Abstract
This report describes a case of Campylobacter fetus prosthetic valve infective endocarditis and discusses the subsequent management. Although C. fetus has a tropism for vascular endothelium, infective endocarditis has rarely been reported. In this patient, despite initial optimal antimicrobial therapy, valve replacement was ultimately required due to ongoing infectious emboli to the brain in the setting of evidence of vegetation enlargement on echocardiogram. The prosthetic valve was replaced, the patient completed a 6-week course of parenteral antibiotics after surgical intervention and he made a full recovery with no long-term neurological sequelae. This case highlights the fact that despite the relatively low prevalence of C. fetus endocarditis, it is associated with a high degree of mortality and valve replacement is often indicated.
Background
Gram-negative endocarditis due to Haemophilus species, Actinobacillus, Cardiobacterium, Eikenella and Kingella species (HACEK) bacteria and non-HACEK organisms is an infrequent, albeit under-recognised occurrence, but is associated with significant morbidity and mortality. While the incidence of HACEK infective endocarditis has remained relatively unchanged over time, recent data suggest an increase in the incidence of non-HACEK Gram-negative endocarditis.1 2 Campylobacter fetus is an uncommon cause of human infection, but may be responsible for severe disease, including infective endocarditis, because of a predilection for bacteraemia and infecting vascular endothelium.3 In general campylobacteriosis presents as a self-limited gastrointestinal illness, but in approximately 0.15% of cases, intestinal disease leads to bacteraemia.3 Although, Campylobacter jejuni and Campylobacter coli are the more common causes of Campylobacter infection, one study, using PCR methods, estimated that 2.4% of cases are caused by C. fetus.4 In spite of the low overall frequency as a causal entity in general campylobacteriosis, in the setting of bacteraemia, C. fetus is a much more commonly detected pathogen, found in 19%–53% of cases.3 We report a case of C. fetus prosthetic valve endocarditis so as to add to the growing body of literature regarding a very rare disease and to improve the general awareness of the management of Gram-negative bacilli endocarditis. To the best of our knowledge, there have only been 25 cases of infective endocarditis due to this organism, and only four of those cases involved a prosthetic valve.5–9
Case presentation
In the early Autumn, a man in his late 70s was admitted to our hospital, with a 10-day history of relapsing fevers, chills, night sweats and malaise. He had travelled to southeast Asia and Europe in the 6 weeks prior to falling ill. During questioning to obtain his history, he recalled eating steak tartare 2 days before symptom onset. An exhaustive review of his recent dietary history was otherwise unrevealing. His medical history was most notable for Streptococcus bovis endocarditis requiring aortic valve replacement 4 years prior. He also had a history of hypertension and hypercholesterolaemia, which were well controlled with prescription medications. He was also taking an aspirin daily, but was not taking any anticoagulants or other over the counter medications. He had no history of chronic gastrointestinal disease or other conditions that may have rendered him vulnerable to recurrent infections. Recent glycated haemoglobin A1c levels confirmed he did not have diabetes mellitus. Notably, he had visited his dentist 4 weeks prior to falling ill and had undergone a routine cleaning. He was prescribed prophylactic amoxicillin at that time.
On presentation, the patient's blood pressure was 115/58 mm Hg, pulse was 54 bpm and temperature was 39.4°C. On physical examination, the patient had a prosthetic second heart sound, but no murmurs were audible on auscultation of the precordium. The rest of the examination was unremarkable including the neurological, eye and dermatological examinations.
Investigations
Initial laboratory findings included a haemoglobin concentration of 12.0 mg/dL and white cell count of 8.8×109 cells/L (laboratory normal range of 4.5–12×109 cells/L). Three thick and thin blood smears for malaria were negative. Serologies for Coxiella burnetii, dengue virus and Borrelia burgdorferi were sent for testing and subsequently returned negative. Antibody and antigen testing for HIV types 1 and 2 also returned negative.
Two sets of aerobic and anaerobic blood cultures were sent to the laboratory. Both sets of blood cultures turned positive and Gram stain revealed Gram-negative rods with a ‘gull-wing’ morphology (figures 1 and 2), suspicious for Campylobacter species. Further initial microbiological work up of the organism was precluded by the fastidious nature of the bacteria and formal microbiological identification would not be available for another 6 days.
Figure 1.
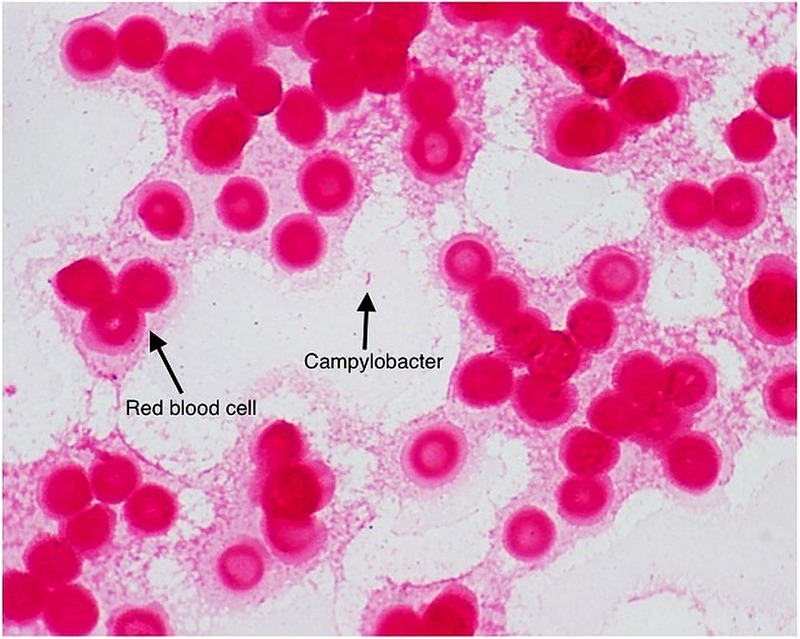
Gram stain with arrow demonstrating a Gram-negative organism, ultimately identified as Campylobacter fetus. A red blood cell is also noted for comparison.
Figure 2.
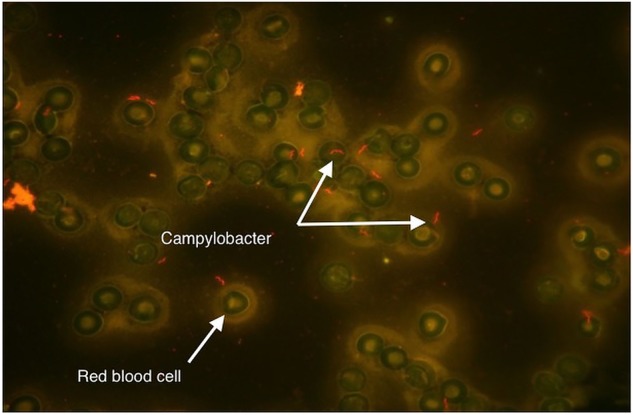
Acridine orange stain with arrows demonstrating an organism showing the classic gull-wing morphology seen with Campylobacter species. A red blood cell is also noted for comparison.
A transoesophageal echocardiogram was performed shortly after admission, given the presence of a prosthetic valve in the setting of fever and bacteraemia, and demonstrated a soft tissue density measuring 1.6×1.0 cm in the left aortic sinus extrinsic to the bioprosthetic valve, confirming prosthetic valve endocarditis.
MRI of the brain and spine were reported normal. CT of the abdomen and pelvis did not demonstrate any acute findings to explain the history of fever, however, CT imaging of the chest was notable for a filling defect within the left coronary sinus, which was suspicious for a prosthetic valvular vegetation.
Differential diagnosis
The initial differential diagnosis was quite broad given the patient was a returning traveller with a fever.10 Given a history of prosthetic valve and consumption of raw meat, particular attention was given to Gram-negative bacteraemia and included infective endocarditis, involving salmonellosis and campylobacteriosis. Malaria, dengue viral infection, chikungunya viral infection, Q-fever and seasonal viral infections were also considered. However, after the return of echocardiographic findings, the differential was quickly narrowed based on these findings and the persistent Gram-negative bacteraemia.
Treatment
The patient was initiated on intravenous ceftriaxone, 2000 mg two times a day, but transitioned to meropenem, 2000 mg intravenous three times a day, on day 3 of hospitalisation in the setting of high-grade fevers and persistent bacteraemia. Antibiotics were chosen for empiric bactericidal activity against Gram-negative organisms. In the setting of a prosthetic valve, there was considerable discussion about treating with both meropenem and an aminoglycoside, in line with national guidelines for endocarditis antimicrobial therapy. However, given the potential toxicities of treating with an aminoglycoside in the setting of relative clinical stability, it was decided that the patient could be treated with meropenem monotherapy pending confirmation of the organism by the microbiologists.
Of note, in the setting of persistent bacteraemia and ongoing fever, a total body positron emission tomography scan was performed on hospital day 4, and no definitive fluorodeoxyglucose avidity was noted in the area of the aortic root or elsewhere in the body. The patient ultimately became afebrile and blood cultures were cleared of bacteria after 5 days of parenteral antibiotics.
On day 7 of hospitalisation, blood cultures from the day of admission were finally identified as C. fetus. Drug susceptibilities later confirmed that the C. fetus isolate was susceptible to both meropenem and ceftriaxone. The patient was evaluated by the cardiac surgeons but valve replacement was deferred per the patient's preference for a trial of medical management. The patient was discharged home to complete a 6-week course of intravenous meropenem.
Outcome and follow-up
Four days after discharge from the hospital, the patient was re-admitted to the hospital following an episode of confusion at home. Repeat brain MRI revealed numerous new emboli, which neuroradiologists determined were most consistent with septic phenomenon. Repeat transoesophageal echocardiogram showed enlargement of the vegetation along the left aortic sinus (figure 3).
Figure 3.
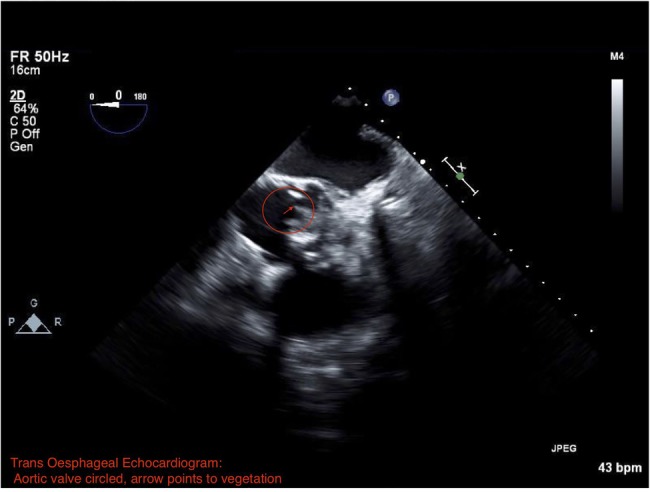
Transoesophageal echocardiogram showing a vegetation along the left aortic sinus (contained within the circle and arrow shows the vegetation and sinus).
The day after hospital admission, the patient was taken to the operating room and underwent prosthetic valve replacement. Intraoperative cultures of the explanted prosthetic valve grew C. fetus. For ease of administration the patient was switched from intravenous meropenem three times a day to 1 g of intravenous ertapenem once daily (against which the isolate was also susceptible) at the time of hospital discharge, for convenience of dosing. He completed a 6-week course of parenteral antibiotics at the time of surgery, and made a full recovery, with no long-term neurological sequelae.
Discussion
We report a case of C. fetus prosthetic valve endocarditis, complicated by local perivalvular extension and central nervous system emboli. C. fetus is a ubiquitous pathogen causing infections in cattle and sheep. It can be transmitted to humans through ingestion of contaminated food or through animal exposure, although definitive data regarding the precise process of transmission is limited by the difficulties in isolating and culturing the Campylobacter species.3 In this case, we suspect that the infection was secondary to ingestion of raw steak, with transintestinal migration of bacteria resulting in bacteraemia and then ultimately prosthetic valve infection. While 75% of C. fetus infections occur in persons with underlying chronic gastrointestinal disease,7 9 our patient had no such history.
Campylobacter species, including C. fetus, are unusual causes of endocarditis. C. fetus is thought to be acquired via direct contact with infected hosts or through ingestion of raw meat, as in this patient.7–9 11 This pathogen may cause a wide variety of clinical manifestations including bacteraemia, pneumonia, meningoencephalitis, pericarditis, septic arthritis and septic abortion,5 7 9 11 12 particularly in immunocompromised hosts, including patients with HIV infection, haematological malignancy, splenectomy, cirrhosis, diabetes mellitus, advanced age, pregnancy and cardiovascular disease with valve abnormalities or medical device implants.5 9 11 Additionally, C. fetus has a predilection for the vascular endothelium and is associated with a higher incidence of thrombophlebitis and mycotic aneurysms of large arteries.5 7 9 12 One explanation for the greater tendency of C. fetus to cause endovascular infections is that it is relatively resistant to the bactericidal activity present in normal human serum,13 since it is covered with a surface (S)-layer protein that functions as a capsule.14 15 Virtually all human isolates of C. fetus possess this S-layer protein which serves to completely disrupt complement binding to these organisms.16
The standard method for detecting Campylobacter species in clinical specimens is by laboratory culture, often by Campylobacter blood agar plates.17 Given the fastidious nature of the organism, incubation blood cultures are routinely extended to 2 weeks when systemic infection is suspected. Growth is optimised between 37 and 42°C with variable pathogenicity at different temperatures dependent on species.17 18 Gram staining of these species demonstrates a fine-curved, gull-wing or spiral, lightly stained Gram-negative appearance. Other methods for the direct detection of Campylobacter in clinical specimens, such as DNA probes and amplification by PCR, have been successful in research studies, and may be more sensitive than traditional cultures for the detection of and typing of these organisms. As access to advanced technology improves, they may be incorporated in rapid diagnostic assays.19–21
The optimal treatment for C. fetus endocarditis is challenging to elucidate, as there are limited numbers of reports in the literature. Approximately 25 cases of infective endocarditis due to C. fetus have been reported since 1955, including four cases that involved a prosthetic valve.5–8 These patients were treated with antibiotics with or without surgical repair of the affected valve.
Traditionally, valve repair or replacement is the cornerstone of treatment for non-HACEK Gram-negative bacilli endocarditis (HACEK represents endocarditis caused by the following genera: Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella or Kingella).1 22 Among prior case reports of patients with C. fetus prosthetic valve endocarditis, three required replacement of the artificial valve and subsequently recovered.5–8 In one case, described by Peetermans et al,5 surgical repair was not pursued due to severe three-vessel coronary artery disease, and the patient subsequently died from complications related to extensive infection. However, in a case reported by Haruyama et al,8 the patient refused surgery and subsequently recovered after prolonged treatment with imipenem/cilastatin and gentamicin. In the current report, surgery was initially deferred but proved to be the definitive therapy after treatment failure with antibiotics alone. While the optimal antimicrobial treatment of choice for C. fetus endocarditis has not been established, available data suggest carbapenems and third-generation cephalosporins are effective against serious C. fetus infections.23 Despite effective antibiotic therapy, mortality may be as high as 40% for C. fetus infective endocarditis managed non-surgically.7 In the current report, surgery proved to be the definitive therapy after treatment failure with antibiotics alone, and the patient went on to do well.
C. fetus is an important, albeit rare, cause of infective endocarditis, especially in patients with underlying valvular pathology and an exposure history of raw meat consumption. Careful consideration of appropriate diagnostic studies and definitive intervention should be considered in patients with this disease.
Learning points.
Campylobacter fetus is a rare, but important, cause of infective endocarditis.
Bacteraemia and cardiovascular infections with C. fetus may be covert in presentation, with non-specific symptoms and physical examination findings.
Suppurative complications of infectious endocarditis may evolve despite appropriate antimicrobial therapy, prompting early consideration for surgical intervention.
Footnotes
Twitter: Follow Michael Reid at @mikereidmd
Contributors: MJAR and PC-H conceived the manuscript. EMS and MJAR wrote the first draft. The other authors contributed to subsequent drafts. PC-H and SMB prepared critical revisions of the article. All the authors contributed to all aspects of the manuscript. All the authors approved publication of the manuscript and reviewed the final form.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Morpeth S, Murdoch D, Cabell CH et al. Non-HACEK gram-negative bacillus endocarditis. Ann Intern Med 2007;147:829–35. 10.7326/0003-4819-147-12-200712180-00002 [DOI] [PubMed] [Google Scholar]
- 2.Reyes MP, Reyes KC. Gram-negative endocarditis. Curr Infect Dis Rep 2008;10:267–74. 10.1007/s11908-008-0044-5 [DOI] [PubMed] [Google Scholar]
- 3.Wagenaar JA, van Bergen MA, Blaser MJ et al. Campylobacter fetus infections in humans: exposure and disease. Clin Infect Dis 2014;58:1579–86. 10.1093/cid/ciu085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullman S, Corcoran D, O'Leary J et al. Emerging dynamics of human campylobacteriosis in Southern Ireland. FEMS Immunol Med Microbiol 2011;63:248–53. 10.1111/j.1574-695X.2011.00847.x [DOI] [PubMed] [Google Scholar]
- 5.Peetermans WE, De Man F, Moerman P et al. Fatal prosthetic valve endocarditis due to Campylobacter fetus. J Infect 2000;41:180–2. 10.1053/jinf.2000.0699 [DOI] [PubMed] [Google Scholar]
- 6.Caramelli B, Mansur AJ, Grinberg M et al. Campylobacter fetus endocarditis on a prosthetic heart valve. South Med J 1988;81:802–3. 10.1097/00007611-198806000-00028 [DOI] [PubMed] [Google Scholar]
- 7.Farrugia DC, Eykyn SJ, Smyth EG. Campylobacter fetus endocarditis: two case reports and review. Clin Infect Dis 1994;18:443–6. 10.1093/clinids/18.3.443 [DOI] [PubMed] [Google Scholar]
- 8.Haruyama A, Toyoda S, Kikuchi M et al. Campylobacter fetus as cause of prosthetic valve endocarditis. Tex Heart Inst J 2011;38:584–7. [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison VA, Lloyd BK, Chia JK et al. Cardiovascular and bacteremic manifestations of Campylobacter fetus infection: case report and review. Rev Infect Dis 1990;12:387–92. 10.1093/clinids/12.3.387 [DOI] [PubMed] [Google Scholar]
- 10.Wilson ME, Weld LH, Boggild A et al. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis 2007;44:1560–8. 10.1086/518173 [DOI] [PubMed] [Google Scholar]
- 11.Guerrant RL, Lahita RG, Winn WC Jr et al. Campylobacteriosis in man: pathogenic mechanisms and review of 91 bloodstream infections. Am J Med 1978;65:584–92. 10.1016/0002-9343(78)90845-8 [DOI] [PubMed] [Google Scholar]
- 12.Montero A, Corbella X, López JA et al. Campylobacter fetus-associated aneurysms: report of a case involving the popliteal artery and review of the literature. Clin Infect Dis 1997;24:1019–21. 10.1093/clinids/24.5.1019 [DOI] [PubMed] [Google Scholar]
- 13.Blaser MJ, Smith PF, Kohler PF. Susceptibility of Campylobacter isolates to the bactericidal activity of human serum. J Infect Dis 1985;151:227–35. 10.1093/infdis/151.2.227 [DOI] [PubMed] [Google Scholar]
- 14.Blaser MJ, Smith PF, Hopkins JA et al. Pathogenesis of Campylobacter fetus infections: serum resistance associated with high-molecular-weight surface proteins. J Infect Dis 1987;155:696–706. 10.1093/infdis/155.4.696 [DOI] [PubMed] [Google Scholar]
- 15.Dworkin J, Blaser MJ. Molecular mechanisms of Campylobacter fetus surface layer protein expression. Mol Microbiol 1997;26:433–40. 10.1046/j.1365-2958.1997.6151958.x [DOI] [PubMed] [Google Scholar]
- 16.Blaser MJ, Smith PF, Repine JE et al. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Invest 1988;81:1434–44. 10.1172/JCI113474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.M'Ikanatha NM, Dettinger LA, Perry A et al. Culturing stool specimens for Campylobacter spp., Pennsylvania, USA. Emerg Infect Dis 2012;18:484–7. 10.3201/eid1803.111266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aroori SV, Cogan TA, Humphrey TJ. The effect of growth temperature on the pathogenicity of Campylobacter. Curr Microbiol 2013;67:333–40. 10.1007/s00284-013-0370-1 [DOI] [PubMed] [Google Scholar]
- 19.Kulkarni SP, Lever S, Logan JM et al. Detection of campylobacter species: a comparison of culture and polymerase chain reaction based methods. J Clin Pathol 2002;55:749–53. 10.1136/jcp.55.10.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson S, Petersen HM, Jespersgaard C et al. Real-time TaqMan polymerase chain reaction-based genus-identification and pyrosequencing-based species identification of Campylobacter jejuni, C. coli, C. lari, C. upsaliensis, and C. fetus directly on stool samples. Diagn Microbiol Infect Dis 2012;74:6–10. 10.1016/j.diagmicrobio.2012.05.029 [DOI] [PubMed] [Google Scholar]
- 21.Ugarte-Ruiz M, Gómez-Barrero S, Porrero MC et al. Evaluation of four protocols for the detection and isolation of thermophilic Campylobacter from different matrices. J Appl Microbiol 2012;113:200–8. 10.1111/j.1365-2672.2012.05323.x [DOI] [PubMed] [Google Scholar]
- 22.Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005;111:e394–434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 23.Neuzil KM, Wang E, Haas DW et al. Persistence of Campylobacter fetus bacteremia associated with absence of opsonizing antibodies. J Clin Microbiol 1994;32:1718–20. [DOI] [PMC free article] [PubMed] [Google Scholar]


