Abstract
Background
Mechanical loading plays an important role in the regulation of extracellular matrix (ECM) homeostasis as well as pathogenesis of intervertebral disc (IVD) degeneration. The human annulus fibrosus (hAF) in the IVD is subjected to contact shear stress during body motion. However, the effects of shear stress on hAF cells remain unclear. This aim of the study was to investigate the expression of the ECM (COLI, COLIII and aggrecan) and matrix metalloproteinase (MMP-1, MMP-3 and ADAMTS-4) genes in hAF cells following fluid-induced shear stress in a custom-fabricated bio-microfluidic device.
Methods
hAF cells were harvested from degenerated disc tissues in routine spine surgery, staged by magnetic resonance imaging, expanded in monolayers and then seeded onto the bio-microfluidic device. The experimental groups were subjected to 1 and 10 dyne/cm2 shear stress for 4 h, and no shear stress was applied to the control group. We used real time polymerase chain reaction for gene expression.
Results
Shear stress of 1 dyne/cm2 exerted an anabolic effect on COLI and COLIII genes and catabolic effects on the aggrecan gene, while 10 dyne/cm2 had an anabolic effect on the COLI gene and a catabolic effect on COLIII and aggrecan genes. The COLI gene was upregulated in a stress-dependent manner. Expression of MMP-1 was significantly higher in the 10 dyne/cm2 group compared to the control group (P < 0.05), but was similar in the control and 1 dyne/cm2 groups. Expression of MMP-3 and ADAMTS-4 were similar in all three groups.
Conclusion
Taken together, hAF cells responded to shear stress. The findings help us understand and clarify the effects of shear stress on IVD degeneration as well as the development of a new therapeutic strategy for IVD degeneration.
Keywords: Intervertebral disc, Annulus fibrosus, Mechanical loading, Shear stress, Bio-microfluidic device, Mechanobiology
Background
The intervertebral disc (IVD) is composed of two distinct components [1, 2], the central gelatinous nucleus pulposus (NP) and the surrounding outer annulus fibrosus (AF). Movement of the spine subjects the bony structure of the spine and the IVD to mechanical loading [2, 3]. Degeneration of the IVD, which has been identified as one of the major sources of back pain [4], has a complex pathogenesis [1, 5]. A number of mechanical factors such as tension [6], compression [7], shear stress [3] and vibration [8] as well as other factors such as aging, genetic and systemic factors have been implicated in the pathogenesis of IVD degeneration [5].
IVD degeneration is characterized by increased degradation of the extracellular matrix (ECM) by locally produced matrix metalloproteinases (MMPs) and aggrecanase which belongs to the ADAMTS family (a disintegrin and metalloproteinase with thrombospondin motifs) [1, 9]. Collagens and aggrecans, which are the major components of the ECM in the IVD, are synthesized by the IVD, and broken down by MMPs and aggrecanases [5] to achieve dynamic equilibrium.
In vitro experiments demonstrated that proper mechanical loading plays an important role in the function and metabolism of IVD cells [2, 6–8, 10]. Epidemiologic studies [11] also revealed that mechanical overloading resulting from bending, twisting and heavy lifting were important risk factors for back pain, IVD degeneration and herniation [5, 11].
IVD degeneration has been shown to include different types of human AF tears or defects (peripheral, circumferential or radiating) [12]. Although AF degeneration seems to occur following NP degeneration [13], biochemical changes in AF are accompanied by IVD degeneration, suggesting that AF may also play an important role in IVD degeneration [12], particularly the initiation of IVD degeneration.
During movement of the spine, the IVD and human AF (hAF) are exposed to multiple, complex, three-dimensional mechanical loading forces, including compression, tension, lateral bending, vibration, shear stress or a combination of these [5, 14]. The change in intradiscal hydrostatic pressure during spine movement also impacts tissue fluid influx and efflux within the IVD [15]. Consequently, hAF cells are constantly subjected to fluid-induced shear stress even without body motion [3]. Fluid-induced shear stress was defined as the tangential component of frictional forces generated at a surface by the flow of a viscous fluid, reported by Chiu and Chien [16]. Although there is growing awareness of the role of mechanical loading on the metabolism of human, caprine and rabbit AF cells [6, 8, 17], the impact of shear stress on hAF cells has yet to be explored.
We hypothesized that shear stress might play a vital role in the expression of ECM and metalloproteinase genes in hAF cells. We investigated the effects of fluid-induced shear stress on the expression of ECM genes (COLI, COLIII and aggrecan), and MMP genes (MMP-1, MMP-3, and ADAMTS-4) of hAF cells harvested from degenerated disc tissues in a custom-fabricated bio-microfluidic device.
Methods
The study was approved by the ethical committee of the Taipei Veterans General Hospital prior to the study. Informed consent for using the disc tissue for the study was obtained before the index operation. Disc tissues were obtained from three female and three male donors (mean age 55 years, range 47–62 years) during disc excision for transforaminal interbody fusion (TLIF) (n = 6) (Table 1). The degenerative status of the discs was classified based on Pfirrmann’s classification using T2-weighted magnetic resonance images [18]. hAF cells were isolated from AF tissues obtained from IVD after TLIF surgery. The most inner and outer layers of AF tissues and cartilaginous endplates were carefully removed, and only the middle layer of AF tissues was used for further enzymatic digestion. hAF tissues were separated from the discs immediately after surgical excision, finely minced, and transported under sterile conditions to the laboratory for primary hAF culture. To obtain hAF cells, tissues were gently agitated in the presence of phosphate-buffered saline (PBS) containing 0.1 % collagenase for 30 min at 37 °C and then at room temperature for another 30 to 40 min. After digestion, the fragmented tissues were passed through filters, centrifuged twice, and cells were resuspended in Dulbecco’s modified Eagle medium (DMEM; Sigma-Aldrich) supplemented with 10 % fetal bovine serum (FBS; HyClone, Logan, UT, USA), 100 U penicillin, 1000 U streptomycin, 2 mm L-glutamine (Invitrogen, Carlsbad, CA, USA), and 1 ng/mL recombinant human fibroblast growth factor-2 (FGF-2; Sigma-Aldrich). The cell suspension was plated in dishes and incubated at 37 °C in a humidified atmosphere of 5 % CO2 and 95 % air. Culture medium was changed twice a week.
Table 1.
Patient demographic data
| Age (years) | Gender | Diagnosis | Operation | |
|---|---|---|---|---|
| Patient 1 | 62 | Male | L 45 listhesis | L 45 interbody fusion |
| Patient 2 | 55 | Male | L 345 listhesis | L 345 interbody fusion |
| Patient 3 | 47 | Male | L 45 listhesis | L 45 interbody fusion |
| Patient 4 | 60 | Female | L 345 listhesis | L 345 interbody fusion |
| Patient 5 | 52 | Female | L 45 listhesis | L 45 interbody fusion |
| Patient 6 | 54 | Female | L 45 listhesis | L 45 interbody fusion |
L Lumbar, listhesis Spondylolisthesis
When hAF cells from degenerated disc tissue (passage 0) were 70–80 % confluent, cells were detached with 0.25 % trypsin–ethylenediaminetetraacetic acid (Gibco BRL), washed twice with PBS, centrifuged at 1000 rpm for 4 min, and cultured in T-75 flasks as described previously (passage 1). When hAF cells reached 80 % confluence, they were harvested and expanded in T-75 flasks (passage 2). Passage 2 of hAF cells which were 80 % confluent were detached with trypsin, washed twice with PBS and cryopreserved in liquid nitrogen in FBS with 10 % dimethyl sulphoxide (DMSO). All experiments were performed with cells from passages 2 and 3 in order to avoid de-differentiation during culture over long periods (p < 6) [19].
We used a modified prototype of a custom-fabricated microfluidic device [20]. The five-layer device comprised a culture chamber, a vacuum region, and bubble traps. The width of the culture chamber was 10 mm, the length was 40 mm and height was 350 μm. The bubble-removal structure in the micro-channel prevented bubbles from being injected into the culture chamber. The microstructure was fabricated with a polymethyl methacrylate (PMMA) adaptor, two layers of glass, and two layers of polydimethylsiloxane (PDMS) (Fig. 1). The top layer of the device, consisting of three adaptors to produce a vacuum, and the medium inlet and outlet, was made of PMMA. The intermediate layer of the device was made of two patterned glass layers and two patterned PDMS layers. PDMS prepolymer (Sylgard 184, DowCorning) was prepared by mixing PDMS base with a curing agent at a 10:1 ratio by weight. The PDMS prepolymer mixture was then poured on the glass mold to a thickness of 350 μm and cured at 60 °C in an oven. Next, the PDMS membrane was patterned using a CO2 laser machine (ILS-II, Laser Tools and Ttechnics Corp.) and the glass was patterned with an ultrasonic drill machine (Lapidary & Sonic Ent.) to make the microfluidic channel, the culture chamber, and the vacuum channel. The microfluidic device was formed by bonding the patterned glass and PDMS using a plasma treatment system (PX-250, Plasma treatment System, America North) and attaching this to the PMMA adaptor using 3 M tape.
Fig. 1.
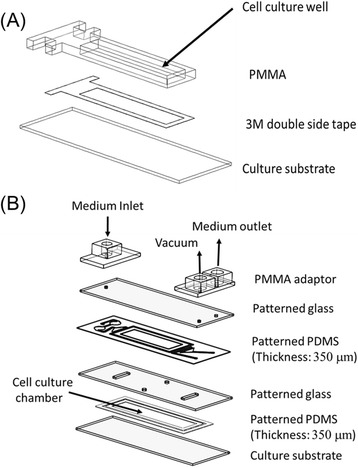
Design of the custom-fabricated microfluidic device. a Schematic representation of the “pre-culture well”. b The assembly of the microfluidic device with a cross-sectional view of the structure. PDMS Polydimethylsiloxane, PMMA Polymethyl methacrylate
Rhodamine 6G was used as a tracing dye to ensure that the flow field on the microfluidic device was uniform and that the flow of the medium into the cell culture region was homogeneous. The flow field in our custom-fabricated chip has been shown to be uniform and steady [20]. Human mesenchymal stem cells (Lonza, PT-2501) were used to test biocompatibility in further experiments.
Fibronectin (Fn) (bovine; Sigma-Aldrich) was diluted in PBS to a working concentration of 100 μg/mL. Diluted Fn (500 μg) was loaded smoothly onto the custom-fabricated bio-microfluidic device and incubated at 37 °C for at least 1 h. The Fn solution was then removed, and the device washed twice with PBS.
hAF cells (8–10 × 104) were seeded onto the fabricated laser-cut dishes and incubated overnight at 37 °C. The microfluidic device was sterilized by injecting it with 75 % ethanol via the plastic pipes and exposing it overnight to ultraviolet radiation. The laser-cut dishes were assembled with the custom-fabricated bio-microfluidic device under sterile conditions. The pumping machine was set up with a 50 mL syringe filled with medium as fluid-induced shear stress from the inlet. The device was placed on a thermal control plate at 37 °C. During the experiment, medium supplied via the 50 mL syringe was injected by the syringe pump into the chamber of the microfluidic device through the pipeline (Fig. 2). There was a continuous flow of medium from the inlet into the microfluidic device, and spent medium flowed out from the outlet to the waste tube. Based on the cell-in-channel model adapted by Farokhzad et al. [19] and Gaver and Kute [21], the maximum shear stress in our chip was calculated to be 1 dyne/cm2 when the flow rate was set to 10.14 mL/h.
Fig. 2.
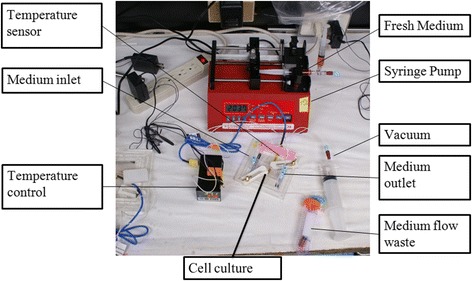
Bio-microfluidic device used for the fluid-induced shear stress experiment
Continuous fluid-induced shear stress of 1 and 10 dyne/cm2 was applied through the microfluidic device for 4 h, respectively. The control group consisted of hAF cells in the device not subjected to shear stress (0 dyne/cm2) (Fig. 3).
Fig. 3.
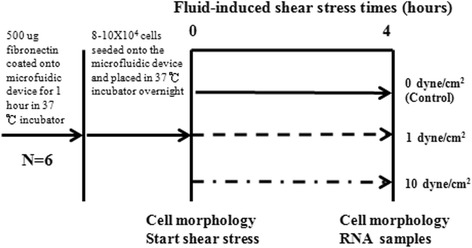
Experimental flowchart. Degenerated hAF cells (n = 6) were seeded onto the bio-microfluidic device and incubated overnight. RNA was extracted from each plate and gene expression evaluated by real-time polymerase chain reaction
The expression of different genes following fluid-induced shear stress was evaluated by quantitative real-time polymerase chain reaction (PCR). Total RNA was extracted from 8–10 × 104 cells using the RNeasy Mini Kit (Qiagen, Stanford, Valencia, CA, USA). RNA samples were used for reverse transcription and subsequent PCR amplification. Quantitative real-time PCR was performed using the LightCycler 480 Real-Time System (Roche Applied Sciences, Mannheim, Germany). Intron spanning primers specific for each targeted gene were designed using the Universal ProbeLibrary Assay Design Center (http://www.roche-applied-science.com) and PCR products were detected using corresponding probes from the Universal ProbeLibrary (Roche) (Table 2). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene was used as an endogenous internal control. All target genes were normalized using the housekeeping gene GAPDH, and analyzed with the 2–△△Ct method. Changes in gene expression were expressed as fold-change.
Table 2.
Primers used in the real-time polymerase chain reaction analysis
| Target gene | Forward primer | Reverse primer | GenBank | Roche |
|---|---|---|---|---|
| accession no. | probe no. | |||
| Housekeeping gene | ||||
| GAPDH | agccacatcgctcagacac | gcccaatacgaccaaatcc | NM_002046 | #60 |
| Anabolic genes | ||||
| COLIA1 | gggattccctggacctaaag | ggaacacctcgctctcc | NM_000088 | #67 |
| COLIIIA1 | ctggaccccagggtcttc | catctgatccagggtttcca | NM_000090 | #20 |
| Aggrecan | cctccccttcacgtgtaaaa | gctccgcttctgtagtctgc | NM_01135 | #76 |
| Catabolic genes | ||||
| MMP-1 | gctaacctttgatgctataactacga | tttgtgcgcatgtagaatctg | NM_001145938.1 | #7 |
| MMP-3 | caaaacatatttctttgtagaggacaa | ttcagctatttgcttgggaaa | NM_002422 | #36 |
| ADAMTS-4 | ccaggcactgggctactact | gaacagggggtcccatcta | NM_005099 | #67 |
ADAMTS-4 A disintegrin and metallopeptidase with thrombospondin motif 4, COLIA1 Collagen type IA1, COLIIIA1 Collagen type IIIA1, GAPDH Glyceraldehyde-3-phosphate dehydrogenase, MMP Matrix metalloproteinase
The relative expression of different genes in the different groups was analyzed by one-way analysis of variance (ANOVA) with the Tukey’s post-hoc test. P < 0.05 indicated statistical significance.
Results
The disc tissues were classified as degeneration grades III or IV based on Pfirrmann’s classification [18]. When grown in monolayers, hAF cells harvested from degenerated disc tissues were astrocytes-like cells with one or three protrusions with a doubling time of 63 ± 12 h (range 52–78 h) (Fig. 4). A pre-test regarding biocompatibility in our custom-fabricated bio-microfluidic device was considered good enough to perform further experiments.
Fig. 4.
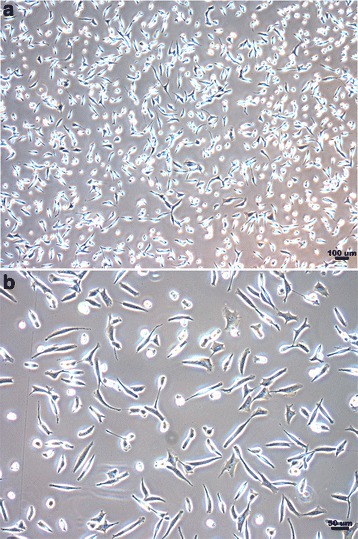
Morphology of hAF cells harvested from degenerated disc tissues. a The scale bar indicates 100 μm. b The scale bar indicates 50 μm. Cells were cultured for 20 days. Cells were spindle-shaped and plastic adherent. The doubling time was 63 ± 12 h (range, 52–78 h)
hAF cells were attached on the Fn-coated bio-microfludic device after overnight incubation at 37 °C, and had a similar morphology to cells cultured in a dish. When the cells were ready, the whole bio-microfluidic device was set up by assembling the laser-cut dishes with the microfluidic chips using vacuum force. Different levels of shear stress were applied (Figs. 3 and 4). Additionally, the morphology of cells seeded on the bio-microfluidic device remained unchanged after being subjected to 1 or 10 dyne/cm2 shear stress for 4 h.
We compared the expression of ECM and metalloproteinase genes in the cells subjected to shear stress with control cells (Figs. 5 and 6). Cells subjected to 1 and 10 dyne/cm2 shear stress had a significantly higher expression of COLI compared to control cells (1.72 ± 0.65- and 2.49 ± 1.06-fold higher, respectively), suggesting that the COLI gene was regulated in a stress-dependent manner. Expression of the COLIII gene was 2.65 ± 0.6 higher than control cells following 1 dyne/cm2 shear stress, but 0.57 ± 0.79-fold lower than the control group following 10 dyne/cm2 shear stress. Expression of the COLIII gene was significantly lower following 10 dyne/cm2 shear stress compared to cells subjected to 1 dyne/cm2. Cells subjected to 1 and 10 dyne/cm2 shear stress had a significantly lower expression of the aggrecan gene compared to the control group (0.65 ± 0.74- and 0.59 ± 0.58-fold lower, respectively). Expression of the aggrecan gene was also significantly lower following 10 dyne/cm2 shear stress compared to cells subjected to 1 dyne/cm2.
Fig. 5.
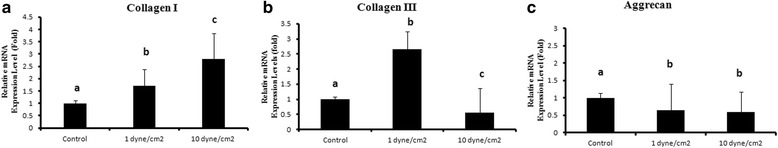
Semi-quantitative PCR for relative quantitation of ECM genes expression after 1 and 10 dyne/cm2 fluid-induced shear stress for 4 h. The control group (0 dyne/cm2) was not subjected to fluid-induced shear stress on the bio-microfluidic device. Semi-quantitative PCR was used to determine relative quantitative gene expression of a COLI, b COLIII and c aggrecan. Data represent mean ± standard deviation (n = 6). Differences were significant between groups with different letters (P < 0.05). Differences were not significant between groups with the same letter
Fig. 6.
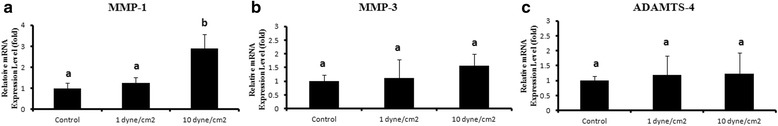
Semi-quantitative PCR for relative quantitation of metalloproteinase genes expression after 1 and 10 dyne/cm2 fluid-induced shear stress for 4 h. Semi-quantitative PCR was used to determine relative quantitative gene expression of a MMP-1, b MMP-3 and c ADAMTS-4. Data represent mean ± standard deviation (n = 6). Differences were significant between groups with different letters (P < 0.05). Differences were not significant between groups with the same letter
Expression of the MMP-1 gene was 2.9 ± 0.76-fold higher in cells subjected to 10 dyne/cm2 shear stress compared to the control group. Expression of the MMP-1 gene was similar between the control and 1 dyne/cm2 groups. There was no significant difference in the expression of the MMP-3 and ADAMTS-4 genes between the three groups.
The above results suggested that 1 dyne/cm2 exerted an anabolic effect on the COLI and COLIII genes but a catabolic effect on the aggrecan gene. It was also possible that 10 dyne/cm2 shear stress exerted an anabolic effect on the COLI gene but a catabolic effect on the COLIII and aggrecan genes.
Discussion
hAF cells are exposed to shear stress during normal physiologic movement of the spine [3], and the mechanical stimulation is important for regulating homeostasis of the AF matrix [2, 3, 6–8, 10, 14]. The disc along with AF tissues which serves as a load-bearing structure is exposed to different types of daily recurring mechanic loads including tension, compression, and shear stress [22]. All these types of mechanical loading may occur simultaneously, resulting in a complex combination loading situation in vivo [22]. Disc cell types and species, loading types, frequency, magnitude and duration are among the key factors thought to play important roles in AF-ECM homeostasis [22].
MMP-1, which belongs to the subclass of collagenases, has been reported to be the most common MMP in the degenerated disc, with 91 % positive staining [23]. MMP-1 activity is highest against collagen type III (> I > II) [23]. Collagen types I and III are distributed in the AF [9]. MMP-3, which plays a major role in disc degeneration, has a broad substrate specificity, and has been shown to degrade proteoglycans, lamin, Fn, gelatins, and collagen types II, III, IV, and V [23]. Degenerative disc is characterized by an upregulation of degradative enzymes such as MMPs, along with a declining ability to produce ECM [4, 5]. Both MMP-1 and -3 have also been found in the AF in the degenerated disc, and this may contribute to the pathogenesis of AF matrix degeneration.
Besides type I and III collagens, the other major ECM component of the AF is proteoglycans, particularly aggrecan. Aggrecan is substituted with several negatively charged glycosaminoglycans (GAGs) which are responsible for its water-binding capacity [1, 5]. ADAMTS-4 (aggrecanase-1), which is a key proteinase for the breakdown of aggrecan, has been shown to play an important role in the pathogenesis of AF degeneration. Several studies reported that mechanical stimuli may impact proteoglycan metabolism in hAF cells. Aggrecan gene expression was inhibited in hAF cells subjected to 10 % cyclic tensile strain at a frequency of 1 Hz for 24 h [17], while 0.4 MPa of compressive stress at 1 Hz for 2 h twice a day up to 7 days had catabolic effects on aggrecan gene expression [24]. Our results indicated that shear stress of 1 and 10 dyne/cm2 resulted in downregulation of aggrecan gene expression.
Shear stress of 1 and 10 dyne/cm2 also affected the expression of COLI in a stress-dependent manner, while the expression of the MMP-3 and ADAMT-4 genes remained unchanged in cells subjected to shear stress of 1 and 10 dyne/cm2 compared to the control group. It is possible that, in addition to MMP-1, -3 and ADAMT-4, other degradative enzymes or mechano-transduction pathways may be involved in the pathogenesis of ECM metabolism following shear stress. Shear stress of 1 dyne/cm2 seemed to have anabolic effects on the expression of the collagen I and III genes in degenerated hAF cells. However, a higher shear stress (10 dyne/cm2) exerted an anabolic effect on COLI expression, and a catabolic effect on COLIII and aggrecan expression.
Mechanically, AF cells bear shear stress during bending or twisting of the trunk; but few studies have investigated the effects of shear stress on the expression of different genes in hAF cells subjected to stress. hAF cells have been shown to respond to laminar shear stress by increasing intracellular calcium levels [3]. Our data differed from a previous report which showed that the expression of the MMP-1 and -3 genes was upregulated in rabbit tendon cells following 1 dyne/cm2 fluid-induced shear stress for 6 h in a specially designed multi-slide flow device [25]. This difference could be due to species variation and differences in the magnitude of force at the cellular level.
Previous reports [11, 26] indicated that excessive loading such as heavy lifting, bending and twisting may play an important role in increased incidence of disc degeneration and herniation. Neidlinger-Wilke et al. [22] reported a baseline intradiscal pressure measuring around 0.1–0.2 MPa under conditions of supine rest, due to the combined effect of muscle loads and osmotic potential of the disc. Shear stress was estimated at 1600 NT at the L5–S1 disc level while stooping or weight lifting [27]. The shear stress applied in our study (1 and 10 dyne) was lower than that seen under physiological conditions. The reason for this was that AF cells may de-attach from the bio-microfludic in vitro following much higher shear stress. The in vitro situation is therefore different from that in vivo with muscles, ligaments and facet articular joint supports.
Our study had a number of limitations which should be considered. Firstly, our results were based on a cell-in-channel model, which did not reflect the real three-dimensional disc environment in vivo during body motion. Secondly, the magnitude of shear stress that hAF cells are subjected to during body motion still needs to be measured, which would validate our results. Thirdly, cells may be detached following fluid-induced shear stress in our chip, which may interfere with our results. Moreover, we still lacked the results of gene expression in healthy hAF cells harvested from discs in post-mortem young donors without prior systemic diseases or spine trauma, which could provide the real control results and allow us to compare between healthy and degenerated discs to understand the role of shear stress in the pathogenesis of disc degeneration.
In summary, we demonstrated that the expressions of ECM and metalloproteinase genes in hAF cells were influenced by fluid-induced shear stress. Although not all aspects of alterations of the mechanical environment of the disc can be extrapolated from the in vivo situation, our results may provide better understanding of the physiology of the degenerated discs and the pathogenesis of disc degeneration following shear stress.
Conclusion
Fluid-induced shear stress regulated ECM and MMP genes expression in hAF cells. The study may clarify the role of shear stress in the patho-mechanism of disc degeneration as well as the development of a new therapeutic strategy for IVD degeneration.
Acknowledgements
The authors wish to thank Shin-Yi Huang from the Biostatistics Task Force, Taipei Veterans General Hospital, for her statistical assistance. We thank the Medical Science & Technology Building of Taipei Veterans General Hospital for providing experimental space and facilities. This work was sponsored by the Ministry of Science and Technology, Taiwan (NSC102-2314-B-07).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PHC performed and analyzed the majority of the experiments and wrote and revised the manuscript. STW helped with disc cell preparation, performed surgery to obtain disc samples and wrote the manuscript. MHY contributed to the study design, and designed and tested the bio-microfluidic chip and drafted manuscript. CLL and MCC performed surgery to obtain disc samples, helped to analyze and interpret the quantitative PCR data, and drafted the manuscript. OKSL designed the whole study conception and drafted and revised the manuscript. All authors read and approved the final manuscript.
References
- 1.Freemont AJ, Watkins A, Le Maitre C, Jeziorska M, Hoyland JA. Current understanding of cellular and molecular events in intervertebral disc degeneration: implications for therapy. J Pathol. 2002;196(4):374–379. doi: 10.1002/path.1050. [DOI] [PubMed] [Google Scholar]
- 2.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 3.Elfervig MK, Minchew JT, Francke E, Tsuzaki M, Banes AJ. IL-1beta sensitizes intervertebral disc annulus cells to fluid-induced shear stress. J Cell Biochem. 2001;82(2):290–298. doi: 10.1002/jcb.1153. [DOI] [PubMed] [Google Scholar]
- 4.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford) 2009;48(1):5–10. doi: 10.1093/rheumatology/ken396. [DOI] [PubMed] [Google Scholar]
- 5.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5(3):120–130. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sowa G, Coelho P, Vo N, Bedison R, Chiao A, Davies C, et al. Determination of annulus fibrosus cell response to tensile strain as a function of duration, magnitude, and frequency. J Orthop Res. 2011;29(8):1275–1283. doi: 10.1002/jor.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korecki CL, Kuo CK, Tuan RS, Iatridis JC. Intervertebral disc cell response to dynamic compression is age and frequency dependent. J Orthop Res. 2009;27(6):800–806. doi: 10.1002/jor.20814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki S, Banes AJ, Weinhold PS, Tsuzaki M, Kawakami M, Minchew JT. Vibratory loading decreases extracellular matrix and matrix metalloproteinase gene expression in rabbit annulus cells. Spine J. 2002;2(6):415–420. doi: 10.1016/S1529-9430(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 9.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 10.Kasra M, Goel V, Martin J, Wang ST, Choi W, Buckwalter J. Effect of dynamic hydrostatic pressure on rabbit intervertebral disc cells. J Orthop Res. 2003;21(4):597–603. doi: 10.1016/S0736-0266(03)00027-5. [DOI] [PubMed] [Google Scholar]
- 11.Hoogendoorn WE, van Poppel MN, Bongers PM, Koes BW, Bouter LM. Systematic review of psychosocial factors at work and private life as risk factors for back pain. Spine. 2000;25(16):2114–2125. doi: 10.1097/00007632-200008150-00017. [DOI] [PubMed] [Google Scholar]
- 12.Osti OL, Vernon-Roberts B, Moore R, Fraser RD. Annular tears and disc degeneration in the lumbar spine. A post-mortem study of 135 discs. J Bone Joint Surg Br. 1992;74(5):678–682. doi: 10.1302/0301-620X.74B5.1388173. [DOI] [PubMed] [Google Scholar]
- 13.Nerlich AG, Schleicher ED, Boos N. 1997 Volvo Award winner in basic science studies. Immunohistologic markers for age-related changes of human lumbar intervertebral discs. Spine. 1997;22(24):2781–2795. doi: 10.1097/00007632-199712150-00001. [DOI] [PubMed] [Google Scholar]
- 14.Setton LA, Chen J. Cell mechanics and mechanobiology in the intervertebral disc. Spine. 2004;29(23):2710–2723. doi: 10.1097/01.brs.0000146050.57722.2a. [DOI] [PubMed] [Google Scholar]
- 15.Ishihara H, McNally DS, Urban JP, Hall AC. Effects of hydrostatic pressure on matrix synthesis in different regions of the intervertebral disk. J Appl Physiol (1985) 1996;80(3):839–846. doi: 10.1152/jappl.1996.80.3.839. [DOI] [PubMed] [Google Scholar]
- 16.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol Rev. 2011;91(1):327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert HT, Hoyland JA, Millward-Sadler SJ. The response of human anulus fibrosus cells to cyclic tensile strain is frequency-dependent and altered with disc degeneration. Arthritis Rheum. 2010;62(11):3385–3394. doi: 10.1002/art.27643. [DOI] [PubMed] [Google Scholar]
- 18.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–1878. doi: 10.1097/00007632-200109010-00011. [DOI] [PubMed] [Google Scholar]
- 19.Farokhzad OC, Khademhosseini A, Jon S, Hermmann A, Cheng J, Chin C, et al. Microfluidic system for studying the interaction of nanoparticles and microparticles with cells. Anal Chem. 2005;77(17):5453–5459. doi: 10.1021/ac050312q. [DOI] [PubMed] [Google Scholar]
- 20.Le Maitre CL, Hoyland JA, Freemont AJ. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9(4):R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaver DP, 3rd, Kute SM. A theoretical model study of the influence of fluid stresses on a cell adhering to a microchannel wall. Biophys J. 1998;75(2):721–733. doi: 10.1016/S0006-3495(98)77562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidlinger-Wilke C, Galbusera F, Pratsinis H, Mavrogonatou E, Mietsch A, Kletsas D, et al. Mechanical loading of the intervertebral disc: from the macroscopic to the cellular level. Eur Spine J. 2014;23(Suppl 3):S333–S343. doi: 10.1007/s00586-013-2855-9. [DOI] [PubMed] [Google Scholar]
- 23.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25(23):3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hee HT, Zhang J, Wong HK. An in vitro study of dynamic cyclic compressive stress on human inner annulus fibrosus and nucleus pulposus cells. Spine J. 2010;10(9):795–801. doi: 10.1016/j.spinee.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 25.Archambault JM, Elfervig-Wall MK, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002;35(3):303–309. doi: 10.1016/S0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 26.Battie MC, Videman T. Lumbar disc degeneration: epidemiology and genetics. J Bone Joint Surg Am. 2006;88(Suppl 2):3–9. doi: 10.2106/JBJS.E.01313. [DOI] [PubMed] [Google Scholar]
- 27.Bazrgari B, Shirazi-Adl A, Arjmand N. Analysis of squat and stoop dynamic liftings: muscle forces and internal spinal loads. Eur Spine J. 2007;16(5):687–699. doi: 10.1007/s00586-006-0240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


