Abstract
We describe three cases of patients with concomitant acute medullary or brainstem multiple sclerosis (MS) lesions and detectable spinal fluid varicella-zoster virus DNA. Herpes simplex virus PCR was also positive in two of the patients. One patient was re-punctured 2 weeks following the relapse, with negative results. The PCR findings greatly delayed correct diagnosis and treatment in all three patients. Based on our cases, we propose that inflammatory medullary and brainstem lesions could result in viral leakage, and possibly viral reactivation, from destroyed sensory neurons, yielding false-positive cerebrospinal fluid PCR results. As this can have diagnostic and therapeutic consequences, further studies are warranted to evaluate the clinical relevance of these findings.
Background
Multiple sclerosis (MS) is an immune-mediated disease of unknown aetiology. Various groups have reported that varicella-zoster virus (VZV) DNA is present in the cerebrospinal fluid (CSF) of patients with MS during exacerbations.1 2 The authors have suggested that reactivation of the VZV could be involved in the pathogenesis of the disease. However, their findings have not been reproduced in other studies.3 4 Thus, the frequency and clinical relevance of PCR positivity in this clinical setting is still uncertain. We report three newly diagnosed patients with MS, with concomitant active medullary or brainstem lesions and detectable spinal fluid DNA from neurotropic viruses, and propose a testable hypothesis for these findings.
Case presentation
Patient 1
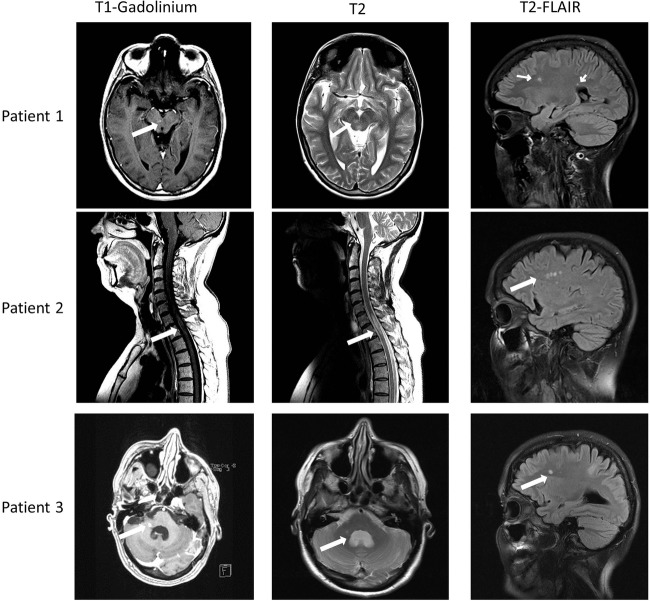
Patient 1, a 29-year-old woman, experienced her first neurological symptom in the form of loss of sensibility in the right side of her face and tongue. MRI detected multiple cerebral T2 lesions, as well as one gadolinium-enhancing (Gd) cerebral lesion and one Gd lesion located in the mesencephalon (figure 1). Spinal fluid analyses were consistent with MS (table 1). She was diagnosed with relapsing–remitting (RR) MS, according to the revised McDonald criteria.5 Intrathecal production of VZV and herpes simplex virus (HSV) IgG was detected, consistent with an MRZ (measles, rubella, and varicella zoster) reaction.6 Owing to diagnostic uncertainty, the spinal fluid was evaluated with PCR, which was positive for VZV DNA. Despite having no symptoms of VZV encephalitis, the PCR findings led to this diagnosis, and she received treatment with acyclovir for 14 days.
Figure 1.
MRI findings from the three patients. All three patients had active gadolinium-enhancing lesions in their brainstem or medulla at the time of diagnosis (arrow, column one). Column two demonstrating T2-weighted images of the same area (arrows) and column three showing T2-fluid attenuation inversion recovery images, consistent with older cerebral multiple sclerosis lesions (arrows).
Table 1.
Clinical and laboratory features of patients
| Patient number | Diagnosis* | Age (year)† | Sex | CSF cells‡ | OCB | White matter lesions | Gd medullary/brainstem lesions | VZV DNA | HSV-1 DNA |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MS | 29 | F | 32 | + | + | + | + | − |
| 2 | CIS | 53 | F | 26 | + | + | + | + | + |
| 3 | MS | 44 | F | 17 | + | + | + | + | + |
*At time of lumbar puncture, according to the revised McDonald criteria.7
†At time of lumbar puncture.
‡White cell counts per cubic millimetre.
CIS, clinical isolated syndrome; CSF, cerebrospinal fluid; F, female; Gd, gadolinium; HSV-1, herpes simplex virus type 1; MS, multiple sclerosis; OCB, oligoclonal bands; VZV, varicella-zoster virus.
Patient 2
Patient 2, a 53-year-old woman, experienced her first neurological symptom in the form of progressive loss of sensibility corresponding to Th4. Cerebral MRI showed multiple cerebral T2 lesions, as well as one Gd-enhancing medullary lesion located between C7 and Th1/Th2 (figure 1). Spinal fluid analyses were consistent with MS (table 1). Intrathecal antibody production of VZV and HSV IgG was positive. Spinal fluid PCR was positive for both VZV DNA and HSV-1. A follow-up MRI 1 month later showed one new medullary Gd lesion, and the patient was ultimately diagnosed with RR-MS.5
Patient 3
Patient 3, a 44-year-old woman, experienced her first neurological symptom in the form of diplopia. Clinical examination confirmed complete right-sided abducens nerve palsy. MRI revealed multiple cerebral T2 lesions, one contrast-enhancing lesion in the right cerebellar peduncle and one in the right temporal cortex (figure 1). Spinal fluid analyses were consistent with MS (table 1) and she was diagnosed according to the revised McDonald criteria.5 Intrathecal production of VZV and HSV IgG was confirmed. PCR was positive for both HSV-1 and VZV DNA. Two weeks after treatment with steroids and acyclovir, the patient was asymptomatic and a follow-up MRI demonstrated regression of the lesions. On re-puncture, PCR was negative for both viruses.
Investigations
VZV antibody detection
Immunoglobulin (Ig)G antibodies to the VZV were measured with Enzygnost Anti-Varicella-Virus/IgG (Siemens Healthcare GmbH, Erlangen, Germany) according to the manufacturer's instructions. Measured CSF and serum VZV-IgG levels were adjusted based on the CSF/serum albumin ratio, and a VZV-IgG ratio was calculated over three dilution steps (1:1–1:100). A CSF/serum ratio above two in the 1:100 dilution was defined as positive.
PCR and melting curve analysis
Uracil-N-glycosylase was added to the CSF samples to avoid contamination. VZV DNA was amplified by LightCycler PCR (Roche, Penzberg, Germany) with primers directed to gene 29. PCR primers were: sense, 5′-TGT CCT AGA GGA GGT TTT ATC TG-3′; antisense, 5′-CAT CGT CTG TAA AGAC TTA ACC AG-3′. The method has previously been described in detail.7 For detection of HSV, real-time PCR with fluorescence assay and the fluorescence resonance energy transfer principle were used. Sequence differences between HSV-2 and HSV-1 were detected by melting curve analysis following PCR amplification, as described previously.8 PCR primers were directed against DNA polymerase and are described in detail by Espy et al.8 The original probe R was replaced by probe A (TIB MOLBIOL GmbH, Berlin, Germany). The methods have been used at our laboratory for many years and are regularly validated against external quality panels.
Outcome and follow-up
We detected spinal fluid VZV DNA in three newly diagnosed patients with MS with brainstem or medullary lesions. Two of the patients were also positive for HSV-1 DNA at the time of relapses. All three patients had clinical symptoms and MRI findings consistent with an acute MS attack—and not with infections from HSV-1 or VZV—and were seropositive for VZV and HSV-1. The PCR findings led to significant diagnostic and therapeutic delay in all three patients.
Discussion
The results of earlier studies assessing the prevalence of spinal fluid viral DNA during MS relapses are conflicting.2 3 Both VZV and HSV are neurotropic viruses, often found dormant in the same sensory ganglia. Based on our findings, we propose that the presence of viral DNA could be dependent on the location of MS lesions. We suggest that the detected viral DNA reflects leakage, or reactivation, of viral particles from destroyed sensory neurons, rather than a true involvement in MS pathogenesis. Should this assumption be correct, one would expect to only detect these viruses in previously infected patients with acute MS plaques affecting medullary or brainstem sensory neurons. This is a hypothesis that could be easily tested in larger material, and would provide an explanation for the discrepant results in prior studies. As PCR examination becomes increasingly available, we expect that this will result in more diagnostic confusion and delay in the future. Establishing the mechanism for these findings will therefore have important diagnostic implications.
Learning points.
False-positive PCR for varicella-zoster virus and herpes simplex virus 1 can be detected in the cerebrospinal fluid of patients with multiple sclerosis (MS), previously infected with the viruses.
This can lead to delayed diagnosis and to unnecessary antiviral treatment.
The cause of these findings is uncertain, but we propose that the detected viral DNA reflects leakage, or reactivation, of viral particles from destroyed sensory neurons, rather than a true involvement in MS pathogenesis.
Since PCR examinations become increasingly available, and are often taken on a routine basis, we expect that this will result in more diagnostic confusion and delay in the future. Establishing the mechanism for these findings will therefore have important diagnostic implications.
Footnotes
Contributors: ØT, ØP and AS were responsible for study concept and design. ØT and AS contributed to acquisition of the data. ØT, ØP and AS were responsible for analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content. ØT contributed to study supervision.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mancuso R, Delbue S, Borghi E et al. . Increased prevalence of varicella zoster virus DNA in cerebrospinal fluid from patients with multiple sclerosis. J Med Virol 2007;79:192–9. 10.1002/jmv.20777 [DOI] [PubMed] [Google Scholar]
- 2.Sotelo J, Martínez-Palomo A, Ordoñez G et al. . Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann Neurol 2008;63:303–11. 10.1002/ana.21316 [DOI] [PubMed] [Google Scholar]
- 3.Burgoon MP, Hammack BN, Owens GP et al. . Varicella zoster virus is not a disease-relevant antigen in multiple sclerosis. Ann Neurol 2009;65:474–9. 10.1002/ana.21605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mancuso R, Hernis A, Cavarretta R et al. . Detection of viral DNA sequences in the cerebrospinal fluid of patients with multiple sclerosis. J Med Virol 2010;82:1051–7. 10.1002/jmv.21764 [DOI] [PubMed] [Google Scholar]
- 5.Polman CH, Reingold SC, Banwell B et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnan M. Does disease-irrelevant intrathecal synthesis in multiple sclerosis make sense in the light of tertiary lymphoid organs? Front Neurol 2014;5:27 10.3389/fneur.2014.00027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espy MJ, Teo R, Ross TK et al. . Diagnosis of varicella-zoster virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol 2000;38:3187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Espy MJ, Uhl JR, Mitchell PS et al. . Diagnosis of herpes simplex virus infections in the clinical laboratory by LightCycler PCR. J Clin Microbiol 2000;38:795–9. [DOI] [PMC free article] [PubMed] [Google Scholar]