Abstract
Background
The comparative effectiveness of percutaneous closure of patent foramen ovale (PFO) plus medical therapy versus medical therapy alone for cryptogenic stroke is uncertain.
Objectives
We performed the first pooled analysis of individual participant data from completed randomized trials comparing PFO closure versus medical therapy in patients with cryptogenic stroke.
Methods
We analyzed data on 2 devices (STARFlex and Amplatzer PFO Occluder) evaluated in 3 trials. The primary composite outcome was stroke, transient ischemic attack (TIA), or death; the secondary outcome was stroke. We used log-rank tests and (unadjusted and covariate-adjusted) Cox regression models to compare device closure versus medical therapy.
Results
Among 2,303 patients, closure was not significantly associated with the primary composite outcome. The difference became significant after covariate adjustment (hazard ratio [HR]: 0.68; p = 0.049). For the outcome of stroke, all comparisons were statistically significant, with unadjusted and adjusted HRs of 0.58 (p = 0.043) and 0.58 (p = 0.044), respectively. In analyses limited to the 2 occluder device trials, the effect of closure was not significant for the composite outcome, but was for the stroke outcome (unadjusted HR: 0.39; p = 0.013. Subgroup analyses did not identify significant heterogeneity of treatment effects. Atrial fibrillation was more common among closure patients.
Conclusions
Among patients with PFO and cryptogenic stroke, closure reduced recurrent stroke and had a statistically significant effect on the composite of stroke, TIA, and death in adjusted but not unadjusted analyses.
Keywords: cryptogenic stroke, meta-analysis, transient ischemic attack
Approximately 30% of ischemic strokes are “cryptogenic,” an etiologically heterogeneous class. Approximately half of patients with cryptogenic stroke <60 years of age have a patent foramen ovale (PFO), nearly double the prevalence in the general population. For these patients, cryptogenic stroke may be caused by paradoxical embolism, in addition to other occult etiologies.
Controversy exists over the preferred management strategy for patients with cryptogenic stroke and PFO. Three randomized clinical trials (RCTs) investigating 2 devices -- STARFlex in CLOSURE I (Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxical Embolism through a Patent Foramen Ovale) (1), and Amplatzer PFO Occluder in RESPECT (Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment) (2) and PC Trial (Percutaneous Closure of Patent Foramen Ovale in Cryptogenic Embolism) (3) -- have now been completed. The trials did not report statistically significant differences between device closure and medical therapy. Meta-analyses using published aggregate data have generally reported results suggestive of a protective effect of closure on stroke or on the composite outcome of recurrent stroke, transient ischemic attack (TIA), or death, but the data have been contradictory as to the statistical significance of these associations (1-5). We performed a meta-analysis of individual participant data to better synthesize data from the 3 randomized trials (6-8).
Individual participant data meta-analysis holds several advantages over meta-analysis using aggregate results extracted from trial publications (9), including the ability to standardize outcome definitions and analyses across studies without any reliance on numerical approximations, which are often necessary when extracting data from publications. Additionally, access to participant-level data allows the use of statistical methods to address missing data, perform covariate-adjusted analyses (which often have greater power than unadjusted analyses for time-to-event outcomes [10-12]), and assess heterogeneity of treatment effects across subgroups.
Methods
We pre-specified our analytical plan and registered the study protocol with PROSPERO, the international prospective register of systematic reviews (CRD42014013895). The Tufts Medical Center Institutional Review Board approved the study.
We used individual participant data from 3 randomized trials that to our knowledge represent the totality of randomized evidence on percutaneously implanted PFO closure devices versus medical therapy in patients with PFO and cryptogenic stroke. The CLOSURE I trial (6) randomized 909 patients ages 18 to 60 years between 2003 and 2008 with a planned follow-up of 2 years. The RESPECT trial (7) randomized 980 patients in the same age range between 2003 and 2011. The trial's primary analysis was performed as planned after the 25th outcome event; the mean duration of follow-up was 2.6 years (range: 0 to 8.1 years). The PC Trial (8) randomized 414 patients <60 years old between 2000 and 2009. The mean duration of follow-up was 4.1 years in the closure group and 4.0 years in the medical therapy group.
Treatments
The CLOSURE I Trial (6) used the STARFlex septal closure system (NMT Medical, Inc., Boston, Massachusetts). After closure, all device patients received an antiplatelet regimen of clopidogrel 75 mg daily for 6 months and aspirin 81 or 325 mg daily for 2 years. The medical therapy group received warfarin with a target international normalized ratio of 2.0 to 3.0; aspirin 325 mg daily (81 mg daily dose was allowed for documented gastrointestinal intolerance); or aspirin 81 mg daily with warfarin. Clopidogrel, ticlopidine, and aspirin plus extended-release dipyridamole were not allowed in the medical group.
The RESPECT Trial (7) used the Amplatzer PFO Occluder (AGA Medical/St. Jude Medical, St. Paul, Minnesota). Patients assigned to closure also received 81 to 325 mg of aspirin and clopidogrel for 1 month after device placement, followed by aspirin monotherapy for 5 months, thereafter at the site investigator's discretion. Patients in the medical therapy group received 1 of 4 permissible medical regimens: aspirin; warfarin; clopidogrel; or aspirin/extended-release dipyridamole. Aspirin with clopidogrel was originally permitted but eliminated in 2006 following changes in the American Heart Association/American Stroke Association (AHA/ASA) practice guidelines (13).
The PC Trial (8) also used the Amplatzer PFO Occluder. For patients randomized to closure, recommended antithrombotic treatment included aspirin (100 to 325 mg/day) for at least 5 to 6 months, plus ticlopidine (250 to 500 mg/day) or clopidogrel (75 to 150 mg/day) for 1 to 6 months. For patients with aspirin intolerance, ticlopidine or clopidogrel was recommended. Antithrombotic treatment in the medical group was left to physician discretion and could include antiplatelet or anticoagulation therapy.
Outcome Definitions and Ascertainment
Our primary outcome was the composite outcome of ischemic stroke, TIA, or death from any cause. We defined ischemic stroke events in accordance with the updated AHA/ASA expert consensus definition (14): a sudden onset neurological deficit in a vascular territory presumably due to focal ischemia; deficits lasting <24 h were TIAs unless accompanied by relevant acute infarcts on neuroimaging, in which case they were classified as ischemic strokes. The PC Trial had originally used a stroke definition that required neurological deficits to persist for >24 h, regardless of neuroimaging changes. For this analysis, we recoded outcome data to conform to the updated stroke definition (corresponding to a composite of PC Trial protocol-defined stroke plus TIA accompanied by neuroimaging changes, assessed blinded to treatment assignment). Our secondary effectiveness outcomes were stroke alone and the composite outcome of ischemic stroke, TIA, or early death from any cause (within 45 days of randomization), similar to the primary outcome definition in both RESPECT and CLOSURE I.
We considered the following safety outcomes, adopting the definitions used in the primary studies: major vascular procedural complication; atrial fibrillation (AF); and major bleeding episode.
Statistical Analysis
Summary descriptive statistics (means with SD and percentages) were generated for patient characteristics in the overall cohort and compared between the 3 component studies. P values testing the null hypothesis of no difference between the 3 studies were obtained either from an analysis of variance F-test (age, body mass index) or from a chi-square test.
Our primary analyses of efficacy estimated intention-to-treat (ITT) effects of PFO closure plus medical therapy versus medical therapy alone. In these analyses, all randomized patients were analyzed according to their assigned treatment groups. We performed additional analyses estimating “as treated” effects by comparing outcomes among patients who underwent device closure (attempted or successful, depending on the trial) compared to control patients. Safety analyses (i.e., analyses of adverse events) primarily estimated “as-treated” effects; ITT safety effects are reported in the Online Appendix. For all analyses, data for patients who discontinued before study completion were included until the time censored.
For time-to-event analyses, we obtained Kaplan-Meier estimates (15) and tested the equality of the survivor functions using the log-rank test stratified by trial. We obtained estimates of the hazard ratio (HR) comparing treatment groups using Cox proportional hazard regression stratified by trial with no covariate adjustment (16). In this analysis, we assumed that each study had a different baseline hazard and that the treatment effect was common across trials. We chose this model because the number of available studies was small, simulation studies at the planning stage indicated that it had reasonable statistical performance, and it is consistent with the statistical analyses performed within each study.
We also performed Cox proportional hazard regression analyses adjusted for the following covariates: age, sex, race, coronary artery disease, diabetes, hypertension, hyperlipidemia, prior stroke, smoking status, index event (stroke vs. TIA), hypermobile septum, and PFO shunt size (large vs. small). Covariates were selected on the basis of prior work suggesting that they predicted stroke recurrence or a PFO-related mechanism in the Risk of Paradoxical Embolism (RoPE) Score (17,18). We used multiple imputation to address missing data in covariates used in adjusted and subgroup analyses.
For safety outcomes, we reported procedural complications in the device arms and, for AF and bleeding events, estimated unadjusted HR comparing treatment groups using Cox regression stratified by trial.
We assessed heterogeneity of treatment effects across levels of baseline characteristics by repeating the efficacy analyses described above including appropriate interactions in Cox regressions. Herein, we report pre-specified subgroups based on age, sex, smoking status, and PFO anatomical features (shunt size and presence of atrial septal aneurysm). One additional subgroup analysis based on the qualifying event (stroke vs. TIA) was added during manuscript revision. Planned subgroup analyses based on the RoPE Score and variations of the score that incorporate recurrence risk will be described in a separate report.
We repeated the analyses described above (stratified log-rank tests and unadjusted and adjusted stratified Cox regressions) using only data from the 2 occluder trials (i.e., excluding data from the CLOSURE I Trial). We also performed stability analyses after excluding each of the other trials in turn.
We defined 2-sided p values < 0.05 as statistically significant. All analyses were performed using SAS (Cary, North Carolina) version 9.4 TS Level 1MI (SAS/Stat 13.1).
Results
This patient-level pooled analysis included data from 2,303 randomized patients followed for a total of 5,849 person-years. Overall, 442 patients (263 in the medical therapy arm; 171 in the device arm) withdrew or were otherwise lost to follow-up before study termination. Characteristics of patients are seen across trials (Table 1) and treatments (Table 2). There were some minor differences across trials. The PC Trial had a higher prevalence of smoking and prior stroke, but a lower prevalence of other vascular risk factors (i.e., diabetes, hypercholesterolemia, or hypertension). Qualifying infarcts in the RESPECT trial were more frequently located superficially on neuroimaging than in CLOSURE I. (Infarct location was unavailable in the PC Trial.) The PC Trial had a higher proportion of patients receiving anticoagulation (instead of antiplatelet) therapy in the medical arm and a lower prevalence of putative high-risk anatomic PFO features (i.e., large shunt or hypermobile interatrial septum) than the other trials. Covariates were generally balanced across treatment groups, except patients assigned to device closure were slightly more likely to have large PFOs.
Table 1. Patient Characteristics Across Individual Trials.
| Variables | TOTAL (N = 2,303) | CLOSURE (n = 909) | PC Trial (n = 414) | RESPECT (n = 980) | p Value*‡ |
|---|---|---|---|---|---|
|
| |||||
| % of data in meta-analysis | 100% | 39% | 18% | 43% | CLOSURE vs. PC Trial vs. RESPECT |
|
| |||||
| Demographic variables | |||||
|
| |||||
| Age | 45.3 ± 9.7 (2,291) | 45.5 ± 9.3 (909) | 44.5 ± 10.2 (414) | 45.4 ± 9.8 (968) | 0.1789 |
| Male | 52.7 (1,213/2,303) | 51.8 (471/909) | 49.8 (206/414) | 54.7 (536/980) | 0.1935 |
| Body mass index | 27.9 ± 5.7 (1,305) | 28.4 ± 5.9 (834) | 26.5 ± 5.2 (391) | 29.4 ± 5.6 (80) | <0.0001 |
| Current smoker | 15.9 (367/2,301) | 15.2 (138/907) | 23.9 (99/414) | 13.3 (130/980) | <0.0001 |
|
| |||||
| Medical history variables | |||||
|
| |||||
| Coronary artery disease | 2.43 (56/2,303) | 2.09 (19/909) | 1.93 (8/414) | 2.96 (29/980) | 0.3622 |
| Diabetes | 6.73 (155/2,303) | 7.81 (71/909) | 2.66 (11/414) | 7.45 (73/980) | 0.0012 |
| Hypercholesterolemia | 39.1 (900/2,303) | 44.1 (401/909) | 27.1 (112/414) | 39.5 (387/980) | <0.0001 |
| Hypertension | 30.3 (697/2,303) | 31.0 (282/909) | 25.8 (107/414) | 31.4 (308/980) | 0.0950 |
| Migraine | 33.4 (770/2,303) | 33.6 (305/909) | 20.5 (85/414) | 38.8 (380/980) | <0.0001 |
| Prior stroke/TIA | 19.6 (451/2,303) | 12.5 (114/909) | 37.4 (155/414) | 18.6 (182/980) | <0.0001 |
|
| |||||
| Echocardiographic variables† | |||||
|
| |||||
| Hypermobile septum | 33.4 (758/2,267) | 35.6 (311/873) | 23.7 (98/414) | 35.6 (349/980) | <0.0001 |
| Large PFO | 61.1 (1,292/2,115) | 61.1 (475/777) | 21.7 (80/369) | 76.1 (737/969) | <0.0001 |
|
| |||||
| Index event variables | |||||
|
| |||||
| Index event of stroke | 89.0 (2,047/2,303) | 72.0 (653/907) | 100 (414/414) | 100 (980/980) | <0.0001 |
| Superficial stroke | 56.5 (995/1,761) | 37.0 (289/782) | Not available | 72.1 (706/979) | <0.0001 |
| Baseline NIHSS | 0.7 ± 1.5 (1,882) | 0.6 ± 1.2 (904) | Not available | 0.8 ± 1.7 (978) | <0.0100 |
| Baseline Rankin | 0.7 ± 0.8 (1,882) | 0.5 ± 0.8 (902) | Not available | 0.8 ± 0.8 (980) | <0.0001 |
|
| |||||
| Treatment variables | |||||
|
| |||||
| Randomized to device closure | 49.9 (1,150/2,303) | 49.2 (447/909) | 49.3 (204/414) | 50.9 (499/980) | 0.7185 |
| Treated with antiplatelets only‡ | 85.2 (1,917/2,250) | 84.7 (770/909) | 80.0 (331/414) | 88.0 (816/927) | 0.0005 |
Values are mean ± SD (n of patients with available data) or % (n/N of patients with available data).
p values testing the null hypothesis of no difference between the 3 groups; obtained either from an analysis of variance F-test (age, body mass index) or from a chi-square test otherwise.
Findings on transesophageal echocardiography.
Among medical arm patients
NIHSS = National Institutes of Health Stroke Scale; PFO = patent foramen ovale; TIA = transient ischemic attack.
Table 2. Patient Characteristics Across Treatment Strategies.
| Variable | Combined Dataset (N = 2,303) | |
|---|---|---|
| Device Closure (n = 1,150) | Medical Therapy (n = 1,153) | |
| Source data | ||
| CLOSURE | 38.9 (447) | 40.1 (462) |
| PC TRIAL | 17.7 (204) | 18.2 (210) |
| RESPECT | 43.4 (499) | 41.7 (481) |
| Clinical variables | ||
| Age | 45.3 ± 9.8 (1,143) | 45.3 ± 9.7 (1,148) |
| Male | 51.6 (593/1,150) | 53.8 (620/1,153) |
| Body mass index | 28.0 ± 5.6 (654) | 27.8 ± 5.8 (651) |
| Current smoker | 17.0 (196/1,150) | 14.9 (171/1,151) |
| Medical history | ||
| Coronary artery disease | 3.1 (36/1,150) | 1.7 (20/1,153) |
| Diabetes | 6.9 (79/1,150) | 6.6 (76/1,153) |
| Hypercholesterolemia | 39.7 (456/1,150) | 38.5 (444/1,153) |
| Hypertension | 31.1 (358/1,150) | 29.4 (339/1,153) |
| Migraine | 34.3 (394/1,150) | 32.6 (376/1,153) |
| Prior stroke/TIA | 19.5 (224/1,150) | 19.7 (227/1,153) |
| Echocardiographic variables* | ||
| Hypermobile septum | 33.6 (380/1,130) | 33.2 (378/1,137) |
| Large PFO | 63.2 (672/1,063) | 58.9 (620/1,052) |
| Index event variables | ||
| Index event of stroke | 89.4 (1027/1,149) | 88.5 (1020/1,152) |
| Superficial stroke | 55.8 (484/867) | 57.2 (511/894) |
| Baseline NIHSS | 0.7 ± 1.6 (942) | 0.7 ± 1.4 (940) |
| Baseline Rankin | 0.7 ± 0.8 (941) | 0.6 ± 0.8 (941) |
Values are % (n), mean ± SD (n of patients with available data), or % (n/N of patients with available data).
Findings on transesophageal echocardiography.
Abbreviations as in Table 1.
A total of 108 composite endpoint events (ischemic stroke/TIA/death) were observed, including 58 ischemic strokes (56 as first events), 54 TIAs (48 as first events), and 7 deaths (4 as first events; 4 classified as early deaths). The rate of stroke was 0.98 per 100 person-years across both arms; the composite event rate was 1.8 per 100 person-years. Annualized event rates by treatment arm are shown in Table 3. Results from trial-specific analyses are shown in Online Table 1.
Table 3. Composite Outcomes and Recurrent Ischemic Stroke (Intention-to-treat Analyses).
| Analysis | Outcome Rate | Log-rank Test | Cox PH Model | Covariate-adjusted Cox PH Model* | |
|---|---|---|---|---|---|
| Device Closure | Medical Therapy | p Value | HR† (95% CI) p Value | HR† (95% CI) p Value | |
| Analyses using data from all 3 trials (N = 2,303) | |||||
| Primary composite outcome | 1.5 (45/3,057) | 2.3 (63/2,792) | 0.0517 | 0.69 (0.47-1.01) 0.0531 | 0.68 (0.46-1.00) 0.0491 |
| Recurrent ischemic stroke | 0.7 (22/3,099) | 1.3 (36/2,839) | 0.0407 | 0.58 (0.34-0.98) 0.0433 | 0.58 (0.34-0.99) 0.0443 |
| Secondary composite outcome (ischemic stroke, TIA, early death) | 1.4 (43/3,057) | 2.2 (61/2,792) | 0.0488 | 0.68 (0.46-1.00) 0.0502 | 0.67 (0.45-1.00) 0.0473 |
| Analyses limited to occluder device trials (N = 1,394)‡ | |||||
| Primary composite outcome | 1.0 (22/2,274) | 1.6 (32/2,021) | 0.0885 | 0.63 (0.36-1.08) 0.0914 | 0.64 (0.37-1.11) 0.1150 |
| Recurrent ischemic stroke | 0.4 (10/2,301) | 1.1 (23/2,044) | 0.0103 | 0.39 (0.19-0.82) 0.0133 | 0.41 (0.20-0.88) 0.0213 |
| Secondary composite outcome (ischemic stroke, TIA, early death) | 0.9 (20/2,274) | 1.6 (32/2,021) | 0.0451 | 0.57 (0.33-1.00) 0.0480 | 0.59 (0.33-1.03) 0.0651 |
Values are % per person-year (events/total person-years) unless otherwise indicated.
Adjusted for age, sex, race, coronary artery disease, diabetes, hypertension, hyperlipidemia, prior stroke, smoking status, index event (stroke versus TIA), hypermobile septum, and PFO shunt size (large versus small).
Adjusted HRs were estimated using Cox PH models combined from 10 multiply imputed datasets. In meta-analyses, source study was included in the model as a stratification term.
Excludes data from the CLOSURE trial.
CI = confidence interval; HR = hazard ratio; PH = proportional hazards; other abbreviations as in Table 1.
The Central Illustration depicts Kaplan-Meier curves for the primary composite outcome and stroke alone. As shown in Table 3, the effect of closure on the composite outcome narrowly missed statistical significance in unadjusted analysis (stratified log-rank p = 0.052; HR: 0.69; 95% confidence interval [CI]: 0.47 to 1.01; p = 0.053). Yet, it was statistically significant in adjusted analysis (HR: 0.68; 95% CI: 0.46 to 1.00; p = 0.049). Effects were similar for the secondary composite outcome, including only early deaths, with the treatment effect being statistically significant for the log-rank test and adjusted Cox regression analysis (p = 0.049 and p = 0.047, respectively), and borderline for the unadjusted Cox model (p = 0.050) (Table 3).
Central Illustration. Patent Foramen Ovale Closure in Stroke Trials: Kaplan-Meier curves comparing device closure versus medical therapy.
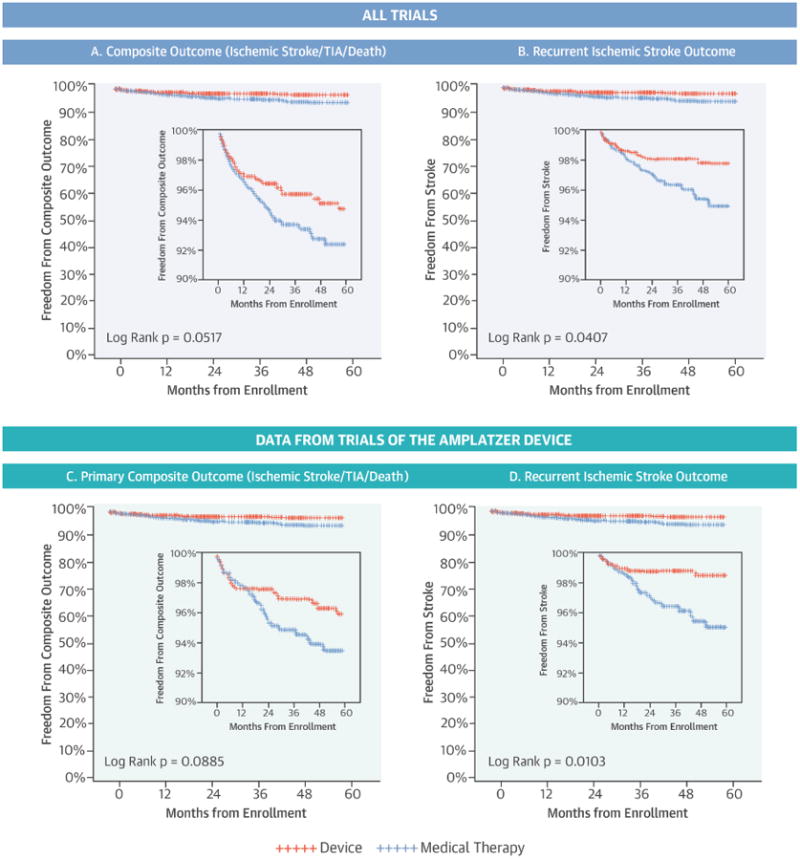
Kaplan-Meier curves comparing device closure (red) versus medical therapy (blue). Results shown for all trials pooled and for Amplatzer device trials only, and for both the primary composite and ischemic stroke outcomes. Secondary composite outcome (including only early death) was statistically significant for both the 3 trial (log rank p-value = 0.0488) and the Amplatzer only analysis (log rank p-value= p = 0.0451).
As shown in Table 3, all analyses for the stroke outcome showed that device closure plus medical therapy was significantly more effective than medical therapy alone, with the unadjusted and adjusted analyses producing identical HR estimates (HR: 0.58; 95% CI: 0.34 to 0.98; p = 0.043 and 0.58; 05% CI 0.34 to 0.99; p = 0.044, respectively).
When the 2 occluder trials were analyzed separately (i.e., excluding data from the CLOSURE I trial), the point estimates of the treatment effect for the composite outcome were lower (more favorable for closure) than those in the overall analysis, but not statistically significant in the smaller sample size of this sub-analysis (1,394 patients; 54 events): unadjusted HR: 0.63; 95% CI: 0.36 to 1.08; p = 0.091. The effect on stroke outcome was stronger than in the overall analysis (unadjusted HR:0.39; 95% CI: 0.19 to 0.82; p = 0.013). The effect of closure on the secondary composite outcome was again stronger than in the overall analysis, and statistically significant for the log-rank test and the unadjusted Cox regression analysis (p = 0.045 and p = 0.048), but not for the adjusted Cox regression analysis (p = 0.065).
As-treated analyses produced point estimates more favorable for closure compared with ITT analyses and were statistically significant for all outcomes, both when all trials were pooled and when analyses were limited to the 2 occluder trials (Table 4).
Table 4. Composite Outcomes and Recurrent Stroke (As-treated Analyses).
| Analysis | Outcome Rate | Log-rank Test | Cox PH Model | Covariate-adjusted Cox PH Model* | |
|---|---|---|---|---|---|
| Device Closure | Medical Therapy | p Value | HR† (95% CI) p Value | HR† (95% CI) p Value | |
| Analyses using data from all 3 trials (N = 2,281) | |||||
| Primary composite outcome | 1.4 (40/2,948) | 2.3 (66/2,877) | 0.025 | 0.64 (0.43-0.95) 0.026 | 0.63 (0.43-0.94) 0.025 |
| Recurrent ischemic stroke | 0.6 (19/2,985) | 1.3 (37/2,929) | 0.023 | 0.53 (0.30-0.92) 0.025 | 0.53 (0.30-0.92) 0.025 |
| Secondary composite outcome (ischemic stroke, TIA, early death) | 1.3 (38/2,948) | 2.2 (64/2,877) | 0.022 | 0.63 (0.42-0.94) 0.023 | 0.63 (0.42-0.94) 0.023 |
| Analyses limited to occluder device trials (N = 1,372)‡ | |||||
| Primary composite outcome | 0.8 (18/2,207) | 1.7 (34/2,063) | 0.018 | 0.51 (0.29-0.90) = 0.020 | 0.51 (0.29-0.91) 0.022 |
| Recurrent ischemic stroke | 0.3 (7/2,230) | 1.2 (24/2,091) | 0.001 | 0.28 (0.12-0.64) 0.003 | 0.28 (0.12-0.66) 0.004 |
| Secondary composite outcome (ischemic stroke, TIA, early death) | 0.7 (16/2,207) | 1.7 (34/2,063) | 0.007 | 0.45 (0.25-0.82) 0.009 | 0.45 (0.25-0.83) 0.010 |
Values are % per person-year (events/total person-years) unless otherwise indicated.
Adjusted for age, sex, race, coronary artery disease, diabetes, hypertension, hyperlipidemia, prior stroke, smoking status, index event (stroke versus TIA), hypermobile septum, and PFO shunt size (large versus small).
Adjusted hazard ratios estimated using Cox PH models combined from ten multiply imputed datasets. In meta-analyses source study was included in the model as a stratification term.
Excludes data from the CLOSURE trial.
Subgroup analysis (Figure 1) did not reveal any statistically significant heterogeneity of treatment effects. Subgroup effects for the ischemic stroke outcome and for the analysis including just the occluder trials are shown in Online Figures 1 through 3.
Figure 1. Subgroup Analysis: Device versus Medical Therapy.
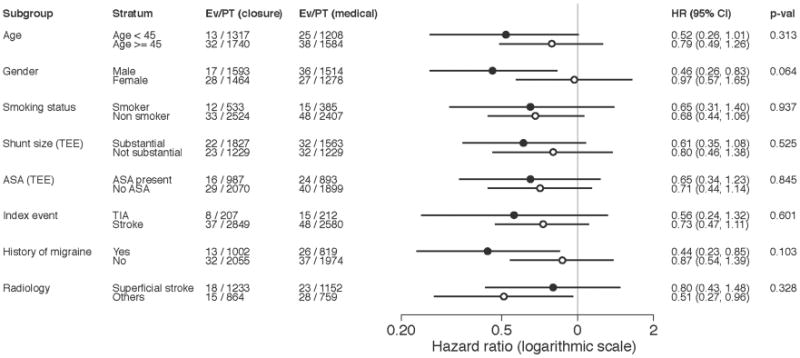
Per unadjusted hazard ratios (HR) for study-stratified Cox proportional hazard, pre-specified subgroup analyses did not show any statistically significant heterogeneity of treatment effect for the primary composite outcome. CI = confidence interval; Ev = event; PT = patient; TEE = transesophageal echocardiography.
Results for safety outcomes are summarized in Table 5. The hazard of AF among patients receiving closure was 3 times as high as that among patients receiving medical therapy (HR: 3.22; 95% CI: 1.76 to 5.90; p < 0.0002). In analyses restricted to the occluder trials, the HR for AF was 1.85 (95% CI: 0.86 to 3.98; p = 0.117). Bleeding rates were not statistically different between treatment groups. Online Table 2 shows safety outcomes analyzed by ITT.
Table 5. Safety Outcomes (As-treated Analyses)*.
| Safety Outcomes | Device Closure | Medical Therapy | HR * (95% CI) p Value† |
|---|---|---|---|
| Analyses using data from all 3 trials (n = 2,281) | n = 1,067 | n = 1,214 | |
| Bleeding | 3.7 (11/2,990) | 3.7 (11/2,941) | 1.11 (0.48-2.56) 0.81 |
| Atrial fibrillation | 1.44 (42/2,915) | 0.48 (14/2,936) | 3.22 (1.76-5.90) 0.0002 |
| Analyses limited to occluder device trials (n = 1,372) ‡ | n = 663 | n = 709 | |
| Bleeding | 0.09 (2/2,240) | 0.14 (3/2,131) | 0.68 (0.11-4.09) 0.67 |
| Atrial fibrillation | 0.87 (19/2,190) | 0.47 (10/2,119) | 1.85 (0.86-3.98) 0.12 |
Values are % per person-year (events/total person-years) unless otherwise indicated.
Unadjusted HRs and p values from Cox PH model; source study was included in the model as a stratification term.
See Table 3 for the data included in as-treated analyses. Data for procedural complications were abstracted from the original study publications (see Appendix) and not available for as-treated analysis.
Excludes data from the CLOSURE trial.
Abbreviations as in Table 3.
Finally, in stability analyses excluding either of the occluder trials (i.e., pairing CLOSURE I with either RESPECT or the PC Trial), treatment effects were no longer statistically significant (Online Table 3).
Discussion
Our study indicates that patients <60 years of age found to have a PFO in the setting of a cryptogenic ischemic stroke have relatively low stroke recurrence rates whether treated with percutaneous device therapy or with medical therapy. Outcome rates were generally lower with percutaneous closure plus medical therapy than with medical therapy alone (Central Illustration); the difference between the 2 treatments was consistently significant for the secondary efficacy outcome of ischemic stroke and for the primary composite outcome in adjusted but not unadjusted analyses.
We interpret these results, based on the totality of available randomized data and all analyses performed, as providing evidence that closure can prevent stroke recurrence in some patients with cryptogenic stroke found to have a PFO. However, AF is increased with closure, though less strongly with the occluder device. These findings suggest that closure with the occluder is a reasonable therapeutic option in the context of informed decision making. Informally, for patients similar to those enrolled in the trials, the annualized rate of ischemic stroke, if treated medically, is approximately 1%; device closure decreases this rate by half. Although there is uncertainty in the estimates, number needed to treat (NNT) over 2.5 years to avert 1 primary composite outcome event was 50 and to avert one ischemic stroke, the NNT was 67. A benefit of this magnitude may be clinically important to some patients and may continue beyond 2.5 years among younger stroke patients with a long life expectancy. We stress that our study results may not be applicable to other devices, particularly non-PFO devices, being used off-label for PFO closure.
While bleeding rates were similar between device and medical therapy arms, rates of AF increased with device closure in the overall analysis. The difference did not reach statistical significance for the occlude device, for which point estimates indicated a potential number needed to harm of 100 by causing 1 additional patient to develop AF over 2.5 years. These results emphasize the importance of meticulously ruling out occult AF as a cause of the index event and the necessity of close initial monitoring of the cardiac rhythm for patients treated with PFO closure and appropriate management of documented atrial fibrillation.
While the effects estimated in our analysis were substantial on the relative-risk scale, and the estimated reduction in absolute risk may be of clinical import, they were smaller than those anticipated in the power calculation of the individual trials. For example, the RESPECT trial was powered to detect a 75% relative risk reduction and the PC Trial was powered to detect an absolute effect of >2% per year. Research completed since the planning of these trials has shown that a substantial portion of index cryptogenic strokes in patients with PFO may be due to mechanisms unrelated to PFO (18,19). Studies have additionally suggested that paradoxical embolism may be a mechanism with an especially low recurrence risk; thus, even in patients with such PFO-related mechanisms, recurrent events may often be unrelated to PFO (6,20). Medical therapies, in theory, can have a protective effect on recurrent strokes for both PFO-related and unrelated events, whereas device closure presumably protects only against the former. Furthermore, our results do not suggest that putative high-risk anatomical features, such as large PFO size or atrial septal aneurysm, are by themselves very useful at discriminating patients likely to benefit from closure from those unlikely to benefit. Thus, even while a consistent signal for an effect on stroke recurrence is seen in our study, the totality of results reported over the last several years should provide some caution against overly simplistic assumptions that paradoxical embolism is the only important mechanism of either index or recurrent stroke in these patients.
Current AHA/ASA guidelines do not support the use of PFO closure among patients with PFO and cryptogenic stroke (21). This recommendation was based on the null results of the 3 trials included in this pooled analysis. Each of these trials was powered to detect very large treatment effects only. In such circumstances, evidence synthesis across trials is appropriate and provides more powerful tests and more precise estimates of the treatment effect than separate analyses of the individual trials (22). Our analysis of all available individual patient data from randomized trials generally suggests that closure plus medical therapy improves outcomes compared with medical therapy alone; however, confidence intervals remained wide, and ongoing and future studies may yet revise these effect estimates.
Our results, particularly with respect to tests of statistical significance, depended on a number of analytic choices, such as using a composite outcome (stroke, TIA, or death) versus using stroke alone; relying on all trials to estimate an overall effect versus trials of different devices separately; and performing an unadjusted versus adjusted analysis. Because the trials' published results were known before this pooled analysis was planned, our analytic choices could not be made completely blinded to those results. Thus, we sought to emulate pretrial reasoning with respect to outcome selection, following widely accepted standard analysis practices, reporting results from all analyses conducted, and interpreting findings based on all available information, including stability and sensitivity analyses.
Our results were minimally affected by whether the composite outcome included all or (similar to RESPECT and CLOSURE I) only early deaths, but these slight differences were of some interest because they affected whether p values were significant at the 0.05 level or borderline nonstatistically significant. In general, clinically informative composite outcomes are those whose components are plausible targets of the intervention; for example, nonstroke fatal events outside the peri-procedural period are unlikely to be affected by closure versus medical therapy. Similarly, our results were minimally affected by whether analyses were performed with or without adjustment for baseline covariates. Analyses adjusted for covariates generally have greater statistical power for time-to-event outcomes compared with unadjusted analyses. The interpretation of such analyses is typically felt to be more relevant for the estimation of patient-level effects and multivariate adjustment of RCT results is a common approach (23). In this study, adjusted and crude analyses gave essentially identical results, despite the nominal significance of only the adjusted analysis for the primary composite outcome.
Whether the endpoint analyzed was a composite outcome or ischemic stroke alone had a somewhat greater effect on our analyses. By definition, composite outcomes have a higher incidence rate than their components and are generally presumed to provide greater statistical power than component outcomes. Nonetheless, about half of the composite outcomes were TIA events, that is, transient neurological symptoms without objective neuroimaging findings subject to diagnostic inaccuracy and all-cause mortality was rare and not pathogenically likely to be modifiable by PFO closure. Thus, ischemic stroke recurrence as a lone endpoint may offer a more reliable outcome with regard to treatment effect, and is an endpoint with greater clinical significance than when joined in a composite with TIA. In our pooled analysis, the benefits of device closure for stroke alone were consistent across all analytical approaches.
In a head-to-head trial comparing the occluder device and the septal closure system, the latter was more commonly associated with device-related complications such as AF and thrombus formation on the device (24); there was also some evidence that the occluder device may more effectively prevent cerebrovascular events (25). A network meta-analysis concluded that the effectiveness of closure depended on the type of device used (5). Thus, it is reasonable to consider the evidence on each device separately. Furthermore, analyses restricted to the occluder device trials are especially relevant to clinical decisions because, of the devices assessed, only the Amplatzer device is available for clinical use (albeit not in the United States); the STARFlex device is no longer manufactured.
This analysis using individual participant data offered several advantages over prior study-level meta-analyses that were based on the published aggregate results. We were able to harmonize outcome definitions across trials and did not have to rely on numerical approximations when extracting data from trial publications; the availability of person-level data also permitted covariate adjustment, proper handling of missing data, and exploration of effect heterogeneity. These differences presumably explained why our results for some analyses were statistically significant where prior study-level meta-analyses were not (1-4,26-28).
Study Limitations
This work has some limitations. First, the pooled analysis inherited any limitations of the component studies. Crossover and loss-to-follow up in the studies was high relative to the number of events and somewhat more common in the medical arm. While the ITT effect is consistently estimated in the presence of noncompliance to assigned treatment, selection bias due to loss to follow-up may have affected our analyses. This analysis did not address questions regarding the optimal antithrombotic regimen in patients with PFO and cryptogenic stroke; medical regimens – both for the comparison group and as cotreatments to device implantation – were heterogeneous within and between the included studies. Observational comparative effectiveness research has shown nonsignificant effects of oral anticoagulation over antiplatelet therapy in these patients (29). Newer agents are now under study in the cryptogenic stroke population (NCT02239120; NCT02313909). Additionally, the trials included in our analysis were designed as prospective, randomized, open, blinded endpoint studies, and it appears this design may have led to a slight imbalance in referral for endpoint adjudication in the PC Trial, though not CLOSURE I or RESPECT (30). Finally, most patients were followed for a short time frame of around 2.5 years and device implantation is essentially a permanent intervention. An observational study comparing closure versus medical therapy using propensity-score matching methods suggested widening benefits over time (31).
Conclusions
In summary, patients with cryptogenic stroke and PFO have relatively low outcome rates with medical therapy with or without device closure; recurrent stroke rates are lower with percutaneously implanted device closure than with medical therapy alone.
Supplementary Material
Appendix Figure 1. Subgroup analysis for recurrent stoke (intention-to-treat analyses)
Appendix Figure 2. Subgroup analysis for composite outcome (Amplatzer device trials)
Appendix Figure 3. Subgroup analysis for recurrent stroke (Amplatzer device trials)
Appendix Table 1. Event rates and effects of closure versus medical therapy for individual trials (intention-to-treat analyses)
Appendix Table 2. Safety Outcomes (intention-to-treat analyses)
Appendix Table 3. Stability analyses for recurrent stroke, composite outcome rates and effects of closure (intention-to-treat analyses)
Perspectives.
Competency In Medical Knowledge
Patients with PFO who develop cryptogenic ischemic stroke face relatively low rates of recurrent cerebral ischemic events during medical therapy with or without device closure, but pooled results of randomized trials show lower stroke rates with percutaneously implanted device closure than with medical therapy alone.
Translational Outlook
Longer-term follow-up of ongoing and completed trials will improve our understanding of the comparative effectiveness of closure versus medical therapy, but comparative studies of various antithrombotic treatment regimens, including those in patients undergoing PFO closure, are needed to address important to knowledge gaps.
Acknowledgments
Funding Sources: This study was supported by the National Institutes of Health (R01 NS062153, R21 NS079826), Patient-Centered Outcomes Research Institute (ME-1306-03758), and PACE Center Funds, Tufts Medical Center.
Disclosures: Dr Kent, Dr. Dahabreh and Ms Ruthazer have no relevant financial conflicts of interest. Dr. Furlan is Principal Investigator of the Closure I trial sponsored by NMT Medical Boston. Dr. Juni is an unpaid steering committee or statistical executive committee member of trials funded by Abbott Vascular, Biosensors, Medtronic and St. Jude Medical. CTU Bern, which is part of the University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, CTU Bern is involved in design, conduct, or analysis of clinical studies funded by Abbott Vascular, Ablynx, Amgen, AstraZeneca, Biosensors, Biotronic, Boehrhinger Ingelheim, Eisai, Eli Lilly, Exelixis, Geron, Gilead Sciences, Nestle´, Novartis, Novo Nordisc, Padma, Roche, Schering-Plough, St Jude Medical, and Swiss Cardio Technologies. Dr. Mattle is supported grants to his institution and speaker bureau: Bayer, Biogen Idec, Boehringer Ingelheim, Bristol-Myers Squibb, Covidien, Daiichi-Sankyo, Genzyme, Merck-Serono, Neuravi, Novartis, Pfizer, Sanofi-Aventis, Teva, St. Jude, Swiss Heart Foundation. Dr. Meier is supported by the following research grants to his institution and personal speaker bureau honoraries: AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Daiichi Sankyo, Eli Lilly, Pfizer, St. Jude Medical. Dr. Thaler is a member of the National Steering Committees of two trials sponsored by St. Jude Medical (RESPECT, ACP Trial) and one trial sponsored by Coherex (WaveCrest). Dr. Kent, as corresponding author, had full access to all data in the study and had final responsibility for the decision to submit for publication. Dr. Saver is an employee of the University of California. The University of California, Regents receive funding for Dr Saver's services as a scientific consultant regarding trial design and conduct to St. Jude Medical, Medtronic/Covidien, Stryker, Neuravi, BrainsGate, Pfizer, Squibb, Boehringer Ingelheim (prevention only), and ZZ Biotech. Dr Saver has served as an unpaid site investigator in multicenter trials run by St. Jude Medical and Gore for which the UC Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled. Dr. Saver serves as an unpaid consultant to Genentech advising on the design and conduct of the PRISMS trial; neither the University of California nor Dr. Saver received any payments for this voluntary service. Dr. Carroll is an employee of the University of Colorado. University Physicians Incorporated of the University of Colorado School of Medicine receives funding from St. Jude Medical, Inc. for Dr. Carroll's services as a scientific consultant and RESPECT steering committee member. Dr. Reisman receives funding from St Jude Medical and Coherex, and is a consultant on the advisory boards for Boston Scientific and Cordis. Dr. Smalling receives funding from St Jude Medical.
This study is supported by National Institutes of Health (R01 NS062153, R21 NS079826), the Patient-Centered Outcomes Research Institute (ME-1306-03758), and PACE center funds from Tufts Medical Center. We would like to thank CLOSURE, RESPECT and PC Trial Investigators, as well as Jennifer S. Lutz, MA, PACE, Tufts Medical Center, for technical support and assistance with manuscript preparation.
Abbreviations
- ITT
intention to treat
- PFO
patent foramen ovale
- RoPE Score
Risk of Paradoxical Embolism Score
- TIA
transient ischemic attack
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kitsios GD, Thaler DE, Kent DM. Potentially Large yet Uncertain Benefits: A Meta-analysis of Patent Foramen Ovale Closure Trials. Stroke. 2013;44:2640–3. doi: 10.1161/STROKEAHA.113.001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan AR, Bin Abdulhak AA, Sheikh MA, et al. Device closure of patent foramen ovale versus medical therapy in cryptogenic stroke: a systematic review and meta-analysis. JACC Cardiovasc Interv. 2013;6:1316–23. doi: 10.1016/j.jcin.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Pineda AM, Nascimento FO, Yang SC, et al. A meta-analysis of transcatheter closure of patent foramen ovale versus medical therapy for prevention of recurrent thromboembolic events in patients with cryptogenic cerebrovascular events. Catheter Cardiovasc Interv. 2013;82:968–75. doi: 10.1002/ccd.25122. [DOI] [PubMed] [Google Scholar]
- 4.Spencer FA, Lopes LC, Kennedy SA, Guyatt G. Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open. 2014;4:e004282. doi: 10.1136/bmjopen-2013-004282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stortecky S, da Costa BR, Mattle HP, et al. Percutaneous closure of patent foramen ovale in patients with cryptogenic embolism: a network meta-analysis. Eur Heart J. 2015;36:120–8. doi: 10.1093/eurheartj/ehu292. [DOI] [PubMed] [Google Scholar]
- 6.Furlan AJ, Reisman M, Massaro J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991–9. doi: 10.1056/NEJMoa1009639. [DOI] [PubMed] [Google Scholar]
- 7.Carroll JD, Saver JL, Thaler DE, et al. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092–100. doi: 10.1056/NEJMoa1301440. [DOI] [PubMed] [Google Scholar]
- 8.Meier B, Kalesan B, Mattle HP, et al. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083–91. doi: 10.1056/NEJMoa1211716. [DOI] [PubMed] [Google Scholar]
- 9.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 10.Hernandez AV, Eijkemans MJ, Steyerberg EW. Randomized controlled trials with time-to-event outcomes: how much does prespecified covariate adjustment increase power? Ann Epidemiol. 2006;16:41–8. doi: 10.1016/j.annepidem.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Hernandez AV, Steyerberg EW, Habbema JD. Covariate adjustment in randomized controlled trials with dichotomous outcomes increases statistical power and reduces sample size requirements. J Clin Epidemiol. 2004;57:454–60. doi: 10.1016/j.jclinepi.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Kent DM, Trikalinos TA, Hill MD. Are unadjusted analyses of clinical trials inappropriately biased toward the null? Stroke. 2009;40:672–3. doi: 10.1161/STROKEAHA.108.532051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacco RL, Adams R, Albers G, et al. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke. Stroke. 2006;37:577–617. doi: 10.1161/01.STR.0000199147.30016.74. [DOI] [PubMed] [Google Scholar]
- 14.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–89. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 17.Kent DM, Thaler DE. The Risk of Paradoxical Embolism (RoPE) Study: Developing risk models for application to ongoing randomized trials of percutaneous patent foramen ovale closure for cryptogenic stroke. Trials. 2011;12:185. doi: 10.1186/1745-6215-12-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kent DM, Ruthazer R, Weimar C, et al. An index to identify stroke-related vs incidental patent foramen ovale in cryptogenic stroke. Neurology. 2013;81:619–25. doi: 10.1212/WNL.0b013e3182a08d59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thaler DE, Ruthazer R, Weimar C, et al. Recurrent stroke predictors differ in medically treated patients with pathogenic vs. other PFOs. Neurology. 2014;83:221–6. doi: 10.1212/WNL.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mono ML, Geister L, Galimanis A, et al. Patent foramen ovale may be causal for the first stroke but unrelated to subsequent ischemic events. Stroke. 2011;42:2891–5. doi: 10.1161/STROKEAHA.111.619577. [DOI] [PubMed] [Google Scholar]
- 21.Kernan WN, Ovbiagele B, Black HR, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 22.Lau J, Antman EM, Jimenez-Silva J, et al. Cumulative meta-analysis of therapeutic trials for myocardial infarction. N Engl J Med. 1992;327:248–54. doi: 10.1056/NEJM199207233270406. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Manca A, Zwarenstein M, et al. A substantial and confusing variation exists in handling of baseline covariates in randomized controlled trials: a review of trials published in leading medical journals. J Clin Epidemiol. 2010;63:142–53. doi: 10.1016/j.jclinepi.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Taaffe M, Fischer E, Baranowski A, et al. Comparison of three patent foramen ovale closure devices in a randomized trial (Amplatzer versus CardioSEAL-STARflex versus Helex occluder) Am J Cardiol. 2008;101:1353–8. doi: 10.1016/j.amjcard.2007.12.040. [DOI] [PubMed] [Google Scholar]
- 25.Hornung M, Bertog SC, Franke J, et al. Long-term results of a randomized trial comparing three different devices for percutaneous closure of a patent foramen ovale. Eur Heart J. 2013;34:3362–9. doi: 10.1093/eurheartj/eht283. [DOI] [PubMed] [Google Scholar]
- 26.Riaz IB, Dhoble A, Mizyed A, et al. Transcatheter patent foramen ovale closure versus medical therapy for cryptogenic stroke: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord. 2013;13:116. doi: 10.1186/1471-2261-13-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hakeem A, Marmagkiolis K, Hacioglu Y, et al. Safety and efficacy of device closure for patent foramen ovale for secondary prevention of neurological events: Comprehensive systematic review and meta-analysis of randomized controlled trials. Cardiovasc Revasc Med. 2013;14:349–55. doi: 10.1016/j.carrev.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Capodanno D, Milazzo G, Vitale L, et al. Updating the evidence on patent foramen ovale closure versus medical therapy in patients with cryptogenic stroke: a systematic review and comprehensive meta-analysis of 2,303 patients from three randomised trials and 2,231 patients from 11 observational studies. EuroIntervention. 2014;9:1342–9. doi: 10.4244/EIJV9I11A225. [DOI] [PubMed] [Google Scholar]
- 29.Kent DM, Dahabreh IJ, Ruthazer R, et al. Anticoagulant versus antiplatelet therapy in patients with cryptogenic stroke and patent foramen ovale: An individual participant data meta-analysis. Euro Heart J. 2015;36:2381–9. doi: 10.1093/eurheartj/ehv252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA. 2010;304:793–4. doi: 10.1001/jama.2010.1161. [DOI] [PubMed] [Google Scholar]
- 31.Wahl A, Juni P, Mono ML, et al. Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation. 2012;125:803–12. doi: 10.1161/CIRCULATIONAHA.111.030494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix Figure 1. Subgroup analysis for recurrent stoke (intention-to-treat analyses)
Appendix Figure 2. Subgroup analysis for composite outcome (Amplatzer device trials)
Appendix Figure 3. Subgroup analysis for recurrent stroke (Amplatzer device trials)
Appendix Table 1. Event rates and effects of closure versus medical therapy for individual trials (intention-to-treat analyses)
Appendix Table 2. Safety Outcomes (intention-to-treat analyses)
Appendix Table 3. Stability analyses for recurrent stroke, composite outcome rates and effects of closure (intention-to-treat analyses)


