Summary
Background
RELM-β has been implicated in airways inflammation and remodelling in murine models. Its possible functions in human airways are largely unknown. The aim was to address the hypothesis that RELM-β plays a role in extracellular matrix deposition in asthmatic airways.
Methods
The effects of RELM-β gene deficiency were studied in a model of allergen exposure in mice sensitised and challenged with Aspergillus fumigatus (Af). RELM-β expression was investigated in bronchial biopsies from asthmatic patients. Direct regulatory effects of RELM-β on human lung fibroblasts were examined using primary cultures and the MRC5 cell line in vitro.
Results
Sensitisation and challenge of wild-type mice with Af-induced release of RELM-β with a time course coincident with that of procollagen in the airways. Af-induced expression of mRNA encoding some, but not all ECM in the lung parenchyma was attenuated in RELM-β−/− mice. RELM-β expression was significantly increased in the bronchial submucosa of human asthmatics compared with controls, and its expression correlated positively with that of fibronectin and α-smooth muscle actin. In addition to epithelial cells, macrophages, fibroblasts and vascular endothelial cells formed the majority of cells expressing RELM-β in the submucosa. Exposure to RELM-β increased TGF-β1, TGF-β2, collagen I, fibronectin, smooth muscle α-actin, laminin α1, and hyaluronan and proteoglycan link protein 1 (Hapl1) production as well as proliferation by human lung fibroblasts in vitro. These changes were associated with activation of ERK1/2 in MRC5 cells.
Conclusion
The data are consistent with the hypothesis that elevated RELM-β expression in asthmatic airways contributes to airways remodelling at least partly by increasing fibroblast proliferation and differentiation with resulting deposition of extracellular matrix proteins.
Keywords: asthma, collagen, fibronectin, RELM-β, remodelling, TGF-β
Introduction
Resistin-like molecule-β (RELM-β), also known as found in inflammatory zone-2 (FIZZ-2) is a protein belonging to the RELM/FIZZ family of cysteine-rich secretory proteins which share homology with resistin. So far, four members have been identified in this family, namely RELM-α (FIZZ-1), RELM-β (FIZZ-2), resistin (FIZZ-3) and RELM-γ [1, 2]. The RELM proteins are 105–114 amino acids in length with three domains: an N-terminal signal sequence, a variable middle portion and a highly conserved C-terminal signature sequence that constitutes nearly half of the molecule [3]. It is speculated that the highly conserved signature region contributes to binding to a related family of receptors that is yet to be discovered. Resistin has been implicated in the pathogenesis of obesity-associated insulin resistance and type 2 diabetes mellitus [4–6]. Human and mouse RELM-β are highly conserved, especially in the C-terminus that is most similar to resistin [3].
RELM-β has been detected in structural cells (epithelial, fibroblast and bronchial smooth muscle) of human airways in the context of scleroderma-associated pulmonary hypertension, consistent with a possible role in vascular remodelling [7]. Similarly, RELM-α has also been described in mice as a hypoxia-inducible mitogenic factor implicated in vascular remodelling and angiogenesis [8]. The fact that RELM-β can be detected in the lumen of the bowel, for example in experimental murine colitis [9], suggests that it is secreted (although this may reflect leakage from blood vessels). In mice, increased expression of RELM-α/FIZZ-1 has been described in association with pulmonary inflammation [10] and RELM-β more specifically with murine models of allergic bronchial inflammation [11]. Although the human RELM-β gene is the closest homologue of murine RELM-α, it is not considered to be orthologous: in fact, the RELM-α gene does not appear to exist in humans [12].
Airways fibrosis, with increased subepithelial matrix deposition is thought to be one fundamental feature of airways remodelling in obstructive airways diseases such as asthma and may at least theoretically compromise reversibility of airways obstruction. Given the aforementioned evidence that RELM-β may be produced in the context of bronchial mucosal inflammation and effect remodelling changes, we hypothesised that RELM-β plays a significant role in bringing about increased deposition of matrix proteins in the airways in asthma by activating local fibroblasts.
To assess the functional contribution of RELM-β to this process, we utilised a single allergen challenge protocol in sensitised mice to examine the alignment of the time course of RELM-β expression and that of procollagen, collagen and matrix protein mRNA expression in the lungs, as well as the effects of RELM-β knockdown. We also examined the expression of RELM-β in the airways of human asthmatic patients as compared to controls and sought correlations with expression of ECM proteins and markers of fibroblast differentiation in vivo. Finally, we assessed the effects of RELM-β on proliferation, myofibroblast differentiation and production of ECM proteins by human airways fibroblasts in vitro.
Materials and methods
Murine model of allergen-induced airways inflammation
Wild-type C57BL/6 (wt) and RELM-β−/− mice used in this study were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and housed under specific pathogen-free conditions. Experiments were performed on animals between 8 and 12 weeks of age. They received water and food ad libitum. The protocol was approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania, where these experiments were performed. The method of sensitisation and challenge with a lyophilised extract of Af has been previously described [13]. Briefly, wild-type mice were sensitised by intraperitoneal injections with 20 μg Af (Hollister-Stier, Spokane, WA, USA) combined with 2 mg alum Al(OH)3 (Imject Alum; Pierce, Rockford, IL, USA) in 100 μL of PBS on days 12 and 7. Nasal challenge was performed on day 0. Separate groups of mice were studied before (day 0) and on days 1, 7 and 10 after the intranasal challenge with Af. RELM-β-gene-deleted mice were raised as previously described [9]. These animals were backcrossed to the C57BL/6 background strain over 10 generations. RELM-β−/− mice appeared to develop normally and bred well, without obvious phenotypic abnormality. Furthermore, these animals showed no alteration in expression of mRNA encoding the other RELM isoforms [9]. Allergen sensitisation and challenge in RELM-β−/− mice were performed in the same manner as with the wild-type mice.
Bronchoalveolar lavage (BAL) was performed as previously described [13]. Total RNA from lung tissue was extracted using the RNeasy Mini Kit (QIAGEN, West Sussex, UK) according to the manufacturer's instructions. Expression of extracellular matrix-related genes was determined by a real-time PCR-based microarray [Extracellular Matrix and Adhesion Molecules PCR Arrays (SA Bioscience Corp., Frederick, MD, USA)] according to the manufacturer's instructions. Results were analysed using the SABioscience PCR Array Data Analysing Software.
Collagen and procollagen assay
Collagen concentrations in murine lung tissues were determined using the SIRCOL collagen assay (Biocolor Ltd., Carrickfergus, Country Antrim BT38 8YF UK) according to the manufacturer's instructions. Briefly, lung tissue samples (approx. 50–100 mg) were homogenised in 0.5 m acetic acid. Sirius red dye binding was determined by absorbance at 540 nm. The amounts of collagen were expressed as μg/mg of total lung protein then as ratios over the naïve control concentrations. Procollagen 1 N-terminal propeptide (PINP) concentrations were measured in BAL fluid using an enzyme immunoassay according to the manufacturer's instructions (Immunodiagnostic Systems Inc., Scottsdale, AZ, USA). In this competitive enzyme immunoassay, BAL samples were concentrated 10 times with Microcon concentrators (Millipore, Billerica, MA, USA) before adding to the antibody-coated plate followed by antibody binding.
Subjects and fibreoptic bronchoscopy
The study was approved by the Ethics Committee of Guy's Hospital, part of King's College London School of Medicine. Each participant provided written informed consent. Asthmatics had a clear history of relevant symptoms, documented reversible airways obstruction (≥12% improvement in FEV1 either spontaneously or after administration of inhaled β2-agonist) and/or histamine PC20 <8 mg/mL measured within 2 weeks prior to biopsy. None had ever smoked and there was no history of other respiratory disease. A total of 41 subjects participated the study, including 23 controls (female/male = 12/11, aged 20–41 years; 15/23 atopic) and 18 asthmatics (female/male = 10/8, aged 20–38 years, 14/18 atopic). Atopy was defined by a positive skin prick test (wheal at 15 min >3 mm in diameter in the presence of positive histamine and negative diluent controls) to extracts of one or more common local aeroallergens. The mean FEV1 of the asthmatics was 80.6 (range 44.6–108.2) % predicted. All subjects were clinically free of respiratory infection and systemic glucocorticoid therapy for at least 1 month prior to the study. Normal control subjects were healthy, lifelong non-smoking volunteers who had no history of lung disease and lung function within the predicted range. No subject was recently receiving therapy for allergic rhinitis (anti-histamine, topical nasal corticosteroid) at the time of the study. Fibreoptic bronchoscopy was performed, and bronchial biopsies were obtained and processed as previously described [14, 15].
Immunohistochemistry and image analysis
RELM-β and collagen I immunoreactivity was detected in sections of bronchial biopsies as previously described [14, 15]. Antibodies included rabbit anti-human RELM-β (1/50), rabbit anti-collagen I (1/2000; Sigma-Aldrich, Gillingham, UK), goat anti-rabbit IgG (1/100; Dako Ltd., Cambridgeshire, UK), horseradish peroxidase (HRP)-conjugated rabbit anti-goat (1/100, Dako), mouse anti-human fibronectin (1/200) and α-SMA (1/400) (both from Sigma). Immunoreactivity was visualised using 3.3′-diaminobenzidine (DAB) (Sigma). Staining following absorption of the primary antibody with the relevant purified human RELM-β (Abcam, Cambridge, UK) or collagen I ligand (Abcam) was used as a negative control. To characterise the phenotypes of cells expressing RELM-β, sequential immunohistochemical staining was performed as previously described [14, 15]. Murine monoclonal antibodies directed against cellular phenotypic markers were purchased from Dako (macrophage CD68, 1/50; endothelial cells CD31, 1/30; mast cell tryptase, 1/50; neutrophil elastase, 1/50), Becton Dickinson (Oxford, UK) (T-cell CD3, 1/10) and Abcam plc (human fibroblasts 5B5, 1/50). A monoclonal antibody against eosinophil major basic protein was a kind gift from A.B. Kay (Faculty of Medicine, Imperial College, London, UK) [15].
Immunostaining was quantified objectively by two independent observers ignorant of the origins of the sections using an Olympus BX40 microscope connected with a Zeiss Vision KS300 imaging system (Carl Zeiss, Germany) [14, 15]. The coefficients of variation between the two observers for all markers examined ranged from 4.1 to 6.4 and 4.5 to 6.9, respectively. Data were expressed as the numbers of RELM-β immunoreactive cells per mm2 of submucosa and the percentages of the total areas of submucosa expressing collagen I, fibronectin and α-SMA immunoreactivity in the biopsy sections.
Fibroblast cell culture
Primary human bronchial fibroblasts were isolated and outgrown in pure lines according to the methods described elsewhere [16]. Cells were maintained in Dulbecco's modified eagle's medium (DMEM) supplemented with 10% v/v heat-inactivated foetal calf serum (FCS) and antibiotics and antimycotics (100 U/mL penicillin, 100 ng/mL streptomycin and 250 ng/mL amphotericin B, Sigma) in 75-cm2 flasks in a 5% CO2/37°C atmosphere until they reached 80% confluence. Cells between passages 3 and 5 ex vivo were used for experiments. Prior to stimulation with RELM-β and other agents in vitro, the cells were maintained in serum-free conditions for 24 h in Ham's F12 medium (Sigma) containing ascorbic acid (40 μg/mL), 2 mm L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin.
MRC5 cells, a human lung normal foetal fibroblast line (CCL-171) (ECACC, European Collection of Cell Cultures, Salisbury, UK) were grown in DMEM (Sigma) supplemented with 10% FCS, 100 U/mL penicillin and 100 ng/mL streptomycin (pH 7.4) at 37°C in a 5% CO2 humidified atmosphere as directed.
Proliferation assays
Cellular proliferation was measured by uptake of MTT ([4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide) according to the manufacturer's instructions (Sigma). Briefly, aliquots of 10,000 cells were seeded in 100 μL serum-free Ham's F12 medium in wells of 96-well plates overnight and then treated with the same medium containing 0.5% FCS and 0, 1, 10 or 100 ng/mL RELM-β (Abcam PLC) for 48 h. For each concentration of RELM-β, proliferation was measured at least in triplicate. After 48 h, MTT solution was added to the culture medium at 10% of the total culture volume according to the manufacturer's instructions. After incubation for a further 4 h, the extinction of the wells at 570 nm wavelength was measured spectrophotometrically and subtracted from that of a blank containing medium buffer alone. The extinction was proportional to the proliferation rate of the cells.
RELM-β-mediated fibroblast proliferation was further determined by measuring BrdU incorporation using a BrdU-ELISA (Roche Molecular Biochemicals, Mannheim, Germany) according to the manufacturer's instructions. Cells were treated with serum-free Ham's F12 medium containing 0.5% FCS and 0, 1, 10 or 100 ng/mL RELM-β for 48 h and then incubated with BrdU labelling solution for a further 4 h. Following BrdU incorporation, cells were incubated for 90 min with diluted peroxidase-conjugated anti-BrdU antibody. Absorbance values were measured at 450 nm using an ELISA reader (Bio-Rad iMark, Richmond, CA, USA).
Western blot analysis
Control and RELM-β-treated primary human bronchial fibroblasts and MRC5 cells were washed with ice-cold PBS, while culture supernatants were retained. Cells were lysed using 50 μL of lysis buffer (25 mm HEPES, 1.5% Triton X-100, 0.1% SDS, 0.5 m NaCl, 5 mm EDTA, 0.1 mm sodium deoxycholate) (Sigma) containing a protease inhibitor cocktail (Roche, Welwyn, UK) and sodium orthovanadate (10 mm, Sigma) per 106 cells. After a 30-min incubation on ice, the lysates were centrifuged at 14 000 g for 15 min at 4°C, and the protein concentrations in the supernatants were determined using the BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Northumberland, UK). Western blotting was performed as previously described [17, 18]. To detect human α-SMA, collagen I, fibronectin, p-ERK1/2 and ERK1/2 proteins, the nitrocellulose membranes were incubated with corresponding antibodies (anti-α-SMA: 1/3000, Sigma; anti-collagen I: 1/10,000, Abcam PLC; anti-fibronectin: 1/15,000, Sigma; anti-ERK1/2: 1/1000 and anti-p-ERK1/2: 1/1,000, Millipore Corporation, Billerica, MA) [17, 18]. The membranes were then incubated with peroxidase-conjugated rabbit anti-mouse (Sigma; 1/80 000 for detection of anti-α-SMA antibody) or peroxidase-conjugated goat anti-rabbit (Sigma; 1/80 000) antibodies for 1.5 h. The blots were stained with the ECL (enhanced luminol-based chemiluminescent) Western Blotting Substrate kit (Abcam) according to the manufacturer's instructions.
To examine how far the effects of RELM-β on human lung fibroblasts might be mediated by secondary TGF-β1 release, a monoclonal mouse anti-human TGF-β1 antibody (clone 9016; R&D Systems, Oxford, UK) was used to neutralise potential endogenous TGF-β1. Cells were treated with the antibody with or without RELM-β, and expression of α-SMA and phosphorylation of ERK were compared.
Real-time PCR
RNA expression in murine lungs was measured as described above. For cultured human cells, RNA was extracted from freshly prepared cell pellets using the QIAGEN RNeasy Mini Kit (QIAGEN, West Sussex, UK). The samples were digested with DNAase I. One nanogram of RNA per sample was reverse transcribed to cDNA using reverse transcriptase (MMLV-RT, Promega, Hampshire, UK) with 100 ng of random hexamer primer. The sequences of the oligonucleotide primers are presented in Table 1. The cycling conditions were 94 °C for 5 min, followed by 94 °C for 50 s, 57 °C for 50 s, 72 °C for 50 s and a final extension with 72 °C for 5 min. The cycle numbers were 20 cycles for 18 S RNA, 25 cycles for TGF-β1, fibronectin, collagen III; 30 cycles for TGF- β2, laminin α1, and hyaluronan and proteoglycan link protein 1 (Hapl1) and 35 cycles for collagen I. Real-time PCR was performed on the ABI Prism 7700 sequence detection system (PE Applied Biosystems, Buckinghamshire, UK) using primers and probes from Applied Biosystems with 18 S RNA or GAPDH as the internal control. An identical threshold cycle (Ct) was applied for each gene of interest. Relative mRNA expression was calculated using the delta Ct method [17, 18].
Table 1.
The sequences of primers used in this study
| Primers | Sequence 5′ to 3′ |
|---|---|
| TGF-β1 | CCTGCA AGACTATCGACATGGAGCACACGGGTTCAGGTACCGCTTCTC |
| TGF-β2 | ATCCCGCCC ACTTTCTACAGACCATCCA AAGCACGCTTCTTCC |
| Collagen 1 | GGCCTCAAGGTATTGCTGGACAGCCAACAGGACCAGCATCACCAGTGC |
| Collagen III | AACCTGGGCAAGCTGGTCCTTCAGCTGGTTGACCATCACTGCCTCGA |
| Fibronectin | CCG TGGGCAACTCTGTCTGCGGCAGTTGTCACAG |
| Laminin α1 | CAGCGAAACCGGAAGCAAGGAGTATCAAGTCGGTGACCAGCC |
| Hyaluronan and proteoglycan link protein 1 | ATCACAAAGCCCAGAGAGCCCACTGCAGCCTCAGTAGGAC |
| I8S rRNA | TGACTCAACACGGGAAACCTCACGGACATCTAAGGGCATCACAGACC |
ELISA for TGF-β1, TGF-β2, Laminin α1, Hapl1 and RELM-β
Isolated primary human bronchial fibroblasts (80% confluent) were serum starved in serum-free medium for 24 h and then cultured with different concentrations of RELM-β in starving medium for a further 48 h. ELISA (enzyme-linked immunosorbent assay) kits were employed to measure active TGF-β1 and TGF-β2 in the culture supernatants according to the manufacturer's instructions (R&D Systems). Limits of detection were 15.4 pg/mL in each case. ELISA kits for measuring human laminin α1 and Hapl1 were purchased from Novus Biologicals LLC (Cambridge, UK) and antibodies-online GmbH (Aachen, Germany). The limits of detection were 10 pg/mL and 19.5 pg/mL, respectively.
To detect murine RELM-β in BAL fluid, ELISA was performed using a kit from Antigenix America (Huntington Station, NY, USA) according to the manufacturer's instructions.
Statistical analysis
Data were analysed using a statistical package (Minitab Release 7; Minitab Inc., State College, PA, USA). For the bronchial biopsies and BAL, the Mann–Whitney U-test was used for between group comparisons. For PCR, proliferation, ELISA and other assays, the data were analysed by one-way ANOVA followed by the Tukey–Kramer multiple comparison test or Dunnett's test. For all tests, P < 0.05 was considered significantly.
Results
Wild and RELM-β−/− mice
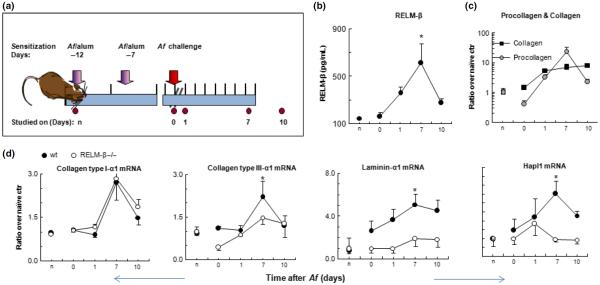
The kinetics of airway remodelling events in response to inflammatory stimulation was studied in mice sensitised and challenged on a single occasion with Aspergillus fumigatus (Af) (Fig. 1). Previous experiments with this model showed that the peak of inflammatory changes in the airways occurs approximately 24 h after nasal challenge and resolves within 7–10 days [19]. ELISA showed that the mean RELM-β protein concentration in BAL fluid was significantly elevated compared with naïve control animals with a peak of 7 days after Af challenge (Fig. 1b, P = 0.007, anova ≤ 0.01), concurrently with elevated expression of lung matrix procollagen (Fig. 1c). Lung matrix collagen content also increased progressively following allergen challenge (Fig. 1c) and showed an 8.0 ± 0.97-fold increase by day 10 (anova < 0.01). Changes in expression of mRNA encoding collagen types I and III showed a similar trend with increases following Af challenge in both wild-type and RELM-β−/− mice, but attenuation in the RELM-β−/− mice was observed only in mRNA encoding type III collagen (Fig. 1d). Interestingly, expression of mRNA encoding laminin-α1 and hyaluronan and proteoglycan link protein 1 (Hapl1) increased in response to Af challenge in the wild-type mice, but the RELM-β−/− mice expressed significantly less laminin-α1 and Hapl1 mRNA compared with the wild-type mice (Fig. 1D, P = 0.0046 and P = 0.049; two-way anova with Bonferroni's correction).
Fig. 1.
Time course of changes in RELM-β expression, procollagen and collagen production and ECM protein mRNA expression in the single challenge murine model. (a) Outline of sensitisation, challenge and sampling protocol in RELM-β−/− and wild-type (wt) C57BL/6 mice (n signifies naïve controls). (b) RELM-β protein concentrations in BAL fluid from wt mice (mean ± SEM of n = 3–6, *P < 0.05; one-way anova). (c) Collagen and procollagen concentrations in lung tissue of wt mice assessed by Sircol assay. Data are expressed as mean ± SEM of fold changes compared with naive control values. (d) Expression of extracellular matrix-related protein mRNA in wild-type and RELM-β-deficient mice (mean ± SEM of n = 3–6). *P < 0.05; one- or two-way anova (comparing wt animals with RELM-β −/− animals) with Bonferroni's correction.
Elevated RELM-β expression is accompanied by airways remodelling and extracellular matrix deposition in the airways of human asthmatic patients
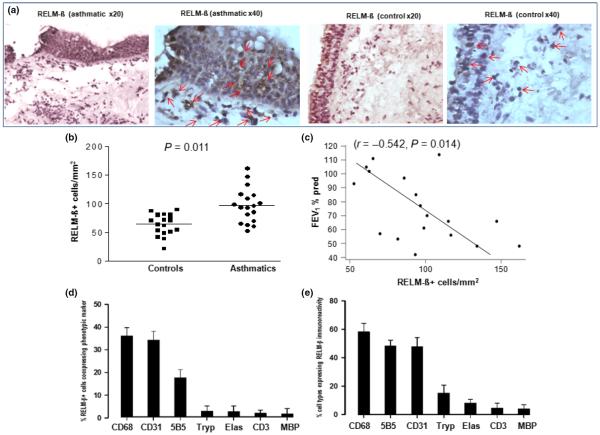
Expression of RELM-β, fibronectin, α-SMA and collagen I was evaluated using immunohistochemistry in sections of bronchial mucosal tissue biopsies obtained from human asthmatic patients and controls. Image analysis showed that the mean numbers of RELM-β+ cells were significantly elevated in the submucosa of the asthmatic patients compared with the controls (P = 0.011) (Fig. 2a,b), while in individual asthmatic patients, the numbers of submucosal RELM-β+ cells inversely correlated with lung function as measured by FEV1 (r = −0.542, P = 0.014, Fig. 2c). Double sequential immunohistochemical staining showed that submucosal RELM-β immunoreactivity colocalised predominantly with CD68+ macrophages, CD31+ vascular endothelial cells and 5B5+ fibroblasts, but much less with other cell types (Fig. 2d,e).
Fig. 2.
(a) Photomicrographs showing immunoreactivity for RELM-β in bronchial biopsy sections from an asthmatic and a control subject. Some RELM-β+ cells are indicated by red arrows. (b) Numbers of RELM-β+ cells in sections of bronchial biopsies from controls (n = 23) and asthmatics (n = 18). (c) Correlation of RELM-β+ cells with lung function (FEV1) of the asthmatics. (d) Mean (±SEM) % of total submucosal RELM-β+ cells accounted by cells of the stated phenotypes. (e) Percentages of total submucosal cells of each phenotype expressing RELM-β (n = 18). CD68, CD68+ macrophages; CD31, CD31+ endothelial cells; 5B5, 5B5+ fibroblasts; Tryp, tryptase+ mast cells; Elas, elastase+ neutrophils; CD3; CD3+ T cells; MBP, MBP+ eosinophils. Mann–Whitney U-test was used for comparison between groups and Spearman's rank-order method with correction was used for the correlations.
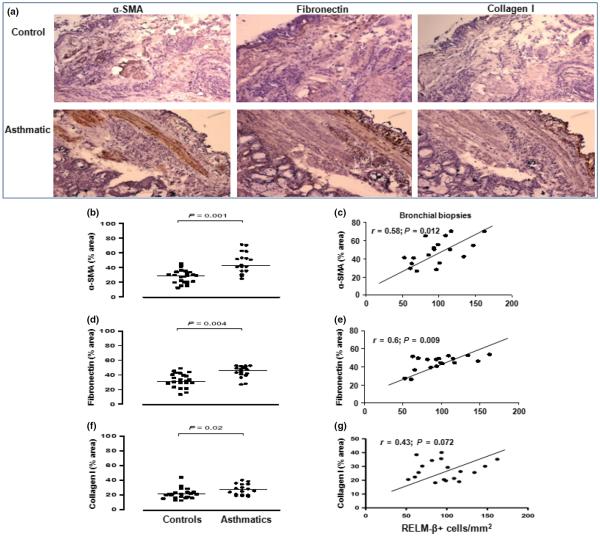
In addition, the mean percentages of the areas of the entire biopsy sections expressing α-SMA, fibronectin and collagen I immunoreactivity were significantly elevated in the asthmatics compared with the controls (Fig. 3b,d,f). The extent of immunoreactivity for RELM-β positively correlated with that of α-SMA (Fig. 2c r = 0.58, P = 0.012), fibronectin (Fig. 3e, r = 0.60, P = 0.009) and, less clearly, collagen I (Fig. 3g, r = 0.43, P = 0.072). A set of representative photomicrographs is shown in Fig. 3a.
Fig. 3.
(a) Photomicrographs showing immunoreactivity for αsmooth muscle actin, fibronectin and collagen I in bronchial biopsy sections from an asthmatic and a control subject. (b, d and f) Global immunoreactivity for α-SMA, fibronectin and collagen I in sections of bronchial biopsies from controls (n = 23) and asthmatics (n = 18). (c, e, g) Correlations between expression of these proteins and RELM-β+ cells in the biopsies from the asthmatics. Mann–Whitney U-test was used for comparison between groups and Spearman's rank-order method with correction was used for the correlations.
RELM-β induces proliferation and expression of α-SMA, collagen, fibronectin, laminin α1 and Hapl1 by human lung fibroblasts
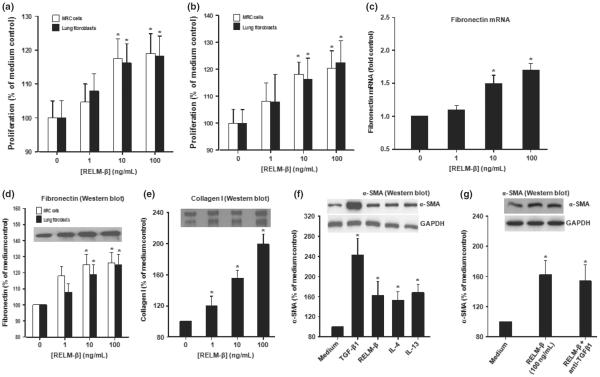
To investigate whether RELM-β can directly affect fibroblast function, we studied isolated primary human lung fibroblasts and the MRC5 human fibroblast line. Exposure of these cells to recombinant RELM-β increased their proliferation in a concentration-dependent fashion over a culture period of 48 h (Fig. 4a,b) (P < 0.05). Further, RELM-β significantly increased production of fibronectin mRNA and protein (Fig. 4c,d), collagen I (Fig. 4e) and α-SMA protein (Fig. 4f) by the primary human cells. This effect of RELM-β was comparable with that of IL-4 and IL-13 at similar concentrations although TGF-β was still more potent (Fig. 4f). Induction of α-SMA by RELM-β was not significantly altered in the presence of a supraphysiological concentration of a functional TGF-β1 blocker (Fig. 4g).
Fig. 4.
RELM-β promotes proliferation of primary human lung and MRC5 fibroblasts, myofibroblast differentiation and extracellular matrix protein production in vitro. (a and b) RELM-β-induced proliferation (48 h) of primary human lung fibroblasts and MRC5 cells (a: MTT assay, b: BrdU assay). (c and d) RELM-β-induced production of fibronectin mRNA (c: real-time PCR, 24 h) and protein (d: Western blot, 48 h) by primary human lung fibroblasts. (e) RELM-β-induced production of collagen I protein (48 h, primary human lung fibroblasts). (f) Western blotting of α-SMA protein induced by cultured primary human lung fibroblasts (48 h) RELM-β, TGF-β1 (positive control), IL-4 and IL-13 (all 10 ng/mL). (g) A blocking anti-TGF-β did not significantly reduce RELM-β-induced production of α-SMA protein. Data are expressed as the mean ± SEM of at least four independent experiments. *P < 0.05 vs. control (Dunnett's test).
RELM-β regulates TGF-β production by human lung fibroblasts
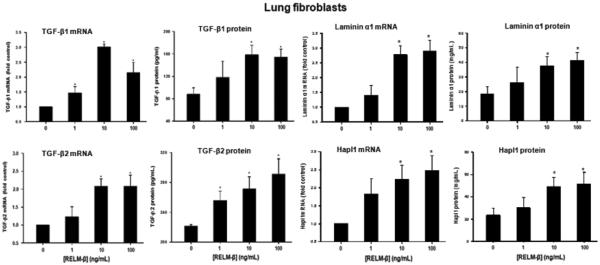
Exposure of primary human airways fibroblasts to RELM-β concentration dependently and significantly increased expression of TGF-β1, TGF-β2, laminin α1 and Hapl1 mRNA and protein (Fig. 5).
Fig. 5.
Effects of RELM-β on TGF-β and extracellular matrix protein expression. RELM-β concentration dependently increased production of TGF-β1, TGF-β2, laminin α1 and Hapl1 mRNA (24 h) and protein (48 h) by cultured primary human fibroblasts as determined by qPCR and ELISA. Data are expressed as the mean ± SEM of at least four independent experiments. *P < 0.05 vs. control (Dunnett's test).
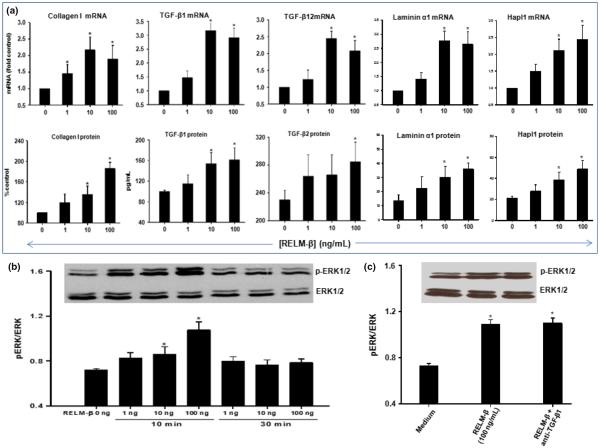
Induction of collagen I, TGF-β1 and TGF-β2 expression by RELM-β is associated with ERK1/2 phosphorylation
As seen in Fig. 6a, RELM-β also concentration dependently increased production of collagen I, TGF-β1, TGF-β2, laminin α1 and Hapl1 mRNA and protein by the human bronchial fibroblast cell line MRC5. We sought evidence of ERK1/2 phosphorylation concomitantly with this. Phosphorylated p44/p42 (pERK1/2) protein was detected using Western blotting of cellular extracts obtained 10 or 30 min after stimulation with different concentrations of RELM-β (Fig. 6b). RELM-β induced concentration-dependent ERK1/2 phosphorylation in MRC5 cells detectable as early as 10 min, but decaying within 30 min after stimulation. Again, blockade of endogenous TGF-β1 did not alter ERK phosphorylation (Fig. 6c).
Fig. 6.
Effects of RELM-β on collagen I, TGF-β1, TGF-β2, laminin α1 and Hapl1 expression were associated with ERK1/2 phosphorylation. (a) Cultured human lung fibroblasts (cell line MRC5) were treated with different concentrations (0, 1, 10 and 100 ng/mL) of RELM-β for 24 h (real-time PCR on expressed RNA) and 48 h (Western blot or ELISA). Data are expressed as the mean ± SEM from at least four independent experiments. *P < 0.05 vs. medium control (Dunnett's test). (b) Phosphorylated p44/p42 (pERK1/2) protein was detected using Western blotting of cellular extracts obtained 10 or 30 min after stimulation with 1, 10 and 100 ng/mL of RELM-β. In the corresponding bottom panel, blots have been probed with antibody to total ERK to show equivalent total protein loading. (c) Blockade of endogenous TGF-β using an anti-TGF-β antibody did not significantly alter RELM-β-induced phosphorylation of pERK1/2. Data are presented as the mean ± SEM of four experiments. *P < 0.05 vs. control (Dunnett's test).
Discussion
RELM-β/FIZZ2 was first identified as a member of the resistin protein family in 2000 [10]. At that time, it was implicated in pulmonary inflammation in animal models. Rothenberg and colleagues [11] then reported that intratracheal administration of RELM-β to mice increased lung collagen deposition and fibrosis, while RELM-β knock-down reduced accumulation of total collagen and goblet cell hyperplasia in an experimental model of allergic airway inflammation [11]. Here, we extend these findings using a single allergen challenge regimen in sensitised animals. While this regimen does not induce airways fibrosis, it does demonstrate that the time course of the changes in expression of RELM-β induced by the challenge coincides with that of activation of remodelling events including increased airways procollagen and collagen production and increased local expression of mRNA encoding a range of extracellular matrix and matrix cross-linking proteins including collagen type I and III, laminin-α1 and Hapl1, while RELM-β knock-down reduces the production of some, but not all of these mRNA species (as one might expect given the wealth of alternative stimuli which may promote the synthesis of these proteins). These studies are clearly consistent with the hypothesis that RELM-β plays a significant role in promoting ECM protein deposition as part of an aberrant `repair process' in allergic airways inflammation. It is of interest that the expression of mRNA encoding collagen I was not attenuated in this model, whereas collagen I is considered a predominant component of ECM protein deposition in human asthma [20–23]. This may represent physiological differences in the deposition of these proteins in man and mouse or, more trivially, a vagary of the particular sensitisation/challenge regimen. Nevertheless, this does not undermine the support of the data for our central hypothesis. It is also of interest that we were able to detect secreted RELM-β protein in airways lumen of these animals. Similarly, in a previous study [18], we were able to detect RELM-β in the airways of human asthmatics.
RELM-β has also been implicated in the mechanism of scleroderma-associated pulmonary hypertension and pulmonary fibrosis in humans [7,24], and we recently showed [18] that it acts on airways epithelial cells to induce mucous hypertrophy and the production of several key remodelling mediators including TGF-β2, EGF and VEGF. The related molecule RELM-α/FIZZ-1, not found in humans, has also been reported to act as an angiogenetic factor under conditions of hypoxia in animals [8].
We have extended these findings in animal models to studies in humans by examining the relationships between the expression of RELM-β and ECM proteins in the airways of human asthmatics as well as the amount of expression of these proteins in the airways of asthmatics as compared to controls. Adding to a previous study [18] in which we demonstrated elevated bronchial epithelial expression of RELM-β in asthmatics compared with controls, we now show that its elevated expression in the submucosa correlates with that of the ECM proteins fibronectin and, less clearly, collagen type I, and also with that of smooth muscle α-actin, a marker of myofibroblast differentiation. These observations are consistent with the hypothesis that RELM-β at least partly regulates ECM lay down in human asthma in vivo. We have further substantiated this hypothesis by showing that RELM-β promotes proliferation, myofibroblast differentiation and secretion of extracellular matrix and matrix binding proteins by human fibroblasts in vitro with a profile comparable to that observed in the murine experiments as well as the key remodelling mediators TGF-β1 and TGF-β2.
In addition to epithelial cells, our data highlight submucosal fibroblast and endothelial structural cells, as well as macrophages and other infiltrating leucocytes as potential sources of RELM-β in human asthmatic airways. In the previous animal study quoted above by Dr Rothenberg and colleagues [11], it was shown that RELM-β, at least at the level of mRNA was expressed most prominently by airways epithelial cells and, to a lesser extent, infiltrating leucocytes in the airways of mice following sensitisation and repeated nasal challenge. Similarly, in a study from Dr Liu and colleagues [24], direct bleomycin challenge by intratracheal injection of the airways was associated with upregulation of RELM-β expression, again principally on epithelial cells. The authors were unable to detect RELM-β mRNA expression in murine fibroblasts in this study. These differences may in part reflect the nature and time course of the inflammatory stimuli used in these models and/or the fact that the physiology of mice and humans differs. Again, these observations do not detract from support for our central hypothesis.
In this and previous study [18], we observed that, in human asthma, airways expression of RELM-β immunoreactivity showed an inverse correlation with lung function (FEV1), supporting a functional link between asthma severity and RELM-β production. On the other hand, at this single cross-sectional time point, we observed no correlations between the expression of RELM-β or smooth muscle α–actin and reversibility of lung function or indeed between baseline FEV1 and reversibility of lung function. This raises doubt about the precise contribution of components of airways `remodelling' changes to irreversible airways obstruction. Indeed, although these and other commonly accepted features of remodelling (such as smooth muscle hypotrophy and hyperplasia, neoangiogenesis and mucous hyperplasia) have been associated with the severity of airways obstruction at a single time point, they are less clearly associated with longevity of disease, suggesting they are possibly not always cumulative and indeed potentially reversible [25, 26].
Dr Grainge and colleagues reported that RELM-β immunoreactivity was elevated in the bronchial mucosa of atopic asthmatics following allergen inhalation challenge, to an extent correlated with increased basement membrane thickness [27]. In the present study, we observed that RELM-β increased expression of TGF-β1 and TGF-β2 by human lung fibroblasts, which have in turn been shown to stimulate fibroblast production of a number of ECM proteins including collagens, fibronectin and tenascin [28, 29]. In asthma, TGF-β-activated fibroblasts appear to be a major source of collagen synthesis [28], and while collagen is a major component of the ECM, there exists evidence that ordered fibrillar collagen lay down requires the additional presence of multimeric fibronectin [30, 31]. Thus, RELM-β may regulate ECM protein lay down in the asthmatic bronchial mucosa in part or entirely by secondary augmentation of local TGF production, although our data clearly suggest that this effect, at least on fibroblasts, is not mediated entirely through elevated TGF-β (TGF-β1) production. Along with our recent demonstration [18] that RELM-β can also regulate production of epithelial and vascular growth factors by airways epithelial cells, it would appear that there is a wide stage for the possible activities of RELM-β in effecting remodelling changes.
We directed our studies to fibroblasts as these are the principal mesenchymal cells contributing to ECM protein lay down in remodelled airways. This is accompanied [24,32] by myofibroblast differentiation. Myofibroblasts may be viewed as a morphological intermediate of fibroblasts and smooth muscle cells which express smooth muscle α-actin and are thought to be predominant contributors to the production of ECM proteins such as collagen [33]. Increased numbers of both fibroblasts and myofibroblasts have been reported in the bronchial mucosa of severe asthmatics [32–34]. Our observations that RELM-β expression in asthmatic airways correlated with α-SMA expression and that RELM-β induced fibroblast proliferation and α-SMA expression in vitro, and others [24] together strongly suggest that RELM-β induces myofibroblast differentiation of fibroblasts in vitro and in vivo. Again, TGF-β1 is possibly the most efficient known inducer of α-SMA protein synthesis in myofibroblasts [35], and although RELM-β was less potent than TGF-β1 in this regard in the present study, it was comparable in potency to IL-13 and IL-4.
In a recent study [18], we showed that RELM-β activates human bronchial epithelial cells through ERK/MAPK-PI3K phosphorylation. A similar mechanism has been reported for RELM-β-mediated enhancement of human aortic smooth muscle cell proliferation [36]. Similarly, in the present study, we have shown that RELM-β clearly induced ERK phosphorylation in human lung fibroblasts early after exposure, suggesting that in both cell types and RELM-β signals at least partly through ERK. Again, blockade of endogenous TGF-β (TGF-β1) did not significantly alter RELM-β-induced ERK phosphorylation, consistent with the hypothesis that RELM-β acts on its own distinct receptors on these target cells, rather than by enhancing TGF-β production. As these receptors are as yet unidentified, it cannot be determined whether ERK phosphorylation occurs directly or indirectly.
In summary, RELM-β is produced in excess in human asthma and has the potential to play a significant functional role in airways remodelling, partially through its effects in promoting proliferation and myofibroblast differentiation of pulmonary fibroblasts along with primary or secondary (via TGF-β production) induction of ECM production. It is a potential molecular target to prevent or reverse ECM protein lay down as well as mucosal hypertrophy in human asthma.
Acknowledgements
The authors are grateful to Kheem Jones, Cherylin Rein-holtz, Helen Bull, May Rabuya and Dr Leonard Siew in the Department of Asthma, Allergy and Respiratory Science, King's College London, for their help recruiting patients, collecting endobronchial biopsies and documenting clinical information. The authors acknowledge financial support from the Medical Research Council (MRC) and Asthma UK. The authors also acknowledge financial support from the Department of Health via a National Institute for Health Research (NIHR) Comprehensive Biomedical Research Centre award to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust. The Haczku Laboratory is supported by R01AI072197; RC1ES018505; P30 ES013508. Dr. Gary Wu (University of Pennsylvania) is acknowledged for providing breeding pairs of the RELM-β−/− mice and the anti RELM-β antibody. Dr Fang CL was funded by MRC. Drs Fang CL, Yin LJ and Huifen Wu were responsible for working in human bronchial biopsies and in vitro. Dr Ghada Eid recruited subjects involved in the study and took the bronchial biopsies. Drs Satish Sharma and Sonia Kierstein were in charge of the animal work, and Drs Angela Haczku, Chris J Corrigan and Sun Ying were the grant' holders from MRC, contributed to the design and analysis of data and with drafting and final approval of the version to be submitted.
Footnotes
Conflict of interest The authors declare no conflict of interest.
References
- 1.Steppan CM, Lazar MA. The current biology of resistin. J Intern Med. 2004;255:439–47. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 2.Asano T, Sakosda H, Fujishiro M, et al. Physiological significance of resistin and resistin-like molecules in the inflammatory process and insulin resistance. Curr Diabetes Rev. 2006;2:449–54. doi: 10.2174/1573399810602040449. [DOI] [PubMed] [Google Scholar]
- 3.Steppan CM, Brown EJ, Wright CM, et al. A family of tissue-specific resistin-like molecules. Proc Natl Acad Sci USA. 2001;98:502–6. doi: 10.1073/pnas.98.2.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel L, Buckels AC, Kinghorn IJ, et al. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun. 2003;300:472–6. doi: 10.1016/s0006-291x(02)02841-3. [DOI] [PubMed] [Google Scholar]
- 5.Steppan CM, Bailey ST, Bhat S, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–12. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 6.Steppan CM, Lazar MA. Resistin and obesity-associated insulin resistance. Trends Endocrinol Metab. 2002;13:18–23. doi: 10.1016/s1043-2760(01)00522-7. [DOI] [PubMed] [Google Scholar]
- 7.Angelini DJ, Su Q, Yamaji-Kegan K, et al. Resistin-like molecule-beta in scleroderma-associated pulmonary hypertension. Am J Respir Cell Mol Biol. 2009;41:553–61. doi: 10.1165/rcmb.2008-0271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng X, Li D, Champion HC, Johns RA. FIZZ-1/RELM-alpha, a novel hypoxia-induced mitogenic factor in lung with vasoconstrictive and angiogenic properties. Circ Res. 2003;92:1065–7. doi: 10.1161/01.RES.0000073999.07698.33. [DOI] [PubMed] [Google Scholar]
- 9.McVay LD, Keilbaugh SA, Wong TM, et al. Absence of bacterially induced RELM-beta reduces injury in the dextran sodium sulfate model of colitis. J Clin Invest. 2006;116:2914–23. doi: 10.1172/JCI28121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holcomb IN, Kabakoff RC, Chan B, et al. FIZZ-1, a novel cysteine-rich secreted protein associated with pulmonary inflammation, defines a new gene family. EMBO J. 2000;19:4046–55. doi: 10.1093/emboj/19.15.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra A, Wang M, Schlotman J, et al. Resistin-like molecule-beta is an allergen-induced cytokine with inflammatory and remodeling activity in the murine lung. Am J Physiol Lung Cell Mol Physiol. 2007;293:L305–13. doi: 10.1152/ajplung.00147.2007. [DOI] [PubMed] [Google Scholar]
- 12.Yang RZ, Huang Q, Xu A, et al. Comparative studies of resistin expression and phylogenomics in human and mouse. Biochem Biophys Res Commun. 2003;310:927–35. doi: 10.1016/j.bbrc.2003.09.093. [DOI] [PubMed] [Google Scholar]
- 13.Kierstein S, Krytska K, Sharma S, et al. Ozone inhalation induces exacerbation of eosinophilic airway inflammation and hyperresponsiveness in allergensensitized mice. Allergy. 2008;63:438–46. doi: 10.1111/j.1398-9995.2007.01587.x. [DOI] [PubMed] [Google Scholar]
- 14.Ying S, O'Connor B, Ratoff J, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–8. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan CJ, Wang W, Meng Q, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–2415. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Pierzchalska M, Szabo Z, Sanak M, Soja J, Szczeklik A. Deficient prostaglandin E2 production by bronchial fibroblasts of asthmatic patients, with special reference to aspirin-induced asthma. J Allergy Clin Immunol. 2003;111:1041–8. doi: 10.1067/mai.2003.1491. [DOI] [PubMed] [Google Scholar]
- 17.Corrigan CJ, Wang W, Meng Q, et al. T-helper cell type 2 (Th2) memory T cell-protentiating cytokine 25 has the potential to promote angiogenesis in asthma. Proc Natl Acad Sci USA. 2011;108:1579–84. doi: 10.1073/pnas.1014241108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fang C, Meng Q, Wu H, et al. Resistin-like molecule-β (RELM-β) is a human airways remodelling mediator. Eur Respir J. 2011;39:458–66. doi: 10.1183/09031936.00107811. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Almeida FA, Kierstein S, et al. Fas ligand neutralization prolongs airway eosinophilia after allergen challenge in a mouse model. Allergy. 2011;67:328–35. doi: 10.1111/j.1398-9995.2011.02763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergeron C, Tulic MK, Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Can Respir J. 2010;17:e85–93. doi: 10.1155/2010/318029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess JK. The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol Ther. 2009;122:19–29. doi: 10.1016/j.pharmthera.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Laitinen A, Altraja A, Kampe M, Linden M, Virtanen I, Laitinen LA. Tenascin is increased in airway basement membrane of asthmatics and decreased by an inhaled steroid. Am J Respir Crit Care Med. 1997;156:951–958. doi: 10.1164/ajrccm.156.3.9610084. [DOI] [PubMed] [Google Scholar]
- 23.Roberts CR, Burke AK. Remodelling of the extracellular matrix in asthma: proteoglycan synthesis and degradation. Can Respir J. 1998;5:48–50. [PubMed] [Google Scholar]
- 24.Liu T, Baek HA, Yu H, et al. FIZZ-2/RELM-β induction and role in pulmonary fibrosis. J Immunol. 2011;187:450–61. doi: 10.4049/jimmunol.1000964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Muhsen S, Johnson JR, Hamid Q. Remodelling in asthma. J Allergy Clin Immunol. 2011;128:451–62. doi: 10.1016/j.jaci.2011.04.047. [DOI] [PubMed] [Google Scholar]
- 26.Kermode JA, Brown NJ, Hardaker KM, et al. The effect of airway remodelling on airway hyper-responsiveness in asthma. Respir Med. 2011;105:1798–804. doi: 10.1016/j.rmed.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Grainge C, Dulay V, Ward J, et al. Resistin-like molecule-β is induced following bronchoconstriction of asthmatic airways. Respirology. 2012;17:1094–100. doi: 10.1111/j.1440-1843.2012.02215.x. [DOI] [PubMed] [Google Scholar]
- 28.Goulet S, Bihl MP, Gambazzi F, Tamm M, Roth M. Opposite effect of corticosteroids and long-acting beta(2)-agonists on serum- and TGF-beta(1)-induced extracellular matrix deposition by primary human lung fibroblasts. J Cell Physiol. 2007;210:167–176. doi: 10.1002/jcp.20836. [DOI] [PubMed] [Google Scholar]
- 29.Andersson LM, Warburton MJ. Intra-cellular degradation of type I collagen and fibronectin in human lung fibroblasts: evidence against degradation in pre-lysosomal compartments. Biochim Biophys Acta. 1995;1268:27–34. doi: 10.1016/0167-4889(95)00038-t. [DOI] [PubMed] [Google Scholar]
- 30.McDonald JA, Kelley DG, Broekelmann TJ. Role of fibronectin in collagen deposition: Fab' to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J Cell Biol. 1982;92:485–92. doi: 10.1083/jcb.92.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magnusson MK, Mosher DF. Fibronectin: structure, assembly, and cardiovascular implications. Arterioscler Thromb Vasc Biol. 1998;18:1363–70. doi: 10.1161/01.atv.18.9.1363. [DOI] [PubMed] [Google Scholar]
- 32.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 33.Johnson PR, Burgess JK, Underwood PA, et al. Extracellular matrix proteins modulate asthmatic airway smooth muscle cell proliferation via an auto-crine mechanism. J Allergy Clin Immunol. 2004;113:690–6. doi: 10.1016/j.jaci.2003.12.312. [DOI] [PubMed] [Google Scholar]
- 34.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–11. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 35.Xu YD, Hua J, Mui A, O'Connor R, Grotendorst G, Khalil N. Release of biologically active TGF-beta1 by alveolar epithelial cells results in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;2:L527–39. doi: 10.1152/ajplung.00298.2002. [DOI] [PubMed] [Google Scholar]
- 36.Calabro P, Samudio I, Willerson JT, Yeh ET. Resistin promotes smooth muscle cell proliferation through activation of extracellular signal-regulated kinase 1/2 and phosphatidylinositol 3-kinase pathways. Circulation. 2004;110:3335–40. doi: 10.1161/01.CIR.0000147825.97879.E7. [DOI] [PubMed] [Google Scholar]