Abstract
A 63-year-old man with a history of non-ischaemic cardiomyopathy presented with acute worsening of heart failure and septic shock. Echocardiogram revealed a large aortic valve vegetation with new onset severe aortic incompetence. Blood cultures grew Granulicatella elegans, for which antimicrobial sensitivities could not be carried out in our lab. Despite antibiotic therapy and aggressive care, the patient's clinical condition worsened and he died. G. elegans, previously grouped under nutrient variant streptococci (NVS), is an extremely rare cause for bacterial infective endocarditis (IE). Unlike with the Viridans group, IE caused by NVS has a very poor outcome and higher mortality rate. The difficulty in isolation of the bacteria in culture, inability to reliably measure antibiotic susceptibility in vitro, frequent treatment failure and complications such as multivalvular involvement, make this an extremely challenging infection to treat. Early detection of the organism, appropriate antibiotics and early surgical management when indicated, are key to management.
Background
This case calls attention to the difficulties in accurately diagnosing and managing infective endocarditis (IE) caused by a rare organism, Granulicatella elegans. There is a lack of literature regarding the course and outcome of IE caused by this organism. There are only seven other case reports of G. elegans IE, to the best of our knowledge. This is also the first reported case of discitis in the setting of G. elegans bacteraemia and endocarditis. Clinicians should be aware of the high complication rate and recommended antibiotic regimen while treating this infection.
Case presentation
A 63-year-old Caucasian man with a history of non-ischaemic cardiomyopathy presented to the emergency room, with worsening shortness of breath, fatigue and malaise of 10 days duration. His other comorbidities included hypertension, paroxysmal atrial fibrillation and stage 3 chronic kidney disease. Cardiomyopathy was diagnosed 2½ years prior to the current admission with an ejection fraction of 30–35%. He also had a defibrillator implanted 8 months before presentation for the cardiomyopathy. Physical examination in the emergency room was significant for a new onset early diastolic decrescendo murmur, signs of congestive heart failure and poor dentition. Soon after admission, he was transferred to the intensive care unit for acute respiratory failure and septic shock.
Vancomycin and ceftriaxone were started as empiric antibiotic therapy. The patient had to be started on pressor support, was mechanically ventilated and underwent continuous venovenous haemofiltration for worsening acute kidney injury. A transoesophageal echo was performed in view of the new murmur, which showed a large aortic valve vegetation and associated new onset moderate to severe aortic incompetence. The patient's clinical condition transiently improved and he was extubated. He also underwent a CT scan of the cervical spine for evaluation of severe neck pain, which demonstrated irregularity and fragmentation of C5 and C6 vertebrae, suggestive of discitis/osteomyelitis.
G. elegans was detected by automated blood culture systems. Antibiotics were changed to ampicillin and ceftriaxone per infectious disease recommendations. Cardiothoracic surgery was consulted but the patient was too unstable for valve replacement surgery. Despite initial improvement, he became progressively hypotensive with worsening congestive heart failure. He died on day 17 of hospital stay.
Investigations
G. elegans was detected by the automated blood culture systems. However, the organism could not be grown to test antimicrobial susceptibilities due to its fastidious nature and the need for special nutrient media, which were not available in our hospital. Subsequent blood cultures were sterile. A transoesophageal echo showed a large 2.3 cm mobile vegetation on the aortic valve (figure 1) affecting its coaptation and associated new onset moderate to severe aortic incompetence (figure 2). The device lead was visualised in the right ventricle and there was no evidence of vegetation on it.
Figure 1.
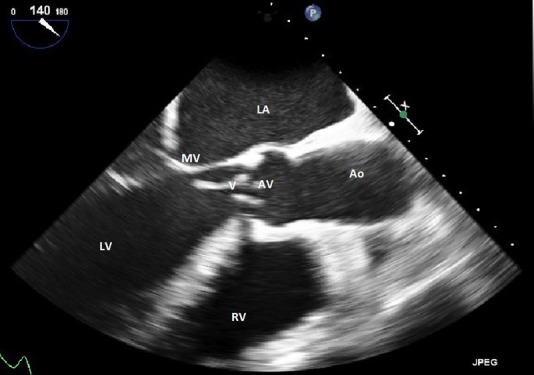
Transoesophageal echocardiogram showing a large 2.3 cm vegetation on the non-coronary cusp of the aortic valve. Left atrium (LA), mitral valve (MV), left ventricle (LV), vegetation (V), aortic valve (AV), aorta (Ao), right ventricle (RV).
Figure 2.
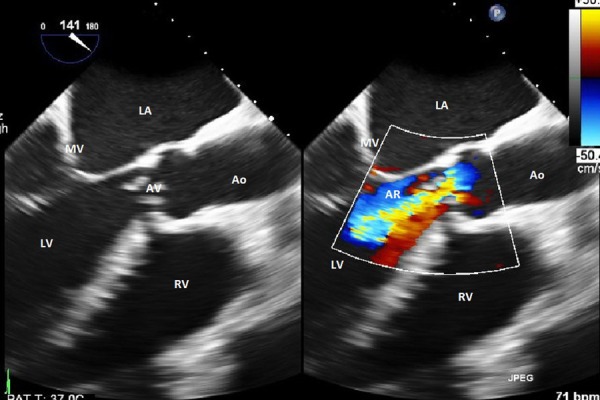
Transoesophageal echocardiogram showing severe aortic regurgitation secondary to mal-coaptation of the aortic valve caused by a large vegetation. Left atrium (LA), mitral valve (MV), left ventricle (LV), aortic regurgitation jet (AR), aortic valve (AV), aorta (Ao), right ventricle (RV).
Treatment
Initial intravenous vancomycin and ceftriaxone treatment was empirically followed by intravenous ampicillin and ceftriaxone. The latter regimen was chosen based on American Heart Association 2005 treatment recommendations for IE, which was current at the time our patient was being treated.
Outcome and follow-up
With the management described above, the patient's clinical condition improved transiently and he was transferred out of the intensive care unit. Infectious disease team was consulted, who recommended that the vancomycin be changed to ampicillin and to continue ceftriaxone. Despite initial improvement, the patient became progressively hypotensive with worsening congestive heart failure. He died on day 17 of hospital stay.
Discussion
G. elegans is an extremely fastidious pleomorphic Gram-positive bacterium, often considered a main culprit in culture-negative endocarditis.1 It was earlier grouped within nutrient variant streptococci (NVS) under the genus Abiotrophia, but later reclassified under genus Granulicatella.2 3 NVS has been reported to cause between 5% and 6% of all cases of streptococcal endocarditis, and about 8% of NVS isolates have been reported to be G. elegans.4 5 G. elegans has been found to be less infective compared to other bacteria in the same Genus, such as Granulicatella adjacens, accounting for its low incidence. This has been postulated to be because of its low binding capacity to extracellular matrix proteins.6 It has also been reported to cause bacteraemia in patients with abdominal infections, and has been isolated from patients with chronic maxillary sinusitis.7 8 It is estimated to be present in about 10% of adult dental plaque, which suggests that the likely source of infection in our patient was his poor dentition.9 Isolation and correct identification of the organism are often arduous tasks because of the fastidious growth requirements and pleomorphism. Identification of G. elegans is best achieved based on a combination of its special phenotypic characteristics and 16S rRNA gene sequencing.10
There is insufficient literature regarding the course and outcome of IE caused specifically by G. elegans. Our patient developed cervical discitis during the course of his illness, likely from seeding during the bacteraemia. This is the first reported case of discitis caused by G. elegans. Other complications that have been described include embolic cerebral infarcts, atrial vegetations, need for early surgical intervention and heart failure.2 11 12 A recent review of 29 cases of Granulicatella species endocarditis by Adam et al, reported the aortic valve to be the most commonly involved valve, and multivalvular involvement in 13% of patients. Incidence of heart failure was 30%, embolism developed in 30% and perivalvular abscess in 11%. The mortality rate was about 17%.13 IE caused by the NVS group as a whole has been reported to have treatment failure rates of 41%, despite the organisms being sensitive to the antibiotics used.14 The high treatment failure rates might be due to the phenomenon of penicillin tolerance or a delay in isolating and identifying the organism, or also a delay in initiation of appropriate antibiotics.15
The American Heart Association 2015 scientific statement for endocarditis treatment recommends that IE caused by the Granulicatella species be treated with a combination regimen that includes ampicillin (12 g/day in divided doses) or penicillin (18–30 million units per day in divided doses or by continuous infusion) plus gentamicin (3 mg/kg/day in 2–3 divided doses). An infectious disease consultation to determine length of therapy is also recommended.16 European Society of Cardiology 2015 guidelines for the management of IE also recommend penicillin G, ceftriaxone or vancomycin for 6 weeks, combined with an aminoglycoside for at least the first 2 weeks.17 Our patient did not receive aminoglycosides in view of acute kidney injury requiring continuous venovenous haemofiltration. He was started on an ampicillin plus ceftriaxone combination based on previous American Heart Association recommendations stating that G. elegans endocarditis should be treated similarly to enterococcal endocarditis.18 The above combination has been used for enterococcal endocarditis but there is no reported clinical experience with it for the Granulicatella species. Failure of this combination in our patient suggests that it might not be effective for Granulicatella species endocarditis, though subsequent cultures were sterile. On the other hand, our patient had other comorbidities and complicated endocarditis to begin with, the presence of which has a poor clinical outcome. As with any case of IE, early surgical intervention should be performed when indicated.19
Learning points.
Granulicatella elegans is a rare cause for infective endocarditis. Practitioners should suspect this organism as a causative agent in culture negative endocarditis.
Identification of G. elegans is best achieved based on a combination of its special phenotypic characteristics and 16S rRNA gene sequencing.
Clinicians should be aware of the high rates of complications associated with it and consider early surgical management when warranted.
Irrespective of antimicrobial sensitivities, G. elegans endocarditis should be treated with a combination of ampicillin or penicillin with an aminoglycoside.
Patients with complicated infectious endocarditis should be referred early and managed in a reference centre. These cases should be discussed with a multidisciplinary ‘endocarditis team’ on a regular basis.
Footnotes
Contributors: SP admitted the patient in the ICU and YA followed the patient until his demise.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Brouqui P, Raoult D. Endocarditis due to rare and fastidious bacteria. Clin Microbiol Rev 2001;14:177–207. 10.1128/CMR.14.1.177-207.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roggenkamp A, Abele-Horn M, Trebesius KH et al. Abiotrophia elegans sp. nov., a possible pathogen in patients with culture-negative endocarditis. J Clin Microbiol 1998;36:100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins MD, Lawson PA. The genus Abiotrophia (Kawamura et al.) is not monophyletic: proposal of Granulicatella gen. nov., Granulicatella adiacens comb. nov., Granulicatella elegans comb. nov. and Granulicatella balaenopterae comb. nov. Int J Syst Evol Microbiol 2000;50(Pt 1):365–9. 10.1099/00207713-50-1-365 [DOI] [PubMed] [Google Scholar]
- 4.Roberts RB, Krieger AG, Schiller NL et al. Viridans streptococcal endocarditis: the role of various species, including pyridoxal-dependent streptococci. Rev Infect Dis 1979;1:955–66. 10.1093/clinids/1.6.955 [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Kanamoto T, Inoue M. Abiotrophia elegans strains comprise 8% of the nutritionally variant streptococci isolated from the human mouth. J Clin Microbiol 1999;37:2553–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada Y. Endocardiac infectivity and binding to extracellular matrix proteins of oral Abiotrophia species. FEMS Immunol Med Microbiol 2000;27:257–61. 10.1111/j.1574-695X.2000.tb01438.x [DOI] [PubMed] [Google Scholar]
- 7.Paju S, Bernstein JM, Haase EM et al. Molecular analysis of bacterial flora associated with chronically inflamed maxillary sinuses. J Med Microbiol 2003;52(Pt 7):591–7. 10.1099/jmm.0.05062-0 [DOI] [PubMed] [Google Scholar]
- 8.Abdul-Redha RJ, Prag J, Sonksen UW et al. Granulicatella elegans bacteraemia in patients with abdominal infections. Scand J Infect Dis 2007;39:830–3. 10.1080/00365540701299624 [DOI] [PubMed] [Google Scholar]
- 9.Ohara-Nemoto Y, Kishi K, Satho M et al. Infective endocarditis caused by Granulicatella elegans originating in the oral cavity. J Clin Microbiol 2005;43:1405–7. 10.1128/JCM.43.3.1405-1407.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang MY, Lin J, Sun SB et al. Identification of Granulicatella elegans from a case of infective endocarditis, based on the phenotypic characteristics and 16S rRNA gene sequence. J Cardiovasc Med (Hagerstow) 2015;16(Suppl 2):S138–40. 10.2459/JCM.0b013e328356a471 [DOI] [PubMed] [Google Scholar]
- 11.Al-Tawfiq JA, Kiwan G, Murrar H. Granulicatella elegans native valve infective endocarditis: case report and review. Diagn Microbiol Infect Dis 2007;57:439–41. 10.1016/j.diagmicrobio.2006.10.005 [DOI] [PubMed] [Google Scholar]
- 12.Casalta JP, Habib G, La Scola B et al. Molecular diagnosis of Granulicatella elegans on the cardiac valve of a patient with culture-negative endocarditis. J Clin Microbiol 2002;40:1845–7. 10.1128/JCM.40.5.1845-1847.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adam EL, Siciliano RF, Gualandro DM et al. Case series of infective endocarditis caused by Granulicatella species. Int J Infect Dis 2015;31:56–8. 10.1016/j.ijid.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 14.Stein DS, Nelson KE. Endocarditis due to nutritionally deficient streptococci: therapeutic dilemma. Rev Infect Dis 1987;9:908–16. 10.1093/clinids/9.5.908 [DOI] [PubMed] [Google Scholar]
- 15.Holloway Y, Dankert J. Penicillin tolerance in nutritionally variant streptococci. Antimicrob Agents Chemother 1982;22:1073–5. 10.1128/AAC.22.6.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a Scientific Statement for Healthcare Professionals from the American Heart Association. Circulation 2015;132:1435–86. 10.1161/CIR.0000000000000296 [DOI] [PubMed] [Google Scholar]
- 17.Authors/Task Force Members, Habib G, Lancellotti P, Antunes MJ et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC)Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 2015;36:3075–128. 10.1093/eurheartj/ehv319 [DOI] [PubMed] [Google Scholar]
- 18.Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 19.Rav-Acha M, Sassa D, Ilan Y et al. [Surgical intervention in infective endocarditis: indications and timing]. Harefuah 2005;144:421–5, 453. [PubMed] [Google Scholar]


