Abstract
Early life stress (ELS) is strongly associated with negative outcomes in adulthood, including reduced motivation and increased negative mood. The mechanisms mediating these relations, however, are poorly understood. We examined the relation between exposure to ELS and reward-related brain activity, which is known to predict motivation and mood, at age 26, in a sample followed since kindergarten with annual assessments. Using functional neuroimaging, we assayed individual differences in the activity of the ventral striatum (VS) during the processing of monetary rewards associated with a simple card-guessing task, in a sample of 72 male participants. We examined associations between a cumulative measure of ELS exposure and VS activity in adulthood. We found that greater levels of cumulative stress during childhood and adolescence predicted lower reward-related VS activity in adulthood. Extending this general developmental pattern, we found that exposure to stress early in development (between kindergarten and grade 3) was significantly associated with variability in adult VS activity. Our results provide an important demonstration that cumulative life stress, especially during this childhood period, is associated with blunted reward-related VS activity in adulthood. These differences suggest neurobiological pathways through which a history of ELS may contribute to reduced motivation and increased negative mood.
Keywords: early life stress, fMRI, ventral striatum, reward, neurodevelopment
Introduction
Early life stress (ELS) is associated with compromised physical and mental development as well as long-term physical and mental difficulties (Shonkoff et al., 2012). Meta-analyses suggest ELS, such as abuse or neglect, is associated with a 68% increase in anxiety and depression (Norman et al., 2012). Although these linkages have been well studied in psychology, epidemiology and other related disciplines, the biological mechanisms mediating such relations are poorly understood. Identifying such mechanisms is important for better conceptualizing, treating and, ultimately, preventing the negative mental health consequences of ELS.
Initial investigations aimed at understanding the neurodevelopmental linkage between ELS and mental health issues have focused on changes in corticolimbic circuitry, specifically the amygdala, which supports recognition and reaction to threat-related stimuli. However, emerging research suggests ELS may also affect functioning of another affective processing network, the corticostriatal circuit (Southwick et al., 2005). Central in this neural circuitry is the ventral striatum (VS), a subcortical structure supporting motivation and action including reward responsiveness and learning (Berridge and Robinson, 2003). VS dysfunction has been theorized to underlie aspects of affective dysfunction including anhedonia and apathy. Neuroimaging studies have reported decreased reward-related VS activity in depressed individuals (Forbes and Dahl, 2012; Pizzagalli, 2014) and a large body of preclinical data has linked ELS to alterations in reward-related neural circuitry, particularly dopaminergic modulation of VS activity (Pani et al., 2000; Matthews and Robbins, 2003).
Despite these suggestive links, only a small number of descriptive studies have noted differences in corticostriatal circuitry associated with ELS, with lower activity found in samples of children and adolescents exposed to early social deprivation (Mehta et al., 2010; Goff et al., 2013) and in adults who had suffered maltreatment (Dillon et al., 2009). More recently, our research group found that emotional neglect, one form of ELS, is related to a significant blunting of reward-related VS activity from ages 13 to 15, and this developmental blunting predicts affective dysregulation in adolescence (Hanson et al., 2015). These neural differences may result from alterations in the hypothalamic–pituitary–adrenal axis or immune system, which commonly occur after ELS (for review, see Nusslock and Miller, 2015). Associated changes in circulating concentrations of cortisol or pro-inflammatory cytokines may subsequently influence signaling pathways, including dopamine, which directly modulate VS function (Kaufman and Charney, 2001; Brenhouse et al., 2013). Resulting alterations in VS function may have profound behavioral consequences, as lower neural activity in the VS is associated with abnormal responsiveness to rewarding stimuli, decreased motivation, and negative mood.
In service of elucidating mechanisms of risk and resilience, past studies have typically focused on a single risk factor (e.g. physical abuse), tying a specific environmental experience to circumscribed neurobiological and psychological alterations. However, multiple forms of ELS might operate similarly, to lead to altered brain development and later behavioral dysfunction. Thus, models of cumulative risk have been advanced to capture broadly the impact of ELS on later negative outcomes (Evans and Kim, 2010, 2012; Evans et al., 2013). Such models have several advantages over single-risk factor approaches and may more quickly advance efforts to identify particularly vulnerable individuals. First, a number of reports in developmental science have found that cumulative indices of ELS exposure are more effective in predicting negative developmental outcomes than single risk factors in isolation (Greenberg et al., 1999; Evans, 2003; Appleyard et al., 2005). Second, single risk factor approaches may overestimate effect magnitude if the factor being examined is correlated with other risk factors (Evans et al., 2013). Third, cumulative risk approaches parallel neurobiological models such as allostatic load and the aggregated dysregulation across neurobiological and psychological domains (Danese and McEwen, 2012).
Past neuroscience research on ELS is limited in the developmental characterizations of observed effects. Cross-sectional research focused on pediatric populations is unable to forecast whether neurobiological alterations are long-lasting, as brain circuitry may reorganize during adolescence and other developmental periods of change (Kolb and Elliott, 1987; Kolb and Gibb, 1991; Mychasiuk et al., 2014). These studies neglect the possibility that the impact of exposure to stress may vary as a function of the age of this experience. Furthermore, most studies in adults have typically used retrospective reports of early adversity collected at the time of neuroimaging. Thus, the measure of ELS might be biased by the effect of current status on recall such that individuals experiencing heightened psychological distress at the time of measurement are both more likely to recall early adversity and to manifest altered neural function. In addition, the lack of timing specificity of most retrospective ELS measures limits our ability to draw conclusions regarding the effects of stress during specific developmental epochs. The timing of stress exposure may be a critical moderator of the effect of ELS on behavioral development (Manly et al., 2001; Conti et al., 2012; Campbell et al., 2014). Neurobiological alterations may also differ depending on the timing of exposure. Research tracking basic neurobiological development and stress exposure suggests important maturational changes pre- and post-puberty. For example, the amygdala reaches peak gray matter volume between 9 and 11 years (Payne et al., 2009; Uematsu et al., 2012), and amygdala gray matter volume is associated with stress occurring at 10–11 years of age, with adversity during this period contributing to larger amygdala volumes in adulthood (Pechtel et al., 2014).
Despite the growing evidence summarized above suggesting the impact of stress on reward-related brain function, research is lacking on the effects of developmental timing on corticostriatal circuitry. This fact is further surprising given a number of studies suggesting that development of the VS may peak at similar times to the amygdala (Ostby et al., 2009; Mills et al., 2014; cf. Raznahan et al., 2014). Understanding the influence of stress during different developmental epochs may inform the search for strategies to offset the negative sequelae of stress and improve resiliency and wellbeing. Utilizing cohorts followed from early childhood may be particularly powerful to fill in these gaps, as stress exposure at specific developmental epochs could be connected to resultant neurobiology in adulthood (Gilliam et al., 2014; Caldwell et al., 2015).
Here, we report on the relation between cumulative stress exposure during distinct periods in childhood and adolescence on reward-related VS activity in young adulthood assayed in a prospective longitudinal study. We hypothesized relatively blunted VS activity as a function of greater amounts of early cumulative stress. Based on past work tracking potential sensitive periods (Pechtel et al., 2014), we also hypothesized that stress early in development (before the age of 9) would have the greatest impact.
To understand potential relations between cumulative stress and reward-related VS activity more fully, we also conducted two exploratory analyses. First, we examined associations between early stress exposure and neural responses to specific valences of feedback (i.e. positive or negative). Based on past theoretical and empirical reports linking lower VS activity to anhedonic features of depression, we predicted lower VS activity to positive feedback in individuals exposed to higher levels of stress. Second, we explored whether different types of cumulative stress would be related to differences in VS activity, focusing on the effects of interpersonal stressors vs physical/non-social adversities. Based on past work focused on the classes of events that precede affective psychopathology (for review, see Hammen, 2005), we hypothesized that cumulative interpersonal stress would have greater influence on reward-related VS activity than would material stress.
Methods
Participants
Our sample was comprised of a subgroup of participants from the Fast Track Program, a prevention trial implemented in the early 1990s to test whether the outcomes of young children at high risk for long-term antisocial behavior could be improved through a multicomponent behavioral intervention. Participants completed annual assessments starting in kindergarten and lasting through Grade 12. The Fast Track Program has been detailed extensively (e.g. Greenberg et al., 1999; The Conduct Problems Prevention Research Group, 1999, 2002) and comprehensive documentation about the study is available at http://www.fasttrackproject.org and in our Supplementary Materials.
Of the 207 male participants enrolled in the Fast Track study from the Durham site, we successfully contacted 148 and invited them to participate in the study. Ten participants declined. MRI screening procedures disqualified an additional 40 participants; exclusion criteria included current drug or alcohol abuse and standard MRI safety exclusions (e.g. metal implants, history of gunshot). Ninety-eight participants met inclusion criteria and provided informed consent to study procedures, in accordance with the Declaration of Helsinki and university research review committee approval and oversight.
These participants completed a neuroimaging protocol assessing brain structure and function. Of these 98 participants, 26 were excluded from fMRI analyses because of (1) excessive motion during the MRI session (n = 1), (2) inadequate behavioral responding during the reward task (n = 12, for additional details, see Supplementary Materials), (3) missing behavioral data (n = 6) and (4) missing data about stress exposure (n = 7). Quality-control cutoffs and additional information about exclusion are detailed in our discussion of each of these measures later in this section. Resulting data from 72 participants were available for current analyses. Of note, the same cohort used in this study has been used to examine the effects of early physical abuse on corticolimbic circuit function (Albert et al., in prep).
The mean age of participants was 26.3 years (s.d. = 1.1). The majority of participants were African American (91.8%). The subsample of men studied here includes participants from the intervention condition (n = 28) of the Fast Track randomized control trial, in addition to participants from the control (n = 29) and normative (n = 15) groups. Intervention and control participants were those individuals who were screened as high-risk for long-term serious violence at the beginning of the project and then assigned randomly to intervention or control conditions, whereas normative participants were selected to represent the entire population (for additional information, see Supplementary Materials). Of important note, although random assignment to intervention had a long-term effect on internalizing and externalizing outcomes in the full sample of 891 participants, there were no effects of the Fast Track intervention on internalizing or externalizing symptomatology in our neuroimaging subsample (internalizing F(2,69) = 0.07, P = 0.9; externalizing F(2,69) = 0.79, P = 0.45; boxplots shown in Supplementary Materials). This was likely due to MRI screening procedures and exclusion criteria. No differences in total cumulative stress exposure or stress exposure in specific developmental epochs were found among the three groups (Total Cumulative Stress F(2,69) = 0.3, P = 0.7; Cumulative Stress During Early Developmental Epoch F(2,69) = 0.7, P = 0.4; Cumulative Stress During Middle Developmental Epoch F(2,69) = 0.08, P = 0.9; Cumulative Stress During Late Developmental Epoch F(2,69) = 1.3, P = 0.27).
Measures
Beginning in kindergarten and lasting through Grade 12, stressful life events were assessed annually via a 16-item parent report instrument, the Life Changes measure (Dodge et al., 1990; Greenberg et al., 1999). This questionnaire assessed major life stressors experienced by the child during the previous year (e.g. move, medical problems, divorce or separation of parents, death of an important person). Each item was weighted 2 for major events and 1 for minor events, based on parental report of the severity of the event for the family. A sum of the items experienced was computed reflecting life events for each year. Z-scores were then computed and normalized for each year for our sample. We also created a composite stress score, averaging these z-scores from Kindergarten to grade 12. We next created developmental epoch specific z-scores for early (Kindergarten-Grade 3), middle (Grade 4–7) and late (Grade 8–12) eras of childhood. This division was motivated by past research on potential sensitive periods on affective brain circuits and to ensure reliable estimation of stress exposure during development (i.e. similar number of scores included in each epoch). For these analyses, participant data were excluded (full list-wise deletion) if stress data were missing from >50% of any developmental time period (n = 7).
As an exploratory analysis, we also divided our measure of cumulative life stress into meaningful clusters of events, across all of development and also during specific developmental epochs. Motivated by the work of Evans et al. (2013),we grouped the questions on the Life Changes measure into interpersonal vs physical/non-social stressors. Interpersonal stressors included events such as the death of an important person or parental divorce, while events such as moving or major home remodeling were grouped as physical/non-social adversities. We computed z-scores for each of these types of events and normalized for each year in our sample. We then averaged these z-scores from Kindergarten to grade 12 and also created developmental-specific epoch z-scores (for early, middle and late developmental epochs).
To rule out potential confounds, the following covariates were included in all analyses: ethnicity (binary-coded of white/not white), and Fast Track treatment group. Further, to probe the specificity of our effect, we also investigated relations between familial socioeconomic status (SES) in grade 1 (based on Hollingshead, 1975, unpublished data) and current SES (based on a factor-score constructed from level of education and full-time employment, see Supplementary Materials). These analyses examined cumulative stress exposure in a continuous fashion, but additional analyses detailed in the Supplementary Materials also focused on potential ‘threshold’ effects, looking at individuals exposed to extremely high levels of stress.
Measures of internalizing and externalizing symptoms were collected at age 26 using the Adult Self-Report (Achenbach and Rescorla, 2003; additional information in the Supplementary Materials). Additional analyses examining stress exposure and reward-related VS activity, while controlling for psychopathology, are also reported in the Supplementary Materials.
VS activity paradigm
To probe reward-related VS activity, participants completed a commonly used, blocked-design fMRI card guessing game involving positive and negative (i.e. win and loss) feedback. During each task trial, participants guessed whether the value of a visually presented card would be higher or lower than 5. After a choice was made, the numerical value of the card was presented and followed by feedback (green upward-facing arrow for positive feedback; red downward-facing arrow for negative feedback). Each of nine randomly-ordered task blocks comprised five trials. Three blocks consisted of predominantly positive feedback (80% correct) and three of predominantly negative feedback (20% correct) interleaved with three control blocks during which participants made alternating button presses at the presentation of an ‘x’ which was followed by an asterisk and a yellow circle. This blocked-design fMRI task has consistently elicited reward-related VS activity in prior studies (Hariri et al., 2006; Gianaros et al., 2011; Nikolova et al., 2013); however, of note, the task’s blocked design does not allow for the parsing of different components of reward processing (i.e. reward anticipation and outcome). Additional information about this task is available in Supplementary Materials.
MRI acquisition, processing and statistical analyses
Structural and functional MRI data were acquired for each participant using a research-dedicated GE MR750 3T scanner (General Electric Healthcare; Waukesha, WI, USA). Specific information about acquisition parameters are detailed in the Supplementary Materials. Pre-processing and analysis of imaging data were conducted using Analysis of Functional Neuroimages (AFNI; http://afni.nimh.nih.gov; Cox, 1996). Individual subject data were realigned to the first volume in the time series, high-pass filtered, percent signal change normalized, aligned to individual subject high-resolution structural images, spatially smoothed using a Gaussian filter set at 6-mm full-width at half-maximum and then analyzed using a general linear model (GLM). Our GLM included separate regressors for each feedback type (positive, negative, control) convolving a stimulus boxcar function with a canonical hemodynamic response function. These first-level GLMs included nuisance covariates of the second-order polynomial used to model the baseline and slow signal drift, six motion estimate covariates and binary flags corresponding to neuroimaging frames with excessive motion (>2 mm). Participants with >10% of total frames censored due to motion were excluded from all analyses (n = 1). Structural images were then normalized to a standard stereotactic space (Montreal Neurological Institute template) using a non-linear diffeomorphic registration algorithm. The resulting warps were applied to all functional data (re-sampled to 2 mm3). Second-level (main-effect) neuroimaging GLMs were then constructed using mixed effects analysis, with subjects as a random factor. An initial (uncorrected) statistical threshold of P < 0.005 was used and false discovery rate correction based on the extent of suprathreshold voxels (i.e. cluster size) was then applied to control for voxel-wise multiple comparisons, yielding a corrected P = 0.05 (similar to past reports, e.g. Bjork et al., 2007;Wager et al., 2008; Gianaros et al., 2013). These results are detailed in Supplementary Table S1.
We focused on our canonical contrast previously used to assess reward-related VS activity, specifically positive > negative feedback for each participant (Hariri et al., 2006; Gianaros et al., 2011; Nikolova et al., 2013). Mean BOLD values from VS clusters exhibiting a main effect of task were then extracted using AFNI. By extracting VS BOLD parameter estimates from the functional clusters activated by our paradigm rather than clusters specifically correlated with our independent variables of interest (i.e. cumulative stress exposure), we limit potential correlation coefficient inflation. This is similar to past reports from our research group (Hyde et al., 2011; Carre et al., 2012;). Supplemental region of interest derivation using the NeuroSynth platform (www.neurosynth.org; Yarkoni et al., 2011) are also described in the Supplementary Materials.
Regression models were then constructed to examine how stress exposure at different time periods related to VS activity. We entered race (binary coded as white or non-white), treatment group (Fast Track intervention or not) and stress exposure (either our measure of cumulative adversity or at specific developmental epochs) as independent variables. Similar exploratory analyses were conducted using interpersonal and physical/non-social stress variables. Relationships between stress exposure and brain activity across the whole brain are detailed in Supplementary Materials.
To understand the effects of stress exposure on reward-related brain function more fully, we also investigated the effects of feedback valence (positive or negative). For these analyses, we extracted the contrasts of positive feedback > control blocks and negative feedback > control blocks for our VS ROI. Due to the brief nature of our task and limited past research studies focused on such effects, these analyses were considered exploratory.
Results
Descriptive statistics for stress exposure
The means for all stress exposure scores were 0 (s.d. = 1), due to z-transformations. For cumulative stress across childhood and adolescence, the range was −1.74 to 3.425. For each developmental epoch, the ranges were as follows: early developmental epoch −1.713 to 3.19, middle developmental epoch −1.753 to 2.55 and late developmental epoch −1.617 to 3.9.
Stress exposure and reward processing
Consistent with prior work, our contrast produced significant reward-related VS activity (see Supplementary Materials for details). There was no effect of the Fast Track intervention on neural responses to reward (Right VS Positive > Negative Feedback β = −0.08, P = 0.54), but we retained intervention group as a covariate in analyses.
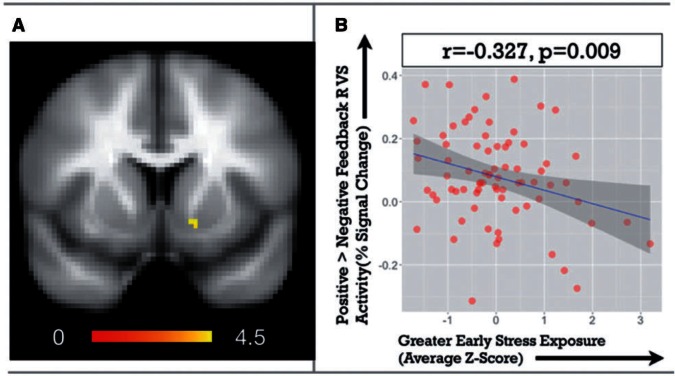
As hypothesized, higher cumulative life stress during childhood and adolescence was associated with blunted VS response to reward (right VS β = −0.26, P = 0.04). Subsequent analyses revealed a specific association between cumulative stress during early childhood and blunted right VS response to reward (β = −0.327, P = 0.009; Figure 1). This association remained significant when controlling for stress exposure later in development (β = −0.32, P = 0.036), which was not significantly associated with VS activity (Middle Developmental Epoch β = 0.015, P = 0.9; Later Developmental Epoch β = 0.08, P = 0.57). A formal test of the difference between correlations indicated the association between VS activity and early stress exposure was different from that between VS activity and stress exposure at other developmental periods (Differences between VS-early stress and VS-middle stress t = −2.81, P = 0.01; differences between VS-early stress and VS-late stress = −3.87, P < 0.001).
Fig. 1.
The left side (A) depicts our right VS region of interest, while the scatterplot for early stress exposure and VS activity is shown on the right side (B). In this scatterplot, right VS activity to positive > negative feedback (as indexed by percent signal change) is shown on the vertical axis and early stress exposure (an average of stress exposure Z-scores) is depicted on the horizontal axis.
Stress exposure and feedback valence
We next examined whether cumulative stress had effects on the processing of positive or negative feedback. Cumulative stress exposure across childhood and adolescence was related to lower right VS signal for positive feedback > control blocks (β = −0.22, P = 0.049). This relation was not seen for right VS signal for negative feedback > control blocks (β = −0.1, P = 0.353). These two correlations were significantly different from one another (t = −3.5, P < 0.001). Similarly, exposure to stress early in development was related to lower right VS signal for positive feedback > control blocks (β = −0.22, P = 0.045). This relation was not seen for right VS signal for negative feedback > control blocks (β = 0.01, P = 0.87) and these two correlations were again significantly different from one another (t = −2.5, P = 0.01).
Analyses focused on specific types of stress
Examining interpersonal vs physical/non-social adversities, we found lower right VS activity in participants exposed to higher interpersonal cumulative life stress across all of childhood and adolescence (β = −0.282, P = 0.02). This relation was not seen for physical/non-social adversities (β = −0.14, P = 0.256), and these two correlations were significantly different from one another (t = −4.46, P < 0.001). Looking at specific developmental epochs, early interpersonal stress was related to lower right VS activity (β = −0.29, P = 0.01), while early physical/non-social adversity was related to lower right VS activity at a trend level (β = −0.248, P = 0.047). These correlations were, however, not statistically different from one another (t = −0.45, P = 0.66). No other significant relations were found for exposure to interpersonal or physical/non-social stress during other developmental epochs (all P’s > 0.18).
Brain–behavior relations
Exploratory analyses focused on brain–behavior relationships did not find any associations between VS activity and adult internalizing or externalizing symptoms (all P’s > 0.25). Additional analyses using ensemble classification techniques and also controlling for current levels of psychopathology are discussed in Supplementary Materials.
Discussion
We found that greater cumulative exposure to stress during childhood was related to lower VS activity during reward processing. Particularly novel, we then found that stress early in development (between kindergarten and grade 3) but not later in development (grades 4 through 7; grades 8 through 12) was specifically associated with this blunted activity. These neural differences may reflect changes in the responding to and processing of important environmental information, specifically reduced engagement with positive stimuli relative to engagement with negative stimuli.
Our results fit well with previous studies charting the impact of ELS on reward-related neural circuitry, specifically the VS. One report in adults found lower VS activity to reward-predicting cues (Dillon et al., 2009), while two reports in pediatric samples have noted similar VS hypoactivity to reward (Mehta et al., 2010; Goff et al., 2013). Our findings also extend prior work (Hanson et al., 2015) by demonstrating a unique effect of early cumulative stress (before the age of 10) on VS activity.
Of note, we found VS activity to positive feedback lower in those exposed to greater levels of stress during childhood and adolescence. This result is consistent with work showing that lower reward-related VS activity explains reductions in positive affect after stressful life events in young adults (Nikolova et al., 2012), as well as theoretical models that posit VS activity may be critically related to psychological aspects of resilience such as optimism (Charney, 2004; Southwick et al., 2005). An inability to maintain positive affect may be one pathway through which ELS conveys risk for affective psychopathology such as depression. Breaking stress exposure into different classes of events, we found strong relationships between interpersonal adversity and decreased VS activity. Again, thinking about affective psychopathology, this finding relates to a large body of research findings that suggest events with greater interpersonal significance often precipitate the onset of depression (Hammen, 2005).
Alterations in dopamine signaling may contribute to the decreased VS activity observed as a function of ELS, which has been linked with alterations in HPA axis and immune system function (for review, see Nusslock and Miller, 2015). Changes in the HPA axis have previously been linked to decreased density in mesolimbic dopamine receptors in the nucleus accumbens, a key subregion of the VS (for review, see Goff and Tottenham, 2014). Similarly, pro-inflammatory cytokine levels can influence dopamine signaling through changes in metabolism and precursor availability in brain (Dunn, 2006).
Our study is not without limitations. First, the work was conducted in an all-male sample, with a portion of the participants undergoing an intervention early in life. While we did not see any associations between this treatment and VS activity, future work should aim to leverage similar longitudinal designs in more heterogeneous samples. That said, our design can be considered advantageous as significant associations were found even after potential clinical mediation. Second, though we found associations between ELS and brain function, we did not find evidence connecting these differences to emergent behavior. This is, however, similar to much of the past neurobiological research on ELS. For example, while a number of studies have reported associations between ELS and functional alterations in the amygdala, few have connected them to negative outcomes, such as self-report measures of negative effect. Such observations may reflect the relative sensitivity of fMRI to subtle alterations in physiology that are not readily detected by distal measures of behavior. It is also possible that latent differences in brain function may manifest as dysfunctional behavior later in time or in other behavioral domains. Third, the experimental paradigm employed here assays only one facet of reward processing. Recent work has noted that such processing is a complex, non-unitary phenomenon (Berridge and Robinson, 2003; Richards et al., 2013). Future work focused on reward anticipation, modulation and other components of reward processing may aid in explaining the effects of ELS and/or connections with different forms of psychopathology. Finally, the effects of ELS may be conveyed through indirect pathways and our neuroimaging measures were cross-sectional in nature. Future prospective examinations with multiple measures of brain functioning could move past such shortcomings, by more rigorously examining developmental trajectories of VS activity and subsequent effects on behavior. In fact, emerging work suggests that trajectories of neurobiological development rather than a snapshot are more closely related to behavior (Shaw et al., 2006).
These limitations notwithstanding, our study suggests that cumulative stress exposure in childhood impacts reward-related brain function. It is possible that blunted VS activity associated with higher cumulative stress in early life represents a potential neural marker of lower positive psychosocial characteristics necessary for resilience to stress (such as hopefulness). Future research is needed to explicate these relations more fully. More generally, this study underscores that a constellation of stressors in children’s lives may affect neurobiological markers of risk and resilience, which may subsequently inform the development of novel targets for intervention and prevention.
Funding
The Fast Track Project was supported by National Institute of Mental Health (NIMH) Grants R18-MH48043, R18-MH50951, R18-MH50952, and R18-MH50953 and by National Institute on Drug Abuse (NIDA) Grant 1-RC1-DA028248-01. The Center for Substance Abuse Prevention and the NIDA also provided support for Fast Track through a memorandum of agreement with the NIMH. This work was also supported in part by Department of Education Grant S184U30002; NIMH Grants K05MH00797 and K05MH01027; and NIDA Grants DA16903, DA015226, DA017589, and DA023026. Support for Dr. Hariri and his laboratory came from NIH grants DA033369 and DA031579. Additional support came from a Postdoctoral Fellowship provided by NIDA through the Center for the Study of Adolescent Risk and Resilience (Grant P30DA023026 to J.L.H.) and a postdoctoral fellowship provided by the National Institute of Child Health and Human Development (Grant T32HD0737625) through the Center for Developmental Science, University of North Carolina at Chapel Hill, to J.L.H.
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Achenbach T.M., Rescorla L.A. (2003). Manual for the ASEBA Adult Forms & Profiles. Burlington, VT: University of Vermont. [Google Scholar]
- Appleyard K., Egeland B., Dulmen M.H.M., Sroufe L.A. (2005). When more is not better: the role of cumulative risk in child behavior outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines , 46(3), 235–45. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. (2003). Parsing reward. Trends in Neurosciences , 26(9), 507–13. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Danube C.L., Hommer D.W. (2007). Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. The Journal of Neuroscience , 27(18), 4839–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse H., Lukkes J., Andersen S. (2013). Early life adversity alters the developmental profiles of addiction-related prefrontal cortex circuitry. Brain Sciences , 3(1), 143–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J.Z.K., Armstrong J.M., Hanson J.L., et al. (2015). Preschool externalizing behavior predicts gender-specific variation in adolescent neural structure. PLoS One , 10(2), e0117453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell F., Conti G., Heckman J.J., et al. (2014). Early childhood investments substantially boost adult health. Science , 343(6178), 1478–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre J.M., Fisher P.M., Manuck S.B., Hariri A.R. (2012). Interaction between trait anxiety and trait anger predict amygdala reactivity to angry facial expressions in men but not women. Social Cognitive and Affective Neuroscience , 7(2), 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney D.S. (2004). Psychobiological mechanisms of resilience and vulnerability: implications for successful adaptation to extreme stress. American Journal of Psychiatry , 161(2), 195–216. [DOI] [PubMed] [Google Scholar]
- Conti G., Hansman C., Heckman J.J., Novak M.F.X., Ruggiero A., Suomi S.J. (2012). Primate evidence on the late health effects of early-life adversity. Proceedings of the National Academy of Sciences of the United States of America , 109(23), 8866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. (1996). AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research , 29(3), 162–73. [DOI] [PubMed] [Google Scholar]
- Danese A., McEwen B.S. (2012). Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiology & Behavior , 106(1), 29–39. [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Holmes A.J., Birk J.L., Brooks N., Lyons-Ruth K., Pizzagalli D.A. (2009). Childhood adversity is associated with left basal ganglia dysfunction during reward anticipation in adulthood. Biological Psychiatry , 66(3), 206–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge K.A., Bates J.E., Pettit G.S. (1990). Mechanisms in the cycle of violence. Science , 250(4988), 1678–83. [DOI] [PubMed] [Google Scholar]
- Dunn A.J. (2006). Effects of cytokines and infections on brain neurochemistry. Clinical Neuroscience Research , 6(1–2), 52–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G.W. (2003). A multimethodological analysis of cumulative risk and allostatic load among rural children. Developmental Psychology , 39(5), 924–33. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. (2010). Multiple risk exposure as a potential explanatory mechanism for the socioeconomic status-health gradient. Annals of the New York Academy of Sciences , 1186(1), 174–89. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Kim P. (2012). Childhood poverty and young adults' allostatic load: the mediating role of childhood cumulative risk exposure. Psychological Science , 23(9), 979–83. [DOI] [PubMed] [Google Scholar]
- Evans G.W., Li D., Whipple S.S. (2013). Cumulative risk and child development. Psychological Bulletin , 139(6), 1342–96. [DOI] [PubMed] [Google Scholar]
- Forbes E.E., Dahl R.E. (2012). Research Review: altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry , 53(1), 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Manuck S.B., Sheu L.K., et al. (2011). Parental education predicts corticostriatal functionality in adulthood. Cerebral Cortex , 21(4), 896–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros P.J., Marsland A.L., Kuan D.C.H., et al. (2013). An inflammatory pathway links atherosclerotic cardiovascular disease risk to neural activity evoked by the cognitive regulation of emotion. Biological Psychiatry, 75(9), 738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam M., Forbes E.E., Gianaros P.J., Erickson K.I., Brennan L.M., Shaw D.S. (2014). Maternal depression in childhood and aggression in young adulthood: evidence for mediation by offspring amygdala—hippocampal volume ratio. Journal of Child Psychology and Psychiatry , 56(10), 1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Tottenham N. (2014). Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS Spectrums , 20(4), 337–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B., Gee D.G., Telzer E.H., et al. (2013). Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience , 249, 129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M.T., Lengua L.J., Coie J.D., Pinderhughes E.E. (1999). Predicting developmental outcomes at school entry using a multiple-risk model: four American communities. The Conduct Problems Prevention Research Group. Developmental Psychology , 35(2), 403–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammen C. (2005). Stress and depression. Annual Review of Clinical Psychology , 1(1), 293–319. [DOI] [PubMed] [Google Scholar]
- Hanson J.L., Hariri A.R., Williamson D.E. (2015). Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biological Psychiatry, 78(9), 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A.R., Brown S.M., Williamson D.E., Flory J.D., De Wit H., Manuck S.B. (2006). Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. Journal of Neuroscience , 26(51), 13213–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde L.W., Gorka A., Manuck S.B., Hariri A.R. (2011). Perceived social support moderates the link between threat-related amygdala reactivity and trait anxiety. Neuropsychologia , 49(4), 651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J., Charney D. (2001). Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Development and Psychopathology , 13(3), 451–71. [DOI] [PubMed] [Google Scholar]
- Kolb B., Elliott W. (1987). Recovery from early cortical damage in rats. II. Effects of experience on anatomy and behavior following frontal lesions at 1 or 5 days of age. Behavioural Brain Research , 26(1), 47–56. [DOI] [PubMed] [Google Scholar]
- Kolb B., Gibb R. (1991). Environmental enrichment and cortical injury: behavioral and anatomical consequences of frontal cortex lesions. Cerebral Cortex , 1(2), 189–98. [DOI] [PubMed] [Google Scholar]
- Manly J.T., Kim J.E., Rogosch F.A., Cicchetti D. (2001). Dimensions of child maltreatment and children's adjustment: contributions of developmental timing and subtype. Development and Psychopathology , 13(4), 759–82. [PubMed] [Google Scholar]
- Matthews K., Robbins T.W. (2003). Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neuroscience and Biobehavioral Reviews , 27(1–2), 45–55. [DOI] [PubMed] [Google Scholar]
- Mehta M.A., Gore-Langton E., Golembo N., Colvert E., Williams S.C.R., Sonuga-Barke E. (2010). Hyporesponsive reward anticipation in the basal ganglia following severe institutional deprivation early in life. Journal of Cognitive Neuroscience , 22(10), 2316–25. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.-L., Clasen L.S., Giedd J.N., Blakemore S.-J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience , 36(3–4), 147–60. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R., Muhammad A., Kolb B. (2014). Environmental enrichment alters structural plasticity of the adolescent brain but does not remediate the effects of prenatal nicotine exposure. Synapse, 68(7), 293–305. [DOI] [PubMed] [Google Scholar]
- Nikolova Y.S., Bogdan R., Brigidi B.D., Hariri A.R. (2012). Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biological Psychiatry , 72(2), 157–63. [DOI] [PubMed] [Google Scholar]
- Nikolova Y.S., Singhi E.K., Drabant E.M., Hariri A.R. (2013). Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes, Brain and Behavior , 12(5), 516–24. [DOI] [PubMed] [Google Scholar]
- Norman R.E., Byambaa M., De R., Butchart A., Scott J., Vos T. (2012). The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Medicine , 9(11), e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock R., Miller G.E. (2015). Early-life adversity and physical and emotional health across the lifespan—a neuroimmune network hypothesis. Biological Psychiatry, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. (2009). Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. Journal of Neuroscience , 29(38), 11772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L., Porcella A., Gessa G.L. (2000). The role of stress in the pathophysiology of the dopaminergic system. Molecular Psychiatry , 5(1), 14–21. [DOI] [PubMed] [Google Scholar]
- Payne C., Machado C.J., Bliwise N.G., Bachevalier J. (2009). Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus , 20(8), 922–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Lyons-Ruth K., Anderson C.M., Teicher M.H. (2014). Sensitive periods of amygdala development: the role of maltreatment in preadolescence. NeuroImage , 97, 236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli D.A. (2014). Depression, stress, and anhedonia: toward a synthesis and integrated model. Annual Review of Clinical Psychology , 10(1), 393–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P.W., Lerch J.P., et al. (2014). Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proceedings of the National Academy of Sciences of the United States of America , 111(4), 1592–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J.M., Plate R.C., Ernst M. (2013). A systematic review of fMRI reward paradigms used in studies of adolescents vs. adults: the impact of task design and implications for understanding neurodevelopment. Neuroscience and Biobehavioral Reviews , 37(5), 976–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Greenstein D., Lerch J., et al. (2006). Intellectual ability and cortical development in children and adolescents. Nature , 440(7084), 676–9. [DOI] [PubMed] [Google Scholar]
- Shonkoff J.P., Garner A.S., Siegel B.S., et al. (2012). The lifelong effects of early childhood adversity and toxic stress. Pediatrics , 129(1), e232–46. [DOI] [PubMed] [Google Scholar]
- Southwick S.M., Vythilingam M., Charney D.S. (2005). The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annual Review of Clinical Psychology , 1(1), 255–91. [DOI] [PubMed] [Google Scholar]
- The Conduct Problems Prevention Research Group. (1999). Initial impact of the Fast Track prevention trial for conduct problems: I. The high-risk sample. Conduct Problems Prevention Research Group. Journal of Consulting and Clinical Psychology , 67(5), 631–47. [PMC free article] [PubMed] [Google Scholar]
- The Conduct Problems Prevention Research Group. (2002). Evaluation of the first 3 years of the Fast Track prevention trial with children at high risk for adolescent conduct problems. Journal of Abnormal Child Psychology , 30(1), 19–35. [DOI] [PubMed] [Google Scholar]
- Uematsu A., Matsui M., Tanaka C., et al. (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One , 7(10), e46970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. (2008). Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron , 59(6), 1037–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T., Poldrack R.A., Nichols T.E., Essen D.C.V., Wager T.D. (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods , 8(8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.