Abstract
Stress elicits activation of the hypothalamic-pituitary-adrenal axis, which leads to enhanced circulating glucocorticoids, as well as impaired gonadotropin secretion and ovarian cyclicity. Here, we tested the hypothesis that elevated, stress-levels of glucocorticoids disrupt ovarian cyclicity by interfering with the preovulatory sequence of endocrine events necessary for the LH surge. Ovarian cyclicity was monitored in female mice implanted with a cholesterol or corticosterone (Cort) pellet. Cort, but not cholesterol, arrested cyclicity in diestrus. Subsequent studies focused on the mechanism whereby Cort stalled the preovulatory sequence by assessing responsiveness to the positive feedback estradiol signal. Ovariectomized mice were treated with an LH surge-inducing estradiol implant, as well as Cort or cholesterol, and assessed several days later for LH levels on the evening of the anticipated surge. All cholesterol females showed a clear LH surge. At the time of the anticipated surge, LH levels were undetectable in Cort-treated females. In situ hybridization analyses the anteroventral periventricular nucleus revealed that Cort robustly suppressed the percentage of Kiss1 cells coexpressing cfos, as well as reduced the number of Kiss1 cells and amount of Kiss1 mRNA per cell, compared with expression in control brains. In addition, Cort blunted pituitary expression of the genes encoding the GnRH receptor and LHβ, indicating inhibition of gonadotropes during the blockage of the LH surge. Collectively, our findings support the hypothesis that physiological stress-levels of Cort disrupts ovarian cyclicity, in part, through disruption of positive feedback mechanisms at both the hypothalamic and pituitary levels which are necessary for generation of the preovulatory LH surge.
Physical and emotional stress can inhibit reproduction in males and females. For example, restraint and/or isolation, a model of psychosocial or emotional stress, reduces mean plasma LH concentrations in numerous species, including in rats, sheep, and monkeys (1–6). In females, stress has been shown to disrupt ovulatory cyclicity, which is dependent on proper feedback control of gonadotropin synthesis and secretion (7, 8). One such mode of gonadotropin secretion is the pulsatile release of LH which dominates during the period of follicular development. Stressors such as restraint or endotoxin, a model of immune or inflammatory stress, reduce the frequency of LH pulses in rats and sheep (3, 9, 10). Decreased GnRH secretion, as well as diminished pituitary responsiveness to GnRH, underlies the reduction in LH pulsatility (11, 12). In addition to the suppression of LH pulses, the surge mode of LH secretion, which governs ovulation in females, is vulnerable to the effects of stress. Isolation-restraint stress can block the preovulatory LH surge in rats, and in sheep, the LH surge can be delayed or blocked by transport in a truck or by infusion of endotoxin (11, 13, 14). A delay in the LH surge has been observed in rhesus monkeys exposed to the combined stress of exercise, food restriction, and movement to a novel environment (8). Despite evidence that stress interferes with ovarian cyclicity in females, the fundamental mechanisms that result in disrupted pulsatile and surge LH secretion are not well understood.
In response to stress, the body reacts with a host of chemical mediators, such as endogenous opioid peptides, cytokines, excitatory and inhibitory amino acids, and hormones of the hypothalamic-pituitary-adrenal (HPA) stress axis, each of which could participate in mediating the effects of stress on reproduction. A few lines of evidence implicate enhanced release of glucocorticoids from the adrenal cortex in mediating suppression of gonadotropin secretion. First, impairment of reproductive function by various stressors, including those perturbing psychosocial, metabolic, and immune function, have all reported to be accompanied by a rise in circulating levels (4, 5, 15–17). In addition, exogenous administration of elevated glucocorticoids, in the absence of stress, disrupts gonadotropin secretion and estrous cyclicity in species ranging from rodents to domestic animals to primates and humans (18–31). Finally, evidence that RU486, a well-used antagonist of both the glucocorticoid receptor (GR) and the progesterone receptor, attenuates the inhibitory effect of immobilization stress on LH secretion in male rats, or psychosocial stress on pituitary responsiveness to GnRH in female sheep, implicates a physiologic role for glucocorticoids in mediating the inhibitory effects of stress on LH secretion (9, 32, 33).
We recently demonstrated that the period between successive estrus phases was lengthened in gonad-intact female mice exposed to daily restraint stress, suggesting a disruption in the preovulatory events necessary to generate the LH surge, ovulation, and subsequent stage of estrus (34). Much of the ovulatory cycle is dominated by negative feedback effects of estradiol and progesterone which restrain the secretion of LH pulses. As the cycle progresses, rising estradiol production by maturing ovarian follicles evokes a positive feedback action upon the hypothalamus and pituitary gland that culminates in generation of a GnRH and subsequent LH surge. Mechanistically, stress could block the LH surge by impairing the pulsatile release of LH which is required to drive the preovulatory increase in estradiol secretion. Alternatively, stress could directly interfere with the neuroendocrine response to this positive feedback estradiol signal at either the hypothalamic or pituitary level. Based on the type of stress and inhibitory mediators involved, it is possible that both modes of secretion are targets for reproductive suppression, although stress-specific pathways are still under investigation.
In this study, we tested the hypothesis that a stress-like elevation in plasma corticosterone (Cort) interferes with the ovulatory cycle by disrupting neuroendocrine generation of the LH surge. We first demonstrate that an elevation in glucocorticoids, mimicking the endogenous level induced by psychosocial stress, arrests intact female mice in diestrus and prevents successive preovulatory events. Based on this response, we elucidated whether there are significant impairments in the neuroendocrine mechanisms necessary for generation of the LH surge by investigating the effect of glucocorticoids on both hypothalamic Kiss1 circuits and anterior pituitary gonadotrope cells during estradiol positive-feedback induction of the LH surge.
Materials and Methods
Animals
Experiments were conducted on adult female C57Bl/6 mice (11–12 wk old) housed in a University of California, San Diego vivarium animal facility under standard conditions. Mice were provided food and water ad libitum and housed in groups of 3–4 during all experiments under a 12-hour light, 12-hour dark cycle (lights off at 6 pm). All experiments were performed in agreement with the National Institutes of Health Animal Care and Use Guidelines and with authorization from the Institutional Animal Care and Use Committee at the University of California, San Diego.
Cort pellet preparation
Cholesterol or Cort-releasing pellets were manufactured in our laboratory by coating silicone-filled Silastic tubing of 1.0-cm length (0.040 in. inner diameter × 0.085 in. outer diameter) with molten steroid, as per Meyer et al (35). Unlike gonadal steroids, Cort release from Silastic capsules is insufficient to elevate circulating levels of steroid to physiologic levels. Rather, implantation of solid or fused corticosteroids pellets have been effective in maintaining viability of adrenalectomized animals (36). Pilot studies were conducted to establish an effective Cort pellet composition (42.5-mg Cort and 7.5-mg cholesterol; Sigma) that would produce a continuous elevation in circulating Cort which would mimic the high physiologic endogenous Cort level we observe in response to isolation/restraint stress in female mice (34). An equivalent weight of pure cholesterol was used to manufacture vehicle (Veh) pellets for implantation in control animals.
Evaluation of estrous cyclicity
At 8 weeks of age, vaginal lavage was performed daily (between 9 and 11 am) with distilled H2O and collected smears were mounted on glass slides for microscopic examination of cell type (37). Smears were classified into one of 4 phases of the estrous cycle: proestrus, estrus, metestrus, or diestrus by 2 investigators, blinded to treatment groups. Female mice exhibiting a 4- to 6-day estrous cycle, including positive classification of all 4 estrous stages, were used in animal experiments. On the morning of metestrus, after demonstration of 3 consecutive estrous cycles, female mice were implanted sc, under isoflurane anesthesia, with a cholesterol or Cort pellet. Estrous cycle length was calculated as the number of days between successive occurrences of estrus. The time spent in each cycle stage was calculated as the proportion of days classified in each cycle stage relative to the length of the baseline or implant period.
LH surge induction
Mice were ovariectomized under isoflurane anesthesia and implanted sc with an estradiol implant that produces a physiological level of circulating estradiol which establishes positive feedback and reliably initiates the GnRH and LH surge (38). Estradiol-containing implants consisted of Silastic tubing of 1.2-cm length (0.125 in. inner diameter × 0.078 in. outer diameter) filled with 0.625-μg 17-β estradiol (Sigma) dissolved in sesame oil (39, 40). Under this hormonal milieu, female mice will normally produce a daily circadian-timed LH surge, occurring each evening exclusively around the time of lights off (39, 41). At the time of ovariectomy, animals were also sc implanted with either a Cort or cholesterol pellet. Mice were killed 2 days after surgery either in the morning (between 10 and 11 am) or in the evening within 30 minutes of lights off (6 pm). The LH surge was defined as a rise in serum LH concentration exceeding 10-fold above the mean (±SEM) morning LH concentration.
Blood, brain, and pituitary gland collection
Mice were anesthetized with isoflurane and blood drawn via retroorbital bleeding just before killing via rapid decapitation. Serum was isolated from the blood samples by centrifugation (5000 rpm × 15 min) and stored at − 20°C. Brains and pituitary glands were collected at killing and immediately frozen on dry ice before being stored at −80°C.
Single- and double-label in situ hybridization (ISH)
Frozen brains were sectioned on a cryostat into 5 sets of 20-μm sections and thaw mounted on Superfrost-plus slides that were stored at −80°C until assay. Single-label ISH for Kiss1, double-label ISH for cfos/Kiss1, and double-label ISH for ERα/Kiss1 were performed as previously described (39, 41–44). For the single-label Kiss1 ISH, 1 set of slides containing the entire AVPV/PeN was fixed in 4% paraformaldehyde, pretreated with acetic anhydride, rinsed in 2× sodium citrate, sodium chloride (SSC), delipidated in chloroform, dehydrated in ethanol, and air dried. Radiolabeled (33P) Kiss1 (0.04 pmol/mL) antisense riboprobes were combined with tRNA, heat denatured, added to hybridization buffer, and applied to each slide (100 μL/slide). Slides were then placed in a 55°C humidity chamber overnight. On the second day, slides were washed in 4× SSC, treated with RNAse A for 30 minutes at 37°C, then in in RNAse buffer without RNAse at 37°C for 30 minutes. After a room temperature wash in 2× SSC, slides were washed in 0.1× SSC at 62°C for 1 hour, dehydrated in ethanols, and air dried. Slides were then dipped in Kodak NTB emulsion, air dried, and stored at 4°C for 3 days before being developed and coverslipped.
For double-label cfos/Kiss1 and ERα/Kiss1 ISH, a similar protocol was used, with slight modifications, using separate slide sets for each ISH. Briefly, antisense radiolabeled (33P) cfos (0.04 pmol/mL) or ERα (0.05 pmol/mL) riboprobe was combined with digoxigenin (DIG)-labeled Kiss1 (1:500) riboprobe and tRNA, heat-denatured, dissolved together in hybridization buffer, and applied to each slide (100 μL/slide) before hybridization at 55°C overnight. After the 62°C washes on day 2, slides were incubated in 2× SSC with 0.05% Triton X-100 containing 3% normal sheep serum for 1 hour at room temperature. Slides were then incubated overnight at room temperature with anti-DIG antibody conjugated to alkaline phosphatase (diluted 1:500 in buffer 1 containing 1% normal sheep serum and 0.3% Triton X-100; Roche). On the third day, slides were washed with buffer 1, incubated with Vector Red alkaline phosphatase substrate (Vector Labs) for 1 hour at room temperature, and then air dried. Once dry, slides were dipped in emulsion, stored at 4°C, and developed 5 or 7 days later (cfos/Kiss or ERα/Kiss1, respectively).
Quantification and analysis of ISH data
ISH slides were analyzed by a person blinded to treatment groups using an automated image processing system used widely for evaluation of Kiss1 cells (Dr Don Clifton, University of Washington). For the single-label Kiss1 slides, an individual blinded to treatments identified all silver grain clusters. After identification of cells, the automated software program counted the number of silver grain clusters, representing cells, as well as the number of silver grains over each cell (a semiquantitative index of mRNA content per cell) (45). Cells were categorized as Kiss1 positive when the number of silver grains in a cluster exceeded that of background by 3-fold (44, 46). A relative measure of total mRNA in the AVPV/PeN region was calculated by multiplying the total number of cells by the number of silver grains per cell (43, 44, 47). For double-label assays, a person blinded to treatments identified red fluorescent DIG-containing cells (Kiss1 cells) under fluorescence microscopy. Once DIG cells were identified, the automated grain-counting software quantified silver grains (representing cfos or ERα mRNA) overlying each DIG cell. Signal to background ratios for individual cells were calculated by the program, and a cell was categorized as double-labeled if its ratio was greater than 3 (42–44, 46); signal to background ratios of less than 3 identified the cell as single-labeled.
Hormone assays
Serum LH levels were measured by the Center for Research in Reproduction Ligand Assay and Analysis Core at the University of Virginia by 2-site sandwich immunoassay using monoclonal antibodies against bovine LH (number 581B7) and the human LH-β subunit (number 5303), as described previously (48–50). Mouse LH reference prep (AFP5306A; provided by Dr A. F. Parlow and the National Hormone and Peptide Program) is used as a standard. The limit of detectability for the mouse LH assay is 0.04 ng/mL. Circulating serum estradiol levels were also measured by the Ligand Assay and Analysis Core using a commercially available Calbiotech Mouse/Rat Estradiol ELISA (catalog number ES180S; Calbiotech) validated for use in mouse (51). Assay sensitivity for this estradiol ELISA was determined to be 3.0 pg/mL. Serum Cort levels were measured using DetectX Corticosterone EIA kit (catalog number K014; Arbor Assays), per the manufacturer's instructions. Assay sensitivity for this Cort ELISA was determined to be 18.6 pg/mL.
Quantitative real-time PCR
Preparation of cDNA from mouse pituitary was performed as previously described (52). RNA was extracted with TRIzol reagent (Invitrogen/GIBCO) according to the manufacturer's instructions, treated to remove contaminating genomic DNA (DNA free; Ambion) and reverse transcribed using iScript First-Strand Synthesis System (Bio-Rad). Quantitative real-time PCR was performed on a CFX Real-Time PCR instrument (Bio-Rad) and used iQ SYBR Green Supermix (Bio-Rad) with specific primers for Gapdh, Lhβ, or Gnrhr:
Lhβ forward, CTGTCAACGCAACTCTGG;
Lhβ reverse, ACAGGAGGCAAAGCAGC;
Gnrhr forward, GCCCCTTGCTGTACAAAG C;
Gnrhr reverse, CCGTCTGCTAGGTAGATCATCC;
Gapdh forward, TGCACCACCAACTGCTTAG;
Gapdh reverse, GGATGCAGGGATGATGGTTC.
The CFX real-time PCR program was as follows: 95°C for 15 minutes, followed by 40 cycles at 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. Threshold cycle (Ct) values for the genes of interest, Lhβ or Gnrhr, were compared with the reference gene, Gapdh, by the 2−ΔΔCt method. Data are represented as mean fold change compared with control ± SEM.
Statistical analyses
Data are expressed as the mean ± SEM for each group. Differences were analyzed by one- or two-way ANOVA followed by Dunnett's multiple comparison test or by a χ2 analysis. Specific details of individual analyses can be found in each figure legend. All statistical analyses were performed by JMP 10.0.0 (SAS Institute), and significance was established as P < .05.
Results
Elevated Cort blocks estrous cyclicity
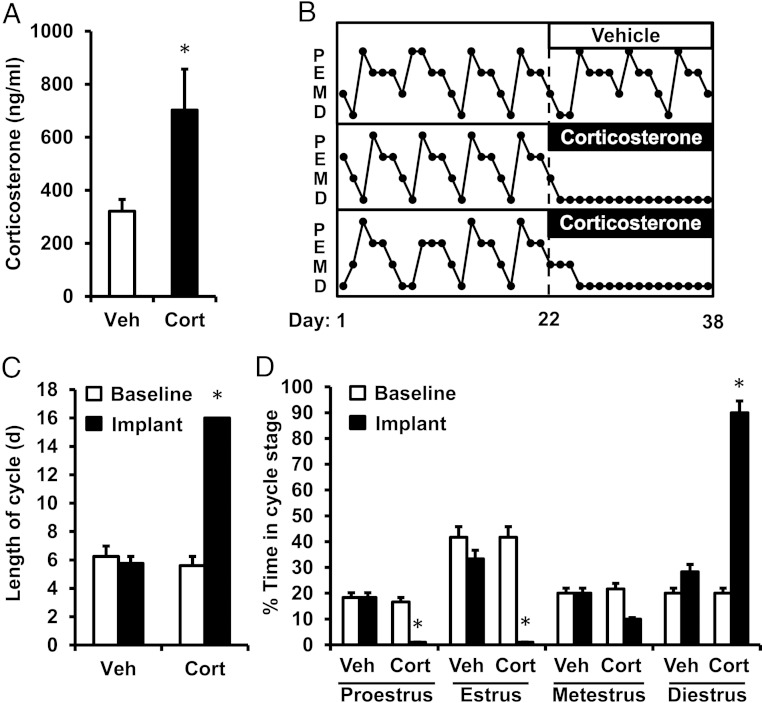
This experiment tested whether a continuous exposure to stress-like levels of glucocorticoids, mimicking the maximal level of Cort that occurs in response to stress (∼700 ng/mL) (53–57), impairs ovarian cyclicity in female mice. On the morning of metestrus, female mice were implanted with a sc pellet containing either cholesterol or Cort (n = 6/group). Circulating levels of Cort remained significantly elevated during the implant period in Cort-treated females compared with cholesterol-treated Veh controls (P < .05) (Figure 1A). Figure 1B illustrates profiles of vaginal histological classification during the baseline control period (d 1–22) and implant period (d 23–38) in 1 female mouse exposed to cholesterol and 2 exposed to Cort. In mice implanted with cholesterol, neither the average cycle length nor the percent time documented in individual cycle stages significantly differed between the baseline and implant periods (Figure 1, C and D, baseline Veh vs implant Veh). In contrast, Cort rapidly and robustly disrupted the pattern of cyclicity in females, resulting in a significant increase in cycle length during the implant period (Figure 1C, baseline Cort vs implant Cort; P < .05). More specifically, females treated with Cort remained in diestrus for 90% of the implant period and failed to progress to estrus (Figure 1D), demonstrating a complete obstruction of ovarian cyclicity in the presence of chronically elevated glucocorticoids.
Figure 1.
Cort disrupts estrous cyclicity in the female mouse. A, Mean serum Cort measured on the final day of implantation in Veh- and Cort-treated female mice. B, Representative profiles depicting estrous cyclicity in female mice, as measured by vaginal cytology, before (d 1–22, baseline) and during the period of implantation (d 23–38, implant) with a cholesterol (Veh, top panel) or Cort pellet (2 lower panels). P, proestrus; E, estrus; M, metestrus; D, diestrus. C, Average estrous cycle length during the baseline and implant periods in female mice treated with Veh or Cort. D, Average time spent in each stage of the cycle during the baseline and implant periods in Veh and Cort mice; n = 6 animals/group. Values were analyzed by one- or two-way ANOVA with group (Veh vs Cort) and time (baseline period vs implant period) as factors. *, P < .001, Veh vs Cort.
Cort blocks the estradiol-induced LH surge
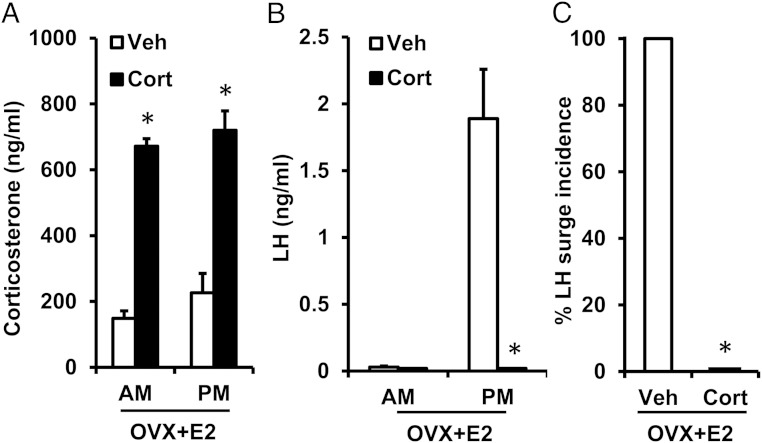
We postulate that impairment of the preovulatory GnRH and LH surge is one mechanism whereby Cort could disrupt estrous cyclicity in female mice. Generation of the LH surge is not only dependent on the increase in estradiol secretion during proestrus, which constitutes the ovarian positive feedback signal, but also on neuroendocrine processing of this positive feedback estradiol signal (ie, the responsiveness of brain and pituitary cells to elevated estradiol). In this experiment, we directly tested the hypothesis that glucocorticoids interfere with the neuroendocrine response to the estradiol positive feedback signal. We used a well-established estradiol-induced LH surge paradigm in the presence or absence of elevated Cort (39, 40). As expected, estradiol-treated females implanted with a Veh pellet displayed low circulating Cort levels in both the morning (am) or evening (pm) of the anticipated LH surge (Figure 2A, open bars), whereas circulating levels of Cort in estradiol-primed females implanted with Cort were significantly elevated at both time points (P < .05) (Figure 2A, closed bars). Morning levels of LH were low in estradiol-primed females treated with either Veh or Cort, as expected, because this is the period of estradiol-induced negative feedback (Figure 2B). All estradiol-treated Veh controls displayed robust LH surges in the evening (Figure 2, B and C). In contrast to this unanimous surge response in estradiol-treated Veh females, none of the estradiol-treated females given Cort displayed an LH surge (Figure 2C). Indeed, LH values in these Cort-treated females were undetectable on the evening of the expected LH surge (Figure 2B), coinciding with elevated circulating levels of Cort that mirror the increment induced endogenously by stress. Circulating levels of estradiol did not differ between estradiol-primed females treated with Cort or Veh (P > .05, Veh vs Cort, 18.6 ± 2.3 vs 16.4 ± 3.4 pg/mL). Collectively, these data demonstrate that stress-like levels of Cort prevent the LH surge response to a positive feedback estradiol signal.
Figure 2.
Cort blocks the estradiol-induced LH surge. Mean (±SEM) serum Cort (ng/mL, A) and LH (ng/mL, B) in adult female mice ovariectomized and exposed to a positive feedback regimen of constant elevated estradiol along with either a cholesterol (Veh) or Cort implant. Two days after surgery, blood was collected in the morning (am; 10 am) or evening (pm; 6 pm) of the expected LH surge in female mice. C, Percentage of female mice, treated with either Veh or Cort, that responded to the estradiol stimulus with an LH surge; n = 6 animals/group. Values were analyzed by two-way ANOVA with group (Veh vs Cort) and time (am vs pm) as factors or χ2 analysis (LH surge incidence). *, P < .001, Veh vs Cort.
Cort inhibits Kiss1 expression and neuronal activation at the time of the LH surge
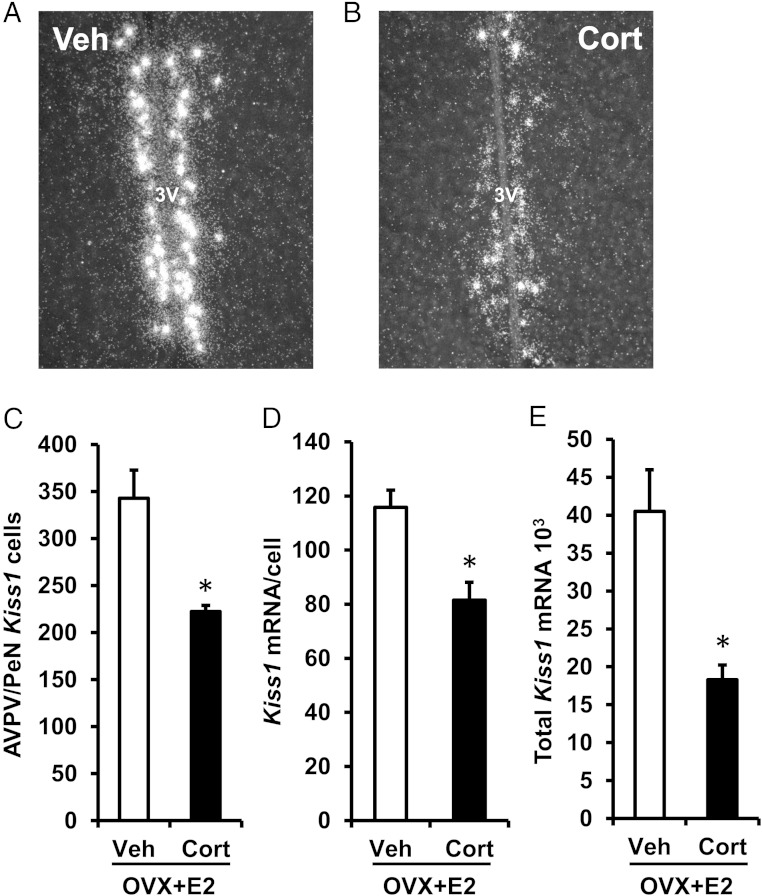
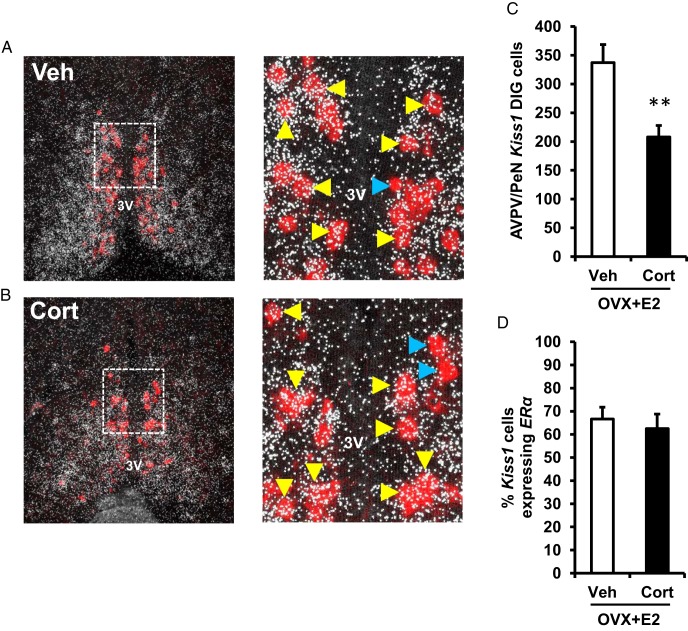
Kisspeptin signaling is obligatory for expression of the LH surge in females. The specific kisspeptin population considered important for processing the positive feedback estradiol signal during generation of the GnRH and LH surge resides in the AVPV and neighboring periventricular nucleus (AVPV/PeN) (58–60). To test the hypothesis that glucocorticoids disrupt kisspeptin (encoded by Kiss1) expression required for the LH surge, we used single-label ISH to investigate whether Cort alters Kiss1 levels in the AVPV/PeN during the estradiol-induced LH surge. Brains collected from estradiol-treated Veh female controls, demonstrated high levels of Kiss1-containing cells, robust Kiss1 mRNA content per cell, and high levels of total Kiss1 expression within the AVPV/PeN region (Figure 3, A and C–E). In contrast, estradiol-treated females given Cort showed significantly fewer detectable Kiss1 cells by 35%, reduced Kiss1 mRNA content per cell by 30%, and diminished total Kiss1 mRNA levels in the AVPV/PeN by 56% compared with estradiol-treated controls given Veh (Figure 3, B and C–E).
Figure 3.
Cort inhibits Kiss1 expression at the time of the LH surge. Representative dark-field photomicrographs showing Kiss1 mRNA (silver grain clusters) expressed in the AVPV/PeN at the time of the expected LH surge (6 pm) in brains collected from female mice exposed to a surge-inducing estradiol stimulus, as well as a pellet containing cholesterol (Veh) (A) or Cort (B). C, Mean (±SEM) number of Kiss1 mRNA-containing cells in the AVPV/PeN of Veh- and Cort-treated females. D, Mean (±SEM) level of Kiss1 mRNA per cell in the AVPV/PeN of Veh- and Cort-treated females. E, Mean (±SEM) total Kiss1 mRNA in the AVPV/PeN of Veh- and Cort-treated females. n = 6 animals per group. Values were analyzed by one-way ANOVA. *, P < .01, Veh vs Cort; 3V, third ventricle.
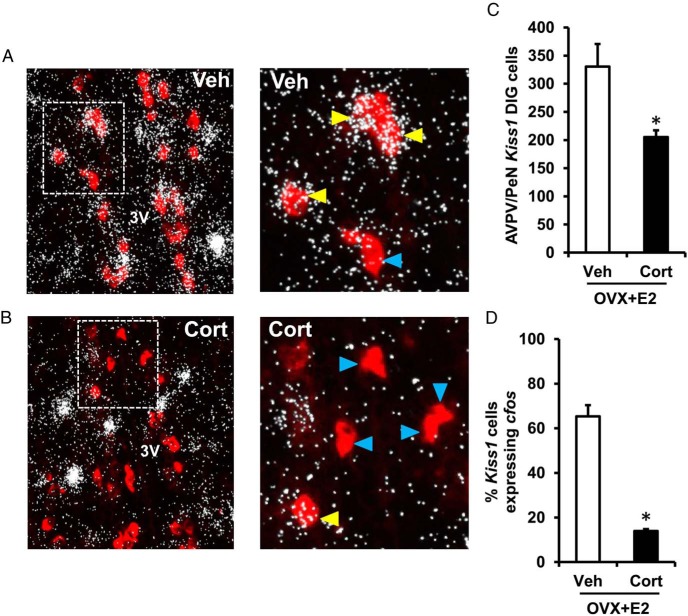
Having found that Cort strongly suppressed Kiss1 expression within the AVPV/PeN at the time of the LH surge, we next investigated whether Cort treatment also lowered levels of Kiss1 neuronal activation, evaluated by coexpression with cfos. In addition to confirming the reduction in AVPV/PeN Kiss1 cell number observed in the single-label analysis (Figure 4C), double-label ISH analysis also revealed that Cort induced a dramatic reduction in total number of AVPV/PeN Kiss1 neurons expressing cfos (Figure 4, A vs B), resulting in a significant decrease in the percentage of Kiss1 neurons showing neuronal activation (Veh vs Cort, 65% vs 14% colocalized with cfos) (Figure 4D). Collectively, these findings demonstrate that glucocorticoid-induced suppression of the estradiol-induced LH surge is associated with disrupted Kiss1 neuronal activation and expression in the AVPV/PeN.
Figure 4.
Cort inhibits neuronal activation of AVPV/PeN Kiss1 cells. Representative low- and high-power photomicrographs showing Kiss1 mRNA-containing cells (red fluorescence) and cfos mRNA (silver grain clusters; representative of neuronal activation) in the AVPV/PeN at the time of the expected LH surge (6 pm) in brains collected from female mice exposed to a surge-inducing estradiol stimulus, as well as a pellet containing cholesterol (Veh) (A) or Cort (B). Dashed box indicates area of higher magnification. Yellow arrowheads denote examples of Kiss1 cells with significant cfos coexpression, as determined by an automated image processing system. Blue arrowheads designate examples of Kiss1 neurons that did not have notable cfos induction. C, Mean (±SEM) number of Kiss1 mRNA-containing cells in the AVPV/PeN of Veh- and Cort-treated females. D, Mean (±SEM) percentage of Kiss1 mRNA-containing cells that coexpress cfos mRNA in the AVPV/PeN of Veh- and Cort-treated females; n = 6 per group. Values were analyzed by one-way ANOVA. *, P < .01, Veh vs Cort; 3V, third ventricle.
Cort does not reduce the level of ERα mRNA expression in Kiss1 cells
The presence of estrogen receptor alpha (encoded by ERα) within AVPV/PeN Kiss1 cells is necessary for enhanced Kiss1 expression during the preovulatory GnRH and LH surge (42, 59, 61). Indeed, knockdown of ERα in Kiss1 neurons prevents the estradiol-induced LH surge and results in complete infertility (62). Based on our finding that AVPV/PeN Kiss1 neurons are less responsive to the estradiol positive feedback signal in the presence of elevated Cort, indicated by lower Kiss1 mRNA expression and reduced neuronal activation (Figures 3 and 4), we next tested the hypothesis that glucocorticoids decrease ERα expression within Kiss1 neurons. We conducted double-label ISH analysis for ERα expression within AVPV/PeN neurons expressing Kiss1 in brains collected during the anticipated LH surge from estradiol-primed females treated with cholesterol or Cort. Steroid receptors are typically expressed at low levels in individual neurons and mRNA staining normally appears more diffuse compared with denser silver grain staining observed for neuropeptide mRNAs (41, 63). Again, the number of AVPV/PeN Kiss1 cells identified in this double-label assay was significantly reduced by Cort as compared with Veh-treated females (Figure 5, A vs B; 38% reduction in Figure 5C). However, the percentage of Kiss1 cells colocalized with ERα was not significantly different between the 2 groups (Veh vs Cort; P > .05) (Figure 5D), suggesting that the effect of Cort to suppress the Kiss1 system during the LH surge is independent of suppressed ERα expression.
Figure 5.
Cort does not reduce the level of ERα mRNA expression in Kiss1 cells. Representative photomicrographs of brains collected at 6 pm from female mice exposed to a surge-inducing estradiol stimulus, as well as a pellet containing cholesterol (Veh) (A) or Cort (B). Low- and high-power photomicrographs show Kiss1 mRNA containing cells (red fluorescence) and ERα mRNA (silver grain clusters) in the AVPV/PeN. Dashed box indicates area of higher magnification presented. Yellow arrowheads denote examples of Kiss1 cells with significant ERα coexpression. Blue arrowheads designate examples of Kiss1 neurons that did not have ERα induction. C, Mean (±SEM) number of Kiss1 mRNA-containing cells in the AVPV/PeN of Veh- and Cort-treated females. D, Mean (±SEM) percentage of Kiss1 mRNA-containing cells that coexpress ERα mRNA in the AVPV/PeN of Veh- and Cort-treated females; n = 6 per group. Values were analyzed by one-way ANOVA. **, P < .01; Veh vs Cort. 3V, third ventricle.
Cort inhibits pituitary gene expression at the time of the LH surge
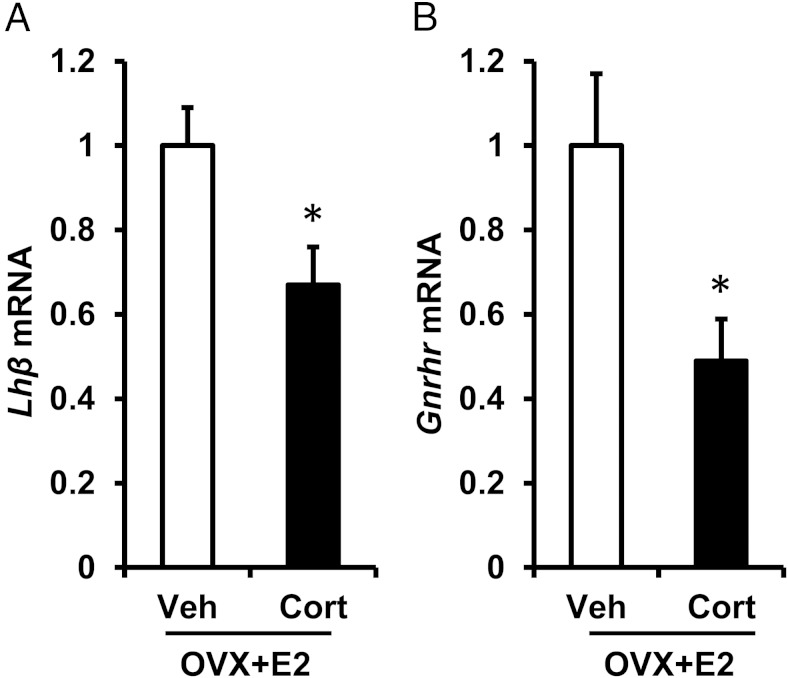
In addition to actions at the central level, glucocorticoids have been shown to inhibit the response of the pituitary gonadotrope to GnRH. We analyzed LHβ and GnRH receptor gene expression (encoded by Lhβ and Gnrhr, respectively) in ovariectomized female mice exposed to the estrogen positive feedback LH surge paradigm with or without Cort. Females were killed in the evening, at the time of the expected LH surge, and the pituitary gland collected for mRNA analysis by quantitative RT-PCR. Cort reduced Lhβ transcript levels by 30% compared with Veh controls (P < .05) (Figure 6A, Veh vs Cort). In addition, Gnrhr expression was reduced by 50% in Cort-treated females (P < .05) (Figure 6B). Collectively, these observations raise the possibility that stress can interfere with the preovulatory LH surge by disrupting the synthesis and secretion of LH at the level of the anterior pituitary gonadotrope cell, in addition to the suppression observed at the level of the hypothalamus.
Figure 6.
Cort inhibits pituitary gene expression at the time of the LH surge. Quantitative RT-PCR analysis of Lhβ (A) and Gnrhr (B) mRNA was performed on individual mouse pituitary glands collected at the time of the expected LH surge (6 pm) from female mice exposed to a surge-inducing estradiol stimulus, as well as a pellet containing cholesterol (Veh) or Cort n = 6 per group. Ct values for Lhβ and Gnrhr were compared with Gapdh using the 2−ΔΔCt method and analyzed by one-way ANOVA. Data are represented as mean fold change ± SEM. *, P < .05, Veh vs Cort.
Discussion
Here, we use a mouse model to investigate mechanisms whereby elevated glucocorticoids suppress the reproductive neuroendocrine axis. We demonstrate that an elevation in the natural occurring glucocorticoid, which is normally enhanced in response to stress, prevents ovulatory cyclicity in intact female mice. Using a hormonal paradigm to generate the estradiol-induced LH surge in ovariectomized female mice, we demonstrate that multiple aspects of the positive feedback response to estradiol are disrupted by elevated glucocorticoids. At the central level, Cort decreased transcriptional activation of AVPV/PeN Kiss1 neurons, as measured by neuronal cfos induction, and prevented enhanced AVPV/PeN Kiss1 expression, including both a reduction in the Kiss1 cell population and mRNA expression level per cell. At the pituitary level, Cort suppressed expression of the genes encoding the GnRH receptor, as well as the LHβ subunit, suggesting impaired pituitary responsiveness to GnRH. Collectively, these experiments indicate multiple mechanisms in which glucocorticoids disrupt the positive feedback action of estradiol to induce the preovulatory LH surge, either of which could contribute to the suppressive effect of Cort on ovulatory cyclicity in female mice.
Early work revealed that the synthetic glucocorticoid, dexamethasone, could block both the natural preovulatory surge and the estradiol-induced LH surge in ovariectomized rats (25, 26, 64), but the neuroendocrine site and mechanism of action was not identified. Our results, using female C57Bl/6 mice, are consistent with these previous findings and confirm the physiologic relevance of the natural glucocorticoid, Cort, as a potential inhibitory intermediate of the effects of stress. Furthermore, our study expands those initial observations by identifying the AVPV/PeN Kiss1 neuron as a central target of Cort-induced suppression of the LH surge. Kiss1 cells in the AVPV/PeN remain quiescent at the time of the anticipated LH surge, indicated by the 80% reduction in cfos expression and 50% suppression in total Kiss1 expression. This population of AVPV/PeN Kiss1 neurons expresses ERα and is thought to relay the estradiol signal to GnRH neurons, resulting in GnRH and LH surge release. In mouse models in which ERα is eliminated from AVPV/PeN Kiss1 neurons, females fail to elicit a surge, identifying this neuronal population as a critical pathway during positive feedback surge generation. Taken together, our data support a mechanism whereby elevated glucocorticoids in female mice inhibit the preovulatory LH surge by impairing neuroendocrine responsiveness of AVPV/PeN Kiss1 neurons to elicit the estradiol-induced surge release of GnRH and LH. It remains to be determined whether the Kiss1 cell population in the arcuate nucleus is also suppressed by glucocorticoids. Elevated estrogen is well known to inhibit arcuate Kiss1 levels (59). Thus, the positive feedback paradigm employed in the current study would be expected to strongly lower Kiss1 expression in the arcuate nucleus in all groups, precluding our ability to accurately assess further potential suppression by glucocorticoids in these females. Future studies employing non-estradiol treated females will be useful to elucidate any contribution of glucocorticoid suppression of the arcuate Kiss1 system.
Evidence in rats demonstrates that a majority of Kiss1 neurons in the AVPV/PeN contain GR (65), the receptor which mediates neuroendocrine actions of stress levels of glucocorticoids, positioning Kiss1 neurons as a direct target for the suppressive actions of glucocorticoids. Our findings do not exclude, however, that cells upstream or downstream (ie, suprachiasmatic nucleus or GnRH, respectively) of the AVPV Kiss1 neurons are also inhibited by Cort during blockade of the surge. Questions regarding the role of other neurons impacted by Cort during blockade of the surge remain exciting, but purely speculative, as the animals models to piece together this pathway do not yet exist. Importantly, the mouse is an extremely useful model to genetically probe the pathways whereby Cort or stress alters reproductive neuroendocrine function. With regard to GnRH neurons, GR is expressed in a population of GnRH neurons (66) and glucocorticoids have been shown to inhibit expression in GT1–7 cells (67), a cell model of GnRH neurons. As for involvement by the suprachiasmatic nucleus, vasoactive intestinal peptide-containing neurons feed into the surge system and are an additional cell population whereby glucocorticoids may alter estradiol-induced responsiveness of the Kiss1 neuron (42). Because the percent Kiss1/ERα coexpression did not differ with treatments, we interpret this to mean that Cort's ability to suppress the kisspeptin system is not due to a reduction of ER expression within Kiss1 cells. However, we cannot exclude that ER signaling within AVPV/PeN Kiss1 neurons may be impaired by glucocorticoids. Further, ERα is highly expressed throughout the anterior hypothalamus and preoptic area, in many neuronal cell types in addition to AVPV/PeN kisspeptin neurons (see Figure 5A), and changes in this steroid receptor may be involved in suppression of the surge via another cell system, such as a neuron population sensing circadian input. Further work is clearly warranted to determine whether the disruptive influence of Cort is expressed at other neuroendocrine sites and the contribution of estradiol or its receptor to the suppression by Cort.
The anterior pituitary gonadotrope cell is a downstream neuroendocrine site in which Cort could prevent the LH surge by reducing LH synthesis and secretion. Evidence in numerous species that glucocorticoids impair pituitary responsiveness to a single GnRH bolus or repeated GnRH pulses supports an inhibitory action of glucocorticoids upon the gonadotrope. Consistent with a direct action upon the gonadotrope, GR is expressed within gonadotrope cells of the mouse and rat (34, 68). Glucocorticoids have been shown to modulate signaling mechanisms downstream of the GnRH receptor, including protein kinase C and cAMP, and blunt transactivation of the Lhβ promoter, either of which may lead to a reduction in gonadotropin gene expression or hormone release (34, 69, 70). The present study demonstrates that both Lhβ as well as Gnrhr mRNA levels are lowered by Cort and that this reduction is associated with undetectable levels of LH at the time of the expected LH surge, consistent with glucocorticoid-induced suppression of gonadotrope responsiveness during the surge.
Our findings suggest that stress levels of glucocorticoids alter multiple aspects of endocrine control necessary for maintenance of cyclicity and fertility. Specifically, our data suggest that Cort 1) suppresses basal LH levels causing females to be arrested in diestrus, preventing proestrus from occurring, and 2) inhibits neural kisspeptin circuits from properly being activated by elevated estradiol (which normally occurs in proestrus). Support for the first point is based on the response of ovarian-intact Cort-treated females to remain in diestrus and not transition to proestrus. Thus, we hypothesize that stress levels of glucocorticoids can impair the basal pulsatile release of LH, which is required to drive the preovulatory increase in estradiol secretion needed to transition to proestrus, when the LH surge would occur. Indeed, this finding has been shown in Cort-treated female sheep (71, 72). At this time, investigating pulsatile LH in mice remains very difficult and technically challenging, precluding analysis of this directly. Support for the second point comes from our present findings that glucocorticoids directly interfere with the neuroendocrine response to a positive feedback estradiol signal at both the hypothalamic or pituitary levels. Thus, glucocorticoids appear to provide inhibition of multiple aspects of the female neuroendocrine reproductive axis by preventing both the transition into proestrus and proper activation of neuroendocrine circuits by proestrus-level estradiol signals.
Our finding that Cort impairs central as well as pituitary mechanisms important for expression of the LH surge raises the question of whether suppression of pituitary responsiveness account for the full blockade of the LH surge? Two lines of evidence suggest that suppression of pituitary responsiveness may not be the sole neuroendocrine site for suppression. First, in studies in sheep where glucocorticoids inhibit pituitary responsiveness to both endogenous and exogenous GnRH pulses, the suppression in LH is maximally reduced by no more than 50% (73, 74). Second, evidence in ovariectomized female mice in which Cort induces acute suppression of mean LH, the reduction in circulating levels again reaches, at most, a 45% reduction (34). Therefore, the lack of any detectable LH leads us to speculate that suppression of the LH surge is due to combined effects at the hypothalamic and pituitary levels.
Another important question raised by the current study is in regard to the relevance of elevated glucocorticoids to stress-induced suppression of reproduction. In this regard, adaptations to stress comprise an initial autonomic activation and release of catecholamines from the sympathetic nervous system, followed by a more delayed and prolonged activation of the HPA axis and enhanced secretion of CRH, arginine vasopressin, ACTH, and glucocorticoids (12–14). Each of these factors potentially mediates reproductive suppression. In actuality, the integrated actions of all of them are likely involved, with the relative importance of each depending on the nature of the stressor (12). In this study, we chose to focus on the natural glucocorticoid in rodents. Importantly, the Cort pellet used in the current study produces a constant elevation in circulating Cort, which differs from an endogenous stress-induced Cort rise that produces negative feedback upon the HPA axis, and returns circulating Cort to basal levels. This Cort paradigm produces a defined circulating level of the natural glucocorticoid in mice, in the absence of stress, allowing us to test the mechanisms whereby this steroid alone disrupts reproductive function. Future studies will use this mouse model not only for investigating mechanisms of suppression, but the relevance of these glucocorticoid-mediated mechanisms to the suppression of reproduction and fertility associated with stress.
In summary, we developed a mouse model to test the effects of stress levels of glucocorticoids on ovulatory cyclicity in female mice. Our results definitively show that Cort alone can inhibit reproductive function in female mice, in part, by interrupting generation of the LH surge response. Our findings point to 2 important mechanisms which could account for the disruption in reproductive neuroendocrine function. At the central level, glucocorticoids impair responsiveness of the AVPV/PeN kisspeptin system to a positive feedback estradiol signal. Coinciding with failure of female mice to respond with an estradiol-induced LH surge, gonadotropin expression is significantly reduced. Either of these inhibitory effects of glucocorticoids could by themselves prevent the LH surge response and may represent mechanisms contributing to menstrual cycle disturbances and infertility that are associated with physical and emotional stress.
Acknowledgments
We thank Dr Al Parlow of the National Hormone and Peptide Program for providing the NIDDK-anti-rBeta LH-IC-2 antibody. We also thank Dr Matthew Poling, Dr Melvin Rouse, and Dr Kristen Tolson for technical assistance with the ISH assays and Dr Kathleen Yip for assistance with the animal experiments. We also thank the members of the Breen Church, Mellon, Kauffman, Lawson, and Thackray laboratories for helpful discussions throughout this work.
This work was supported by the National Institutes of Health Grant R00 HD060947 (to K.M.B.) and the National Science Foundation Grant IOS-1457226 (to A.S.K.). Serum hormone assays were performed by The University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Center for Translational Research in Reproduction and Infertility Grant P50-HD28934.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AVPV/PeN
- anteroventral periventricular nucleus–periventricular nucleus continuum
- Cort
- corticosterone
- Ct
- threshold cycle
- DIG
- digoxigenin
- ERa
- Estrogen receptor alpha
- GR
- glucocorticoid receptor
- HPA
- hypothalamic-pituitary-adrenal
- in.
- inches
- ISH
- in situ hybridization
- Veh
- vehicle.
References
- 1. Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O'Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18:602–610. [DOI] [PubMed] [Google Scholar]
- 2. Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA. 2009;106:11324–11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wagenmaker ER, Breen KM, Oakley AE, Tilbrook AJ, Karsch FJ. Psychosocial stress inhibits amplitude of gonadotropin-releasing hormone pulses independent of cortisol action on the type II glucocorticoid receptor. Endocrinology. 2009;150:762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilbrook AJ, Turner AI, Clarke IJ. Effects of stress on reproduction in non-rodent mammals: the role of glucocorticoids and sex differences. Rev Reprod. 2000;5:105–113. [DOI] [PubMed] [Google Scholar]
- 5. Tilbrook AJ, Turner AI, Clarke IJ. Stress and reproduction: central mechanisms and sex differences in non-rodent species. Stress. 2002;5:83–100. [DOI] [PubMed] [Google Scholar]
- 6. Chen MD, Ordög T, O'Byrne KT, Goldsmith JR, Connaughton MA, Knobil E. The insulin hypoglycemia-induced inhibition of gonadotropin-releasing hormone pulse generator activity in the rhesus monkey: roles of vasopressin and corticotropin-releasing factor. Endocrinology. 1996;137:2012–2021. [DOI] [PubMed] [Google Scholar]
- 7. Battaglia DF, Krasa HB, Padmanabhan V, Viguié C, Karsch FJ. Endocrine alterations that underlie endotoxin-induced disruption of the follicular phase in ewes. Biol Reprod. 2000;62:45–53. [DOI] [PubMed] [Google Scholar]
- 8. Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–E276. [DOI] [PubMed] [Google Scholar]
- 9. Breen KM, Oakley AE, Pytiak AV, Tilbrook AJ, Wagenmaker ER, Karsch FJ. Does cortisol acting via the type II glucocorticoid receptor mediate suppression of pulsatile luteinizing hormone secretion in response to psychosocial stress? Endocrinology. 2007;148:1882–1890. [DOI] [PubMed] [Google Scholar]
- 10. Kinsey-Jones JS, Li XF, Knox AM, et al. Down-regulation of hypothalamic kisspeptin and its receptor, Kiss1r, mRNA expression is associated with stress-induced suppression of luteinising hormone secretion in the female rat. J Neuroendocrinol. 2009;21:20–29. [DOI] [PubMed] [Google Scholar]
- 11. Battaglia DF, Bowen JM, Krasa HB, Thrun LA, Viguié C, Karsch FJ. Endotoxin inhibits the reproductive neuroendocrine axis while stimulating adrenal steroids: a simultaneous view from hypophyseal portal and peripheral blood. Endocrinology. 1997;138:4273–4281. [DOI] [PubMed] [Google Scholar]
- 12. Williams CY, Harris TG, Battaglia DF, Viguié C, Karsch FJ. Endotoxin inhibits pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2001;142:1915–1922. [DOI] [PubMed] [Google Scholar]
- 13. Dobson H, Tebble JE, Phogat JB, Smith RF. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J Reprod Fertil. 1999;116:1–8. [DOI] [PubMed] [Google Scholar]
- 14. Roozendaal MM, Swarts JJ, van Maanen JC, Wiegant VM, Mattheij JA. Inhibition of the LH surge in cyclic rats by stress is not mediated by opioids. Life Sci. 1997;60:735–742. [DOI] [PubMed] [Google Scholar]
- 15. Rivier C, Rivest S. Effect of stress on the activity of the hypothalamic-pituitary-gonadal axis: peripheral and central mechanisms. Biol Reprod. 1991;45:523–532. [DOI] [PubMed] [Google Scholar]
- 16. Ferin M. Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. [DOI] [PubMed] [Google Scholar]
- 17. Dobson H, Smith RF. What is stress, and how does it affect reproduction? Anim Reprod Sci. 2000;60–61:743–752. [DOI] [PubMed] [Google Scholar]
- 18. Dubey AK, Plant TM. A suppression of gonadotropin secretion by cortisol in castrated male rhesus monkeys (Macaca mulatta) mediated by the interruption of hypothalamic gonadotropin-releasing hormone release. Biol Reprod. 1985;33:423–431. [DOI] [PubMed] [Google Scholar]
- 19. Vreeburg JT, de Greef WJ, Ooms MP, van Wouw P, Weber RF. Effects of adrenocorticotropin and corticosterone on the negative feedback action of testosterone in the adult male rat. Endocrinology. 1984;115:977–983. [DOI] [PubMed] [Google Scholar]
- 20. Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol. 2006;27:233–245. [DOI] [PubMed] [Google Scholar]
- 21. Saketos M, Sharma N, Santoro NF. Suppression of the hypothalamic-pituitary-ovarian axis in normal women by glucocorticoids. Biol Reprod. 1993;49:1270–1276. [DOI] [PubMed] [Google Scholar]
- 22. Turner AI, Hemsworth PH, Canny BJ, Tilbrook AJ. Sustained but not repeated acute elevation of cortisol impaired the luteinizing hormone surge, estrus, and ovulation in gilts. Biol Reprod. 1999;61:614–620. [DOI] [PubMed] [Google Scholar]
- 23. Hagino N, Watanabe M, Goldzieher JW. Inhibition by adrenocorticotrophin of gonadotrophin-induced ovulation in immature female rats. Endocrinology. 1969;84:308–314. [DOI] [PubMed] [Google Scholar]
- 24. Smith ER, Johnson J, Weick RF, Levine S, Davidson JM. Inhibition of the reproductive system in immature rats by intracerebral implantation of cortisol. Neuroendocrinology. 1971;8:94–106. [DOI] [PubMed] [Google Scholar]
- 25. Baldwin DM, Sawyer CH. Effects of dexamethasone on LH release and ovulation in the cyclic rat. Endocrinology. 1974;94:1397–1403. [DOI] [PubMed] [Google Scholar]
- 26. Baldwin DM. The effect of glucocorticoids on estrogen-dependent luteinizing hormone release in the ovariectomized rat and on gonadotropin secretin in the intact female rat. Endocrinology. 1979;105:120–128. [DOI] [PubMed] [Google Scholar]
- 27. Sakakura M, Takebe K, Nakagawa S. Inhibition of luteinizing hormone secretion induced by synthetic LRH by long-term treatment with glucocorticoids in human subjects. J Clin Endocrinol Metab. 1975;40:774–779. [DOI] [PubMed] [Google Scholar]
- 28. McAndrews JM, Ringstrom SJ, Dahl KD, Schwartz NB. Corticosterone in vivo increases pituitary follicle-stimulating hormone (FSH)-β messenger ribonucleic acid content and serum FSH bioactivity selectively in female rats. Endocrinology. 1994;134:158–163. [DOI] [PubMed] [Google Scholar]
- 29. Ringstrom SJ, Schwartz NB. Cortisol suppresses the LH, but not the FSH, response to gonadotropin-releasing hormone after orchidectomy. Endocrinology. 1985;116:472–474. [DOI] [PubMed] [Google Scholar]
- 30. Ringstrom SJ, Suter DE, Hostetler JP, Schwartz NB. Cortisol regulates secretion and pituitary content of the two gonadotropins differentially in female rats: effects of gonadotropin-releasing hormone antagonist. Endocrinology. 1992;130:3122–3128. [DOI] [PubMed] [Google Scholar]
- 31. Rosen H, Dalkin A, Haisenleder D, Friberg RD, Ortolano G, Barkan A. Dexamethasone alters responses of pituitary gonadotropin-releasing hormone (GnRH) receptors, gonadotropin subunit messenger ribonucleic acids, and gonadotropins to pulsatile GnRH in male rats. Endocrinology. 1991;128:654–660. [DOI] [PubMed] [Google Scholar]
- 32. Briski KP, Vogel KL, McIntyre AR. The antiglucocorticoid, RU486, attenuates stress-induced decreases in plasma-luteinizing hormone concentrations in male rats. Neuroendocrinology. 1995;61:638–645. [DOI] [PubMed] [Google Scholar]
- 33. Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25:973–981. [DOI] [PubMed] [Google Scholar]
- 34. Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26:1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meyer JS, Micco DJ, Stephenson BS, Krey LC, McEwen BS. Subcutaneous implantation method for chronic glucocorticoid replacement therapy. Physiol Behav. 1979;22:867–870. [DOI] [PubMed] [Google Scholar]
- 36. Segaloff A. The gradation of effectiveness and absorption of desoxycorticosterone acetate pellets by dilution with cholesterol. Endocrinology. 1950;47:320–325. [DOI] [PubMed] [Google Scholar]
- 37. Becker KL. Principles and Practice of Endocrinology and Metabolism. 2nd ed Philadelphia, PA: Lippincott; 1995. [Google Scholar]
- 38. Bronson FH. The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology. 1981;108:506–516. [DOI] [PubMed] [Google Scholar]
- 39. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bronson FH, Vom Saal FS. Control of the preovulatory release of luteinizing hormone by steroids in the mouse. Endocrinology. 1979;104:1247–1255. [DOI] [PubMed] [Google Scholar]
- 41. Stephens SB, Tolson KP, Rouse ML, et al. Absent progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology. 2015;156:3091–3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kauffman AS, Sun Y, Kim J, Khan AR, Shu J, Neal-Perry G. Vasoactive intestinal peptide modulation of the steroid-induced LH surge involves kisspeptin signaling in young but not in middle-aged female rats. Endocrinology. 2014;155:2222–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kim J, Tolson KP, Dhamija S, Kauffman AS. Developmental GnRH signaling is not required for sexual differentiation of kisspeptin neurons but is needed for maximal Kiss1 gene expression in adult females. Endocrinology. 2013;154:3273–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Semaan SJ, Kauffman AS. Daily successive changes in reproductive gene expression and neuronal activation in the brains of pubertal female mice. Mol Cell Endocrinol. 2015;401:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology. 1990;52:581–588. [DOI] [PubMed] [Google Scholar]
- 46. Di Giorgio NP, Semaan SJ, Kim J, et al. Impaired GABAB receptor signaling dramatically up-regulates Kiss1 expression selectively in nonhypothalamic brain regions of adult but not prepubertal mice. Endocrinology. 2014;155:1033–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Navarro VM, Castellano JM, McConkey SM, et al. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300:E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fallest PC, Trader GL, Darrow JM, Shupnik MA. Regulation of rat luteinizing hormone b gene expression in transgenic mice by steroids and a gonadotropin-releasing hormone antagonist. Biol Reprod. 1995;53:103–109. [DOI] [PubMed] [Google Scholar]
- 49. Matteri RL, Roser JF, Baldwin DM, Lipovetsky V, Papkoff H. Characterization of a monoclonal antibody which detects luteinizing hormone from diverse mammalian species. Domest Anim Endocrinol. 1987;4:157–165. [DOI] [PubMed] [Google Scholar]
- 50. Haavisto AM, Pettersson K, Bergendahl M, Perheentupa A, Roser JF, Huhtaniemi I. A supersensitive immunofluorometric assay for rat luteinizing hormone. Endocrinology. 1993;132:1687–1691. [DOI] [PubMed] [Google Scholar]
- 51. Haisenleder DJ, Schoenfelder AH, Marcinko ES, Geddis LM, Marshall JC. Estimation of estradiol in mouse serum samples: evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGillivray SM, Thackray VG, Coss D, Mellon PL. Activin and glucocorticoids synergistically activate follicle-stimulating hormone β-subunit gene expression in the immortalized LβT2 gonadotrope cell line. Endocrinology. 2007;148:762–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Breen KM, Davis TL, Doro LC, et al. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology. 2008;149:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Harada A, Shiosaka S, Ishikawa Y, Komai S. Acute stress increases neuropsin mRNA expression in the mouse hippocampus through the glucocorticoid pathway. Neurosci Lett. 2008;436:273–277. [DOI] [PubMed] [Google Scholar]
- 55. Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ. Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun. 2008;22:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Prince CR, Anisman H. Situation specific effects of stressor controllability on plasma corticosterone changes in mice. Pharmacol Biochem Behav. 1990;37:613–621. [DOI] [PubMed] [Google Scholar]
- 57. Solomon MB, Loftspring M, de Kloet AD, et al. Neuroendocrine function after hypothalamic depletion of glucocorticoid receptors in male and female mice. Endocrinology. 2015;156:2843–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V). Brain Res Rev. 2008;57:277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 60. Robertson JL, Clifton DK, de la Iglesia HO, Steiner RA, Kauffman AS. Circadian regulation of Kiss1 neurons: implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology. 2009;150:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Miller BH, Olson SL, Levine JE, Turek FW, Horton TH, Takahashi JS. Vasopressin regulation of the proestrous luteinizing hormone surge in wild-type and Clock mutant mice. Biol Reprod. 2006;75:778–784. [DOI] [PubMed] [Google Scholar]
- 62. Dubois SL, Acosta-Martínez M, DeJoseph MR, et al. Positive, but not negative feedback actions of estradiol in female mice require estrogen receptor α (ERα) in kisspeptin neurons. Endocrinology. 2015;156:1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cao J, Patisaul HB. Sex-specific expression of estrogen receptors α and β and Kiss1 in the postnatal rat amygdala. J Comp Neurol. 2013;521:465–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol. 2006;256:40–48. [DOI] [PubMed] [Google Scholar]
- 65. Takumi K, Iijima N, Higo S, Ozawa H. Immunohistochemical analysis of the colocalization of corticotropin-releasing hormone receptor and glucocorticoid receptor in kisspeptin neurons in the hypothalamus of female rats. Neurosci Lett. 2012;531:40–45. [DOI] [PubMed] [Google Scholar]
- 66. Ahima RS, Harlan RE. Glucocorticoid receptors in LHRH neurons. Neuroendocrinology. 1992;56:845–850. [DOI] [PubMed] [Google Scholar]
- 67. Chandran UR, Attardi B, Friedman R, Dong KW, Roberts JL, DeFranco DB. Glucocorticoid receptor-mediated repression of gonadotropin-releasing hormone promoter activity in GT1 hypothalamic cell lines. Endocrinology. 1994;134:1467–1474. [DOI] [PubMed] [Google Scholar]
- 68. Kononen J, Honkaniemi J, Gustafsson JA, Pelto-Huikko M. Glucocorticoid receptor colocalization with pituitary hormones in the rat pituitary gland. Mol Cell Endocrinol. 1993;93:97–103. [DOI] [PubMed] [Google Scholar]
- 69. Li PS. Modulation by cortisol of luteinizing hormone secretion from cultured porcine anterior pituitary cells: effects on secretion induced by phospholipase C, phorbol ester and cAMP. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:107–112. [DOI] [PubMed] [Google Scholar]
- 70. Suter DE, Schwartz NB, Ringstrom SJ. Dual role of glucocorticoids in regulation of pituitary content and secretion of gonadotropins. Am J Physiol. 1988;254:E595–E600. [DOI] [PubMed] [Google Scholar]
- 71. Oakley AE, Breen KM, Clarke IJ, Karsch FJ, Wagenmaker ER, Tilbrook AJ. Cortisol reduces gonadotropin-releasing hormone pulse frequency in follicular phase ewes: influence of ovarian steroids. Endocrinology. 2009;150:341–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Breen KM, Billings HJ, Wagenmaker ER, Wessinger EW, Karsch FJ. Endocrine basis for disruptive effects of cortisol on preovulatory events. Endocrinology. 2005;146:2107–2115. [DOI] [PubMed] [Google Scholar]
- 73. Breen KM, Karsch FJ. Does cortisol inhibit pulsatile luteinizing hormone secretion at the hypothalamic or pituitary level? Endocrinology. 2004;145:692–698. [DOI] [PubMed] [Google Scholar]
- 74. Breen KM, Stackpole CA, Clarke IJ, et al. Does the type II glucocorticoid receptor mediate cortisol-induced suppression in pituitary responsiveness to gonadotropin-releasing hormone? Endocrinology. 2004;145:2739–2746. [DOI] [PubMed] [Google Scholar]