Abstract
β-Cell compensation is an essential mechanism by which β-cells increase insulin secretion for overcoming insulin resistance to maintain euglycemia in obesity. Failure of β-cells to compensate for insulin resistance contributes to insulin insufficiency and overt diabetes. To understand the mechanism of β-cell compensation, we characterized the role of forkhead box O1 (FoxO1) in β-cell compensation in mice under physiological and pathological conditions. FoxO1 is a key transcription factor that serves as a nutrient sensor for integrating insulin signaling to cell metabolism, growth, and proliferation. We showed that FoxO1 improved β-cell compensation via 3 distinct mechanisms by increasing β-cell mass, enhancing β-cell glucose sensing, and augmenting β-cell antioxidative function. These effects accounted for increased glucose-stimulated insulin secretion and enhanced glucose tolerance in β-cell-specific FoxO1-transgenic mice. When fed a high-fat diet, β-cell-specific FoxO1-transgenic mice were protected from developing fat-induced glucose disorder. This effect was attributable to increased β-cell mass and function. Furthermore, we showed that FoxO1 activity was up-regulated in islets, correlating with the induction of physiological β-cell compensation in high-fat-induced obese C57BL/6J mice. These data characterize FoxO1 as a pivotal factor for orchestrating physiological adaptation of β-cell mass and function to overnutrition and obesity.
Insulin resistance is characterized by inept responsiveness of peripheral tissues to insulin in obesity and type 2 diabetes. To overcome insulin resistance, β-cells augment insulin synthesis and secretion. Such an adaptive response, termed β-cell compensation, is critical for peripheral tissues to override insulin resistance for maintaining euglycemia in obesity (1). β-Cell compensation culminates in insulin hypersecretion, which is orchestrated through the expansion of β-cell mass and/or up-regulation of insulin synthesis (2). β-Cell compensation ensues in both humans and rodents with increased adiposity (1, 3–5). Failure of β-cells to compensate for insulin resistance results in insulin insufficiency and overt diabetes (2, 6). To date, it remains elusive how β-cells compensate for insulin resistance in obesity and what causes β-cell failure in diabetes.
Although glucose and insulin impact β-cell compensation, the underlying mechanism remains elusive. In response to hyperglycemia, β-cells undergo proliferation, contributing to β-cell mass expansion in rodents (4, 7–9). Glucose also stimulates β-cell replication in human islets engrafted under the kidney capsule of diabetic mice (10). This effect seems to depend on increased glycolysis in β-cells (11), as β-cell deficiency of glucokinase (GK), a key function in glucose sensing and glycolysis, compromises β-cells to undergo cell expansion (12). Likewise, genetic depletion of insulin receptor substrate 2 (Irs2) impairs the ability of β-cells to undergo compensatory hyperplasia in response to insulin resistance, contributing to premature diabetes in mice (13, 14). It follows that disruption of glucose sensing or interception of insulin signaling in islets incapacitates β-cells to compensate for insulin resistance. The underlying mechanisms are poorly understood.
Forkhead box O1 (FoxO1) belongs to the FoxO family that is characterized by a highly conserved DNA binding motif, termed “FoxO” domain (15). FoxO1 acts as a substrate of protein kinase B to mediate insulin action on the expression of genes involved in cell survival, proliferation, metabolism, and differentiation. FoxO1 is expressed mainly in β-cells with little expression in exocrine cells in the pancreas (16). There is clinical evidence that FOXO1 variants are associated with β-cell dysfunction, impaired glucose tolerance, and increased risk of diabetes in humans (17). Preclinical studies show that embryonic FoxO1 deletion impairs glucose-stimulated insulin secretion (GSIS) or causes β-cell degranulation and dedifferentiation in aged mice (18–20). These data, although underscoring the importance of FoxO1 in maintaining β-cell fate and function, fail to reconcile with earlier observations that FoxO1 seems deleterious to β-cell function (16, 21–24). Indeed, Kawamori et al (25) show that FoxO1 somewhat promotes pancreatic and duodenal homeobox 1 (Pdx1) nuclear export and this effect inhibits Pdx1 activity and diminishes insulin synthesis in MIN6 cells. Kitamura et al (21) report that FoxO1 antagonizes FoxA2 binding to the Pdx1 promoter and inhibits FoxA2-mediated induction of Pdx1 expression and insulin synthesis in βTC-3 cells. In contrast, Al-Masri et al (26) show that Pdx1 and FoxO1 colocalize in the nucleus of β-cells in both rodent and human pancreas, consistent with the observation that the expression profile of FoxO1 closely parallels that of Pdx1 in islets during the pancreas development (23). Kitamura et al (27) demonstrate that FoxO1 is acetylated in response to hyperglycemia or H2O2, resulting in its nuclear localization in βTC-3 cells. This effect contributes to the induction of neurogenic differentiation factor (NeuroD) and v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A (MafA), augmenting insulin synthesis/secretion in β-cells (27). These apparent controversies warrant further investigation into the role of FoxO1 in β-cells.
In this study, our goal is to determine whether FoxO1 augments or deteriorates β-cell compensation. To address this fundamental question, we generated β-cell FoxO1-transgenic mice, in which FoxO1 is expressed from the rat insulin II promoter (RIP). Unchecked FoxO1 activity, resulting from its constitutively active allele, is associated with endoplasmic reticulum (ER) stress, which can cause β-cell dysfunction (22, 28, 29). Indeed, adenovirus-mediated expression of constitutively active FoxO1 mutant results in metabolic diapause with a concomitant reduction in glucose use and insulin secretion in INS-1 cells (24). Thus, to avoid potential deleterious effects of unbridled FoxO1 activity on β-cell mass and function, we chose to express wild-type (WT) FoxO1 in islets in transgenic mice. We showed that FoxO1 improved β-cell compensation for insulin resistance via multiple mechanisms by expanding β-cell mass, enhancing β-cell glucose sensing and augmenting β-cell antioxidative function. As a result, β-cell FoxO1-transgenic mice were protected from developing fat-induced glucose disorder. Furthermore, we showed that β-cell FoxO1 activity was up-regulated in response to overnutrition, contributing to physiological induction of β-cell compensation for insulin resistance in WT C57BL/6J obese mice. Together these data indicate that FoxO1 is instrumental for orchestrating the physiological adaptation of β-cell mass and function to overnutrition and obesity.
Materials and Methods
Mice
C57BL/6J mice were obtained from The Jackson Laboratory. Mice were fed standard rodent chow and water ad libitum with a 12-hour light, 12-hour dark cycle in a barrier facility. β-Cell-specific FoxO1 transgenic mice were generated using the RIP for driving the mouse FoxO1 cDNA. The KpnI-XbaI DNA fragment containing the RIP-directed FoxO1 cDNA was isolated from pHD382 and microinjected into the pronuclei of C57 B6C3F1/J ova. We obtained a total of 24 founder generation mice from 7 pregnant female mice. Transgenic mice were identified by PCR analysis using primers (forward 5′-AAGAGCGTGCCCTACTTCAA-3′ and reverse 5′-CTCTTGCCCAGACTGGAGAG-3′). We confirmed 6 founder mice, 2 of which failed to transmit the transgene to their progenies, and 1 of which did not exhibit any gross phenotype. Three lines were associated with elevated FoxO1 expression in islets, displaying enhanced glucose tolerance. Because the progenies of these 3 founder mice had equivalent levels of FoxO1 transgene expression in islets (by 2-to 3-fold vs control littermates), we chose 1 line (founder number 4) for further characterization. This transgenic line was rederived into C57BL/6J background, followed by crossing with C57BL/6J for 7 generations. The resulting progenies were used in this study. To induce obesity, FoxO1-tg and control littermates (8 wk old) were fed a high-fat diet (fat content, 60 kcal%; Research Diet) for 8 weeks. All experiments were performed in male mice, to avoid the potential impact of hormonal fluctuation associated with the estrous cycle on β-cell function in female mice. All procedures were approved by the institutional animal care and usage committee of University of Pittsburgh.
Glucose tolerance test
Glucose tolerance test was performed in mice as described (30). Mice were fasted for 16 hours, followed ip injection of glucose (2 g/kg) and determination of blood glucose levels.
Insulin tolerance test
Mice were injected ip with regular human insulin (0.75 IU/kg; Eli Lilly and Co), followed by determination of blood glucose levels (31).
Glucose-stimulated insulin secretion
Aliquots (25 μL) of tail vein blood were sampled before and 15 minutes after ip injection of glucose (2 g/kg) to 16-hour fasted mice for determining plasma insulin levels using the ultrasensitive insulin ELISA (ALPCO).
Islet isolation and GSIS
Mice were euthanized, followed by pancreatic intraductal infusion of 3-mL cold Hanks' buffer containing 1.95 mg/mL of collagenase-V (Sigma). The pancreas was procured for islet isolation, as described (32). To measure GSIS, islets were cultured in RPMI 1640 medium overnight, followed by incubation in Krebs buffer containing 2.8mM glucose for 30 minutes. Islets were induced by shifting culture medium from 2.8mM to 20mM glucose concentrations. Aliquots (50 μL) of culture medium were collected at 0 and 30 minutes for determining insulin concentrations.
Human pancreatic tissues
Human pancreatic tissues were obtained from deceased organ donors via the Center for Organ Procurement and Education, Pittsburgh, PA) with consent in accordance with guidelines of the Committee for Oversight of Research Involving the Dead of University of Pittsburgh, as reported (33). Human fetal pancreatic tissues (14- and 21-wk fetal age) were collected according to protocols approved by the Health Sciences Research Ethics Board at the University of Western Ontario in accordance with guidelines of the Canadian Council on Health Sciences Research Involving Human Subjects, as described (26).
Fat mass determination
Fat mass and lean mass of mice were determined using the EchoMRI-100 system (Echo Medical Systems).
Energy expenditure determination
Mice were placed individually in metabolic cages with free access to food and water in the Oxymax Lab Animal Monitoring System (Columbus Instruments). After acclimation for 2 days, oxygen consumption and respiratory exchange ratio of mice were determined during a 48-hour period.
Immunoblot assay
Mice were euthanized after 16-hour fast for tissue procurement. The pancreas or hypothalamus was homogenized in 400-μL Mammalian protein extract reagent supplemented with 4-μL Halt Protease Inhibitor Cocktail (Pierce) for the preparation of nuclear and cytoplasmic proteins. Aliquots (20 μg) of proteins were subjected to immunoblot analysis, using rabbit anti-FoxO1 and mouse anti-β-actin antibodies, as described (31).
RNA isolation and real-time quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
RNA isolation from islets (200–300) was performed using the TRIzol reagent (Invitrogen). Real-time qRT-PCR was used for quantifying mRNA concentrations using the Roche LightCycler-RNA amplification kit (Roche Diagnostics), as described (34). As tabulated in Supplemental Table 1, all primers were obtained from Integrated DNA Technologies.
Immunofluorescent microscopy
Mice were euthanized after 16-hour fast. The pancreas was retrieved and fixed in 4% paraformaldehyde for 16 hours at 4°C, followed by incubation in 30% sucrose overnight at 4°C. Cyrosections (8 μm) were immunostained with rabbit antiinsulin B (sc-7838; Santa Cruz Biotechnology, Inc) or guinea pig anti-Pdx1 (ab47308; Abcam) or rabbit anti-FoxO1 (2880S; Cell Signaling). After washing with PBS buffer containing 0.5% Tween 20, sections were incubated with fluorescein isothiocyanate-conjugated goat antirabbit IgG, or cyanine dye 3-conjugated goat antirabbit IgG, or cyanine dye 3-conjugated donkey antiguinea pig IgG (Jackson ImmunoResearch). The nuclei of cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich), before visualization in the Aviovert 200 fluorescent microscope (Zeiss).
β-Cell mass
Images of pancreas sections immunostained by antiinsulin antibody were subjected to morphometric analysis using MetaMorph Image Analysis software (Molecular Devices). β-Cell area out of total pancreas area per section was determined in 3–5 nonconsecutive sections for determining β-cell mass, as described (20).
In vivo 5-bromo-2-deoxyuridine (BrdU) labeling
FoxO1-tg and WT littermates (male, 4 wk old) were fed high-fat diet for 1 week, followed by ip injection with thymidine analog, BrdU (100 mg/kg) daily for 4 consecutive days. Mice were killed for procuring the pancreas. Cryosections (8 μm) of the pancreas were subjected to immunohistochemistry using anti-BrdU antibody (ab#6326; Abcam) and antiinsulin antibody (sc-7838; Santa Cruz Biotechnology, Inc), followed by immunofluorescent microscopy. BrdU-positive β-cells out of total β-cells in islets in 3–5 nonconsecutive pancreas sections were determined.
Chromatin immunoprecipitation (ChIP) assay
ChIP assay was performed as described (30). Pancreata procured from fasted mice was subjected to ChIP assay for determining FoxO1 interaction with the cyclin D3 (Ccnd3) promoter, using anti-FoxO1 or anti-β-galactosidase IgG as control. The immunoprecipitates were subjected to PCR analysis using the primers (5′-TCTAAAACTGCAAGCAGACC-3′ and 5′-CCCCTAGCACTACTTACATT-3′) flanking the consensus FoxO1 site (−880/−700 nt) within the Ccnd3 promoter. As input control, aliquots of cell lysates (10 μL) before immunoprecipitation were analyzed in the same PCR assay. As off-target control, the immunoprecipitates were subjected to PCR assay, using the primers (5′-GCTTCTCCTAGGACTCGCTAAC-3′ and 5′-CATGTGCGGCTTGATCTCCT-3′) flanking the mouse Ccnd3 cDNA region (−61/+175 nt). To assay FoxO1 binding to the glucose transporter 2 (Glut2) promoter by ChIP assay, we used the primers 5′-GATGTGCGCCACCGCATCTG-3 and 5′-CAGCATCGGTTATCTGTGTG-3′ that flank the proximal region (−638/−318 nt) of the mouse Glut2 promoter. As off-target control, we used the primers (5′-ACCAAAGAGTAAACATGTTG-3′ and 5′-AGAGTATAGGTGGAACTCTG-3′) flanking the distal region devoid of FoxO1 consensus sites (−5004/−4649 nt) in the mouse Glut2 promoter. Likewise, ChIP assay was performed to visualize FoxO1 binding to the mouse superoxide dismutase 1 (Sod1) promoter DNA, using the primers (5′-CTGTGAACATCCACTTCTGT-3′ and 5′-GCTTTATTTTCTCATGATGT-3′) that flank the FoxO1 consensus site (−610/−584 nt) within the Sod1 promoter. As off-target control, the primers (5′-GTACCAGTGCAGGACCTCAT-3′ and 5′-CGCAATCCCAATCACTCCAC-3′) are complementary to the mouse Sod1 coding region (172/460 nt). Similarly, ChIP assay was conducted to assess FoxO1 binding to the mouse catalase (Cat) promoter DNA, using the primers (5′-GTGACAGAGTCTCAAAGTCT-3′ and 5′- TGCTGGTGTTCTCTGACCTC-3′) that flank the FoxO1 consensus site (−367/−340 nt) within the mouse Cat promoter. The off-target primers (5′-GTCCAGTGCGCTGTAGATGT-3′ and 5′-GCGTGTAGGTGTGAATTGCG-3′) are complementary to the mouse Cat coding region (1266/1540 nt).
INS-1 cell culture
INS-1 cells were cultured as described (35). For adenoviral transduction, INS-1 cells were incubated in the presence of Adv-FoxO1 or Adv-Empty control vector (100 plaque forming unit [pfu]/cell), as described (28). After a 24-hour incubation, cells were cultured in the absence or presence of H2O2 (100μM) (Sigma-Aldrich) for 2 hours. Cells were subjected to real-time qRT-PCR analysis for determining prosurvival and apoptotic gene expression.
Luciferase reporter assay
We used the dual luciferase reporter system (Promega) for determining promoter activity. To clone the Ccnd3 promoter (1.5 kb), we subjected mouse genomic DNA to PCR assay using the primers (5′-TCCTGGGGTCCTGAGGTCCTGAGT-3′ and 5′-ACTCGAGCTGGAAGCTGAGCAGGA-3′) that flank the mouse Ccnd3 promoter (−1468/+25 nt). The Ccnd3 promoter DNA was cloned into the pGL3-Basic plasmid (Promega) encoding the luciferase reporter gene. Likewise, the mouse Sod1 promoter was cloned, using the primers (5′-CTGTGAACATCCACTTCTGT-3′ and 5′-GCCACGGAGCTTTTATAGGC-3′) that flank the 600-bp Sod1 promoter (−600/+1 nt). The mouse Cat promoter was cloned, using the primers (5′-GTGACAGAGTCTCAAAGTCT-3′ and 5′-AGGCTCCACCAATCAGCACC-3′) that flank the 750-bp Cat promoter (−750/+1). To determine promoter activity, INS-1 cells were transduced in 12-well plates with 100 pfu/cell of Adv-FoxO1 or Adv-Empty vector, followed by transfection with 2-μg of pCcnd3-Luc encoding the mouse Ccnd3 promoter-directed luciferase reporter system. Plasmid pGL3-basic encoding promoter-less luciferase was used as control for determining the baseline luciferase activity in INS-1 cells. pGL4.75 expressing Renilla luciferase (Promega) was included as control for normalizing transfection efficiency. After a 24-hour incubation, cells were subjected to dual luciferase activity assay for determining Ccnd3 promoter activity. Likewise, the effect of FoxO1 on Sod1 and Cat promoter activities was determined.
Statistics
Statistics of data were analyzed by Student's t test and were validated by ANOVA, using the JMP statistics software. Dunnett's post hoc tests were performed to determine the significance between FoxO1-tg and WT groups. Data are expressed as mean ± SEM. P < .05 were considered statistically significant.
Results
β-Cell FoxO1-transgenic expression improves glucose metabolism
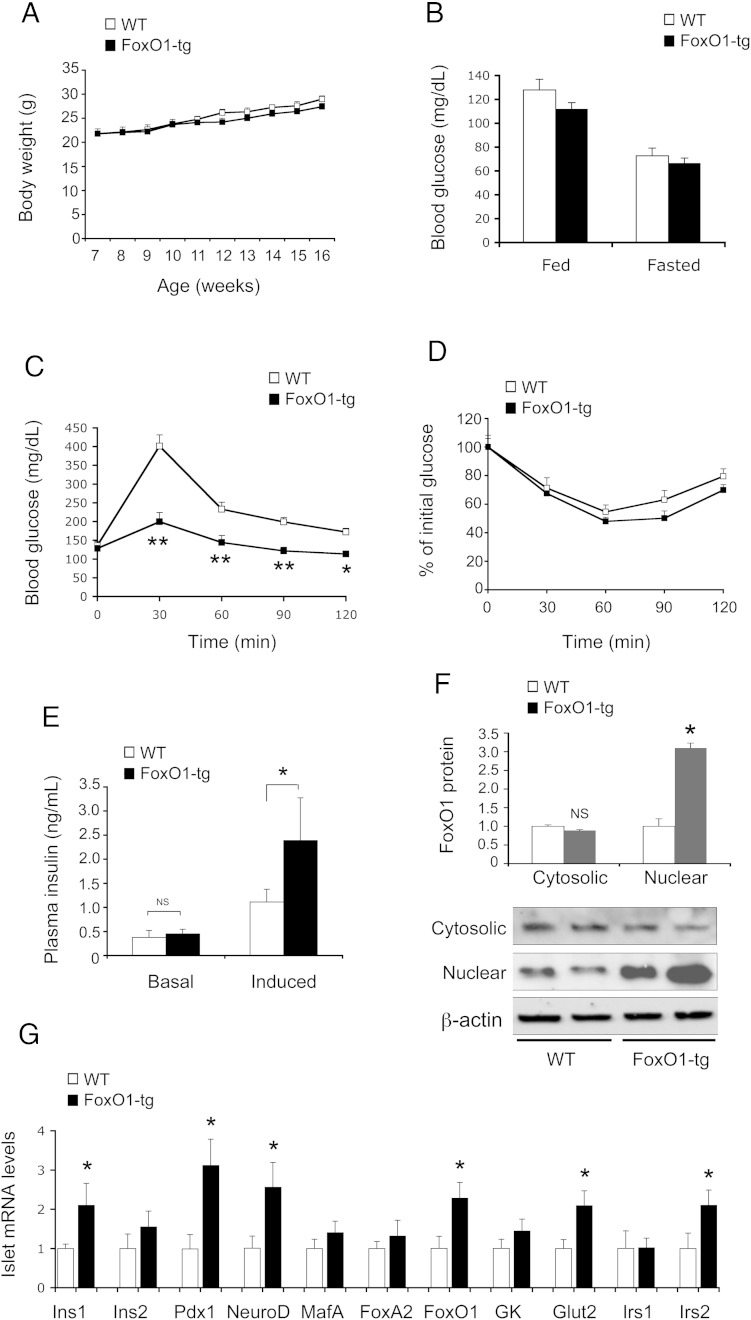
β-Cell FoxO1-transgenic (FoxO1-tg, n = 10) and WT (n = 10) littermates had a similar weight gain (Figure 1A) and blood glucose levels (Figure 1B). In response to glucose challenge, FoxO1-tg displayed significantly improved glycemic profile (Figure 1C). This effect was not due to altered insulin sensitivity, as FoxO1-tg and control mice maintained similar blood glucose profiles in response to insulin tolerance (Figure 1D). To account for improved postprandial glucose profiles in FoxO1-tg mice, we determined plasma insulin levels before and 15 minutes after ip glucose injection. FoxO1-tg mice had significantly higher GSIS without alterations in basal insulin secretion (Figure 1E), consistent with their enhanced glucose tolerance (Figure 1C). To determine RIP-directed FoxO1-transgenic expression, we subjected islets from FoxO1-tg and WT littermates (n = 8, 16 wk old) to anti-FoxO1 immunoblot analysis. We detected a 3-fold induction of nuclear FoxO1 protein levels in islets in FoxO1-tg vs control mice (Figure 1F).
Figure 1.
Effect of FoxO1 on glucose metabolism and β-cell function in FoxO1-transgenic mice on regular chow. A, Body weight. B, Blood glucose levels. C, Glucose tolerance test. Mice were fasted for 5 hours and were ip injected with glucose (2 g/kg), followed by determining blood glucose levels at different times. D, Insulin tolerance test. E, Basal and GSIS. Mice were fasted for 16 hours, followed by ip injection of glucose (2 g/kg). Plasma insulin levels were determined before (basal condition) and 15 minutes after glucose injection (induced condition). Data in A–E were obtained from FoxO1-tg and WT littermates (male, n = 10/group) on regular chow at 16 weeks of age. F, Islet FoxO1 protein levels. G, Islet mRNA levels. FoxO1-tg and WT littermates (male, 17 wk old, n = 10/group) were euthanized after a 16-hour fasting for isolating islets, which were subjected to real-time RT-PCR analysis using 18S RNA as control or immunoblot assay using rabbit anti-FoxO1 antibody, using anti-β-actin as control. *, P < .05 and **, P < .001 vs WT control. NS, not significant.
To preclude the possibility that enhanced glucose tolerance was secondary to altered central FoxO1 expression, we determined FoxO1 expression in the brain. Hypothalamic FoxO1 at mRNA and protein levels was unchanged in FoxO1-tg mice (Supplemental Figure 1). FoxO1 protein levels in the brain were similar, correlating with the lack of changes in food intake in FoxO1-tg vs WT mice (Supplemental Figure 1).
FoxO1 gain-of-function enhances β-cell function
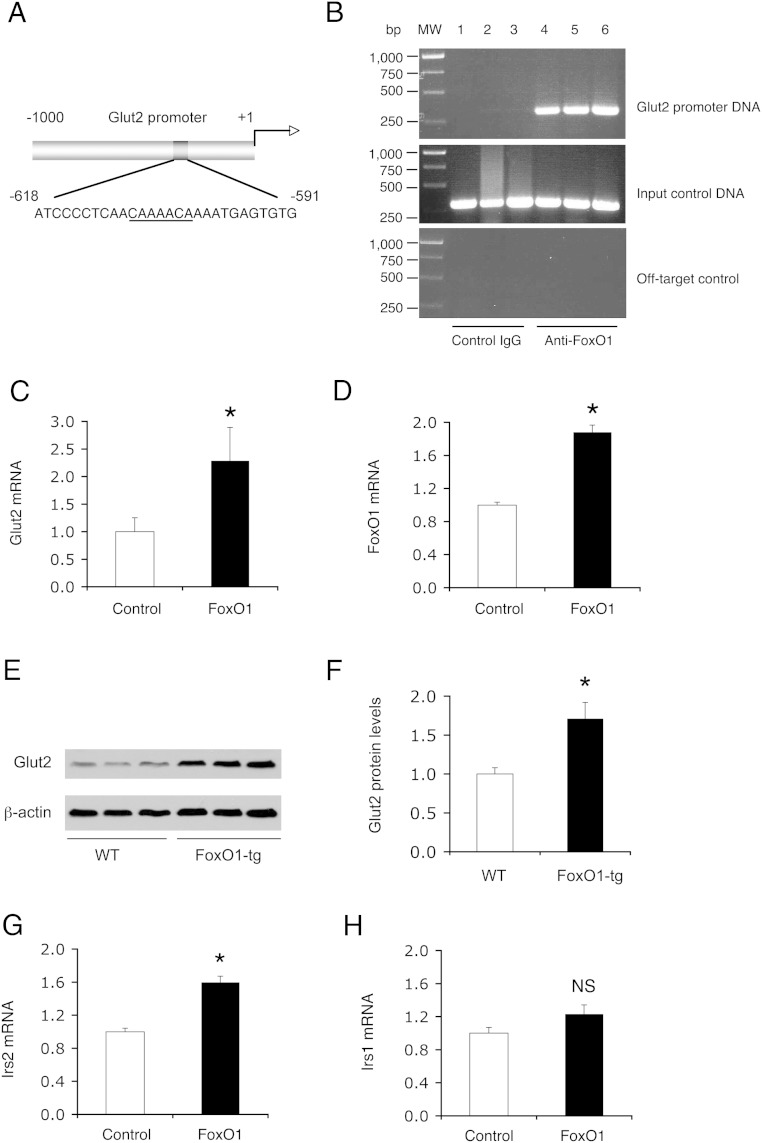
To understand the mechanism underlying FoxO1-mediated induction of GSIS, we profiled the expression of β-cell genes, whose functions are critical for β-cell glucose sensing, and insulin synthesis and secretion (Figure 1G). FoxO1-tg mice exhibited 2-fold elevation in insulin 1 (Ins1) gene expression, accompanied by significant up-regulation of Pdx1 and NeuroD, 2 key transcription factors in insulin synthesis. These effects correlated with a 2.5-fold increase in FoxO1 mRNA levels (Figure 1G) and a 3-fold increase in FoxO1 nuclear protein levels in FoxO1-tg islets (Figure 1F). FoxO1-tg production also raised islet mRNA levels of Glut2 and Irs2 (Figure 1G), 2 components instrumental for β-cell glucose sensing and insulin signaling (13, 14). In contrast, islet expression of Ins2 gene, Irs1, GK, FoxA2, and MafA remained unchanged in FoxO1-tg mice.
FoxO1-transgenic mice are protected from fat-induced glucose disorder
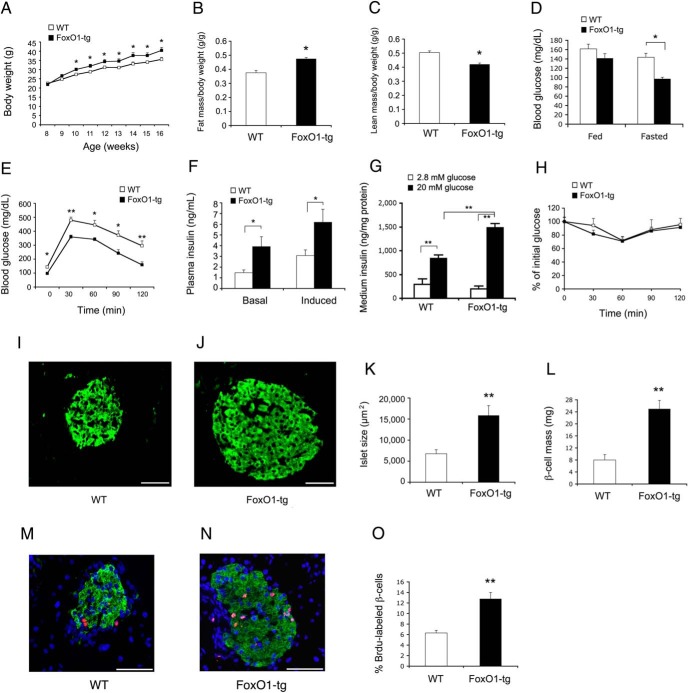
To determine the ability of FoxO1-tg islets to compensate for insulin resistance, we fed FoxO1-tg and WT littermates (n = 10/group) high-fat diet for 8 weeks. High-fat feeding induced obesity in both groups, with slightly greater weight gain in FoxO1-tg mice (Figure 2A). FoxO1-tg mice had a small increase in fat mass (Figure 2B) and a corresponding decrease in lean mass (Figure 2C). FoxO1-tg mice exhibited significantly lower fasting blood glucose levels (Figure 2D) and enhanced glucose tolerance (Figure 2E), correlating with significant induction of both basal and GSIS in FoxO1-tg mice (Figure 2F). We recapitulated this finding ex vivo, showing that FoxO1-tg islets were associated with significantly higher GSIS in culture (Figure 2G). No differences in insulin tolerance (Figure 2H) and food intake (Supplemental Figure 2) were detected in FoxO1-tg vs WT mice. To account for the small increase in weight gain in FoxO1-tg mice, we subjected high-fat-fed FoxO1-tg and WT littermates to metabolic cage studies for determining energy homeostasis. FoxO1-tg mice had a small nonsignificant reduction in mean oxygen consumption rates without alterations in respiratory exchange ratio, when compared with WT littermates (Supplemental Figure 2).
Figure 2.
Effect of FoxO1 on glucose metabolism and β-cell function in FoxO1-transgenic mice on high-fat diet. FoxO1-tg and WT littermates (male, 8 wk old, n = 10) were fed a high-fat diet for 8 weeks. A, Body weight. B, Fat mass determined by MRI. C, Lean mass. D, Blood glucose levels. E, Glucose tolerance test. F, Basal and GSIS. G, Ex vivo GSIS. Aliquots of islets (n = 50) isolated from high-fat-fed FoxO1-tg and WT littermates (16 wk old, n = 3/group) were cultured in RPMI 1640 medium overnight, followed by shifting culture medium from 2.8mM to 20mM glucose concentrations. Insulin levels were determined in culture medium collected at 30 minutes in low- vs high-glucose concentrations and were normalized to total islet proteins. H, Insulin tolerance test. Pancreas tissues of high-fat-fed WT (I) and FoxO1-tg (J) mice (n = 10) were subjected to antiinsulin immunohistochemistry for determining islet size (K) and islet mass (L). Additionally, high-fat-fed WT and FoxO1-tg mice (male, 5 wk old, n = 4/group) were treated with BrdU (ip 100 mg/kg daily) for 4 days. Pancreata of WT (M) and FoxO1-tg (N) mice were subjected to anti-BrdU immunohistochemistry for determining BrdU-positive β-cells out of total β-cells per islet (O). *, P < .05 and **, P < .005 vs WT control. Scale bar, 50 μm.
FoxO1 augments β-cell compensation for insulin resistance
To address the mechanism underlying FoxO1-mediated induction of β-cell compensation, we performed antiinsulin immunohistochemistry on the pancreas, demonstrating that FoxO1-tg islets were significantly larger in size than WT controls (Figure 2, I–K). FoxO1-tg mice had a 3-fold increase in β-cell mass (Figure 2L). This effect was not secondary to β-cell hypertrophy (Supplemental Figure 2), but due to β-cell hyperplasia, culminating in 2-fold induction of β-cell replication in high-fat-fed FoxO1-tg mice (Figure 2, M–O), as determined by in vivo BrdU-labeling assay.
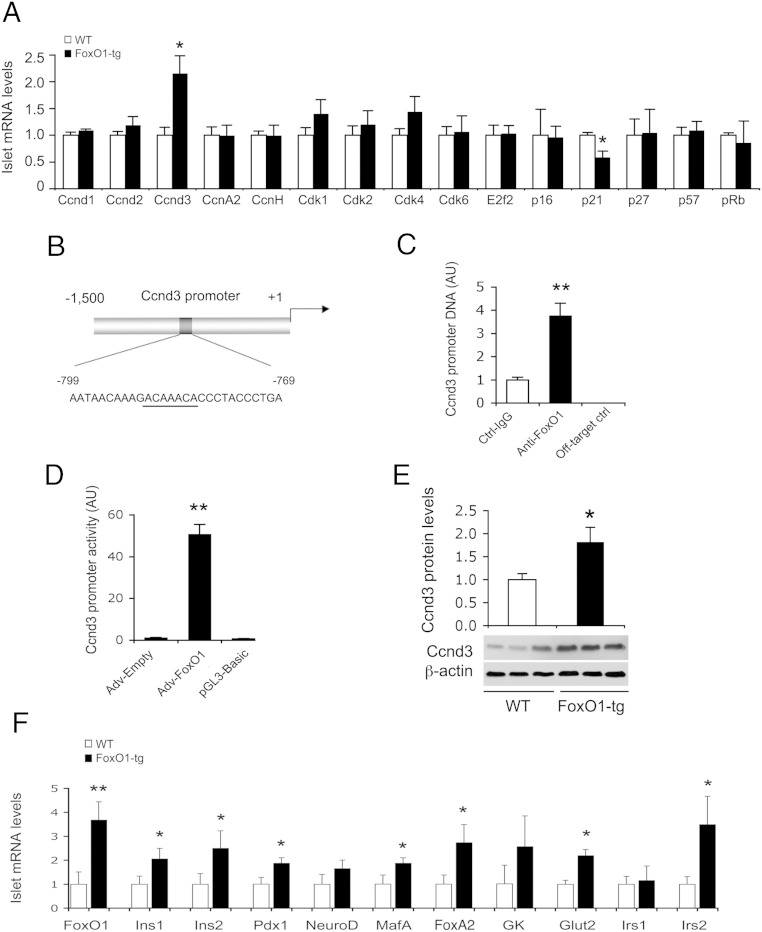
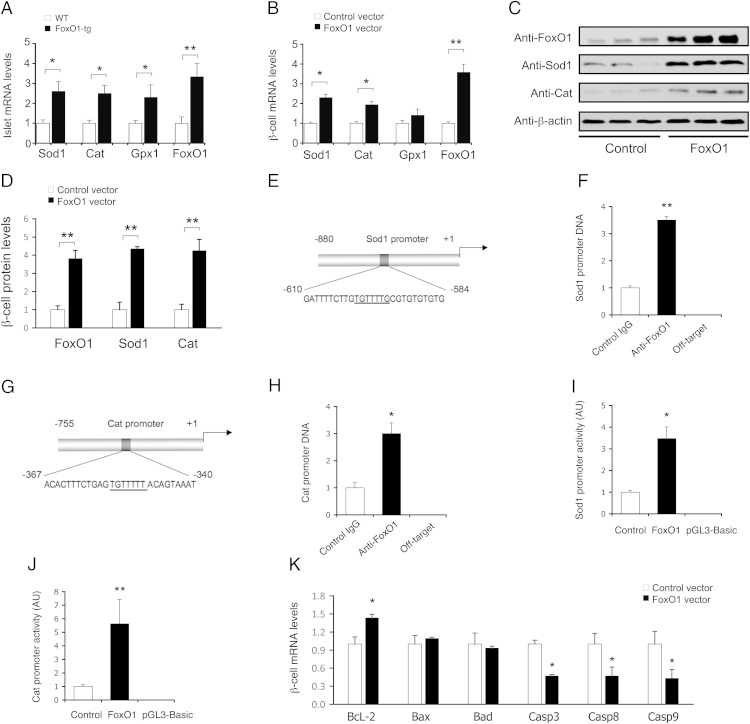
To gain mechanistic insights into FoxO1-mediated induction of β-cell mass and function, we profiled islet expression of genes involved in β-cell replication, insulin synthesis and secretion, and glucose-sensing. FoxO1-tg production selectively up-regulated Ccnd3 and down-regulated cyclin-dependent kinase inhibitor 1 (p21) (Figure 3A). Ccnd3 acts in complex with cyclin-dependent kinase (Cdk)4 or Cdk6 to promote cell cycle G1/S transition, whereas p21 functions as a Cdk inhibitor of cell cycle progression (36). To determine whether Ccnd3 gene is a FoxO1 target, we performed ChIP assay, demonstrating that FoxO1 was capable of binding to its consensus site in the Ccnd3 promoter (Figure 3, B and C). This effect translated into a significant induction of Ccnd3 promoter activity, as determined using the Ccnd3 promoter-directed luciferase reporter system in INS-1 cells (Figure 3D). Immunoblot analysis further confirmed that Ccnd3 protein levels were significantly increased in FoxO1-tg islets (Figure 3E).
Figure 3.
Effect of FoxO1 on islet mRNA expression in FoxO1-transgenic mice on high-fat diet. FoxO1-tg and WT littermates (male, 8 wk old, n = 10/group) were killed under fasting conditions after 8 weeks of high-fat feeding. Islets were subjected to real-time qRT-PCR analysis using 18S RNA as control. A, Islet mRNA profiles of genes in cell-cycle G1/S transition. B, The mouse Ccnd3 promoter. Underlined are nucleotides corresponding to the consensus FoxO1 binding site. C, ChIP assay for FoxO1-Ccnd3 promoter interaction. D, Ccnd3 promoter activity in INS-1 cells, as determined by luciferase reporter assay; n = 3. E, Ccnd3 protein levels. Aliquots of islets (n = 150/mouse) from high-fat-fed FoxO1-tg and WT littermates (n = 3/group) were subjected to anti-Ccnd3 immunoblot analysis, using anti-β-actin as control. The amounts of Ccnd3 relative to β-actin proteins in islets were determined. F, Islet mRNA profiles of genes in β-cell mass and function regulation. *, P < .05 and **, P < .005 vs WT control.
FoxO1-tg mice also had significantly higher Ins1 and Ins2 expression in islets (Figure 3F). This effect correlated with a significant induction in Pdx1, MafA, FoxA2, and Glut2 expression in islets, consistent with increased GSIS in FoxO1-tg mice (Figure 3F). NeuroD and GK expression remained unchanged in islets of FoxO1-tg mice. FoxO1-transgenic production also resulted in selective induction of Irs2, but not Irs1, expression in islets, underpinning the notion that FoxO1 targets the Irs2 gene for trans-activation (28, 37).
FoxO1 contributes to physiological β-cell compensation for dietary obesity
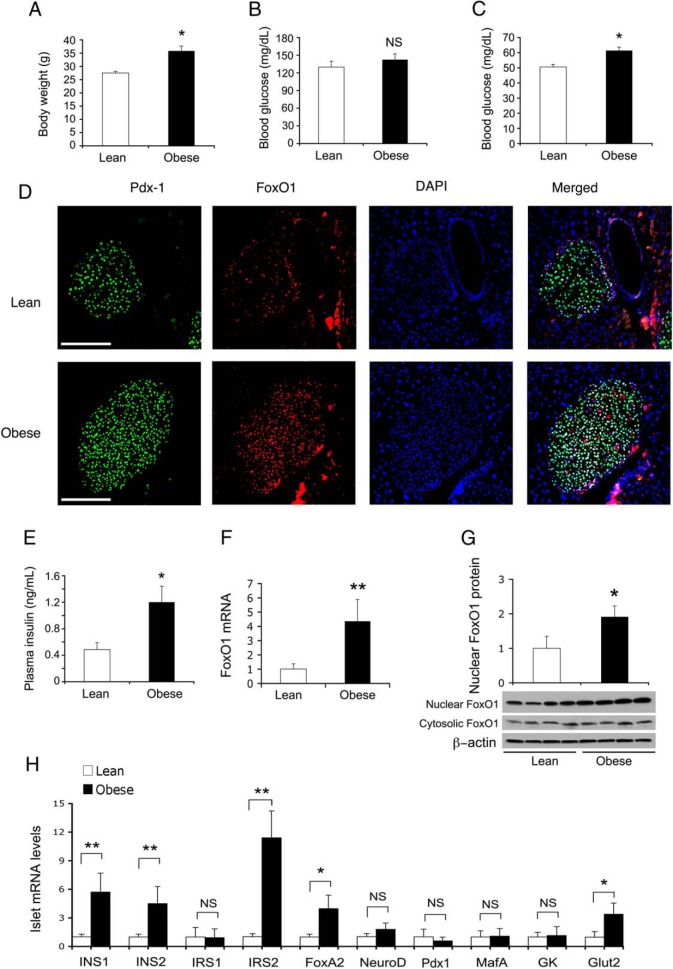
To address whether FoxO1 contributes to physiological β-cell compensation for overnutrition, we determined β-cell FoxO1 expression in lean vs dietary obese mice (n = 7/group). C57BL/6J mice fed high-fat developed obesity (Figure 4A), with a concomitant induction of glucose intolerance, hypertriglyceridemia and hypercholesterolemia (Supplemental Figure 3). Nonetheless, obese mice maintained fed and fasting blood glucose levels within the physiological range (Figure 4, B and C). These effects were attributable to the compensatory induction of β-cell hyperplasia and insulin hypersecretion in obese mice (Figure 4, D and E), consistent with the notion that insulin resistance begets β-cell compensation (1, 3, 5, 38–40).
Figure 4.
FoxO1 contributes to physiological β-cell compensation for insulin resistance in obese mice. C57BL/6J mice (male, 6 wk old, n = 7/group) were fed regular chow or high-fat diet for 10 weeks, followed by determining body weight and blood glucose metabolism. A, Body weight. B, Fed blood glucose levels. Fed blood glucose levels were determined in mice under ad libitum condition. C, Fasting blood glucose levels. Fasting blood glucose levels were determined after a 16-hour fasting. D, Immunohistochemistry of pancreas for visualizing Pdx1 and FoxO1 proteins in islets. E, Fasting plasma insulin levels. Mice were fasted for 16 hours for determining fasting plasma insulin levels. F, FoxO1 mRNA levels in islets. G, FoxO1 protein levels in islets. H, Islet mRNA levels. Mice were killed at 17 weeks of age for isolating islets. Handpicked islets (150–200 islets per mouse) were subjected to real-time qRT-PCR analysis using 18S RNA as control or immunoblot assay using β-actin as control for determining FoxO1 and β-cell mRNA levels as well as FoxO1 nuclear vs cytoplasmic protein levels. *, P < .05 and **, P < .001 vs control. NS, not significant. Scale bar, 50 μm.
To determine FoxO1 contribution to β-cell compensation in diet-induced obesity, we subjected islets from lean and obese mice to real-time qRT-PCR analysis, revealing a significant induction of β-cell FoxO1 expression at both mRNA and nuclear protein levels in obese mice (Figure 4, F and G). Obese mice also exhibited significant up-regulation of Ins1, Ins2, Glut2, Irs2, and FoxA2 in islets (Figure 4H), consistent with the compensatory increase in insulin synthesis and secretion in response to dietary obesity. In contrast, Irs1, MafA, NeuroD, GK, and Pdx1 mRNA levels in islets remained unchanged in obese vs lean mice (Figure 4H).
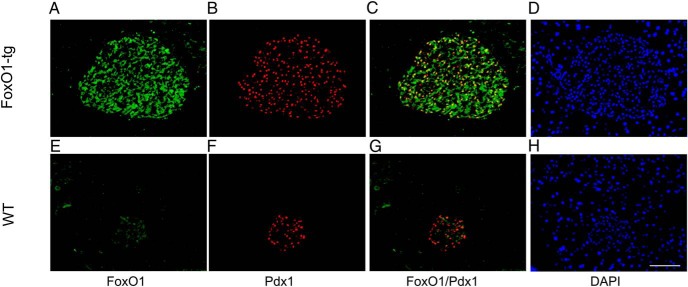
FoxO1 acts in parallel with Pdx1 in β-cells
There is anecdotal evidence that FoxO1 antagonizes Pdx1 somewhat by promoting Pdx1 translocation from the nucleus to cytoplasm (25). This observation is made in MIN6 cells with vector-mediated production of a constitutively active FoxO1 mutant known to cause ER stress (29). To resolve this issue and elucidate the molecular interplay between FoxO1 and Pdx1 in β-cells, we performed anti-Pdx1 and anti-FoxO1 dual immunohistochemistry. Pdx1 was localized in the nucleus of β-cells in FoxO1-tg and WT mice (n = 6/group) (Figure 5). Similar results were recapitulated in islets of C57BL/6J mice (Figure 4D). To corroborate these findings, we transduced INS-1 cells with FoxO1 vector, followed by studies of Pdx1 expression and its subcellular distribution. Elevated FoxO1 production neither affected Pdx1 expression nor altered Pdx1 subcellular redistribution in INS-1 cells (Supplemental Figure 4). These findings were corroborated by anti-Pdx1 immunocytochemistry, showing that FoxO1 production did not affect Pdx1 nuclear localization (Supplemental Figure 4).
Figure 5.
FoxO1 and Pdx1 colocalize in mouse islets. Male FoxO1-tg (n = 6, 8 wk old) and age/sex-matched WT littermates (n = 6) fed high-fat diet were killed at 20 weeks of age. The pancreas was sectioned for anti-FoxO1 (A and E) and anti-Pdx1 (B and F) immunohistochemistry. C and G, Merged images for illustrating FoxO1 and Pdx1 colocalization in the nucleus of islet cells. D and H, Nuclei of cells stained by DAPI. Scale bar, 50 μm.
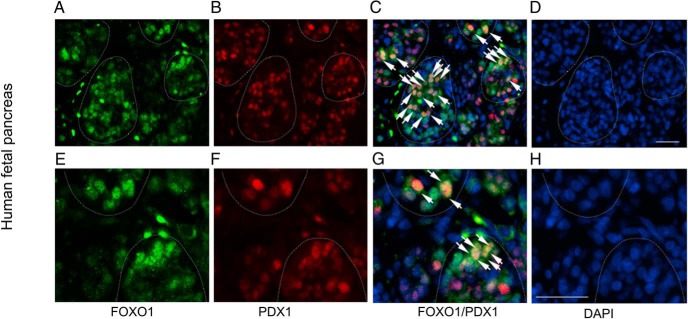
To bolster the above findings, we revealed that PDX1 and FOXO1 colocalized in the nucleus of islets in 21-week-old human fetal pancreas (Figure 6). These results were reproduced in 14-week-old human fetal pancreas (Supplemental Figure 5). We examined FOXO1 and PDX1 localization in mature islets of human pancreas, demonstrating that PDX1 colocalized with FOXO1 in β-cells (Supplemental Figure 5). These data suggest that FOXO1 functions in parallel with PDX1 in β-cells, arguing against the assertion that FOXO1 counteracts Pdx1 by promoting PDX1 nuclear exclusion in β-cells (25). Indeed, FOXO1 has been shown to cooperate with PDX1 in prenatal β-cell differentiation during embryonic development (20, 26).
Figure 6.
FOXO1 and PDX1 colocalize in human fetal islets. Frozen sections of human fetal pancreas (21 wk old) were coimmunostained with anti-FOXO1 (A) and anti-PDX1 (B) antibodies. Merged images were shown in C. Nuclei of cells were stained with DAPI (D). E–H, Same sections visualized at high resolution. Islets were outlined by dash lines. Islet cells that were positively stained for both FOXO1 and PDX1 in the nucleus were marked by arrows. Scale bar, 100 μm.
FoxO1 signaling through Glut2 improves β-cell glucose sensing
Islets with elevated FoxO1 activity had increased Glut2 expression. This finding was reproduced in FoxO1-tg mice on regular chow and high-fat conditions as well as in dietary obese C57BL/6J mice, spurring the hypothesis that the Glut2 gene is a FoxO1 target. Implicit in this postulation is that there is a conserved FoxO1 binding site in the Glut2 promoter (Figure 7A). To address this hypothesis, we determine FoxO1 association with Glut2 promoter by ChIP assay, demonstrating that FoxO1 was bound to β-cell Glut2 promoter (Figure 7B). This effect translated into a significant induction of Glut2 expression in FoxO1-expressing INS-1 cells (Figure 7, C and D). Using immunoblot assay, we detected a significant induction of Glut2 protein levels in FoxO1-tg islets (Figure 7, E and F). FoxO1 also stimulated β-cell expression of Irs2 (Figure 7G), but not Irs1 (Figure 7H), expression in INS-1 cells, corroborating that the Irs2 gene is a FoxO1 target (28, 37).
Figure 7.
Effect of FoxO1 on Glut2 and Irs2 expression in β-cells. A, Schematic depiction of the mouse Glut2 promoter. The nucleotides of the FoxO1 consensus site were underlined. B, ChIP assay. C57BL/6J mice (male, 10 wk old) were euthanized after a 16-hour fasting, and the pancreases were subjected to ChIP assay using preimmune IgG (n = 3) and anti-FoxO1 antibody (n = 3). The immunoprecipitates were subjected to PCR analysis for detecting FoxO1-bound Glut2 promoter DNA using specific primers flanking the proximal region (−638/−318 nt) of the mouse Glut2 promoter. As input control, aliquots of pancreas cell lysates before immunoprecipitation were subjected to PCR analysis. As off-target control, the immunoprecipitates were analyzed by PCR assay using a pair of primers flanking the distal region (−5004/−4649 nt) of the mouse Glut2 promoter. In addition, INS-1 cells were transduced with Adv-FoxO1 and Adv-Empty control vector (100 pfu/cell). Each condition was run in triplicate. After a 24-hour incubation, cells were processed for real-time qRT-PCR analysis using 18S RNA as control. C, Glut2 mRNA levels. D, FoxO1 mRNA levels. E, Anti-Glut2 immunoblot. Aliquots of islets (n = 150/mouse) from high-fat-fed FoxO1-tg and WT littermates (n = 3/group, 16 wk old) were subjected to anti-Glut2 immunoblot analysis, using anti-β-actin as control. F, Islet Glut2 protein levels. The amount of Glut2 proteins was determined after normalizing to β-actin protein in islets. G, Irs2 mRNA levels. H, Irs1 mRNA levels. *, P < .05 vs control. NS, not significant.
FoxO1 augments β-cell antioxidative function
To understand the mechanism by FoxO1 preserved β-cell mass and function, we determined the effect of FoxO1 on β-cell antioxidative function. When compared with WT littermates (n = 6), FoxO1-tg mice (n = 6) had significantly increased islet expression of Sod1, Cat, and glutathione peroxidase 1 (Gpx1), 3 key enzymes for defending oxidative stress (Figure 8A). We reproduced these findings in INS-1 cells with increased FoxO1 activity. FoxO1 gain-of-function promoted β-cell expression of Sod1 and Cat at mRNA and protein levels (Figure 8, B–D). Furthermore, we showed that FoxO1 enhanced Sod1 and Cat promoter activities via selective binding to the Sod1 and Cat promoters (Figure 8, E–H). These effects translated into significant induction of β-cell Sod1 and Cat promoter activities (Figure 8, I and J). To determine whether FoxO1-mediated induction of antioxidative function would confer a cytoprotective effect on β-cells, we subjected INS-1 cells with and without FoxO1 production to H2O2-mediated oxidative stress. Elevated FoxO1 production resulted in up-regulation of prosurvival factor B-cell leukemia/lymphoma 2 and down-regulation of proapoptotic genes including Caspase 3, Caspase 8, and Caspase 9 in INS-1 cells (Figure 8K).
Figure 8.
Effect of FoxO1 on β-cell antioxidative function. A, Effect of FoxO1 on β-cell antioxidative gene expression. Islets from FoxO1-tg and WT littermates (male, 10 wk old, n = 6/group) were subjected to real-time qRT-PCR analysis for determining Sod1, Cat, Gpx1, and FoxO1 mRNA levels. B, Effect of FoxO1 on antioxidative gene expression in INS-1 cells. INS-1 cells were transduced with Adv-Empty and Adv-FoxO1 vectors (100 pfu/cell). C, Immunoblot of INS-1 cells pretransduced with Adv-Empty and Adv-FoxO1 vectors. D, Effect of FoxO1 on antioxidative protein levels in INS-1 cells. After a 24-hour incubation, cells were subjected to real-time qRT-PCR and immunoblot assays. E, The mouse Sod1 promoter. F, ChIP analysis of FoxO1-Sod1 promoter association. G, The mouse Cat promoter. H, ChIP analysis of FoxO1-Cat promoter interaction. Pancreatic islets were subjected to ChIP assay using anti-FoxO1 or anti-β-galactosidase IgG for determining FoxO1-bound DNA. As off-target control, immunoprecipitated DNA was analyzed by real-time PCR, using primers that are complementary to the mouse Sod1 or Cat coding region. I, Sod1 promoter activity. J, Cat promoter activity. The mouse Sod1 (−880/+1 nt) or Cat (−755/+1 nt) promoter was cloned into the pGL3-basic luciferase reporter system for determining Sod1 or Cat promoter activity in INS-1 cells that were pretransduced with Adv-FoxO1 or Adv-Empty vector (100 pfu/cell). K, Effect of FoxO1 on apoptotic gene expression in INS-1 cells. INS-1 cells pretransduced with Adv-FoxO1 and Adv-Empty vectors were subjected to H2O2 treatment, followed by real-time qRT-PCR analysis of prosurvival and proapoptotic gene expression. Data were from 3–5 independent experiments. *, P < .05 and **, P < .001 vs control.
Discussion
Type 2 diabetes results from β-cell failure, culminating in the disability of β-cells to compensate for insulin resistance in at-risk subjects with obesity. A vast majority of obese individuals are able to ward off diabetes, due to increased β-cell compensation. Nonetheless the underlying mechanism that integrates β-cell compensation with obesity and insulin resistance remain elusive. Here, we showed that such a compensatory mechanism of β-cells is governed by FoxO1, known as a nutrient sensor to integrate insulin signaling to cell metabolism, survival, proliferation and differentiation (15). FoxO1 gain-of-function augmented β-cell compensation, as reflected by the induction of β-cell mass expansion and GSIS. These effects correlated with significant up-regulation of key functions involved in insulin synthesis and secretion in FoxO1-tg islets. FoxO1-tg mice, despite diet-induced obesity, were protected from fasting hyperglycemia and glucose intolerance. In response to overnutrition, β-cell FoxO1 activity was up-regulated, contributing to physiological induction of β-cell compensation for insulin resistance in obese C57BL/6J mice. These data characterize FoxO1 as an important factor for physiological adaptation of β-cell mass and function to dietary obesity.
In keeping with this view, Müssig et al (17) report that genetic FOXO1 variants are associated with β-cell dysfunction, glucose intolerance and increased risk of diabetes in humans. Talchai et al (18, 20) show that embryonic FoxO1 depletion results in the loss of β-cell function and identity, as FoxO1-depleted β-cells undergo β-cell degranulation and dedifferentiation in response to metabolic stress. Although the mechanisms underlying β-cell degranulation and dedifferentiation remain to be detailed, these results reinforce the idea that FoxO1 is critical for preserving functional β-cell mass.
β-Cell compensation is also manifested by enhanced glucose-sensing in response to insulin resistance. Here, we showed that FoxO1 stimulated β-cell expression of Glut2, a glucose sensor that acts along with GK to catalyze the rate-limiting step in GSIS. Glut2 deficiency is linked to abnormal GSIS and impaired β-cell compensation in mice (41). Glut2 loss-of-function attenuates the ability of islets to respond to glucose, contributing in part to impaired GSIS in diabetic db/db mice (42, 43). Humans with genetic GLUT2 mutations are associated with transient neonatal diabetes, fasting hyperglycemia and risk of developing diabetes (44). In this context, we showed that FoxO1 augments β-cell Glut2 expression. We reproduced these results in INS-1 cells as well as in FoxO1-tg mice and dietary obese C57BL/6J mice. Furthermore, we showed that FoxO1 targets the Glut2 promoter for trans-activation in β-cells. These data suggest that FoxO1 signaling through Glut2 may play an important role in improving β-cell glucose sensing in the face of insulin resistance for maintaining GSIS in obesity. In support of this notion, embryonic FoxO1 deletion attenuates β-cell Ca2+-dependent insulin secretion, resulting in premature diabetes in mice (19). FoxO1 deficiency is associated with reduced β-cell Glut2 expression, coinciding with impaired GSIS in diabetic db/db mice (20).
Furthermore, FoxO1 promoted β-cell expression of Sod1, Cat, and Gpx1, contributing to the induction of antioxidative function in islets. We recapitulated these findings in INS-1 cells, demonstrating that INS-1 cells with elevated FoxO1 production exhibited significantly increased B-cell leukemia/lymphoma 2 expression and decreased expression of proapoptotic genes, including Caspases 3, 8, and 9, in response to H2O2-elicited oxidative stress. FoxO1 may confer a cytoprotective effect on β-cells. In accordance with our findings, Kitamura et al (27) show that FoxO1 is acetylated, resulting in its nuclear localization in βTC-3 cells in response to hyperglycemia or H2O2. This effect accounts for increased FoxO1 activity in protecting β-cells against oxidative damage (27). Hughes et al (45) report that FoxO1 promotes the expression of DNA repair enzyme growth arrest and DNA damage-inducible protein-α, protecting INS-1 cells from nitric oxide-elicited DNA damage. Kibbe et al (46) show that FoxO1 protects β-cells from glucolipotoxicity by inhibiting the expression of thioredoxin-interacting protein, whose overproduction is linked to β-cell apoptosis in diabetes. Interestingly, such a cytoprotective action of FoxO1 is evolutionally conserved. The FoxO1 ortholog Daf16 is up-regulated along with its nuclear localization in response to oxidative stress in Caenorhabditis elegans (47). This effect contributes to the induction of Sod expression, protecting against oxidative damage and improving the longevity in C. elegans. Although oxidative stress secondary to glucolipotoxicity contributes to β-cell dysfunction in diabetes (48–51), the molecular basis that links oxidative stress to β-cell dysfunction remains elusive. Our data along with previous results underscore the importance of FoxO1 in preserving β-cell mass in response to metabolic stress.
Apart from its cytoprotective effect on functional β-cell mass, FoxO1 gain-of-function was associated with β-cell mass expansion in response to overnutrition and metabolic stress. This finding was reproduced in high-fat-fed FoxO1-tg mice and obese C57BL/6J mice. One potential mechanism is that FoxO1 participates directly in β-cell mass regulation. Consistent with this interpretation, we showed that FoxO1 enhanced β-cell expression of Ccnd3, a G1/S-specific cyclin that acts in complex with Cdk4 or Cdk6 to promote cell proliferation (36). Indeed, pharmacological up-regulation of Ccnd3 activity promotes β-cell proliferation, contributing to the reversal of hyperglycemia in db/db mice (52). Moreover, β-cell Ccnd3 expression is up-regulated, correlating with postnatal β-cell mass expansion in humans (53). FoxO1 is also shown to promote INS-1 cell proliferation in vitro (54). Our data together with previous findings suggest that FoxO1 signaling through Ccnd3 may play a significant role in regulating β-cell replication.
Alternatively, FoxO1 contributes to β-cell mass expansion via an indirect mechanism. Sharma et al (55) recently showed that in response to overnutrition, β-cells sense unmet insulin demand via activation of ER stress. Although unresolved ER stress causes β-cell apoptosis, moderate ER stress serves as a physiological signal for triggering β-cell proliferation (55). FoxO1 is known to modulate ER stress in responsible to metabolic stress (28, 29). It is plausible that increased FoxO1 activity, resulting from overnutrition and insulin resistance, links ER stress to β-cell mass expansion in dietary obesity. Further investigation is warranted to characterize the role of FoxO1 in regulating β-cell replication in response to metabolic stress. Consistent with FoxO1-mediated induction of β-cell mass expansion, we showed that FoxO1-transgenic production resulted in down-regulation of p21, known as a Cdk inhibitor of cell cycle progression (36). Although FoxO1 has been shown to suppress gene expression via a trans-repression mechanism by counteracting other nuclear factors (46, 56, 57), studies are needed to determine whether this FoxO1-mediated inhibition of β-cell p21 expression derived from direct or indirect mechanisms.
Although Irs2 is required for β-cell compensation, the underlying mechanism is poorly understood. We showed that Irs2 is significantly up-regulated in islets of FoxO1-tg and dietary obese C57BL/6J mice, and in INS-1 cells with elevated FoxO1 production. β-Cell Irs2 is also up-regulated by FoxO3, a homologue of FoxO1 (58). These data substantiate the idea that FoxO1 activity is tightly regulated via the Irs2-mediated feedback mechanism (Supplemental Figure 6). Unbridled FoxO1 activity, resulting from a dislodged Irs2-FoxO1 feedback loop, contributes to undue ER stress, metabolic diapause, and cellular apoptosis (28, 37). Our data underscore the importance of the Irs2-FoxO1 feedback loop in physiological β-cell compensation. This model provides important insights to the observations that Irs2 deficiency is associated with β-cell decompensation in mice (13, 14). Although not reported in those studies (13, 14), it is predicted that FoxO1 activity becomes unchecked, due to the loss of Irs2-mediated feedback control in Irs2-deficient islets. It is anticipated that selective inhibition of FoxO1 activity would ameliorate β-cell decompensation secondary to Irs2 deficiency. Indeed, FoxO1 haploinsufficiency recovers β-cell mass and function and rescues the diabetic phenotype in Irs2-deficient mice (59).
We noted that FoxO1-tg mice, as opposed to WT littermates, had a slight increase in weight gain, despite similar food intake on high-fat diet. To account for this observation, we performed metabolic cage studies. Although FoxO1-tg mice had relatively lower oxygen consumption, the difference did not reach a significant level when compared with that in WT littermates, arguing against the interpretation that the slight weight gain was attributable to reduced energy expenditure in FoxO1-tg mice. It is noteworthy that high-fat-fed FoxO1-tg mice had significantly higher basal and GSIS. It is plausible that the small increase in body weight was secondary to the anabolic action of chronic hyperinsulinemia in FoxO1-tg mice. Consistent with this interpretation, we showed that FoxO1-tg mice had increased fat mass, relative to WT littermates, in response to high-fat feeding.
Our data contradict with results from Kitamura and coworkers (60), who showed that Pdx1 promoter-directed transgenic production of the constitutively active FoxO1-ADA mutant resulted in reduced β-cell mass, accompanied by islet hypervascularization and polycystic pancreas. Despite increased microvasculature in islets, FoxO1-ADA-transgenic islets were associated with reduced GSIS. In a follow-up study, they used a genetic knock-in approach to achieve transgenic FoxO1-ADA production in both hypothalamus and pancreas (61), demonstrating that FoxO1-ADA-transgenic mice were hyperphagic and obese, developing insulin resistance and glucose intolerance. These effects were accompanied by increased β-cell mass, due to increased β-cell replication in FoxO1-ADA-transgenic mice. Despite significant increases in β-cell mass and islet size, FoxO1-ADA-transgenic islets were associated with reduced GSIS and glucose intolerance with unknown mechanisms.
It is noted that Kitamura and coworkers (60) achieved β-cell transgenic production of FoxO1-ADA, a constitutively active FoxO1 mutant that harbors nonphosphorylable amino acid residues (Ala or Asp) in replace of Thr24, Ser256, and Ser319, 3 amino acid residues that are phosphorylated by protein kinase B in response to insulin (15). As a result, FoxO1-ADA is permanently localized in the nucleus regardless of insulin action. This effect contributes to constitutive transcription activity of FoxO1 in promoting the expression of target genes in different pathways. It has been shown that unchecked FoxO1 activity, resulting from the constitutively active FoxO1-ADA allele, is associated with ER stress and metabolic diapause, which can impair GSIS and perturb β-cell function (22, 28, 29). To avoid the potential deleterious effect of FoxO1-ADA on β-cell function, we have chosen to express WT FoxO1 in our transgenic mice. Unlike FoxO1-ADA that is insensitive to insulin action, WT FoxO1 activity is regulated by insulin and physiological cues. This fundamental difference in FoxO1-ADA vs WT FoxO1 may account for some of the discrepancies between our data and their previous findings.
Talchai et al (62) showed that genetic ablation of FoxO1 in Neurog3-expressing enteroendocrine progenitor cells gives rise to insulin-positive cells with glucose-stimulated secretion of insulin and C-peptide with a kinetics that is analogous to native β-cells. This finding was reproduced in cultured pluripotent stem cells derived from human gut organoids (63). Thus, apart from its role in regulating β-cell mass and function, FoxO1 plays a role in governing trans-differentiation of endocrine progenitor cells to β-cells. These results seem counterintuitive, given the evidence that FoxO1 is indispensable for maintaining β-cell characteristics (20). Further investigation is needed to understand the mechanism by which FoxO1 loss-of-function converts gut endocrine progenitor cells to β-cells.
We must acknowledge the limitation associated with the leaky RIP system used in our studies. Although we did not detect a significant increment in FoxO1 protein levels in the hypothalamus and brain, we could not rule out the likelihood that residual FoxO1 expression from the leaky RIP system in the brain would affect β-cell function and glucose metabolism in FoxO1-tg mice. To address the concern, we performed functional studies on islets ex vivo, demonstrating that FoxO1-tg islets were associated with increased GSIS, consistent with enhanced glucose tolerance in FoxO1-tg mice. Nonetheless, these results could not exclude the possibility that FoxO1 derived from the leaky RIP system in the brain could impact glucose metabolism in vivo. Indeed, hypothalamic FoxO1 overproduction promotes food intake, resulting in excessive weight gain and reduced energy expenditure in mice (64). Conversely, hypothalamic FoxO1 depletion inhibits satiety and reduces food intake, contributing to leanness and improved glucose homeostasis in mice (65). Thus, cautions must be excised in interpreting the potential impact of leaky FoxO1 production in the brain on weight gain and glucose metabolism in FoxO1-tg mice.
In conclusion, our studies shed light on the missing link connecting β-cell compensation to overnutrition and obesity (Supplemental Figure 7). FoxO1 promotes β-cell compensation via 3 distinct mechanisms by increasing β-cell mass and function, enhancing β-cell glucose sensing and augmenting β-cell antioxidative function. Such FoxO1-dependent β-cell compensatory mechanism is vital for physiological adaptation of β-cell mass and function to overnutrition for overriding insulin resistance and maintaining euglycemia in obesity. β-Cell decompensation, characterized by dwindling functional β-cell mass in the face of unyielding insulin resistance, presages the onset of diabetes in obesity. Our studies suggest that FoxO1 is a potential target of therapeutics for enhancing β-cell compensation and preventing β-cell failure in at-risk subjects with morbid obesity.
Acknowledgments
We thank Dr Christopher Newgard (Duke University) for providing INS-1 cells and Dr Kevin A. Kelley (Mount Sinai School of Medicine, New York) for assistance in generating RIP-FoxO1 transgenics; Dr Yousef El-Gohary for assistance in immunohistochemistry on human islets; and Dr Domenico Accili (Columbia University, New York) for providing FoxO1 vectors.
Author contributions: T.Z., D.H.K., and H.H.D. conducted all phases of studies in the manuscript; X.X. and G.G. performed immunohistochemistry on mouse and human pancreas tissues; S.L. performed real-time qRT-PCR analysis of islets; Z.G. and R.M. performed ex vivo studies in islets and INS-1 cells; V.C.-N., J.Y., and H.H. conducted studies in FoxO1-tg mice; R.W. performed studies in human fetal pancreas tissues; R.B. determined blood chemistry; J.C.A.-P. and A.G.-O. performed studies in lean and dietary obese C57BL/6J mice; H.H.D. contributed to the study design, analysis, and interpretation of data and final draft of the manuscript; and H.H.D. is the guarantor of this study, taking full responsibility for the integrity of data and accuracy of data analysis.
This work was supported by the National Institutes of Health Grant R01DK098437. T.Z. was supported by the Cochrane-Weber Foundation of Children's Hospital of Pittsburgh. J.Y. was the recipient of Grant-in-Aid Scholarship 24-3549 from the Japan Society for the Promotion of Science.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BrdU
- 5-bromo-2-deoxyuridine
- Cat
- catalase
- Ccnd3
- cyclin D3
- Cdk
- cyclin-dependent kinase
- ChIP
- chromatin immunoprecipitation
- DAPI
- 4′,6-diamidino-2-phenylindole
- ER
- endoplasmic reticulum
- FoxO1
- forkhead box O1
- GK
- glucokinase
- Glut2
- glucose transporter 2
- GSIS
- glucose-stimulated insulin secretion
- Ins1
- insulin 1
- Irs2
- insulin receptor substrate 2
- MafA
- v-maf musculoaponeurotic fibrosarcoma oncogene family, protein A
- NeuroD
- neurogenic differentiation factor
- Pdx1
- pancreatic and duodenal homeobox 1
- p21
- cyclin-dependent kinase inhibitor 1
- pfu
- plaque forming unit
- qRT-PCR
- quantitative reverse-transcription polymerase chain reaction
- RIP
- rat insulin II promoter
- Sod1
- superoxide dismutase 1
- WT
- wild type.
References
- 1. Sachdeva MM, Stoffers DA. Minireview: meeting the demand for insulin: molecular mechanisms of adaptive postnatal β-cell mass expansion. Mol Endocrinol. 2009;23:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stamateris RE, Sharma RB, Hollern DA, Alonso LC. Adaptive β-cell proliferation increases early in high-fat feeding in mice, concurrent with metabolic changes, with induction of islet cyclin D2 expression. Am J Physiol Endocrinol Metab. 2013;305:E149–E159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alonso LC, Yokoe T, Zhang P, et al. Glucose infusion in mice: a new model to induce β-cell replication. Diabetes. 2007;56:1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Topp BG, Atkinson LL, Finegood DT. Dynamics of insulin sensitivity, -cell function, and -cell mass during the development of diabetes in fa/fa rats. Am J Physiol Endocrinol Metab. 2007;293:E1730–E1735. [DOI] [PubMed] [Google Scholar]
- 6. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-Cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52:102–110. [DOI] [PubMed] [Google Scholar]
- 7. Bonner-Weir S, Deery D, Leahy JL, Weir GC. Compensatory growth of pancreatic β-cells in adult rats after short-term glucose infusion. Diabetes. 1989;38:49–53. [DOI] [PubMed] [Google Scholar]
- 8. Topp BG, McArthur MD, Finegood DT. Metabolic adaptations to chronic glucose infusion in rats. Diabetologia. 2004;47:1602–1610. [DOI] [PubMed] [Google Scholar]
- 9. Pechhold K, Koczwara K, Zhu X, et al. Blood glucose levels regulate pancreatic β-cell proliferation during experimentally-induced and spontaneous autoimmune diabetes in mice. PLoS One. 2009;4:e4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levitt HE, Cyphert TJ, Pascoe JL, et al. Glucose stimulates human β cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Porat S, Weinberg-Corem N, Tornovsky-Babaey S, et al. Control of pancreatic β cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Terauchi Y, Takamoto I, Kubota N, et al. Glucokinase and IRS-2 are required for compensatory β cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117:246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Withers DJ, Gutierrez JS, Towery H, et al. Disruption of IRS-2 causes type 2 diabetes in mice. Nature. 1998;391:900–904. [DOI] [PubMed] [Google Scholar]
- 14. Kubota N, Tobe K, Terauchi Y, et al. Disruption of insulin receptor substrate 2 causes type 2 diabetes because of liver insulin resistance and lack of compensatory β-cell hyperplasia. Diabetes. 2000;49:1880–1889. [DOI] [PubMed] [Google Scholar]
- 15. Accili D, Arden KC. FoxOs at the crossroads of cellular metabolism, differentiation, and transformation. Cell. 2004;117:421–426. [DOI] [PubMed] [Google Scholar]
- 16. Kitamura T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013;9:615–623. [DOI] [PubMed] [Google Scholar]
- 17. Müssig K, Staiger H, Machicao F, et al. Association of common genetic variation in the FOXO1 gene with β-cell dysfunction, impaired glucose tolerance, and type 2 diabetes. J Clin Endocrinol Metab. 2009;94:1353–1360. [DOI] [PubMed] [Google Scholar]
- 18. Talchai SC, Accili D. Legacy effect of Foxo1 in pancreatic endocrine progenitors on adult β-cell mass and function. Diabetes. 2015;64:2868–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim-Muller JY, Zhao S, Srivastava S, et al. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metab. 2014;20:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150:1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kitamura T, Nakae J, Kitamura Y, et al. The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic β cell growth. J Clin Invest. 2002;110:1839–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okamoto H, Hribal ML, Lin HV, Bennett WR, Ward A, Accili D. Role of the forkhead protein FoxO1 in β cell compensation to insulin resistance. J Clin Invest. 2006;116:775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kitamura T, Kitamura YI, Kobayashi M, et al. Regulation of pancreatic juxtaductal endocrine cell formation by FoxO1. Mol Cell Biol. 2009;29:4417–4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Buteau J, Shlien A, Foisy S, Accili D. Metabolic diapause in pancreatic β-cells expressing a gain-of-function mutant of the forkhead protein Foxo1. J Biol Chem. 2007;282:287–293. [DOI] [PubMed] [Google Scholar]
- 25. Kawamori D, Kaneto H, Nakatani Y, et al. The forkhead transcription factor Foxo1 bridges the JNK pathway and the transcription factor PDX-1 through its intracellular translocation. J Biol Chem. 2006;281:1091–1098. [DOI] [PubMed] [Google Scholar]
- 26. Al-Masri M, Krishnamurthy M, Li J, et al. Effect of forkhead box O1 (FOXO1) on β cell development in the human fetal pancreas. Diabetologia. 2010;53:699–711. [DOI] [PubMed] [Google Scholar]
- 27. Kitamura YI, Kitamura T, Kruse JP, et al. FoxO1 protects against pancreatic β cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. [DOI] [PubMed] [Google Scholar]
- 28. Kamagate A, Kim DH, Zhang T, et al. FoxO1 links hepatic insulin action to endoplasmic reticulum stress. Endocrinology. 2010;151:3521–3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez SC, Tanabe K, Cras-Méneur C, Abumrad NA, Bernal-Mizrachi E, Permutt MA. Inhibition of Foxo1 protects pancreatic islet β-cells against fatty acid and endoplasmic reticulum stress-induced apoptosis. Diabetes. 2008;57:846–859. [DOI] [PubMed] [Google Scholar]
- 30. Kim DH, Perdomo G, Zhang T, et al. FoxO6 integrates insulin signaling with gluconeogenesis in the liver. Diabetes. 2011;60:2763–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Qu S, Altomonte J, Perdomo G, He J, et al. Aberrant Forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Su D, Zhang N, He J, et al. Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes. 2007;56:2274–2283. [DOI] [PubMed] [Google Scholar]
- 33. Sklavos MM, Bertera S, Tse HM, et al. Redox modulation protects islets from transplant-related injury. Diabetes. 2010;59:1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamagate A, Qu S, Perdomo G, et al. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest. 2008;118:2347–2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schisler JC, Jensen PB, Taylor DG, et al. The Nkx6.1 homeodomain transcription factor suppresses glucagon expression and regulates glucose-stimulated insulin secretion in islet β cells. Proc Natl Acad Sci USA. 2005;102:7297–7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes. 2013;62:2450–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Puig O, Tjian R. Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 2005;19:2435–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meier JJ, Butler AE, Saisho Y, et al. β-Cell replication is the primary mechanism subserving the postnatal expansion of β-cell mass in humans. Diabetes. 2008;57:1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Georgia S, Hinault C, Kawamori D, et al. Cyclin D2 is essential for the compensatory β-cell hyperplastic response to insulin resistance in rodents. Diabetes. 2010;59:987–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Balcazar N, Sathyamurthy A, Elghazi L, et al. mTORC1 activation regulates β-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J Biol Chem. 2009;284:7832–7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Del Guerra S, Lupi R, Marselli L, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. [DOI] [PubMed] [Google Scholar]
- 42. Thorens B, Wu YJ, Leahy JL, Weir GC. The loss of GLUT2 expression by glucose-unresponsive β cells of db/db mice is reversible and is induced by the diabetic environment. J Clin Invest. 1992;90:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo S, Dai C, Guo M, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sansbury FH, Flanagan SE, Houghton JA, et al. SLC2A2 mutations can cause neonatal diabetes, suggesting GLUT2 may have a role in human insulin secretion. Diabetologia. 2012;55:2381–2385. [DOI] [PubMed] [Google Scholar]
- 45. Hughes KJ, Meares GP, Hansen PA, Corbett JA. FoxO1 and SIRT1 regulate β-cell responses to nitric oxide. J Biol Chem. 2011;286:8338–8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kibbe C, Chen J, Xu G, Jing G, Shalev A. FOXO1 competes with carbohydrate response element-binding protein (ChREBP) and inhibits thioredoxin-interacting protein (TXNIP) transcription in pancreatic β cells. J Biol Chem. 2013;288:23194–23202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC. Functional interaction between β-catenin and FOXO in oxidative stress signaling. Science. 2005;308:1181–1184. [DOI] [PubMed] [Google Scholar]
- 48. Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet β cells in diabetes. J Biol Chem. 2004;279:42351–42354. [DOI] [PubMed] [Google Scholar]
- 49. Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. [DOI] [PubMed] [Google Scholar]
- 50. Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and β-cell dysfunction. Endocr Rev. 2008;29:351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meares GP, Fontanilla D, Broniowska KA, Andreone T, Lancaster JR, Jr, Corbett JA. Differential responses of pancreatic β-cells to ROS and RNS. Am J Physiol Endocrinol Metab. 2013;304:E614–E622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhao Z, Choi J, Zhao C, Ma ZA. FTY720 normalizes hyperglycemia by stimulating β-cell in vivo regeneration in db/db mice through regulation of cyclin D3 and p57(KIP2). J Biol Chem. 2012;287:5562–5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Köhler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of β-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab. 2011;300:E221–E230. [DOI] [PubMed] [Google Scholar]
- 54. Ai J, Duan J, Lv X, et al. Overexpression of FoxO1 causes proliferation of cultured pancreatic β cells exposed to low nutrition. Biochemistry. 2010;49:218–225. [DOI] [PubMed] [Google Scholar]
- 55. Sharma RB, O'Donnell AC, Stamateris RE, et al. Insulin demand regulates β cell number via the unfolded protein response. J Clin Invest. 2015;125:3831–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dowell P, Otto TC, Adi S, Lane MD. Convergence of peroxisome proliferator-activated receptor γ and Foxo1 signaling pathways. J Biol Chem. 2003;278:45485–45491. [DOI] [PubMed] [Google Scholar]
- 57. Qu S, Su D, Altomonte J, Kamagate A, et al. PPARα mediates the hypolipidemic action of fibrates by antagonizing FoxO1. Am J Physiol Endocrinol Metab. 2007;292:E421–E434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tsunekawa S, Demozay D, Briaud I, et al. FoxO feedback control of basal IRS-2 expression in pancreatic β-cells is distinct from that in hepatocytes. Diabetes. 2011;60:2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakae J, Biggs WH, 3rd, Kitamura T, et al. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. [DOI] [PubMed] [Google Scholar]
- 60. Kikuchi O, Kobayashi M, Amano K, et al. FoxO1 gain of function in the pancreas causes glucose intolerance, polycystic pancreas, and islet hypervascularization. PLoS One. 2012;7:e32249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim HJ, Kobayashi M, Sasaki T, et al. Overexpression of FoxO1 in the hypothalamus and pancreas causes obesity and glucose intolerance. Endocrinology. 2012;153:659–671. [DOI] [PubMed] [Google Scholar]
- 62. Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012;44:406–412, S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bouchi R, Foo KS, Hua H, et al. FOXO1 inhibition yields functional insulin-producing cells in human gut organoid cultures. Nat Commun. 2014;5:4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kim MS, Pak YK, Jang PG, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. [DOI] [PubMed] [Google Scholar]
- 65. Ren H, Orozco IJ, Su Y, et al. FoxO1 target Gpr17 activates AgRP neurons to regulate food intake. Cell. 2012;149:1314–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]