Summary
NF-κB, a key activator of inflammation primes the NLRP3-inflammasome for activation by inducing pro-IL-1β and NLRP3 expression. NF-κB, however, also prevents excessive inflammation and restrains NLRP3-inflammasome activation through a poorly defined mechanism. We now show that NF-κB exerts its anti-inflammatory activity by inducing delayed accumulation of the autophagy receptor p62/SQSTM1. External NLRP3-activating stimuli trigger a form of mitochondrial (mt) damage that is caspase-1- and NLRP3-independent and causes release of direct NLRP3-inflammasome activators, including mtDNA and mtROS. Damaged mitochondria undergo Parkin-dependent ubiquitin conjugation and are specifically recognized by p62, which induces their mitophagic clearance. Macrophage-specific p62 ablation causes pronounced accumulation of damaged mitochondria and excessive IL-1β-dependent inflammation, enhancing macrophage death. Therefore, the “NF-κB-p62-mitophagy” pathway is a macrophage-intrinsic regulatory loop through which NF-κB restrains its own inflammation-promoting activity and orchestrates a self-limiting host response that maintains homeostasis and favors tissue repair.
Introduction
Macrophages are sentinels that detect foreign invaders and sterile tissue damage. Upon encounter of pathogen-associated (PAMPs) or damage-associated (DAMPs) molecular patterns, macrophages initiate an acute but transient host response whose ultimate goal is clearance of foreign organisms and cellular debris and restoration of tissue integrity and function (Kotas and Medzhitov, 2015; Meylan et al., 2006). Indeed, while interfering with innate immunity, macrophage ablation also results in excessive tissue damage (Brenner et al., 2013). Thus, proper control of macrophage activation is pivotal to restoration of tissue integrity and function.
Macrophages sense PAMPs and DAMPs via Toll-like (TLRs) and Nod-like (NLRs) receptors, which control inflammation through transcriptional and post-transcriptional mechanisms, respectively (Meylan et al., 2006). Unlike TLRs, which directly recognize their agonists, most NLRs involved in inflammasome activation and IL-1β/IL-18 production are not bona fide receptor proteins (Vance, 2015; von Moltke et al., 2013). For instance, NLRP3 (NLR family, pyrin domain containing 3), which binds the adaptor ASC (apoptosis-associated speck-like protein containing a CARD domain) to induce pro-caspase-1 recruitment, autoactivation and pro-IL-1β processing, responds to highly diverse stimuli, including ATP, bacterial toxins, micro-crystalline substances, lipid particles, bacteria and viruses, none of which binds NLRP3 directly (Elliott and Sutterwala, 2015; Lamkanfi and Dixit, 2014). Some of these stimuli act via the purinergic receptor P2X7 and others are thought to induce plasma membrane damage, but all of them promote NLRP3 association with ASC, inducing formation of the NLRP3-inflammasome complex through indirect mechanisms that involve mitochondrial (mt) signals (Elliott and Sutterwala, 2015; Zhong et al., 2013). Inflammasome-activating signals include mtDNA, reactive oxygen species (mtROS) or cytosolic presentation of cardiolipin, all of which were proposed to serve as direct activators of the NLRP3:ASC:pro-Caspase-1 complex. Essential for NLRP3-inflammasome activation is transcription factor NF-κB, which acts downstream TLRs and other immune receptors (Vallabhapurapu and Karin, 2009). While inducing numerous inflammatory chemokines, cytokines, and cytokine precursors, including pro-IL-1β, NF-κB also induces NLRP3, and is therefore important for inflammasome priming and assembly (Schroder and Tschopp, 2010). Surprisingly, NF-κB also has anti-inflammatory functions and its activity is needed for preventing premature and excessive NLRP3-inflammasome activation in macrophages as well as inhibition of neutrophil proteases, that also process pro-IL-1β (Greten et al., 2007). Treatment of mice with potent IKKβ inhibitors or myeloid-specific IKKβ ablation enhances IL-1β production and mortality in response to LPS challenge and microbial infections (Greten et al., 2007). IKKβ ablation or inhibition also results in neutrophilia (neutrophil accumulation), another dangerous condition causing extensive tissue damage (Hsu et al., 2011). Enhanced inflammation and neutrophilia were observed in human subjects that were given IKKβ inhibitors, resulting in termination of clinical development programs focused on such compounds. The precise molecular mechanism underlying NF-κB-mediated inhibition of NLRP3-inflammasome activation remains obscure.
A recent study suggested that NF-κB promotes autophagy (Criollo et al., 2010), a quality control process that negatively regulates NLRP3-inflammsome activation (Nakahira et al., 2011; Saitoh et al., 2008; Zhou et al., 2011) through a yet-to-be defined mechanism. Postulating that the anti-inflammatory effect of NF-κB could be mediated through autophagy, we searched for NF-κB-regulated gene products involved in autophagy. We thus examined expression of all five known autophagy receptors (Lazarou et al., 2015), namely: NBR1, NDP52, p62/SQSTM1, optineurin (OPTN) and TAX1BP1, in LPS-primed macrophages and found that only p62 was upregulated upon NF-κB activation. p62, a highly conserved protein, is a multifunctional signaling scaffold and chaperon that binds polyubiquitinated proteins and damaged organelles and targets them to autophagosomal clearance via its ubiquitin association domain (UBA) and LC3 binding motif (LIR), respectively (Komatsu et al., 2012). Upon stimulation of primed macrophages with NLRP3 agonists, p62 is recruited to damaged mitochondria that release signals causing NLRP3-inflammasome activation. By eliminating such signal-producing mitochondria, p62 prevents excessive inflammasome activation, which, in addition to IL-1β release, triggers macrophage death (Miao et al., 2010), thereby compromising macrophage-mediated immunity, tissue repair and healing. Therefore, the “NF-κB-p62/SQSTM1-mitophagy” pathway provides an essential regulatory loop through which NF-κB orchestrates a reparative inflammatory response and prevents excessive collateral damage.
Results
LPS induces NF-κB-dependent p62/SQSTM1 expression in macrophages
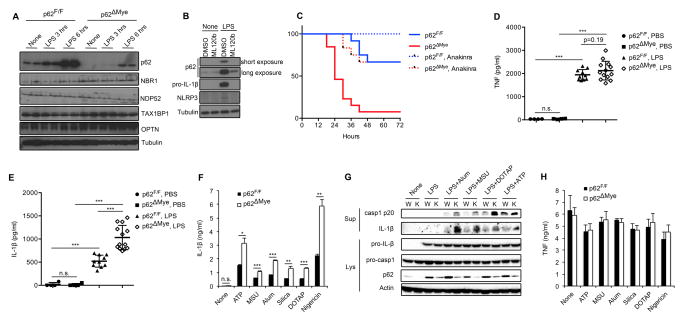
We examined whether NF-κB attenuates NLRP3-inflammasome activation in macrophages by inducing genes whose products promote autophagy. We examined expression of five known autophagy receptors (NBR1, NDP52, p62/SQSTM1, OPTN and TAX1BP1) in the presence or absence of LPS, a TLR4 ligand that activates NF-κB and primes macrophages for NLRP3-inflammasome activation. Expression of all five autophagy receptors was low in wild-type (WT) macrophages but LPS treatment led to strong and selective p62 mRNA and protein induction (Figure 1A and S1A). Notably, LPS-induced p62 continues to accumulate for at least 6 hrs during the priming step and lags behind NLRP3 and pro-IL-1β, whose accumulation was completed within the first 3 hrs of priming (Figure 1A and S1B). However, similar to NLRP3 and pro-IL-1β, induction of p62 was NF-κB-dependent because IKKβ inhibition by ML120b prevented it (Figure 1B).
Figure 1. p62, induced on NF-κB activation, suppresses NLRP3-inflammasome dependent IL-1β production.
(A) Immunoblot (IB) analysis of autophagy receptors in wild-type (p62F/F) and p62ΔMye BMDM stimulated with LPS (200 ng/ml). (B) Wild-type BMDM were incubated with or without LPS in the absence or presence of IKKβ inhibitor ML120b and expression of p62, pro-IL-1β, and NLRP3 was IB analyzed. (C) Survival of p62F/F or p62ΔMye mice injected with intraperitoneal (i.p.) LPS (30 mg/kg) without or with anakinra (50 mg/kg), n=11–13. (D, E) 12-weeks old p62F/F or p62ΔMye mice were i.p. injected with LPS and their sera collected 3 hrs later and analyzed by ELISA for TNF (D) and IL-1β (E). (F, H) Release of IL-1β (F) or TNF (H) from LPS-primed p62F/F or p62ΔMye BMDM that were stimulated with the indicated NLRP3 agonists. Data are averages ± s.d. (n=3). (G) IB analysis of caspase-1 and pro-IL-1β processing in LPS-primed WT (W) or p62-deficient (K) iBMDM stimulated as indicated. Data are representative of three independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
Macrophage p62 ablation enhances IL-1β production
We investigated whether p62 could mediate the inhibitory effect of NF-κB on the NLRP3-inflammasome. We crossed p62F/F and LysM-Cre mice to generate LysM-Cre/p62F/F mice (hereafter p62ΔMye), which lack p62 in mature myeloid cells, including macrophages and neutrophils. Intraperitoneal (i.p.) LPS injection resulted in approximately 35% mortality in WT mice, but close to 100% mortality was seen in p62ΔMye mice (Figure 1C). Importantly, the effect of myeloid-specific p62 deletion on sepsis-induced mortality is similar to that of IKKβ ablation in the same compartment (Greten et al., 2007). LPS-induced mortality is mediated by TNF and IL-1β, but circulating TNF in LPS-injected p62ΔMye mice was barely higher than in WT mice (Figure 1D). By contrast, p62ΔMye mice exhibited a two-fold increase in circulating IL-1β after LPS treatment (Figure 1E). No strain-specific differences in basal TNF or IL-1β were observed. Blocking IL-1 signaling with anakinra, an IL-1β receptor antagonist, prevented septic shock and improved survival in both WT and p62ΔMye mice (Figure 1C), indicating a central and critical role for IL-1β-IL-1R signaling in the pathogenesis of excessive inflammation.
Unlike TNF, whose synthesis and secretion are inflammasome-independent, secretion of mature IL-1β by macrophages requires inflammasome-dependent caspase-1 activation. Indeed, stimulation of WT and p62ΔMye bone marrow-derived macrophages (BMDM) with different NLRP3 inflammasome activators, including ATP, urea microcrystals (MSU), alum, silica microparticles, liposomes (DOTAP) and nigericin, led to 2–3-fold higher IL-1β secretion by p62ΔMye macrophages than p62F/F macrophages (Figures 1F and S1C), indicating that enhanced IL-1β secretion is retained ex vivo and likely to be related to enhanced inflammasome activation. Importantly, small hairpin RNA (shRNA)-mediated p62 silencing in WT (C57Bl/6) immortalized BMDM (Hornung et al., 2008) led to a similar enhancement of pro-IL-1β processing and IL-1β secretion upon incubation with the inflammasome activators used above (Figure 1G and S1D). Thus, the results are not unique to knockout mice. Elevated IL-1β secretion was accompanied by enhanced caspase-1 activation in p62ΔMye BMDM (Figure S1C, E). Fittingly, ATP-induced pyroptosis, an inflammatory form of cell death mediated by caspase-1, was exacerbated in p62-deficient macrophages relative to WT counterparts (Figure S1F). p62 ablation had no effect on NLRP3, ASC or pro-IL-1β expression after LPS treatment (Figure S1G), and did not affect TNF induction by LPS alone or together with NLRP3-inflammasome activators (Figures 1H and S1H). BMDM from a global p62-KO mice also displayed elevated IL-1β secretion after stimulation with NLRP3 agonists (Figure S1I). Moreover, p62 ablation did not have a significant effect on AIM2- or NLRP1b-inflammasome activities (Figure S1J,K), suggesting the effect is specific to the NLRP3-inflammasome. In p62-deficient macrophages, IKKβ inhibition did not cause a further increase in caspase-1 activation (Figure S1L).
Inflammasome activators recruit p62 to mitochondria
Inflammasome activators were suggested to affect mitochondrial membrane integrity or enhance mtROS production (Elliott and Sutterwala, 2015), and p62 can recognize damaged mitochondria and may promote their autophagic clearance (Geisler et al., 2010), although this function of p62 has been disputed (Lazarou et al., 2015). We therefore examined whether inflammasome activators affect the subcellular distribution of p62 and/or induce its mitochondrial translocation. In non-stimulated macrophages, p62 displayed diffuse cytoplasmic distribution, but diverse NLRP3 inflammasome activators induced p62-containing aggregates that were either colocalized with or adjacent to mitochondria (Figure 2A,B). Cell fractionation confirmed these results. In LPS-primed macrophages, very little p62 co-sedimented with mitochondria, but after incubation with ATP or nigericin, much more p62 was present in the mitochondrial fraction, which now also contained lipidated LC3 (LC3II) and Parkin (Figure 2C). These results suggest that NLRP3-inflammasome agonists stimulate mitochondrial damage, which results in p62 recruitment.
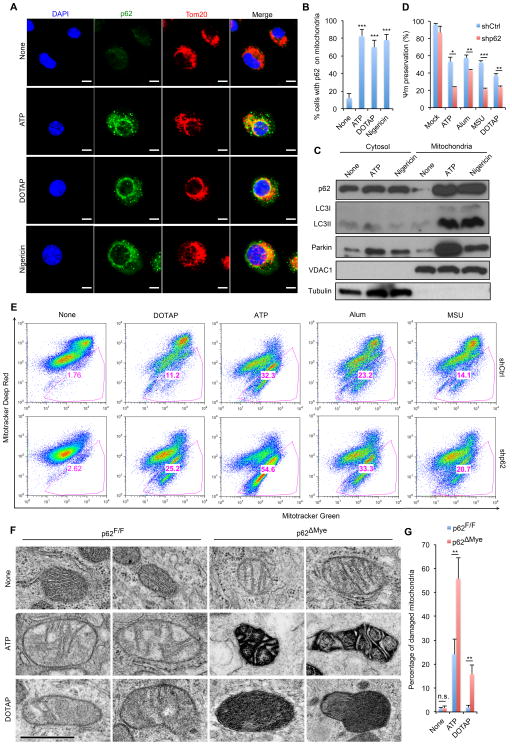
Figure 2. NLRP3 agonists promote mitochondrial damage and p62 recruitment.
(A) Intracellular distribution of p62 and mitochondria (Tom20) in LPS-primed BMDM stimulated with NLRP3 agonists examined by confocal microscopy (Scale bars, 5 μm) and (B) quantitated by counting cells with p62 aggregation on mitochondria. Averages ± s.d. represent the ratio of cells with mitochondrial p62 aggregates to total cells in each field. (n=6). (C) Subcellular distribution of p62, LC3II, and Parkin before and after NLRP3-inflammasome activation. Data are representative of three independent experiments. (D) NLRP3 agonist-induced changes in mitochondrial membrane potential (Ψm) in LPS-primed WT (shCtrl) or p62-deficient (shp62) iBMDM were measured by TMRM fluorescence. Data are averages ± s.d.(n=3). (E) Flow cytometric analysis of mitochondrial status in macrophages challenged with NLRP3 agonists. Gates represent cells with damaged mitochondria. (F, G) Electron micrographs of mitochondria in LPS-primed p62F/F or p62ΔMye BMDM after incubation with NLRP3 agonists. Shown are representative examples of normal, partially damaged and heavily damaged mitochondria. Scale bars: 500 nm. (G) quantification of damaged mitochondria in (F). Results are averages ± s.e.m. (n=8). *, p<0.05; **, p<0.01; ***, p<0.001.
Damaged mitochondria accumulate in p62-deficient macrophages
Mitophagy mediates clearance of damaged mitochondria (Lazarou et al., 2015). To test more directly whether NLRP3-inflammasome agonists trigger mitochondrial damage, we measured mitochondrial membrane potential. All tested NLRP3 agonists induced loss of mitochondrial membrane potential (ΔΨm), and this was exacerbated in p62-deficient macrophages (Figure 2D). To confirm these findings, we used a fluorescence-based assay and flow cytometry to quantitate mitochondrial damage. Consistent with the above results, inflammasome activators induced appearance of damaged mitochondria and their effect was considerably enhanced in p62-deficient macrophages (Figure 2E). Using electron microscopy (EM) we directly assessed mitochondrial integrity and state in WT and p62ΔMye BMDM. Whereas ATP, and to a lesser extent, DOTAP induced accumulation of a few swollen mitochondria in WT cells, this effect was strongly enhanced in p62ΔMye BMDM, which contained many highly damaged, electron-dense mitochondria (Figure 2F,G). Accumulation of damaged mitochondria in p62-deficient macrophages correlated with enhanced mtROS production and elevated mtDNA release (Figure S2A,B). These results confirm that NLRP3 agonists induce mitochondrial damage, resulting in mtROS and mtDNA release. Unlike previous studies, in which p62 deficiency only affected the subcellular distribution of mitochondria (Narendra et al., 2010; Okatsu et al., 2010), p62 ablation results in substantial accumulation of damaged mitochondria in macrophages stimulated with NLRP3 agonists. By promoting clearance of damaged mitochondria, p62 attenuates NLRP3-inflammasome activation.
It was suggested that NLRP3-inflammasome-dependent caspase-1 activation is responsible for mitochondrial damage (Yu et al., 2014), thus placing mitochondria downstream of caspase-1. However, numerous other studies had reached the opposite conclusion and placed mitochondrial damage upstream to caspase-1(Elliott and Sutterwala, 2015). We addressed this controversy using Nlrp3−/−, Asc−/− and Casp1−/− macrophages, which are defective in inflammasome activation. Despite the defect, all types of inflammasome-deficient macrophages exhibited mitochondrial damage comparable to WT, when incubated with NLRP3-inflammasome agonists (Figure S2C–E), confirming that mitochondrial damage is upstream to and independent of caspase-1 activation. These results also confirmed that NLRP3 is not the direct site of action of the inflammasome activators tested in these experiments.
To validate these conclusions, we took advantage of a tamoxifen inducible Nlrp3 knock-in mouse strain, that expresses a human Muckle-Wells syndrome NLRP3(A350V) variant that is constitutively active towards caspase-1 (Brydges et al., 2013). BMDM isolated from these mice released considerable amounts of IL-1β upon LPS priming, without the need for secondary stimulation with NLRP3 agonists (Figure S2F). However, macrophages containing genetically activated NLRP3(A350V) did not exhibit mitochondrial damage and enhanced mROS production after LPS stimulation (Figure S2G,H), supporting the notion that mitochondrial damage induced by external NLRP3 agonists is caspase-1 independent.
Parkin is required for p62 mitochondrial recruitment
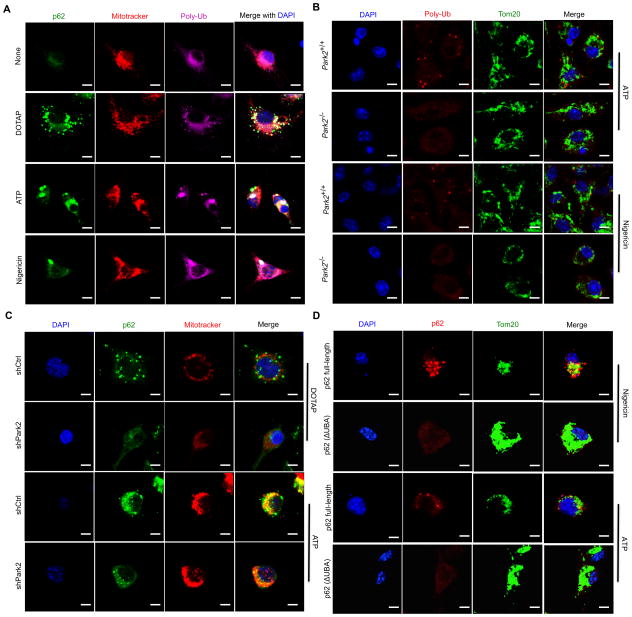
p62 binds damaged mitochondria through its UBA domain (Komatsu et al., 2012). Macrophage treatment with NLRP3-inflammasome agonists induced formation of poly-ubiquitin (poly-Ub) aggregates, most of which colocalized with p62 puncta on mitochondria (Figures 3A and S3A). Induction of mitochondrial poly-Ub is likely to depend on the E3 ligase Parkin (Lazarou et al., 2015). Indeed, no mitochondrial poly-Ub was detected in Park2−/− (Park2 encodes Parkin) macrophages, which were also defective in mitochondrial p62 recruitment (Figures 3B,C and S3B,C). We examined whether ectopically expressed p62 is also recruited to mitochondria upon macrophage stimulation. Whereas full-length WT p62 underwent mitochondrial recruitment after stimulation with NLRP3 agonists, the UBA-deficient construct, p62(ΔUBA), did not (Figures 3D and S3D). Thus, mitochondrial recruitment of p62 after exposure to NLRP3 agonists requires Parkin-dependent decoration of damaged mitochondria with poly-Ub chains that are recognized by the p62 UBA domain.
Figure 3. Mitochondrial p62 recruitment is Parkin dependent.
(A) Intracellular distribution of p62, polyubiquitin (poly-Ub) and mitochondria (Mitotracker) in LPS-primed WT BMDM stimulated with NLRP3 agonists determined by confocal microscopy. Scale bars: 5 μm. (B) Mitochondrial poly-Ub decoration examined by confocal microscopy in LPS-primed Park2+/+ or Park2−/− BMDM stimulated with NLRP3 agonists. Scale bars: 5 μm. (C) Mitochondrial recruitment of p62 in LPS-primed WT (shCtrl) or Parkin-deficient (shPark2) iBMDM stimulated with NLRP3 agonists. Scale bars: 5 μm. (D) Subcellular distribution of human p62 in LPS-primed, NLRP3 agonist stimulated p62-deficient BMDMs transduced with human p62-full length or p62(ΔUBA) constructs. Scale bars: 5 μm.
Parkin restricts buildup of damaged mitochondria
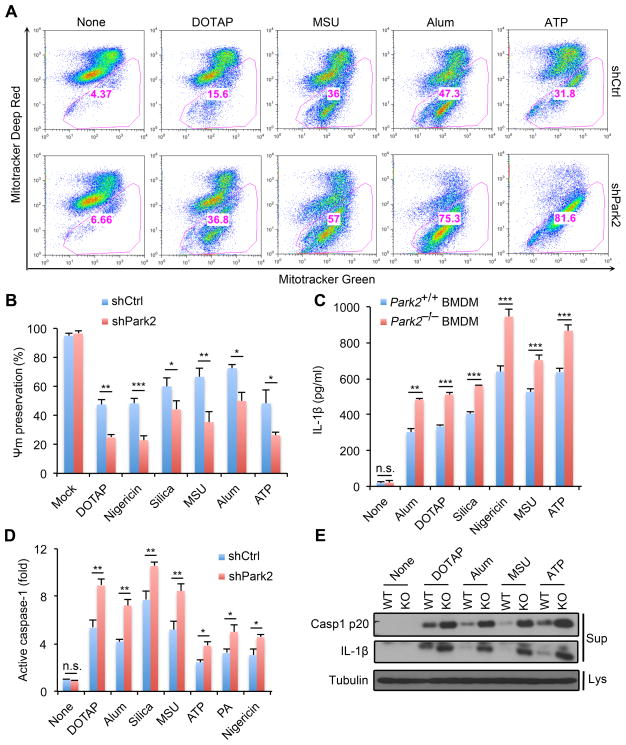
By decorating damaged mitochondria with poly-Ub chains, Parkin targets them to mitophagic clearance (Eiyama and Okamoto, 2015). Accordingly, Parkin-deficient macrophages exhibited enhanced accumulation of damaged mitochondria after treatment with NLRP3 agonists (Figure 4A). The Parkin deficiency also enhanced Ψm loss upon stimulation with NLRP3 agonists, and augmented mtDNA release and mtROS production (Figures 4B and S4A,B). Although the Parkin deficiency did not affect NLRP3, ASC, pro-caspase-1 or pro-IL-1β expression (Figure S4C), it enhanced IL-1β release upon stimulation with inflammasome activators, without affecting TNF secretion (Figures 4C and S4D). NLRP3 agonist-induced caspase-1 activation was also elevated in Parkin-deficient macrophages (Figure 4D,E). Thus, just like p62, Parkin limits accumulation of damaged mitochondria in macrophages stimulated with NLRP3 inflammasome activators and thereby attenuates caspase-1 activation and IL-1β secretion.
Figure 4. Parkin limits accumulation of damaged mitochondria after inflammasome activation.
(A) Flow cytometry of mitochondrial status in WT (shCtrl) or Parkin-deficient (shPark2) iBMDM stimulated with NLRP3 agonists. Gates represent cells with damaged mitochondria. (B) NLRP3 agonist-induced changes in mitochondrial membrane potential (Ψm) in LPS-primed WT (shCtrl) or p62-deficient (shp62) iBMDM measured by TMRM fluorescence. Data are averages ± s.d. (n=3). (C) IL-1β release by LPS-primed Park2+/+ or Park2−/− BMDM stimulated with NLRP3 agonists. Data are averages ± s.d. (n=3). (D) Caspase-1 activity in LPS-primed WT (shCtrl) or Parkin-deficient (shPark2) iBMDM stimulated with NLRP3 agonists. Results are averages ± s.d. (n=3). (E) IB analysis of cleaved caspase-1 and IL-1β secretion to cell culture supernatants (Sup) or tubulin in cell lysates (Lys) in LPS-primed Park2+/+ or Park2−/− BMDM stimulated with NLRP3 agonists. Data are representative of 3 independent experiments. *, p<0.05; **, p<0.01; ***, p<0.001.
ATG7 is needed for clearance of p62-bound mitochondria
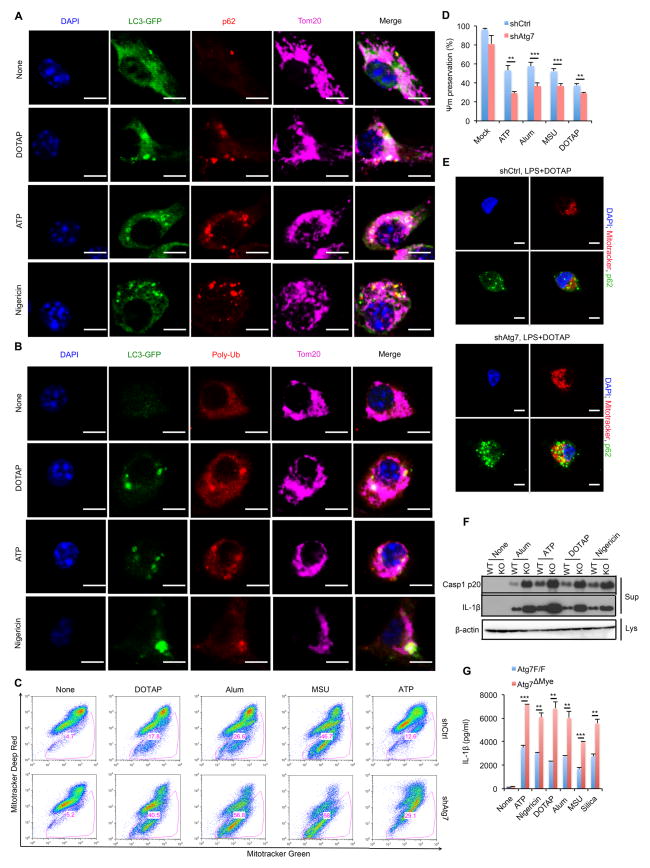
To better understand the role of the autophagic machinery in clearing damaged mitochondria, we conducted additional imaging experiments. LC3 puncta, which indicate autophagosome formation, colocalized with mitochondria, p62, and poly-Ub after NLRP3 inflammasome activation (Figures 5A,B and S5A,B). ATG7 deletion had no pronounced effect on NLRP3, ASC, pro-caspase-1 or pro-IL-1β in LPS-stimulated macrophages (Figure S5C), although it enhanced accumulation of damaged mitochondria after stimulation with NLRP3 agonists, increased mitochondrial depolarization and augmented mtROS production and mtDNA release (Figures 5C,D and S5D,E). As expected, ATG7 deficiency enhanced p62 accumulation (Figure S5C) and more p62 was recruited to mitochondria in stimulated cells (Figure 5E), but these damaged mitochondria were not cleared (Figure 5C), leading to elevated caspase-1 activity and IL-1β secretion (Figures 5F,G and S5F,G) without affecting TNF release (Figure S5H,I). In contrast to a previous study (Shi et al., 2012), ATG7 deletion had no significant effect on AIM2-inflammasome activation (Figure S5J). This discrepancy is likely due to the previous reliance on autophagy inhibitors (Shi et al., 2012) that probably have off-target effects. Activation of the NLRP1b-inflammasome was also unaffected by ATG7 ablation (Figure S5K). These results suggest that autophagy specifically inhibits activation of the NLRP3-inflammasome.
Figure 5. Autophagy mediates clearance of p62-bound mitochondria.
(A, B) Intracellular distribution of LC3, p62, poly-Ub and mitochondria in LPS-primed LC3-GFP BMDM stimulated with NLRP3 agonists examined by confocal microscopy. (C) Flow cytometric analysis of mitochondrial status in LPS-primed WT (shCtrl) or Atg7-deficient (shAtg7) iBMDM after NLRP3 agonist stimulation. Gates – cells with damaged mitochondria. (D) NLRP3 agonist-induced changes in mitochondrial membrane potential (Ψm) in LPS-primed WT (shCtrl) or Atg7-deficient (shAtg7) iBMDM. Data are averages ± s.d. (n=3). (E) Intracellular distribution of p62 and mitochondria in LPS-primed shCtrl or shAtg7 iBMDM stimulated with NLRP3 agonists. (F) IB analysis of caspase-1 p20 and mature IL-1β in culture supernatants (Sup) and β-actin in cell lysates (Lys) of LPS-primed Atg7F/F (WT) and Atg7ΔMye (KO) BMDM stimulated as indicated. Data are representative of three independent experiments. (G) IL-1β secretion by above cells measured by ELISA. Results are averages ± s.d. (n=3). *, p<0.05; **, p<0.01; ***, p<0.001.
Eliminating mitochondrial signals attenuates IL-1β release
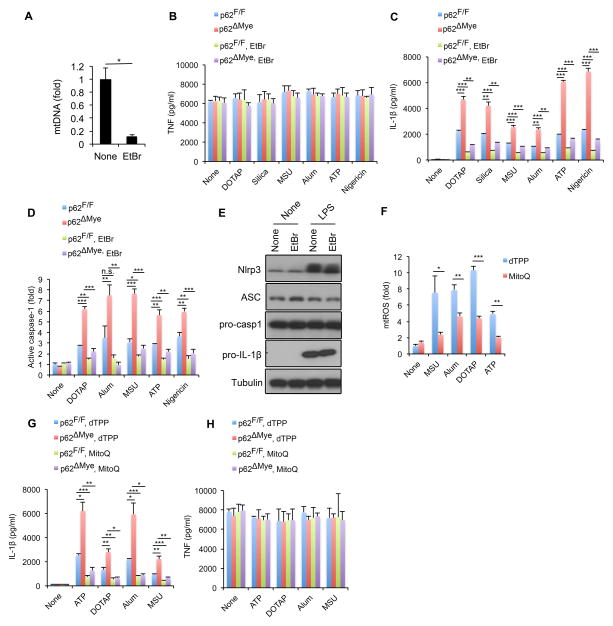
Damaged mitochondria release or display signals, such as mtROS, mtDNA and cardiolipin, that promote NLRP3-inflammasome activation (Elliott and Sutterwala, 2015). We reasoned that elimination of these signals may attenuate IL-1β release from p62-deficient macrophages. To test this hypothesis, we treated WT or p62-deficient macrophages with low dose ethidium bromide (EtBr), which depletes mtDNA and blocks mtROS production (Nakahira et al., 2011). Although not affecting TNF production, EtBr treatment, which reduced mtDNA by approximately 90%, attenuated excessive IL-1β release by p62-deficient macrophages stimulated with inflammasome activators and inhibited caspase-1 activation (Figure 6A–D). EtBr treatment had no effect on expression of pro-IL-1β or inflammasome components (Figure 6E). Similar results were obtained by treating macrophages with chloramphenicol (Figure S6A,B), an antibiotic that targets mitochondrial protein synthesis, thus depleting mitochondria-derived inflammasome-activating signals (Shimada et al., 2012). We also tested whether sequestering mtROS would reverse the effect of p62 deficiency. Treatment of macrophages with the mitochondrial-specific antioxidant Mito-Q (Chernyak et al., 2006) partially reduced NLRP3 agonist-induced mtROS production and release of IL-1β without affecting TNF release (Figure 6F–H). These results collectively indicate that eliminating mitochondria-derived signals attenuates IL-1β release in p62ΔMye macrophages.
Figure 6. Elimination of mitochondrial signals prevents excessive IL-1β production by p62-deficient macrophages.
(A) Relative concentrations of mtDNA in p62F/F and p62ΔMye BMDM before and after 4 days of ethidium bromide (EtBr) treatment. Results are averages ± s.d. (n=3). (B–D) TNF (B) IL-1β (C) release and caspase-1 activity (D) in p62F/F and p62ΔMye BMDM pre-treated with EtBr or not that were co-stimulated with LPS and different NLRP3 agonists. Results are averages ± s.d. (n=3). (E) IB analysis of pro-IL-1β, NLRP3, ASC, and pro-caspase-1 in lysates of EtBr-pretreated WT BMDM before and after 4 hrs of LPS stimulation. Data are representative of three independent experiments. (F) Relative mtROS amounts determined by MitoSOX staining of LPS-primed WT BMDM stimulated with NLRP3 agonists in the presence of dTPP or Mito-Q. (G,H) Release of IL-1β (F) and TNF (G) from LPS-primed p62F/F and p62ΔMye BMDM; treated with Mito-Q or vehicle (dTPP) before addition of NLRP3 agonists. Results are averages ± s.d. (n=3).
Although the p62-autophagy pathway was suggested to attenuate inflammasome activation by targeting inflammasome components to autophagic degradation (Shi et al., 2012), we did not observe significant elevation in amounts of inflammasome components in p62ΔMye or Atg7ΔMye macrophages relative to WT cells after stimulation with ATP (Figure S6C,D). We reason that the “NLRP3-ASC-pro-caspase-1” complex cannot be effectively degraded in lysosomes because inflammasome aggregates are highly resistant to lysosomal degradation and can be secreted out of the cell (Baroja-Mazo et al., 2014; Franklin et al., 2014). Enhanced inflammasome secretion may account for the decrease in NLRP3 after a 2 hr stimulation of p62ΔMye or Atg7ΔMye macrophages with LPS + ATP.
As our results suggest that the NF-κB-p62-mitophagy pathway acts upstream to caspase-1 and restricts its activation, we reasoned that genetic activation of NLRP3 in the absence of any external NLRP3 agonists should lead to caspase-1 activation that cannot be inhibited by this pathway. Indeed, treatment of BMDM that expressed genetically activated NLRP3(A350V) with ML120b, which blocks p62 induction, did not further enhance caspase-1 activity (Figure S6E). Similarly, knocking down of p62 had no effect on IL-1β secretion by LPS-treated NLRP3(A350V) BMDM (Figure S6F) although it enhanced nigericin-induced IL-1β release from WT BMDM (Figure 6SG). These results indicate that the genetically activated NLRP3-inflammasome is refractory to the “NF-κB-p62-mitophagy” negative regulatory loop.
p62 attenuates sterile inflammation and fulminant hepatitis
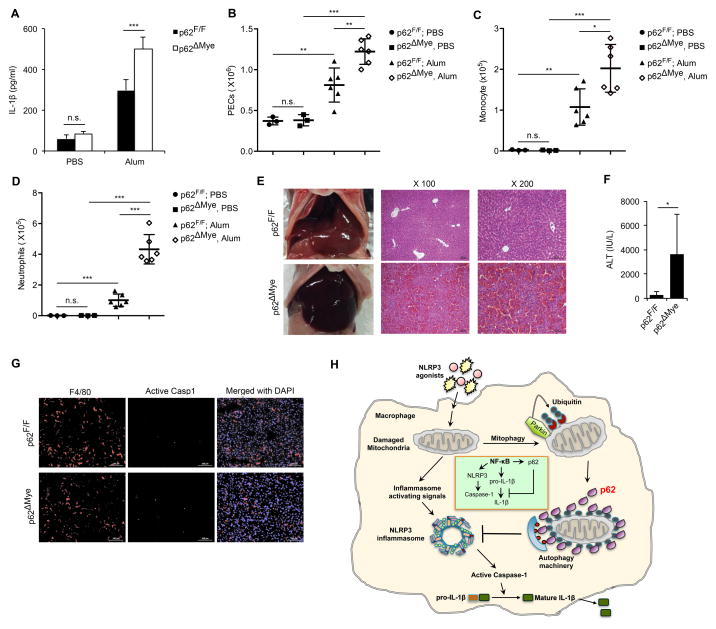
To further examine the physiological function of p62, we used two mouse models of NLRP3-inflammasome dependent inflammation (Eisenbarth et al., 2008; Kim and Lee, 2013). We employed alum-induced peritonitis, in which alum was i.p. injected into WT or p62ΔMye mice. More peritoneal IL-1β was induced in p62ΔMye mice, which exhibited elevated neutrophil and monocyte infiltration relative to WT counterparts (Figure 7A–D). Similar results were seen in global p62 KO mice (Figure S7A–D), confirming that global p62 ablation has a similar phenotype to the macrophage-specific deletion in respect to control of NLRP3-inflammasome activity. In a second model, we induced fulminant hepatitis with LPS plus D-galactosamine. p62ΔMye mice displayed enhanced liver damage and elevated caspase-1 activation in liver macrophages relative to WT mice (Figure 7E–G). Notably, as p62 ablation in neutrophils did not affect IL-1β secretion induced by NLRP3 agonists (Figure S7E), the phenotypes seen in p62ΔMye mice are due to p62 deletion in macrophages.
Figure 7. p62 inhibits inflammasome-dependent sterile inflammation.
(A) Peritoneal IL-1β in p62F/F or p62ΔMye mice 4 hrs after i.p. injection of alum or PBS. n=3–6. (B–D) Alum-induced peritoneal infiltration of PEC (B), monocytes (CD11b+Ly6C+Ly6G−) (C) and neutrophils (CD11b+Ly6G+F4/80−) (D) in p62F/F and p62ΔMye mice 16 hrs after alum or PBS injection. (E) Representative liver appearance and histology of p62F/F or p62ΔMye mice i.p. injected with LPS plus D-galactosamine. (F) Serum ALT in above mice. (G) Fluorescent staining of F4/80, active caspase-1, and DAPI in livers of p62F/F and p62ΔMye mice after LPS+ D-gal challenge. *, p<0.05; **, p<0.01; ***, p<0.001. (H) Schematic representation of key findings. NF-κB induces expression of p62, which negatively regulates caspase-1 activation via mitophagic elimination of mitochondria that release NLRP3-inflammasome activating signals.
We generated Atg7ΔMye mice, which, similar to p62ΔMye mice, exhibited enhanced IL-1β production and decreased survival after i.p. injection of LPS (Figure S7F–H). Thus, ATG7 also has a protective role in acute septic inflammation. Moreover, upon alum challenge, Atg7ΔMye mice displayed enhanced IL-1β production and neutrophil/monocyte recruitment relative to WT mice (Figure S7I–L). Similarly, Atg7ΔMye mice exhibited elevated caspase-1 activation in liver macrophages and enhanced liver damage relative to WT counterparts after LPS + D-galactosamine challenge (Figure S7M–O). These results confirm that autophagy limits excessive inflammation by restraining NLRP3-inflammasome activation.
Discussion
This study identifies p62 as the missing link that allows NF-κB to inhibit NLRP3-inflammasome activation. Our results demonstrate that the “NF-κB-p62-mitophagy” pathway represents a key regulatory loop through which NF-κB orchestrates NLRP3-inflammasome activation and cytokine release, thereby focusing macrophage-mediated immunity on eliminating infectious agents while preventing long-lasting tissue inflammation and damage due to excessive IL-1β secretion and inflammatory macrophage death (Figure 7H). Moreover, our results reaffirm the importance of mitochondrial damage in control of NLRP3-inflammasome activation.
In addition to being the primary transcriptional activator of inflammatory genes (Barnes and Karin, 1997), NF-κB is responsible for keeping NLRP3-inflammasome activation and IL-1β production in check (Greten et al., 2007). Although the mechanistic basis for this unexpected IKKβ/NF-κB function was heretofore obscure, the findings have turned out to be clinically relevant as excessive inflammation was observed in human volunteers that were given highly specific and potent IKKβ inhibitors. Unfortunately, this precipitated the termination of further clinical development of this group of NF-κB antagonists. In addition to excessive IL-1 production, myeloid-specific IKKβ ablation results in IL-1-dependent neutrophilia and tissue damage (Hsu et al., 2011). By examining the ability of autophagy receptors to link NF-κB signaling to inhibition of inflammasome activation, we found that only p62, but not other receptors, was upregulated upon IKKβ/NF-κB activation. Furthermore, p62 induction was found to account for the inflammasome inhibitory function of NF-κB, as it promotes the mitophagic clearance of damaged mitochondria that emit NLRP3-inflammasome activating signals (Figure 7H). These signals, which are induced by a bewildering collection of unrelated stimuli, include mtROS, mtDNA and cardiolipin (Elliott and Sutterwala, 2015). Yet, generation of these more direct NLRP3 activators does not require NLRP3 itself, its association with ASC, or caspase-1 activation. Furthermore, in p62-deficient macrophages IKKβ inhibition no longer enhances caspase-1 activation. Notably, the kinetics of p62 accumulation in LPS-stimulated macrophages lag behind those of NLRP3 and pro-IL-1β, further supporting the notion that p62 induction is only involved in elimination of direct inflammasome activating signals and does not affect NLRP3-inflammasome assembly (Lu et al., 2014) or expression of inflammasome components. As inflammasome activation may be a digital, all-or-none, process (Liu et al., 2014), the “NF-κB-p62-mitophagy” pathway may only control the number of macrophages in which the NLRP3-inflammasome triggers caspase-1 activation.
p62 is a multifunctional protein that is expressed at low amounts by most healthy cells and tissues. Protein aggregates containing p62, however, referred to as Mallory-Denke bodies or hyaline granules, accumulate under stress, and are a characteristic pathological feature of chronic liver inflammation and cancer (Stumptner et al., 2007). p62-containing aggregates also accumulate in chronic pancreatitis, which is attenuated upon p62 ablation in pancreatic epithelial cells (Li et al., 2013). Whole body p62 ablation also attenuates liver inflammation and damage caused by disruption of autophagy (Komatsu et al., 2007), but it is not clear in which cells p62 exerts its primary pro-inflammatory effects. Disruption of autophagic protein degradation results in accumulation of p62, which can activate NF-κB via its TRAF6 binding motif (Moscat and Diaz-Meco, 2009). However, p62 may also inhibit TRAF6 signaling (Kim and Ozato, 2009) and promote caspase-3 activation and cell death (Komatsu et al., 2012). Given the involvement of p62 in pancreatitis and liver inflammation, the likely pathogenic role of macrophages in these diseases, which could be mediated through either TNF or IL-1β, and the conflicting reports about the effect of p62 on TRAF6 signaling, we directly investigated the function of myeloid p62 in acute LPS-induced inflammation. LPS from enteric bacteria, reaching the liver and pancreas through the portal circulation, is thought to play important roles in the pathogenesis of chronic hepatitis, liver fibrosis, liver cancer and pancreatitis (Henao-Mejia et al., 2012; Seki and Schwabe, 2015; Vonlaufen et al., 2007). To our surprise, myeloid-specific p62 deletion strongly enhanced LPS-induced inflammation and mortality due to unrestrained inflammasome activation, without an effect on pro-IL-1β or NLRP3-inflammasome component expression. Collectively, these findings reinforce the notion that p62 is a multifunctional protein with distinct cell-type and context-specific roles.
Damaged mitochondria are recognized by the E3 ubiquitin ligase Parkin, which decorates their outer membrane proteins with poly-Ub chains (Eiyama and Okamoto, 2015). p62 binds poly-Ub chains through its UBA domain and can direct protein aggregates and organelles bearing such modifications to autophagic clearance (Komatsu et al., 2012). However, the mitophagic function of p62 has been questioned, because in certain cells it only modulated the subcellular distribution of mitochondria that were damaged by treatment with uncouplers of oxidative phosphorylation and its ablation had no effect on their autophagic clearance (Lazarou et al., 2015; Narendra et al., 2010; Okatsu et al., 2010). By contrast, our results clearly demonstrate that in macrophages, p62 is an essential mediator of mitophagic elimination of mitochondria that were damaged by treatment with NLRP3 agonists. Since LPS-induced macrophage activation is associated with a marked and selective increase in p62 expression, it is plausible that only in cells that express much more p62 than other mitophagy receptors, p62 becomes the rate-limiting mediator of mitophagy. Alternatively, mitochondrial uncouplers (e.g. CCCP), which are commonly used to study mitophagy, may induce a different pattern of mitochondrial outer membrane protein ubiquitination than the one induced by classical NLRP3 agonists. Such differences in the composition or distribution of ubiquitin chains may be sensed by specific autophagy receptors, such that only p62 recognizes mitochondria that have been damaged with NLRP3 agonists. Although production of catalytically active caspase-1, induced by canonical NLRP3 agonists (e.g. ATP), was proposed to enhance mitochondrial damage (Yu et al., 2014), we found that NLRP3 agonists induced mitochondrial damage independently of NLRP3-inflammasome components, including caspase-1. Furthermore, mutational activation of NLRP3 did not induce any detectable mitochondrial damage, although it activated caspase-1. Thus, mitochondrial damage occurs upstream to NLRP3 and independently of it, and its removal through mitophagy prevents NLRP3-dependent inflammasome activation. Most likely, different experimental conditions account for the difference between our results and those of Yu et al., and when macrophages are incubated with suboptimal amounts of NLRP3 agonists, caspase-1 activation may be needed to enhance mitochondrial damage at later time points. However, recent studies suggest that inflammasome-mediated caspase-1 activation is an all-or-none process, further ruling out the operation of a caspase-1-dependent positive feedback loop. Of note, little or no mitochondrial damage was observed in macrophages that express the constitutively active NLRP3(A350V) protein, even though these cells secreted copious amounts of IL-1β.
p62 was originally identified as a protein kinase Cζ binding protein that promotes NF-κB activation through its TRAF binding motif, thereby enhancing conventional inflammatory responses mediated by IL-1 or TNF (Moscat and Diaz-Meco, 2009). Since NF-κB stimulates p62 gene transcription, p62 can be part of a feed-forward loop that amplifies NF-κB-dependent inflammation. However, our results indicate just the opposite – p62 is responsible for preventing excessive inflammasome activation. Since unrestricted caspase-1 activation results in macrophage death in addition to IL-1β and IL-18 release, the absence of proper safety loops, such as the one mediated by NF-κB and p62, can lead to macrophage loss and uncontrolled neutrophil accumulation. This highly dangerous condition has been detected in mice lacking either IKKβ (Hsu et al., 2011) or p62 in myeloid cells. However, it should be noted that the mere upregulation of p62 does not inhibit NLRP3 inflammasome activation; ATG7-deficient macrophages accumulate large amounts of p62, but in the absence of the autophagic machinery p62 cannot restrain inflammasome activation.
While being responsive to various foreign toxins and irritants, the NLRP3-inflammasome is activated by DAMPs, such as ATP and uric acid, natural cellular constituents that are released upon sterile tissue damage. Under such conditions, the antimicrobial function of IL-1β may not be essential and the goal of the sterile inflammatory response is to stimulate regenerative processes. DAMPs and other NLRP3 agonists elicit mitochondrial damage and thereby trigger production of NLRP3-inflammasome-activating signals which are removed through p62-mediated mitophagy, whose major goal is to dampen inflammation caused by tissue damage and initiate regeneration. Thus, NF-κB activation, which increases transcription of genes encoding essential inflammatory mediators, also ensures that the beneficial inflammatory response will not be excessive, thus avoiding chronic inflammatory disease and parainflammation caused by cell and tissue stress (Medzhitov, 2008). Self-limiting inflammation is also important for initiating regeneration, which restores integrity of epithelial barriers and thereby attenuates PAMP and DAMP availability (Taniguchi et al., 2015). Notably, Parkin-mediated mitophagy, in which p62 participates, is utilized by all metazoan phagocytes, from fly to human, to eliminate ingested bacteria (Manzanillo et al., 2013). Thus, the “NF-κB-p62”-dependent anti-inflammatory module described above could be an evolutionary relic of the xenophagy pathway, which also limits PAMP availability. Mitochondria have likely originated from prokaryotic intracellular parasites that have adapted to their eukaryotic hosts, which tolerate them as long as they do not pose danger or are altered in such a way that makes them perceived as “non-self”. Failed xenophagy increases microbial load, which is likely to be dealt with through IL-1β/IL-18-promoted innate immune responses.
Dysregulation of the NLRP3-inflammasome is frequently associated with diverse inflammatory, metabolic and malignant diseases, including gouty arthritis, Alzheimer’s disease, obesity, type II diabetes and colorectal cancer (Lamkanfi and Dixit, 2012). Therefore, proper control of NLRP3-inflammasome activity is critical for preventing disease development. Mutations in genes that encode components of the Parkin-dependent mitophagy pathway were detected in patients with Parkinson disease (Pickrell and Youle, 2015). Although Parkinsonism entails age-related degeneration of dopaminergic neurons, it is plausible that excessive inflammasome-mediated IL-1β production by microglia may exacerbate neuronal cell death and inflammation. Another age-related disease linked to the NLRP3-inflammasome is macular degeneration (Tarallo et al., 2012). In fact, many degenerative diseases may be associated with accumulation of damaged and defective mitochondria, which can be the major cause of parainflammation (Medzhitov, 2008; Wallace, 2005). The aging process itself is linked to elevated low-grade inflammation or parainflammation, characterized by constitutive production of low amounts of IL-1β, TNF and IL-6 (Chung et al., 2009; Licastro et al., 2005). We suggest that the NLRP3-inflammasome activation and insufficient mitophagy link accumulation of damaged mitochondria to the para-inflammatory state discussed above. Normal, healthy aging may be compromised and greatly enhanced by accumulation of somatically mutated mitochondria (Wallace, 2005), which are more likely to release mtROS and fragmented mtDNA that act as direct NLRP3-inflammasome activators. Enhanced caloric intake may further accelerate this process through mTORC1-dependent attenuation of autophagy.
Experimental procedures
Please see online Supplementary Information for detailed experimental procedures.
Mice
LysM-Cre mice were from Jackson Laboratories and crossed with p62F/F and Atg7F/F mice (Komatsu et al., 2005; Muller et al., 2013) to generate p62ΔMye and Atg7ΔMye mice, respectively. p62−/− mice were previously described (Rodriguez et al., 2006). Nlrp3A350V/+CreT mice were as previously described (Brydges et al., 2013).
Cell culture and stimulation
Primary bone marrow-derived macrophages (BMDM) were generated as described (Hornung et al., 2008). For stimulation, BMDM were LPS-primed and then stimulated with NLRP3 agonists, AIM2 agonist poly(dA:dT) or NLRP1b agonist B. anthracis. Macrophages with a constitutively active mutant NLRP3 were generated by treating BMDM from Nlrp3A350V/+CreT mice with 1 μM 4-hydroxytamoxifen.
Enzyme-linked immunosorbent assay
Paired antibodies and standard recombinant mouse IL-1β (R&D Systems) and TNF (eBioscience) were used to determine cytokines concentrations according to manufacturer’s instructions.
Measurement of active caspase-1 by FLICA assay
The levels of active caspase-1 were quantified using a fluorescent probe (FLICA™ FAM-YVAD-FMK, ImmunoChemistry Technologies) that specifically recognized active caspase-1. Fluorescence intensity was measured with a FilterMax F5 multimode plate reader (Molecular Devices).
Measurements of Mitochondrial membrane potential and ROS
Mitochondrial membrane potential (Ψm) was measured using TMRM as previously described(Shimada et al., 2012). Mitochondrial ROS were measured using MitoSOX (Life Technologies) following the manufacturer’s instructions.
Immunofluorescent staining and confocal microscopy
BMDM were primed with LPS, and then treated with NLRP3 agonists. Cells were then fixed, permeabilized and blocked. After incubating overnight with primary antibodies, secondary fluorescent antibodies (Alexa-488, -594 or -647, from Life Technologies or Jackson Laboratories) were added and DAPI was used for nuclear counterstaining. Samples were imaged through a SP5 confocal microscope (Leica).
Statistics
All data are shown as means ± s.d. or means ± s.e.m. as indicated. Statistical analysis was performed using a two-tailed Student’s t-test or log-rank test. For all tests, P-values lower than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank Karin lab members for helpful discussions, and eBioscience, Cell Signaling Technologies, Santa Cruz Technologies, and Life Technologies for gifts of antibodies and other reagents. Z.Z. was supported by Cancer Research Institute (CRI) Irvington postdoctoral fellowship; S.S. was supported by fellowships from CRI-Irvington and German Research Foundation (SH721/1-1); J.W. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research. Research was supported by grants from the NIH (AI043477 and CA163798) to M.K., (AA020172 and DK085252) to E.S., (ES010337) to M.K. and E.S., (HL087023) to A.B.G., (AI52430) to H.M.H., and (CA132847, CA172025) to J.M., Leukemia and Lymphoma Society SCOR (20132569) to Tom Kipps and M.K. and the Alliance for Lupus Research (257214) to M.K., who is an American Cancer Research Professor and holds the Ben and Wanda Hildyard Chair for Mitochondrial and Metabolic Diseases.
Footnotes
Author Contributions
Z.Z. and M.K. conceived the project. Z.Z. designed the study and performed most of the experiments. A.U., S.L. and E.S-L., J.W. and F.H. performed immunoblot analysis. Z.Z. and E.S-L. performed confocal microscopy analysis. Z.Z., D.B, and G.P. performed EM analysis. Z.Z. and J.W. performed p62 reconstitution experiments. S.A. performed B. anthracis infection experiment. Z.Z. and S.L. conducted septic shock experiments. A.U. performed fulminant hepatitis experiments. Z.Z. and S.S. did the peritonitis experiments with assistance from A.U.. A.G. provided Park2−/− bone marrow cells. E.S. provided Atg7ΔMye mice. M.H.E. provided analytical tools and suggestions. M.M, and H.H. provided Nlrp3A350V/+CreT mice and suggestions. M. T. D-M and J.M. provided p62F/F mice and p62 constructs. Z.Z. and M.K. wrote the manuscript with inputs from all authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A, Martin-Sanchez F, Gomez AI, Martinez CM, Amores-Iniesta J, Compan V, Barbera-Cremades M, Yague J, Ruiz-Ortiz E, Anton J, et al. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- Brenner C, Galluzzi L, Kepp O, Kroemer G. Decoding cell death signals in liver inflammation. J Hepatol. 2013;59:583–594. doi: 10.1016/j.jhep.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyak BV, Izyumov DS, Lyamzaev KG, Pashkovskaya AA, Pletjushkina OY, Antonenko YN, Sakharov DV, Wirtz KW, Skulachev VP. Production of reactive oxygen species in mitochondria of HeLa cells under oxidative stress. Biochim Biophys Acta. 2006;1757:525–534. doi: 10.1016/j.bbabio.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criollo A, Senovilla L, Authier H, Maiuri MC, Morselli E, Vitale I, Kepp O, Tasdemir E, Galluzzi L, Shen S, et al. The IKK complex contributes to the induction of autophagy. EMBO J. 2010;29:619–631. doi: 10.1038/emboj.2009.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiyama A, Okamoto K. PINK1/Parkin-mediated mitophagy in mammalian cells. Curr Opin Cell Biol. 2015;33:95–101. doi: 10.1016/j.ceb.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Elliott EI, Sutterwala FS. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, Brenker C, Nordhoff M, Mirandola SR, Al-Amoudi A, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nat Immunol. 2014;15:727–737. doi: 10.1038/ni.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, Goktuna SI, Neuenhahn M, Fierer J, Paxian S, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130:918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Enzler T, Seita J, Timmer AM, Lee CY, Lai TY, Yu GY, Lai LC, Temkin V, Sinzig U, et al. IL-1beta-driven neutrophilia preserves antibacterial defense in the absence of the kinase IKKbeta. Nat Immunol. 2011;12:144–150. doi: 10.1038/ni.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Ozato K. The sequestosome 1/p62 attenuates cytokine gene expression in activated macrophages by inhibiting IFN regulatory factor 8 and TNF receptor-associated factor 6/NF-kappaB activity. J Immunol. 2009;182:2131–2140. doi: 10.4049/jimmunol.0802755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee SM. NLRP3 inflammasome activation in D-galactosamine and lipopolysaccharide-induced acute liver failure: role of heme oxygenase-1. Free Radic Biol Med. 2013;65:997–1004. doi: 10.1016/j.freeradbiomed.2013.08.178. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kageyama S, Ichimura Y. p62/SQSTM1/A170: physiology and pathology. Pharmacol Res. 2012;66:457–462. doi: 10.1016/j.phrs.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al. Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell. 2007;131:1149–1163. doi: 10.1016/j.cell.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Ueno T, Iwata J, Murata S, Tanida I, Ezaki J, Mizushima N, Ohsumi Y, Uchiyama Y, et al. Impairment of starvation-induced and constitutive autophagy in Atg7-deficient mice. J Cell Biol. 2005;169:425–434. doi: 10.1083/jcb.200412022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotas ME, Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Inflammasomes and their roles in health and disease. Annu Rev Cell Dev Biol. 2012;28:137–161. doi: 10.1146/annurev-cellbio-101011-155745. [DOI] [PubMed] [Google Scholar]
- Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Sliter DA, Kane LA, Sarraf SA, Wang C, Burman JL, Sideris DP, Fogel AI, Youle RJ. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524:309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Wu X, Holzer RG, Lee JH, Todoric J, Park EJ, Ogata H, Gukovskaya AS, Gukovsky I, Pizzo DP, et al. Loss of acinar cell IKKalpha triggers spontaneous pancreatitis in mice. J Clin Invest. 2013;123:2231–2243. doi: 10.1172/JCI64498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licastro F, Candore G, Lio D, Porcellini E, Colonna-Romano G, Franceschi C, Caruso C. Innate immunity and inflammation in ageing: a key for understanding age-related diseases. Immun Ageing. 2005;2:8. doi: 10.1186/1742-4933-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Yamaguchi Y, Shirasaki Y, Shikada K, Yamagishi M, Hoshino K, Kaisho T, Takemoto K, Suzuki T, Kuranaga E, et al. Single-cell imaging of caspase-1 dynamics reveals an all-or-none inflammasome signaling response. Cell Rep. 2014;8:974–982. doi: 10.1016/j.celrep.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schroder GF, Fitzgerald KA, Wu H, Egelman EH. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanillo PS, Ayres JS, Watson RO, Collins AC, Souza G, Rae CS, Schneider DS, Nakamura K, Shiloh MU, Cox JS. The ubiquitin ligase parkin mediates resistance to intracellular pathogens. Nature. 2013;501:512–516. doi: 10.1038/nature12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–1004. doi: 10.1016/j.cell.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Lee SJ, Jastroch M, Kabra D, Stemmer K, Aichler M, Abplanalp B, Ananthakrishnan G, Bhardwaj N, Collins S, et al. p62 links beta-adrenergic input to mitochondrial function and thermogenesis. J Clin Invest. 2013;123:469–478. doi: 10.1172/JCI64209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy MF, Diez-Guerra FJ, Flores JM, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signaling adapter p62. Cell Metab. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Shenderov K, Huang NN, Kabat J, Abu-Asab M, Fitzgerald KA, Sher A, Kehrl JH. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat Immunol. 2012;13:255–263. doi: 10.1038/ni.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumptner C, Fuchsbichler A, Zatloukal K, Denk H. In vitro production of Mallory bodies and intracellular hyaline bodies: the central role of sequestosome 1/p62. Hepatology. 2007;46:851–860. doi: 10.1002/hep.21744. [DOI] [PubMed] [Google Scholar]
- Taniguchi K, Wu LW, Grivennikov SI, de Jong PR, Lian I, Yu FX, Wang K, Ho SB, Boland BS, Chang JT, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarallo V, Hirano Y, Gelfand BD, Dridi S, Kerur N, Kim Y, Cho WG, Kaneko H, Fowler BJ, Bogdanovich S, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–859. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- Vance RE. The NAIP/NLRC4 inflammasomes. Curr Opin Immunol. 2015;32:84–89. doi: 10.1016/j.coi.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Moltke J, Ayres JS, Kofoed EM, Chavarria-Smith J, Vance RE. Recognition of bacteria by inflammasomes. Annu Rev Immunol. 2013;31:73–106. doi: 10.1146/annurev-immunol-032712-095944. [DOI] [PubMed] [Google Scholar]
- Vonlaufen A, Xu Z, Daniel B, Kumar RK, Pirola R, Wilson J, Apte MV. Bacterial endotoxin: a trigger factor for alcoholic pancreatitis? Evidence from a novel, physiologically relevant animal model. Gastroenterology. 2007;133:1293–1303. doi: 10.1053/j.gastro.2007.06.062. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T. Inflammasome activation leads to Caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci U S A. 2014;111:15514–15519. doi: 10.1073/pnas.1414859111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Zhai Y, Liang S, Mori Y, Han R, Sutterwala FS, Qiao L. TRPM2 links oxidative stress to NLRP3 inflammasome activation. Nat Commun. 2013;4:1611. doi: 10.1038/ncomms2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.