Abstract
Highly active antiretroviral therapy (HAART) is associated with multiple metabolic disorders, including lipodystrophy, dyslipidaemia and insulin resistance. HIV/HAART-associated lipodystrophy syndrome (HALS) is characterised by subcutaneous fat wasting, central fat accumulation and increased risk of diabetes. Thiazolidinediones are considered a promising treatment for HALS, because they improve insulin sensitivity and increase subcutaneous fat mass. In previous studies, pioglitazone increased overall fat mass in patients with HALS but whether fat distribution changes remains unclear. We describe a HALS patient with diabetes treated with pioglitazone. Prior to pioglitazone therapy, he had hollowed cheeks, loss of fat in the extremities and abdominal obesity. 18 months after starting pioglitazone and switching his HAART regimens, T1-weighted MRI showed obvious increases in the subcutaneous fat mass of the neck and upper trunk, but no changes in the cheeks and extremities. Pioglitazone therapy for HALS could increase subcutaneous fat mass in non-lipoatrophic but not in lipoatrophic regions.
Background
Improvements in antiretroviral therapy, especially highly active antiretroviral therapy (HAART), have dramatically reduced mortality associated with HIV.1 However, HAART is also associated with multiple metabolic disorders, including lipodystrophy, dyslipidaemia and insulin resistance.2 3 These metabolic disorders constitute HIV/HAART-associated lipodystrophy syndrome (HALS). HALS is accompanied by disorders in fat metabolism, manifested as subcutaneous fat wasting and central fat accumulation.2 3 Patients with HALS are at increased risk of diabetes due to the adverse effects of HAART and lipodystrophy.4 5
Thiazolidinediones are antidiabetic drugs that improve insulin sensitivity via agonism of peroxisome proliferator-activated γ (PPARγ), a key regulator of fat metabolism.6 In patients with type 2 diabetes and impaired glucose tolerance, thiazolidinedione therapy increases fat mass in subcutaneous but not visceral body regions.7–9 PPARγ expression is reduced in patients with HALS.10 Thus, thiazolidinediones are considered promising treatment for HALS.10 A meta-analysis reported that pioglitazone increases body mass index (BMI) in patients with HALS.11 However, whether fat distribution changes with pioglitazone therapy remains unclear.
We describe a patient with HALS with diabetes who was treated with pioglitazone. His subcutaneous fat mass increased heterogeneously during pioglitazone therapy.
Case presentation
A 46-year-old man with chronic hepatitis B was diagnosed with HIV infection in 1996. As a result of HIV infection, he developed cytomegalovirus retinitis and became blind. He was treated with indinavir 800 mg three times a day starting in 1997, with the addition of lamivudine 150 mg twice daily and stavudine 40 mg twice daily in 1998. In 1999, indinavir was discontinued and nevirapine 200 mg twice daily was started. The patient's CD4 cell count increased and HIV viral load became undetectable with HAART. Figure 1A shows the patient's face at the start of HAART. Subsequently, he observed changes in his body fat distribution, with loss of subcutaneous fat and gain of abdominal fat.
Figure 1.
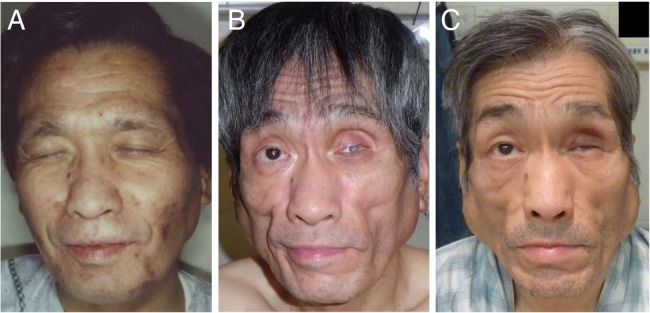
Photographs of the patient's face at the start of highly active antiretroviral therapy (HAART) in 1988 (A), at the start of pioglitazone therapy and change of HAART regimen in 2012 (B), and after 18 months of pioglitazone therapy (C).
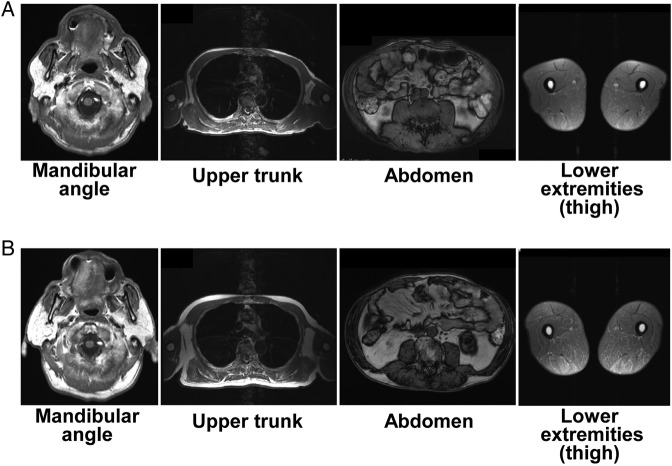
In 2009, he developed diabetes (random plasma glucose, 297 mg/dL; glycated haemoglobin (HbA1c), 8.5%). He began to take glimepiride in 2010 and sitagliptin in 2011 to control hyperglycaemia, but his blood glucose levels remained high. In 2012, he consulted our clinic for diabetic management. During the initial visit to our clinic, he had hollowed cheeks, loss of fat in the extremities and abdominal obesity (figure 1B). His BMI was 21.8 kg/m2 (height, 1.68 m; weight, 61.5 kg). T1-weighted MRI revealed very low subcutaneous fat mass, especially in the extremities, in contrast to relatively high visceral fat mass (figure 2A). While on glimepiride 2 mg once daily and sitagliptin 50 mg once daily, the patient's HbA1c was 8.4%, fasting plasma glucose was 122 mg/dL, fasting insulin was 29.7 μU/L, urinary C peptide reactivity was 257 μg/day, fasting leptin was 2.7 ng/mL, triglycerides were 331 mg/dL and total cholesterol was 291 mg/dL (table 1). We diagnosed his metabolic disorders as diabetes in association with HALS. He also presented with subclinical hypothyroidism.
Figure 2.
T1-weighted MRIs of body fat mass at the start of pioglitazone therapy and change of highly active antiretroviral therapy regimen in 2012 (A), and after 18 months of pioglitazone therapy (B).
Table 1.
Changes in the patient's metabolic parameters at each follow-up
| After changes in treatment regimen |
|||||
|---|---|---|---|---|---|
| Initial visit | 3 months | 6 months | 12 months | 18 months | |
| Body weight (kg) | 61.5 | 53.7 | 57.6 | 61.6 | 63.6 |
| BMI (kg/m2) | 21.8 | 19.0 | 20.4 | 21.8 | 22.5 |
| HbA1c (%) | 8.4 | 5.8 | 6.6 | 8.5 | 9.4 |
| Plasma glucose (mg/dL) | |||||
| Fasting | 122 | 106 | |||
| At random | 107 | 152 | 225 | ||
| Fasting insulin (μU/L) | 29.7 | 28.2 | |||
| Urinary CPR (μg/day) | 257 | 143 | |||
| Fasting leptin (ng/mL) | 2.7 | 6.3 | |||
| Triglyceride (mg/dL) | 331 | 237 | 396 | 327 | 238 |
| Total cholesterol (mg/dL) | 291 | 220 | 276 | 245 | 198 |
| HDL cholesterol (mg/dL) | 44 | 58 | 58 | 52 | 57 |
| LDL cholesterol (mg/dL) | 181 | 115 | 139 | 128 | 93 |
| AST (U/L) | 63 | 27 | 29 | 48 | 50 |
| ALT (U/L) | 45 | 20 | 19 | 38 | 44 |
| Serum creatinine (mg/dL) | 1.18 | 1.49 | 1.29 | 1.35 | 1.25 |
| TSH (μU/mL) | 15.5 | 0.80 | 1.86 | 5.96 | 4.04 |
| Free T4 (ng/dL) | 1.02 | 1.17 | 1.02 | 1.12 | 1.10 |
| Free T3 (pg/mL) | 2.9 | 2.8 | 3.0 | 3.0 | 2.6 |
| CD4 cell count (/μL) | 279 | 204 | 204 | 263 | 184 |
ALT, alanine transaminase; AST, aspartate transaminase; CPR, C peptide reactivity; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; T3, tri-iodothyronine; T4, thyroxine; TSH, thyroid-stimulating hormone.
Treatment
We discontinued glimepiride and sitagliptin and started pioglitazone 45 mg once daily. Around the same time, the patient's HAART regimen was switched to lamivudine 300 mg once daily, abacavir 600 mg once daily and raltegravir 400 mg twice daily. We administered levothyroxine 25 μg once daily for treating subclinical hypothyroidism.
Outcome and follow-up
The patient's glycaemic control improved with the treatment described above and after complying with dietary restrictions. Three months after changes in his treatment regimen, his body weight decreased to 53.7 kg (BMI, 19.0 kg/m2) and his HbA1c dropped to 5.8%. We started pravastatin 10 mg once daily to treat dyslipidaemia. Subsequently, his body weight and HbA1c gradually increased with inappropriate dietary restriction (table 1). Twelve months after starting pioglitazone, we added alogliptin 25 mg once daily to his treatment regimen for glycaemic control. Eighteen months after starting pioglitazone, his glycaemic control worsened again (HbA1c, 9.4%). He observed swelling around the bilateral mandibular angles (figure 1C). T1-weighted MRI revealed that the swelling consisted mostly of subcutaneous adipose tissue (figure 2B). MRI also showed that the subcutaneous fat mass of the neck and upper trunk was obviously increased, but that of the cheeks and extremities was unchanged (figure 2B). We forewent assessment of changes in total body fat mass using dual-energy X-ray absorptiometry.
Discussion
The present case report shows that pioglitazone therapy increased subcutaneous fat mass in a heterogeneous manner in a patient with HALS with diabetes. In previous studies, pioglitazone increased fat mass in patients with diabetes8 and those with HALS.11 However, whether fat distribution changes with pioglitazone therapy in patients with HALS remains unclear.
A previous study described patients with familial partial lipodystrophy on long-term thiazolidinedione therapy.12 The case report demonstrated that thiazolidinediones (troglitazone and rosiglitazone) do not reverse subcutaneous fat loss in lipoatrophic regions, but increase overall adiposity due to excessive fat accumulation in non-lipoatrophic regions.12 In our patient, pioglitazone therapy increased subcutaneous fat mass in non-lipoatrophic regions, but not in lipoatrophic regions.
Previous clinical studies have analysed whether thiazolidinediones are efficacious in treating HALS. Rosiglitazone was examined in many clinical studies evaluating changes in insulin sensitivity and body fat composition. Most studies demonstrated that rosiglitazone improves insulin sensitivity.13–18 However, it remains controversial whether rosiglitazone increases fat mass, since several studies reported a positive association between rosiglitazone and increased fat mass14 16 19 while other studies found no association.13 18 20 In contrast, relatively few clinical studies have examined pioglitazone. One meta-analysis reported that pioglitazone increases BMI despite unchanged glycaemic control in patients with HALS.11
Slama et al conducted the largest study analysing whether pioglitazone 30 mg daily improves lipodystrophy in 130 patients with HALS. They found that pioglitazone significantly increases fat mass in the extremities of patients treated without stavudine, but not in those treated with stavudine,21 which suggests that pioglitazone increases body fat mass, but stavudine affects the efficacy of pioglitazone therapy on HALS. Recent clinical studies implicate the use of thymidine nucleotide reverse transcriptase inhibitors (NRTIs), including stavudine, as a strong risk factor for HALS.22–24 In a previous study, switching from NRTIs to abacavir increased fat mass in the extremities of patients with HALS.25 Therefore, in the present case, the switch in HAART regimens might have also increased subcutaneous fat mass.
Although further investigation is needed, our findings suggest that pioglitazone therapy for HALS could increase subcutaneous fat mass in non-lipoatrophic but not in lipoatrophic regions. Pioglitazone therapy is considered a promising treatment for HALS, to improve insulin sensitivity and increase subcutaneous fat mass. However, in our case, pioglitazone therapy is not beneficial for reversing subcutaneous fat distribution. We believe that the present case raises a question about the use of thiazolidinediones in HALS to improve lipoatrophy.
Learning points.
Thiazolidinediones are considered a promising treatment for HIV/highly active antiretroviral therapy-associated lipodystrophy syndrome (HALS).
In previous studies, pioglitazone increased fat mass in patients with HALS; however, whether fat distribution changes remains unclear.
In our patient, pioglitazone was considered to have increased subcutaneous fat mass in non-lipoatrophic but not in lipoatrophic regions.
Our findings suggest that pioglitazone therapy is not beneficial for reversing subcutaneous fat distribution in HALS.
Acknowledgments
The authors thank H Nakamura (Center for Postgraduate Training, Nara Medical University), Y Yamada (Center for Infectious Diseases, Nara Medical University), F Nakamura (Center for Infectious Diseases, Nara Medical University) and all members of Center for Diabetes Care and Research, Nara Medical University Hospital, for the patient's care.
Footnotes
Contributors: SO, MK and HI designed the report, collected data and analysed the findings. All the authors wrote and edited the manuscript, and approved the final version.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Bhaskaran K, Hamouda O, Sannes M et al. Changes in the risk of death after HIV seroconversion compared with mortality in the general population. JAMA 2008;300:51–9. 10.1001/jama.300.1.51 [DOI] [PubMed] [Google Scholar]
- 2.Brown TT. Approach to the human immunodeficiency virus-infected patient with lipodystrophy. J Clin Endocrinol Metab 2008;93:2937–45. 10.1210/jc.2008-1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain SS, Ramteke KB, Raparti GT et al. Pathogenesis and treatment of human immunodeficiency virus lipodystrophy. Indian J Endocrinol Metab 2012;16(Suppl 1):S20–6. 10.4103/2230-8210.94250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009;50:499–505. 10.1097/QAI.0b013e31819c291b [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez AD, Balasubramanyam A. Dysregulation of glucose metabolism in HIV patients: epidemiology, mechanisms, and management. Endocrine 2012;41:1–10. 10.1007/s12020-011-9565-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn CR, Chen L, Cohen SE. Unraveling the mechanism of action of thiazolidinediones. J Clin Invest 2000;106:1305–7. 10.1172/JCI11705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carey DG, Cowin GJ, Galloway GJ et al. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes Res 2002;10:1008–15. 10.1038/oby.2002.137 [DOI] [PubMed] [Google Scholar]
- 8.Smith SR, De Jonge L, Volaufova J et al. Effect of pioglitazone on body composition and energy expenditure: a randomized controlled trial. Metab Clin Exp 2005;54:24–32. 10.1016/j.metabol.2004.07.008 [DOI] [PubMed] [Google Scholar]
- 9.Punthakee Z, Almeras N, Despres JP et al. Impact of rosiglitazone on body composition, hepatic fat, fatty acids, adipokines and glucose in persons with impaired fasting glucose or impaired glucose tolerance: a sub-study of the DREAM trial. Diabet Med 2014;31:1086–92. 10.1111/dme.12512 [DOI] [PubMed] [Google Scholar]
- 10.Edgeworth A, Treacy MP, Hurst TP. Thiazolidinediones in the treatment of HIV/HAART-associated lipodystrophy syndrome. AIDS Rev 2013;15:171–80. [PubMed] [Google Scholar]
- 11.Sheth SH, Larson RJ. The efficacy and safety of insulin-sensitizing drugs in HIV-associated lipodystrophy syndrome: a meta-analysis of randomized trials. BMC Infect Dis 2010;10:183 10.1186/1471-2334-10-183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simha V, Rao S, Garg A. Prolonged thiazolidinedione therapy does not reverse fat loss in patients with familial partial lipodystrophy, Dunnigan variety. Diabetes Obes Metab 2008;10:1275–6. 10.1111/j.1463-1326.2008.00978.x [DOI] [PubMed] [Google Scholar]
- 13.Sutinen J, Hakkinen AM, Westerbacka J et al. Rosiglitazone in the treatment of HAART-associated lipodystrophy—a randomized double-blind placebo-controlled study. Antivir Ther (Lond) 2003;8:199–207. [PubMed] [Google Scholar]
- 14.Hadigan C, Yawetz S, Thomas A et al. Metabolic effects of rosiglitazone in HIV lipodystrophy: a randomized, controlled trial. Ann Intern Med 2004;140:786–94. 10.7326/0003-4819-140-10-200405180-00008 [DOI] [PubMed] [Google Scholar]
- 15.Tomazic J, Karner P, Vidmar L et al. Effect of metformin and rosiglitazone on lipid metabolism in HIV infected patients receiving protease inhibitor containing HAART. Acta Dermatovenerol Alp Pannonica Adriat 2005;14:99–105. [PubMed] [Google Scholar]
- 16.Mulligan K, Yang Y, Wininger DA et al. Effects of metformin and rosiglitazone in HIV-infected patients with hyperinsulinemia and elevated waist/hip ratio. Aids 2007;21:47–57. 10.1097/QAD.0b013e328011220e [DOI] [PubMed] [Google Scholar]
- 17.Silic A, Janez A, Tomazic J et al. Effect of rosiglitazone and metformin on insulin resistance in patients infected with human immunodeficiency virus receiving highly active antiretroviral therapy containing protease inhibitor: randomized prospective controlled clinical trial. Croat Med J 2007;48:791–9. 10.3325/cmj.2007.6.791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindler K, Rieger A, Tura A et al. The effect of rosiglitazone on insulin sensitivity, beta cell function, bone mineral density, and body composition in hiv-positive patients on highly-active antiretroviral therapy (HAART). Horm Metab Res 2009;41:573–9. 10.1055/s-0029-1202779 [DOI] [PubMed] [Google Scholar]
- 19.Tungsiripat M, Bejjani DE, Rizk N et al. Rosiglitazone improves lipoatrophy in patients receiving thymidine-sparing regimens. Aids 2010;24:1291–8. 10.1097/QAD.0b013e328339e274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carr A, Workman C, Carey D et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet 2004;363:429–38. 10.1016/S0140-6736(04)15489-5 [DOI] [PubMed] [Google Scholar]
- 21.Slama L, Lanoy E, Valantin MA et al. Effect of pioglitazone on HIV-1-related lipodystrophy: a randomized double-blind placebo-controlled trial (ANRS 113). Antivir Ther (Lond) 2008;13:67–76. [PubMed] [Google Scholar]
- 22.Mallal SA, John M, Moore CB et al. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. Aids 2000;14:1309–16. 10.1097/00002030-200007070-00002 [DOI] [PubMed] [Google Scholar]
- 23.Saint-Marc T, Partisani M, Poizot-Martin I et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: preliminary results of the LIPOCO study. Aids 2000;14:37–49. 10.1097/00002030-200001070-00005 [DOI] [PubMed] [Google Scholar]
- 24.Hammond E, McKinnon E, Nolan D. Human immunodeficiency virus treatment-induced adipose tissue pathology and lipoatrophy: prevalence and metabolic consequences. Clin Infect Dis 2010;51:591–9. 10.1086/655765 [DOI] [PubMed] [Google Scholar]
- 25.Martin A, Smith DE, Carr A et al. Reversibility of lipoatrophy in HIV-infected patients 2 years after switching from a thymidine analogue to abacavir: the MITOX Extension Study. Aids 2004;18:1029–36. 10.1097/00002030-200404300-00011 [DOI] [PubMed] [Google Scholar]