Abstract
Young people frequently socialize together in contexts that encourage risky decision making, pointing to a need for research into how susceptibility to peer influence is related to individual differences in the neural processing of decisions during sequentially escalating risk. We applied a novel analytic approach to analyze EEG activity from college-going students while they completed the Balloon Analogue Risk Task (BART), a well-established risk-taking propensity assessment. By modeling outcome-processing-related changes in the P200 and feedback-related negativity (FRN) sequentially within each BART trial as a function of pump order as an index of increasing risk, our results suggest that analyzing the BART in a progressive fashion may provide valuable new insights into the temporal neurophysiological dynamics of risk taking. Our results showed that a P200, localized to the left caudate nucleus, and an FRN, localized to the left dACC, were positively correlated with the level of risk taking and reward. Furthermore, consistent with our hypotheses, the rate of change in the FRN was higher among college students with greater self-reported resistance to peer influence.
Keywords: peer influence, risk taking, Balloon Analogue Risk Task, EEG, feedback-related negativity
Introduction
Because young people are generally healthy, the greatest challenges to their well-being are self-inflicted and preventable inasmuch as they result from decisions to engage in behaviors that increase the risk of harm (Dahl, 2004; Steinberg, 2007). College students in particular are more likely to engage in potentially harmful behaviors like binge drinking, drinking and driving, and risky sexual behavior than both older adults (O’Malley and Johnston, 2002; Wechsler et al., 2003; Simons et al., 2005; Turchik and Garske, 2008; Crowley et al., 2009) and adolescents (Willoughby et al., 2013). Critically, risky decisions are typically made in social settings where peer pressure to conform is prevalent (Simons et al., 2005; Turrisi et al., 2006; Willoughby and Carroll, 2009), creating a need for research into how peer influence susceptibility is related to individual differences in the processing of risky decision making.
Although numerous studies have investigated neural components associated with risk-related outcome processing (e.g. Fein and Chang, 2008; Euser et al., 2011, 2013), to the best of our knowledge, no study has assessed changes in these components as a function of sequentially escalating risk and the capacity to resistance to peer influence (RPI; Steinberg and Monahan, 2007). In order to address this limitation, we adapted the Balloon Analogue Risk Task (BART), a risk-taking task that has been shown to correlate with ‘real-world’ risk taking in multiple populations (Lejuez et al., 2002, 2003, 2004; Aklin et al., 2005), and developed a novel EEG/ERP analysis strategy incorporating the moderating role of self-reported RPI (Steinberg and Monahan, 2007).
The BART involves a series of trials within which risk taking is assessed by calculating a participant’s voluntary exit point across a series of incrementally increasing risk levels. At each level, the participant must decide whether to continue ‘pumping up’ a virtual balloon to gain a small monetary reward at the risk of the balloon popping and losing all rewards accumulated that trial, or to terminate the trial and bank that trial’s earnings. Prior research indicates that BART participants typically engage in a series of risk evaluations within each trial (Wallsten et al., 2005; Pleskac and Wershbale, 2014), making the BART a potentially unique risk-related decision-making measure for investigating the neurophysiological underpinnings of sequentially escalating risk.
To link our findings with factors relevant to our target population, our study incorporated a validated measure of RPI (Steinberg and Monahan, 2007) that has been shown in fMRI research to be sensitive to observation of emotion-laden actions in early adolescence (Grosbras et al., 2007). Peer influence consistently predicts engagement in specific risk-taking-related activities such as binge drinking (Weitzman et al., 2003; Eisenberg et al., 2014), risky drinking (Simons-Morton et al., 2005) and drug use (Bahr et al., 2005; Clark and Lohéac, 2007). Although previous work in this area (e.g. Cavalca et al., 2013) has not shown a direct relationship between RPI and behavioral differences in risk taking on the BART, neuroimaging work with young adolescents has shown low RPI to be associated with reduced connectivity between brain regions involved with inhibitory control (dorsal premotor cortex) and decision-making (dorsolateral prefrontal cortex) (Grosbras et al., 2007). These findings suggest that RPI should also be related to individual variability in neural activation during decision-making when risk is escalating.
In our study, we employed an adaptation of the BART that was suited to the exploration of early ERP components. We focused our analysis on two early components that have typically emerged in risk-taking tasks and have been shown to be highly sensitive to risk-parameter-related manipulations, the feedback-related negativity (FRN) (e.g. Kóbor et al., 2015; Takács et al., 2015; Yau et al., 2015) and the P200 (e.g. Polezzi et al., 2008; Xu et al., 2011; Schuermann et al., 2012).
The first component, the FRN, is a negative deflection typically observed at frontocentral sites maximal at 200–300 ms post-feedback onset (Miltner et al., 1997). Holroyd and Coles (2002) proposed that the FRN represents a reward-prediction error component that is more positive when outcomes are better than expected and more negative when they are worse. Although some researchers argue that the FRN is an unsigned outcome deviance evaluation component (see Oliveira et al., 2007; Hauser et al., 2014), Sambrook and Goslin’s (2015) grand-average meta-analysis of more than 25 FRN studies supported Holroyd and Coles’s (2002) interpretation by showing the FRN to be more positive for unexpected relative to expected positive outcomes (Eppinger et al., 2009; Hämmerer et al., 2010; Mason et al., 2012; Zottoli and Grose-Fifer, 2012).
From a signed reward prediction perspective, given that our analysis focused on positive feedback trials where the balloon did not pop, and the fact that the level of risk was steadily increasing, we expected a linear increase in the FRN with increasing risk. This hypothesis assumes, however, that participant expectations of negative outcomes increased in tandem with risk. If participants had no changes in their expectations or were consistently expecting the best outcome, no modulation in the FRN would likely be observed. Based on the FRN’s predicted role as a signed index of outcome expectation, and its likely neural source in the dorsal anterior cingulate cortex (dACC; Hauser et al., 2014), a region proposed to have an important role in the cognitive control of risk taking (Steinberg, 2008), we hypothesized that changes in the FRN with escalating risk would be moderated by RPI.
Specifically, we hypothesized that participants reporting greater RPI would have outcome expectations more consistent with rising BART risk levels, exhibiting increasing positive FRNs upon receiving positive feedback at higher levels of risk. This prediction draws on models arguing that maturation in cognitive control systems drives risk-taking behavior regulation (Steinberg, 2008). Prior findings suggest that adolescents high in RPI possess higher connectivity between control and decision-related brain areas (Grosbras et al., 2007) and that peer influence on the BART is moderated by cognitive impulsivity (Cavalca et al., 2013).
The second target component, the cognitive P200, is a positive ERP component occurring in the 150–280 ms post-stimulus interval (Carretié et al., 2001). Although P200 activity is associated with sensory processing, higher-order cognitive processing has also been shown to moderate neural activity within the P200 time window. Polezzi et al. (2008), for example, found an outcome-related P200 component to be more positive for feedback on uncertain as opposed to certain outcomes in a two choice (certain vs uncertain) selection paradigm. Similarly, Schuermann et al. (2012) used a low- vs high-risk paradigm to show a more positive P200 for feedback on high-risk choices independent of outcome valence. Taken as a whole, these findings support the hypothesis that the P200 plays a role in the neural coding of outcome predictability such that it is more positive at higher levels of unpredictability and risk.
In contrast with the FRN, it is less clear whether RPI should moderate the P200. A moderating role would imply that individuals either particularly vulnerable or particularly RPI are less calibrated to risk levels. Neither of these hypotheses is supported by past investigations (Cavalca et al., 2013) or general risk-perception findings across older and younger populations, which are likely to vary systematically in regard to susceptibility to peer influence (Beyth-Marom et al., 1993; Millstein and Halpern-Felsher, 2002). Accordingly, RPI was not hypothesized to modulate the P200 and thus the encoding of outcome predictability.
Methods
Participants
Thirty-one participants (18 females, four left-handed, mean age = 20.03, s.d. = 1.78, range 18–26) were recruited from a large Midwestern university. The local institutional review board approved all study procedures and subjects earned up to $10 on the task (mean = $9.82). Three participants did not meet the minimum criterion of having at least 12 clean ERP trials and were removed from the dataset, leaving a total of 28 participants (16 females, 4 left-handed, mean age = 20.21, s.d. = 1.77) in the final sample.
Procedure
Participants completed an online questionnaire outside the lab prior to the experiment that included questions pertaining to demographics as well as the 10-item RPI Scale (Cronbach’s α = 0.75; Steinberg and Monahan, 2007). All participants were tested individually in the experimental session with one experimenter monitoring the participant for possible movement artifacts and a second experimenter monitoring the real-time EEG waveforms.
BART task procedure
The BART is a well-established risk-taking propensity measure (Lejuez et al., 2002) that is correlated with ‘real-world’ risk taking in multiple populations (e.g. Cazzell et al., 2012). To adapt this task for ERP collection and to control for the fact that neural activity within both the P200 and FRN time period is influenced by visual characteristics of stimuli (Pierret et al., 1994), we modified the BART in the following ways: First, a fixed 600-ms time window between feedback and subsequent responses was set (i.e. participant could only respond 600 ms after feedback). Second, in order to control for the confounding of sequentially increasing balloon size with each ‘pump’ in the first condition, a second condition was added where the on-screen balloon started out initially large (equivalent to a balloon inflated with 20 pumps) and was subsequently compressed. The two conditions were randomized and differentiated by balloon color (red = inflation, blue = compression) and initial size. This modification ensured that effects due to changes in image size should move in opposite directions when compared across conditions. In the task instructions, participants were explicitly told that both the inflation and compression conditions could lead to explosions. Participants were also given four practice trials, two inflation and two compression, to gain familiarity with the input controls before beginning the actual task. One of the two practice trials in each condition was set to explode after two pumps to ensure that participants were aware that over-pumping in either condition could lead to explosions.
The experimental task consisted of 25 inflation and 25 compression trials. Participants were instructed to press a button on a button box to either inflate or compress the balloon on screen, accumulating $0.05 on that trial for each button press. The participant could stop pumping a balloon, transfer the accumulated monies to a permanent bank and proceed to the next trial at any time. If a balloon exploded, all accumulated monies on that trial were lost and the participant proceeded to the next trial. On each pump, the balloon was set to explode randomly with an a priori probability of 1/(n − number of prior attempts) with n = 20 being the maximum number of pumps allowed in both the inflation and compression conditions. Participants were not informed of this probability structure and were told that their task was to pump/compress the balloon to make it as large/small as they could without popping it.
EEG collection procedure
The EEG data were recorded using a 256 high-density AgCl electrode Hydrocel Geodesic Sensor Net connected to a high-input impedance NetAmps 300 amplifier (Electrical Geodesics Inc.; EGI, Eugene, OR, USA) using Netstation version 4.4.2. Recordings were collected using a vertex sensor (Cz), later re-referenced to an average reference. Electrode impedances were below 60 kΩ, a level appropriate for the high impedance system used. The data were analogue filtered from 0.1 to 100 Hz and digitized at 250 Hz.
EEG preprocessing
In order to explore within-trial changes in feedback components as a function of escalating risk, the ongoing EEG on each BART trial was segmented into individual pumps. ERPs associated with the positive feedback screen (i.e. balloon size change) for each of those pump decisions provided a measure of each participant’s neural response to feedback on their first pump decision, their second pump and so on. To ensure that each response average was calculated from an equal number of segments, trials with at least five responses were selected and segmented to just those first five responses. Segmentation was locked to feedback onset for each 600 ms segment, beginning 100 ms before onset and continuous for 500 ms thereafter.
Segments were re-referenced using ERP PCA Toolkit (Dien, 2010) to an average reference configuration and baseline corrected using the 100 ms pre-stimulus average. The average number of trials used to compute each of the five response averages was 18.6 (s.d. = 2.6) and 18.2 (s.d. = 2.7) in the inflation and compression conditions, respectively.
Segments were then digitally filtered in EEGlab (Delorme and Makeig, 2004) using a 0.3–30 Hz zero-phase shift finite impulse response bandpass filter. EEGlab’s Automatic Artifact Removal (AAR) toolbox (Gomez-Herrero et al., 2006) was then used to remove ocular and electromyographic artifacts using spatial filtering and blind source separation. Bad channels were then identified and interpolated using the ERP PCA Toolkit’s preprocessing functions (Dien, 2010). Bad channels were identified across the entire session via their poor overall correlation (<0.40) between neighboring channels and identified within each segment via either unusually high differences between an electrode’s average voltage and that of their neighbors (>30 µV) or as extreme voltage differences within the electrode (>100 µV min to max). A channel was also marked as bad for the entire session if >20% of its segments were classified as being bad. All identified bad channels were replaced using whole head spline interpolation. After the bad channels were identified and interpolated, trials with >10% interpolated channels were removed from the analysis set.
Results
Behavioral results
In the final trimmed EEG sample, risk-taking levels in the inflation and compression conditions were correlated (r(28) = 0.38, P = 0.045). Participants made an average of 2.43 pumps (s.d. = 0.95) in the inflation condition and 2.25 pumps (s.d. = 0.91) in the compression condition and did not significantly differ from each other, t(30) = 0.93, P = 0.36. Participants successfully cashed out on 86.87% (s.d. = 0.04%) of trials across conditions. Consistent with Cavalca et al. (2013), there was no significant relationship between RPI and risk level in either condition (inflation: r(28) = 0.031, P = 0.876, compression: r(28) = 0.231, P = 0.238).
ERP analysis
To assess the possibility of a noise confound wherein later BART pump responses had a poorer signal-to-noise ratio than earlier ones, the noise in each ERP average was estimated by inverting the polarity of every other trial that contributed to that average. Inverted and un-inverted trials were then re-averaged (Schimmel, 1967) so that consistent ERP signals cancelled out, leaving noise. Pump order was then regressed onto this noise estimate separately by condition. No significant relationship between noise and pump order in either the inflation (F(1,123) = 1.33, P = 0.25) or compression (F(1,123) = 1.97, P = 0.16) conditions was found.
The ERP components were then quantified using temporal–spatial PCA using the ERP PCA Toolkit version 2.43 (Dien, 2010). First, temporal PCA was performed using all time points from each participant’s averaged ERP as variables, and condition and recording sites as observations. Promax rotation was used and 34 temporal factors (the majority representing noise) were extracted based on a 99% variance-accounted-for criterion. The spatial distribution of these factors was then reduced using spatial ICA. This ICA used all recording sites as variables and considered participants, conditions and temporal factor scores as observations. Infomax rotation identified nine spatial factors based on parallel analysis. The covariance matrix and Kaiser normalization were used for both the temporal PCA and spatial ICA steps. To facilitate interpretation, the waveforms for each temporal–spatial factor were reconstructed (i.e. converted to microvolts) by multiplying the factor loadings with their respective factor scores (Dien, 2012).
Based on past ERP work in the area, two ERP components were selected for quantitative analysis based on their topography and temporal time course: First, a posterior component spanning 150–275 ms post-stimulus (peaking at 212 ms), henceforth referred to as a P200; second, a central component spanning 240–300 ms post-stimulus (peaking at 256 ms), henceforth referred to as an FRN. No other components with non-artifactual topographies and sources within the typically observed time windows of the P200 and FRN were observed.
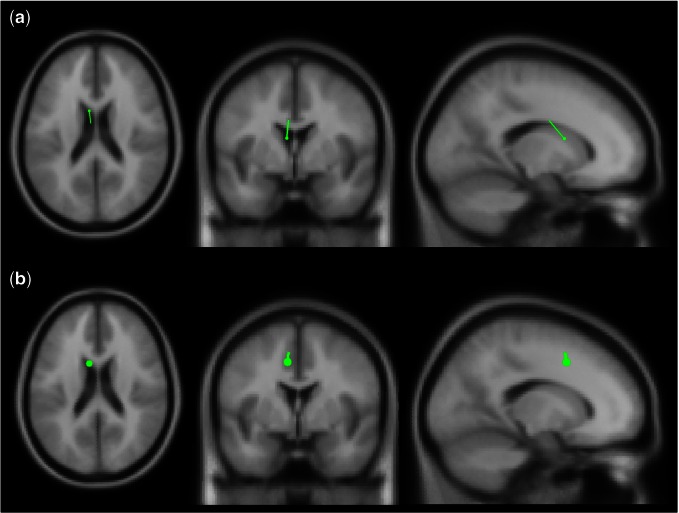
Source localization of the neural sources of these factors was conducted by specifying a pair of hemispheric dipoles (mirrored in position but not orientation) in Oostenveld et al.’s (2010) Fieldtrip using a four-shell model, with a single-dipole model being applied should the two dipoles run into each other. A grid scan first produced a rough estimate of the best starting position after an iterative algorithm identified the position of maximum fit using maximum likelihood (Lütkenhöner, 1998). P200 component source localization was best accounted for by a single dipole model that identified the left caudate nucleus as the most likely neural generator, as shown in Figure 1a. While the source of the decision-making-related P200 is generally not well established, localization of decision-processing-related activity during the P200 time window (i.e. 200 ms) to the left caudate has been demonstrated in recent simultaneous EEG-fMRI localization work (Walz et al., 2013). In addition, Polezzi et al. (2008) localized the P200 in their outcome-predictability-encoding paradigm to the inferior frontal gyrus, an area that has been shown to have functional interactions with the caudate (Robinson et al., 2012; Kireev et al., 2015). Source localization of the neural generator for the FRN factor, Figure 1b, was also best represented by a single dipole model. This model identified the left dACC (Broadmann area 32) as the most likely neural generator for this component, consistent with past EEG (Luu et al., 2003) and simultaneous EEG-fMRI FRN localization studies (e.g., Hauser et al., 2014).
Fig. 1.
Single dipole source localization of (a) the neural generator of the P200 component in the left caudate nucleus and (b) the FRN in the left dACC.
Although localization of ERP components to deep brain sources such as the caudate is a point of slight controversy (e.g., Cohen et al., 2011), there is evidence to suggest that it is possible to record electrical dipoles from the caudate and its neighboring regions using EEG (Rektor, 2002, 2008; Jung et al., 2007), even with only a limited number of trials (Attal et al., 2009; Foti et al., 2011).
P200 temporal–spatial factor results
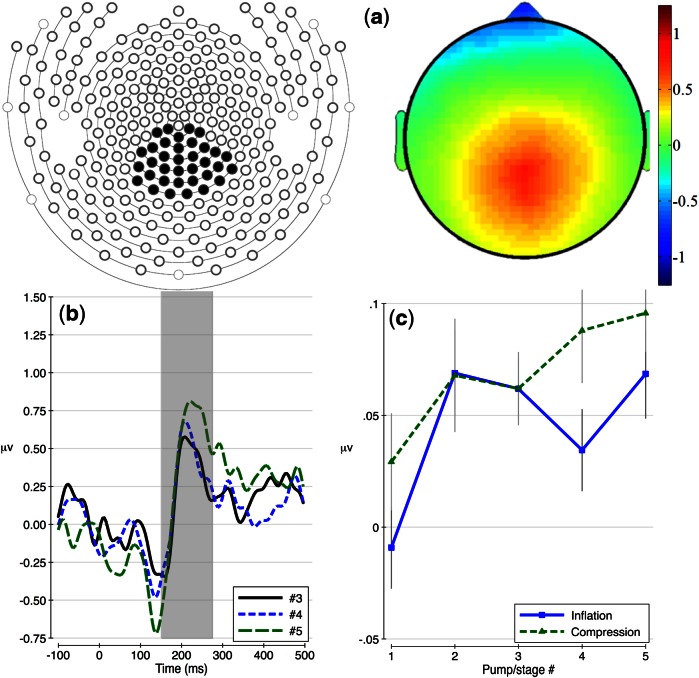
ICA scalp topographies and waveforms depicting the raw ERPs (i.e. before PCA) for the last three responses (i.e. pumps 3, 4 and 5), averaged across conditions and electrodes with loadings above 0.6 on the P200 factor are presented in Figure 2a and b. The relationship between pump order and factor voltage scores on this component was modeled using a random intercept model that allowed individuals to differ in average voltage for this component. A positive relationship between pump order and P200 factor voltage was found for both inflation (F(1,123) = 4.81, P = 0.03) and compression (F(1,123) = 7.29, P = 0.01) conditions. Raw factor voltage means alongside ±1 standard error bars are presented in Figure 2c.
Fig. 2.
P200 temporal–spatial factor results (a) represented as scalp topographies, (b) waveforms prior to PCA and (c) raw factor voltage means with ±1 standard error bars.
RPI was then added to the model as a fully interacting covariate. RPI did not have a significant main effect on P200 factor voltage (inflation: F(1,26) = 0.66, P = 0.43, compression: F(1,26) = 3.72, P = 0.07) and did not significantly moderate the relationship between pump order and factor voltage (inflation: F(1,110) = 2.32, P = 0.13, compression: F(1,110) = 2.27, P = 0.14).
FRN temporal–spatial factor results
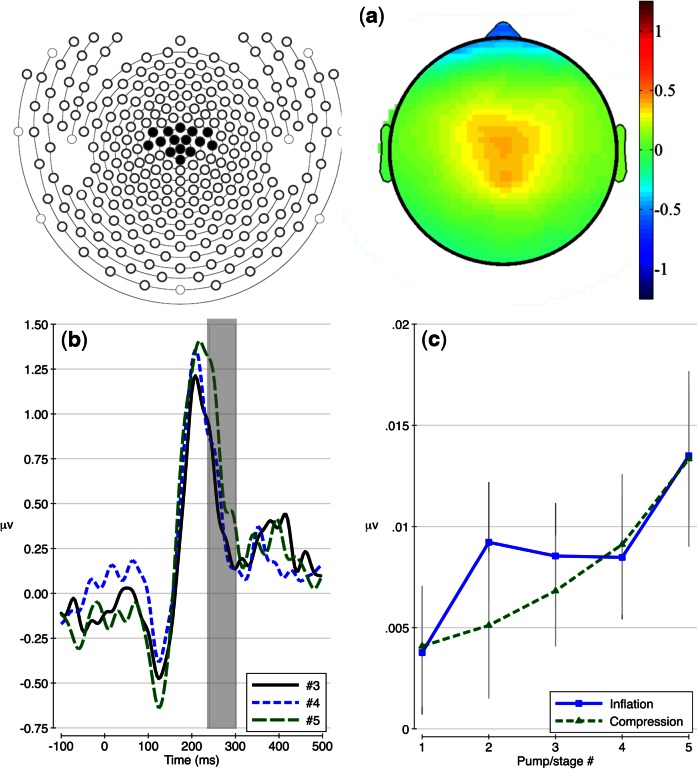
ICA scalp topographies and waveforms depicting the raw ERPs (i.e. before PCA) for the last three responses, averaged across conditions and electrodes with loadings above 0.6 on the FRN factor are shown in Figure 3a and b. The relationship between pump order and factor voltage scores on this component was modeled using a random intercept model, which allowed individuals to differ in average voltage for this component. A positive relationship between pump order and factor voltage was found for both conditions (inflation: F(1,123) = 6.03, P = 0.02, compression: F(1,123) = 5.97, P = 0.02). Raw factor voltage means with ±1 standard error bars are presented in Figure 3c.
Fig. 3.
FRN temporal–spatial factor results (a) represented as scalp topographies, (b) waveforms prior to PCA and (c) raw factor voltage means with ±1 standard error bars.
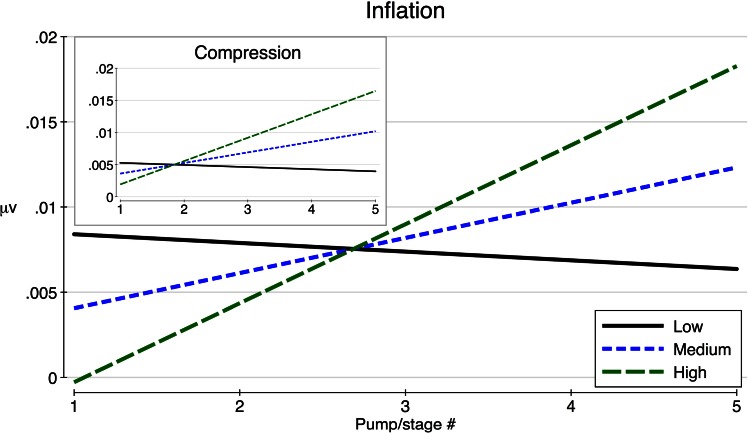
RPI was then added as a fully interacting covariate. RPI did not have a significant main effect on FRN factor voltage (inflation: F(1,26) = 2.63, P = 0.12, compression: F(1,26) = 0.58, P = 0.45). Moderation of the relationship between pump order and FRN amplitude by RPI was statistically significant in the inflation condition, F(1,110) = 6.41, P = 0.01, and showed a trend toward significance (in the expected direction) in the compression condition, F(1,110) = 2.66, P = 0.10. In both conditions, the direction of moderation was positive, such that as hypothesized higher levels of RPI were associated with a higher rate of increase in FRN factor voltage. This relationship is depicted in Figure 4 for high (+1 s.d.), medium (mean) and low (−1 s.d.) RPI levels. At lower levels of RPI, little to no increase in the FRN was predicted in either condition, as shown in Figure 4. Predictions were consistent across inflation and compression conditions.
Fig. 4.
Relationship between balloon pumps and FRN voltage change, moderated by low (−1 s.d.), medium (mean) and high (+1 s.d.) RPI.
Discussion
The aim of this study was to determine how escalating risk-taking levels are reflected in outcome-related ERPs in conjunction with the possible moderating role of RPI. Two ERP components were more positive at higher levels of risk taking, a P200 component source localized to the left caudate nucleus and an FRN component localized to the left dACC. Consistent with our hypotheses, the P200 was not moderated by RPI while the FRN was.
Past studies have shown the P200 to be more positive at higher levels of unpredictability and risk (Polezzi et al., 2008; Schuermann et al., 2012), which is particularly interesting given the localization of the observed P200 component to the caudate nucleus. Activity in the caudate nucleus has been linked with goal-directed behavior and reward-based learning, specifically in regard to both outcome evaluation and the support of appropriately switching behavioral strategies in response to changing contingencies and/or goals (see Grahn et al., 2008 for a review; Haruno et al., 2004). In past feedback-related P200 findings (Polezzi et al., 2008; Schuermann et al., 2012), activity in the left caudate has been associated with feedback processing (Yacubian et al., 2007), specifically in regard to response-related as opposed to observation-based feedback (Cincotta and Seger, 2007). These experimental findings have also been linked with real-world outcomes. Reduced activity in the left caudate during risk taking has been observed among individuals with high, as opposed to low or moderate, social drinking levels (Bednarski et al., 2012). With these findings in mind, it is plausible that the observed feedback-related P200 component activity in our study reflects cognitive control mechanisms related to feedback monitoring and possibly the moderation of appropriate behavioral strategies based on changing reward-to-risk ratios. While no significant moderation of RPI was observed in regard to the P200 effect, given the trend toward significance in some of the observed P-values, it may interesting to investigate this possible relationship in future research with a larger sample size.
The FRN also showed a linear increase with higher risk levels, but was significantly moderated by self-reported RPI such that the FRN was virtually absent at lower RPI levels. The localization of the FRN to the dACC is consistent with both past ERP FRN localization work (Hauser et al., 2014) and the demonstrated decision-making role of the dACC in regard to reward probability and risk in fMRI tasks (Smith et al., 2009). Based on the interpretation of the FRN as a signed reward prediction error component (for a meta-analytic review, see Sambrook and Goslin, 2015), it is possible that individuals in our study who report being highly RPI were more likely to anticipate negative outcomes in regard to risk taking, which would partially account for their ability to RPI to engage in risky behaviors. Alternatively, previous study has shown that FRNs may not be observed when outcome expectations are not being formed (Bismark et al., 2013), in which case individuals who report being highly susceptible to peer influence may simply be failing to consider the potential consequences of their actions. Accordingly, they may be more readily influenced into making risky decisions. A third possibility is that individuals highly susceptible to peer influence may consistently expect more positive outcomes. Dissociating these different explanatory mechanisms is an important goal for future research.
Taken together, the P200 and FRN activity patterns are consistent with a ‘hot–cold’ dual-system risk-taking model. This model views risk taking to be a result of two neural systems, a phylogenetically older, more affective socioemotional system and a phylogenetically younger, more deliberate cognitive-control system (Cohen, 2005; Ernst et al., 2006; Steinberg, 2007, 2008; Casey et al., 2008; Geier and Luna, 2009). The affective system is considered to be fairly automatic and spontaneous and is believed to be driven by, among other regions, midbrain dopaminergic centers. The deliberative system relies on higher-order brain structures including the ACC and PFC (Steinberg, 2008). Developmentally, the affective system is considered to be initially more assertive with the deliberative system gaining strength as adolescents’ age into adulthood. Eventually, arousal-driven inclinations toward risk taking can be modulated more efficiently in late adolescence and adulthood (Giedd, 2004; Casey et al., 2008; Steinberg, 2008; but see Pfeifer and Allen, 2012 for an opposing view). Our college student sample may represent a transitional period in this developmental trajectory with individuals particularly RPI on average demonstrating evidence of more mature control mechanisms.
Our source localization results are also in line with the dual-system model, particularly in regard to cognitive control. The P200 component localized to regions associated with feedback processing and appropriate behavioral strategy selection and regulation (Haruno et al., 2004, Cincotta and Seger, 2007; Yacubian et al., 2007; Grahn et al., 2008). The FRN in turn localized to the dACC. The dACC is known to play an important role within the reward network (Rushworth et al., 2004, 2011; Beckmann et al., 2009; Haber and Knutson, 2009;) and is also proposed to play an important role in response evaluation and adaptation (Williams et al., 2004). Together these two components may represent activity related to the moderation of affective motivation or behavioral strategies based on outcome expectation and feedback processing.
Our findings are also congruent with more fine-grained multiple-system models. These models (e.g. St Onge et al., 2012; see Phelps et al., 2014 for a review) propose multiple modular neural circuits to be differentially recruited in the decision-making process depending on various decision and individual difference-related variables. In this framework, observed changes in the P200 may reflect general activation in neural circuits involved with outcome encoding while the moderating effect of RPI on the FRN may be suggestive of individual differences in the engagement of systems involved in forming outcome expectations. Further research would help clarify whether our findings are reflective of (1) differences in the neural systems being activated, (2) variability in activity in the relevant systems or (3) some combination of the two.
Interestingly, there were minor differences in the regularity of voltage increases in these two components across the inflation and compression conditions, with the inflation condition being less consistent. This result may be due to size-related confounds in the visual system that are present in the inflation but not compression condition. Nevertheless, both the consistent moderation of the FRN component as a function of RPI and the source localization of both the FRN and P200 components support the idea that observed changes in these components are related to higher-order cognitive processing.
In summary, our findings suggest that feedback evaluation and monitoring have an important role in sequential risk taking among college students with additional control mechanisms related to outcome expectation coming into play among individuals with a higher level of RPI. Our results also suggest that analyzing BART data in a sequential fashion, capturing its progressive structure, may provide valuable insights into the temporal neurophysiological dynamics of risk-taking behavior in different populations and across developmental stages.
Given known age-related differences in the neural underpinnings of risk taking (e.g. West et al., 2014), future studies should explore the moderating effects of age on the effects observed in this study. It is also uncertain whether the current findings are specific to RPI or whether they apply more broadly to cognitive control in general in line with work by Kóbor et al. (2015), Fein and Chang (2008) and Yau et al. (2015). Future research addressing these issues, in addition to isolating variables that moderate changes in the P200, would be of significant value. Given our findings regarding the role of RPI in moderating risk-related outcome processing, future work could also extend these findings by directly incorporating peer influence or feedback to increase social-ecological validity (e.g. Parkinson et al., 2012).
Acknowledgements
The authors express their gratitude to Caitlin Hudac, Allison Skinner and the Undergraduate Research Assistants who provided research support over the course of the project.
Funding
This study was supported by generous contributions from the University of Nebraska-Lincoln Office of Research & Economic Development, College of Arts & Sciences, Sociology Department, and Center for Brain, Biology, and Behavior with special thanks to Dennis Molfese and the Developmental Brain Laboratory for training and gratis use of equipment.
Conflict of interest. None declared.
References
- Aklin W.M., Lejuez C.W., Zvolensky M.J., Kahler C.W., Gwadz M. (2005). Evaluation of behavioral measures of risk taking propensity with inner city adolescents. Behaviour Research and Therapy, 43(2), 215–28. [DOI] [PubMed] [Google Scholar]
- Attal Y., Bhattacharjee M., Yelnik J., et al. (2009). Modelling and detecting deep brain activity with MEG and EEG. IRBM, 30(3), 133–8. [DOI] [PubMed] [Google Scholar]
- Bahr S.J., Hoffmann J.P., Yang X. (2005). Parental and peer influences on the risk of adolescent drug use. Journal of Primary Prevention, 26(6), 529–51. [DOI] [PubMed] [Google Scholar]
- Beckmann M., Johansen-Berg H., Rushworth M.F.S. (2009). Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. The Journal of Neuroscience, 29(4), 1175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarski S.R., Erdman E., Luo X., Zhang S., Hu S., Li C.-S.R. (2012). Neural processes of an indirect analog of risk taking in young nondependent adult alcohol drinkers—an fMRI study of the stop signal task. Alcoholism: Clinical and Experimental Research, 36(5), 768–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyth-Marom R., Austin L., Fischhoff B., Palmgren C., Jacobs-Quadrel M. (1993). Perceived consequences of risky behaviors: adults and adolescents. Developmental Psychology, 29(3), 549–63. [Google Scholar]
- Bismark A.W., Hajcak G., Whitworth N.M., Allen J.J.B. (2013). The role of outcome expectations in the generation of the feedback-related negativity. Psychophysiology, 50(2), 125–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretié L., Mercado F., Tapia M., Hinojosa J.A. (2001). Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology, 41(1), 75–85. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124(1), 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalca E., Kong G., Liss T., Reynolds E.K., Schepis T.S., Lejuez C.W., Krishnan-Sarin S. (2013). A preliminary experimental investigation of peer influence on risk-taking among adolescent smokers and non-smokers. Drug and Alcohol Dependence, 129(1–2), 163–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazzell M., Li L., Lin Z.-J., Patel S.J., Liu H. (2012). Comparison of neural correlates of risk decision making between genders: an exploratory fNIRS study of the Balloon Analogue Risk Task (BART). NeuroImage, 62(3), 1896–911. [DOI] [PubMed] [Google Scholar]
- Cincotta C.M., Seger C.A. (2007). Dissociation between striatal regions while learning to categorize via feedback and via observation. Journal of Cognitive Neuroscience , 19(2), 249–65. [DOI] [PubMed] [Google Scholar]
- Clark A.E., Lohéac Y. (2007). “It wasn’t me, it was them!” Social influence in risky behavior by adolescents. Journal of Health Economics, 26(4), 763–84. [DOI] [PubMed] [Google Scholar]
- Cohen J.D. (2005). The vulcanization of the human brain: a neural perspective on interactions between cognition and emotion. Journal of Economic Perspectives , 19(4), 3–24. [Google Scholar]
- Cohen M.X., Cavanagh J.F., Slagter H.A. (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: temporospatial principal components analysis and source localization of the feedback negativity: commentary. Human Brain Mapping, 32(12), 2270–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley M.J., Wu J., Crutcher C., Bailey C.A., Lejuez C.W., Mayes L.C. (2009). Risk-taking and the feedback negativity response to loss among at-risk adolescents. Developmental Neuroscience, 31(1–2), 137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. (2004). Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences , 1021(1), 1–22. [DOI] [PubMed] [Google Scholar]
- Delorme A., Makeig S. (2004). EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Dien J. (2010). The ERP PCA toolkit: an open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods, 187(1), 138–45. [DOI] [PubMed] [Google Scholar]
- Dien J. (2012). Applying principal components analysis to event-related potentials: a tutorial. Developmental Neuropsychology , 37(6), 497–517. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Golberstein E., Whitlock J.L. (2014). Peer effects on risky behaviors: new evidence from college roommate assignments. Journal of Health Economics , 33, 126–38. [DOI] [PubMed] [Google Scholar]
- Eppinger B., Mock B., Kray J. (2009). Developmental differences in learning and error processing: evidence from ERPs. Psychophysiology, 46(5), 1043–53. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine, 36(3), 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser A.S., Evans B.E., Greaves-Lord K., Huizink A.C., Franken I.H.A. (2013). Parental rearing behavior prospectively predicts adolescents’ risky decision-making and feedback-related electrical brain activity. Developmental Science , 16(3), 409–27. [DOI] [PubMed] [Google Scholar]
- Euser A.S., Meel C.S., van, Snelleman M., Franken I.H.A. (2011). Acute effects of alcohol on feedback processing and outcome evaluation during risky decision-making: an ERP study. Psychopharmacology, 217, 111–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G., Chang M. (2008). Smaller feedback ERN amplitudes during the BART are associated with a greater family history density of alcohol problems in treatment-naïve alcoholics. Drug & Alcohol Dependence , 92(1–3), 141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D., Weinberg A., Dien J., Hajcak G. (2011). Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: response to commentary. Human Brain Mapping, 32(12), 2267–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahn J.A., Parkinson J.A., Owen A.M. (2008). The cognitive functions of the caudate nucleus. Progress in Neurobiology , 86(3), 141–55. [DOI] [PubMed] [Google Scholar]
- Geier C., Luna B. (2009). The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior , 93(3), 212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N. (2004). Structural magnetic resonance imaging of the adolescent brain. Annals of the New York Academy of Sciences, 1021(1), 77–85. [DOI] [PubMed] [Google Scholar]
- Gomez-Herrero G., De Clercq W., Anwar H., et al. (2006). Automatic removal of ocular artifacts in the EEG without an EOG reference channel. In: Proceedings of the 7th Nordic Signal Processing Symposium, 2006. NORSIG 2006. 130–3. [Google Scholar]
- Grosbras M.H., Jansen M., Leonard G., et al. (2007). Neural mechanisms of resistance to peer influence in early adolescence. The Journal of Neuroscience, 27(30), 8040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2009). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer D., Li S.C., Müller V., Lindenberger U. (2010). Life span differences in electrophysiological correlates of monitoring gains and losses during probabilistic reinforcement learning. Journal of Cognitive Neuroscience, 23(3), 579–92. [DOI] [PubMed] [Google Scholar]
- Haruno M., Kuroda T., Doya K., et al. (2004). A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. Journal of Neuroscience , 24(7), 1660–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser T.U., Iannaccone R., Stämpfli P., et al. (2014). The feedback-related negativity (FRN) revisited: new insights into the localization, meaning and network organization. NeuroImage, 84, 159–68. [DOI] [PubMed] [Google Scholar]
- Holroyd C.B., Coles G. (2002). The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. [DOI] [PubMed] [Google Scholar]
- Jung K.-Y., Seo D.-W., Na D.L., et al. (2007). Source localization of periodic sharp wave complexes using independent component analysis in sporadic Creutzfeldt–Jakob disease. Brain Research, 1143, 228–37. [DOI] [PubMed] [Google Scholar]
- Kireev M., Medvedeva N., Korotkov A., Medvedev S. (2015). Functional interactions between the caudate nuclei and inferior frontal gyrus providing deliberate deception. Human Physiology , 41(1), 22–6. [PubMed] [Google Scholar]
- Kóbor A., Takács Á., Janacsek K., Németh D., Honbolygó F., Csépe V. (2015). Different strategies underlying uncertain decision making: higher executive performance is associated with enhanced feedback-related negativity. Psychophysiology , 52(3), 367–77. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Aklin W.M., Zvolensky M.J., Pedulla C.M. (2003). Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence, 26(4), 475–9. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Read J.P., Kahler C.W., et al. (2002). Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART). Journal of Experimental Psychology: Applied, 8(2), 75–84. [DOI] [PubMed] [Google Scholar]
- Lejuez C.W., Simmons B.L., Aklin W.M., Daughters S.B., Dvir S. (2004). Risk-taking propensity and risky sexual behavior of individuals in residential substance use treatment. Addictive Behaviors, 29(8), 1643–7. [DOI] [PubMed] [Google Scholar]
- Lütkenhöner B. (1998). Dipole source localization by means of maximum likelihood estimation. I. Theory and simulations. Electroencephalography and Clinical Neurophysiology, 106(4), 314–21. [DOI] [PubMed] [Google Scholar]
- Luu P., Tucker D.M., Derryberry D., Reed M., Poulsen C. (2003). Electrophysiological responses to errors and feedback in the process of action regulation. Psychological Science, 14(1), 47. [DOI] [PubMed] [Google Scholar]
- Mason L., O’Sullivan N., Bentall R.P., El-Deredy W. (2012). Better than I thought: positive evaluation bias in hypomania. PLoS One , 7(10), e47754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millstein S.G., Halpern-Felsher B.L. (2002). Perceptions of risk and vulnerability. The Journal of Adolescent Health , 31(1 Suppl), 10–27. [DOI] [PubMed] [Google Scholar]
- Miltner W.H., Braun C.H., Coles M.G. (1997). Event-related brain potentials following incorrect feedback in a time-estimation task: evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience , 9(6), 788–98. [DOI] [PubMed] [Google Scholar]
- Oliveira F.T.P., McDonald J.J., Goodman D. (2007). Performance monitoring in the anterior cingulate is not all error related: expectancy deviation and the representation of action-outcome associations. Journal of Cognitive Neuroscience , 19(12), 1994–2004. [DOI] [PubMed] [Google Scholar]
- O’Malley P.M., Johnston L.D. (2002). Epidemiology of alcohol and other drug use among American college students. Journal of Studies on Alcohol and Drugs, Supplement , 14, 23–39. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. (2010). FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience, 2011, e156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson B., Phiri N., Simons G. (2012). Bursting with anxiety: adult social referencing in an interpersonal Balloon Analogue Risk Task (BART). Emotion , 12(4), 817–26. [DOI] [PubMed] [Google Scholar]
- Pfeifer J.H., Allen N.B. (2012). Arrested development? Reconsidering dual-systems models of brain function in adolescence and disorders. Trends in Cognitive Sciences, 16(6), 322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E.A., Lempert K.M., Sokol-Hessner P. (2014). Emotion and decision making: multiple modulatory neural circuits. Annual Review of Neuroscience , 37, 263–87. [DOI] [PubMed] [Google Scholar]
- Pierret A., Peronnet F., Echallier J.F. (1994). A behavioral and electrophysiological study of the comparison of size-discrepant shapes. Journal of Physiology-Paris , 88(5), 279–90. [DOI] [PubMed] [Google Scholar]
- Pleskac T.J., Wershbale A. (2014). Making assessments while taking repeated risks: A pattern of multiple response pathways. Journal of Experimental Psychology: General, 143(1), 142–62. [DOI] [PubMed] [Google Scholar]
- Polezzi D., Lotto L., Daum I., Sartori G., Rumiati R. (2008). Predicting outcomes of decisions in the brain. Behavioural Brain Research, 187(1), 116–22. [DOI] [PubMed] [Google Scholar]
- Rektor I. (2002). Scalp-recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators—a hypothesis. Clinical Neurophysiology , 113(12), 1998–2005. [DOI] [PubMed] [Google Scholar]
- Rektor I. (2008). The cognitive event-related potentials in the basal ganglia: invasive studies. In:Inoue, Y., Ikeda, A., editors. Event-Related Potentials in Patients with Epilepsy: From Current State to Future Prospects. Montrouge:. John Libbey Eurotext, 177–90. [Google Scholar]
- Robinson J.L., Laird A.R., Glahn D.C., et al. (2012). The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. NeuroImage , 60(1), 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F.S., Noonan M.P., Boorman E.D., Walton M.E., Behrens T.E. (2011). Frontal cortex and reward-guided learning and decision-making. Neuron , 70(6), 1054–69. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F.S., Walton M.E., Kennerley S.W., Bannerman D.M. (2004). Action sets and decisions in the medial frontal cortex. Trends in Cognitive Sciences, 8(9), 410–7. [DOI] [PubMed] [Google Scholar]
- Sambrook T.D., Goslin J. (2015). A neural reward prediction error revealed by a meta-analysis of ERPs using great grand averages. Psychological Bulletin, 141(1), 213–35. [DOI] [PubMed] [Google Scholar]
- Schimmel H. (1967). The (±) reference: accuracy of estimated mean components in average response studies. Science , 157, 92–4. [DOI] [PubMed] [Google Scholar]
- Schuermann B., Endrass T., Kathmann N. (2012). Neural correlates of feedback processing in decision-making under risk. Frontiers in Human Neuroscience, 6, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L., Lantz V., Klichine S., Ascolese L. (2005). Drinking games, binge drinking and risky sexual behaviors among college students. Journal of Alcohol & Drug Education, 49(3), 23. [Google Scholar]
- Simons-Morton B., Lerner N., Singer J. (2005). The observed effects of teenage passengers on the risky driving behavior of teenage drivers. Accident Analysis & Prevention , 37(6), 973–82. [DOI] [PubMed] [Google Scholar]
- Smith B.W., Mitchell D.G.V., Hardin M.G., et al. (2009). Neural substrates of reward magnitude, probability, and risk during a wheel of fortune decision-making task. NeuroImage, 44(2), 600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Onge J.R., Stopper C.M., Zahm D.S., Floresco S.B. (2012). Separate prefrontal-subcortical circuits mediate different components of risk-based decision making. Journal of Neuroscience, 32(8), 2886–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2007). Risk taking in adolescence new perspectives from brain and behavioral science. Current Directions in Psychological Science, 16(2), 55–9. [Google Scholar]
- Steinberg L. (2008). A social neuroscience perspective on adolescent risk-taking. Developmental Review , 28(1), 78–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L., Monahan K.C. (2007). Age differences in resistance to peer influence. Developmental Psychology, 43(6), 1531–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takács Á., Kóbor A., Janacsek K., Honbolygó F., Csépe V., Németh D. (2015). High trait anxiety is associated with attenuated feedback-related negativity in risky decision making. Neuroscience Letters , 600, 188–92. [DOI] [PubMed] [Google Scholar]
- Turchik J.A., Garske J.P. (2008). Measurement of sexual risk taking among college students. Archives of Sexual Behavior, 38(6), 936–48. [DOI] [PubMed] [Google Scholar]
- Turrisi R., Mallett K.A., Mastroleo N.R., Larimer M.E. (2006). Heavy drinking in college students: who is at risk and what is being done about it? The Journal of General Psychology, 133(4), 401–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsten T.S., Pleskac T.J., Lejuez C.W. (2005). Modeling behavior in a clinically diagnostic sequential risk-taking task. Psychological Review, 112(4), 862–80. [DOI] [PubMed] [Google Scholar]
- Walz J.M., Goldman R.I., Carapezza M., Muraskin J., Brown T.R., Sajda P. (2013). Simultaneous EEG-fMRI reveals temporal evolution of coupling between supramodal cortical attention networks and the brainstem. Journal of Neuroscience , 33(49), 19212–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H., Lee J.E., Nelson T.F., Lee H. (2003). Drinking and driving among college students. American Journal of Preventive Medicine, 25(3), 212–8. [DOI] [PubMed] [Google Scholar]
- Weitzman E.R., Nelson T.F., Wechsler H. (2003). Taking up binge drinking in college: the influences of person, social group, and environment. Journal of Adolescent Health, 32(1), 26–35. [DOI] [PubMed] [Google Scholar]
- West R., Tiernan B.N., Kieffaber P.D., Bailey K., Anderson S. (2014). The effects of age on the neural correlates of feedback processing in a naturalistic gambling game. Psychophysiology, 51(8), 734–45. [DOI] [PubMed] [Google Scholar]
- Williams Z.M., Bush G., Rauch S.L., Cosgrove G.R., Eskandar E.N. (2004). Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neuroscience, 7(12), 1370–5. [DOI] [PubMed] [Google Scholar]
- Willoughby B.J., Carroll J.S. (2009). The impact of living in co-ed resident halls on risk-taking among college students. Journal of American College Health, 58(3), 241–6. [DOI] [PubMed] [Google Scholar]
- Willoughby T., Good M., Adachi P.J.C., Hamza C., Tavernier R. (2013). Examining the link between adolescent brain development and risk taking from a social–developmental perspective. Brain and Cognition, 83(3), 315–23. [DOI] [PubMed] [Google Scholar]
- Xu Q., Shen Q., Chen P., Ma Q., Sun D., Pan Y. (2011). How an uncertain cue modulates subsequent monetary outcome evaluation: an ERP study. Neuroscience Letters, 505(2), 200–4. [DOI] [PubMed] [Google Scholar]
- Yacubian J., Sommer T., Schroeder K., Gläscher J., Braus D.F., Büchel C. (2007). Subregions of the ventral striatum show preferential coding of reward magnitude and probability. NeuroImage , 38(3), 557–63. [DOI] [PubMed] [Google Scholar]
- Yau Y.H.C., Potenza M.N., Mayes L.C., Crowley M.J. (2015). Blunted feedback processing during risk-taking in adolescents with features of problematic internet use. Addictive Behaviors , 45, 156–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zottoli T.M., Grose-Fifer J. (2012). The feedback-related negativity (FRN) in adolescents. Psychophysiology, 49(3), 413–20. [DOI] [PubMed] [Google Scholar]