Abstract
The effectiveness of a drug is dependent on accumulation at the site of action at therapeutic levels, however, challenges such as rapid renal clearance, degradation or non-specific accumulation requires drug delivery enabling technologies. Albumin is a natural transport protein with multiple ligand binding sites, cellular receptor engagement, and a long circulatory half-life due to interaction with the recycling neonatal Fc receptor. Exploitation of these properties promotes albumin as an attractive candidate for half-life extension and targeted intracellular delivery of drugs attached by covalent conjugation, genetic fusions, association or ligand-mediated association. This review will give an overview of albumin-based products with focus on the natural biological properties and molecular interactions that can be harnessed for the design of a next-generation drug delivery platform.
Keywords: Human serum albumin (HSA), Drugs, Albumin-binding, Albumin fusions, Half-life extension, Intracellular delivery, Neonatal Fc receptor (FcRn), Molecular medicine, Targeted drug delivery
Background
The therapeutic efficiency of a drug is dependent on the availability at the target site at a concentration and frequency that maximises the therapeutic action and minimizes side-effects to the patient. Therapeutic drugs are often low-molecular weight molecules that result in non-specific distribution, with a molecular weight below the renal filtration threshold resulting in rapid renal clearance and concomitant short plasma circulatory time [1, 2].
Drug delivery technology has been utilised to overcome these obstacles. The standard method to extend the circulatory half-life of drugs, particularly peptide and protein-based, is by PEGylation using poly (ethylene glycol) (PEG) conjugation [3]. The PEGylation approach for drug delivery applications has proved to be effective with a large number of marketed drugs, for example, Adagen® (pegademase bovine) and Pegasys® (PEG-interferon alfa-2α) [4]. Drawbacks to PEGylation, however, include accumulation of high molecular weight PEG in tissues such as the liver [5] and the necessity for chemical conjugation of the drug. An alternative strategy is incorporation in nanoscale carriers (nanocarriers) of a size range that enables transit across tissue and cellular barriers [6]. Examples include liposomes, polymeric nanoparticles, dendrimers, and solid lipid nanoparticles [6–9]. A requirement for complex designs that includes surface engineering to reduce host foreign body responses, whilst maintaining cellular targeting capabilities, and possible toxicological issues due to non-specific accumulation of synthetic material would seemingly restrict clinical application in the short-term. This is exemplified by the limited number of nanocarrier-based marketed products. Albumin is an attractive next-generation “self” drug delivery approach. It is the most abundant plasma protein involved in transport of nutrients in the body facilitated by its multiple binding sites and circulatory half-life of ~19 days [10]. It is crucial, however, to understand its biological interactions in order to harness its properties towards drug delivery solutions.
Biological properties of albumin
Albumin is the most abundant plasma protein in human blood (35–50 g/L human serum) with a molecular weight of 66.5 kDa [11]. It is synthesised in the liver hepatocytes with ~ 10–15 g of albumin produced and released into the vascular space daily [10, 12]. Circulation in the blood proceeds for an extended period of ~ 19 days [10, 13, 14]. This long half-life is thought mainly due to neonatal Fc receptor (FcRn)-mediated recycling, and the Megalin/Cubilin-complex rescue from renal clearance. Termination of the circulation is typically caused by catabolism of albumin in organs such as the skin and muscles [2, 12]. Modifications of albumin, for instance by non-enzymatic glycosylation, is thought to trigger lysosomal degradation [10, 15, 16]. Albumin contains multiple hydrophobic binding pockets and naturally serves as a transporter of a variety of different ligands such as fatty acids and steroids as well as different drugs [10]. Furthermore, the surface of albumin is negatively charged [10] making it highly water-soluble.
Structure, domains and binding sites
The overall three-dimensional structure of human serum albumin (HSA), shown by X-ray crystallography, is heart-shaped (Fig. 1) [17]. Structurally, albumin consists of three homologous domains I, II, and III. Each domain contains two sub-domains (A and B), which contains 4 and 6 α-helices, respectively. The two main drug binding sites are named Sudlow site I and Sudlow site II [18]. Site I, positioned in subdomain IIA, reversibly binds the anticoagulant drug warfarin [19, 20]. In the subdomain IIIA Sudlow Site II is located. It is known as the benzodiazepine binding site and diazepam, which is used in the treatment of anxiety, binds with high affinity [19]. Site I and site II are the primary binding sites though it has been found that some drugs bind elsewhere in the protein [18, 21, 22].
Fig. 1.
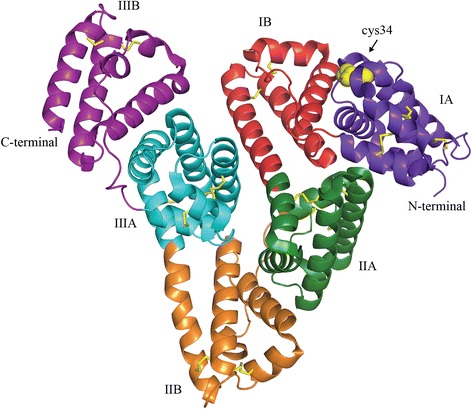
Crystal structure of human serum albumin. The illustration shows the tertiary structure of human serum albumin in complex with stearic acid (PDB 1e7e). The three domains of albumin are shown in purple (IA), red (IB), green (IIA), orange (IIB), blue (IIIA), and violet (IIIB). Yellow sticks depicture disulfide bridges, and yellow spheres highlight the available cysteine 34 in domain IA
Drugs and drug metabolites can also bind covalently to albumin. Glucuronidation of drugs as part of metabolism, often occurs to drugs having a carboxylic acid group resulting in acid glucuronides [19]. These acid glucuronide metabolites can bind covalently to HSA [23]. This can occur by nucleophilic attack from NH2, OH or SH in a protein to the acyl carbon of the glucuronide, giving a covalent attachment of drug to protein without retention of the glucuronide moiety. Another mechanism is the migration of the acyl group from position 1 in the sugar ring to 2, 3, or 4 position leading to tautomerism of the sugar ring. Aldehyde in the open tautomer structure reacts with a lysine group in the protein resulting in a covalent attachment of drug to protein with a glucuronic acid in between [19, 23, 24]. Covalent binding to albumin will naturally affect the clearance and metabolic destiny of such drugs. Drug metabolites such as furosemide, salicylic acid, and Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) like ibuprofen react covalently with HSA [19].
Albumin contains 35 cysteine residues of which 34 form disulfide bridges internally in the structure. These contribute to the high stability of albumin. The availability of a free cysteine residue at position 34 (cys34) for covalent attachment of drugs is an attractive feature for drug delivery as it holds a free thiol group (−SH) accounting for 80 % of thiols in the plasma [17]. Cys34 is located on the outer surface of albumin distant from the main interior drug binding sites and has, therefore, been a focus for covalent conjugation of drugs [11, 25, 26].
Albumin cellular receptors and engagement
Interaction with cellular receptors is responsible for albumin’s recycling, cellular transcytosis and hyphenate if word on two lines. Receptors include glycoproteins Gp60, Gp30 and Gp18, a secreted protein acidic and rich in cysteine (SPARC), the Megalin/Cubilin complex, and the neonatal Fc receptor (FcRn) [27–33]. Understanding the interaction with these cellular receptors is crucial for specific delivery of drug cargoes.
Gp60 receptor
The Gp60 receptor, named because its molecular size of 60 kDa, also referred to as albondin, is a vascular endothelial membrane protein, which acts to increase membrane permeability for receptor-mediated uptake of circulating proteins [32, 34–38]. Binding of proteins such as albumin to the Gp60 receptor is proposed to activate the membrane protein caveolin-1, which induces the formation of a caveolae vesicle. The caveolae then migrate through the cytoplasm, fuses with the basolateral membrane and releases material from the caveolae into the interstitium. Gp60, therefore, is thought to facilitate cellular transcytosis of albumin and redirect albumin from lysosomal degradation [36–43].
In 1986 work from Ghitescu et al. confirmed albumin-binding surface receptor engagement in capillary endothelium in mouse lung, heart and diaphragm by showing albumin-gold complexes were adsorbed at specific bindings sites associated with the plasmalemmal vesicles [44]. Work by Schnitzer and Oh, showed ~ 50 % of albumin transport was facilitated by binding to Gp60, while fluid-phase transport via vesicles or transport through intercellular junctions, performed the remaining transport [36, 40, 45]. This was found by in situ and in vitro studies of albumin transport across lung microvascular endothelium. Albumin binding to the cell surface was almost completely inhibited by anti-Gp60 antibodies [40, 46].
Secreted protein, acidic and rich in cysteine (SPARC) receptor
Secreted protein, acidic and rich in cysteine (SPARC) also known as osteonectin or basement-membrane 40, is an albumin binding protein located in the extracellular matrix and is expressed by a variety of cells including fibroblasts and endothelial cells and associated with tissue growth and cell movement and/or proliferation [47–53]. SPARC has been hypothesized to enhance tumour uptake of an albumin-based nanoparticle system of nab-paclitaxel (Abraxane®) though direct evidence remains to be elucidated [54].
Gp18 and Gp30 receptor
The Gp18 and Gp30 are cell surface glycoproteins with molecular weights of 18 and 30 kDa, respectively. Gp18 and Gp30 are expressed in endothelium cell membranes, in particular in the liver [55] and peritoneal macrophages [10, 56]. Whilst Gp60 serves to rescue albumin from degradation, it has been shown that Gp18 and Gp30 bind to modified albumin, for instance gold-labelled albumin or formaldehyde-treated albumin [27, 31, 34, 36, 42, 45]. Gp18 and Gp30 will then direct the modified albumin to lysosomal degradation, possibly as a safety mechanism to remove old, damaged or altered albumin [40, 42, 45]. This was demonstrated by the study of Schnitzer et al. using a cell-based study of rat epididymal fat pads by investigating binding, uptake and degradation [42]. Albumin modified by formaldehyde, maleic anhydride or gold-attachment was shown to bind Gp18 and Gp30 with higher affinity than native albumin [42]. Modification of native albumin is thought to occur through oxidation or non-enzymatic glycosylation as a means of protection or simply due to normal aging or a disease-mediated reaction, for instance such as oxidation from inflammation or hyperglycation in diabetes [10, 40, 45, 57]. Hence, it appears that Gp18 and Gp30 are important for degradation of modified albumin, as altered albumin not only binds to Gp18 and Gp30 but are also internalized and degraded [42, 46]. Native albumin does not avidly bind to the Gp18 and Gp30 receptors, but binds to the aforementioned Gp60 receptor, which is responsible for transcytosis of albumin through endothelium [10]. Investigations of albumin interactions with Gp18 and Gp30 receptors have not been extensively explored, yet it has been shown that modified albumin is degraded faster than native albumin [10, 45] and that chemically modified bovine serum albumin (BSA) shows 1000-fold higher affinity for Gp18 and Gp30 compared to native bovine serum albumin [31]. In summary, these results suggest that the receptors Gp18 and Gp30 are responsible for the degradation of modified albumins and are, therefore, known as scavenger receptors.
Megalin/Cubilin receptor
Cubilin is a glycoprotein expressed in the apical endocytic compartments of kidney proximal tubules, anchored to the membrane at the N-terminal by a α-helix. Cubilin lacks a transmembrane segment as well as a cytoplasmic domain, therefore, it depends on another membrane protein, Megalin, to facilitate endocytosis. Megalin has an extracellular domain, a transmembrane segment as well as a cytoplasmic tail. The binding site for albumin on Megalin, to our knowledge, has not been identified, yet, the functional role of the Megalin/Cubilin complex in reabsorption in the kidneys has been extensively studied. Reabsorption of filtered proteins in the kidney occurs by receptor-mediated endocytosis in the hyphenate if the word on two lines tubule. The receptors responsible for mediating the reabsorption are Cubilin and Megalin, both shown to bind albumin [29, 30]. As albumin binds to Cubilin and Megalin, it is likely that the Megalin/Cubilin complex is responsible for the receptor-mediated endocytosis and rescuing of albumin from renal excretion. Studies in Cubilin-deficient mice, as well as in humans with a mutation in a Cubilin gene [58], show a decrease in albumin uptake [59, 60]. The uptake of albumin in Megalin-, Cubilin- and double-knock out mice was completely inhibited that indicates these receptors are needed for the uptake of albumin [59, 60].
In a study by Weyer et al. using Megalin/Cubilin deficient mice, 125I-labelled murine albumin was used to investigate the uptake in the kidney and urinary excretion of intact albumin as well as its fragments by using size-exclusion chromatography [61]. For control mice all albumin was eluted as fragments, whereas the Megalin/Cubilin-deficient mice showed a decreased albumin uptake in the kidneys, as well as decreased degradation, together with an increased excretion of intact labelled albumin. An albumin conjugate, only fluorescent when intracellularly degraded, was used to visualize the degradation in proximal tubular cells after intravenous injection. Proximal tubular cells in control mice were positive, while there was an absence of fluorescence in Megalin/Cubilin deficient mice that indicated an Megalin/Cubilin-mediated endocytosis mechanism also plays a role in the intracellular degradation of albumin in the proximal tubular cells [61]. Furthermore, a study by Zhai et al. using a double labelling strategy of fluorescent albumin and antibodies against either of the two receptors Megalin or Cubilin, showed a correlation between Megalin and Cubilin expression and the uptake of albumin that supports a role in reabsorption of albumin [62].
Neonatal Fc receptor (FcRn)
A major role of the neonatal Fc receptor (FcRn) is in placenta and proximal small intestine transport of IgG from mother to fetus [63]. FcRn is a glycoprotein comprising of a MHC-class I-like heavy chain, consisting of three extracellular domains (α1, α2, and α3), which is non-covalently associated with a β2-microglobulin (β2m) light chain. The light chain is necessary for the function of FcRn [64]. The heavy chain is connected to a transmembrane element that continues into the cytoplasm.
It has been revealed that a lower amount of Immunoglobulin G (IgG) antibodies were present in the blood of β2m deficient mice and that immunization of the mice showed decreased immune responses probably due to degradation of IgG caused by a lack of diversion from lysosomal degradation facilitated by FcRn [65–67]. This indicates, therefore, that FcRn plays a role in adults as well as in the neonatal state. FcRn is distributed in many tissues including vascular endothelium as well as the gut, lungs and kidney [63]. The first evidence for albumin/FcRn binding was co-elution of bovine albumin and soluble human FcRn on a human IgG-coupled column [28], also suggesting that both IgG and albumin could simultaneously bind FcRn. Work by the same group revealed that the serum concentration of albumin in FcRn deficient mice was reduced compared to wild-type mice and that FcRn-deficient mice had shortened half-life of albumin [28].
Domain III was first suggested as the primary binding site for FcRn [68, 69]. However, a study of FcRn binding to recombinant domain III alone showed a ten-fold weaker FcRn binding compared to non-recombinant albumin [68, 70]. In the same study a docking model of human FcRn in complex with human albumin revealed FcRn interactions with two loops in the N-terminal of domain I, in addition to the interactions in domain III [68]. Site-directed mutagenesis of specific residues residing in the loops in domain I resulted in an altered affinity to FcRn [71]. Co-crystallization studies of human FcRn in complex with human albumin supports involvement of both domain III and domain I in FcRn binding [72, 73]. In vitro studies have shown that albumin binding is dependent on the presence of a conserved histidine residue in FcRn (His166) [74, 75]. X-ray crystallography data revealed a loop surrounding the His166 at acidic pH. Hence, the theory of a pH-sensitive loop stabilized by the protonated His166 was proposed [76, 77]. Furthermore, the loops were shown to contain four conserved tryptophan residues that resulted in reduced or loss of binding to albumin when mutated [72, 78]. This indicates that the binding of albumin is not only pH dependent but also hydrophobic and that both domain I and III are involved in FcRn interaction.
A cellular FcRn-mediated recycling pathway was first proposed for IgG by Brambell in 1965 [79]. Later the hypothesis that albumin recycling was carried out by the same mechanism was proposed [80]. It is widely accepted that FcRn is responsible for IgG half-life extension by a mechanism of increased binding at low pH (<6.5) within the endosomes and recycling and release into the extracellular space at physiological pH. The first indications for FcRn involvement in albumin recycling were revealed in 2003 by Chaudhury et al. [28]. The authors confirmed the hypothesis of a single receptor responsible for the half-life regulation of albumin in the same manner as for IgG [81] by showing FcRn-albumin binding and a shortened life-span of albumin in FcRn-deficient mice.
Albumin-based drug delivery strategies
The natural transport function, multiple ligand binding sites, and cellular interactions provides rational for the exploitation of albumin for drug delivery. The ability to covalent and non-covalently attach drugs or expression of albumin-drug fusions provides a range of design options (Fig. 2) that has been taken into clinical trials or on the market (Table 1).
Fig. 2.
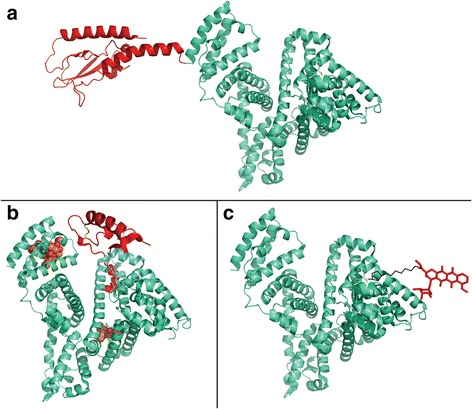
Albumin-based drug delivery strategies. a Albumin fusion-based drugs, in light green (HSA) and in red (fusion peptide) (modified PDB 1e7e + 3IOL). b Albumin associating drugs; upper left binding of paclitaxel (from PDB1JFF), upper right binding of insulin detemir (Levemir®) (insulin from PDB1ZNI, Myristic acid from PDB1H9Z), lower panel binding of the weakly associated warfarin (PDB1H9Z). c Covalent conjugation of a drug to albumin via the available Cys34 (modified PDB 1e7e + 1I1E)
Table 1.
A selection of albumin-based systems in clinical trials and marketed products
| Attachment | Name | Disease | Drug type | Clinical status | Company | Ref |
|---|---|---|---|---|---|---|
| Non-covalent/reversible association | Levemir® | Diabetes type 1 and 2 | Insulin detemir | Marketed | Novo Nordisk | [123, 124] |
| Victoza® | Diabetes type 2 | GLP-1 | Marketed | Novo Nordisk | [123] | |
| Ozoralizumab | Rheumatoid arthritis | Antibody derivative | Phase II completed | Ablynx | [90] | |
| Covalent | MTX-HSA | Cancer and autoimmune diseases | Methotrexate | Phase II | Access Pharmaceuticals Inc. | [26, 119, 125, 126] |
| Aldoxorubicin | Cancer | Doxorubicin | Phase I completed | CytRx, Inc. | [110, 127] | |
| CJC-1134 | Diabetes type 2 | Exendin-4 | Phase II | ConjuChem | [11, 128–131] | |
| Genetic fusion | Eperzan/Tanzeum | Diabetes type 2 | GLP-1 | Marketed | Glaxo Smith Kline | [132–134] |
| N/A | Hemophilia | FVIIa | Phase I completed | CSL Behring GmbH | [135–138] | |
| N/A | Hemophilia B | rIX-FP | Phase III completed | CSL Behring GmbH | [139] | |
| Albuferon®/Zalbin/Jouleferon | Hepatitis C | INFalpha-2b | Phase III completed, Development ceased | Human Genome Sciences in collaboration with Novartis | [11, 140] | |
| Micro-/Nanoparticle | Abraxane® | Cancer | Paclitaxel | Marketed | Celgene | [141] |
| ABI-008 | Cancer | Docetaxel | Phase I/II | Celgene | [95] | |
| ABI-009 | Cancer | Rapamycin | Phase I/II | Celgene | [96] | |
| ABI-010 | Cancer | HSP90 Inhibitor | Withdrawn before enrollment | Celgene | [97] | |
| 99mTc-Albures | Diagnostic purpose | Technetium-99 | Marketed | GE Healthcare | ||
| 99mTc-Nanocoll | Diagnostic purpose | Technetium-99 | Marketed | GE Healthcare |
Albumin-associated drugs
Albumin binds to endogenous ligands such as fatty acids; however, it also interacts with exogenous ligands such as warfarin, penicillin and diazepam. As the binding of these drugs to albumin is reversible the albumin-drug complex serves as a drug reservoir that can enhance the drug biodistribution and bioavailability. Incorporation of components that mimic endogenous albumin-binding ligands, such as fatty acids, has been used to potentiate albumin association and increase drug efficacy. Examples include Levemir® (Insulin detemir) and Victoza® (Liraglutide) manufactured by Novo Nordisk for the treatment of diabetes. Levemir® is a myristic acid modified insulin analog. While for Victoza® a palmitic acid is attached to a glucagon-like peptide-1 agonist. On injection the fatty acid moiety binds to albumin and dissociates over time and, therefore, enhances the bioavailability and distribution. Levemir® has been shown to improve glycaemic control and resulted in limited serious adverse drug reactions that was evaluated in a large multi-national follow up data study after 14 weeks in which the safety and efficacy was assessed of 20,531 patients with type 1 or 2 diabetes [82]. Victoza® went through 8 phase III trials to evaluate the efficacy and safety of Victoza® as a monotherapy or as a combination therapy. Victoza® resulted in improvements in both hemoglobin A1c and fasting plasma glucose (FPG) [83–89]. Benefits of those insulin analogues by albumin-binding are an extended time of action profile compared to conventional basal insulin such as neutral protamine Hagedorn (NPH) that peak before 8 h of injection [82].
Another category that utilises specific-binding to albumin is nanobodies. Ablynx has developed ATN-103, now known as Ozoralizumab, which is a trivalent antibody having two peptides, one to interact with TNF-α, and the other, albumin. In collaboration with Pfizer, Ozoralizumab has completed Phase II studies in patients with rheumatoid arthritis [90]. Five different dosing groups were compared to placebo treatment and the highest dose of Ozoralizumab (80 mg every 4 weeks) improved the ACR20 response compared to placebo in week 16 [91].
An alternative strategy to specific ligand binding is non-specific association of albumin. Albumin has hydrophobic binding domains in which drugs such as warfarin and diazepam can bind. Abraxane® is an established albumin-based nanoparticle system produced by Celgene and is used in the treatment of cancer. It is proposed to be an albumin-bound nanoparticle of about 130 nm in which the outer layer consists of albumin while the inner core contains the water insoluble cytotoxic agent paclitaxel [92]. It has been shown to be less toxic to its free drug counterpart paclitaxel and also exhibits higher anti-tumour activity compared to free paclitaxel [92]. SPARC has been hypothesized to support tumour uptake of Abraxane®. A preliminary study showing that SPARC-positive cancer patients had a higher response to an Abraxane®, supports the hypothesis that SPARC mediated accumulation of albumin in tumours increases the effectiveness of albumin-bound paclitaxel [54]. In contrast, a study from 2014 on genetically modified SPARC-deficient mice did not show any difference in uptake of Abraxane® into tumours [93]. The uptake mechanism of Abraxane® in cells remains to be elucidated, yet, Desai et al. have proposed that Gp60 and SPARC work in combination [54] suggesting Abraxane® is transported across the endothelial barrier by binding to Gp60 and subsequent caveolae-mediated transcytosis into the tumour interstitium where SPARC enhances the uptake of Abraxane into tumour cells [54]. Celgene has a portfolio of albumin-based nanoparticles for cancer treatment, which have been presented in a report by Desai [94]. In this report, preclinical studies of ABI-008 and ABI-009 are described. ABI-008 contains the active drug docetaxel. It has completed phase I/II [95] and showed anti-tumour effects in preclinical studies using xenograft studies of prostate and colon tumours as reviewed by Desai [94]. Likewise, ABI-009 in which the active drug is rapamycin proved to be effective against colon and breast tumours in xenograft studies and exhibited low toxicology and good efficacy [94]. To our knowledge it has reached a combined phase I and phase II study in the treatment of non-muscle invasive bladder cancer [96]. ABI-010 contains a Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin (17-AAG). Hsp90 is a chaperone that helps to fold signaling proteins involved in cancer; hence, it is an interesting candidate for cancer treatment. A phase I trial was planned for ABI-010 in a combination treatment with Abraxane® for different hematological malignancies though it has been withdrawn prior to enrollment [97].
In addition to albumin-based nanoparticle therapeutics, diagnostic nanoparticles have been developed. 99mTc-Albures and 99mTc-Nanocoll are both albumin aggregated particles containing the metastable nuclear isotope of technetium-99 that have been used for various diagnostics purposes in cancer and infectious diseases [98, 99]. In a study of 59 patients with peripheral joint pain, 99mTc-nanocolloid scintigraphy showed that the scan was able to detect 82 % of the clinically assessed joint disease in a group with arthralgia [100]. In a study of rheumatoid arthritis comparing clinical assessment with 99mTc-nanocolloid scans, 79 % of clinically positive joints were detected by the scan [101].
Albumin-fusions
An elegant approach to combine protein-based drugs with albumin, is genetically fusion to the N- or C-terminal or both ends of the albumin. The protein gene is connected to that of albumin and expressed in a suitable expression host, typically yeast, resulting in a single fused protein. It is, however, necessary that the linker and fused moiety do not interfere with the folding of albumin so it retains its functionality and long half-life.
The product albiglutide (Eperzan®/Tanzeum®) manufactured by GlaxoSmithKline for the treatment of type II diabetes, is a GLP-1 receptor agonist developed by fusion of two human GLP-1 repeats to recombinant human albumin [102, 103]. In eight phase III studies also known as the Harmony program, the efficacy and safety profile of albiglutide has been studied. A detailed review by Woodward et al. shows that weekly dosing of albiglutide showed lowered glycated hemoglobin, reductions in fasting plasma glucose and weight loss in patients with type II diabetes [104].
Albuferon®, also known as albinterferon, is an interferon α-2b fused to albumin that went into phase III studies for treatment of Hepatitis infections. In the phase IIb study of a combination therapy of ribavirin and albinterferon to treat hepatitis C virus, patients given albinterferon of 900 μg and 1200 μg every 2 weeks showed the same sustained virologic response as the standard treatment of PEGylated interferon α-2a (Pegasys®) 180 μg every week [105]. In the phase III studies albinterferon was equal to standard treatment of PEGylated interferon α-2a though treatment discontinuation due to adverse effects which were 4.1 %, 10.4 % and 10.0 % for PEGylated interferon α-2a, albinterferon 1200 μg and albinterferon 900 μg respectively [106, 107]. As of October 2010 FDA issued a complete response letter and Novartis and Human Genome Sciences, Inc. decided to stop further development of the drug [108].
Covalent attached drugs
A standard approach is chemical conjugation of the drug to either lysines, tyrosines, or the free SH-group on the cys34. The free thiol group on cys34 has been widely used, for instance by reacting with a maleimide linker from prodrugs, which have been intravenously injected [109, 110]. Covalent attachment of drugs, however, requires a release mechanism from albumin. In the group of Kratz, this was solved by introducing an acid sensitive hydrazone linker that is thought to be cleaved upon delivery at tumour sites due to an acidic extracellular environment or inside endosomes or lysosomes after cellular uptake [11, 109]. The group of Kratz modified doxorubicin with maleimides and demonstrated in situ conjugation with cys34 of endogenous albumin after intravenous injection. This is based on 70 % of the endogenous pool of albumin contributing to free thiols. In vivo studies performed by the same group revealed that doxorubicin maleimide derivatives were superior to free doxorubicin with regards to anti-tumour efficacy and toxicity in three different animal models (RENCA, MDA-MB 435 and MCF-7) [111, 112]. This work by the group of Kratz was taken further and Aldoxorubicin (also known as INNO-206 or DOXO-EMCH) produced by CytRx is a doxorubicin conjugate containing an acid-sensitive linker. Upon administration the linker is thought to bind to circulating albumin and is, therefore, transported to the tumour site where the acidic environment will cleave the linker and release doxorubicin to exert its action. Aldoxorubicin was shown to be superior to doxorubicin in a Phase IIb study involving 126 patients for treatment of soft tissue sarcoma [113]. CytRx has initiated a phase III global trial of their anti-cancer drug Aldoxorubicin for soft tissue sarcoma, and phase II studies and below are ongoing for treatment of small cell lung cancer, HIV-related Kaposi’s sarcoma and late-stage glioblastoma [114]. CytRx are also studying Aldoxorubicin combination treatments, for instance Ifosfamide for patients with soft tissue sarcoma and Gemcitabine to treat metastatic solid tumours [114].
Lau et al. used maleimide conjugation to link small interfering RNA (siRNA) to endogenous albumin. Using SMCC, a thiol-reactive group was incorporated terminally in the siRNA able to react to the free cys34 on circulating albumin [115]. Ex vivo results indicated a fast reaction of maleimide-activated siRNA with cys34 on albumin, and after 1 h maximal conjugation was reached. Furthermore, in vivo work showed that siRNA-albumin was still detectable after 4 h, whilst non-activated siRNA was not after 30 min [115]. In vivo silencing of mice treated with activated siRNA (1 mg/kg) resulted in significantly reduced levels of the myocardium target gene IGF-IR mRNA compared to vehicle treated or nonactivated siRNA [115]. Hence, siRNA-albumin conjugates may be useful for gene silencing in tissues.
Ehrlich et al. have conjugated an Y2R-peptide to albumin to enhance its circulation time [116]. The Y2R-peptide is a potential obesity drug as it acts on the Y2 receptor located in the hypothalamus and peripheral nervous system and is, therefore, thought to reduce appetite. The Y2R-peptide was modified using different linkers (succinimidyl 4-[N-maleimidomethyl]cyclohexane-1-carboxylate (SMCC), 6-maleimidohexanoic acid N-hydroxysuccinimide ester (MHS), and N-[γ-maleimidobutyryloxy]-sulfosuccinimide ester (GMBS) before attachment to albumin. One of the most active albumin conjugates in vitro (HSA-MH-Y2R) showed a significant reduction in food uptake after 24 h of 37 % [116].
Methotrexate human serum albumin (MTX-HSA) is a covalent attached methotrexate to lysine residues in albumin. In a study by Stehle et al. it was found that the drug loading ratio to albumin affected the tumour targeting properties in a rat tumour model [117]. Though, it was thought that more MTX attached to HSA would increase the therapeutic effect, it was found that a low molecular ratio of 1:1 resulted in the highest tumour targeting properties such as high tumour uptake, long half-life and low liver uptake rates [117]. Phase I studies of MTX-HSA in cancer patients applied at a ratio of 1:1.3 did not result in any severe side-effects and was in general well tolerated by the patients, therefore, showing a good toxicology profile [118]. MTX-HSA was used in combination with cisplatin in treatment of patients with bladder cancer in a phase II study. One patient showed a partial response and another showed complete response out of seven patients resulting in 27 % response rate [119]. To our knowledge, MTX-HSA has not been taken further for clinical studies.
Conclusion and future perspectives
Exploitation of the natural properties of ligand binding and transport have been utilised for albumin-based drug delivery, with a focus on drug half-life extension. A drug construct design incorporating binding ligands is a simple, but elegant, approach used for commercial reversible binding drugs Levemir® and Victoza®. A more elaborate non-reversible strategy is development of albumin covalent conjugated drugs. The availability of a free thiol at cys34 in domain I allows site-specific conjugation distant from the main FcRn binding site in domain III and Hyphenate if the word is on two lines binding pockets, a chemoselectivity not possible when conjugation is performed to the multiple lysines distributed throughout albumin. Thiol-maleimide conjugation is the dominant method employed to attach drugs; however, the susceptibility of the maleimide bond to serum breakdown in the bloodstream due to thiol exchange reactions may require alternative chemistries [120]. Pre-hydrolysis of the maleimide-conjugate prior to thiol exposure to create a stable open-ring structure is a promising approach [121]. The application of albumin fusions containing a therapeutic protein is a strategy that circumvents the requirement for covalent conjugation. Eperzan®/Tanzeum® is now on the market, with the number of albumin fusion products expected to rise. The application of engineered recombinant albumins with different affinity to FcRn shown in non-human primates to tune the drug pharmacokinetic profile is an exciting next-generation approach [122]. Interaction with a range of cellular receptors such as Gp18, Gp30 and Gp60 may potentiate cellular entry for intracellular drug delivery applications. A greater understanding of the intracellular pathway of albumin, however, is needed in order to optimise albumin-based intracellular drug delivery approaches.
Albumins inherent transport properties and cellular receptor engagement promotes albumin as a natural molecular medicine, greater control of these properties is key to further harness nature to cure disease.
Acknowledgements
This work was supported by the Danish Innovation fund grant 102-2014-3.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
KH, MTL, MK and MLH all contributed to the review. All authors read and approved the final manuscript.
Contributor Information
Maja Thim Larsen, Email: maja.thim.larsen@inano.au.dk.
Matthias Kuhlmann, Email: kuhlmann@inano.au.dk.
Michael Lykke Hvam, Email: mhvam@inano.au.dk.
Kenneth A. Howard, Email: kenh@inano.au.dk
References
- 1.Markovsky E, Baabur-Cohen H, Eldar-Boock A, Omer L, Tiram G, Ferber S, et al. Administration, distribution, metabolism and elimination of polymer therapeutics. J Control Release. 2012;161(2):446–60. doi: 10.1016/j.jconrel.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 2.Sleep D, Cameron J, Evans LR. Albumin as a versatile platform for drug half-life extension. Biochim Biophys Acta. 2013;1830(12):5526–34. doi: 10.1016/j.bbagen.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252(11):3582–6. [PubMed] [Google Scholar]
- 4.Rajender Reddy K, Modi MW, Pedder S. Use of peginterferon alfa-2a (40 KD) (Pegasys) for the treatment of hepatitis C. Adv Drug Deliv Rev. 2002;54(4):571–86. doi: 10.1016/S0169-409X(02)00028-5. [DOI] [PubMed] [Google Scholar]
- 5.Pasut G, Veronese FM. Polymer–drug conjugation, recent achievements and general strategies. Prog Polym Sci. 2007;32(8–9):933–61. doi: 10.1016/j.progpolymsci.2007.05.008. [DOI] [Google Scholar]
- 6.Howard KA, Dong M, Oupicky D, Bisht HS, Buss C, Besenbacher F, et al. Nanocarrier stimuli-activated gene delivery. Small. 2007;3(1):54–7. doi: 10.1002/smll.200600328. [DOI] [PubMed] [Google Scholar]
- 7.Wilczewska AZ, Niemirowicz K, Markiewicz KH, Car H. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–37. doi: 10.1016/S1734-1140(12)70901-5. [DOI] [PubMed] [Google Scholar]
- 8.Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Advanced drug delivery reviews. 2009;61(9):710-20. doi:10.1016/j.addr.2009.04.001. [DOI] [PubMed]
- 9.Peer D. Harnessing RNAi nanomedicine for precision therapy. Mol Cell Ther. 2014;2:5. doi: 10.1186/2052-8426-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters T. All about albumin: biochemistry, genetics, and medical applications. San Diego, Calif: Academic; 1996. [Google Scholar]
- 11.Kratz F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132(3):171–83. doi: 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Garcovich M, Zocco MA, Gasbarrini A. Clinical use of albumin in hepatology. Blood Transfus. 2009;7(4):268–77. doi: 10.2450/2008.0080-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson CL, Chaudhury C, Kim J, Bronson CL, Wani MA, Mohanty S. Perspective – FcRn transports albumin: relevance to immunology and medicine. Trends Immunol. 2006;27(7):343–8. doi: 10.1016/j.it.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim J, Hayton WL, Robinson JM, Anderson CL. Kinetics of FcRn-mediated recycling of IgG and albumin in human: Pathophysiology and therapeutic implications using a simplified mechanism-based model. Clin Immunol. 2007;122(2):146–55. doi: 10.1016/j.clim.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris MA, Preddy L. Glycosylation accelerates albumin degradation in normal and diabetic dogs. Biochem Med Metab Biol. 1986;35(3):267–70. doi: 10.1016/0885-4505(86)90082-4. [DOI] [PubMed] [Google Scholar]
- 16.Williams SK, Devenny JJ, Bitensky MW. Micropinocytic ingestion of glycosylated albumin by isolated microvessels: possible role in pathogenesis of diabetic microangiopathy. Proc Natl Acad Sci U S A. 1981;78(4):2393–7. doi: 10.1073/pnas.78.4.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quinlan GJ, Martin GS, Evans TW. Albumin: biochemical properties and therapeutic potential. Hepatology. 2005;41(6):1211–9. doi: 10.1002/hep.20720. [DOI] [PubMed] [Google Scholar]
- 18.Sudlow G, Birkett DJ, Wade DN. The characterization of two specific drug binding sites on human serum albumin. Mol Pharmacol. 1975;11(6):824–32. [PubMed] [Google Scholar]
- 19.Kragh-Hansen U, Chuang VT, Otagiri M. Practical aspects of the ligand-binding and enzymatic properties of human serum albumin. Biol Pharm Bull. 2002;25(6):695–704. doi: 10.1248/bpb.25.695. [DOI] [PubMed] [Google Scholar]
- 20.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S. Crystal structure analysis of warfarin binding to human serum albumin: anatomy of drug site I. J Biol Chem. 2001;276(25):22804–9. doi: 10.1074/jbc.M100575200. [DOI] [PubMed] [Google Scholar]
- 21.Sjoholm I, Ekman B, Kober A, Ljungstedt-Pahlman I, Seiving B, Sjodin T. Binding of drugs to human serum albumin:XI. The specificity of three binding sites as studied with albumin immobilized in microparticles. Mol Pharmacol. 1979;16(3):767–77. [PubMed] [Google Scholar]
- 22.Bhattacharya AA, Curry S, Franks NP. Binding of the general anesthetics propofol and halothane to human serum albumin. High resolution crystal structures. J Biol Chem. 2000;275(49):38731–8. doi: 10.1074/jbc.M005460200. [DOI] [PubMed] [Google Scholar]
- 23.Benet LZ, Spahn-Langguth H, Iwakawa S, Volland C, Mizuma T, Mayer S, et al. Predictability of the covalent binding of acidic drugs in man. Life Sci. 1993;53(8):L141–6. doi: 10.1016/0024-3205(93)90279-C. [DOI] [PubMed] [Google Scholar]
- 24.Williams AM, Dickinson RG. Studies on the reactivity of acyl glucuronides--VI. Modulation of reversible and covalent interaction of diflunisal acyl glucuronide and its isomers with human plasma protein in vitro. Biochem Pharmacol. 1994;47(3):457–67. doi: 10.1016/0006-2952(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 25.Bertucci C, Domenici E. Reversible and covalent binding of drugs to human serum albumin: methodological approaches and physiological relevance. Curr Med Chem. 2002;9(15):1463–81. doi: 10.2174/0929867023369673. [DOI] [PubMed] [Google Scholar]
- 26.Kratz F, Abu Ajaj K, Warnecke A. Anticancer carrier-linked prodrugs in clinical trials. Expert Opin Investig Drugs. 2007;16(7):1037–58. doi: 10.1517/13543784.16.7.1037. [DOI] [PubMed] [Google Scholar]
- 27.Schnitzer JE, Oh P. Antibodies to SPARC inhibit albumin binding to SPARC, gp60, and microvascular endothelium. Am J Physiol. 1992;263(6 Pt 2):H1872–9. doi: 10.1152/ajpheart.1992.263.6.H1872. [DOI] [PubMed] [Google Scholar]
- 28.Chaudhury C, Mehnaz S, Robinson JM, Hayton WL, Pearl DK, Roopenian DC, et al. The major histocompatibility complex-related Fc receptor for IgG (FcRn) binds albumin and prolongs its lifespan. J Exp Med. 2003;197(3):315–22. doi: 10.1084/jem.20021829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, et al. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. J Clin Investig. 2000;105(10):1353–61. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. Am J Physiol. 1996;271(4 Pt 2):F900–7. doi: 10.1152/ajprenal.1996.271.4.F900. [DOI] [PubMed] [Google Scholar]
- 31.Schnitzer JE, Sung A, Horvat R, Bravo J. Preferential interaction of albumin-binding proteins, gp30 and gp18, with conformationally modified albumins. Presence in many cells and tissues with a possible role in catabolism. J Biol Chem. 1992;267(34):24544–53. [PubMed] [Google Scholar]
- 32.Ghinea N, Fixman A, Alexandru D, Popov D, Hasu M, Ghitescu L, et al. Identification of albumin-binding proteins in capillary endothelial cells. J Cell Biol. 1988;107(1):231–9. doi: 10.1083/jcb.107.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sage H, Johnson C, Bornstein P. Characterization of a novel serum albumin-binding glycoprotein secreted by endothelial cells in culture. J Biol Chem. 1984;259(6):3993–4007. [PubMed] [Google Scholar]
- 34.Ghinea N, Eskenasy M, Simionescu M, Simionescu N. Endothelial albumin binding proteins are membrane-associated components exposed on the cell surface. J Biol Chem. 1989;264(9):4755–8. [PubMed] [Google Scholar]
- 35.Schnitzer JE, Carley WW, Palade GE. Albumin interacts specifically with a 60-kDa microvascular endothelial glycoprotein. Proc Natl Acad Sci U S A. 1988;85(18):6773–7. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schnitzer JE, Oh P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J Biol Chem. 1994;269(8):6072–82. [PubMed] [Google Scholar]
- 37.Tiruppathi C, Finnegan A, Malik AB. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proc Natl Acad Sci U S A. 1996;93(1):250–4. doi: 10.1073/pnas.93.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. Am J Physiol. 1992;262(1 Pt 2):H246–54. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- 39.Iancu C, Mocan L, Bele C, Orza AI, Tabaran FA, Catoi C, et al. Enhanced laser thermal ablation for the in vitro treatment of liver cancer by specific delivery of multiwalled carbon nanotubes functionalized with human serum albumin. Int J Nanomedicine. 2011;6:129–41. doi: 10.2147/IJN.S15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnitzer JE. Update on the cellular and molecular basis of capillary permeability. Trends Cardiovasc Med. 1993;3(4):124–30. doi: 10.1016/1050-1738(93)90012-U. [DOI] [PubMed] [Google Scholar]
- 41.Schnitzer JE, Allard J, Oh P. NEM inhibits transcytosis, endocytosis, and capillary permeability: implication of caveolae fusion in endothelia. Am J Physiol. 1995;268(1 Pt 2):H48–55. doi: 10.1152/ajpheart.1995.268.1.H48. [DOI] [PubMed] [Google Scholar]
- 42.Schnitzer JE, Bravo J. High affinity binding, endocytosis, and degradation of conformationally modified albumins. Potential role of gp30 and gp18 as novel scavenger receptors. J Biol Chem. 1993;268(10):7562–70. [PubMed] [Google Scholar]
- 43.Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–75. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- 44.Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986;102(4):1304–11. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merlot AM, Kalinowski DS, Richardson DR. Unraveling the mysteries of serum albumin-more than just a serum protein. Front Physiol. 2014;5:299. doi: 10.3389/fphys.2014.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnitzer J, Oh P. Antibodies to the albumin binding protein, albondin, inhibit transvascular transport of albumin in the rat lung. FASEB Journal. 1993;7(3–4):A902.
- 47.Sage H, Vernon RB, Funk SE, Everitt EA, Angello J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca + 2-dependent binding to the extracellular matrix. J Cell Biol. 1989;109(1):341–56. doi: 10.1083/jcb.109.1.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19(7):569–80. doi: 10.1016/S0945-053X(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 49.Jacob K, Webber M, Benayahu D, Kleinman HK. Osteonectin promotes prostate cancer cell migration and invasion: a possible mechanism for metastasis to bone. Cancer Res. 1999;59(17):4453–7. [PubMed] [Google Scholar]
- 50.Kato Y, Sakai N, Baba M, Kaneko S, Kondo K, Kubota Y, et al. Stimulation of motility of human renal cell carcinoma by SPARC/Osteonectin/BM-40 associated with type IV collagen. Invasion Metastasis. 1998;18(2):105–14. doi: 10.1159/000024503. [DOI] [PubMed] [Google Scholar]
- 51.Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8(2):163–73. [PubMed] [Google Scholar]
- 52.Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, et al. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996;50(6):1978–89. doi: 10.1038/ki.1996.520. [DOI] [PubMed] [Google Scholar]
- 53.Pichler RH, Bassuk JA, Hugo C, Reed MJ, Eng E, Gordon KL, et al. SPARC is expressed by mesangial cells in experimental mesangial proliferative nephritis and inhibits platelet-derived-growth-factor-medicated mesangial cell proliferation in vitro. Am J Pathol. 1996;148(4):1153–67. [PMC free article] [PubMed] [Google Scholar]
- 54.Desai N, Trieu V, Damascelli B, Soon-Shiong P. SPARC Expression Correlates with Tumor Response to Albumin-Bound Paclitaxel in Head and Neck Cancer Patients. Transl Oncol. 2009;2(2):59–64. doi: 10.1593/tlo.09109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ottnad E, Via DP, Frubis J, Sinn H, Friedrich E, Ziegler R, et al. Differentiation of binding sites on reconstituted hepatic scavenger receptors using oxidized low-density lipoprotein. Biochem J. 1992;281(Pt 3):745–51. doi: 10.1042/bj2810745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang H, Yang Y, Steinbrecher UP. Structural requirements for the binding of modified proteins to the scavenger receptor of macrophages. J Biol Chem. 1993;268(8):5535–42. [PubMed] [Google Scholar]
- 57.Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320(14):915–24. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 58.Storm T, Emma F, Verroust PJ, Hertz JM, Nielsen R, Christensen EI. A patient with cubilin deficiency. N Engl J Med. 2011;364(1):89–91. doi: 10.1056/NEJMc1009804. [DOI] [PubMed] [Google Scholar]
- 59.Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, et al. Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol. 2010;21(11):1859–67. doi: 10.1681/ASN.2010050492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weyer K, Storm T, Shan J, Vainio S, Kozyraki R, Verroust PJ, et al. Mouse model of proximal tubule endocytic dysfunction. Nephrol Dial Transplant. 2011;26(11):3446–51. doi: 10.1093/ndt/gfr525. [DOI] [PubMed] [Google Scholar]
- 61.Christensen EI, Birn H, Storm T, Weyer K, Nielsen R. Endocytic receptors in the renal proximal tubule. Physiology. 2012;27(4):223–36. doi: 10.1152/physiol.00022.2012. [DOI] [PubMed] [Google Scholar]
- 62.Zhai XY, Nielsen R, Birn H, Drumm K, Mildenberger S, Freudinger R, et al. Cubilin- and megalin-mediated uptake of albumin in cultured proximal tubule cells of opossum kidney. Kidney Int. 2000;58(4):1523–33. doi: 10.1046/j.1523-1755.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- 63.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 64.Zhu X, Peng J, Raychowdhury R, Nakajima A, Lencer WI, Blumberg RS. The heavy chain of neonatal Fc receptor for IgG is sequestered in endoplasmic reticulum by forming oligomers in the absence of beta2-microglobulin association. Biochem J. 2002;367(Pt 3):703–14. doi: 10.1042/bj20020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roopenian DC, Christianson GJ, Sproule TJ, Brown AC, Akilesh S, Jung N, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170(7):3528–33. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 66.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci U S A. 1996;93(11):5512–6. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghetie V, Hubbard JG, Kim JK, Tsen MF, Lee Y, Ward ES. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26(3):690–6. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 68.Andersen JT, Dalhus B, Cameron J, Daba MB, Plumridge A, Evans L, et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nat Commun. 2012;3:610. doi: 10.1038/ncomms1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Andersen JT, Daba MB, Sandlie I. FcRn binding properties of an abnormal truncated analbuminemic albumin variant. Clin Biochem. 2010;43(4–5):367–72. doi: 10.1016/j.clinbiochem.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 70.Bern M, Sand KM, Nilsen J, Sandlie I, Andersen JT. The role of albumin receptors in regulation of albumin homeostasis: Implications for drug delivery. J Control Release. 2015;211:144–62. doi: 10.1016/j.jconrel.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Sand KM, Bern M, Nilsen J, Dalhus B, Gunnarsen KS, Cameron J, et al. Interaction with both domain I and III of albumin is required for optimal pH-dependent binding to the neonatal Fc receptor (FcRn) J Biol Chem. 2014;289(50):34583–94. doi: 10.1074/jbc.M114.587675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmidt MM, Townson SA, Andreucci AJ, King BM, Schirmer EB, Murillo AJ, et al. Crystal structure of an HSA/FcRn complex reveals recycling by competitive mimicry of HSA ligands at a pH-dependent hydrophobic interface. Structure. 2013;21(11):1966–78. doi: 10.1016/j.str.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 73.Oganesyan V, Damschroder MM, Cook KE, Li Q, Gao C, Wu H, et al. Structural insights into neonatal Fc receptor-based recycling mechanisms. J Biol Chem. 2014;289(11):7812–24. doi: 10.1074/jbc.M113.537563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry. 2006;45(15):4983–90. doi: 10.1021/bi052628y. [DOI] [PubMed] [Google Scholar]
- 75.Andersen JT, Dee Qian J, Sandlie I. The conserved histidine 166 residue of the human neonatal Fc receptor heavy chain is critical for the pH-dependent binding to albumin. Eur J Immunol. 2006;36(11):3044–51. doi: 10.1002/eji.200636556. [DOI] [PubMed] [Google Scholar]
- 76.West AP, Jr, Bjorkman PJ. Crystal structure and immunoglobulin G binding properties of the human major histocompatibility complex-related Fc receptor(,) Biochemistry. 2000;39(32):9698–708. doi: 10.1021/bi000749m. [DOI] [PubMed] [Google Scholar]
- 77.Mezo AR, Sridhar V, Badger J, Sakorafas P, Nienaber V. X-ray crystal structures of monomeric and dimeric peptide inhibitors in complex with the human neonatal Fc receptor, FcRn. J Biol Chem. 2010;285(36):27694–701. doi: 10.1074/jbc.M110.120667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sand KM, Dalhus B, Christianson GJ, Bern M, Foss S, Cameron J, et al. Dissection of the neonatal Fc receptor (FcRn)-albumin interface using mutagenesis and anti-FcRn albumin-blocking antibodies. J Biol Chem. 2014;289(24):17228–39. doi: 10.1074/jbc.M113.522565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malkinson M. The transmission of passive immunity to Escherichia coli from mother to young in the domestic fowl (Gallus domesticus) Immunology. 1965;9(4):311–7. [PMC free article] [PubMed] [Google Scholar]
- 80.Schultze HE, Heremans JF. Molecular biology of human proteins: with special reference to plasma proteins. Nature and Metabolism of Extracellular Proteins, vol 1. New York Elsevier 1966.
- 81.Andersen JT, Sandlie I. The versatile MHC class I-related FcRn protects IgG and albumin from degradation: implications for development of new diagnostics and therapeutics. Drug Metab Pharmacokinet. 2009;24(4):318–32. doi: 10.2133/dmpk.24.318. [DOI] [PubMed] [Google Scholar]
- 82.Dornhorst A, Luddeke HJ, Sreenan S, Koenen C, Hansen JB, Tsur A, et al. Safety and efficacy of insulin detemir in clinical practice: 14-week follow-up data from type 1 and type 2 diabetes patients in the PREDICTIVE European cohort. Int J Clin Pract. 2007;61(3):523–8. doi: 10.1111/j.1742-1241.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 83.Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26(3):268–78. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nauck M, Frid A, Hermansen K, Shah NS, Tankova T, Mitha IH, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32(1):84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met + TZD) Diabetes Care. 2009;32(7):1224–30. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met + SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374(9683):39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 88.Pratley RE, Nauck M, Bailey T, Montanya E, Cuddihy R, Filetti S, et al. Liraglutide versus sitagliptin for patients with type 2 diabetes who did not have adequate glycaemic control with metformin: a 26-week, randomised, parallel-group, open-label trial. Lancet. 2010;375(9724):1447–56. doi: 10.1016/S0140-6736(10)60307-8. [DOI] [PubMed] [Google Scholar]
- 89.Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010;33(6):1300–3. doi: 10.2337/dc09-2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ablynx. http://www.ablynx.com/rd-portfolio/clinical-programmes/ozoralizumab/. Accessed 13 Nov 2015.
- 91.Ablynx. http://hugin.info/137912/R/1516627/452958.pdf. Accessed Nov 13 2015.
- 92.Desai N, Trieu V, Yao Z, Louie L, Ci S, Yang A, et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clin Cancer Res. 2006;12(4):1317–24. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 93.Neesse A, Frese KK, Chan DS, Bapiro TE, Howat WJ, Richards FM, et al. SPARC independent drug delivery and antitumour effects of nab-paclitaxel in genetically engineered mice. Gut. 2014;63(6):974–83. doi: 10.1136/gutjnl-2013-305559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Desai N. Nab technology: a drug delivery platform utilizing endothelial gp60 receptor-based transport and tumour-derived SPARC for targeting. Drug Delivery report. 2007; 37–41.
- 95.Clinical Trials NCT00477529. https://www.clinicaltrials.gov/ct2/ct2/show/NCT00477529. 916 Accessed Nov 13 2015.
- 96.Clinical Trials NCT02009332. https://www.clinicaltrials.gov/ct2/ct2/show/NCT02009332. 918 Accessed Nov 13 2015.
- 97.Clinical Trials NCT00820768. https://www.clinicaltrials.gov/ct2/ct2/show/NCT00820768?920term=ABI-010&rank=1. Accessed Nov 13 2015.
- 98.Rink T, Heuser T, Fitz H, Schroth HJ, Weller E, Zippel HH. Lymphoscintigraphic sentinel node imaging and gamma probe detection in breast cancer with Tc-99 m nanocolloidal albumin: results of an optimized protocol. Clin Nucl Med. 2001;26(4):293–8. doi: 10.1097/00003072-200104000-00002. [DOI] [PubMed] [Google Scholar]
- 99.Wang YF, Chuang MH, Chiu JS, Cham TM, Chung MI. On-site preparation of technetium-99 m labeled human serum albumin for clinical application. Tohoku J Exp Med. 2007;211(4):379–85. doi: 10.1620/tjem.211.379. [DOI] [PubMed] [Google Scholar]
- 100.Adams BK, Al Attia HM, Khadim RA, Al Haider ZY. 99Tc(m) nanocolloid scintigraphy: a reliable way to detect active joint disease in patients with peripheral joint pain. Nucl Med Commun. 2001;22(3):315–8. doi: 10.1097/00006231-200103000-00008. [DOI] [PubMed] [Google Scholar]
- 101.Liberatore M, Clemente M, Iurilli AP, Zorzin L, Marini M, Di Rocco E, et al. Scintigraphic evaluation of disease activity in rheumatoid arthritis: a comparison of technetium-99 m human non-specific immunoglobulins, leucocytes and albumin nanocolloids. Eur J Nucl Med. 1992;19(10):853–7. doi: 10.1007/BF00168160. [DOI] [PubMed] [Google Scholar]
- 102.Rosenstock J, Reusch J, Bush M, Yang F, Stewart M, Albiglutide SG. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–6. doi: 10.2337/dc09-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bush MA, Matthews JE, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab. 2009;11(5):498–505. doi: 10.1111/j.1463-1326.2008.00992.x. [DOI] [PubMed] [Google Scholar]
- 104.Woodward HN, Anderson SL. Once-weekly albiglutide in the management of type 2 diabetes: patient considerations. Patient Prefer Adherence. 2014;8:789–803. doi: 10.2147/PPA.S53075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zeuzem S, Yoshida EM, Benhamou Y, Pianko S, Bain VG, Shouval D, et al. Albinterferon alfa-2b dosed every two or four weeks in interferon-naive patients with genotype 1 chronic hepatitis C. Hepatology. 2008;48(2):407–17. doi: 10.1002/hep.22403. [DOI] [PubMed] [Google Scholar]
- 106.Zeuzem S, Sulkowski MS, Lawitz EJ, Rustgi VK, Rodriguez-Torres M, Bacon BR, et al. Albinterferon Alfa-2b was not inferior to pegylated interferon-alpha in a randomized trial of patients with chronic hepatitis C virus genotype 1. Gastroenterology. 2010;139(4):1257–66. doi: 10.1053/j.gastro.2010.06.066. [DOI] [PubMed] [Google Scholar]
- 107.Colvin RA, Tanwandee T, Piratvisuth T, Thongsawat S, Hui AJ, Zhang H, et al. Randomized, controlled pharmacokinetic and pharmacodynamic evaluation of albinterferon in patients with chronic hepatitis B infection. J Gastroenterol Hepatol. 2015;30(1):184–91. doi: 10.1111/jgh.12671. [DOI] [PubMed] [Google Scholar]
- 108.Prescription Drug Information ISe. http://www.drugs.com/history/zalbin.html. Accessed 13 Nov 2015.
- 109.Kratz F. DOXO-EMCH (INNO-206): the first albumin-binding prodrug of doxorubicin to enter clinical trials. Expert Opin Investig Drugs. 2007;16(6):855–66. doi: 10.1517/13543784.16.6.855. [DOI] [PubMed] [Google Scholar]
- 110.Kratz F. A clinical update of using albumin as a drug vehicle - a commentary. J Control Release. 2014;190:331–6. doi: 10.1016/j.jconrel.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 111.Kratz F, Warnecke A, Scheuermann K, Stockmar C, Schwab J, Lazar P, et al. Probing the cysteine-34 position of endogenous serum albumin with thiol-binding doxorubicin derivatives. Improved efficacy of an acid-sensitive doxorubicin derivative with specific albumin-binding properties compared to that of the parent compound. J Med Chem. 2002;45(25):5523–33. doi: 10.1021/jm020276c. [DOI] [PubMed] [Google Scholar]
- 112.Kratz F, Muller-Driver R, Hofmann I, Drevs J, Unger C. A novel macromolecular prodrug concept exploiting endogenous serum albumin as a drug carrier for cancer chemotherapy. J Med Chem. 2000;43(7):1253–6. doi: 10.1021/jm9905864. [DOI] [PubMed] [Google Scholar]
- 113.Chawla SP, Papai Z, Mukhametshina G, Sankhala K, Vasylyev L, Fedenko A, et al. First-Line Aldoxorubicin vs Doxorubicin in Metastatic or Locally Advanced Unresectable Soft-Tissue Sarcoma: A Phase 2b Randomized Clinical Trial. JAMA Oncol. 2015;1(9):1271–80. doi: 10.1001/jamaoncol.2015.3101. [DOI] [PubMed] [Google Scholar]
- 114.CytRx. http://www.cytrx.com/aldoxorubicin. Accessed 13 Nov 2015.
- 115.Lau S, Graham B, Cao N, Boyd BJ, Pouton CW, White PJ. Enhanced extravasation, stability and in vivo cardiac gene silencing via in situ siRNA-albumin conjugation. Mol Pharm. 2012;9(1):71–80. doi: 10.1021/mp2002522. [DOI] [PubMed] [Google Scholar]
- 116.Ehrlich GK, Michel H, Truitt T, Riboulet W, Pop-Damkov P, Goelzer P, et al. Preparation and characterization of albumin conjugates of a truncated peptide YY analogue for half-life extension. Bioconjug Chem. 2013;24(12):2015–24. doi: 10.1021/bc400340z. [DOI] [PubMed] [Google Scholar]
- 117.Stehle G, Sinn H, Wunder A, Schrenk HH, Schutt S, Maier-Borst W, et al. The loading rate determines tumor targeting properties of methotrexate-albumin conjugates in rats. Anti Cancer Drugs. 1997;8(7):677–85. doi: 10.1097/00001813-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 118.Hartung G, Stehle G, Sinn H, Wunder A, Schrenk HH, Heeger S, et al. Phase I trial of methotrexate-albumin in a weekly intravenous bolus regimen in cancer patients. Phase I Study Group of the Association for Medical Oncology of the German Cancer Society. Clin Cancer Res. 1999;5(4):753–9. [PubMed] [Google Scholar]
- 119.Bolling C, Graefe T, Lubbing C, Jankevicius F, Uktveris S, Cesas A, et al. Phase II study of MTX-HSA in combination with cisplatin as first line treatment in patients with advanced or metastatic transitional cell carcinoma. Investig New Drugs. 2006;24(6):521–7. doi: 10.1007/s10637-006-8221-6. [DOI] [PubMed] [Google Scholar]
- 120.Tumey LN, Charati M, He T, Sousa E, Ma D, Han X, et al. Mild method for succinimide hydrolysis on ADCs: impact on ADC potency, stability, exposure, and efficacy. Bioconjug Chem. 2014;25(10):1871–80. doi: 10.1021/bc500357n. [DOI] [PubMed] [Google Scholar]
- 121.Fontaine SD, Reid R, Robinson L, Ashley GW, Santi DV. Long-term stabilization of maleimide-thiol conjugates. Bioconjug Chem. 2015;26(1):145–52. doi: 10.1021/bc5005262. [DOI] [PubMed] [Google Scholar]
- 122.Andersen JT, Dalhus B, Viuff D, Ravn BT, Gunnarsen KS, Plumridge A, et al. Extending serum half-life of albumin by engineering neonatal Fc receptor (FcRn) binding. J Biol Chem. 2014;289(19):13492–502. doi: 10.1074/jbc.M114.549832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elsadek B, Kratz F. Impact of albumin on drug delivery--new applications on the horizon. J Control Release. 2012;157(1):4–28. doi: 10.1016/j.jconrel.2011.09.069. [DOI] [PubMed] [Google Scholar]
- 124.Home P, Kurtzhals P. Insulin detemir: from concept to clinical experience. Expert Opin Pharmacother. 2006;7(3):325–43. doi: 10.1517/14656566.7.3.325. [DOI] [PubMed] [Google Scholar]
- 125.Wunder A, Muller-Ladner U, Stelzer EH, Funk J, Neumann E, Stehle G, et al. Albumin-based drug delivery as novel therapeutic approach for rheumatoid arthritis. J Immunol. 2003;170(9):4793–801. doi: 10.4049/jimmunol.170.9.4793. [DOI] [PubMed] [Google Scholar]
- 126.Stehle G, Wunder A, Sinn H, Schrenk HH, Schutt S, Frei E, et al. Pharmacokinetics of methotrexate-albumin conjugates in tumor-bearing rats. Anti Cancer Drugs. 1997;8(9):835–44. doi: 10.1097/00001813-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 127.Clinical Trials NCT01706835. https://www.clinicaltrials.gov/ct2/ct2/show/NCT01706835?1016term=aldoxorubicin&rank=5. Accessed Nov 13 2015.
- 128.Baggio LL, Huang Q, Cao X, Drucker DJ. An albumin-exendin-4 conjugate engages central and peripheral circuits regulating murine energy and glucose homeostasis. Gastroenterology. 2008;134(4):1137–47. doi: 10.1053/j.gastro.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 129.Leger R, Thibaudeau K, Robitaille M, Quraishi O, van Wyk P, Bousquet-Gagnon N, et al. Identification of CJC-1131-albumin bioconjugate as a stable and bioactive GLP-1(7–36) analog. Bioorg Med Chem Lett. 2004;14(17):4395–8. doi: 10.1016/j.bmcl.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 130.Clinical Trials NCT00638716. https://www.clinicaltrials.gov/ct2/ct2/show/NCT00638716?term=1026conjuchem&rank=2. Accessed Nov 18 2015.
- 131.ConjuChem. http://www.conjuchem.com/pipeline/cjc-1134-pc. Accessed Nov 18 2015.
- 132.Poole RM, Nowlan ML. Albiglutide: first global approval. Drugs. 2014;74(8):929–38. doi: 10.1007/s40265-014-0228-2. [DOI] [PubMed] [Google Scholar]
- 133.Baggio LL, Huang Q, Brown TJ, Drucker DJ. A recombinant human glucagon-like peptide (GLP)-1-albumin protein (albugon) mimics peptidergic activation of GLP-1 receptor-dependent pathways coupled with satiety, gastrointestinal motility, and glucose homeostasis. Diabetes. 2004;53(9):2492–500. doi: 10.2337/diabetes.53.9.2492. [DOI] [PubMed] [Google Scholar]
- 134.Glaxo Smith Kline. http://www.gsk.com/en-gb/media/press-releases/2014/gsk-receives-us-approval-for-once-weekly-type-2-diabetes-treatment-tanzeum-albiglutide/. Accessed Nov 13 2015.
- 135.Golor G, Bensen-Kennedy D, Haffner S, Easton R, Jung K, Moises T, et al. Safety and pharmacokinetics of a recombinant fusion protein linking coagulation factor VIIa with albumin in healthy volunteers. J Thromb Haemost. 2013;11(11):1977–85. doi: 10.1111/jth.12409. [DOI] [PubMed] [Google Scholar]
- 136.Schulte S. Use of albumin fusion technology to prolong the half-life of recombinant factor VIIa. Thromb Res. 2008;122(Suppl 4):S14–9. doi: 10.1016/S0049-3848(08)70029-X. [DOI] [PubMed] [Google Scholar]
- 137.Weimer T, Wormsbacher W, Kronthaler U, Lang W, Liebing U, Schulte S. Prolonged in-vivo half-life of factor VIIa by fusion to albumin. Thromb Haemost. 2008;99(4):659–67. doi: 10.1160/TH07-08-0525. [DOI] [PubMed] [Google Scholar]
- 138.Clinical Trials NCT01542619. https://www.clinicaltrials.gov/ct2/ct2/show/NCT01542619?1050term=albumin+fusion+VIIa&rank=1. Accessed Nov 18 2015.
- 139.Clinical Trials NCT01496274. https://www.clinicaltrials.gov/ct2/ct2/show/NCT01496274?1052term=rIX-FP&rank=3. Accessed Dec 2 2015.
- 140.http://www.drugs.com. http://www.drugs.com/nda/zalbin_101005.html. Accessed Nov 18 2015.
- 141.Abraxane. http://www.abraxane.com. Accessed Nov 13 2015.


