Abstract
Eluxadoline, an orally active mixed μ opioid receptor (μOR) agonist δ opioid receptor (δOR) antagonist developed for the treatment of diarrhea-predominant irritable bowel syndrome, normalizes gastrointestinal (GI) transit and defecation under conditions of novel environment stress or post-inflammatory altered GI function. Furthermore, compared to loperamide, which is used to treat non-specific diarrhea, the effects of eluxadoline on GI transit occur over a wider dosage range. However, the mechanisms of action of eluxadoline are unclear. In this study, we compared the ability of eluxadoline and loperamide to activate G-protein- and β-arrestin-mediated signaling at μOR homomers or μOR-δOR heteromers in heterologous cells. We also examined the ability of both compounds to reduce castor oil induced diarrhea in wild type (WT) and mice lacking δOR. We find that eluxadoline is more potent than loperamide in eliciting G-protein activity and β-arrestin recruitment in μOR expressing cells. However, in cells expressing μOR-δOR heteromers, the potency of eluxadoline is higher, but its maximal effect is lower than that of loperamide. Moreover, in these cells the signaling mediated by eluxadoline but not loperamide is reduced by μOR-δOR heteromer-selective antibodies. We find that in castor oil-induced diarrhea eluxadoline is more efficacious compared to loperamide in WT mice, and δOR appears to play a role in this process. Taken together these results indicate that eluxadoline behaves as a potent μOR agonist in the absence of δOR, while in the presence of δOR eluxadoline’s effects are mediated through the μOR-δOR heteromer.
Keywords: μOR-δOR heteromer, Anti-diarrhea, Eluxadoline, Loperamide, Irritable bowel syndrome
1. Introduction
Opioid receptors are therapeutic targets for the treatment of pain. Morphine, the prototypic opioid, targets the mu opioid receptor (μOR) and is clinically preferred for the treatment of chronic pain [1]. However, chronic morphine administration leads to a number of side-effects including development of analgesic tolerance and constipation. Studies seeking to decrease the side-effects associated with chronic morphine use found that delta opioid receptor (δOR) antagonists could enhance morphine-induced analgesia while preventing the development of tolerance to this drug [2-6] which suggested interactions between μOR and δOR. These interactions were examined using cells heterologously expressing either μOR or δOR or a combination of both receptors and showed that δOR selective antagonists, irrespective of their nature (peptidic or non-peptidic), could enhance μOR selective ligand binding and signaling only in cells co-expressing both receptors [7,8]. Moreover, these in vitro studies showed that the δOR antagonist decreased the dissociation rate of radioligand bound to μOR [9]. These data supported the idea that the δOR antagonist allosterically enhances μOR ligand binding leading to potentiation of μOR-mediated signaling and antinociception. One way in which allosteric modulation of μOR properties by δOR could occur is via the formation of μOR-δOR heteromers; μOR-δOR heteromerization is supported by studies using antibodies that selectively target the heteromer [10] or TAT peptides that can disrupt the formation of μOR-δOR heteromers [11]. Ligands targeting μOR-δOR heteromers either by having μOR agonist/δOR antagonist activity such as bivalent ligands or ligands possessing mixed μOR agonist and δOR antagonist activity have been generated [12-17]. Studies using a bivalent ligand comprising of a μOR agonistic pharmacophore separated by a 21-atom spacer arm from a δOR antagonistic pharmacophore (MDAN21) [15,17] showed that it exhibited 100-times higher antinociceptive potency compared to morphine without significant development of tolerance or dependence [15]. Similarly, studies using ligands possessing mixed μOR agonist/δOR antagonist activity show that their chronic administration leads to lesser side-effects compared to morphine [13]. Taken together these results suggest that targeting the μOR-δOR heteromer could lead to the development of drugs that are likely to have lower side effects than drugs targeting μOR alone.
As mentioned above, one of the severe side-effects associated with chronic morphine use is constipation; this suggests that opioid receptors in the gastrointestinal (GI) tract could be targeted for the treatment of GI tract disorders [18] such as diarrhea. This led to the development of loperamide, a peripherally active μOR agonist, as a therapeutic agent for the treatment of diarrhea [19,20]. However, one of the side-effects associated with the use of loperamide is the development of constipation [21,22]. The possibility that drugs having μOR agonist/δOR antagonist activity could have lesser side effects led to the synthesis of eluxadoline [14,16]. Recent studies show that eluxodaline is a locally acting μOR agonist/δOR antagonist that can normalize GI transit in stressed animals over a wide dose range [16]. Eluxadoline has limited systemic bioavailability which could potentially reduce its effects on the central nervous system and consequently prevent the development of side-effects associated with therapies currently used to treat irritable bowel syndrome with diarrhea (IBS-d). Currently, eluxadoline has completed Phase II [23] and is undergoing Phase III clinical trials for treatment of IBS-d. While in vivo preclinical studies indicate that eluxadoline modulates GI motility and decreases intestinal pain or visceral hyperalgesia without the constipation associated with drugs that activate μOR [16], its mechanism of action is not clear. Since eluxadoline is a mixed μOR agonist/δOR antagonist [14,16,23], it is possible that it may mediate its effects by targeting μOR-δOR heteromers. Therefore, in this study we examined the mechanism of the in vitro effects of eluxadoline by comparing its activity in cell lines (using an assay that specifically examines heteromer signaling) and in tissues from wild-type (WT) and knockout mice (δOR−/− or μOR−/−). Furthermore, we evaluated the extent to which eluxadoline affects GI transit in WT and δOR−/− mice in a castor oil induced model of diarrhea. We find that eluxadoline-mediated signaling can be significantly, albeit partially, blocked by an μOR-δOR heteromer selective antibody in cells co-expressing both receptors. We also find that eluxadoline is more effective in blocking castor oil-induced diarrhea in WT mice as compared to δOR−/− mice. These results suggest that eluxadoline, at least in part, mediates its effects by targeting μOR-δOR heteromers.
2. Methods
2.1. Cell culture
μβgalOR and μβgalOR-δOR expressing U2OS cells were a kind gift from DiscoveRx (Fremont, CA, USA). μβgalOR cells expressing μOR tagged with a ProLink/β-galactosidase (βgal) donor (PK) fragment at the C-terminal region and β-arrestin tagged with a complementary βgal activator (EA) fragment were grown in MEM alpha (Life Technologies, Grand Island, NY, USA) containing 10% FBS (Biowest SAS, Nuaille, France), streptomycin-penicillin (Life Technologies), 500 μg/ml geneticin (Life Technologies) and 250 μg/ml hygromycin (Life Technologies). μβgalOR-δOR cells expressing wild-type δOR, μOR tagged with the PK fragment at the C-terminal region and β-arrestin tagged with the EA fragment were grown in MEM alpha containing 10% FBS, streptomycin-penicillin, 500 μg/ml geneticin, 250 μg/ml hygromycin and 0.25 μg/ml puromycin (Life Technologies).
2.2. [35S]GTPγS binding
Membranes were prepared from the spinal cord of either WT (Jackson Laboratories, Sacramento, CA, USA), δOR−/− (Charles River Laboratories, Kingston, NY, USA), μOR−/− (a gift from Dr. Charles Mobbs, Ichan School of Medicine at Mount Sinai, NY, USA) or from the ileal longitudinal muscle (containing myenteric plexus) of WT mice as described previously [24,25]. Membranes (10 or 20 μg) were subjected to a [35S]GTPγS binding assay using DAMGO (R&D Systems, Minneapolis, USA), loperamide (Toronto Research Chemicals Inc., Ontario, Canada), eluxadoline (Furiex, Morrisville, NC, USA) (0–10 μM final concentration) in the presence or absence of TIPPψ (10 nM final concentration) (a gift from Dr. Peter Schiller, Institut de Reserches Cliniques de Montreal, Montreal, ON, Canada) as described previously [25]. EC50 and Emax were calculated using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
2.3. β-arrestin recruitment assay
U2OS cells expressing either μβgalOR or μβgalOR-δOR were plated in each well (5000 cells) of a 96-well white clear bottom plate in 100 μl of media. Next day, cells were treated with either DAMGO, loperamide, eluxadoline (0–10 μM final concentration) in the absence or presence of the δOR antagonist, TIPPψ (10 nM final concentration) (a gift from Dr. Peter Schiller) or in the absence or presence of antibodies (1 μg/well) to either μOR, μOR-δOR (generated as reported in [26]) or cannabinoid receptor type1-angiotensin II receptor type 1 heteromer (CB1R-AT1R) (generated as reported in [27]) for 60 min at 37 °C. β-arrestin recruitment was measured using the PathHunter Chemiluminescence detection kit as described in the manufacturer’s protocol (DiscoveRx, Fremont, CA, USA). EC50 and Emax were calculated using Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
2.4. Animals
Male C57BL/6 WT and δOR−/− mice (25–35 g; 6–12 weeks old) were obtained from either Jackson Laboratories (Sacramento, CA, USA; WT mice) or Charles River Laboratories (Kingston, NY, USA; δOR−/− mice). All mice were maintained on a 12-h light:12-h dark cycle with rodent chow and water available ad libitum, and housed in groups of five until testing. Animal studies were carried out according to protocols approved by the Icahn School of Medicine at Mount Sinai Animal Care and Use Committee.
2.5. Drug administration
Loperamide (Toronto Research Chemicals, Inc., Ontario, Canada) and eluxadoline (Furiex, Morrisville, NC, USA) were dissolved in 0.5% methylcellulose and 2% DMSO in water. Corresponding vehicle was used for control group. Mice were administered these drugs orally (p.o.). Naltrexone (R&D Systems, Minneapolis, USA) was dissolved in saline and administered intraperitoneally (i.p.). For chronic treatment with eluxadoline and loperamide, mice were treated with these drugs at the dose of 10 mg/kg (p.o.) once a day for 5 days. On the 6th day, mice were euthanized by cervical dislocation and ileum was collected (3–4 mice/sample) for ELISA assay.
2.6. Castor oil-induced diarrhea
Mice were placed in new absorbent lined bottomed cages with no access to food and water for 2 h before the test. Immediately before the test, the absorbent liner was discarded, and a fresh pre-weighed liner was placed in the cage. Diarrhea was induced by oral administration of castor oil (0.6 ml/mouse) (ACROS Organics, Geel, Belgium) to WT or δOR−/− mice. Stools were scored (diarrhea score of 0 = normal; 1 = wet and irregular shape; or 2 = shapeless) and weighed over a 4-h period as described previously [28,29]. After every hour, the absorbent liner was weighed, and another pre-weighed liner was placed in the cage. Diarrhea score represents the most marked change in feces for individual mice during a 4-h period. Loperamide and eluxadoline were administered orally 15 min before the castor oil administration. When naltrexone (10 mg/kg, i.p.) was used, it was administered 20 min before loperamide or eluxadoline administration. Body weight was measured before and 4 h after castor oil administration.
2.7. ELISA assay
Ileal longitudinal muscle (containing myenteric plexus) was prepared as described previously [24]. Membranes (10 μg) from mouse ileal longitudinal muscle were subjected to an ELISA assay using rat anti-μOR antibody (1:500), rat anti-δOR antibody (1:500) (generated as reported in [26]) or mouse anti-μOR-δOR heteromer selective antibody (1:100) as primary antibodies and anti-mouse IgG (1:1000) (Vector laboratories, Inc., Burlingame, CA., USA) or anti-rat IgG (1:1000) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA) coupled to horseradish peroxidase as secondary antibodies as described previously [30]. ELISA for each sample was performed in triplicate.
2.8. Statistical analysis
The data were expressed as means ± S.E.M. Student’s t-test or one-way ANOVA and multiple-comparison test (Student–Newman–Keuls test or Dunnett’s test) were used to analyze the data. A difference was considered to be significant at p < 0.05.
3. Results
3.1. Eluxadoline-mediated signaling
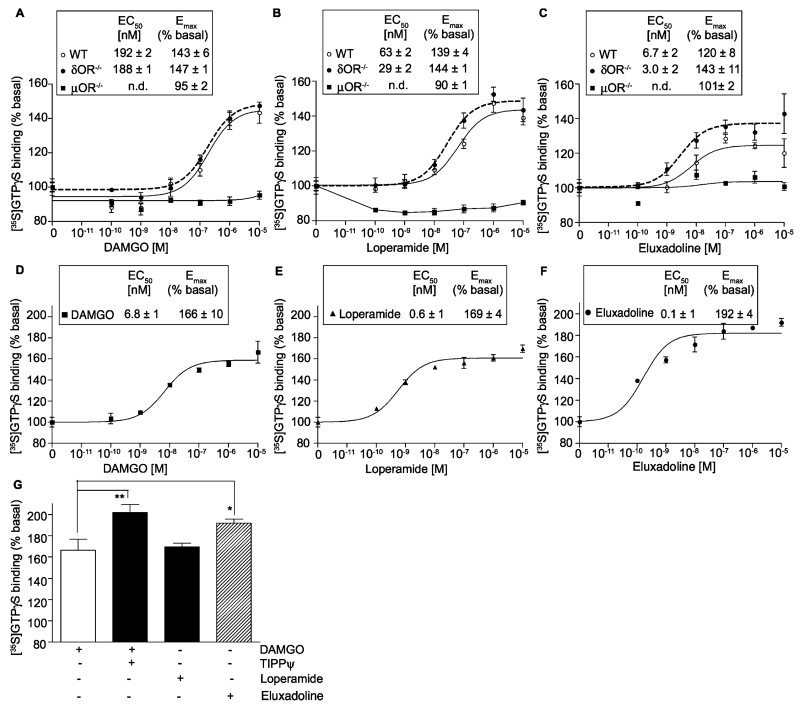
Although phase II clinical studies show that eluxadoline, a locally acting mixed μOR agonist and δOR antagonist, is effective in the treatment of IBS-d patients [23] very little is known about its cellular mechanism of action through heteromers. Therefore, we determined the signaling of eluxadoline and compared it to that of DAMGO, a selective μOR agonist, and of loperamide, a peripherally active μOR agonist used in the treatment of diarrhea [19,20]. For this, we measured G protein activity by carrying out [35S]GTPγS binding assays using spinal cord membranes from WT, δOR−/− or μOR−/− mice. We find that DAMGO, the selective μOR agonist, dose dependently increases [35S]GTPγS binding to spinal cord membranes from WT (EC50 ~ 192 nM; Emax ~ 143%) and from δOR−/− (EC50 ~ 188 nM; Emax ~ 147%) but not from μOR−/− mice (Fig. 1A). Similarly, loperamide, the peripherally active μOR agonist, dose dependently increases [35S]GTPγS binding to spinal cord membranes from WT (EC50 ~ 63 nM; Emax ~ 139%), δOR−/− (EC50 ~ 29 nM; Emax ~ 144%) but not from μOR−/− mice (Fig. 1B). Interestingly, although eluxadoline like DAMGO and loperamide did not increase [35S]GTPγS binding to membranes from μOR−/− mice, it induced a dose dependent increase in [35S]GTPγS binding to spinal cord membranes from WT mice with a higher potency (EC50 ~ 7 nM) and lower efficacy (Emax ~ 120%) than seen with either DAMGO or loperamide (Fig. 1C). Moreover, in membranes from δOR−/− mice the efficacy of eluxadoline (Emax of ~143%) was higher than in WT membranes (Emax of ~120%) and similar to that seen with DAMGO in δOR−/− mice (Emax of ~147%) (Fig. 1A and C). These results suggest that a portion of eluxadoline-mediated signaling in the presence of both μOR and δOR could be due to activation of μOR-δOR heteromers since in the absence of δOR it behaves like a pure μOR agonist.
Fig. 1.
Effect of DAMGO, loperamide and eluxadoline on G-protein activation. (A–C) Membranes (10 μg) from spinal cords of WT, μOR−/− and δOR−/− mice were subjected to a [35S]GTPγS binding assay using DAMGO (A), loperamide (B), and eluxadoline (C) (0–10 μM final concentration) as described in Section 2. (D–G) Membranes (20 μg) from the ileum of WT mice were subjected to a [35S]GTPγS binding assay using DAMGO (D and G), loperamide (E and G), and eluxadoline (F and G) (0–10 μM final concentration) in the presence or absence of TIPPψ (10 nM final concentration) as described in Section 2. (G) Represents Emax (% of basal) obtained with 10 μM final concentration of DAMGO (±10 nM final concentration of TIPPψ), eluxadoline or loperamide. Basal values determined in the absence of the agonist were taken as 100%. Results are the mean ± S.E.M. n= 3–9. n.d., Not determined. *p < 0.05; **p < 0.01, Dunnett’s test.
Since eluxadoline is effective in the treatment of IBS-d [23] we also carried out [35S]GTPγS binding assays using ileal membranes from WT animals. We find that eluxadoline, DAMGO and loperamide cause dose-dependent increases in [35S]GTPγS binding (Fig. 1D–F). Interestingly, we find that in ileal membranes a combination of the δOR antagonist, TIPPψ, with the μOR agonist, DAMGO, or eluxadoline (a μOR agonist/δOR antagonist) give a higher increase in [35S]GTPγS binding compared to either DAMGO or loperamide (μOR agonists) (Fig. 1G). These results would suggest the presence of μOR-δOR heteromers in ileal tissue that could contribute to the effectiveness of eluxadoline in the treatment of IBS-d.
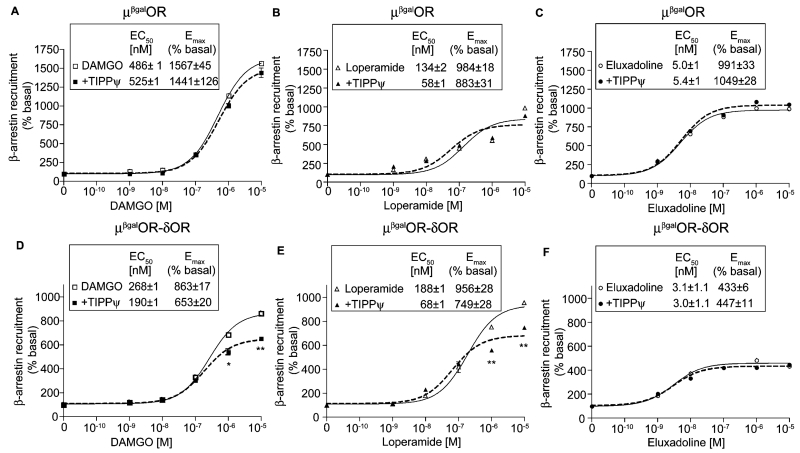
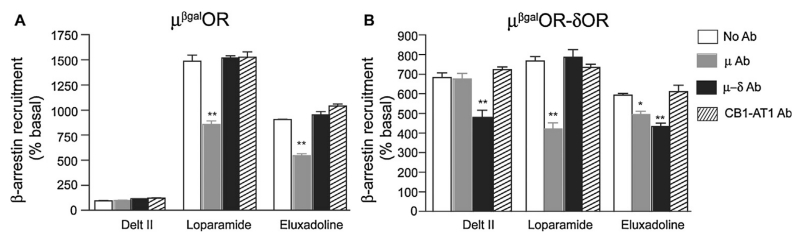
A common feature of GPCR signaling is that following receptor activation, the receptor is phosphorylated by GRKs and this leads to the recruitment of β-arrestin to the phosphorylated receptor, cessation of G-protein mediated signaling and induction of β-arrestin-mediated signaling (reviewed in [31]). Moreover, several studies in the last decade show that some agonists preferentially signal via one signaling pathway (for e.g., G protein-mediated) versus another (for e.g., β-arrestin-mediated) leading to biased agonism (reviewed in [31]). We therefore compared the ability of DAMGO, loperamide and eluxadoline to induce β-arrestin recruitment in cells expressing either μOR homomers (μβgalOR cells) or μOR-δOR heteromers (μORβgal-δOR cells) using an enzyme complementation assay that we recently used to identify and characterize compounds that preferentially activate the μOR-δOR heteromer [26]. Using this assay, we find that eluxadoline is more potent (i.e., lower EC50) at inducing β-arrestin recruitment than DAMGO or loperamide in μβgalOR (Fig. 2A–C) or in μORβgal-δOR cells (Fig. 2D–F). Interestingly, eluxadoline is less efficacious than DAMGO or loperamide in μORβgal-δOR cells suggesting that it’s intrinsic δOR antagonistic activity could affect its ability to induce β-arrestin recruitment (Fig. 2D–F). This is supported by our previous observation that the δOR antagonist, TIPPψ, decreased DAMGO-mediated β-arrestin recruitment only in μORβgal-δOR cells but not in μβgalOR cells [26]. We therefore examined the effect of TIPPψ on DAMGO-, loperamide- or eluxadoline-mediated β-arrestin recruitment. We find that TIPPψ has no effect on β-arrestin recruitment induced by either DAMGO, loperamide or eluxadoline in μβgalOR cells (Fig. 2A–C). However, in cells expressing μORβgal-δOR, TIPPψ decreased DAMGO- or loperamide-induced β-arrestin recruitment but not that induced by eluxadoline (Fig. 2D–F). The lack of effect of TIPPψ on eluxadoline-mediated β-arrestin recruitment in μORβgal-δOR cells could be because eluxadoline already exhibits antagonistic activity at δOR. In this context, it is interesting to note that in μORβgal-δOR cells the efficacy of eluxadoline to elicit β-arrestin recruitment is 50% lower (Emax ~ 433%) than seen with DAMGO or loperamide (Emax ~ 863% and 956%, respectively). Together these results indicate that a component of eluxadoline’s effects is different from DAMGO or loperamide and is possibly through activation of μOR-δOR heteromers. We tested this by using μOR-δOR heteromer-selective antibodies that we have generated and previously shown that they can block heteromer-mediated binding and signaling [10,26]. Also, we had previously reported that activation of δOR can lead to β-arrestin recruitment to μOR in μORβgal-δOR cells and this can be blocked by μOR-δOR heteromer-selective antibodies [26]. In agreement with these observations we find that deltorphin II (Delt II; R&D Systems, Minneapolis, USA), the δOR selective agonist, induces β-arrestin recruitment to μOR in μORβgal-δOR cells (Fig. 3B) but not to μβgalOR cells (Fig. 3A) and this can be blocked by μOR-δOR heteromer-selective antibodies and not by μOR-selective antibodies or by antibodies selective to an unrelated heteromer, i.e., the CB1R-AT1R heteromer (Fig. 3B). We find that loperamide-mediated β-arrestin recruitment both in μβgalOR (Fig. 3A) and μORβgal-δOR cells (Fig. 3B) is significantly blocked by μOR-selective antibodies but not by antibodies selective for either the μOR-δOR or CB1R-AT1R heteromer. This supports that loperamide exerts its effects by activating μOR. In the case of eluxadoline, we find that its ability to induce β-arrestin recruitment is blocked by μOR-selective antibodies by ~40% in μβgalOR cells (Fig. 3A) and by ~17% in μORβgal-δOR cells (Fig. 3B). In addition, we find that μOR-δOR heteromer-selective antibodies block 27% of eluxadoline-mediated β-arrestin recruitment in μORβgal-δOR cells (Fig. 3B) but have no effect in μβgalOR cells (Fig. 3A); no effect of CB1R-AT1R heteromer-selective antibodies on eluxadoline-mediated β-arrestin recruitment was observed in either μβgalOR (Fig. 3A) or μORβgal-δOR cells (Fig. 3B). Taken together these results suggest that in cells co-expressing μOR and δOR a portion of eluxadoline-mediated signaling occurs via activation of μOR-δOR heteromers while in cells expressing only μOR eluxadoline functions as a μOR agonist.
Fig. 2.
Effect of DAMGO, loperamide and eluxadoline on β-arrestin recruitment. Cells (5000/well) expressing either μβgalOR (A–C) or μβgalOR-δOR (D–F) were treated with either DAMGO (A and D), loperamide (B and E), eluxadoline (C and F) (0–10 μM final concentration) in the absence or presence of the δOR antagonist, TIPPψ (10 nM final concentration) for 60 min at 37 °C and β-arrestin recruitment was measured as described in Section 2. Results are the mean ± S.E.M. n = 4–12. *p < 0.05, **p < 0.01, vs. absence of TIPPψ, t-test.
Fig. 3.
Effect of μOR-δOR heteromer-selective antibody on eluxadoline-mediated signaling. Cells (5000 cells) expressing either μβgalOR (A) or μβgalOR-δOR (B) were treated with either deltorphin II (Delt II), loperamide or eluxadoline (1 μM final concentration) in the absence or presence of antibodies (Ab, 1 μg/well) to either μOR, μOR-δOR heteromer or CB1R-AT1R heteromer for 60 min at 37 °C and β-arrestin recruitment was measured as described in Section 2. Results are the mean ± S.E.M. n = 4. *p < 0.05, **p < 0.01, vs. no Ab treatment for each group, Dunnett’s test.
3.2. Castor oil-induced diarrhea
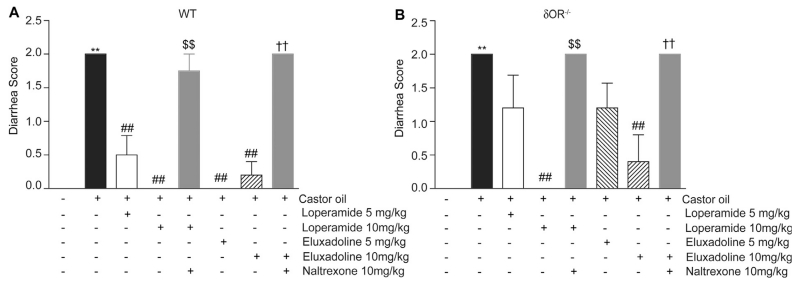
Previous studies showed that eluxadoline can normalize GI transit over a wider dose range compared to loperamide in WT mice [16]. Although WT mice express both μOR and δOR in myenteric plexus [32], not much is known about the contribution of each receptor type or of μOR-δOR heteromers to the effects of eluxadoline on GI transit. To investigate this we used WT and δOR−/− mice and compared the effects of eluxadoline and loperamide on castor oil induced diarrhea. We find that oral administration of castor oil induces severe diarrhea (i.e., higher score) both in WT (Fig. 4A) and δOR−/− mice (Fig. 4B). The castor oil-induced diarrhea in WT mice was reduced by administration of 5 or 10mg/kg of either loperamide or eluxadoline (Fig. 4A). Moreover, this reduction in castor oil-induced diarrhea by either loperamide or eluxadoline at 10 mg/kg were not observed if the animals were pre-treated with the μOR antagonist, naltrexone (Fig. 4A); studies have reported that antagonizing the activity of opioid receptors, including μOR, has no effect on castor oil induced diarrhea [33,34]. Interestingly, in δOR−/− mice a 5 mg/kg dose of either loperamide or eluxadoline did not significantly reduce castor oil-induced diarrhea (Fig. 4B); a higher dose of 10 mg/kg was required to completely eliminate diarrhea in the case of loperamide and partially eliminate in the case of eluxadoline (Fig. 4B).
Fig. 4.
Effect of loperamide and eluxadoline on castor oil-induced diarrhea. Diarrhea was induced by oral administration of castor oil (0.6 ml/mouse) in WT (A) or δOR−/− (B) mice. Stools were scored for diarrhea (0 = normal; 1 = wet and irregular shape; or 2 = shapeless) for 4 has described in Section 2. Loperamide and eluxadoline (5 or 10 mg/kg) were administered orally 15 min before the castor oil administration. Naltrexone (10 mg/kg, i.p.) was administered 20 min before loperamide or eluxadoline administration. Results are the mean ± S.E.M. n = 3–6. **p < 0.01, vs. vehicle control; ##p < 0.01, vs. castor oil alone; $$p < 0.01, vs. castor oil + loperamide (10 mg/kg, p.o.); ††p < 0.01, vs. castor oil + eluxadoline (10 mg/kg, p.o), Student–Newman–Keuls test.
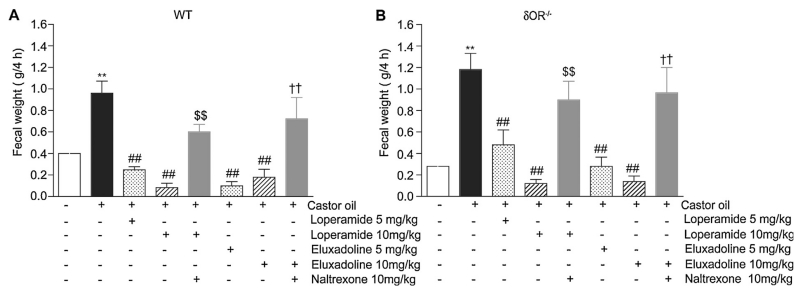
Next, we correlated in the same animals the diarrheal scores with fecal output by measuring fecal weight. We find that castor oil administration significantly increases the fecal output in WT (Fig. 5A) and δOR−/− mice (Fig. 5B). In addition, we find that in WT mice, treatment with either loperamide or eluxadoline significantly reduces the castor oil induced fecal output and the values were below that seen with controls administered with vehicle instead of castor oil (Fig. 5A). These effects were blocked by coadministration of naltrexone (Fig. 5A). Similar results were obtained in δOR−/− mice (Fig. 5B) although a 5 mg/kg dose caused a less pronounced decrease in fecal output compared to that seen in WT animals for both loperamide and eluxadoline (Fig. 5A and B). The fact that a 5 mg/kg dose of either loperamide or eluxadoline significantly decreased fecal output in δOR−/− mice but had no significant effect on the diarrhea scores (Fig. 4B) in the same animals could be due to the qualitative nature of the diarrhea score. Taken together these results showing that eluxadoline (at 5 mg/kg) produced a lower blockade of diarrhea and fecal output in δOR−/− mice compared to WT mice suggests that δOR and/or μOR-δOR heteromers could contribute to its anti-diarrheal effect in WT mice. Moreover, a dose-response effect with eluxadoline is observed in δOR−/− but not in WT mice which would again indicate that δOR activity may modulate eluxadoline’s effects on μOR in WT mice and that eluxadoline behaves as a μOR agonist in mice that lack δOR.
Fig. 5.
Effect of loperamide and eluxadoline on castor oil-induced diarrhea (fecal output). Diarrhea was induced by oral administration of castor oil (0.6 ml/mouse) in WT (A) or δOR−/− (B) mice. Stools were collected and weighed during 4 h as described in Section 2. Loperamide and eluxadoline (5 or 10 mg/kg) were administered orally 15 min before the castor oil administration. Naltrexone (10 mg/kg, i.p.) was administered 20 min before loperamide or eluxadoline administration. Results are the mean ± S.E.M. n = 3–6. **p < 0.01, vs. vehicle control; ##p < 0.01, vs. castor oil alone; $$p < 0.01, vs. castor oil + loperamide (10 mg/kg, p.o.); ††p < 0.01, vs. castor oil + eluxadoline (10 mg/kg, p.o), Student–Newman–Keuls test.
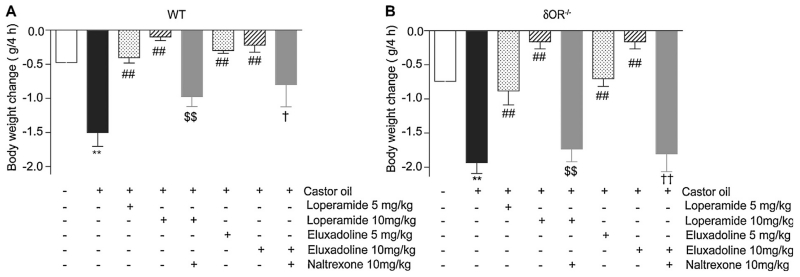
Next we examined the changes in body weight in WT and δOR−/−mice and found that in both groups castor oil-induced diarrhea led to decreases in body weight (Fig. 6A and B) and this was blocked by loperamide and eluxadoline. Both loperamide and eluxadoline effects could be partly blocked by naltrexone. Although not statistically significant, the amount of body weight change in δOR−/− appeared more robust compared to WT mice. Interestingly, the effect of naltrexone was more pronounced in δOR−/− mice (Fig. 6). The fact that 5 mg/kg eluxadoline was less effective in δOR−/− mice as compared to the WT mice is consistent with the results with diarrhea score and fecal output and further support a role for δOR (or μOR-δOR heteromer) in this effect in WT mice.
Fig. 6.
Effect of loperamide and eluxadoline on castor oil-induced body weight change. Diarrhea was induced by oral administration of castor oil (0.6 ml/mouse) in WT (A) or δOR−/− (B) mice. Body weight was measured before and 4 h after castor oil administration. Loperamide and eluxadoline (5 or 10 mg/kg) were administered orally 15 min before the castor oil administration. Naltrexone (10 mg/kg, i.p.) was administered 20 min before loperamide or eluxadoline administration. Results are the mean ± S.E.M. n = 3–6. **p < 0.01, vs. vehicle control; ##p < 0.01, vs. castor oil alone; $$p < 0.01, vs. castor-oil + loperamide (10 mg/kg, p.o.); †p < 0.05, ††p < 0.01, vs. castor oil + eluxadoline (10 mg/kg, p.o), Student–Newman–Keuls test.
3.3. Chronic eluxadoline treatment
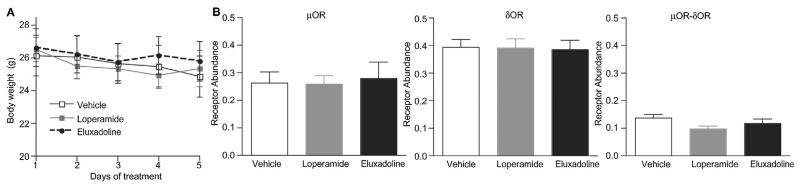
We have previously reported/noted that chronic treatment with morphine under a paradigm that leads to the development of tolerance causes an increase in μOR-δOR heteromer expression in different brain regions [10]. Therefore, in this study we examined whether μOR-δOR heteromers are present in myenteric neurons from GI tissue and ascertained the effect of long-term treatment with loperamide or eluxadoline on receptor expression and on body weight. We find that administration of these compounds at the dose of 10 mg/kg/day for 5 days did not lead to significant changes in body weight (Fig. 7A), indicating that repeated oral treatment with either drug would not affect the nutrient absorptive function in intestine. In addition, using antibodies selective for either μOR, δOR or μOR-δOR heteromers we can detect the presence of μOR, δOR or μOR-δOR heteromers in mouse ileal longitudinal muscle (containing the myenteric plexus) (Fig. 7B). In vehicle treated animals levels of δOR appear to be the highest, followed by levels of μOR and μOR-δOR heteromers. These results support findings by a study reporting co-localization of μOR and δOR in myenteric neurons [32]. In addition, we find that chronic treatment with loperamide or eluxadoline does not induce significant changes in the levels of μOR, δOR or μOR-δOR heteromer levels in ileal longitudinal muscle preparations containing myenteric plexus from WT mice.
Fig. 7.
Effect of chronic treatment of loperamide and eluxadoline on body weight (A) and on receptor expression levels in ileal longitudinal muscle (B). Mice were treated with loperamide or eluxadoline (10 mg/kg, p.o., once a day for 5 days), or with 0.5% methylcellulose (0.1 ml/10 g; vehicle). (A) Body weight was measured immediately before the daily administration. Results are the mean ± S.E.M. n= 6–7. (B) On 6th day, ileum was collected (3–4 mice/sample). Membranes (10 μg) from mouse ileal longitudinal muscle (containing the myenteric plexus) were subjected to an ELISA assay in as described in Section 2. Tissues from 3 to 4 individual animals were pooled and collected as one sample. ELISA was performed in triplicate. Results are the mean ± S.E.M. n = 5.
4. Discussion
Several studies have examined the pharmacological profiles of μOR-δOR heteromers (reviewed in [35,36]). Among them a few have demonstrated that occupancy of δOR enhances μOR activity. Thus δOR selective antagonists were shown to enhance μOR ligand binding, μOR ligand-mediated signaling and μOR-mediated (i.e., morphine-mediated) analgesia [7-9,37]. Furthermore, studies showed that mice with reduced δOR levels (through the use of antisense oligonucleotides) or δOR−/− mice did not develop tolerance or dependence to morphine [38,39]. Together these studies indicate that the use of δOR antagonists could lead to a decrease in the adverse effects associated with in vivo administration of μOR agonists. Based on this possibility a number of ligands were synthesized that have dual μOR agonist and δOR antagonist activity [13-15,17,26,40,41]. The earliest studies with a single compound possessing mixed μOR agonistic and δOR antagonistic activities involved peptide ligands [42]. These peptides were found to produce potent antinociception with reduced tolerance compared to morphine and no physical dependence was observed upon chronic administration [42]. Among the non-peptide ligands possessing mixed μOR agonistic and δOR antagonistic activities, 14-alkoxypyridomorphinans have been reported to induce potent antinociception but diminished tolerance development as compared to morphine [13]. However, to date it is not clear as to whether these compounds exert their effects by binding to individual μOR or δOR or by targeting the μOR-δOR heteromer.
In this study, we find that loperamide, a μOR agonist, is more potent but as efficacious as DAMGO (a peptidic μOR agonist) in promoting [35S]GTPγS binding while being more potent but less efficacious at recruiting β-arrestin in μβgalOR cells, suggesting that it exhibits bias towards G-protein mediated signaling. Biased signaling, i.e., the ability of some agonists to preferentially signal via one signaling pathway (for e.g., G protein-mediated) versus another (for e.g., β-arrestin-mediated) is being increasingly reported for G-protein coupled receptors (reviewed in [31]). Interestingly, eluxadoline, a mixed μOR agonist/δOR antagonist acts as a μOR agonist in the absence of δOR, but exerts some of its effects via the μOR-δOR heteromer in the presence of δOR. Importantly, blockade of eluxadoline-mediated signaling by μOR-δOR heteromer selective antibody supports the idea that this compound targets μOR-δOR heteromers. Furthermore, the in vivo findings of the differences in anti-diarrheal effect of lower dose of eluxadoline between WT and δOR−/− mice is consistent with the notion that, eluxadoline, at least in part, targets μOR-δOR heteromers.
Previous studies that tested the anti-diarrheal effects of eluxadoline in either novel-environment stressed mice or in intracolonic mustard oil-induced intestinal inflammatory model found that it reduces GI transit and fecal output [16]. Consistent with this we find that eluxadoline exhibits similar effects on castor oil induced diarrhea (Figs. 4 and 5). However, in the present study we find that the lower dose (5 mg/kg) of eluxadoline slightly reduced fecal output to below the vehicle control in WT mice (i.e., induced constipation). This is in contrast to previous reports showing that eluxadoline even at doses of 5–25 mg/kg did not cause constipation in the novel-environment stressed mice model of diarrhea [16]. These differences could be due to the differences in the stressor used in the studies (novel environment vs. castor oil) and the time period for measuring fecal output since Wade et al. (2012) measured for only 1 h after the novel-environment stress, while we measured for 4 h after castor oil injection. Castor oil is known to release ricinoleic acid followed by alterations in ion transport and water flux in the intestine [43-46] leading to increases in fecal output or diarrhea. In contrast, novel environment stress is a form of psychological stress that is accompanied by behavioral changes like grooming, rearing and sniffing [47-49]. It is known that the novel-environment-induced increase in fecal output is mediated by an increase in corticotropin-releasing hormone and thyrotropin-releasing hormone and by activation of cholinergic and serotonergic neurons [50]. It is possible that these differences as well as changes in the levels and/or activity of intestinal μOR-δOR heteromers under these two assay conditions are responsible for the observed differences.
Relatively few studies have examined the levels of opioid receptor proteins in the GI tract. Using enhanced green fluorescent protein (eGFP)-tagged δOR (δOReGFP) expressing mice, the distribution of δOR was found to be confined to enteric neurons and fibers within the muscularis externa; submucosal plexus and myenteric plexus [32]. This study also showed that, in the myenteric ganglia, over 80% of δOReGFP positive myenteric neurons co-expressed μOR, and 60% of μOR positive neurons co-expressed δOReGFP [32]. This is consistent with a previous study that showed co-localization of μOR and δOR in myenteric neurons by immunohistochemistry [51]. These results suggest that μOR-δOR heteromers are present in the myenteric neurons. In this study, we detected the presence of μOR-δOR heteromers in ileal longitudinal muscle of mice that includes myenteric neurons [24] using μOR-δOR heteromer-selective antibodies. Together these results support the presence of μOR-δOR heteromers in ileal tissue.
Studies have shown that chronic morphine treatment leads to increase in μOR-δOR heteromer levels in select brain regions [10]. Moreover, μOR-δOR heteromerization changes morphine-mediated signaling from G-protein-into β-arrestin mediated which could contribute to side-effects such as the development of analgesic tolerance [11,37,52]. Interestingly, β-arrestin2 knockout mice exhibit less morphine-induced constipation than their WT counterparts [53], indicating an involvement of β-arrestin mediated signaling (potentially via μOR-δOR heteromers) in the constipating effects of morphine [54]. However, chronic morphine administration does not lead to development of tolerance to the constipating side-effect [55-58]. This led us to wonder if intestinal μOR, δOR, and μOR-δOR heteromer levels are altered following chronic treatment with drugs. In this study we did not detect significant changes in μOR, δOR, and μOR-δOR heteromer levels in the intestine following chronic treatment with either loperamide or eluxadoline compared to controls treated with vehicle (Fig. 7). However, preliminary studies detect a slight increase albeit not significant in μOR-δOR heteromer levels in ileum following chronic morphine administration (data not shown). This would suggest a differential regulation of μOR-δOR heteromer function in brain and gut, which is consistent with what has been previously reported [54].
The detection of μOR-δOR heteromers in mouse ileal tissue together with the anti-diarrheal effect of eluxadoline and in vitro data showing that eluxadoline-mediated signaling is reduced by μOR-δOR heteromer-selective antibodies indicates that eluxadoline, at least in part, mediates its effects by targeting μOR-δOR heteromers in the intestine. This would suggest that intestinal μOR-δOR heteromers could be a potential therapeutic target for the treatment of GI tract disorders including IBS-d. However, additional studies examining the level and changes in the localization of μOR-δOR heteromers in the human GI tract following IBS-d are required to demonstrate that the μOR-δOR heteromer is a novel therapeutic target for the treatment of this disorder.
In this study, we find that in the absence of δOR, eluxadoline behaves as a potent μOR agonist. However, co-expression of μOR and δOR alters the signaling profile of eluxadoline that can be partly blocked by μOR-δOR heteromer-selective antibodies. Thus, the actions of eluxadoline could, at least in part, be due to targeting of μOR-δOR heteromers in the gut. In addition, we find that eluxadoline can block castor oil-induced diarrhea in WT mice and this is attenuated in δOR−/− mice indicating the involvement of δOR probably through μOR-δOR heteromerization in the in vivo effects of eluxadoline.
Acknowledgements
We would like to thank Dr. Erin Bobeck for critically reading the manuscript, and Dr. Charles Mobbs for the gift of μOR−/− spinal cords. This study was supported in part by funds from DA-R3708863 and Furiex (to L.D.).
Abbreviations
- GI
gastrointestinal
- IBS-d
irritable bowel syndrome with diarrhea
- μOR
mu opioid receptor
- δOR
delta opioid receptor
- βgal
beta-galactosidase
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- DAMGO
[d-Ala2, N-MePhe4, Gly-ol]-enkephalin
- CB1R
cannabinoid receptor type1
- AT1R
angiotensin II receptor type 1
- ELISA
enzyme-linked immunosorbent assay
- EC50
50% effective concentration
- Emax
maximum effective concentration
- WT
wild-type
- −/−
knockout
- eGFP
enhanced green fluorescent protein
References
- [1].Hall EJ, Sykes NP. Analgesia for patients with advanced disease: I. Postgrad Med J. 2004;80:148–54. doi: 10.1136/pgmj.2003.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Abul-Husn NS, Sutak M, Milne B, Jhamandas K. Augmentation of spinal morphine analgesia and inhibition of tolerance by low doses of mu- and delta-opioid receptor antagonists. Br J Pharmacol. 2007;151:877–87. doi: 10.1038/sj.bjp.0707277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Porreca F, Heyman JS, Mosberg HI, Omnaas JR, Vaught JL. Role of mu and delta receptors in the supraspinal and spinal analgesic effects of [D-Pen2, D-Pen5]enkephalin in the mouse. J Pharmacol Exp Therap. 1987;241:393–400. [PubMed] [Google Scholar]
- [4].Szentirmay AK, Kiraly KP, Lenkey N, Lacko E, Al-Khrasani M, Friedmann T, et al. Spinal interaction between the highly selective mu agonist DAMGO and several delta opioid receptor ligands in naive and morphine-tolerant mice. Brain Res Bull. 2013;90:66–71. doi: 10.1016/j.brainresbull.2012.09.006. [DOI] [PubMed] [Google Scholar]
- [5].Vaught JL, Takemori AE. Differential effects of leucine and methionine en-kephalin on morphine-induced analgesia, acute tolerance and dependence. J Pharmacol Exp Therap. 1979;208:86–90. [PubMed] [Google Scholar]
- [6].Vaught JL, Takemori AE. A further characterization of the differential effects of leucine enkephalin, methionine enkephalin and their analogs on morphine-induced analgesia. J Pharmacol Exp Therap. 1979;211:280–3. [PubMed] [Google Scholar]
- [7].Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci USA. 2004;101:5135–9. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: a role in opiate synergy. J Neurosci Off J Soc Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gomes I, Ijzerman AP, Ye K, Maillet EL, Devi LA. G protein-coupled receptor heteromerization: a role in allosteric modulation of ligand binding. Mol Pharmacol. 2011;79:1044–52. doi: 10.1124/mol.110.070847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated code-gradation. Neuron. 2011;69:120–31. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- [12].Ananthan S. Opioid ligands with mixed mu/delta opioid receptor interactions: an emerging approach to novel analgesics. AAPS J. 2006;8:E118–25. doi: 10.1208/aapsj080114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ananthan S, Saini SK, Dersch CM, Xu H, McGlinchey N, Giuvelis D, et al. 14-Alkoxy- and 14-acyloxypyridomorphinans: mu agonist/delta antagonist opi-oid analgesics with diminished tolerance and dependence side effects. J Med Chem. 2012;55:8350–63. doi: 10.1021/jm300686p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Breslin HJ, Diamond CJ, Kavash RW, Cai C, Dyatkin AB, Miskowski TA, et al. Identification of a dual delta OR antagonist/mu OR agonist as a potential therapeutic for diarrhea-predominant Irritable Bowel Syndrome (IBS-d) Bioorg Med Chem Lett. 2012;22:4869–72. doi: 10.1016/j.bmcl.2012.05.042. [DOI] [PubMed] [Google Scholar]
- [15].Daniels DJ, Lenard NR, Etienne CL, Law PY, Roerig SC, Portoghese PS. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc Natl Acad Sci USA. 2005;102:19208–13. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wade PR, Palmer JM, McKenney S, Kenigs V, Chevalier K, Moore BA, et al. Modulation of gastrointestinal function by MuDelta, a mixed micro opioid receptor agonist/micro opioid receptor antagonist. Br J Pharmacol. 2012;167:1111–25. doi: 10.1111/j.1476-5381.2012.02068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yekkirala AS, Kalyuzhny AE, Portoghese PS. An immunocytochemical-derived correlate for evaluating the bridging of heteromeric mu-delta opioid proto-mers by bivalent ligands. ACS Chem Biol. 2013 doi: 10.1021/cb400113d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen W, Chung HH, Cheng JT. Opiate-induced constipation related to activation of small intestine opioid mu2-receptors. World J Gastroenterol: WJG. 2012;18:1391–6. doi: 10.3748/wjg.v18.i12.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cann PA, Read NW, Holdsworth CD, Barends D. Role of loperamide and placebo in management of irritable bowel syndrome (IBS) Digest Dis Sci. 1984;29:239–47. doi: 10.1007/BF01296258. [DOI] [PubMed] [Google Scholar]
- [20].Talley NJ. Evaluation of drug treatment in irritable bowel syndrome. Br J Clin Pharmacol. 2003;56:362–9. doi: 10.1046/j.1365-2125.2003.01966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hovdenak N. Loperamide treatment of the irritable bowel syndrome. Scand J Gastroenterol Suppl. 1987;130:81–4. doi: 10.3109/00365528709091004. [DOI] [PubMed] [Google Scholar]
- [22].Meuris B. Observational study of travelers’ diarrhea. J Travel Med. 1995;2:11–5. doi: 10.1111/j.1708-8305.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- [23].Dove LS, Lembo A, Randall CW, Fogel R, Andrae D, Davenport JM, et al. Eluxadoline benefits patients with irritable bowel syndrome with diarrhea in a phase 2 study. Gastroenterology. 2013;145:329–3380. doi: 10.1053/j.gastro.2013.04.006. [DOI] [PubMed] [Google Scholar]
- [24].Rang HP. Stimulant actions of volatile anaesthetics on smooth muscle. Br J Pharmacol Chemother. 1964;22:356–65. doi: 10.1111/j.1476-5381.1964.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gomes I, Filipovska J, Devi LA. Opioid receptor oligomerization. Detection and functional characterization of interacting receptors. Methods Mol Med. 2003;84:157–83. doi: 10.1385/1-59259-379-8:157. [DOI] [PubMed] [Google Scholar]
- [26].Gomes I, Fujita W, Gupta A, Saldanha AS, Negri A, Pinello CE, et al. Identification of a mu-delta opioid receptor heteromer-biased agonist with antinociceptive activity. Proc Natl Acad Sci USA. 2013;110:12072–77. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rozenfeld R, Gupta A, Gagnidze K, Lim MP, Gomes I, Lee-Ramos D, et al. AT1R-CB(1)R heteromerization reveals a new mechanism for the pathogenic properties of angiotensin II. EMBO J. 2011;30:2350–63. doi: 10.1038/emboj.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moriya R, Shirakura T, Hirose H, Kanno T, Suzuki J, Kanatani A. NPYY2 receptor agonist PYY(3-36) inhibits diarrhea by reducing intestinal fluid secretion and slowing colonic transit in mice. Peptides. 2010;31:671–5. doi: 10.1016/j.peptides.2009.11.005. [DOI] [PubMed] [Google Scholar]
- [29].Poggioli R, Benelli A, Arletti R, Cavazzuti E, Bertolini A. K+ channel openers delay intestinal transit and have antidiarrheal activity. Eur J Pharmacol. 1995;287:207–9. doi: 10.1016/0014-2999(95)00658-3. [DOI] [PubMed] [Google Scholar]
- [30].Gupta A, Decaillot FM, Gomes I, Tkalych O, Heimann AS, Ferro ES, et al. Conformation state-sensitive antibodies to G-protein-coupled receptors. J Biol Chem. 2007;282:5116–24. doi: 10.1074/jbc.M609254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Gomes I, Wardman J, Stockton SD, Devi LA. G protein-coupled receptor signaling. Morgan & Claypool Publishers LLC; San Rafael: 2013. [Google Scholar]
- [32].Poole DP, Pelayo JC, Scherrer G, Evans CJ, Kieffer BL, Bunnett NW. Localization and regulation of fluorescently labeled delta opioid receptor, expressed in enteric neurons of mice. Gastroenterology. 2011;141:982–91. e1–8. doi: 10.1053/j.gastro.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Croci T, Landi M, Emonds-Alt X, Le Fur G, Maffrand JP, Manara L. Role of tachykinins in castor oil diarrhoea in rats. Br J Pharmacol. 1997;121:375–80. doi: 10.1038/sj.bjp.0701130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Marcais-Collado H, Uchida G, Costentin J, Schwartz JC, Lecomte JM. Naloxone-reversible antidiarrheal effects of enkephalinase inhibitors. Eur J Pharmacol. 1987;144:125–32. doi: 10.1016/0014-2999(87)90510-3. [DOI] [PubMed] [Google Scholar]
- [35].Fujita W, Gomes I, Devi LA. Revolution in GPCR signaling: opioid receptor heteromers as novel therapeutic targets. Br J Pharmacol. 2014 doi: 10.1111/bph.12798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fujita W, Gomes I, Devi LA. Mu-Delta opioid receptor heteromers: new pharmacology and novel therapeutic possibilities. Br J Pharmacol. 2014 doi: 10.1111/bph.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rozenfeld R, Devi LA. Receptor heterodimerization leads to a switch in signaling: beta-arrestin2-mediated ERK activation by mu-delta opioid receptor heterodimers. FASEB J Off Publ Fed Am Soc Exp Biol. 2007;21:2455–65. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Sanchez-Blazquez P, Garcia-Espana A, Garzon J. Antisense oligodeoxynucleotides to opioid mu and delta receptors reduced morphine dependence in mice: role of delta-2 opioid receptors. J Pharmacol Exp Therap. 1997;280:1423–31. [PubMed] [Google Scholar]
- [39].Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–52. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- [40].Harvey JH, Long DH, England PM, Whistler JL. Tuned-affinity bivalent ligands for the characterization of opioid receptor heteromers. ACS Med Chem Lett. 2012;3:640–4. doi: 10.1021/ml300083p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lenard NR, Daniels DJ, Portoghese PS, Roerig SC. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur J Pharmacol. 2007;566:75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- [42].Schiller PW, Fundytus ME, Merovitz L, Weltrowska G, Nguyen TM, Lemieux C, et al. The opioid mu agonist/delta antagonist DIPP-NH(2)[Psi] produces a potent analgesic effect, no physical dependence, and less tolerance than morphine in rats. J Med Chem. 1999;42:3520–6. doi: 10.1021/jm980724+. [DOI] [PubMed] [Google Scholar]
- [43].Ammon HV, Phillips SF. Inhibition of ileal water absorption by intraluminal fatty acids. Influence of chain length, hydroxylation, and conjugation of fatty acids. J Clin Investig. 1974;53:205–10. doi: 10.1172/JCI107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Ammon HV, Thomas PJ, Phillips SF. Effects of oleic and ricinoleic acids on net jejunal water and electrolyte movement. Perfusion studies in man. J Clin Investig. 1974;53:374–9. doi: 10.1172/JCI107569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bright-Asare P, Binder HJ. Stimulation of colonic secretion of water and electrolytes by hydroxy fatty acids. Gastroenterology. 1973;64:81–8. [PubMed] [Google Scholar]
- [46].Tunaru S, Althoff TF, Nusing RM, Diener M, Offermanns S. Castor oil induces laxation and uterus contraction via ricinoleic acid activating prostaglandin EP3 receptors. Proc Natl Acad Sci USA. 2012;109:9179–84. doi: 10.1073/pnas.1201627109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Angrini M, Leslie JC, Shephard RA. Effects of propranolol, buspirone, pCPA, reserpine, and chlordiazepoxide on open-field behavior. Pharmacol Biochem Behav. 1998;59:387–97. doi: 10.1016/s0091-3057(97)00457-7. [DOI] [PubMed] [Google Scholar]
- [48].Stam R, Croiset G, Akkermans LM, Wiegant VM. Effects of novelty and conditioned fear on small intestinal and colonic motility and behaviour in the rat. Physiol Behav. 1995;58:803–9. doi: 10.1016/0031-9384(95)00137-8. [DOI] [PubMed] [Google Scholar]
- [49].Stam R, Croiset G, Akkermans LM, Wiegant VM. Behavioural and intestinal responses to novelty in rats selected for diverging reactivity in the open field test. Behav Brain Res. 1997;88:231–8. doi: 10.1016/s0166-4328(97)00046-6. [DOI] [PubMed] [Google Scholar]
- [50].Okano S, Nagaya H, Inatomi N. Novelty stress increases fecal pellet output in Mongolian gerbils: effects of several drugs. J Pharmacol Sci. 2005;98:411–8. doi: 10.1254/jphs.fp0050353. [DOI] [PubMed] [Google Scholar]
- [51].Gray AC, Coupar IM, White PJ. Comparison of opioid receptor distributions in the rat ileum. Life Sci. 2006;78:1610–6. doi: 10.1016/j.lfs.2005.07.048. [DOI] [PubMed] [Google Scholar]
- [52].Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–8. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- [53].Raehal KM, Walker JK, Bohn LM. Morphine side effects in beta-arrestin 2 knockout mice. J Pharmacol Exp Therap. 2005;314:1195–201. doi: 10.1124/jpet.105.087254. [DOI] [PubMed] [Google Scholar]
- [54].Bohn LM, Raehal KM. Opioid receptor signaling: relevance for gastrointestinal therapy. Curr Opin Pharmacol. 2006;6:559–63. doi: 10.1016/j.coph.2006.06.007. [DOI] [PubMed] [Google Scholar]
- [55].Holzer P, Ahmedzai SH, Niederle N, Leyendecker P, Hopp M, Bosse B, et al. Opioid-induced bowel dysfunction in cancer-related pain: causes, consequences, and a novel approach for its management. J Opioid Manage. 2009;5:145–51. doi: 10.5055/jom.2009.0015. [DOI] [PubMed] [Google Scholar]
- [56].Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic non-malignant pain: systematic review of randomised trials of oral opioids. Arthritis Res Ther. 2005;7:R1046–51. doi: 10.1186/ar1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Tuteja AK, Biskupiak J, Stoddard GJ, Lipman AG. Opioid-induced bowel disorders and narcotic bowel syndrome in patients with chronic non-cancer pain. Neurogastroenterol Motility Off J Eur Gastrointestinal Motility Soc. 2010;22:424–30. e96. doi: 10.1111/j.1365-2982.2009.01458.x. [DOI] [PubMed] [Google Scholar]
- [58].Ross GR, Gabra BH, Dewey WL, Akbarali HI. Morphine tolerance in the mouse ileum and colon. J Pharmacol Exp Therap. 2008;327:561–72. doi: 10.1124/jpet.108.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]