receptor expression resulting in dysfunction of transduced fat pad and impaired whole body glucose tolerance (36). To avoid high level of Cre-associated toxicity in the hypothalamus, we performed a titer titration experiment in wild-type mice and found bilateral injection of Rec2-Cre/GFP at the dose of 1 × 109 vg per site showed no difference of body weight or food intake compared with mice receiving Rec2-YFP (data not shown). Thus Rec2-Cre/GFP at this dose was injected to the hypothalamus of the male VGFflpflox/flpflox at 6 weeks of age with YFP as control. GFP fluorescence (from fusion protein to Cre) was observed in both the arcuate nucleus (Arc) and the ventromedial hypothalamus (VMH) (Figure 2A), confirming that the transgene expression was largely confined in the hypothalamus. Confocal microscopy showed that Cre/GFP was colocalized with both NPY (Figure 2E) and POMC (Figure 2F) indicating that the physiological readout was based on the overall lack of VGF in both anorexigenic and orexigenic circuits. Western blotting showed VGF protein in the hypothalamic lysates as a doublet at 80 and 90 kDa (Figure 2C) (27). Quantification of Western blotting showed knockdown in both bands with approximately 80% decrease in the upper band, and approximately 50% decrease in the lower band (Figure 2D). The VGF knockdown was verified by quantitative RT-PCR showing approximately 40% decrease of Vgf mRNA (Figure 2B). Interestingly, neither Mc4r nor Bdnf expression was affected by Vgf knockdown (Figure 2B).
Figure 2.
Hypothalamic-specific knockdown of VGF. A, Representative GFP fluorescence of rAAV-Cre/GFP-injected VGFflpflox/flpflox mice. Scale bar, 100 μm. 3V, third ventricle. B, Gene expression profile of the hypothalamus. n = 8 YFP, n = 9 Cre. C, Western blot analysis of hypothalamic lysates using antibody targeting the carboxy-terminal region of full-length VGF and cleaved peptides. D, Quantification of VGF Western blotting, n = 4 per group. E, Colocalization of Cre/GFP (green) with NPY (red). F, Colocalization of Cre/GFP (green) with POMC (red). Scale bar, 20 μm. Data are mean ± SEM; *, P < .05.
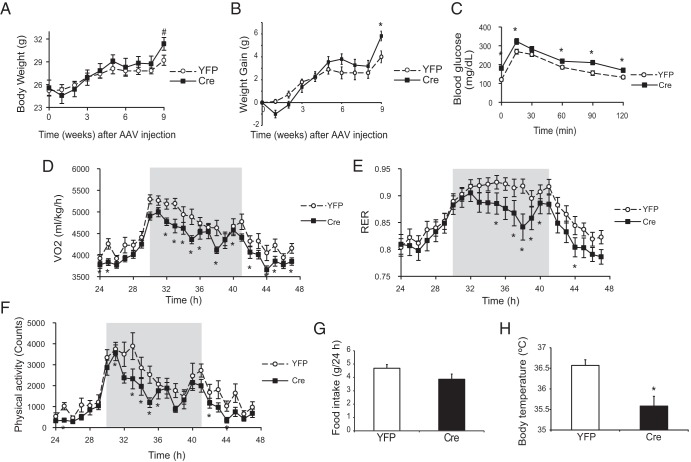
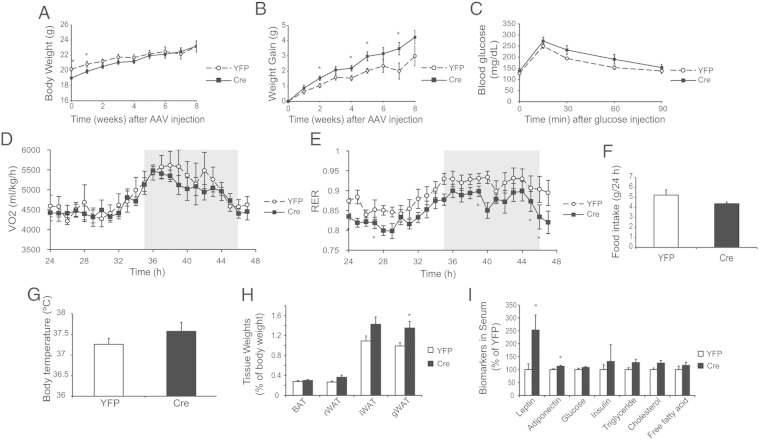
Male VGFflpflox/flpflox mice receiving Cre showed gradually increased weight gain compared with YFP mice (Figure 3, A and B). Body temperature of Cre-injected mice was decreased 6 weeks after AAV injection (Figure 3H). Glucose tolerance was impaired in the mice receiving Cre 5 weeks after AAV injection (Figure 3C). Indirect calorimetry was performed 6.5 weeks after AAV injection. Cre-injected mice showed reductions in oxygen consumption (Figure 3D), respiratory exchange ratio (RER) (Figure 3E), and locomotor activity (Figure 3F) mostly in the dark phase. Food intake was not changed (Figure 3G).
Figure 3.
Characterization of the metabolic phenotypes of male VGFflpflox/flpflox mice receiving rAAV-Cre. A, Body weight. B, Weight gain. C, Glucose tolerance test 5 weeks after rAAV injection. Oxygen consumption (VO2) (D), RER (E), physical activity (F), and food consumption (G) measured by CLAMS. Shaded area, dark phase. H, Rectal temperature measured 6 weeks after rAAV-Cre injection. Data are mean ± SEM, n = 10 YFP, n = 11 Cre; *, P < .05; #, P = .06.
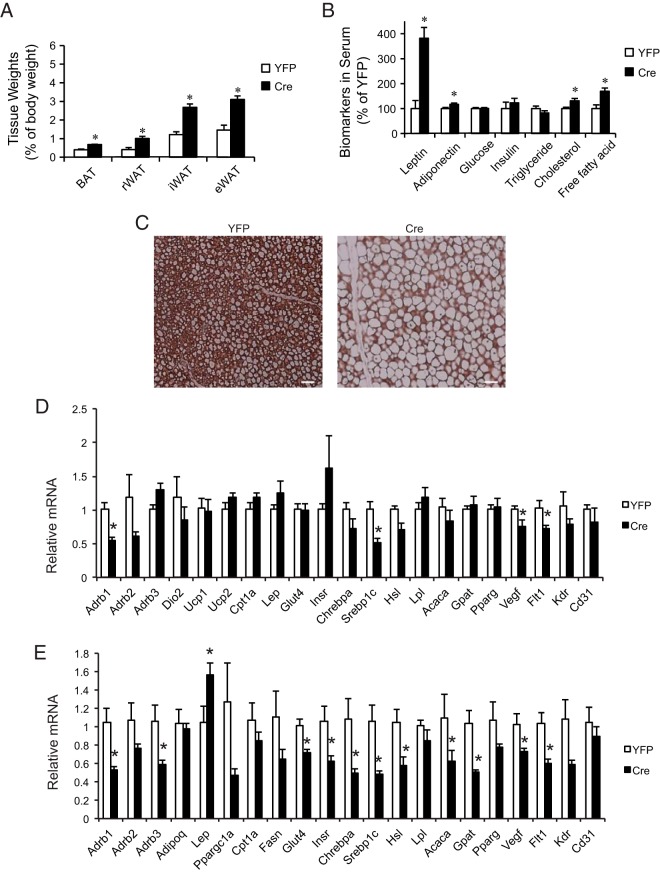
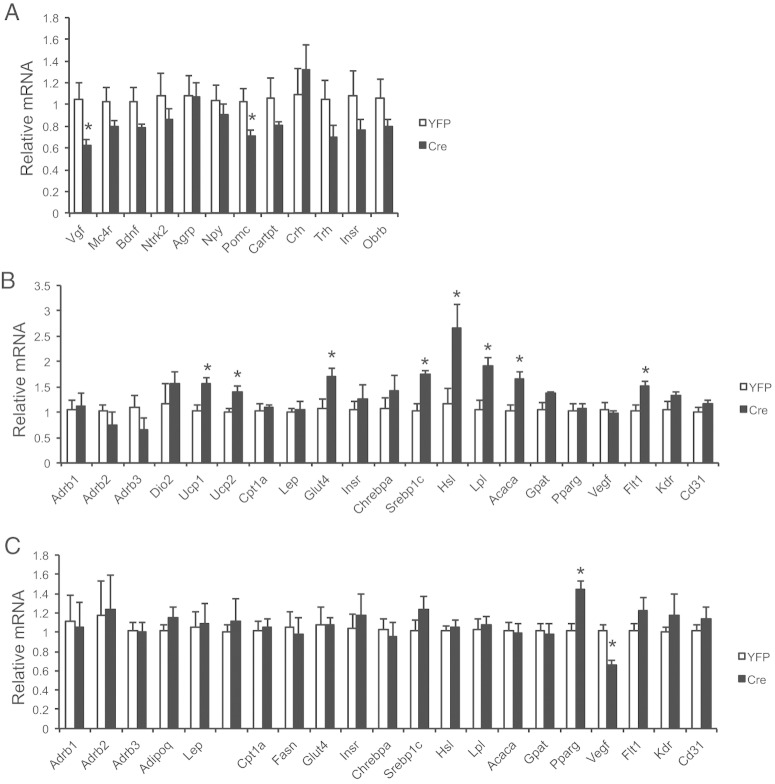
Cre-injected male VGFflpflox/flpflox mice showed significantly increased adiposity 9 weeks after Cre injection (Figure 4A). BAT and sc and visceral WAT depots were all enlarged compared with YFP control (Figure 4A). Serum biomarker analysis showed elevated levels of leptin, adiponectin, cholesterol, and free fatty acid (Figure 4B). The BAT of Cre mice was enlarged but appeared pale. Immunohistochemistry showed substantial loss of UCP1 protein, the key thermogenic protein (42), in the BAT of Cre mice (Figure 4C). However, gene expression profiling of the BAT showed limited changes at mRNA level (Figure 4D). In contrast, the epididymal WAT (eWAT) depot showed extensive gene expression changes (Figure 4E).
Figure 4.
Modulation of adipose tissue by hypothalamic VGF knockdown. A, Adiposity at killing 9 weeks after rAAV-Cre injection. n = 10 YFP, n = 11 Cre. B, Serum biomarkers. n = 5 YFP, n = 6 Cre. C, UCP1 immunohistochemistry of BAT. Scale bar, 40 μm. D, Gene expression profile of BAT. n = 5 YFP, n = 5 Cre. E, Gene expression profile of eWAT. n = 5 YFP, n = 5 Cre. Abbreviations: eWAT, epididymal WAT; iWAT, inguinal WAT; rWAT, retroperitoneal WAT. Data are mean ± SEM; *, P < .05.
Multiple β-adrenergic receptors (Adrb1 in BAT; Adrb1 and Adrb3 in eWAT), sterol regulatory element-binding protein (Srebp1), and Vegf and its receptor 1 (Flt1) were all down-regulated in both the BAT and eWAT after Vgf knockdown. Additionally, eWAT exhibited significant decrease in mRNA expression of glycemic regulators such as glucose transporter type 4 (Glut4) and insulin receptor (Insr) (Figure 4E). Several genes involved in lipid metabolism including both lipogenic genes eg, carbohydrate responsive element-binding protein α (Chrebpa), Srebp1c, and glycerol-3-phosphate acyltransferase (Gpat), and lipolytic genes eg, hormone sensitive lipase (Hsl) and acetyl-Co-A carboxylase 1 (Acaca) were down-regulated (Figure 4E). In addition, leptin (Lep) mRNA level was increased in eWAT of Cre mice consistent with the elevated level of leptin in the circulation (Figure 4E).
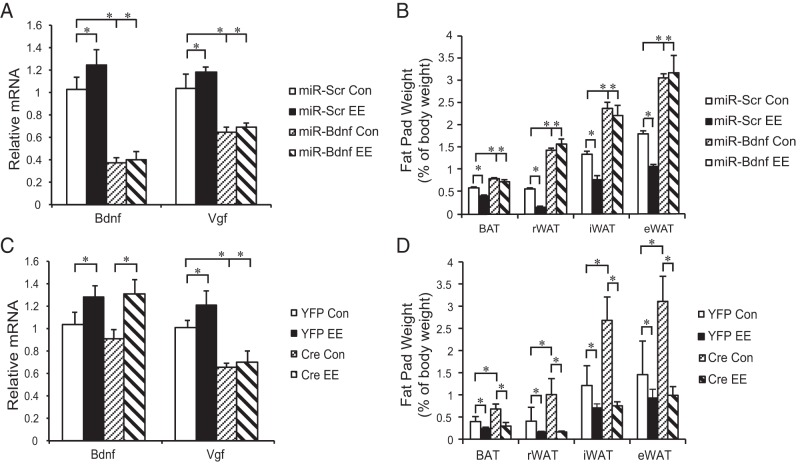
VGF knockdown fails to block the EE-induced phenotypes
Our previous studies have demonstrated that inhibition of the EE-induced hypothalamic BDNF up-regulation using a microRNA targeting BDNF (miR-Bdnf) completely blocks the key effects of EE such as cancer inhibition and browning of WAT (3, 5). Here, we used the same approach to verify the role of BDNF in EE's effects on adiposity and Vgf expression. Male wild-type mice were randomly assigned to receive AAV-miR-Bdnf or AAV-miR-scr targeting a scrambled sequence that does not interfere with any known genes. Half of each AAV vector-injected group was housed in either EE or control housing for 4 weeks. In the mice receiving miR-scr, EE led to up-regulation of both Bdnf and Vgf in the hypothalamus (Figure 5A), as well as decreased adiposity (Figure 5B). miR-Bdnf robustly suppressed Bdnf and Vgf expression in control housing, and completely prevented the EE-induced up-regulation in the hypothalamus (Figure 5A). miR-Bdnf resulted in obesity in control housing and completely blocked the EE-induced decrease of adiposity (Figure 5B).
Figure 5.
Hypothalamic knockdown of Bdnf or Vgf in EE. microRNA targeting Bdnf blocked EE-induced Bdnf and Vgf up-regulation (A) and decrease of adiposity (B), n = 8 per group. Cre-loxP-mediated knockdown of Vgf failed to block the EE-induced Bdnf up-regulation (C) or leanness (D). n = 5 per group. Con, control housing; EE, EE housing; rWAT, retroperitoneal WAT; iWAT, inguinal WAT. Data are mean ± SEM; *, P < .05.
Similar knockdown approach was used to investigate the role of hypothalamic VGF in EE-induced leanness. VGFflpflox/flpflox mice were randomly assigned to receive AAV-Cre or AAV-YFP. Each group was split to live in EE or control housing for 4 weeks. VGF knockdown blocked the EE-induced up-regulation of Vgf but did not inhibit the EE-induced Bdnf up-regulation in the hypothalamus (Figure 5C). Although VGF knockdown resulted in obesity in control housing, it failed to block the EE-induced decrease of adiposity (Figure 5D).
Female mice display blunted response to hypothalamic knockdown of VGF
Previous reports have noted gender differences with regards to the role of VGF in metabolism (27), and also BDNF (43). Therefore, female VGFflpflox/flpflox mice were also subjected to Cre-mediated hypothalamic VGF knockdown. Quantitative reverse transcription PCR revealed similar knockdown in females, an approximately 40% decrease at mRNA level (see Figure 7A). Parallel to the male study, metabolic analyses were completed on the same timeline to determine the effect of VGF depletion in adult female mice. Interestingly, the drops of body temperature and reduced oxygen consumption observed in male mice (Figure 3, D and H) were not found in Cre-injected female mice (Figure 6, D and G). Moreover, the impairment of glucose tolerance was not significant in female mice (Figure 6C). VGF knockdown in females led to milder effects on adiposity or serum biomarkers (Figure 6, H and I) than that in the male mice (Figure 4, A and B). Gene expression analysis of the BAT and gonadal WAT displayed a distinct profile (Figure 7, B and C) compared with the males (Figure 4, D and E).
Figure 7.
Gene expression profiles of female VGFflpflox/flpflox mice receiving rAAV-Cre. A, Hypothalamus. B, BAT. C, Perigonadal WAT. Data are mean ± SEM, n = 5 YFP, n = 6 Cre; *, P < .05.
Figure 6.
Characterization of the metabolic phenotypes of female VGFflpflox/flpflox mice receiving rAAV-Cre. A, Body weight. B, Weight gain. C, Glucose tolerance test 5 weeks post-rAAV injection. D, Oxygen consumption (VO2). E, Respiratory exchange ration (RER). F, Food consumption measured by CLAMS. Shaded area = dark phase. G, Rectal temperature measured 6 weeks after rAAV-Cre injection. H, Adiposity at killing 9 weeks after rAAV-Cre injection. I, Serum biomarkers. Data are mean ± SEM, n = 10 YFP, n = 12 Cre; *, P < .05.
Discussion
Our pharmacological and genetic data demonstrated that VGF in the hypothalamus was responsive to environmental demand, likely downstream of BDNF, and involved in the MC4R pathway regulating energy homeostasis. Several lines of evidence support this notion. 1) EE is an accepted model of eustress that introduces mild and controllable challenges and is associated with beneficial effects on health and resistance to diseases including obesity and cancer (3, 5, 6, 35, 44, 45). EE up-regulated Vgf expression in the hypothalamus after the up-regulation of Bdnf. 2) Hypothalamic Vgf expression often parallel to the changes of Bdnf. Food deprivation down-regulated the expression of both Bdnf and Vgf in the hypothalamus while had no effects in cortex. MC4R agonist MT II up-regulated both Bdnf and Vgf also in the hypothalamus not the cortex. 3) BDNF regulated Vgf expression in the hypothalamus. Acute infusion of BDNF protein or long-term overexpression of BDNF in the hypothalamus up-regulated Vgf expression. In contrast, inhibition of BDNF signaling via heterozygous knockout, miR-Bdnf-mediated knockdown, or dominant-negative TrkB all down-regulated Vgf expression. 4) Hypothalamic VGF knockdown had no significant impact on Bdnf expression. 5) Hypothalamic Bdnf up-regulation was essential for EE-induced leanness, which was completely blocked by miR-Bdnf. In contrast, prevention of EE-induced Vgf up-regulation failed to attenuate the decrease of adiposity or Bdnf up-regulation, suggesting a minor role of VGF in the HSA axis, with respect to EE. Previous studies examined the effects of fasting on hypothalamic VGF expression by in situ hybridization (22, 33, 46). However, results have been varied and some are discrepant to our finding by quantitative reverse transcription PCR on block dissection of the hypothalamus. The discrepancy is very likely dependent on the hypothalamic nuclei examined, and possibly different VGF transcripts responded to fasting distinctly in discrete nuclei. Indeed, differential fasting-induced expression of Vgf mRNA and protein in the medial Arc (Vgf increased in NPY neurons) compared with more lateral areas of the Arc (Vgf decreased in POMC neurons) has previously been reported (25, 46). In addition, BDNF levels are reduced by fasting in the nearby VMH (43), a region included in our block dissection, suggesting that relatively robust Vgf down-regulation due to reduced BDNF levels in VMH may have resulted in an overall reduction in Vgf levels with fasting in our tissue sample. Nonetheless, the dissection method used in this study was consistent across all the experiments allowing sufficient understanding of the relationship between Bdnf and Vgf, as well as the relative roles of them in the HSA axis.
Knockdown of VGF specifically in the hypothalamus of adult male mice led to disturbance of energy balance with the following characteristics: increase of adiposity; lower core body temperature; impaired glucose tolerance; decrease of energy expenditure without change of food intake; elevated levels of leptin, cholesterol, and fatty acid in circulation; “whitening” of BAT; and suppression of the expression of genes involved in lipid metabolism and glucose metabolism in WAT. This phenotype of hypothalamic knockdown was mostly opposite to the germline global knockout mice (22) but instead largely consistent to the functions of a VGF-derived peptide TLQP-21 observed in the adult animals (28). In comparison, knockdown in adult hippocampus and global VGF knockout in the germline result in very similar effects on contextual fear memory and depressive behavior (34 and S.R.J. Salton, unpublished data), suggesting different developmental roles for VGF in hypothalamic and hippocampal circuits, and potentially the function of distinct bioactive VGF peptides in the hypothalamus during development and in the adult. Furthermore, the results of our knockdown study are supportive to the findings that hypothalamic VGF was up-regulated by the HSA axis activation and often mirrored BDNF in the same regions of the hypothalamus. Vgf gene produces a peptide precursor that is further processed to generate several mature peptides with distinct functions (31, 33). To add more complexity, 2 receptors have been identified that bind TLQP-21, the G protein-coupled complement 3a complement receptor (47), and the gC1q complement receptor (48), whereas whether other VGF-derived peptides act on distinct receptors remains unknown. Future investigations on the levels of various VGF-derived peptides and their functions in response to environmental and energy demands will advance the elucidation of the mechanisms.
The metabolic phenotype of the hypothalamic VGF knockdown was gender-dependent. The decreased energy expenditure and significant impairment of glucose tolerance were not observed in female mice although the level of VGF knockdown in the hypothalamus was similar to that in the male mice. The gender difference concerning VGF has been found in other models. For instance, male humanized VGF knockin mice fed high-fat diet most consistently demonstrated modulation of energy expenditure, whereas the females showed the greatest change in body weight and adiposity when fed standard chow. Additionally, male VGF C-terminal mutated mice showed significant gene expression changes in muscle, whereas females showed no changes (27). Moreover, VGF-derived peptide TLQP-21 has been shown to increase energy expenditure and lipolysis by increasing sympathetic tone in adipose tissues (28, 29). These effects of TLQP-21 were also more pronounced in the males. Lastly, sexually dimorphic regulation of BDNF expression in rat VMH by dietary intervention has been reported (43), with fasted males but not females showing reduced VMH BDNF expression, which was associated with more robust decreases in % body weight and % body fat in fasted males compared with fasted females. These data suggest distinct BDNF regulation and/or VMH circuitry in males and females, which could result in downstream differences between fasted males and females in their hypothalamic VGF expression levels and function. These differences may be reflected in the distinct metabolic phenotypes we noted here in males and females after hypothalamic VGF down-regulation. The mechanisms controlling gender-specific hypothalamic BDNF and VGF expression require further investigation.
In summary, hypothalamic VGF was regulated by the physical and social environments, and likely involved in the melanocortin pathway downstream of BDNF. Modest knockdown of VGF specifically in the hypothalamus of adult male mice led to obesity and impaired glycemic control, whereas females were resistant to the disturbance of metabolic regulation. Although hypothalamic VGF played an important role in energy homeostasis of adult mice, it was not essential for the HSA axis in contrast to the critical role of BDNF.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA166590 and CA163640 and The Comprehensive Cancer Center fund (L.C.) and by National Institutes of Health Grants DK071308, MH086499, and MH083496 and grants from the Diabetes Action Research and Education Foundation (S.R.J.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP
- anteroposterior
- Arc
- arcuate nucleus
- BAT
- brown adipose tissue
- BDNF
- brain-derived neurotrophic factor
- Cre/GFP
- Cre recombinase fused with GFP
- DV
- dorsal ventral
- EE
- environmental enrichment
- eWAT
- epididymal WAT
- HSA
- hypothalamic-sympathoneural-adipocyte
- MC4R
- melanocortin 4 receptor
- ML
- medial lateral
- MT II
- melanotan-II
- NPY
- neuropeptide Y
- POMC
- proopiomelanocortin
- rAAV
- recombinant adeno-associated virus
- UCP1
- uncoupling protein 1
- VMH
- ventromedial hypothalamus
- WAT
- white adipose tissue
- YFP
- yellow fluorescent protein.
References
- 1. World Health Organization. Obesity and overweight. Fact Sheet No. 311. Geneva, Switzerland: World Health Organization; 2015. [Google Scholar]
- 2. Batsis JA, Nieto-Martinez RE, Lopez-Jimenez F. Metabolic syndrome: from global epidemiology to individualized medicine. Clin Pharmacol Ther. 2007;82:509–524. [DOI] [PubMed] [Google Scholar]
- 3. Cao L, Liu X, Lin EJ, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cao L, Lin EJ, Cahill MC, Wang C, Liu X, During MJ. Molecular therapy of obesity and diabetes by a physiological autoregulatory approach. Nat Med. 2009;15:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao L, Choi EY, Liu X, et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;35:331–345. [DOI] [PubMed] [Google Scholar]
- 7. Liu X, McMurphy T, Xiao R, Slater A, Huang W, Cao L. Hypothalamic gene transfer of BDNF inhibits breast cancer progression and metastasis in middle age obese mice. Mol Ther. 2014;22:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. During MJ, Liu X, Huang W, et al. Adipose VEGF links the white-to-brown fat switch with environmental, genetic, and pharmacological stimuli in male mice. Endocrinology. 2015;156:2059–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levi A, Eldridge JD, Paterson BM. Molecular cloning of a gene sequence regulated by nerve growth factor. Science. 1985;229:393–395. [DOI] [PubMed] [Google Scholar]
- 10. van den Pol AN, Bina K, Decavel C, Ghosh P. VGF expression in the brain. J Comp Neurol. 1994;347:455–469. [DOI] [PubMed] [Google Scholar]
- 11. van den Pol AN, Decavel C, Levi A, Paterson B. Hypothalamic expression of a novel gene product, VGF: immunocytochemical analysis. J Neurosci. 1989;9:4122–4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Snyder SE, Pintar JE, Salton SR. Developmental expression of VGF mRNA in the prenatal and postnatal rat. J Comp Neurol. 1998;394:64–90. [PubMed] [Google Scholar]
- 13. Snyder SE, Salton SR. Expression of VGF mRNA in the adult rat central nervous system. J Comp Neurol. 1998;394:91–105. [PubMed] [Google Scholar]
- 14. Salton SR, Ferri GL, Hahm S, et al. VGF: a novel role for this neuronal and neuroendocrine polypeptide in the regulation of energy balance. Front Neuroendocrinol. 2000;21:199–219. [DOI] [PubMed] [Google Scholar]
- 15. Alder J, Thakker-Varia S, Bangasser DA, et al. Brain-derived neurotrophic factor-induced gene expression reveals novel actions of VGF in hippocampal synaptic plasticity. J Neurosci. 2003;23:10800–10808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fan W, Dinulescu DM, Butler AA, Zhou J, Marks DL, Cone RD. The central melanocortin system can directly regulate serum insulin levels. Endocrinology. 2000;141:3072–3079. [DOI] [PubMed] [Google Scholar]
- 17. Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. [DOI] [PubMed] [Google Scholar]
- 18. Giordano A, Frontini A, Murano I, et al. Regional-dependent increase of sympathetic innervation in rat white adipose tissue during prolonged fasting. J Histochem Cytochem. 2005;53:679–687. [DOI] [PubMed] [Google Scholar]
- 19. Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1613–R1621. [DOI] [PubMed] [Google Scholar]
- 20. Song CK, Jackson RM, Harris RB, Richard D, Bartness TJ. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1467–R1476. [DOI] [PubMed] [Google Scholar]
- 21. Voss-Andreae A, Murphy JG, Ellacott KL, et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. [DOI] [PubMed] [Google Scholar]
- 22. Hahm S, Mizuno TM, Wu TJ, et al. Targeted deletion of the Vgf gene indicates that the encoded secretory peptide precursor plays a novel role in the regulation of energy balance. Neuron. 1999;23:537–548. [DOI] [PubMed] [Google Scholar]
- 23. Watson E, Fargali S, Okamoto H, et al. Analysis of knockout mice suggests a role for VGF in the control of fat storage and energy expenditure. BMC Physiol. 2009;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fargali S, Scherer T, Shin AC, Sadahiro M, Buettner C, Salton SR. Germline ablation of VGF increases lipolysis in white adipose tissue. J Endocrinol. 2012;215:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahm S, Fekete C, Mizuno TM, et al. VGF is required for obesity induced by diet, gold thioglucose treatment, and agouti and is differentially regulated in pro-opiomelanocortin- and neuropeptide Y-containing arcuate neurons in response to fasting. J Neurosci. 2002;22:6929–6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Watson E, Hahm S, Mizuno TM, et al. VGF ablation blocks the development of hyperinsulinemia and hyperglycemia in several mouse models of obesity. Endocrinology. 2005;146:5151–5163. [DOI] [PubMed] [Google Scholar]
- 27. Sadahiro M, Erickson C, Lin WJ, et al. Role of VGF-derived carboxy-terminal peptides in energy balance and reproduction: analysis of “humanized” knockin mice expressing full-length or truncated VGF. Endocrinology. 2015;156:1724–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bartolomucci A, La Corte G, Possenti R, et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc Natl Acad Sci USA. 2006;103:14584–14589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Possenti R, Muccioli G, Petrocchi P, et al. Characterization of a novel peripheral pro-lipolytic mechanism in mice: role of VGF-derived peptide TLQP-21. Biochem J. 2012;441:511–522. [DOI] [PubMed] [Google Scholar]
- 30. Stephens SB, Schisler JC, Hohmeier HE, et al. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 2012;16:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartolomucci A, Possenti R, Levi A, Pavone F, Moles A. The role of the vgf gene and VGF-derived peptides in nutrition and metabolism. Genes Nutr. 2007;2:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Toshinai K, Yamaguchi H, Kageyama H, et al. Neuroendocrine regulatory peptide-2 regulates feeding behavior via the orexin system in the hypothalamus. Am J Physiol Endocrinol Metab. 2010;299:E394–E401. [DOI] [PubMed] [Google Scholar]
- 33. Brancia C, Cocco C, D'Amato F, et al. Selective expression of TLQP-21 and other VGF peptides in gastric neuroendocrine cells and modulation by feeding. J Endocrinol. 2010;207:329–341. [DOI] [PubMed] [Google Scholar]
- 34. Lin WJ, Jiang C, Sadahiro M, et al. VGF and its C-terminal peptide TLQP-62 regulate memory formation in hippocampus via a BDNF-TrkB-dependent mechanism. J Neurosci. 2015;35:10343–10356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827–835. [DOI] [PubMed] [Google Scholar]
- 36. Liu X, Magee D, Wang C, et al. Adipose tissue insulin receptor knockdown via a new primate-derived hybrid recombinant AAV serotype. Mol Ther Methods Clin Dev. 2014;1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔC(T)) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 38. Xu B, Goulding EH, Zang K, et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6:736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lyons WE, Mamounas LA, Ricaurte GA, et al. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA. 1999;96:15239–15244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benoit SC, Schwartz MW, Lachey JL, et al. A novel selective melanocortin-4 receptor agonist reduces food intake in rats and mice without producing aversive consequences. J Neurosci. 2000;20:3442–3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Enriori PJ, Evans AE, Sinnayah P, et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5:181–194. [DOI] [PubMed] [Google Scholar]
- 42. Nicholls DG, Locke RM. Thermogenic mechanisms in brown fat. Physiol Rev. 1984;64:1–64. [DOI] [PubMed] [Google Scholar]
- 43. Liu X, Zhu Z, Kalyani M, Janik JM, Shi H. Effects of energy status and diet on Bdnf expression in the ventromedial hypothalamus of male and female rats. Physiol Behav. 2014;130:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Slater A, Cao L. A protocol for housing mice in an enriched environment. J Vis Exp. 2015;(100):e52874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. [DOI] [PubMed] [Google Scholar]
- 46. Saderi N, Buijs FN, Salgado-Delgado R, et al. A role for VGF in the hypothalamic arcuate and paraventricular nuclei in the control of energy homeostasis. Neuroscience. 2014;265:184–195. [DOI] [PubMed] [Google Scholar]
- 47. Hannedouche S, Beck V, Leighton-Davies J, et al. Identification of the C3a receptor (C3AR1) as the target of the VGF-derived peptide TLQP-21 in rodent cells. J Biol Chem. 2013;288:27434–27443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen YC, Pristerá A, Ayub M, et al. Identification of a receptor for neuropeptide VGF and its role in neuropathic pain. J Biol Chem. 2013;288:34638–34646. [DOI] [PMC free article] [PubMed] [Google Scholar]