summary
An important underlying mechanism that contributes to autoimmunity is the loss of inhibitory signaling in the immune system. Sialic acid-recognizing Ig superfamily lectins or Siglecs are a family of cell surface proteins largely expressed in hematopoietic cells. The majority of Siglecs are inhibitory receptors expressed in immune cells that bind to sialic acid containing ligands and recruit SH2-domain containing tyrosine phosphatases to their cytoplasmic tails. They deliver inhibitory signals that can contribute to the constraining of immune cells and thus protect the host from autoimmunity. The inhibitory functions of CD22/Siglec-2 and Siglec-G and their contributions to tolerance and autoimmunity, primarily in the B lymphocyte context, are considered in some detail in this review. The relevance to autoimmunity and unregulated inflammation of modified sialic acids, enzymes that modify sialic acid, and other sialic acid binding proteins are also reviewed.
Keywords: Sialic acids, autoimmunity, Siglecs, CD22, SIAE, CMAH
Introduction
Sialic acids are structurally unique N- or O-substituted derivatives of neuraminic acid, a nine-carbon keto sugar, that in some cells occupy the interface between the host and commensal/pathogenic microorganisms (Fig. 1) (1, 2). The term sialic acid is also loosely used to refer to the most common member of this group found in humans, i.e. N-acetylneuraminic acid (Neu5Ac). In animals, sialic acids are typically attached to the ends of glycan chains. Sialic-acid bearing glycans form a key component of the glycocalyx and are expressed on the cell surface as components of membrane glycoproteins or of a class of glycolipids called gangliosides. They are also highly enriched in mucins secreted by epithelial surfaces. They are especially abundant in vertebrates, and being present on the cell surface, are critically involved in cell-cell interactions and in cell signaling. It has been suggested that their presence also may function as an inhibitory “self-signal” to the vertebrate immune system and in this context, they have been referred to as self-associated molecular patterns, or SAMPs (3). This nomenclature follows the more widely-accepted description of pathogen associated molecular patterns (PAMPs) and damage associated molecular patterns (DAMPs), categories of molecules that can contribute to innate immunity and the induction of adaptive immunity (4). The potential relevance of SAMPs to tolerance and autoimmunity will be discussed later in the review. It has been argued that some microbial pathogens of vertebrates may have acquired the means to display sialic acids on their surface in order to mimic their hosts and thus avoid immune detection (5). These pathogens evade the immune system, either by scavenging host sialic acids or synthesizing them de novo, in some instances by evolving entirely novel pathways in a remarkable case of convergent evolution (6). Occasionally, sialic acids on microbial pathogens may trigger the generation of pathogenic auto-antibodies against host sialic acids (7).
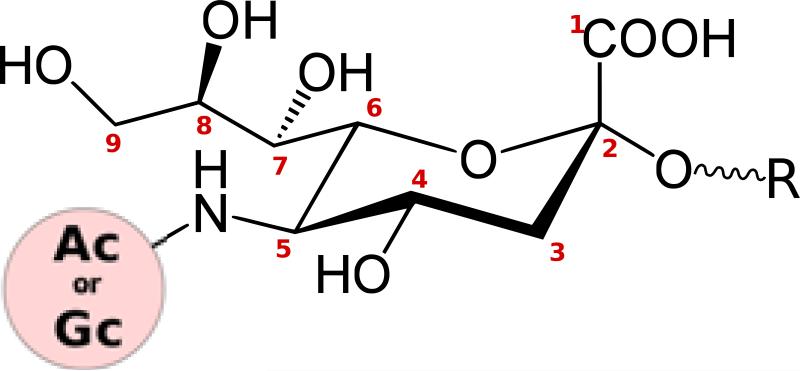
Fig. 1. Structure and diversity of sialic acids.
The core structure of sialic acid comprises a pyranose ring and a glycerol tail. The 5-carbon position can carry an N-Acetyl (Ac) or N-Glycolyl (Gc) group. Several types of O-substitutions have been discovered at carbon positions 4, 7, 8, and 9, giving rise to an enormous diversity of sialic acids. Carbon 2 forms glycosidic linkages with various sugars (R).
Siglecs (sialic acid-recognizing Ig superfamily lectins) are a category of cell surface proteins that bind sialic acids (8-10). Siglecs contain Ig domains with sites that bind sialic acid containing glycans (thereby contributing to the categorization of these proteins as lectins), a transmembrane domain and a cytosolic domain that contains signaling motifs. Siglecs are abundant in vertebrates, are primarily expressed on immune cells, and perform a number of different functions, including cell signaling and adhesion. The cytosolic domains of some Siglecs contain signaling motifs called immunoreceptor tyrosine-based inhibitory motifs (ITIMs), while a few Siglecs contain immunoreceptor tyrosine based activating motifs, commonly referred to as ITAMS. ITIM-containing Siglecs function as inhibitory receptors in the immune system, and their loss of function has been linked to autoimmunity in several mouse models. The most well-studied among these inhibitory Siglecs are CD22 (also called Siglec-2) and Siglec-G. They are expressed primarily on B cells and have non-redundant roles in preventing B cell-mediated autoimmunity. The ligands of CD22 and Siglec-G are also expressed on T cells, and the possible implications of these ligands being available in trans will also be discussed in this review.
Sialic acids have been linked to autoimmunity in the context of the CD22 pathway. The biology of CD22 is complex and this molecule has been referred to on one occasion as an inhibitory enigma (11). The function of this Siglec has been dissected over the past two decades with a variety of genetic knockouts and knockins in both cell lines and mice, the latter in different mouse genetic backgrounds. Although several inconsistencies remain unresolved, a more coherent picture of the mode of action of this Siglec is now emerging (Table 1). The biology of Siglec ligands appears to be far more complex than the Siglecs themselves. Sialic acid containing ligands are subject to various modifications that alter their binding specificities and more than one protein and/or glycoforms may function as ligands for any given Siglec. Indeed, we have a very limited understanding of how the availability of sialic acid ligands in their various forms modulate Siglec function, both in physiological conditions and in disease. An added layer of complexity stems from the fact that, in addition to CD22, there are many Siglecs that also bind sialic acid containing ligands. Mutations that alter the abundance or structure of sialic acids therefore result in wide-ranging and pleiotropic manifestations.
Table 1.
Phenotypes of genetically altered mice with presumed alterations in the CD22 pathway
| B-cell populations | B-cell surface phenotype | Signalling (anti-IgM) | Mouse | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouse strain | Genetic Background | MZ | B1 | Recirc. | sIgM | MHC II | CD22 | Ca2+ | Prolif. | Apop. | Serum IgM | Td | Ti | AutoAb | Reference |
| CD22−/− | mixed 129 × B6 (from 129 ES cells) | ↓ | ↑ | ↓ | ↓ | ↑ | n.a. | ↑ | ↓ | ↑ | ↑ | ← → | ↓ | Yes (1 of 3 lines) | 46, 133, 134 |
| CD22−/− | B6, (from B6 ES cells) | ↓ | ← → | ↓ | ↓ | ↑ | n.a. | ↑ | ← → | ↑ | ↑ (> 5 mo) | ← → | ↓ | No | 30 |
| CD22Δ1-2 | Backcrossed to B6 for 6 generations. | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ← → | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | 55 |
| CD22AA | ↓ | ↑ | ↓ | ↓ | ↑ | ↓ | ← → | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | 55 | |
| CD22a.B6 | B6 (congenic with NZB CD22a allotype) | ← → | n.d. | n.d. | ↓ | ↑ | ↓ | ← → | ↓ | n.d. | n.d. | n.d. | n.d. | Yes (Coombs Ab) | 65 |
| CD22 R130E | B6 | ↓ | ← → | ↓ | n.d. | n.d. | ← → | ↓ | n.d. | n.d. | ← → | ← → | ← → | n.d. | 39 |
| CD22 ITIM mutant | B6 | ↓ | ← → | ↓ | n.d. | n.d. | ← → | ↑ | n.d. | n.d. | ↑ | ↑ | ← → | n.d. | 39 |
| ST6Gal I−/− | Backcrossed to B6 for 6 generations from 129. | ↓ | ← → | ↓ | ↓ ↓ | ← → | ↓ | ↓ | ↓ | n.d. | ↓ | ↓ | ↓ | n.d. | 65, 135, 136 |
| ST6Gal I−/− × CD22−/− | ↓ | ← → | ↓ | ↓ ↓ | n.d | n.a. | ↑ | ↓ | n.d. | n.d. | n.d. | n.d. | n.d. | 137 | |
| SIAE−/− | mixed 129 × B6 (from 129 ES cells) | ↓ | n.d. | ↓ | ← → | n.d | ← → | ↑ | ↓ | ↑ | ↑ | ↓ | ↑ | Yes | 19 |
| CMAH−/− | 2 lines: B6, mixed 129/B6 | ↓ | n.d. | ↓ | ← → | ← → | ← → | ↑ | ↑ | n.d. | ← → | ← → | ↑ | n.d. | 91, 138 |
Note: This an expanded and updated version of a summary table originally presented in a review by Smith et al (11). Td = T-dependent; Ti = T-independent; AutoAb = autoantibody; B6 = C57/BL6; sIgM = surface IgM; n.d = not determined; n.a. = not applicable.
Extrapolating findings from mouse models to human disease is not always straightforward, especially when it comes to pathways involving sialic acids. There are two common animal sialic acids, N-acetyl sialic acid (Neu5Ac) and N-glycolyl sialic acid (Neu5Gc), that differ by a single oxygen atom at the 5-position that is irreversibly added by an enzyme called cytidine monophospho-N-acetyl neuraminic acid hydroxylase (CMAH) (12). Most mammals express an abundance of Neu5Gc in the majority of their tissues. Notably, humans lack the ability to synthesize Neu5Gc because of an inactivating exon deletion in the CMAH gene that was acquired upon diverging from our last common ancestor with the African great apes about two million years ago (13). The loss of functional CMAH from the human genome was immediately followed by rapid compensatory changes in multiple human Siglecs, some of which may still be ongoing. Remarkably, human-specific pathogens and commensals are unique in their ability to display Neu5Ac on their surfaces, and their human tissue receptors have a preference for Neu5Ac over Neu5Gc. An unfortunate consequence of the species-specificity of sialic acids and Siglecs for the immunologist is that the biology of Siglecs and sialic acids in mouse and humans are very different. Indeed, Siglecs are among the fastest evolving genes in humans and have undergone numerous gene conversions and pseudogenization events; furthermore, expression profiles of Siglecs have been dramatically altered in human hematopoietic cells, possibly as an evolutionary attempt to adapt to their changing ligands. This evolutionary arms race between sialic acids, Siglecs, and pathogens, has been extensively studied by the groups of Varki, Angata, and others (12, 14-16).
In this review, we have presented an overview of the biology of sialic acids and Siglecs especially as they relate to autoimmunity and inflammation. We have focused on pathways linked to CD22 (Siglec-2) and Siglec-G, and briefly speculate on the potential role of other proteins that also bind to sialic acids, such as selectins and factor H, in the context of autoimmunity.
Structure of mammalian sialic acids
Most mammalian sialic acids are derived from the “primary” sialic acid, N-acetyl neuraminic acid (Neu5Ac) (17). Neu5Ac can be converted to N-glycolyl neuraminic acid (Neu5Gc) by CMAH, an enzyme that is lacking in humans. Neu5Ac and Neu5Gc form two core sialic acids that can carry one or more additional substitutions, typically ester linkages at the 4, 6, 7 or 9-OH positions (Fig. 1). O-acetylation is the most commonly observed sialic acid substitution. Varying combinations of substitutions give rise to an enormous structural diversity of sialic acids and over 50 types of sialic acids have been reported. This review will discuss the 9-O-acetyl substitution in some depth, as it is a common sialic acid modification seen in both humans and in rodents. While the hemagglutinins of certain viruses like influenza types A and B recognize terminal sialic acid moieties on host glycans, the hemagglutinins of certain viruses, such as the influenza C virus, are specific for 9-O-acetyl sialic acid, and have been extensively used to study the role of this sialic acid modification in the immune system (18). 9-O-acetylation of sialic acids has been linked to autoimmunity, both in humans and in mouse models, possibly because it impacts Siglec function (19, 20). 9-O-acetyl groups are added to sialic acids by sialic acid O-acetyl transferases (discussed briefly in a later section), but the contribution of these poorly characterized enzymes to autoimmunity has not yet been studied.
Sialic acids are typically found at the terminal positions of the branches of N-glycans, O-glycans, and glycosphingolipids (gangliosides), and occasionally cap side chains of glycophosphatidyl inositol (GPI) anchors. Glycosidically bound sialic acids typically form an α-glycosidic linkage between the C-2 position of sialic acids and the C-3 or C-6 positions of underlying galactose residues or the C-6 position of pre-terminal N-acetylgalactosamine residues (Fig. 2). Sialic acids can also occupy internal positions within glycans, the most common being when one sialic acid residue is attached to another, typically at the C-8 position. The linkages between sialic acid and the underlying sugars are established by specific sialyltransferase enzymes using CMP-sialic acids as high energy donors. Variability in sialic acid linkages to the underlying glycan sugars imparts an additional layer of diversity to the sialic acids. Lectins that recognize sialic acids often have a preference for a particular sialic acid with a specific linkage. There is evidence suggesting that enzymes that add sialic acids to glycans (sialyltransferases) and remove sialic acids from glycans (neuraminidases) are synthesized in a tightly regulated manner, suggesting their potential involvement in controlling Siglec ligand abundance.
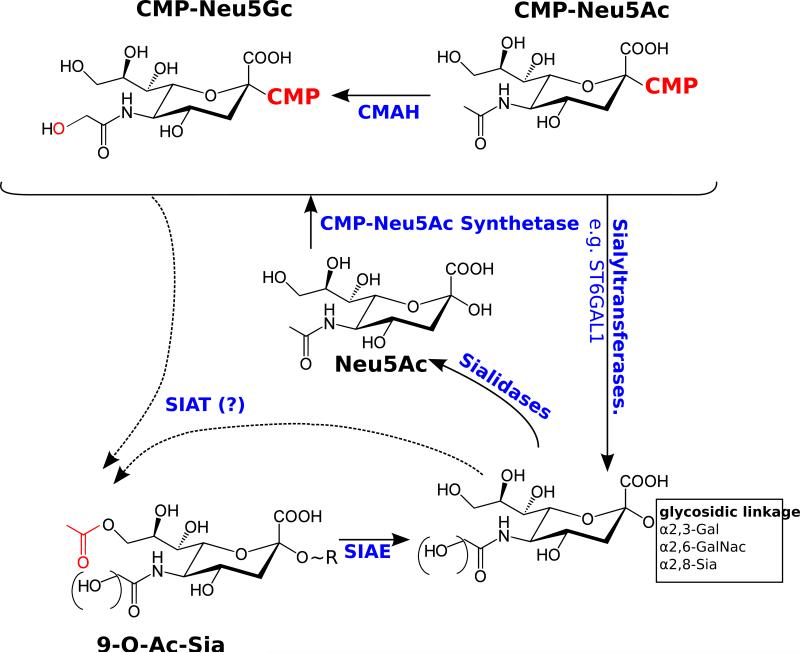
Fig. 2. Sialic acid modifying enzymes discussed in this review.
Sialic acids are conjugated to cytidine monophosphate by CMP-5-N-acetylneuraminic acid synthetase. The CMP-sialic acid conjugates act as high energy donors for sialyltransferase reactions. There are numerous sialyltransferases in the mammalian genome, each specific for a particular linkage. ST6GAL1 is discussed in the review. Sialidases cleave sialic acids from glycans. Sialic acid acetyl esterase (SIAE) in an enzyme - perhaps one of many - that removes the 9-O-acetyl group from sialic acid bearing gycans. The identity of sialic acid O-acetyl transferases (SOAT) has not been established. CASD1 is a strong candidate for such an enzyme, but it is likely that multiple SOAT enzymes exist that add 9-O-acetyl group to specific sialic acid bearing glycans and/or the CMP-sialic acid precursor.
Siglecs and alterations in adaptive immunity linked to autoimmunity
Siglecs are I-type lectins of the immunoglobulin superfamily, and like antibodies, have one V-set (variable-like) domain and one or more C2-set (constant-like) domains. For a detailed discussion of the biology of Siglecs, we direct the readers to excellent reviews by Crocker, Paulson, Varki, and others (8-10, 21, 22). Siglecs are expressed primarily on hematopoietic cells. Several Siglecs contain ITIM motifs and are involved in negative regulation of the cell type in which they are expressed. For instance, Siglec-F (murine) and Siglec-8 (human) are expressed on eosinophils, and ligating Siglec-8 with an agonistic antibody is a proposed therapeutic strategy for allergic asthma as it can trigger eosinophil apoptosis (23). Defects in CD22 and Siglec-G, which are expressed on B cells, have been linked to autoimmunity and are discussed in detail in this review (24). An antibody against CD22 (epratuzumab) has shown significant benefits in the treatment of SLE due to its immunomodulatory activity (25).
CD22 (also known as Siglec-2), binds to α2,6-linked sialic acids that cap a lactosamine moiety (26, 27). These terminal sugars are a common structural motif among N-glycans and are highly expressed on soluble and surface-associated glycoproteins, including IgM. Although CD22 ligands are abundant and present on several hematopoietic cell types and endothelial cells, their ability to bind CD22 in a physiologic context is unclear (28, 29). CD22 is expressed at low levels early in B cell development and its expression is highest on mature B cells (30); it is downregulated in plasma cells by Blimp-1 (31). CD22 can suppress signaling through the B cell antigen receptor (30), and though some CD22 molecules on B cells are associated with the BCR (32), the mechanism of their association is controversial. Both glycan-dependent and glycan-independent associations have been demonstrated in different experimental settings. Following crosslinking of the BCR, the ITIM tyrosines of CD22 are rapidly phosphorylated by the Src-family tyrosine protein kinase Lyn, resulting in the recruitment of the tyrosine phosphatase SHP-1, a negative regulator of B cell signaling (33-35). Indeed, B cells from mice deficient in CD22 display an increase in BCR-induced Ca2+ signaling, attributed to the inability to recruit SHP-1 to the BCR signaling complex (30). Interestingly, increased calcium flux is observed only in splenic Cd22−/− B cells, while peritoneal Cd22−/− B-1 cells exhibit no change compared to wild-type upon BCR crosslinking (30, 36). Cd22−/− B cells also show an increase in tyrosine phosphorylation of the signaling molecules, SLP65, CD19 and Vav, activated downstream of the BCR (37, 38). Corroborating this model, Cd22 mutant knock-in mice that are unable to phosphorylate the CD22 ITIMs and recruit SHP-1 (because critical ITIM tyrosine residues have been mutated) phenocopy Cd22 knockout mice (39). Additionally, CD22 has also been described to activate the Ca2+ efflux pump PMCA-4 in a SHP-1-dependent manner (40). As such, the absence of CD22 may perturb both the initiation and termination of Ca2+ flux in B cells.
Given these data, it is thought that regulation of BCR signaling through CD22 may be critical for the modulation of peripheral B cell tolerance, as almost a third of B cells that exit the bone marrow are autoreactive (41). In the absence of inhibitory signals, B cells can trigger systemic autoimmunity, as evidenced by studies from mouse models. Mice that are deficient in the inhibitory receptor FcγRIIb, as well as mice that lack the kinase Lyn (that phosphorylates ITIM motifs in CD22 and FcγRIIb) or the phosphatase SHP-1 (specifically deleted in the B lineage), all develop autoimmunity (42-44). B cells are hyperactive in Cd22-deficient mice, as has been observed in multiple independent knockout mouse lines (Table 1). However, Cd22 deficiency causes systemic autoimmunity only in certain permissive backgrounds. For instance, although Cd22−/− mice generated in the C57BL/6 background do not develop any signs of autoimmunity despite evidence for B cell hyperactivation (30), the presence of the Yaa (Y-linked autoimmune accelerator) locus, which carries a duplication of the TLR7 gene, is sufficient to induce signs of autoimmunity in C57BL/6 mice, even with haploinsufficiency of Cd22 (Cd22+/−) (45). In the C57BL/6 background, Cd22 null mice have been shown to develop anti-DNA antibodies as they age but they do not develop lupus. Autoantibodies have been noted in other Cd22−/− mouse lines from mixed genetic backgrounds (46), suggesting that while the lack of CD22 may break tolerance, additional epistatic modifier genes influence the development of frank disease.
Although the role of CD22 as an inhibitory regulator of BCR signaling is well established, the impact of CD22 ligands on CD22 signaling is not fully understood. Conflicting results have been obtained, and indeed it is not possible to reconcile all published results. Table 1 lists the features of genetically altered mice with demonstrated or suspected defects in CD22 function.
One of the issues that arises while considering the role of the availability or modification of CD22 ligands in modulating the action of this inhibitory Siglec is the ability of cis-ligands of CD22 on B cells to “mask” the ligand binding site of CD22 (47). Since the concentration of α2,6-linked sialic acids on the B-cell surface is estimated to be 100-fold higher than the Kd of CD22 (0.1–0.3 mM) for α2,6-linked sialic acid (48, 49), the majority of CD22 ligand binding sites are predicted to be occupied by glycoprotein ligands on the same cell under physiological conditions. The net result is that the high concentration of sialic acids on the surface of B cells (cis ligands), mask the binding sites of CD22 and make them unavailable to external or trans ligands. However, the masked state of Siglecs is in a dynamic equilibrium with cis and trans ligands (Fig. 3) (50). Thus, an external probe or a cell surface bearing high affinity or high avidity sialic acid ligands can compete for the binding domains of CD22, even in its masked state (50). The cis ligands of CD22 can also be outcompeted in physiological conditions. When high levels of α2-6-sialylated ligands are available in trans, CD22 redistributes to the points of cell contact and engages its ligands in trans (48). The effect of such re-localization of CD22 during cell-cell interactions on BCR triggering has yet not been studied. It is speculated that this phenomenon could be important for altering B-cell activation thresholds, especially in lymphoid tissues.
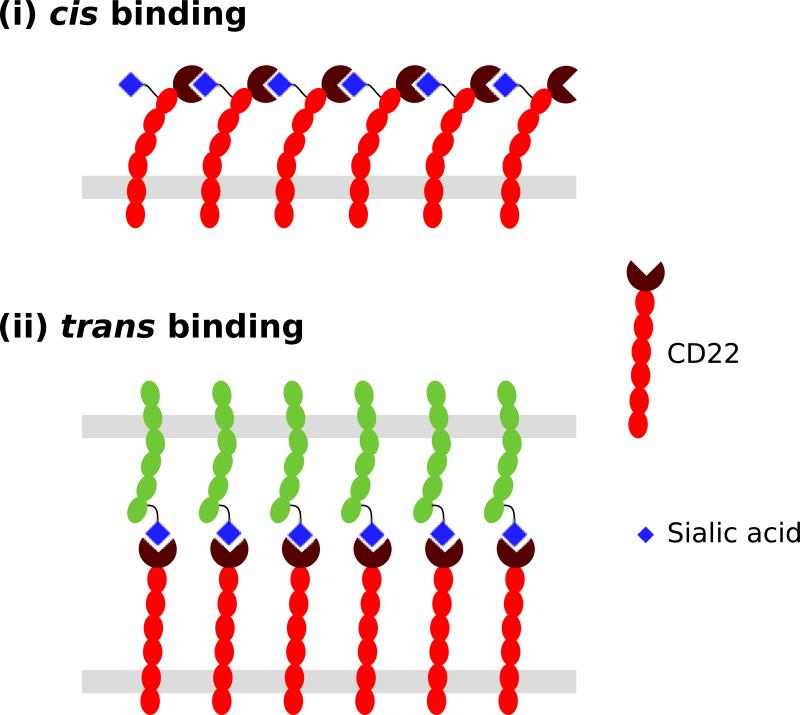
Fig. 3. Cis and trans interactions between CD22 and its ligands.
(i) CD22 can engage its ligands in cis on the same cell. A sialic acid bearing glycan on CD22 itself is the primary physiological cis ligand of CD22. (ii) Trans interactions of CD22 with glycan ligands presented on other cells or glycoproteins are also possible.
Photo-affinity crosslinking experiments with B cells suggest that the CD22 ligand binding site is masked by α2,6 linked sialic acids present on other molecules of CD22 under physiological conditions (51). Thus, in resting B cells, CD22 is thought to be largely sequestered away from the BCR in homo-oligomeric clusters (Fig. 4). This view is compatible with the “picket-fence” model of BCR signaling where BCR molecules on resting B cells are constrained within membrane microdomains by cytoskeletal elements until B cell activation (52, 53). Dynamic changes in the cytoskeleton upon BCR activation increase the cell-surface mobility of BCR molecules and perhaps free up additional BCR molecules to interact with CD22. Interestingly, treatment with the actin depolymerization agent, latrunculin, causes the BCR and CD22 to interact in an α2,6-linked sialic acid independent manner (39).
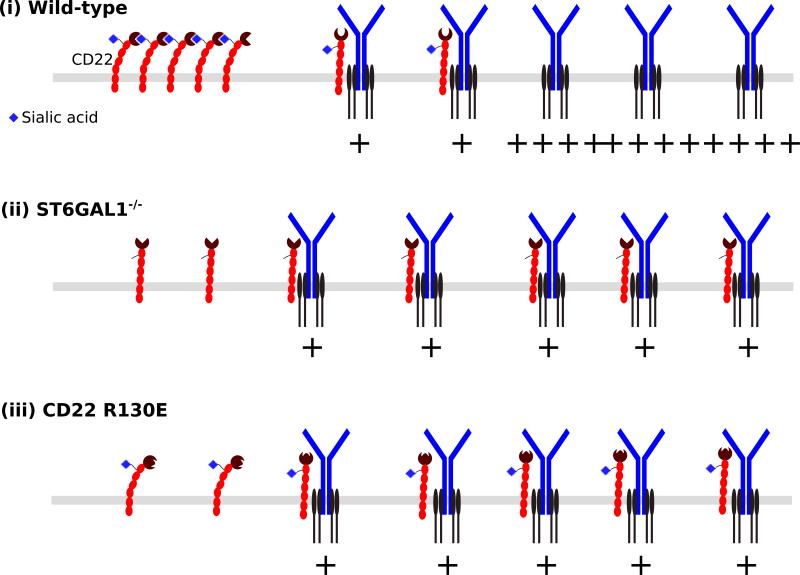
Fig. 4. Model of CD22 action.
(i) The majority of CD22 molecules are sequestered away from the BCR by cis interactions. (ii) Loss of CD22 ligands as in ST6GAL1−/− mice disrupts cis interactions, freeing up more CD22 to interact with and inhibit the BCR in a glycan-independent manner. (iii) The R130E mutation in the CD22 ligand-binding domain, that disrupts glycan-binding, also disrupts cis interactions among CD22 moleules, resulting in greater BCR signal inhibition.
It is not clear whether the interaction between CD22 and the BCR is glycan-dependent, glycan-independent or both (32, 54, 55). Crosslinking experiments with the Daudi cell line (a human B cell line) suggest that at least some physiological interactions between the BCR and CD22 are glycan-independent (56). At the same time, photo-affinity methods have been used to demonstrate that both soluble CD22-Fc or CD22 that is expressed on the cell surface have the potential to interact with IgM in trans in a glycan-dependent manner (57). Antigen complexes with soluble IgM have also been shown to be a potential natural trans-ligand of CD22 (58). The physiological implications of these interactions remain to be worked out, but it is possible that they constitute a natural feedback loop that regulates B cell activation, considering that engineered immunogens coupled to high affinity/avidity CD22 ligands induce antigen-specific tolerance (59). In addition, CD22 may interact with other glycoproteins on the B cell surface such as CD45 (56). Thus, any alteration in the ligands of CD22 could impact B cells through multiple mechanisms, and may also affect other inhibitory Siglecs, such as Siglec-G, that are also expressed on B cells. Even in the absence of BCR crosslinking, treatment with pervanadate, an irreversible phosphatase inhibitor, can cause a B cell activation/phosphorylation pattern that is similar in intensity to BCR crosslinking, suggesting that tonic signaling in resting B cells is held in check by phosphatase activity at baseline (60, 61). Interestingly, pervanadate treatment induces the co-localization of the BCR and CD22 in a manner that is dependent on the CD22 ligand binding domain, implying the role of a glycan-mediated interaction, a result that is in stark contrast to the glycan-independent interaction observed with latrunculin treatment (39). It is unclear if this result has an obvious physiological counterpart and it should be investigated further. Thus, at least in some circumstances, CD22 interaction with the BCR appears to be dependent on its ligand-binding domain.
B cells from St6gal1 deficient mice lack CD22 ligands and exhibit suppressed BCR signaling (62). St6gal1−/− B cells also showed a redistribution of the BCR to clathrin-rich microdomains containing most of the CD22, resulting in an increased association of CD22 and the BCR (Fig. 4). The suppression of BCR signaling appears to be mediated by the unfettered activity of CD22, as the phenotype of the St6gal1−/− Cd22−/− mice is similar to that of Cd22−/− mice (63). However, none of the studies with mutant mice bearing genetically engineered variants of CD22 or its ligands can distinguish between the direct effects of the CD22 mutations and the potential compensatory changes in BCR signaling that may occur in vivo during B cell development. Thus, it is intriguing that contradictory results were obtained with novel sialoside ligands of CD22, which have a nanomolar affinity for human CD22 (64). Surprisingly, treatment with these CD22-binding sialosides induces increased calcium flux upon BCR crosslinking in Daudi cells, in which CD22 is highly masked by cis ligands.
Several mouse strains bearing CD22 with a defective ligand-binding domain have been studied. It has been appreciated that a number of lupus-prone mouse strains, such as NZW and BXSB, express an allele of CD22 called CD22a, which is subtly altered in ligand binding, as the first Ig domain in the CD22 molecule carries a six amino acid deletion as well as multiple point mutations (65). Ligand binding ability is clearly not entirely lost because it can be stained with the oligomeric SAAP probe after sialidase treatment. In addition to the mutations in the carbohydrate-binding domain, this allele also carries a mutation in a Cd22 intron that results in aberrant splicing, altering the pool of CD22 protein that is expressed in B cells. When the CD22a allele was crossed into the C57BL/6 background, B cells appear chronically activated (increased MHC-II expression), suggesting that ligand binding was necessary for the suppression of autoimmunity. The total levels of CD22 overall were also reduced. Due to the complicated nature of the effects of the CD22a allele on the CD22 protein and mRNA splicing and its subtle impact on ligand binding, these results are hard to interpret in an explicit manner.
Multiple groups have generated mice with a targeted inactivation of the CD22 ligand-binding domain. The Tedder laboratory generated two mutant lines Cd22AA and Cd22Δ1-2, bearing Cd22 alleles which completely lack the ability to bind α2,6-linked sialic acids (55). B cells from these mice also appear to be in a chronically activated state (increased MHC-II expression). Although the authors conclude that there is no change in calcium flux following BCR activation, there does appear to be a mild reduction in Ca2+ flux compared to wild-type for both Cd22AA and Cd22Δ1-2 B cells (with a high dose - 40 μg/mL - of anti-IgM Fab2). B cells from these mice also happen to exhibit reduced levels of CD22, and it is possible that the results were confounded by insufficient levels of unmasked CD22 available for BCR inhibition.
To further investigate this issue, the Nitschke laboratory recently generated additional Cd22 knockin lines that express wild-type levels of CD22. They showed that in mice bearing a Cd22 R130E knockin mutation, which abrogates the ability of CD22 to bind to α2,6-linked sialic acids, B cells exhibit decreased calcium flux upon BCR crosslinking as well as an increased association between CD22 and the BCR in resting B cells (39). The result is reminiscent of the findings with St6gal1 knockout mice, and is compatible with the notion that CD22 is sequestered away from the BCR in homo-multimeric clusters in resting B cells in a ligand-dependent manner (Fig. 4). Increased availability of unsequestered CD22 R130E may allow a greater number of CD22 R130E molecules to associate with the BCR in a sialic acid ligand-independent manner as has been suggested in some studies (56), and dampen B cell activation. Upon activation, B cells from Cd22 R130E knockin mice exhibit increased phosphorylation of CD22 and higher SHP-1 recruitment. Interestingly, this group also generated mice with a targeted mutation of the CD22 ITIM motifs in the same genetic background, and the B cells in these mice are in a hyperactive state, phenocopying CD22 deficient mice. These results are consistent with the model of CD22 action described in Fig. 4.
Siglec-G is another inhibitory Siglec that is expressed on B cells; like CD22, it is believed that its cis-ligands modulate its ability to inhibit BCR signaling. It can recognize both α2,3- and α2,6-linked sialic acids, while its human homolog, Siglec-10, has a slight preference for α2,6-linked sialic acids (66, 67). CD24 functions as a cis ligand of Siglec-G on dendritic cells in some contexts, and the function of the CD24/Siglec-G complex as a sensor of endogenous DAMPs is described in a later section. The specific glycoprotein ligands of Siglec-G on B cells have not yet been clearly defined. Like Cd22, when Siglec-G is deleted, the development of autoimmunity is background dependent. Siglec-G deficiency does not cause spontaneous autoimmunity in Balb/c mice (68). Although Siglec-G is expressed on all B cells, Siglec-G deficiency in Balb/c mice causes a selective increase in B-1a cell numbers, and increased calcium flux in B-1a cells upon IgM crosslinking. B2 cells are not affected, perhaps because of a redundant role with CD22 (68). Interestingly, however, when Siglec-G is deleted from the lupus-prone MRL/lpr background (69) or when it is deleted from the Cd22-deficient background (generating a double-deficient mouse), the mice develop an SLE-like disease, characterized by the production of high-affinity autoantibodies and glomerulonephritis, suggesting that Siglec-G helps to maintain B cell tolerance (70). Additionally, Siglec-G deficiency in the C57BL/6 background results in increased autoantibody production and mild glomerulonephritis in aged mice (71). The fact that deficiency in both CD22 and Siglec-G is required for the onset of autoimmunity suggests functional redundancy between these two Siglec molecules (70). However, there are some differences between CD22 and Siglec-G.
Intriguingly, knockin mice with mutations in the ligand binding domain of Siglec-G and CD22 yield opposite phenotypes. B cells from Siglec-G R120E knockin mice exhibit a phenotype similar to that seen in Siglec-G deficient mice, and B-1a cells are selectively hyperactive in both these mice (72). Siglec-G is associated with the BCR in resting B-1a and B2 cells, as well as upon activation with IgM crosslinking or pervanadate treatment (73). However, the association of Siglec-G with IgM is completely lost in Siglec-G R120E knockin mice, both in B-1 and B-2 cells. Unlike CD22, it appears that Siglec-G is unable to interact with the BCR in a ligand-independent fashion. However, only B-1a cells show an increased calcium flux in the absence of Siglec-G, perhaps because CD22 plays a redundant role in B2 cells. The molecular basis for the differences between B-1 and B-2 cells in these studies is not fully understood.
Interestingly, in all mice with genetic alterations of the CD22 pathway that either increase or decrease BCR inhibition, a loss of marginal zone (MZ) B cells is observed (Table 1). We have argued that the loss of MZ B cells in Cd22 deficient mice is linked to the enhanced signaling of the B cell receptor during development, which may drive a stronger follicular B cell fate at the expense of MZ B cells (9). However, it is unclear why MZ B cells are lost in St6gal1−/− mice or CD22 R130E knockin mice, where B cell signaling is dampened presumably by increased CD22 activity. Whatever the reason, a drastic alteration in the B cell repertoire likely occurs when Cd22 is altered, since MZ B cells are lost. Whether this alteration in the repertoire is a major factor in the development of autoimmunity remains to be explored.
The inhibitory Siglecs on B cells can be therapeutically exploited, and recent studies suggest that high avidity sialoside ligands for CD22 and Siglec-G conjugated to antigens can be used to induce antigen-specific B cell tolerance (74, 75). Mice expressing membrane bound hen egg lysozyme (mHEL) and anti-HEL BCR were used to explore the role of CD22 and Siglec-G in inducing tolerance against membrane-bound antigens. In this model, both CD22 and Siglec-G are recruited to the immunological synapse formed between B cells and target cells co-expressing membrane antigens and natural ligands of CD22 and Siglec-G (76). This results in the induction of tolerance to membrane antigens by inhibiting BCR signaling in a B-cell intrinsic manner, due to Lyn and Bim-dependent deletion of the antigen-reactive B cells. An antibody against CD22 (epratuzumab) has demonstrated immunomodulatory potential of therapeutic benefit in SLE and Sjögren's syndrome, while providing only moderate B cell depletion, in contrast to rituximab, an anti-CD20 antibody (25). Epratuzumab is a non-blocking antibody that does not hamper ligand binding by CD22. One proposed mechanism of action of epratuzumab is that it unmasks CD22 by sterically inihibiting cis interactions, perhaps allowing for the formation of tolerance-inducing immunological synapses with endothelial cells, which co-express a high density of CD22 ligands and membrane-bound immune complexes (77).
Sialic acid modifications
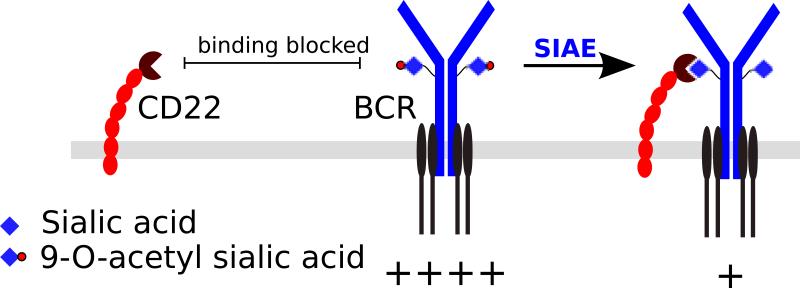
9-O-acetylation is a commonly observed modification of sialic acids that can inhibit Siglec binding (78-80). O-acetyl groups can be added to sialic acids by poorly characterized acetyl-transferases, and removed by sialic acid acetyl-esterases (81). In the case of 9-O-acetylation of sialic acid, it has been shown using microsomal extracts of bovine submandibular glands, a tissue that is rich in 9-O-acetyl sialic acids, that enzymatic O-acetylation of sialic acids initially occurs in the 7-OH position and the acetyl moiety subsequently migrates to the 9-OH position at neutral pH in a time dependent manner (82). Although migration of the O-acetyl group from 7-OH to the 9-OH position can occur spontaneously (83), the migration from the 7-OH to the 9-OH position was inhibited by heat inactivation of the microsomal extract, suggesting the possible involvement of an enzymatic “migrase”, but such an enzyme has not been directly demonstrated. It is likely that there are multiple O-acetyl transferases and esterases, perhaps with varying specificities for particular types of sialic acid bearing glycans. CASD1 is one such O-acetyl transferase, but it has not yet been definitively demonstrated if CASD1 O-acetylates a specific subset of sialic acid bearing glycans or if it directly acetylates their biosynthetic precursor, i.e. CMP-sialic acid (84). SIAE (sialic acid acetyl esterase) is an O-acetyl esterase, and mice deficient in SIAE have a B cell hyperactivation phenotype (19). Murine B cells lacking SIAE exhibit increased calcium flux upon BCR stimulation (19) and functionally defective mutations in SIAE have been linked to human autoimmunity (20).
Given that (i) sialic acid 9-O-acetylation levels are increased on B cells in SIAE deficient mice, (ii) α2,6 linked sialic acids are the preferred ligands of CD22, (iii) CD22 ligands can be masked by 9-O-acetylation (78), and (iv) Siae deficiency broadly phenocopies Cd22 knockout mice, it was speculated that SIAE is a regulator of the CD22 pathway. It was surmised that SIAE deficiency may interfere with the interaction of CD22 with the BCR or with other sialoglycoproteins (85) by increasing 9-O-acetylation of sialic acid ligands (Fig. 5). However, it is still unclear if the association between CD22 and IgM is dependent on α2,6 sialic acid bearing glycans for its action, let alone what the consequences of increasing the 9-O-acetylation of these sialic acids are. This model of SIAE function in B cells is inconsistent with data from knockin mice bearing mutations targeting the ligand-binding domain of CD22 (Fig. 4) (39). Data from the Cd22 R130E knockin mouse model suggest that disrupting the ability of CD22 to bind to its cis ligands suppresses B cell signaling. However, SIAE deficient mice have the opposite phenotype of B cell hyperactivation, despite the possibly analogous interference in the ability of CD22 to bind its cis ligands due to their increased 9-O-acetylation of sialic acid. While alterations in the CD22 pathway alone may be insufficient to explain the findings in SIAE knockout mice, a potential non-redundant role for Siglec-G cannot be excluded, as increased 9-O-acetylation of sialic acids may hamper binding to both CD22 and Siglec-G. However, Siglec-G appears to have a non-redundant role primarily in B-1a cells (68, 73) and a satisfactory explanation of the findings in SIAE deficient mice is still lacking. The links between rare genetic variants of Siae and human autoimmune disease are intriguing but remain to be more fully evaluated (20, 86-90). While heterozygous loss-of-function variants of SIAE may be enriched in autoimmune subjects, the final word on this issue will require functional characterization of all variants of Siae in tens of thousands of disease subjects and controls in order to statistically validate the relevance of this gene in disease.
Fig. 5. Proposed model for SIAE action.
9-O-acetylation of sialic acid glycans on the BCR complex can be expected to inhibit inhibitory glycan-dependent interactions between CD22 and BCR. SIAE can remove the 9-O-acetyl moieties, thereby allowing CD22-mediated inhibition of BCR signaling. Note that this model is inconsistent with the model of CD22 action depicted in Fig. 4.
In many ways, the Siae knockout mice resemble mice with CMAH deficiency (91). B cells from both Siae and Cmah knockout mice are hyperactive and exhibit increased Ca2+ flux upon BCR crosslinking. Marginal zone B cells are lacking in both, a feature that is consistent with the B cell hyperactivation phenotype. Although a direct link to CD22 has not been established, the following lines of circumstantial evidence suggested the involvement of CD22: murine CD22 is specific for Neu5Gc, i.e. the product of CMAH (92), and specifically Neu5Gc that lacks the 9-O-acetyl modification (79), i.e. the predicted product of SIAE. Thus, lack of either CMAH or SIAE would be predicted to reduce the availability of preferred CD22 ligands.
The inactivation of CD22 ligands in either Cmah or Siae knockout mice would be expected to phenocopy the selective inactivation of the CD22 ligand-binding domain, which results in blunted B cell activation, a phenotype that is also reminiscent of the St6gal1 knockout (62). In light of these contradictory findings, we believe that the phenotypes of the Cmah and Siae knockout mice deserve closer re-examination, and possible involvement of other Siglecs or selectins should be considered. Ca2+ flux data suggest that the phenotype of Cmah knockout B cells is cell-intrinsic. However, this does not exclude the possibility that the B cell phenotype is developmental, i.e. arises from compensatory changes in the levels of kinases, phosphatases and other signaling molecules, which alter the BCR signaling threshold or amplitude. Perhaps one could exclude this possibility by assessing if treatment with Neu5Gc can correct the defect in CMAH deficient B cells. Inducible deletion of Siae or Cmah in mature B cells or the development of pharmacologic inhibitors can also help clarify this issue. Variability in the genetic backgrounds of these mice has also been pointed out as a potential concern. Although Cmah deficient mice have been evaluated in at least two different genetic backgrounds (91, 93), Siae knockout mice have been studied only in the 129/Sv x B6 background (backcrossed into B6 for 10 generations) (19). We have now generated an independent knockout allele of Siae in a pure B6 background and are currently analyzing these mice.
In the mouse germinal center, B cells downregulate CMAH expression and this results in a switch in their sialic acid moieties from NeuGc to Neu5Ac (91). Indeed, GL7 - an antibody used to detect mouse germinal center B cells by flow cytometry - recognizes α2,6-linked Neu5Ac (91). Since α2,6-linked Neu5Gc is the preferred ligand for mouse CD22, perhaps the downregulation of CMAH in mice results in physiological unmasking of CD22 in the germinal center, and creates an opportunity for CD22-dependent trans interactions with non-B cells present in the germinal center such as follicular helper T cells or FDCs; these speculations have not been verified. Interestingly, the glycan epitope recognized by human CD22 (α2-6-sialylated 6-sulfo-N-acetyllactosamine) is also downregulated in the germinal center, resulting in unmasking of CD22 in human germinal center B cells (94). These observations suggest that, much like CMAH in mice, the enzyme responsible for generating the epitope recognized by human CD22 (a 6-O-sulfotransferase) is also downregulated in the germinal center. Although the precise identity of the 6-O-sulfotransferase is not known, it does appear to be an astonishing case of convergent evolution affecting the ligand rather than the Siglec, arising to compensate for the loss of CMAH during human evolution. The α2-6-sialylated 6-sulfo-N-acetyl-lactosamine determinant was also more strongly expressed in the lumen of high endothelial venules of secondary lymphoid tissues than on B-lymphocytes, suggesting an additional role for CD22 in B-cell interaction with blood vessels and trafficking. It would make teleological sense if the threshold for BCR signaling is increased in the germinal center, as would be predicted from the unmasking of CD22 in order to enable selection of BCRs with a higher affinity for antigen that could signal despite the increased signaling thresholds. One could test this hypothesis by forcing the expression of CMAH in the B lineage and preventing the downregulation of CMAH in mouse B cells. Although it is clear that CMAH levels are reduced upon B cell activation, alterations in the levels of SIAE during B cell activation and development have not yet been fully characterized.
Other ways in which sialic acids are linked to autoimmunity
There are a number of other ways in which sialic acids have been linked to autoimmunity and chronic inflammation, and some of them are discussed in this section. We will first discuss the role of Neu5Gc as pseudo-self-antigens in humans, consider the role of SAMPs in setting an inhibitory tone, especially in innate immune cells, the incorporation of sialic acids into microbes to evade immunity, the role of sialic acids in the therapeutic use of intravenous immunoglobulins in autoimmune diseases, and the role of sialic acid interactions in selectin function in the context of autoimmunity.
Neu5Gc and chronic inflammation
Humans lost CMAH when they diverged from their last common ancestor with the great apes (12). This event is considered an evolutionary landmark, as it led to a dramatic upheaval in the subsequent evolution of human sialic acid binding proteins in an attempt to re-adapt to the changes in their endogenous ligands. Thus, Siglecs are among the most rapidly evolving gene families in humans. The majority of human Siglecs are encoded on chromosome 19 and mouse Siglec genes are present in a syntenic region on chromosome 7. The Siglec genes have undergone rapid evolution in sequence, gene conversions, changes in gene expression and pseudogenization events, and it is indeed possible that this process is still ongoing (12). Human autoimmunity could be a transitory phenomenon in the evolutionary timescale of ongoing and potentially incomplete adaptation of human Siglecs to their self-ligands. Humans cannot synthesize endogenous Neu5Gc because of the lack of CMAH, but are constantly exposed to this sialic acid in their environment. Dietary Neu5Gc, which is abundant in red meat, can be incorporated into tissues (95). Since this occurs after infancy, Neu5Gc represents a foreign antigen that is incorporated into self structures. Neu5Gc is therefore called a xeno-autoantigen in this context and anti-Neu5Gc antibodies can be considered xeno-autoantibodies (96). Although dietary Neu5Gc is poorly immunogenic, there is evidence that incorporation of dietary Neu5Gc into the cell walls of commensal bacteria may convert Neu5Gc into a more immunogenic form (96). Anti-Neu5Gc antibodies have been linked to a variety of specific human diseases that are not seen in the great apes including cardiovascular disease and cancer (97-99). These studies, pioneered by the Varki group, suggest that dietary red meat provides the immunogens that result in the generation of IgG antibodies specific for Neu5Gc, and these antibodies target Neu5Gc that has been incorporated into host glycoproteins, and thus induce chronic inflammation (100).
Studies on Cmah knockout mice have been performed using an immunization step and oral feeding of Neu5Gc to mimic what is seen in humans (99). It is likely that there are a number of mechanisms that contribute to the epidemiologic link seen between the consumption of red meat and chronic inflammation, heart disease, and cancer (101, 102). One of these mechanisms is likely the incorporation of dietary Neu5Gc into host proteins and lipids as a xeno-autoantigen and the induction of a cross reactive immune response by either dietary Neu5Gc containing glycoproteins or gut commensals/pathogens that incorporate dietary Neu5Gc into their cell walls. In the mouse model, passive transfer of anti-Neu5Gc IgG recreates the chronic inflammatory phenotype. The inflammatory process has not yet been studied in mechanistic detail - whether it is mediated by Fc receptor activation on phagocytes, complement activation, or by an ADCC-like process remains unclear. The parallels with accepted mechanisms in well-established humoral autoimmune conditions are striking.
Sialic acid mediated tolerance, SAMPs, and immune responses to sialic acid containing microbes
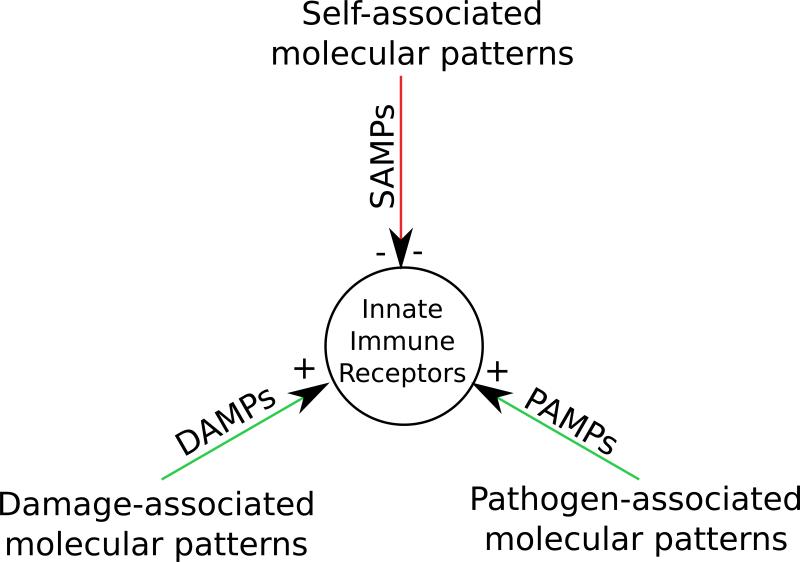
Most microbes lack the expression of sialylated glycans on their surfaces, in contrast to vertebrates, which express sialic acid at high levels. It has been postulated that these terminal sugars behave as self-associated molecular patterns (SAMPs) and provide an important tolerogenic signal in mammals (3). Sialic acids may function as SAMPs because they can ligate Siglecs, either in “cis” or in “trans” on innate immune cells and thus maintain an inhibitory tone. Unlike DAMPs or PAMPs, which activate innate immunity and prime dendritic cells to activate T cells, SAMPs are only thought to provide a normal inhibitory state in innate immune cells preventing excessive inflammation (Fig. 6). It should be noted that some Siglecs are activating receptors, and sialic acids may function as SAMPs, especially when they are a part of a glycan motif that is specifically recognized by inhibitory Siglecs. In line with this idea and emphasizing the importance of immune recognition of sialic acids, it has been appreciated that a number of pathogenic microorganisms have evolved mechanisms to evade immune detection by mimicking host cells by incorporating sialic acids onto their cell surface. Indeed, sialic acids are rare in bacteria that are not vertebrate pathogens or commensals, which have evolved numerous ways to assimilate and/or synthesize sialic acids (6). In some instances, such molecular mimicry between bacterial pathogens and the host can trigger autoimmunity. For instance, Campylobacter jejuni expresses ganglioside mimics in its capsule and the antibody response against C. jejuni lipo-oligosaccharides generated in the context of enteritis can cross-react against host neural gangliosides, GM1 and GD1, causing Guillain-Barre syndrome (7). The presence of a high concentration of sialic acids in the environment may also act as a cue for bacteria that they are in a vertebrate host, and may aid in their switch to a host-specific gene expression program.
Fig. 6. Self-associated molecular patterns.
Self-associated molecular patterns (SAMPs) are postulated to be self-molecules that inhibit innate immune receptors, unlike DAMPs and PAMPs provide activating signals.
Factor H, autoantibodies and inflammatory disorders
Factor H is a complement inhibitory protein that recognizes sialic acids on the host cell surface in the presence of C3b and accelerates the decay of C3b on host cell surfaces. Other polyanions such as heparan sulfate glycosaminoglycans can also promote the binding of factor H to cell surfaces. Rare inherited mutations in the C-terminal domains that are involved in sialic acid recognition, or autoantibodies against factor H that interfere with its complement regulatory function, can result in atypical hemolytic uremic syndrome or contribute to age-related macular degeneration (103, 104). Interestingly, about 17% of subjects with age-related macular degeneration also carry a polymorphism in factor H (Y402H), but the molecular basis for this association does not appear to involve SAMPs. The Y402H polymorphism compromises the ability of factor H to bind and shield inflammatory oxidized phospholipids, which function as DAMPs rather than SAMPs in this context (105). While it is clear that SAMPs play a critical role in mediating host-pathogen or host-commensal interactions, their impact on the maintenance of tolerance or autoimmunity needs further investigation.
Sialic acids and the therapeutic use of intravenous immunoglobulins
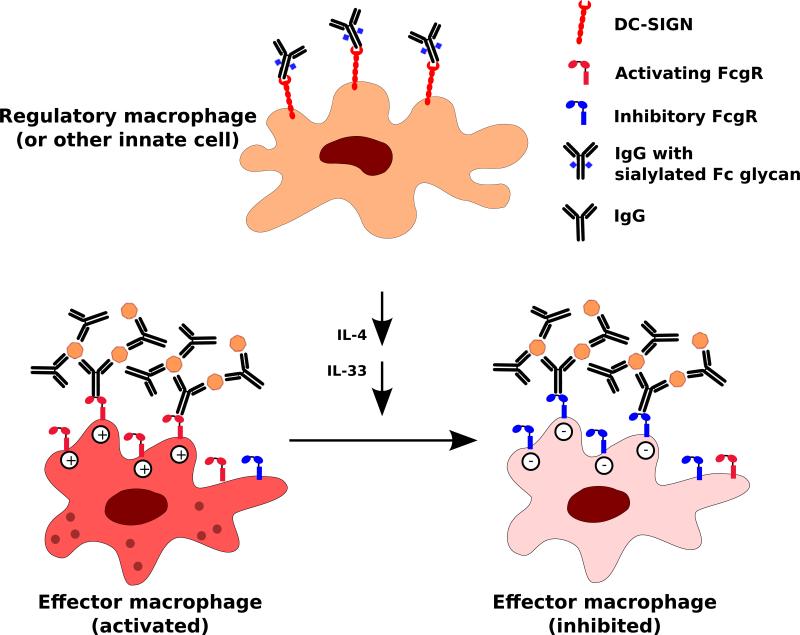
The immunosuppressant activity of intravenous immunoglobulins (IVIg) has been used to treat autoimmune conditions for decades. A number of mechanisms may contribute to IVIg activity, including direct competition with pathogenic immunoglobulins. However, work from the Ravetch laboratory suggests that the prominent mechanism of action involves a complex biantennary N-glycan in the IgG Fc domain that is conserved in all IgG subclasses (Fig. 7) (106, 107). When this glycan terminates in an α2,6-linked sialic acid, it imparts a regulatory function to IgG antibody. This sialoglycan alters the conformation of the IgG Fc domain, such that it results in the loss of ability to bind canonical (type 1) Fc receptors, and instead promotes interaction with the type 2 Fc receptors such as the Dendritic Cell Specific Intracellular adhesion molecule 3 Grabbing Non-integrin (DC-SIGN). Signaling through type 2 Fc receptors on regulatory macrophages, dendritic cells, or other unidentified innate immune cells triggers a potent anti-inflammatory cascade involving the production of cytokine mediators such as IL-33 and IL-4 (106). These cytokines ultimately alter the ratio of inhibitory to activating type 1 Fc receptors on effector macrophages, and also contribute to regulatory T cell activation (108). Thus, IVIg can be used to treat both antibody and T-cell mediated autoimmunity.
Fig. 7. Immunodulatory role of Fc glycan sialylation.
IgGs with sialylated Fc glycans engage DC-SIGN on regulatory macrophages or other innate immune cells. This interaction, via a cascade of events involving the production of secreted mediators such as IL-4, IL-33, alters the balance of activating and inhibitory FcγRs on effector macrophages. Signaling through inhibitory FcγRs predominates, resulting in the dampening of inflammation.
Less than 10% of IgG antibodies in healthy individuals are sialylated, and these levels are markedly reduced during inflammatory conditions, including the autoimmune diseases rheumatoid arthritis and Wegener's granulomatosis. Indeed, IgG from individuals with chronic inflammatory conditions have an increased fraction of Fc glycans lacking terminal sialic acid. It is possible that altered glycosylation contributes to the pathogenicity of autoantibodies from patients with autoimmune disorders. While the mechanism of the immunosuppressant action of the IgG Fc sialoglycan has been well worked out, it remains unclear how the sialylation of the IgG Fc glycan is regulated in physiological conditions. It is possible that a combination of sialyltransferase or sialidase activities are involved, but there is currently very little known about the mechanisms by which glycosylation of the IgG Fc glycan is regulated. It is also unclear if sialylation of Fc glycans is altered after IgG secretion.
CD24, Siglec-G, and HMGB1
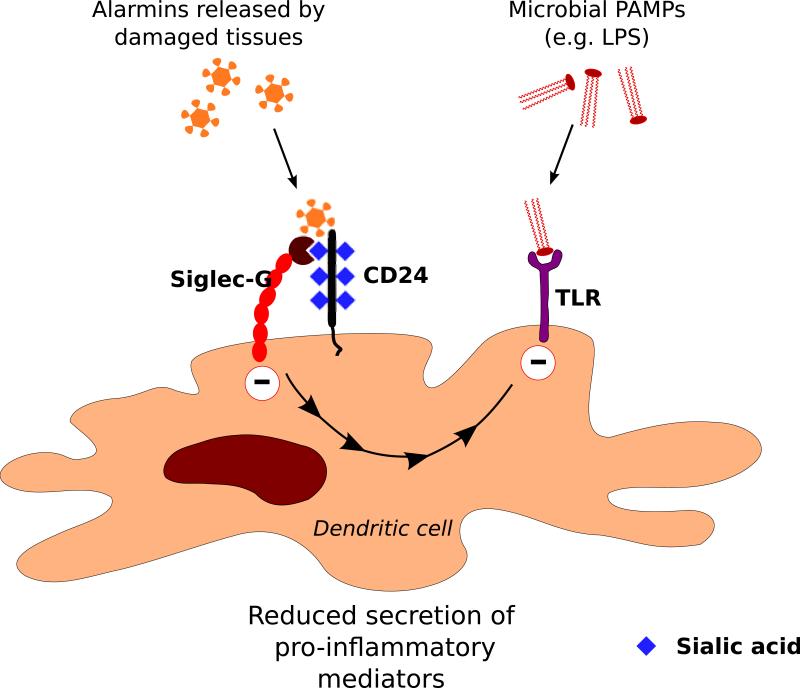
Siglec-G and its cis ligand CD24, a GPI-linked sialoglycoprotein, are both expressed on dendritic cells. Mice that lack CD24 or Siglec-G are susceptible to a sublethal challenge of acetaminophen and die from a severe liver injury within 20 hours of acetaminophen administration (109). Dendritic cells express both CD24 and Siglec-G, and the absence of either molecule results in insufficient negative regulation of the response to the danger signal, HMGB1, released in response to acetaminophen-induced tissue damage. HMGB1 can interact with the CD24/Siglec-G complex in a manner that requires both CD24 and Siglec-G. Siglec-G was also shown to function as the signal transducer for endogenous danger signals (alarmins), HSP70 and HSP90, in a CD24-dependent manner (Fig. 8). Presumably inhibitory signaling by Siglec-G dampens inflammation following acetaminophen-induced liver damage and is cytoprotective in the liver (110). Siglec-G can inhibit dendritic cell responses to PAMPs as well as DAMPs. Sepsis in an intestinal perforation model was shown to be exacerbated by the disruption of the Siglec-G/CD24 complex by microbial sialidases, and sialidase inhibitors that can preserve the Siglec-G/CD24 interaction may have therapeutic potential in the management of sepsis (66).
Fig. 8. Recognition of enodogenous danger signals by the Siglec-G/CD24 complex.
CD24 is a cis ligand of Siglec-G on dendritic cells. The Siglec-G/CD24 complex recognizes endogenous danger signals (alarmins) resulting in inhibitory signaling mediated by Siglec-G that dampens immune activation. It can also suppress TLR-mediated signaling in response to PAMPs such as LPS.
HMGB1 is an alarmin that is released by necrotic cells and can also be secreted by DCs and macrophages stimulated with pro-inflammatory cytokines such as TNFα (111, 112). Serum levels of HMGB1 are increased in several autoimmune conditions, and it may play a role in promoting inflammation in many of these diseases. However, HMGB1 has a number of binding partners in addition to CD24 such as RAGE, thrombospondin, and CXCL12, and it can indirectly interact with TLRs (113). Furthermore, CD24 occurs in a variety of glycoforms of molecular weights ranging from 20 to 70 kDa, and the specific glycoforms that interact with Siglec-G or HMGB1 are likely to be cell-type and context dependent and have yet to be explicitly defined. Although some polymorphisms in CD24 have been linked to autoimmunity, it is not clear if they impact glycosylation and ultimately Siglec-G binding (114).
Mice deficient in CD24 are protected from experimental autoimmune encephalitis and experimental autoimmune thyroiditis. CD24 deficiency in thymic epithelial cells and possibly thymic dendritic cells causes clonal deletion of T cells with an autoreactive TCR transgene (2D2) that normally escape negative selection but has no effect on a TCR transgene specific for ovalbumin (115). Thus, CD24 may have an impact on central tolerance mechanisms. Although Siglec-G is known to be expressed on some dendritic cell subsets, its expression on dendritic cells or epithelial cells in the thymus has not been evaluated. Surprisingly, transfer of syngeneic T cells into a lymphopenic CD24 deficient host was shown to result in excessive and indeed lethal homeostatic proliferation, suggesting that CD24 dampens the activation of T cells by APCs (116). However, a possible alternate explanation could be that the release of alarmins such as HMGB1 resulting from the irradiation used to induce lymphopenia, may have allowed the recipient CD24 knockout dendritic cells to cause an exaggerated donor T cell proliferative response akin to the lethal response to the acetaminophen-injury seen in CD24 knockout mice (109). This possibility may be excluded using unirradiated Cd24−/− Rag−/− mice as lymphopenic recipients or antibody-mediated neutralization of HMGB1. The direct contribution of the glycan ligands of Siglec-G to the above described phenomena has not been fully addressed. While the possible role of the CD24/Siglec-G axis in regulating tolerance or the effects of DAMPs in autoimmune disease remains an interesting possibility, it is far from established.
Sialic acids, selectins, and autoimmunity
Aside from their role in acting as Siglec ligands, sialic acids are also a component of sialyl LewisX - the carbohydrate ligand for the selectins, a family of transmembrane cell adhesion molecules that are structurally homologous to C-type lectins (117-119). Selectins are expressed on the surface of leukocytes and activated endothelial cells that regulate the exit of leukocytes from the circulation and into inflamed tissue. During inflammation, the flow of blood through the vasculature slows considerably, allowing leukocytes to contact and engage adhesion receptors on the endothelium, and then roll along the endothelial wall of the blood vessel (120). This important initiating step of leukocyte tethering and adhesion is mediated by the binding of selectins expressed by leukocytes or endothelial cells to glycans containing the sialyl LewisX motif on the endothelial cells or leukocytes, respectively. Although the sialyl LewisX tetrasaccharide is a critical determinant of selectin ligand-binding, additional modifications on the glycan or on the glycoprotein bearing this glycan motif are required for efficient selectin binding as mentioned below. Leukocyte rolling is followed by adhesion and extravasation through the endothelium to the site of inflammation. Thus, it is reasonable to think that perturbations in this process may predispose to autoimmunity.
There are a few examples of the involvement of selectins and their ligands in autoimmunity. In the case of L-selectin, it has been observed that in vivo antibody blockade in pre-diabetic mice alleviates subsequent development of insulitis and diabetes in non-obese diabetic (NOD) mice, a result suggesting that preventing leukocyte adhesion to the endothelial venules in the pancreatic islets at the early stages could thwart the development of autoimmune disease (121). Similarly, antibody blockade of L-selectin prevents experimental allergic neuritis, a model of Guillain-Barre syndrome, in rats (122). However, genetic ablation of L-selectin in several diabetic mouse backgrounds (including NOD, NOD.scid, and BDC2.5 TCR-transgenic mice) or non-diabetic backgrounds (SJL, C57BL/6) did not render the mice more resistant to diabetes onset (123-125). Other selectins, however, may have a less tenuous role in autoimmunity. The protein TIM-1 (T cell immunoglobulin and mucin domain 1) was identified as a P-selectin ligand, that is especially important for the tethering and rolling of Th1 and Th17 cells. Studies in mice deficient in the O-glycosylated TIM-1 mucin domain, showed that TIM-1 mediates adhesion of activated T cells, including adhesion of these T cells in the inflamed venules of the central nervous system in experimental autoimmune encephalomyelitis (EAE) (126). The precise structure of the TIM-1 glycans that bind P-selectin are yet to be determined. Although it is known that α1,3 fucosylation and the sulfation of tyrosine residues on PSGL-1 is required, sialylation does not appear to be required for the binding of P-selectin to TIM-1 (126). Another well-studied ligand for P-selectin, PSGL-1 (P-selectin glycoprotein ligand-1) has been shown to be involved in the negative regulation of autoimmunity through Tregs, as a deficiency in PSGL-1 increases the severity of EAE (127). In the absence of PSGL-1, Tregs were selectively unable to suppress EAE and effector T cell proliferation, demonstrating a role for PSGL-1 in Tregs inhibiting T cell activation in the late stages of the immune response to self-antigen. PSGL-1 deficient mice also develop systemic autoimmunity with some characteristics of systemic sclerosis (128). The presence of the sialyl-LewisX glycan, as well as sulfation of key tyrosine residues on PSGL-1, is critical for selectin binding. Although the sialyl LewisX motif is widely seen in several glycoproteins, not all of them are bona fide selectin ligands (129, 130). It was recently shown that the sialyl-LewisX glycan is highly specific for suppression-competent “effector” T regulatory cells in humans and can be used to distinguish them from other FoxP3+ T cells, but the function of this glycan in this context is not yet understood (131). Knowledge of the structural requirements for selectin binding has led to the development of glycomimetic inhibitors of selectins, which will pave the way for therapeutic targeting of this pathway in inflammatory disorders and also help unravel the role of selectin ligands in autoimmune disease (132).
Conclusions
Our current knowledge of sialic acids, sialic acid modifying enzymes and Siglecs in autoimmunity is in its infancy. Investigations on the regulation of sialic acid biology and autoimmunity have focused largely on B cells, and the role of sialic acid containing ligands in regulating T cells and dendritic cells needs further examination. Genetic polymorphisms in Cd22 and rare variants of Siae have been linked to autoimmunity, but these alleles may contribute to a very small fraction of the population level genetic risk to autoimmune disease. Nevertheless both sialic acid binding lectins as well as sialic acid modifying enzymes are considered druggable targets, and sialoside inhibitors as well as antibodies targeting Siglecs on B cells have demonstrated immunomodulatory potential. There remain many gaps in our knowledge about the mechanism of action of Siglecs and the therapeutics used to target Siglecs and Siglec-linked signaling. Clearly, sialic acid bearing glycans profoundly influence BCR signaling as well as several other cellular processes of relevance to autoimmunity, and more efforts are needed towards a better understanding of the underlying molecular mechanisms.
ACKNOWLEDGMENTS
This work was supported by NIH grants to S.P. (U19AI110495, R01AI064930) and V.M. (K08AI113163). We thank Ajit Varki for helpful discussions.
REFERENCES
- 1.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Curr Opin Struct Biol. 2009;19:507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varki A. Sialic acids in human health and disease. Trends Mol Med. 2008;14:351–360. doi: 10.1016/j.molmed.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A. Since there are PAMPs and DAMPs, there must be SAMPs? Glycobiology. 2011;21:1121–1124. doi: 10.1093/glycob/cwr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 5.Varki A, Gagneux P. Multifarious roles of sialic acids in immunity. Ann N Y Acad Sci. 2012;1253:16–36. doi: 10.1111/j.1749-6632.2012.06517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. Diversity of microbial sialic acid metabolism. Microbiol Mol Biol Rev. 2004;68:132–153. doi: 10.1128/MMBR.68.1.132-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 8.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillai S, Netravali IA, Cariappa A, Mattoo H. Siglecs and immune regulation. Annual review of immunology. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varki A, Angata T. Siglecs--the major subfamily of I-type lectins. Glycobiology. 2006;16:1R–27R. doi: 10.1093/glycob/cwj008. [DOI] [PubMed] [Google Scholar]
- 11.Walker JA, Smith KG. CD22: an inhibitory enigma. Immunology. 2008;123:314–325. doi: 10.1111/j.1365-2567.2007.02752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010;107(Suppl 2):8939–8946. doi: 10.1073/pnas.0914634107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou HH, et al. Inactivatin of CMP-N-acetylneuraminic acid hydroxylase occurred prior to brain expansion during human evolution. Proc Natl Acad Sci USA. 2002;99:11736–11741. doi: 10.1073/pnas.182257399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angata T, Varki NM, Varki A. A second uniquely human mutation affecting sialic acid biology. J Biol Chem. 2001;276:40282–40287. doi: 10.1074/jbc.M105926200. [DOI] [PubMed] [Google Scholar]
- 15.Angata T, Margulies EH, Green ED, Varki A. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. doi: 10.1073/pnas.0404833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103:7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varki A. Essentials of glycobiology. Cold Spring Harbor Laboratory Press {a}; 10 Skyline Drive, Plainview, New York 11803, USA: 1999. [Google Scholar]
- 18.Klein A, Krishna M, Varki NM, Varki A. 9-O-acetylated sialic acids have widespread but selective expression: analysis using a chimeric dual-function probe derived from influenza C hemagglutininesterase. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7782–7786. doi: 10.1073/pnas.91.16.7782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cariappa A, et al. B cell antigen receptor signal strength and peripheral B cell development are regulated by a 9-O-acetyl sialic acid esterase. J Exp Med. 2009;206:125–138. doi: 10.1084/jem.20081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Surolia I, et al. Functionally defective germline variants of sialic acid acetylesterase in autoimmunity. Nature. 2010;466:243–247. doi: 10.1038/nature09115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paulson JC, Macauley MS, Kawasaki N. Siglecs as sensors of self in innate and adaptive immune responses. Annals of the New York Academy of Sciences. 2012;1253:37–48. doi: 10.1111/j.1749-6632.2011.06362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schwarz F, Fong JJ, Varki A. Human-specific evolutionary changes in the biology of siglecs. Advances in experimental medicine and biology. 2015;842:1–16. doi: 10.1007/978-3-319-11280-0_1. [DOI] [PubMed] [Google Scholar]
- 23.Kiwamoto T, Kawasaki N, Paulson JC, Bochner BS. Siglec-8 as a drugable target to treat eosinophil and mast cell-associated conditions. Pharmacology & therapeutics. 2012;135:327–336. doi: 10.1016/j.pharmthera.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angata T. Associations of genetic polymorphisms of Siglecs with human diseases. Glycobiology. 2014;24:785–793. doi: 10.1093/glycob/cwu043. [DOI] [PubMed] [Google Scholar]
- 25.Leonard JP, Goldenberg DM. Preclinical and clinical evaluation of epratuzumab (anti-CD22 IgG) in B-cell malignancies. Oncogene. 2007;26:3704–3713. doi: 10.1038/sj.onc.1210370. [DOI] [PubMed] [Google Scholar]
- 26.Powell LD, Sgroi D, Sjoberg ER, Stamenkovic I, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta carry N-linked oligosaccharides with alpha-2,6-linked sialic acids that are required for recognition. J Biol Chem. 1993;268:7019–7027. [PubMed] [Google Scholar]
- 27.Powell LD, Jain RK, Matta KL, Sabesan S, Varki A. Characterization of sialyloligosaccharide binding by recombinant soluble and native cell-associated CD22. Evidence for a minimal structural recognition motif and the potential importance of multisite binding. J Biol Chem. 1995;270:7523–7532. doi: 10.1074/jbc.270.13.7523. [DOI] [PubMed] [Google Scholar]
- 28.Engel P, et al. The same epitope on CD22 of B lymphocytes mediates the adhesion of erythrocytes, T and B lymphocytes, neutrophils, and monocytes. J Immunol. 1993;150:4719–4732. [PubMed] [Google Scholar]
- 29.Hanasaki K, Varki A, Powell LD. CD22-mediated cell adhesion to cytokine-activated human endothelial cells. Positive and negative regulation by alpha 2-6-sialylation of cellular glycoproteins. The Journal of biological chemistry. 1995;270:7533–7542. doi: 10.1074/jbc.270.13.7533. [DOI] [PubMed] [Google Scholar]
- 30.Nitschke L, Carsetti R, Ocker B, Kohler G, Lamers MC. CD22 is a negative regulator of B-cell receptor signalling. Current biology : CB. 1997;7:133–143. doi: 10.1016/s0960-9822(06)00057-1. [DOI] [PubMed] [Google Scholar]
- 31.Shaffer AL, et al. Blimp-1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity. 2002;17:51–62. doi: 10.1016/s1074-7613(02)00335-7. [DOI] [PubMed] [Google Scholar]
- 32.Leprince C, Draves KE, Geahlen RL, Ledbetter JA, Clark EA. CD22 associates with the human surface IgM-B-cell antigen receptor complex. Proc Natl Acad Sci USA. 1993;90:3236–3240. doi: 10.1073/pnas.90.8.3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cornall RJ, et al. Polygenic autoimmune traits: Lyn, CD22, and SHP-1 are limiting elements of a biochemical pathway regulating BCR signaling and selection. Immunity. 1998;8:497–508. doi: 10.1016/s1074-7613(00)80554-3. [DOI] [PubMed] [Google Scholar]
- 34.Smith KG, Tarlinton DM, Doody GM, Hibbs ML, Fearon DT. Inhibition of the B cell by CD22: a requirement for Lyn. J Exp Med. 1998;187:807–811. doi: 10.1084/jem.187.5.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doody GM, et al. A role in B cell activation for CD22 and the protein tyrosine phosphatase SHP. Science. 1995;269:242–244. doi: 10.1126/science.7618087. [DOI] [PubMed] [Google Scholar]
- 36.Lajaunias F, et al. Differentially regulated expression and function of CD22 in activated B-1 and B-2 lymphocytes. J Immunol. 2002;168:6078–6083. doi: 10.4049/jimmunol.168.12.6078. [DOI] [PubMed] [Google Scholar]
- 37.Gerlach J, et al. B cell defects in SLP65/BLNK-deficient mice can be partially corrected by the absence of CD22, an inhibitory coreceptor for BCR signaling. Eur J Immunol. 2003;33:3418–3426. doi: 10.1002/eji.200324290. [DOI] [PubMed] [Google Scholar]
- 38.Sato S, Jansen PJ, Tedder TF. CD19 and CD22 expression reciprocally regulates tyrosine phosphorylation of Vav protein during B lymphocyte signaling. Proc Natl Acad Sci USA. 1997;94:13158–13162. doi: 10.1073/pnas.94.24.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller J, et al. CD22 ligand-binding and signaling domains reciprocally regulate B-cell Ca2+ signaling. Proc Natl Acad Sci USA. 2013;110:12402–12407. doi: 10.1073/pnas.1304888110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, McLean PA, Neel BG, Okunade G, Shull GE, Wortis HH. CD22 attenuates calcium signaling by potentiating plasma membrane calcium-ATPase activity. Nat Immunol. 2004;5:651–657. doi: 10.1038/ni1072. [DOI] [PubMed] [Google Scholar]
- 41.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 42.Chan VW, Meng F, Soriano P, DeFranco AL, Lowell CA. Characterization of the B lymphocyte populations in Lyn-deficient mice and the role of Lyn in signal initiation and down-regulation. Immunity. 1997;7:69–81. doi: 10.1016/s1074-7613(00)80511-7. [DOI] [PubMed] [Google Scholar]
- 43.Takai T, Ono M, Hikida M, Ohmori H, Ravetch JV. Augmented humoral and anaphylactic responses in Fc gamma RII-deficient mice. Nature. 1996;379:346–349. doi: 10.1038/379346a0. [DOI] [PubMed] [Google Scholar]
- 44.Pao LI, et al. B cell-specific deletion of protein-tyrosine phosphatase Shp1 promotes B-1a cell development and causes systemic autoimmunity. Immunity. 2007;27:35–48. doi: 10.1016/j.immuni.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 45.Mary C, et al. Dysregulated expression of the Cd22 gene as a result of a short interspersed nucleotide element insertion in Cd22a lupus-prone mice. J Immunol. 2000;165:2987–2996. doi: 10.4049/jimmunol.165.6.2987. [DOI] [PubMed] [Google Scholar]
- 46.O'Keefe TL, Williams GT, Batista FD, Neuberger MS. Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J Exp Med. 1999;189:1307–1313. doi: 10.1084/jem.189.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razi N, Varki A. Masking and unmasking of the sialic acid-binding lectin activity of CD22 (Siglec-2) on B lymphocytes. Proc Natl Acad Sci USA. 1998;95:7469–7474. doi: 10.1073/pnas.95.13.7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc Natl Acad Sci USA. 2004;101:6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. Sialoside specificity of the siglec family assessed using novel multivalent probes: identification of potent inhibitors of myelin-associated glycoprotein. J Biol Chem. 2003;278:31007–31019. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 50.Collins BE, et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol. 2006;177:2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- 51.Han S, Collins BE, Bengtson P, Paulson JC. Homomultimeric complexes of CD22 in B cells revealed by protein-glycan cross-linking. Nat Chem Biol. 2005;1:93–97. doi: 10.1038/nchembio713. [DOI] [PubMed] [Google Scholar]
- 52.Harwood NE, Batista FD. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb Perspect Biol. 2011:3. doi: 10.1101/cshperspect.a002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J Cell Biol. 2002;157:1071–1081. doi: 10.1083/jcb.200202050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peaker CJ, Neuberger MS. Association of CD22 with the B cell antigen receptor. Eur J Immunol. 1993;23:1358–1363. doi: 10.1002/eji.1830230626. [DOI] [PubMed] [Google Scholar]
- 55.Poe JC, et al. CD22 regulates B lymphocyte function in vivo through both ligand-dependent and ligand-independent mechanisms. Nat Immunol. 2004;5:1078–1087. doi: 10.1038/ni1121. [DOI] [PubMed] [Google Scholar]
- 56.Zhang M, Varki A. Cell surface sialic acids do not affect primary CD22 interactions with CD45 and surface IgM nor the rate of constitutive CD22 endocytosis. Glycobiology. 2004;14:939–949. doi: 10.1093/glycob/cwh126. [DOI] [PubMed] [Google Scholar]
- 57.Ramya TN, et al. In situ trans ligands of CD22 identified by glycan-protein photocross-linking-enabled proteomics. Mol Cell Proteomics. 2010;9:1339–1351. doi: 10.1074/mcp.M900461-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adachi T, et al. CD22 serves as a receptor for soluble IgM. Eur J Immunol. 2012;42:241–247. doi: 10.1002/eji.201141899. [DOI] [PubMed] [Google Scholar]
- 59.Macauley MS, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123:3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wienands J, Larbolette O, Reth M. Evidence for a preformed transducer complex organized by the B cell antigen receptor. Proc Natl Acad Sci USA. 1996;93:7865–7870. doi: 10.1073/pnas.93.15.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Monroe JG. ITAM-mediated tonic signalling through pre-BCR and BCR complexes. Nat Rev Immunol. 2006;6:283–294. doi: 10.1038/nri1808. [DOI] [PubMed] [Google Scholar]
- 62.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proc Natl Acad Sci USA. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Collins BE, Smith BA, Bengtson P, Paulson JC. Ablation of CD22 in ligand-deficient mice restores B cell receptor signaling. Nat Immunol. 2006;7:199–206. doi: 10.1038/ni1283. [DOI] [PubMed] [Google Scholar]
- 64.Prescher H, Schweizer A, Kuhfeldt E, Nitschke L, Brossmer R. Discovery of multifold modified sialosides as human CD22/Siglec-2 ligands with nanomolar activity on B-cells. ACS Chem Biol. 2014;9:1444–1450. doi: 10.1021/cb400952v. [DOI] [PubMed] [Google Scholar]
- 65.Nitschke L, et al. Expression of aberrant forms of CD22 on B lymphocytes in Cd22a lupus-prone mice affects ligand binding. International immunology. 2006;18:59–68. doi: 10.1093/intimm/dxh349. [DOI] [PubMed] [Google Scholar]
- 66.Chen GY, et al. Amelioration of sepsis by inhibiting sialidase-mediated disruption of the CD24-SiglecG interaction. Nature biotechnology. 2011;29:428–435. doi: 10.1038/nbt.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duong BH, et al. Decoration of T-independent antigen with ligands for CD22 and Siglec-G can suppress immunity and induce B cell tolerance in vivo. The Journal of experimental medicine. 2010;207:173–187. doi: 10.1084/jem.20091873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann A, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. doi: 10.1038/ni1480. [DOI] [PubMed] [Google Scholar]
- 69.Bokers S, et al. Siglec-G deficiency leads to more severe collagen-induced arthritis and earlier onset of lupus-like symptoms in MRL/lpr mice. J Immunol. 2014;192:2994–3002. doi: 10.4049/jimmunol.1303367. [DOI] [PubMed] [Google Scholar]
- 70.Jellusova J, Wellmann U, Amann K, Winkler TH, Nitschke L. CD22 x Siglec-G double-deficient mice have massively increased B1 cell numbers and develop systemic autoimmunity. J Immunol. 2010;184:3618–3627. doi: 10.4049/jimmunol.0902711. [DOI] [PubMed] [Google Scholar]
- 71.Muller J, et al. Siglec-G Deficiency Leads to Autoimmunity in Aging C57BL/6 Mice. J Immunol. 2015;195:51–60. doi: 10.4049/jimmunol.1403139. [DOI] [PubMed] [Google Scholar]
- 72.Hutzler S, et al. The ligand-binding domain of Siglec-G is crucial for its selective inhibitory function on B1 cells. Journal of immunology. 2014;192:5406–5414. doi: 10.4049/jimmunol.1302875. [DOI] [PubMed] [Google Scholar]
- 73.Hutzler S, et al. The ligand-binding domain of Siglec-G is crucial for its selective inhibitory function on B1 cells. J Immunol. 2014;192:5406–5414. doi: 10.4049/jimmunol.1302875. [DOI] [PubMed] [Google Scholar]
- 74.Pfrengle F, Macauley MS, Kawasaki N, Paulson JC. Copresentation of antigen and ligands of Siglec-G induces B cell tolerance independent of CD22. Journal of immunology. 2013;191:1724–1731. doi: 10.4049/jimmunol.1300921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macauley MS, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. The Journal of clinical investigation. 2013;123:3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macauley MS, Paulson JC. Siglecs induce tolerance to cell surface antigens by BIM-dependent deletion of the antigen-reactive B cells. Journal of immunology. 2014;193:4312–4321. doi: 10.4049/jimmunol.1401723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chang CH, Wang Y, Gupta P, Goldenberg DM. Extensive crosslinking of CD22 by epratuzumab triggers BCR signaling and caspase-dependent apoptosis in human lymphoma cells. mAbs. 2015;7:199–211. doi: 10.4161/19420862.2014.979081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sjoberg ER, Powell LD, Klein A, Varki A. Natural ligands of the B cell adhesion molecule CD22 beta can be masked by 9-O-acetylation of sialic acids. J Cell Biol. 1994;126:549–562. doi: 10.1083/jcb.126.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 80.Shi WX, Chammas R, Varki NM, Powell L, Varki A. Sialic acid 9-O-acetylation on murine erythroleukemia cells affects complement activation, binding to I-type lectins, and tissue homing. J Biol Chem. 1996;271:31526–31532. doi: 10.1074/jbc.271.49.31526. [DOI] [PubMed] [Google Scholar]
- 81.Tiralongo J, Schauer R. The Enigma of Enzymatic Sialic Acid O-Acetylation. Trends in Glycoscience and Glycotechnology. 2004;16:1–15. [Google Scholar]
- 82.Vandamme-Feldhaus V, Schauer R. Characterization of the enzymatic 7-O-acetylation of sialic acids and evidence for enzymatic O-acetyl migration from C-7 to C-9 in bovine submandibular gland. J Biochem. 1998;124:111–121. doi: 10.1093/oxfordjournals.jbchem.a022069. [DOI] [PubMed] [Google Scholar]
- 83.Kamerling JP, Schauer R, Shukla AK, Stoll S, Van Halbeek H, Vliegenthart JF. Migration of O-acetyl groups in N,O-acetylneuraminic acids. Eur J Biochem. 1987;162:601–607. doi: 10.1111/j.1432-1033.1987.tb10681.x. [DOI] [PubMed] [Google Scholar]
- 84.Arming S, et al. The human Cas1 protein: a sialic acid-specific O-acetyltransferase? Glycobiology. 2011;21:553–564. doi: 10.1093/glycob/cwq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pillai S, Cariappa A, Pirnie SP. Esterases and autoimmunity: the sialic acid acetylesterase pathway and the regulation of peripheral B cell tolerance. Trends Immunol. 2009;30:488–493. doi: 10.1016/j.it.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chellappa V, et al. M89V Sialic acid Acetyl Esterase (SIAE) and all other non-synonymous common variants of this gene are catalytically normal. PloS one. 2013;8:e53453. doi: 10.1371/journal.pone.0053453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gan EH, MacArthur K, Mitchell AL, Pearce SH. The role of functionally defective rare germline variants of sialic acid acetylesterase in autoimmune Addison's disease. European journal of endocrinology / European Federation of Endocrine Societies. 2012;167:825–828. doi: 10.1530/EJE-12-0579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirschfield GM, et al. Association of primary biliary cirrhosis with variants in the CLEC16A, SOCS1, SPIB and SIAE immunomodulatory genes. Genes and immunity. 2012;13:328–335. doi: 10.1038/gene.2011.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hunt KA, et al. Rare and functional SIAE variants are not associated with autoimmune disease risk in up to 66,924 individuals of European ancestry. Nature genetics. 2012;44:3–5. doi: 10.1038/ng.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto M, et al. A missense single-nucleotide polymorphism in the sialic acid acetylesterase (SIAE) gene is associated with anti-PIT-1 antibody syndrome. Endocrine journal. 2014;61:641–644. doi: 10.1507/endocrj.ej13-0539. [DOI] [PubMed] [Google Scholar]
- 91.Naito Y, et al. Germinal center marker GL7 probes activation-dependent repression of N-glycolylneuraminic acid, a sialic acid species involved in the negative modulation of B-cell activation. Mol Cell Biol. 2007;27:3008–3022. doi: 10.1128/MCB.02047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelm S, Schauer R, Manuguerra JC, Gross HJ, Crocker PR. Modifications of cell surface sialic acids modulate cell adhesion mediated by sialoadhesin and CD22. Glycoconjugate journal. 1994;11:576–585. doi: 10.1007/BF00731309. [DOI] [PubMed] [Google Scholar]
- 93.Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kimura N, et al. Human B-lymphocytes express alpha2-6-sialylated 6-sulfo-N-acetyllactosamine serving as a preferred ligand for CD22/Siglec-2. The Journal of biological chemistry. 2007;282:32200–32207. doi: 10.1074/jbc.M702341200. [DOI] [PubMed] [Google Scholar]
- 95.Banda K, Gregg CJ, Chow R, Varki NM, Varki A. Metabolism of vertebrate amino sugars with N-glycolyl groups: mechanisms underlying gastrointestinal incorporation of the non-human sialic acid xeno-autoantigen N-glycolylneuraminic acid. J Biol Chem. 2012;287:28852–28864. doi: 10.1074/jbc.M112.364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Taylor RE, et al. Novel mechanism for the generation of human xeno-autoantibodies against the nonhuman sialic acid N-glycolylneuraminic acid. J Exp Med. 2010;207:1637–1646. doi: 10.1084/jem.20100575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pham T, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Samraj AN, Laubli H, Varki N, Varki A. Involvement of a non-human sialic Acid in human cancer. Front Oncol. 2014;4:33. doi: 10.3389/fonc.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Samraj AN, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci USA. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Padler-Karavani V, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sinha R, Cross AJ, Graubard BI, Leitzmann MF, Schatzkin A. Meat intake and mortality: a prospective study of over half a million people. Arch Intern Med. 2009;169:562–571. doi: 10.1001/archinternmed.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan A, et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med. 2012;172:555–563. doi: 10.1001/archinternmed.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Atkinson JP, Goodship TH. Complement factor H and the hemolytic uremic syndrome. J Exp Med. 2007;204:1245–1248. doi: 10.1084/jem.20070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raychaudhuri S, et al. A rare penetrant mutation in CFH confers high risk of age-related macular degeneration. Nat Genet. 2011;43:1232–1236. doi: 10.1038/ng.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shaw PX, et al. Complement factor H genotypes impact risk of age-related macular degeneration by interaction with oxidized phospholipids. Proc Natl Acad Sci USA. 2012;109:13757–13762. doi: 10.1073/pnas.1121309109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pincetic A, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nature immunology. 2014;15:707–716. doi: 10.1038/ni.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Anthony RM, Nimmerjahn F, Ashline DJ, Reinhold VN, Paulson JC, Ravetch JV. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fiebiger BM, Maamary J, Pincetic A, Ravetch JV. Protection in antibody- and T cell-mediated autoimmune diseases by antiinflammatory IgG Fcs requires type II FcRs. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2385–2394. doi: 10.1073/pnas.1505292112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen GY, Tang J, Zheng P, Liu Y. CD24 and Siglec-10 selectively repress tissue damage-induced immune responses. Science. 2009;323:1722–1725. doi: 10.1126/science.1168988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Segura T, Volk MJ, Shea LD. Substrate-mediated DNA delivery: role of the cationic polymer structure and extent of modification. Journal of controlled release : official journal of the Controlled Release Society. 2003;93:69–84. doi: 10.1016/j.jconrel.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Wang H, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 112.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 113.Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. HMGB1 and RAGE in inflammation and cancer. Annual review of immunology. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- 114.Tan Y, Zhao M, Xiang B, Chang C, Lu Q. CD24: from a Hematopoietic Differentiation Antigen to a Genetic Risk Factor for Multiple Autoimmune Diseases. Clinical reviews in allergy & immunology. 2015 doi: 10.1007/s12016-015-8470-2. [DOI] [PubMed] [Google Scholar]
- 115.Carl JW, Jr., et al. Autoreactive T cells escape clonal deletion in the thymus by a CD24-dependent pathway. Journal of immunology. 2008;181:320–328. doi: 10.4049/jimmunol.181.1.320. [DOI] [PubMed] [Google Scholar]
- 116.Li O, et al. Massive and destructive T cell response to homeostatic cue in CD24-deficient lymphopenic hosts. The Journal of experimental medicine. 2006;203:1713–1720. doi: 10.1084/jem.20052293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tsuboi S, Fukuda M. Roles of O-linked oligosaccharides in immune responses. BioEssays : news and reviews in molecular, cellular and developmental biology. 2001;23:46–53. doi: 10.1002/1521-1878(200101)23:1<46::AID-BIES1006>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 118.Cummings RD, Smith DF. The selectin family of carbohydrate-binding proteins: structure and importance of carbohydrate ligands for cell adhesion. BioEssays : news and reviews in molecular, cellular and developmental biology. 1992;14:849–856. doi: 10.1002/bies.950141210. [DOI] [PubMed] [Google Scholar]
- 119.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annual review of biochemistry. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 120.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 121.Yang XD, Karin N, Tisch R, Steinman L, McDevitt HO. Inhibition of insulitis and prevention of diabetes in nonobese diabetic mice by blocking L-selectin and very late antigen 4 adhesion receptors. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:10494–10498. doi: 10.1073/pnas.90.22.10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Archelos JJ, Fortwangler T, Hartung HP. Attenuation of experimental autoimmune neuritis in the Lewis rat by treatment with an antibody to L-selectin. Neuroscience letters. 1997;235:9–12. doi: 10.1016/s0304-3940(97)00692-7. [DOI] [PubMed] [Google Scholar]
- 123.Friedline RH, Wong CP, Steeber DA, Tedder TF, Tisch R. L-selectin is not required for T cell-mediated autoimmune diabetes. Journal of immunology. 2002;168:2659–2666. doi: 10.4049/jimmunol.168.6.2659. [DOI] [PubMed] [Google Scholar]
- 124.Mora C, Grewal IS, Wong FS, Flavell RA. Role of L-selectin in the development of autoimmune diabetes in non-obese diabetic mice. International immunology. 2004;16:257–264. doi: 10.1093/intimm/dxh036. [DOI] [PubMed] [Google Scholar]
- 125.Uboldi C, Doring A, Alt C, Estess P, Siegelman M, Engelhardt B. L-Selectin-deficient SJL and C57BL/6 mice are not resistant to experimental autoimmune encephalomyelitis. European journal of immunology. 2008;38:2156–2167. doi: 10.1002/eji.200838209. [DOI] [PubMed] [Google Scholar]
- 126.Angiari S, et al. TIM-1 glycoprotein binds the adhesion receptor P-selectin and mediates T cell trafficking during inflammation and autoimmunity. Immunity. 2014;40:542–553. doi: 10.1016/j.immuni.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Angiari S, Constantin G. Regulation of T cell trafficking by the T cell immunoglobulin and mucin domain 1 glycoprotein. Trends Mol Med. 2014;20:675–684. doi: 10.1016/j.molmed.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 128.Perez-Frias A, et al. Development of an autoimmune syndrome affecting the skin and internal organs in P-selectin glycoprotein ligand 1 leukocyte receptor-deficient mice. Arthritis & rheumatology. 2014;66:3178–3189. doi: 10.1002/art.38808. [DOI] [PubMed] [Google Scholar]
- 129.Varki A. Selectin ligands: will the real ones please stand up? The Journal of clinical investigation. 1997;100:S31–35. [PubMed] [Google Scholar]
- 130.Rho JH, et al. Discovery of sialyl Lewis A and Lewis X modified protein cancer biomarkers using high density antibody arrays. Journal of proteomics. 2014;96:291–299. doi: 10.1016/j.jprot.2013.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Miyara M, et al. Sialyl Lewis x (CD15s) identifies highly differentiated and most suppressive FOXP3high regulatory T cells in humans. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:7225–7230. doi: 10.1073/pnas.1508224112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ernst B, Magnani JL. From carbohydrate leads to glycomimetic drugs. Nature reviews Drug discovery. 2009;8:661–677. doi: 10.1038/nrd2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Otipoby KL, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 134.Sato S, et al. CD22 is both a positive and negative regulator of B lymphocyte antigen receptor signal transduction: altered signaling in CD22-deficient mice. Immunity. 1996;5:551–562. doi: 10.1016/s1074-7613(00)80270-8. [DOI] [PubMed] [Google Scholar]
- 135.Ghosh S, Bandulet C, Nitschke L. Regulation of B cell development and B cell signalling by CD22 and its ligands alpha2,6-linked sialic acids. International immunology. 2006;18:603–611. doi: 10.1093/intimm/dxh402. [DOI] [PubMed] [Google Scholar]
- 136.Hennet T, Chui D, Paulson JC, Marth JD. Immune regulation by the ST6Gal sialyltransferase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:4504–4509. doi: 10.1073/pnas.95.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grewal PK, et al. ST6Gal-I restrains CD22-dependent antigen receptor endocytosis and Shp-1 recruitment in normal and pathogenic immune signaling. Molecular and cellular biology. 2006;26:4970–4981. doi: 10.1128/MCB.00308-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Molecular and cellular biology. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]