Abstract
Although neuroblastoma is a common solid organ malignancy in children, primary pancreatic neuroblastoma is a rare entity in children, with very few cases reported in the literature. The case discusses the presentation of a 21-month-old female presenting to the neurology clinic with ataxia and erratic eye movements. Our case illustrates the computed tomography, ultrasound, and scintigraphic findings of primary pancreatic neuroblastoma presenting as opsoclonus–myoclonus syndrome. Computed tomography and ultrasound demonstrated a vascular, enhancing mass in the pancreatic body clearly separate from the adrenal gland. Metaiodobenzylguanidine scan demonstrates focal intense uptake in the pancreatic body. The patient's diagnosis was confirmed with biopsy, and her malignancy responded well to conventional chemotherapy. The case is important in that it demonstrates the unusual imaging appearance of a primary pancreatic neuroblastoma.
Key words: Neuroblastoma, Opsoclonus-myoclonus syndrome, Oncology, Pediatric radiology
Introduction
Neuroblastoma is the third most common pediatric malignancy and the most common extracranial solid organ malignancy in children [1]. Approximately 30-35% of neuroblastomas are extra-adrenal in origin and located in the retroperitoneum. However, these tumors can arise from neural crest cells anywhere in the body. Although a common tumor in general in children, primary pancreatic neuroblastoma is quite rare in children and very few cases have been reported in the literature since 1969, several of which represent pancreatic involvement following relapse or disease progression [2], [3], [4], [5], [6], [7], [8]. We discuss a rare case of neuroblastoma arising primarily from the pancreas and illustrate the findings on computed tomography (CT), ultrasound, and Metaiodobenzylguanidine (MIBG) scan imaging.
Case report
A full-term 21-month-old female presented to the pediatric neurology clinic with chronic ataxia. The symptoms began 9 months before presentation in the neurology clinic. Nine months before presentation, the patient was admitted to the neurology service and was diagnosed with acute cerebellar ataxia. Per the family, the patient never recovered, and the patient did not relearn to walk until 15 months. The patient walked with an unsteady gait and had intermittent jerks while moving. In addition, the patient also had spells of erratic eye movements starting 3 months before presentation in the neurology clinic, occurring several times per day. There were no clinical manifestations or family history of Beckwith–Wiedemann syndrome or neurofibromatosis type 1.
Physical examination demonstrated absent deep tendon reflexes, a downgoing Babinski sign, ataxic wide-based gait, mild hypotonia, and dysmetria when grasping for objects. Two prior brain magnetic resonance imaging studies with and without the use of intravenous contrast (Figs. 1A-D) and electroencephalogram were within normal limits. Given the broad differential for ataxia with areflexia and myoclonus, a thorough laboratory investigation was performed. All of the patient's initial laboratory results were within normal limits with the exception of an elevated urine vanillylmandelic acid, which measured 26.7 mg/g creatinine (normal value, 0-18.8 mg/g creatinine).
Fig. 1.
(A-D) Sagittal T1 (A), axial T2 fluid-attenuation inversion recovery (FLAIR) (B), axial T2 turbo spin echo (TSE) (C), and axial postcontrast T1 (D) images of the brain demonstrate a normal appearing cerebellum.
Thus, a CT of the chest, the abdomen, and the pelvis were performed for the suspicion of neuroblastoma and opsoclonus–myoclonus syndrome. The CT was performed using the routine protocol at our institution using 23 mL of intravenously injected Optiray-320 and imaged 60 seconds following injection in portal venous phase. No oral contrast was administered. The CT revealed a 3.6 × 3.2 × 2.8 cm enhancing mass in the body and the tail of the pancreas encasing the celiac axis (Figs. 2A and B). Please note, the high-attenuation appearance of the kidneys in Figures 2A and B is secondary to window width and/or level. A confirmatory abdominal ultrasound was performed, which demonstrated a lobular, heterogeneous, well-circumscribed mass in the region of the pancreatic tail with increased vascularity on color Doppler images (Figs. 3A and B). Follow-up laboratory studies also were notable for an elevated serum epinephrine at 129 pg/mL (normal value, 0-80 pg/mL). Given the presumed diagnosis of a neuroblastoma, a nuclear medicine MIBG scan was performed. The patient was intravenously injected with 5.2 mCi of I-123 MIBG and imaged at 24 hours postinjection. The images demonstrated intense focal uptake in the region of the pancreatic tail without evidence of distant metastasis (Fig. 4). Although the patient was deemed unresectable based on the results of the imaging, the patient ultimately underwent surgical biopsy for pathologic conformation. The final pathology indicated a poorly differentiated neuroblastoma infiltrating adjacent pancreatic parenchyma (Figs. 5A and B). The tumor was classified as unfavorable histology by the Shimada index and did not demonstrate amplification of the n-myc oncogene. The patient underwent chemotherapy and follow-up CT scans demonstrated decrease in tumor size, with no CT evidence of residual or recurrent disease at 10 months (Figs. 6A-C).
Fig. 2.
(A and B) Axial computed tomography image of the abdomen demonstrating a heterogeneously enhancing mass in the pancreatic body and tail (A) with encasement of the celiac axis (B).
Fig. 3.
(A and B) Ultrasound images demonstrate a heterogeneous mass in the pancreatic body and/or tail (A) with increased vascularity on color Doppler that encases nearby vessels (B).
Fig. 4.
A 24-hour delayed I-123 MIBG images demonstrated focal intense tracer uptake in the region of the pancreatic body and/or tail without metastatic disease.
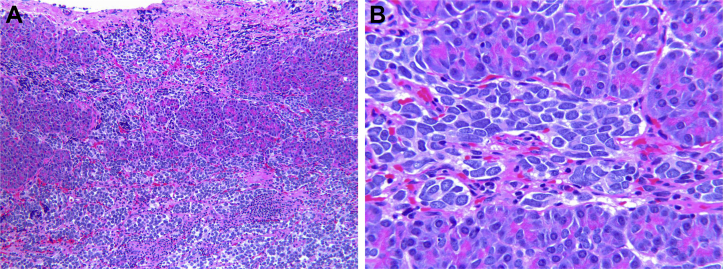
Fig. 5.
(A and B) Histopathology demonstrating sheets of neuroblastoma cells infiltrating pancreatic parenchyma (hematoxylin and eosin stain, × 33 [A], and × 132 [B]).
Fig. 6.
(A-C) Postchemotherapy contrast-enhanced axial (A and B) and coronal (C) computed tomography images of the abdomen and the pelvis demonstrate no evidence of residual disease.
Discussion
Neuroblastoma is the most common solid organ extracranial childhood malignancy and arises from primitive neural crest cells [1]. The typical location of these tumors is the adrenal glands, but neuroblastoma can arise from neural crest cells anywhere in the body, the most commonly the extra-adrenal retroperitoneum and posterior mediastinum. The presence of a neuroblastoma can be suggested with increased urine catecholamines, but ultimately advanced imaging such as CT or magnetic resonance imaging of the chest, the abdomen, and the pelvis is required to search for the primary tumor and for staging if a mass is detected. The diagnosis can also be confirmed by MIBG imaging, which may demonstrate occult metastases. There are 2 well-documented paraneoplastic syndromes associated with neuroblastoma: opsoclonus–myoclonus syndrome and watery diarrhea with hypokalemia syndrome [1]. The exact pathophysiology of opsoclonus–myoclonus syndrome is not known, but speculated to be an autoimmune phenomenon. Although only 2% of neuroblastomas present with opsoclonus–myoclonus syndrome, the clinical diagnosis should prompt a search for neuroblastoma, with approximately 50% of patients presenting with opsoclonus–myoclonus syndrome having neuroblastoma [1]. Watery diarrhea with hypokalemia syndrome is caused by excess secretion of vasoactive intestinal peptides and catecholamines [1].
Primary pancreatic neuroblastoma remains a very rare manifestation of a common disease. However, given the frequency it is encountered in pediatric radiology, it remains important for the radiologist to consider neuroblastoma in any organ with neural crest progenitor cells. As demonstrated in this case, multimodality imaging with radiography, CT, ultrasound, and nuclear medicine is the mainstay of diagnosis, staging, and tracking response to therapy. Imaging features of primary pancreatic neuroblastoma do not differ significantly from neuroblastomas arising in typical locations, so any lesion suspicious for neuroblastoma should be imaged with an appropriate modality and ultimately biopsied if there is any clinical doubt. Elevated urine catecholamines can suggest the presence of neuroblastoma, but a work-up should be undertaken in any patient presenting with either of the afore-mentioned paraneoplastic syndromes.
References
- 1.Blickman J.G., Parker B.R., Barnes P.D. 3rd ed. Mosby Elsevier; Philadelphia, PA: 2009. Pediatric radiology: the requisites. [Google Scholar]
- 2.Kumar H.R., Sandoval J.A., Lovell M.A., Fenton L.Z., Bealer J.F. Primary pancreatic neuroblastoma: an unusual tumor in infancy. J Pediatr Surg. 2010;45(3):642–646. doi: 10.1016/j.jpedsurg.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 3.Hoeffel J.C., Galloy M.A., Schmitt C., Schmitt M. Neuroblastoma of the tail of the pancreas in childhood. Br J Radiol. 1991;64(758):167–168. doi: 10.1259/0007-1285-64-758-167. [DOI] [PubMed] [Google Scholar]
- 4.Galifer R.B., Marguerite G., Balmes M. Ganglioneuroblastome de la tête du pancreas: presentation d'un cas chez un garçon de 2 ans. Chir Pédiatr. 1983;24:396–400. [PubMed] [Google Scholar]
- 5.Bienayme S., Grosse P. Tumeurs malignes du pancréas chez l'enfant. Présentation de 2 cas. Ann Chir Infant. 1975;17:131–155. [Google Scholar]
- 6.Peychlova J. Neuroblastom pankreatu novorozence. Cesk Patol. 1973;9:213–215. [PubMed] [Google Scholar]
- 7.Romualdi C. Su di un caso di ittero da neurinoma del pancreas in una bambina di undici anni trattato con duodeno cefalo-pancreatomia. Riv Chir Pediatr. 1969;11:278–291. [Google Scholar]
- 8.Rosenbaum D.G., Abromson S.J., DeLappe E., Teruya-Feldstein J., La Quaglia M.P. Pancreatic involvement in neuroblastoma with radiologic-pathologic correlation: a single-institution experience. AJR Am J Roentgenol. 2013;201(1):W141–W146. doi: 10.2214/AJR.12.9618. [DOI] [PubMed] [Google Scholar]