Abstract
Interstitial lung disease (ILD) is a rare adverse effect of nitrofurantoin and can range from benign infiltrates to a fatal condition. Nitrofurantoin acts via inhibiting the protein synthesis in bacteria by helping reactive intermediates and is known to produce primary lung parenchymal injury through an oxidant mechanism. Stopping the drug leads to complete recovery of symptoms. In this report, we present a case of nitrofurantoin-induced ILD with the recovery of symptoms and disease process after stopping the drug.
Background
Multitudes of drugs are now known to cause lung diseases, and nitrofurantoin is one of them. It entered the market in 1953. It is commonly used for the treatment of acute or recurrent symptomatic urinary tract infection (UTI) and prophylaxis of recurrent UTI.1 Nitrofurantoin is found to have a clinical efficacy equivalent to that of trimethoprim-sulfamethoxazole, ciprofloxacin and amoxicillin, for treatment of UTI.2 Nitrofurantoin-induced pulmonary toxicity was first reported in 1962; since then, hundreds of similar cases have been reported.3 From an analysis of 921 cases of adverse reactions to nitrofurantoin, acute pulmonary reaction constituted about 43% of all the adverse reactions reported. While allergic reactions were (42%), the remaining cases fell into any of the four smaller groups: chronic pulmonary reactions, liver damage, blood dyscrasia or neuropathy.4 Few acute and chronic pulmonary reactions to nitrofurantoin have turned out to be fatal.3 5–7 The discontinuation of nitrofurantoin is associated with almost complete resolution of symptoms in most of the cases. So, it is necessary to consider nitrofurantoin-induced pulmonary reaction early in the course of the disease to prevent fatal outcome.8
We have a large body of evidence that nitrofurantoin leads to lung disease, besides, there have been fatalities reported due to failure in considering this condition in the differential diagnosis of interstitial lung disease (ILD), thus causing a delay in discontinuation of the drug. It is necessary to recognise the uncommon adverse effects of commonly used drugs, to prevent the unnecessary hardship the patient has to go through, in addition to the financial burden associated with these effects and, most importantly, to prevent fatalities. In this report, we present a case of chronic pulmonary reaction to nitrofurantoin in a patient on long-term nitrofurantoin therapy.
Case presentation
A 37-year-old woman with a medical history significant for recurrent UTI, attention deficit disorder and premature menopause, presented with coughing spells she had developed over 6 weeks. According to the patient, her coughing spells were severe and associated with chest tightness. The cough was dry and nocturnal, occurring mostly between 20:00 and 22:00. She had no associated chest pain, haemoptysis, night sweats, fever, weight loss, legs swelling, orthopnoea, paroxysmal nocturnal dyspnoea, or history of travel or sick contact. She had a history of recurrent UTI for which she had been on nitrofurantoin 100 mg daily at bedtime for the past 2 years.
Other medications included nortriptyline 100 mg at bedtime, dextroamphetamine/amphetamine XR 10 mg as needed, and albuterol inhaler as needed, prescribed for her cough, which provided only minimal relief. The patient denied smoking and took alcohol occasionally; she worked as a registered nurse and lived with her husband, two healthy children and a dog.
On physical examination, she was not in acute distress, had normal vital signs and her oxygen saturation was 96% on room air. Lung examination revealed fine inspiratory crackles in both lung bases posteriorly; she had a normal heart, abdomen and extremities examination.
Initially, the patient was treated as having a case of community-acquired pneumonia. She was given a prescription of levofloxacin for 7 days; however, she did not feel better after completing the full course of antibiotic, so the primary care physician ordered a chest X-ray, which showed bilateral interstitial infiltrates, thus, he referred her to the pulmonologist for further management.
Investigations
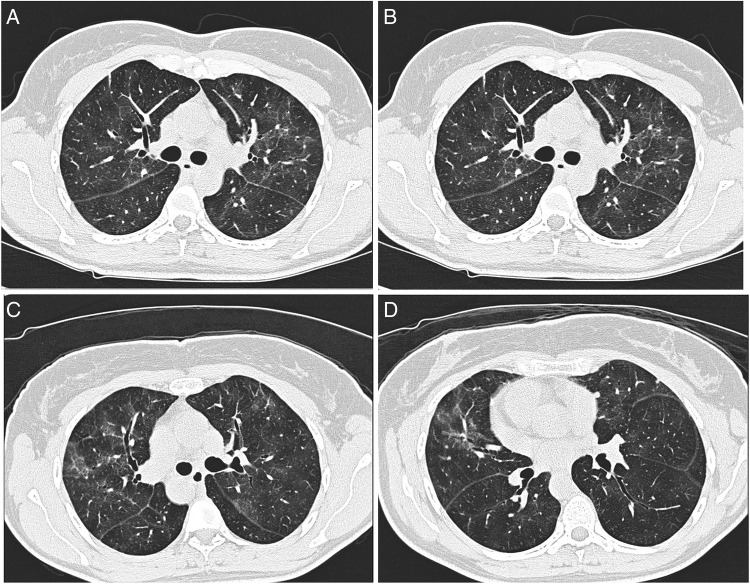
White cell count: 11.8×109/L, neutrophils 73% and eosinophils 1%. Chest X-ray showed diffuse bilateral interstitial prominence. High-resolution CT (HRCT) scan showed patchy ground-glass densities throughout the lung parenchyma with minimal septal thickening in the upper and mid-lung fields figure 1(A–D). Pulmonary function tests (PFTs) showed forced vital capacity (FVC) of 2.1 L, which was 60% of that predicted for her age and sex. Forced expiratory volume in the 1 second (FEV1), of 1.78 L, was 66% of predicted, while the FEV1/FVC ratio of 85% was consistent with the restrictive pattern. Postbronchodilator readings showed no significant change. Diffusion capacity was 60% of predicted. Lung volumes and capacities reduced to less than 80% of normal value.
Figure 1.
(A–D) HRCT showing high attenuation patchy ground-glass opacities with minimal interlobular septal thickening.
Differential diagnosis
The differential diagnosis includes atypical pneumonia, hypersensitivity pneumonitis, cryptogenic organising pneumonia, granulomatous lung disease and various causes of interstitial lung disease. Owing to the lengthy history (2 years) of administration of nitrofurantoin to prevent recurrent UTI, the possibility of nitrofurantoin-induced lung injury was also considered.
There was no history of exposure to any agricultural dusts, bioaerosols or microorganisms (fungal, bacterial or protozoal)—nor to any particular reactive chemical species—to suggest hypersensitivity pneumonitis.
Considering cryptogenic organising pneumonia, it usually affects patients in their fifth to sixth decades and often responds to treatment with corticosteroid therapy.
The patient had no history or manifestations of connective tissue diseases such as systemic lupus erythematosus, rheumatoid arthritis, scleroderma or vasculitis. She reported neither skin rash, renal involvement, haematuria, proteinuria nor haemoptysis.
Chest imaging (HRCT) revealed ground-glass densities throughout lung parenchyma, with no dominant pattern of zonal distribution. In contrast to patients with idiopathic pulmonary fibrosis characterised by irregular linear opacities in addition to honeycombing, both were undistinctive features in patients with nitrofurantoin-induced lung disease.
Treatment
The patient was asked to stop taking nitrofurantoin and to continue taking albuterol inhaler for symptomatic relief.
Outcome and follow-up
After 10 days of stopping nitrofurantoin, the patient reported resolution of cough and only minimal chest tightness on exertion. Overall, she reported significant improvement in her general condition. She was scheduled to have a repeat CT and PFT after 3 months, but due to personal issues declined the tests, however, she attended the pulmonology office and reported no symptoms after 3 months.
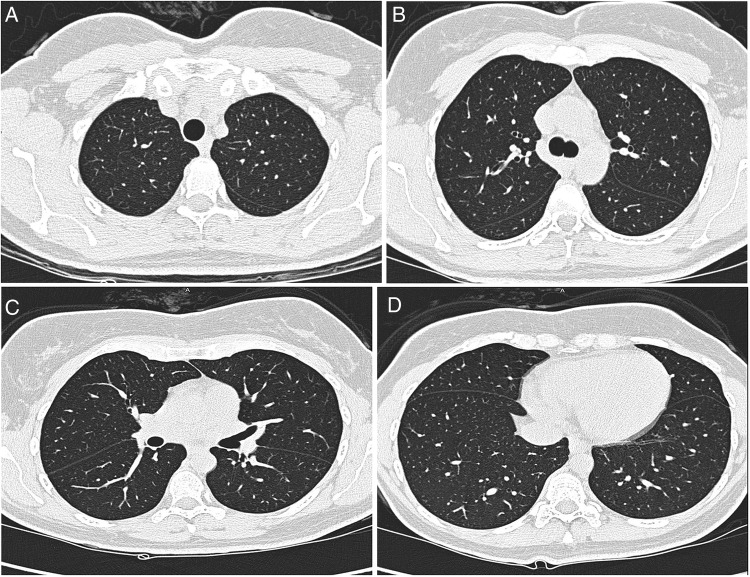
After 6 months, repeat PFT showed FVC of 2.93 L, 96% of predicted, FEV1 2.56 L, 96% of predicated and FEV1/FVC ratio of 87%. Diffusion capacity was 86% of predicted. Airway resistance studies and flow volume loop were normal in appearance. Repeat HRCT of the chest showed almost complete resolution of the abnormalities that had been seen in the previous scan figure 2(A–D). The plan was to perform lung biopsy if the patient did not improve on discontinuing nitrofurantoin.
Figure 2.
(A–D) Follow-up HRCT of the chest (after 6 months of withdrawal of nitrofurantoin) showing complete radiological resolution of the abnormalities previously seen.
Discussion
Nitrofurantoin-induced lung injury can be acute, subacute or chronic; it usually presents with a dry cough and dyspnoea.7–12 Acute pulmonary reactions appear after a mean of 8.7 days from starting nitrofurantoin therapy, symptoms of subacute and chronic pulmonary reactions develop after between 1 and 6 months of treatment, respectively.10 However, in an analysis of 921 reported cases by Holmberg et al, 47% of cases of chronic respiratory disease occurred after more than 12 months of therapy; in the same study, women outnumbered men in all types of reactions to nitrofurantoin except for blood dyscrasia.4 5
Chronic eosinophilic pneumonia and cryptogenic organising pneumonia also have been associated with nitrofurantoin.13 14 Lung fibrosis is the most common finding associated with long-term nitrofurantoin therapy.4 12 15–17
Different mechanisms cause acute and chronic pulmonary reactions to nitrofurantoin. In acute pulmonary reactions, histopathological examination of the lung typically reveals vasculitis, mild interstitial inflammation, eosinophilia, reactive type II pneumocytes, focal haemorrhage and small organising microthrombi.10 18–20 In contrast, diffuse interstitial fibrosis is observed in chronic reactions.11 Vascular sclerosis, fibrosis and thickening of the alveolar septa, and interstitial inflammation, are common features.10
Initial symptoms in nitrofurantoin reactions have been characterised by fever (70%), dyspnoea (34%), exanthema (28%), dry cough (26%), fatigue (12%), flu-like symptoms (9%), cyanosis (4%), Jaundice (3%) and weight loss (2%).4 6 Acute pulmonary reactions usually present with dry cough (66%), fever (80%), increased erythrocyte sedimentation rate (80%) and eosinophilia (80%).7 In contrast, patients with a chronic form of reaction usually presented with dyspnoea (73%), dry cough (63%) and fatigue (37%).4 7 10 Pulmonary toxicity caused by nitrofurantoin is very rare and estimated to be around 0.0013% of the therapeutic course.17
In a retrospective study of 18 patients with chronic nitrofurantoin-induced lung injury, CT chest findings were bilateral areas of ground-glass attenuation with no dominant pattern of zonal distribution. In contrast to patients with idiopathic pulmonary fibrosis, irregular linear opacities were uncommon in patients with nitrofurantoin-induced lung disease. Besides, honeycombing was typically absent.5 9
Though most cases are reversible on discontinuation of medication, the overall mortality in the largest analysis available so far for nitrofurantoin toxicity was 1.19%.4
There is not enough evidence to prove the role of glucocorticoid for the treatment.21 Some studies found that superoxide and hydrogen peroxide production, as well as nicotinamide adenine dinucleotide phosphate (NADPH) oxidation, might be the cause of lung toxicity induced by nitrofurantoin.19 The in vivo study showed a reduction in injury of lung tissue when incubated with nitrofurantoin and antioxidant in comparison with nitrofurantoin alone.20 The nitrofurantoin preparation that contained vitamin C caused less hypersensitivity reaction. Vitamin C might affect the immunological reactivity or protect the capillary wall, and, therefore, it was initially added to nitrofurantoin therapy, to reduce the possible toxic effect of the drug at the tissue level, in addition to having an acidifying effect on the urine, thus enhancing the impact of the drug.10
Learning points.
Nitrofurantoin-induced pulmonary reactions can present similarly to most other commonly encountered respiratory diseases.
It is crucial to have a high index of suspicion to avoid missing such cases, thus a good review of the medication history is necessary to make the diagnosis.
Discontinuation of nitrofurantoin is enough for complete recovery in most instances. It is also important to remember that, if not discontinued in time, it can be fatal.
Patients on long-term use of nitrofurantoin should be regularly monitored for adverse pulmonary effects.
There is not enough evidence to prove the role of glucocorticoid for the treatment of nitrofurantoin-induced lung injury. Nitrofurantoin preparations that contain vitamin C might cause fewer hypersensitivity reactions.
Acknowledgments
The authors would like to thank the other physicians who were involved in patient care: Dr Syed Sattar, MD, and Dr Thomas M Varkey, MD.
Footnotes
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Blass B. Basic principles of drug discovery and development. Elsevier, 2015. [Google Scholar]
- 2.Huttner A, Verhaegh EM, Harbarth S et al. Nitrofurantoin revisited: a systematic review and meta-analysis of controlled trials. J Antimicrob Chemother 2015;70:2456–64. 10.1093/jac/dkv147 [DOI] [PubMed] [Google Scholar]
- 3.Israel HI, Diamond P. Recurrent pulmonary infiltration and pleural effusion due to nitrofurantoin sensitivity. N Engl J Med 1962;266:1024–6. 10.1056/NEJM196205172662002 [DOI] [Google Scholar]
- 4.Holmberg L, Boman G, Böttiger LE et al. Adverse reactions to nitrofurantoin. Analysis of 921 reports. Am J Med 1980;69:733–8. 10.1016/0002-9343(80)90443-X [DOI] [PubMed] [Google Scholar]
- 5.Mendez JL, Nadrous HF, Hartman TE et al. Chronic nitrofurantoin-induced lung disease. Mayo Clin Proc 2005;80:1298 10.4065/80.10.1298 [DOI] [PubMed] [Google Scholar]
- 6.Mullerpattan JB, Dagaonkar RS, Shah HD et al. Fatal Nitrofurantoin lung. J Assoc Physicians India 2013;61:758–60. [PubMed] [Google Scholar]
- 7.Holmberg L, Boman G. Pulmonary reactions to nitrofurantoin. 447 cases reported to the Swedish adverse Drug Reaction Committee 1966–1976. Eur J Respir Dis 1981;62:180–9. [PubMed] [Google Scholar]
- 8.Schattner A, Von der WAlde J, Kozak N et al. Nitrofurantoin-Induced Immune-Mediated lung and liver disease. Am J Med Sci 1999;317:336–40. 10.1016/S0002-9629(15)40536-1 [DOI] [PubMed] [Google Scholar]
- 9.Padley SP, Adler B, Hansell DM et al. High resolution computed tomography of drug induced lung disease. Clin Radiol 1992;46:232–6. 10.1016/S0009-9260(05)80161-8 [DOI] [PubMed] [Google Scholar]
- 10.Sovijari AR, Lemola M, Stenius B et al. Nitrofurantoin-induced acute, subacute and chronic pulmonary reactions. Scand J Respir Dis 1977;58:41–50. [PubMed] [Google Scholar]
- 11.Hainer BL, White AA. Nitrofurantoin pulmonary toxicity. J Fam Pract 1981;13:817. [PubMed] [Google Scholar]
- 12.Goemaere NN, Grijm K, van Hal P et al. Nitrofurantoin-induced pulmonary fibrosis: a case report. J Med Case Rep 2008;2:169 10.1186/1752-1947-2-169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cameron RJ, Kolbe J, Wilsher ML et al. Bronchiolitis obliterans organizing pneumonia associated with the use of nitrofurantoin. Thorax 2000;55:249–51. 10.1136/thorax.55.3.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martins RR, Marchiori E, Viana SL et al. Chronic esonophilic pneumonia secondary to long term use of nitrofurantoin: high-resolution tomography findings. J Bras Pneumol 2008;34:181–4. 10.1590/S1806-37132008000300009 [DOI] [PubMed] [Google Scholar]
- 15.Willcox PA, Maze SS, Sandler M et al. Pulmonary fibrosis following long term nitrofurantoin therapy. S Afr Med J 1982;61:714–17. [PubMed] [Google Scholar]
- 16.Pérez Herms S, Castellanos Acosta R, Guzmán Fernández A et al. Pulmonary fibrosis secondary to nitrofurantoin. Arch Esp Urol 1992;45:577–8. [PubMed] [Google Scholar]
- 17.Jambeih R, Flesher J, Lin JJ. Severe nitrofurantoin-induced lung toxicity. Kansas J Med 2011;4:6–10. [Google Scholar]
- 18.D'Arcy PF. Nitrofurantoin. Drug Intell Clin Pharm 1985;19:540–7. [DOI] [PubMed] [Google Scholar]
- 19.Sasame HA, Boyd MR. Superoxide and hydrogen peroxide production and NADPH oxidation stimulated by nitrofurantoin in lung microsomes: possible implications for toxicity. Life Sci 1979;24:1091–6. 10.1016/0024-3205(79)90042-0 [DOI] [PubMed] [Google Scholar]
- 20.Martin WJ., II Nitrofurantoin: evidence for the oxidant injury of lung parenchymal cells. Am Rev Respir Dis 1983;127:482–6. 10.1164/arrd.1983.127.4.482 [DOI] [PubMed] [Google Scholar]
- 21.Robinson BW. Nitrofurantoin-induced interstitial pulmonary fibrosis. Presentation and outcome. Med J Aust 1983;1:72–6. [PubMed] [Google Scholar]