Abstract
Gata2 is a zinc finger transcription factor that is important in hematopoiesis and neuronal development. However, the roles of Gata2 in the mesenchymal lineages are poorly understood. In vitro studies suggest that Gata2 modulates adipocyte differentiation and mesenchymal stem cell (MSC) proliferation. To systematically determine the in vivo functions of Gata2 in the MSC lineage commitment and development, we have generated three mouse models in which Gata2 is specifically deleted in MSCs, adipocytes, or osteoblasts. During the MSC expansion stage, Gata2 promotes proliferation and attenuates differentiation; thereby Gata2 loss in MSCs results in enhanced differentiation of both adipocytes and osteoblasts. During the differentiation stage, Gata2 also plays MSC-independent roles to impede lineage commitment; hence, Gata2 loss in adipocyte or osteoblast lineages also augments adipogenesis and osteoblastogenesis, respectively. These findings reveal Gata2 as a crucial rheostat of MSC fate to control osteoblast and adipocyte lineage development.
Mesenchymal stem cells (MSCs) are multipotent stromal cells that have the potential to differentiate into several lineages such as osteoblasts and adipocytes (1). There are several key transcription factors that commit and guide MSCs to specific fate: in the osteoblast lineage, runt-related transcription factor 2 (Runx2) orchestrates the activation of downstream genes that control the early stage of osteoblastogenesis; in the adipocyte lineage, CCAAT/enhancer binding proteins (CEBPs) are indispensable for triggering the initiation of adipogenesis, whereas peroxisome proliferator-activated receptor-γ (PPARγ) activation promotes adipocyte maturation (2). MSCs can be isolated from the bone marrow, expanded, and differentiated into different lineages ex vivo. Thus, they present exciting potential for stem cell therapy to restore and replace damaged mesenchymal tissues (3, 4).
Gata2 is a zinc finger transcription factor that plays crucial roles in hematopoietic development. Gata2 homozygous null embryos die during embryogenesis due to a failure in blood cell generation (5), and Gata2 haploinsufficiency resulted in altered integrity of the definitive hematopoietic stem cell (HSC) compartment, leading to a significant reduction in HSC number (6). Gata2 deficiency has been implicated in many human diseases. For example, 84% of GATA2-deficient patients develop myelodysplastic syndrome, in which the ability of bone marrow to make blood cells is compromised. In addition, these patients are also more susceptible to acute myeloid leukemia, chronic myelomonocytic leukemia, immunodeficiency, and vascular/lymphatic dysfunction (7).
Although Gata2 regulation of HSCs has been well studied, whether Gata2 plays a functional role in MSCs or its sublineages is largely unknown. Most studies to date have been done in adipocyte lineage using cell lines such as 3T3-F442A or TBR343 preadipocytic cell lines. Tong et al (8) found that Gata2 inhibits preadipocyte-to-adipocyte transition by suppressing PPARγ and interacting with CEBP family transcription factors (9). In humans, it was reported that patients with aplastic anemia express lower Gata2 and higher PPARγ in bone marrow cells, which could explain the fatty marrow symptom (10). Recently another in vitro study showed that Gata2 knockdown decreased cell proliferation and increased the number of cells in G1/G0 phase (11). However, very little is known about how Gata2 regulates MSCs and their potential to differentiate into sublineages such as osteoblasts and adipocytes in a physiological context. In particular, it is essentially unknown whether and how Gata2 regulates osteoblastogenesis. Furthermore, no in vivo model has been established to study whether Gata2 is functionally significant for MSC renewal and differentiation. In this study, we created mouse models in which Gata2 is specifically deleted in MSCs, adipocytes, or osteoblasts to dissect its physiological roles in mesenchymal lineage development.
Materials and Methods
Mice
Gata2 flox mice have been previously described (12). Transgenic mice for paired related homeobox 1 (Prx1)-Cre (13), adiponectin-Cre (14), and osteocalcin-Cre (15) were from the Jackson Laboratory. Prx1 is a transcription coactivator expressed in early limb bud mesenchyme and in a subset of craniofacial mesenchyme. Prx1-Cre mice are widely used to target mesenchymal stem cells to study mesenchymal specific gene functions. Mice were fed standard chow containing 4% fat ad libitum unless stated otherwise. All studies were conducted with 2-month-old male littermates. Sample size estimate was based on power analyses performed using SAS 9.3 TS X64_7PRO platform at the University of Texas Southwestern Medical Center Biostatistics Core. With the observed group differences and the relatively small variation of the in vivo measurements, n = 4 and n = 3 will provide more than 90% and more than 80% power at the type I error rate of 0.05 (two sided test), respectively. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center.
Bone analyses
Histomorphometry of femoral sections was conducted using the BIOQUANT software (Bioquant) as previously described (16, 17). Alkaline phosphatase (ALP) staining of osteoblasts was performed using an ALP staining kit containing fast blue RR salt (Sigma). Oil Red O (ORO) staining of adipocytes was performed as described (18). An ELISA of a serum bone formation marker amino-terminal propeptide of type I collagen (P1NP) and a microcomputed tomography (μCT) were performed as previously described (16, 17).
Ex vivo bone marrow MSC expansion and differentiation
For the MSC expansion, mouse bone marrow cells were purified with 100 μm cell strainer (19, 20) and then cultured for 3–7 days in MSC media (mouse MesenCult proliferation kit; StemCell Technologies Inc). Osteoblasts were differentiated from marrow MSCs as described (21). Briefly, MSCs were cultured in α-MEM containing 10% fetal bovine serum, 5 mM β-glycerophosphate, and 100 μg/mL ascorbic acid (mineralization medium) for 9 days. Mature osteoblasts were identified as ALP+ cells using fast red violet LB salt (Sigma). For adipocyte differentiation, MSCs were cultured in adipogenesis medium (MesenCult basal medium + mouse MesenCult adipogenic stimulatory supplement; StemCell Technologies) for 7 days. Mature adipocytes were identified as ORO+ cells. Differentiation was quantified by the mRNA expression of marker genes using RT-quantitative PCR.
5-Bromo-2′-deoxyuridine (BrdU) cell proliferation assay
Bone marrow cells were cultured for 4 days in MSC medium. The cells were serum starved overnight and then restimulated with serum for 6 hours to induce the S phase, during which BrdU was provided in the culture medium. Cell proliferation was quantified as BrdU incorporation using the BrdU ELISA assay in the BrdU cell proliferation assay kit (GE Healthcare Life Sciences).
TOP-flash/FOP-flash reporter assay
To quantify β-catenin activity, human embryonic kidney-293 cells were transfected with a T cell factor (TOP-flash) luciferase reporter or a mutant T cell factor (FOP-flash)-negative control reporter together with a cytomegalovirus-βgal reporter (as internal control for transfection efficiency) as well as an expression plasmid for a constitutively active β-catenin (β-catenin-δex3) or vector control as we previously described (22). All transfections were performed using FuGENE HD (Roche) (n = 6) and repeated for at least three times. Reporter assays were conducted 48 hours after transfection. Data are presented as TOP to FOP ratio.
RNA expression analyses
RNA expression was analyzed by RT-quantitative PCR. RNA was extracted with TRIZOL (Invitrogen), reverse transcribed into cDNA using an ABI high capacity cDNA RT kit (Invitrogen), and then analyzed using real-time quantitative PCR (SYBR Greener; Invitrogen) with gene-specific primers (Table 1) in triplicate. All RNA expression was normalized by mitochondrial ribosomal protein L19, one of the most stable and reliable endogenous control genes (23).
Table 1.
Mouse Primers for RT-PCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Gata2 | CCTGGCGCATAACTACATGGA | GAGTCGAGATGGTTGAAGAAGACA |
| Fas | GCAGACATGCTGTGGATCTG | AGTTTCATGAACCCGCCTC |
| Bad | GAGCAACATTCATCAGCAGG | TAAGCTCCTCCTCCATCCCT |
| Bax | TTGCTGATGGCAACTTCAAC | GAGGAAGTCCAGTGTCCAGC |
| Bcl-2 | AGTACCTGAACCGGCATCTG | GCTGAGCAGGGTCTTCAGAG |
| Bcl-6 | AGTTTCTAGGAAAGGCCGGA | ACTAGCGTGCCGGGTAAACT |
| Adiponectin | CAGTGGATCTGACGACACCAA | GAACAGGAGAGCTTGCAACAGT |
| PPARg2 | GCTGATGCACTGCCTATGAGC | CGGAGAGGTCCACAGAGCTG |
| UCP1 | AAGCTGTGCGATGTCCATGT | AAGCCACAAACCCTTTGAAAA |
| Osteocalcin | TGACAAAGCCTTCATGTCCA | ATAGCTCGTCACAAGCAGGG |
| Col1a1 | CGTCTGGTTTGGAGAGAGCAT | GGTCAGCTGGATAGCGACATC |
| Runx2 | GCTCACGTCGCTCATCTTG | ACACCGTGTCAGCAAAGC |
| L19 | GGTCTGGTTGGATCCCAATG | CCCATCCTTGATCAGCTTCCT |
Abbreviations: L19, mitochondrial ribosomal protein L19.
Statistical analyses
Statistical analyses were performed with a Student's t test and represented as mean ± SD (*, P < .05; **, P < .01; ***, P < .005; ****, P < .001; n.s., nonsignificant).
Results
Gata2 expression is differentially regulated during MSC proliferation and differentiation
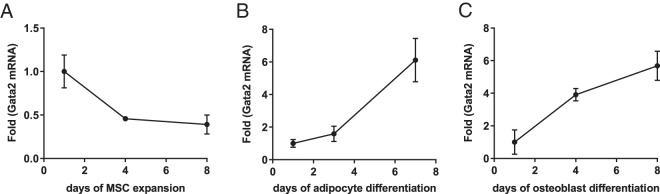
To study how Gata2 expression changes during MSC proliferation, we collected bone marrow MSCs and expanded them in culture. We found that Gata2 expression decreased as MSCs proliferated (Figure 1A). To study how Gata2 expression changes during differentiation, we treated the expanded MSCs with cocktails that trigger adipogenesis or osteoblastogenesis. The results show that the Gata2 level steadily increased during both adipocyte and osteoblast differentiation (Figure 1, B and C). These results suggest that Gata2 is likely important for both MSC proliferation and differentiation into different lineages such as adipocytes and osteoblasts. Furthermore, given Gata2's opposite expression pattern during proliferation vs differentiation, it could play distinct and independent roles in MSC expansion and lineage commitment.
Figure 1.
Gata2 decreases during MSC expansion but increases during differentiation. A, Gata2 expression decreased during MSC proliferation (n = 3). B, Gata2 expression increased during adipocyte differentiation (n = 3). C, Gata2 expression increased during osteoblast differentiation (n = 3). Error bars, SD.
Gata2 deletion in early mesenchymal lineage decreases MSC proliferation
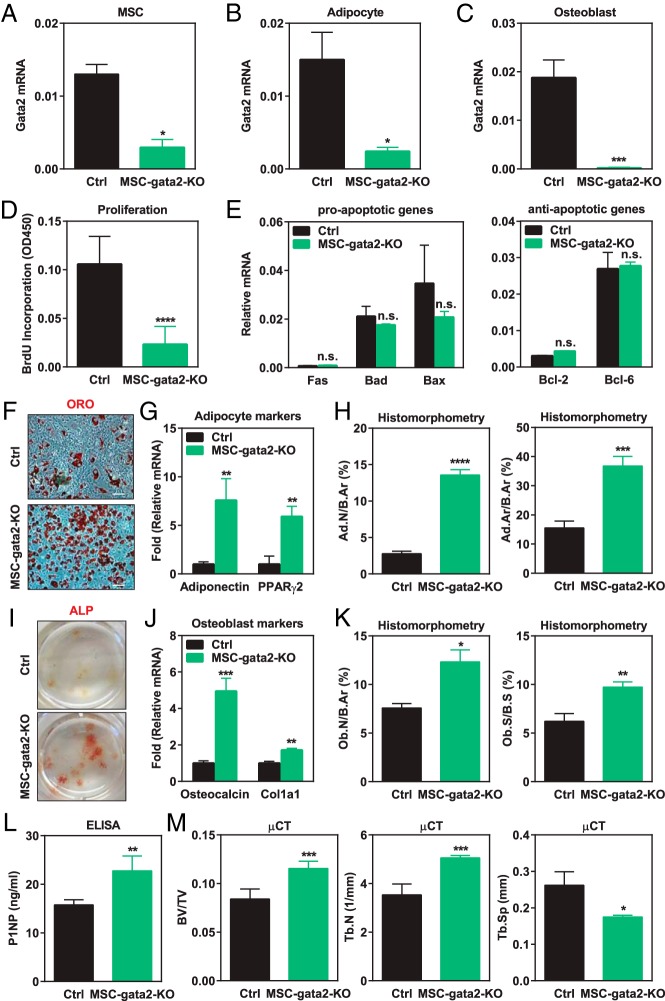
To study how Gata2 affects early mesenchymal lineage in vivo, we generated MSC Gata2 conditional knockout (MSC-gata2-KO) mice by crossing Gata2 flox mice (12) with the previously established Prx1-Cre transgenic mice (13). By comparing MSC-gata2-KO mice with littermate control mice, we found that Prx1-Cre successfully depleted Gata2 expression in all three mesenchymal populations: MSCs, adipocytes, and osteoblasts (Figure 2, A–C). As a result, MSC proliferation was markedly decreased, measured by BrdU incorporation (Figure 2D). This is consistent with the in vitro observations by Kamata et al (11) that Gata2 small interfering RNA knockdown in human MSCs reduced cell proliferation and down-regulated genes in cell cycle and DNA replication. Moreover, we found that Gata2 loss does not affect MSC apoptosis because the expression of proapoptotic genes such as Fas, Bad, and Bax or antiapoptotic genes such as Bcl-2 and Bcl-6 was unaltered (Figure 2E). These findings indicate that Gata2 promotes MSC proliferation.
Figure 2.
Gata2 in MSC promotes proliferation to prevent differentiation. A–C, Gata2 level was significantly decreased in MSC (A), adipocyte (B), and osteoblast (C) ex vivo cultures derived from MSC-gata2-KO bone marrow compared with littermate controls (n = 3). D, MSCs from MSC-gata2-KO mice (n = 5) exhibited reduced proliferation compared with controls (n = 10), measured by BrdU incorporation. E, MSCs from MSC-gata2-KO mice displayed unaltered expression of proapoptotic and antiapoptotic genes (n = 3). F and G, Adipocyte differentiation was enhanced for MSCs from MSC-gata2-KO mice, shown by the increased ORO staining (F) and the higher expression of adipogenic markers such as adiponectin and PPARγ2 (G) (n = 3). H, Femurs from MSC-gata2-KO mice exhibited more marrow fat, shown by the higher adipocyte number/bone area (Ad.N/B.Ar) and adipocyte area/bone area (Ad.Ar/B.Ar) (n = 6). I and J, Osteoblast differentiation was enhanced for MSCs from MSC-gata2-KO mice, shown by the increased ALP staining (I) and the higher expression of osteoblastogenic markers such as osteocalcin and Col1a1 (J) (n = 3). K, Femurs from MSC-gata2-KO mice displayed higher osteoblast number/bone area (Ob.N/B.Ar) and osteoblast surface/bone surface (Ob.S/B.S) (n = 6). L, ELISA analysis showed that serum bone formation marker P1NP was higher in MSC-gata2-KO mice (n = 6). M, μCT analysis of the trabecular bone in proximal tibiae showed that MSC-gata2-KO mice exhibited higher bone mass (n = 4). BV/TV, bone volume/tissue volume ratio; Ctrl, control; Tb.N, trabecular number; Tb.Sp, trabecular separation. Error bars, SD.
Gata2 deletion in early mesenchymal lineage enhances differentiation
We next examined how Gata2 loss in MSCs affects adipocyte and osteoblast differentiation. There are two possibilities: MSC Gata2 deletion may inhibit differentiation by reducing the number of progenitors due to decreased proliferation; alternatively, MSC Gata2 deletion may promote differentiation by dampening proliferation, thereby facilitating the proliferation-to-differentiation switch. Our results supported the second hypothesis and showed that Gata2 loss in MSCs increased the differentiation of both adipocytes and osteoblasts. In ex vivo experiments, adipocyte differentiation cultures from MSC-gata2-KO bone marrow exhibited stronger ORO staining (Figure 2F) as well as higher expression of adipocyte markers such as adiponectin and PPARγ2 (Figure 2G) compared with control differentiation cultures. Consistent with this observation, ORO staining of mouse femur sections showed more marrow fat in vivo (Figure 2H). Similarly, MSC Gata2 deletion also enhanced osteoblastogenesis shown by the stronger ALP staining (Figure 2I) as well as elevated osteoblast markers such as osteocalcin and Collagen, type I, alpha 1 (Figure 2J) in ex vivo differentiation cultures. In accordance with this finding, ALP staining of mouse femur sections showed increased osteoblast number and surface in vivo (Figure 2K); ELISA showed an increased serum bone formation marker P1NP (Figure 2L); μCT showed higher bone volume and trabecular number with a lower trabecular separation (Figure 2M). Together these data support that at the MSC level, Gata2 is a physiologically significant regulator that promotes proliferation and attenuates differentiation.
Gata2 deletion in adipocyte lineage promotes adipocyte differentiation
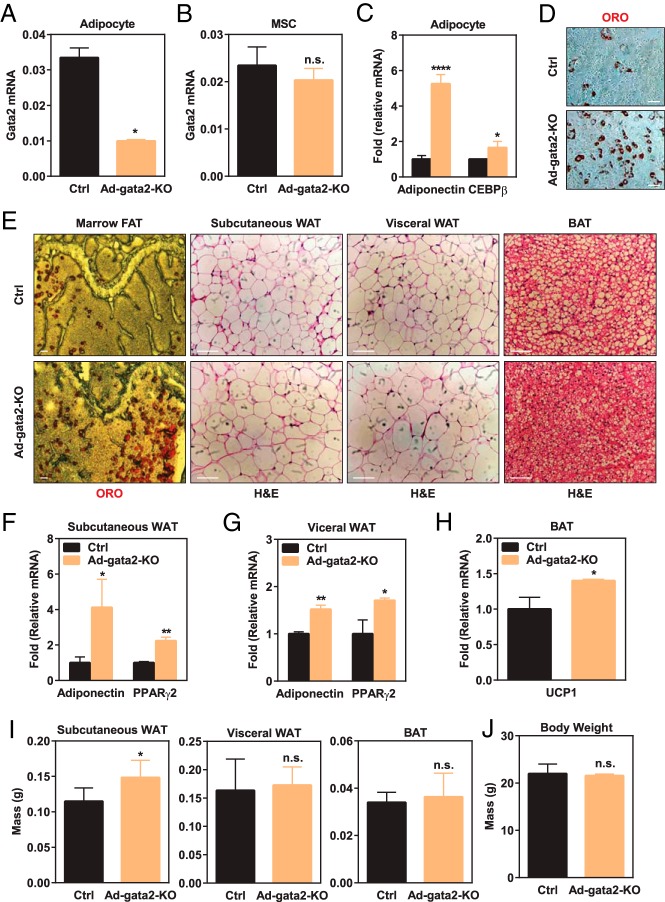
Because the expression of Gata2 increases during adipocyte differentiation whereas it decreases during MSC proliferation, we decided to investigate whether Gata2 plays a synergistic or opposite role in the adipose lineage that may be independent of those in MSC proliferation. To achieve this goal, we created adipocyte-specific Gata2 conditional knockout (Ad-gata2-KO) mice driven by the previously established adiponectin-Cre (14). The adiponectin-Cre driver significantly reduced Gata2 expression in the adipocyte lineage (Figure 3A), whereas it did not affect Gata2 expression in MSCs (Figure 3B). As a result, ex vivo adipogenesis was augmented in the Ad-gata2-KO cultures compared with controls, shown by the increased expression of adipoctye markers such as adiponectin and CEBPβ (Figure 3C) as well as more ORO+ cells (Figure 3D).
Figure 3.
Gata2 in adipocyte lineage suppresses adipogenesis. A and B, Gata2 expression was significantly diminished in adipocytes (A) but not MSCs (B) derived from the bone marrow of Ad-gata2-KO mice (n = 3). C and D, Ex vivo bone marrow adipocyte differentiation was enhanced for Ad-gata2-KO mice compared with littermate controls, shown by the higher expression of adipogenic markers such as adiponectin and CEBPβ (C), and more ORO staining in the differentiation cultures (D) (n = 3). E, Ad-Gata2-KO mice exhibited enhanced adipogenesis in vivo, shown by the more abundant marrow adipocytes and larger adipocytes in subcutaneous and visceral WAT as well as more differentiated BAT. Representative images for ORO or hematoxylin and eosin staining are shown. Scale bars, 25 μm. F and G, Expression of WAT markers such as adiponectin and PPARγ2 was higher in the sc WAT (F) and visceral WAT (G) from Ad-gata2-KO mice compared with controls (n = 3). H, Expression of BAT markers such as UCP1 was higher in the BAT from Ad-gata2-KO mice compared with controls (n = 3). I, Subcutaneous WAT from Ad-gata2-KO mice was heavier compared with controls, whereas visceral WAT and BAT weights were similar (n = 3). J, Body weight was not significantly altered in Ad-gata2-KO mice (n = 3). Ctrl, control. Error bars, SD.
In vivo analysis by ORO staining revealed that femur sections from Ad-gata2-KO mice exhibited more abundant marrow fat compared with littermate control mice (Figure 3E). In addition to marrow adipocytes, there are other types of adipose tissue such as brown adipose tissue (BAT) and peripheral white adipose tissue (WAT) that display different gene expression profile and functions in the body. Thus, we decided to examine how Gata2 deletion affects peripheral WAT and BAT development in vivo. Hematoxylin and eosin staining showed that both sc WAT and visceral WAT from Ad-gata2-KO mice contained more mature adipocytes compared with the littermate control mice, evidenced by the larger size, indicating an enhanced WAT adipogenesis (Figure 3E). Furthermore, BAT from Ad-gata2-KO mice exhibited less lipid droplets, indicating an augmented BAT development (Figure 3E). In support of the histology observations, gene expression analysis showed that both sc WAT and visceral WAT showed significantly higher expression of adipose markers such as adiponectin and PPARγ2 (Figure 3, F and G). Moreover, BAT from Ad-gata2-KO mice also expressed a higher level of uncoupling protein-1 (UCP1), a marker for thermogenesis (Figure 3H). Subcutaneous WAT mass was significantly higher in the Ad-gata2-KO mice compared with the controls, whereas visceral WAT mass, BAT mass, or body weight did not show significant differences (Figure 3, I and J). These in vivo observations further support the notion that Gata2 loss in the adipocyte lineage enhances adipogenesis.
Given that the adiponectin-Cre driver specifically deleted Gata2 in adipocyte but not in MSCs (Figure 3A), these findings indicate that Gata2 has a secondary function in the adipocyte lineage to further suppress adipogenesis. Our genetic and in vivo observations are in line with the report by Tong et al (8, 9), which shows that Gata2 inhibits preadipocyte to adipocyte transition in vitro by suppressing PPARγ and CEBPs, as well as the report by Tsai et al (24), which shows that Gata2 also inhibits BAT differentiation in vitro by suppressing both UCP1 and peroxisomal proliferator-activated receptor-γ coactivator 1α promoters.
Gata2 deletion in osteoblast lineage promotes osteoblast differentiation
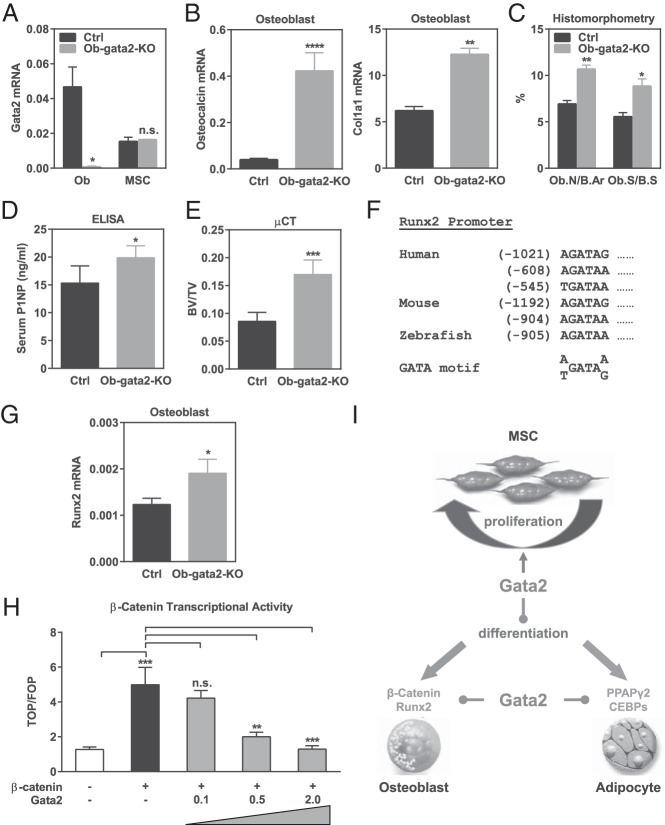
We have uncovered a novel function of Gata2 to impede osteoblastogenesis. This is partially attributed to the ability of Gata2 to sustain MSC proliferation and thereby prevent differentiation. Because the expression of Gata2 increases during osteoblast differentiation whereas it decreases during MSC proliferation, we decided to further investigate whether Gata2 plays a synergistic or opposite role in the osteoblast lineage that may be independent of those in MSCs. To achieve this goal, we generated osteoblast-specific conditional knockout (Ob-gata2-KO) mice driven by the previously established osteocalcin-Cre (15). Our results showed that the osteocalcin-Cre driver successfully lowered Gata2 expression in osteoblast without affecting Gata2 levels in MSCs (Figure 4A). Ex vivo bone marrow differentiation assays revealed that Ob-gata2-KO cultures displayed enhanced osteoblastogenesis, shown by the significantly higher expression of osteoblast marker genes such as Col1a1 and osteocalcin (Figure 4B). In vivo analysis also showed that osteoblast number and surface were significantly greater in the femurs sections from Ob-gata2-KO mice compared with controls (Figure 4C), leading to an increased bone formation (Figure 4D) and a higher bone mass (Figure 4E). Together these findings suggest that Gata2 loss in the osteoblast lineage facilitates osteoblast differentiation.
Figure 4.
Gata2 in osteoblast lineage suppresses osteoblastogenesis. A, Gata2 expression was significantly diminished in osteoblasts (left) but not MSCs (right) derived from the bone marrow of Ob-gata2-KO mice (n = 3). B, Ex vivo bone marrow osteoblast differentiation was enhanced for Ob-gata2-KO mice compared with littermate controls, shown by the higher expression of osteoblast markers such as osteocalcin and Col1a1 (n = 3). C, Femurs from Ob-gata2-KO mice displayed higher osteoblast number/bone area (Ob.N/B.Ar) and osteoblast surface/bone surface (Ob.S/B.S) (n = 6). D, ELISA analysis showed that serum bone formation marker P1NP was higher in Ob-gata2-KO mice (n = 6). E, μCT analysis of the trabecular bone in proximal tibiae showed that Ob-gata2-KO mice exhibited higher bone mass (n = 4). BV/TV, bone volume/tissue volume ratio. F, Multiple GATA binding motifs were identified in the Runx2 promoter in human, mouse, and zebrafish. Examples are shown. G, Runx2 expression was increased in osteoblasts differentiated from the bone marrow of Ob-gata2-KO mice (n = 3). H, Gata2 dose dependently suppressed β-catenin activity in a transient transfection and TOP/FOP reporter assay (n = 6). I, A working model for how Gata2 regulates mesenchymal cell fate. Gata2 in the early mesenchymal lineage promotes MSC proliferation and suppresses terminal differentiation. Gata2 in the committed osteoblast and adipocyte lineages also inhibit differentiation by suppressing key transcription factors: in the osteoblast lineage, Gata2 down-regulates β-catenin and Runx2; in the adipocyte lineage, Gata2 attenuates PPARγ2 and CEBPs. Ctrl, control.
We next sought to explore the mechanism of how Gata2 in the osteoblast lineage inhibits osteoclast differentiation. Runx2 is a key transcription factor that promotes osteoblastogenesis. We found that both human and mouse Runx2 promoters contain multiple GATA binding motifs, such as the examples shown in Figure 4F, suggesting that Runx2 may be a Gata2 target gene. Indeed, we found that Gata2 loss led to an increased Runx2 mRNA expression in osteoblasts (Figure 4G), indicating that Gata2 inhibition of osteoblastogenesis is partially mediated by repressing Runx2 transcription. In addition to Runx2, Wnt/β-catenin signaling also boosts osteoblast differentiation (25). To determine the effects of Gata2 on β-catenin activity, we performed transient transfection and a TOP/FOP β-catenin reporter assay. Compared with vector control, Gata2 significantly decreased β-catenin activity in a dose-dependent manner (Figure 4H), indicating that Gata2 inhibition of osteoblastogenesis also may be contributed by suppressing β-catenin signaling.
Discussion
In this study, we have provided genetic and in vivo evidence that Gata2 is a crucial and physiologically significant rheostat of MSC fate and mesenchymal lineage development. Moreover, we have uncovered multiple cellular and molecular mechanisms that confer Gata2 regulation. In MSCs, Gata2 sustains stem cell proliferation to prevent differentiation (Figure 4J). In lineage-committed cells, Gata2 further inhibits differentiation by impeding key transcription factors and signaling pathways: in adipocytes, Gata2 suppresses PPARγ and CEBPs; in osteoblasts, Gata2 suppresses Runx2 and β-catenin (Figure 4I). The notion that Gata2 targets Runx2 expression is further supported by our analysis of the Encyclopedia of DNA Elements chromatin immunoprecipitation-sequencing database (26), which shows that Gata2 can bind to multiple sites at the human Runx2 promoter and enhancer region. Together these findings provide important new insights to MSC function and cellular differentiation, with potential implications in tissue regeneration and degenerative diseases.
Gata2's expression pattern is controlled by its upstream regulators, whereas its roles in proliferation and differentiation are related to its positive or negative effects on downstream targets. These two readouts do not necessarily correlate with or contradict each other. Our observation that Gata2 decreases during MSC expansion yet promotes proliferation suggests that Gata2 expression in early MSCs is required for MSC proliferation, but its expression may be down-regulated by a negative feedback loop at the late stage of MSC expansion. Similarly, our observation that Gata2 increases during differentiation yet suppresses adipogenesis and osteoblastogenesis is consistent with our findings that Gata2 is a negative regulator that inhibits positive transcription factors such as PPARγ, CEBPs, Runx2, and β-catenin, as our data and model have shown; therefore, the Gata2 level must be low at the beginning of the differentiation to derepress these positive transcription factors and trigger the differentiation program, but Gata2 expression gradually bounces back to timely resolve the signaling and cease the differentiation possibly due to a negative feedback loop at late stage of differentiation; as a result, Gata2 loss enhances differentiation. This dynamic regulation has been shown in many other cell types. For example, the level of the master proosteoclastogenic transcription factor Nuclear Factor of Activated T-Cells, Cytoplasmic, Calcineurin-Dependent 1 is high at the early stage of osteoclast differentiation, yet its expression decreases during the late stage of osteoclast differentiation (27–29). In addition, we have recently shown that the expression of the nuclear receptor Nur77 increases during osteoclastogenesis, yet it suppresses osteoclast differentiation by triggering Nuclear Factor of Activated T-Cells, Cytoplasmic, Calcineurin-Dependent 1 degradation (28).
Gata2 functions in MSCs may exert an impact beyond mesenchymal lineages. MSCs, osteoblasts, and adipocytes have all been shown to help to form and support the HSC niche (30, 31). Therefore, Gata2 in MSC and mesenchymal cell types may indirectly regulate HSCs and hematopoietic lineages. Gata2 loss in mesenchymal lineages may disrupt the HSC niche, further worsening the dysregulation of blood cells caused by Gata2 loss in hematopoietic lineages. Furthermore, marrow fat infiltrations are common features in both myeloplastic syndrome and aplastic anemia, both of which have been related to Gata2 deficiency. In these cases, Gata2 loss in the mesenchymal cell compartment could exacerbate the deficiency in blood cells by up-regulating marrow adipocyte differentiation.
In addition to adipocyte and osteoblast, other lineages such as chondrocyte and myocyte can also be differentiated from MSCs. A recent study suggests that Gata2 dysregulation may be a marker for skeletal muscle hypertrophy (32). In future studies, it will be interesting to see whether and how Gata2 can also regulate these other mesenchymal lineages. Knowledge of how Gata2 controls MSC expansion, lineage commitment, and cellular differentiation will educate us to design better strategies for stem cell therapy and regenerative medicine. To this end, K-7174 and K-11706 are two compounds that can inhibit Gata2 activity (33), which may hold exciting potential as novel bone anabolic agents for the treatment of skeletal degenerative diseases such as osteoporosis.
Acknowledgments
Y.W. is a Virginia Murchison Linthicum Scholar in Medical Research.
This work was supported in part by National Institutes of Health Grant R01 DK089113 (to Y.W.), Cancer Prevention and Research Institute of Texas Grant RP130145 (to Y.W.), Department of Defense Breast Cancer Research Program Idea Award BC122877 (to Y.W.), March of Dimes Grant 6-FY13-137 (to Y.W.), The Welch Foundation Grant I-1751 (to Y.W.), and the University of Texas Southwestern Endowed Scholar Startup Fund (to Y.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALP
- alkaline phosphatase
- BAT
- brown adipose tissue
- BrdU
- 5-bromo-2′-deoxyuridine
- CEBP
- CCAAT/enhancer binding protein
- μCT
- microcomputed tomography
- FOP-flash
- mutant T cell factor negative control reporter
- HSC
- hematopoietic stem cell
- MSC
- mesenchymal stem cell
- ORO
- Oil Red O
- P1NP
- amino-terminal propeptide of type I collagen
- PPARγ
- peroxisome proliferator-activated receptor-γ
- Prx1
- paired related homeobox 1
- Runx2
- runt-related transcription factor 2
- TOP-flash
- T cell factor reporter
- UCP1
- uncoupling protein-1
- WAT
- white adipose tissue.
References
- 1. Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–74. [DOI] [PubMed] [Google Scholar]
- 2. James AW. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica. 2013;2013:684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bornes TD, Adesida AB, Jomha NM. Mesenchymal stem cells in the treatment of traumatic articular cartilage defects: a comprehensive review. Arthritis Res Ther. 2014;16(5):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Perdisa F, Gostynska N, Roffi A, Filardo G, Marcacci M, Kon E. Adipose-derived mesenchymal stem cells for the treatment of articular cartilage: a systematic review on preclinical and clinical evidence. Stem Cells Int. 2015;2015:597652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson KD, Hsu AP, Ryu MJ, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. J Clin Invest. 2012;122(10):3692–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodrigues NP, Janzen V, Forkert R, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106(2):477–484. [DOI] [PubMed] [Google Scholar]
- 7. Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tong Q, Dalgin G, Xu H, Ting CN, Leiden JM, Hotamisligil GS. Function of GATA transcription factors in preadipocyte-adipocyte transition. Science. 2000;290(5489):134–138. [DOI] [PubMed] [Google Scholar]
- 9. Tong Q, Tsai J, Tan G, Dalgin G, Hotamisligil GS. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol Cell Biol. 2005;25(2):706–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu Y, Takahashi Y, Wang Y, et al. Downregulation of GATA-2 and overexpression of adipogenic gene-PPARγ in mesenchymal stem cells from patients with aplastic anemia. Exp Hematol. 2009;37(12):1393–1399. [DOI] [PubMed] [Google Scholar]
- 11. Kamata M, Okitsu Y, Fujiwara T, et al. GATA2 regulates differentiation of bone marrow-derived mesenchymal stem cells. Haematologica. 2014;99(11):1686–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haugas M, Lillevali K, Hakanen J, Salminen M. Gata2 is required for the development of inner ear semicircular ducts and the surrounding perilymphatic space. Dev Dyn. 2010;239(9):2452–2469. [DOI] [PubMed] [Google Scholar]
- 13. Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. [DOI] [PubMed] [Google Scholar]
- 14. Eguchi J, Wang X, Yu S, et al. Transcriptional control of adipose lipid handling by IRF4. Cell Metab. 2011;13(3):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277(46):44005–44012. [DOI] [PubMed] [Google Scholar]
- 16. Krzeszinski JY, Wei W, Huynh H, et al. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17. Wei W, Dutchak PA, Wang X, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc Natl Acad Sci USA. 2012;109(8):3143–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR γ protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21(15):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wei W, Zeve D, Suh JM, et al. Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol. 2011;31(23):4706–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wei W, Zeve D, Wang X, et al. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor γ-expressing bone marrow cell population. Mol Cell Biol. 2011;31(23):4692–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krum SA, Miranda-Carboni GA, Hauschka PV, et al. Estrogen protects bone by inducing Fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008;27(3):535–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jin Z, Wei W, Dechow PC, Wan Y. HDAC7 inhibits osteoclastogenesis by reversing RANKL-triggered β-catenin switch. Mol Endocrinol. 2013;27(2):325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McNeill RE, Miller N, Kerin MJ. Evaluation and validation of candidate endogenous control genes for real-time quantitative PCR studies of breast cancer. BMC Mol Biol. 2007;8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tsai J, Tong Q, Tan G, Chang AN, Orkin SH, Hotamisligil GS. The transcription factor GATA2 regulates differentiation of brown adipocytes. EMBO Rep. 2005;6(9):879–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Case N, Rubin J. β-catenin—a supporting role in the skeleton. J Cell Biochem. 2010;110(3):545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerstein MB, Kundaje A, Hariharan M, et al. Architecture of the human regulatory network derived from ENCODE data. Nature. 2012;489(7414):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim JH, Kim K, Jin HM, et al. Negative feedback control of osteoclast formation through ubiquitin-mediated down-regulation of NFATc1. J Biol Chem. 2010;285(8):5224–5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li X, Wei W, Huynh H, Zuo H, Wang X, Wan Y. Nur77 prevents excessive osteoclastogenesis by inducing ubiquitin ligase Cbl-b to mediate NFATc1 self-limitation. eLife. 2015;4:e07217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. [DOI] [PubMed] [Google Scholar]
- 30. Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011;117(20):5281–5288. [DOI] [PubMed] [Google Scholar]
- 31. Valtieri M, Sorrentino A. The mesenchymal stromal cell contribution to homeostasis. J Cell Physiol. 2008;217(2):296–300. [DOI] [PubMed] [Google Scholar]
- 32. Musaro A, McCullagh KJ, Naya FJ, Olson EN, Rosenthal N. IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature. 1999;400(6744):581–585. [DOI] [PubMed] [Google Scholar]
- 33. Nakano Y, Imagawa S, Matsumoto K, et al. Oral administration of K-11706 inhibits GATA binding activity, enhances hypoxia-inducible factor 1 binding activity, and restores indicators in an in vivo mouse model of anemia of chronic disease. Blood. 2004;104(13):4300–4307. [DOI] [PubMed] [Google Scholar]