Abstract
Humans’ closest living relatives are bonobos (Pan paniscus) and chimpanzees (Pan troglodytes), yet these great ape species differ considerably from each other in terms of social behavior. Bonobos are more tolerant of conspecifics in competitive contexts and often use sexual behavior to mediate social interactions. Chimpanzees more frequently employ aggression during conflicts and actively patrol territories between communities. Regulation of emotional responses is facilitated by the amygdala, which also modulates social decision-making, memory and attention. Amygdala responsiveness is further regulated by the neurotransmitter serotonin. We hypothesized that the amygdala of bonobos and chimpanzees would differ in its neuroanatomical organization and serotonergic innervation. We measured volumes of regions and the length density of serotonin transporter-containing axons in the whole amygdala and its lateral, basal, accessory basal and central nuclei. Results showed that accessory basal nucleus volume was larger in chimpanzees than in bonobos. Of particular note, the amygdala of bonobos had more than twice the density of serotonergic axons than chimpanzees, with the most pronounced differences in the basal and central nuclei. These findings suggest that variation in serotonergic innervation of the amygdala may contribute to mediating the remarkable differences in social behavior exhibited by bonobos and chimpanzees.
Keywords: brain evolution, serotonin, amygdala, bonobo, chimpanzee
Introduction
Bonobos (Pan paniscus) and chimpanzees (Pan troglodytes) are the closest living relatives of humans, sharing over 98% DNA similarity with us. The bonobo and chimpanzee lineages diverged from each other ∼2 million years ago and, consequently, these species resemble one another in many aspects of genetics, anatomy and development (Goodall, 1986; Wrangham, 1993; de Waal, 1997; Prüfer et al., 2012). However, bonobos and chimpanzees are characterized by a very different constellation of social behaviors, both in the wild and captivity. Bonobos display tolerance and cooperation when interacting over limited food resources (Wrangham, 1993; de Waal, 1997; Hare et al., 2007; Wobber et al., 2010b; Woods and Hare, 2011) and exhibit little overt aggressive competition over mates or hostility among neighboring communities (Wrangham, 1993). Bonobos mediate stress through sexual interactions, which often occur face-to-face (Wrangham, 1993; de Waal, 1997; Woods and Hare, 2011). Chimpanzees, on the other hand, tend to display more aggressive behaviors, with males in particular engaging in agonistic encounters within and between groups (Goodall, 1986; Hare et al., 2007). Lethal inter-group aggression is significantly more common among wild chimpanzees than bonobos (Wilson et al., 2014). For example, parties comprised of mostly male chimpanzees have been observed to engage in patrols of the boundary of their territories and to chase back or attack other chimpanzees from neighboring groups (Goodall, 1986; Wrangham, 1999; Watts et al., 2006; Wilson et al., 2014). Similar territorial patrolling has not been reported in bonobos.
The amygdala is one of the principal areas of the brain that is important in regulating social behavior through processing of aversive emotional stimuli that elicit fear and anxiety (LeDoux, 1996). In non-human primates, amygdala lesions amplify aggressive reactivity and lead to a decrease in sociality and dominance rank position (Kling and Brothers, 1992; Fried et al., 1997; Adolphs, 1999). In humans, dysfunction of the amygdala is implicated in a number of cognitive disorders that affect social behavior, such as autism, schizophrenia and extreme chronic aggression (Reynolds, 1992; Adolphs, 1999; Davidson, 2000; Dalton et al., 2005; Meyer-Lindenberg et al., 2006; Schumann and Amaral, 2006; Kennedy and Adolphs, 2012; Markota et al., 2014). Individuals with amygdala damage can recognize conditioned social signals but have difficulty mapping stimuli to emotional categories (Adolphs, 1999; Kennedy and Adolphs, 2012). Amygdala lesions in humans can result in reduced sensitivity to social cues and poor reasoning about interpersonal interactions, influencing the ability to judge personal space violations, strangers’ approachability or trustworthiness and ‘theory of mind’ related to emotional state (Adolphs et al., 1998; Stone et al., 2002, 2003; Kennedy et al., 2009). These social impairments may reflect a more general involvement of the amygdala in memory, decision-making, fear, environmental appraisal and tuning emotional responses to external stimuli (Amaral et al., 1992; Davis, 1992; Rolls, 1992; Packard et al., 1994; LeDoux, 1996; Adolphs, 1999).
The amygdala is comprised of several distinct nuclear or cortical structures (Price et al., 1987). The lateral, basal and accessory basal nuclei of the amygdala are commonly referred to as the basolateral or deep nuclei and grouped together due to their strong neocortical interconnectivity (Price et al., 1987). The lateral nucleus is the primary recipient of sensory cortical input (Stefanacci and Amaral, 2002; LeDoux, 2007; Freese and Amaral, 2009), while the basal nucleus is the main source of amygdalofugal projections to myriad brain structures (Amaral, 2003; Freese and Amaral, 2009). The central nucleus provides the major output to regions of the autonomic nervous system (Price et al., 1987) and can modulate these targets to initiate physiological responses to threatening stimuli (LeDoux, 2007; Freese and Amaral, 2009).
The serotonergic system also plays a key role in regulating neuronal responses to social stimuli and the amygdala contains a high density of serotonin (5-HT) receptors (Stuart et al., 1986; Murphy et al., 1998; Lesch et al., 2012; Albert et al., 2014). Data from a variety of mammalian species demonstrate that low levels of serotonin (5-HT) in the cerebral spinal fluid and various brain regions is associated with increased aggression, anxiety, fearfulness and depression (Hassanain et al., 2003; Cervantes and Delville, 2007, 2009; Jacobs et al., 2007; Meyer-Lindenberg et al., 2006; Wang et al., 2013; Markota, 2014). Pharmacological manipulation of serotonin signal transduction in the amygdala of rodents and primates produces changes in conditioned fear and stress responses, and genetically modified expression of molecules in the serotonin pathway in animal models have an impact on the structural development, neurochemistry and function of the amygdala (reviewed in Asan et al., 2013). Furthermore, differential expression of the serotonin transporter (SERT) has been linked to within-species variation in sensitivity to social cues, vigilance to social threats, risk avoidance, responsiveness to changes in reward contexts and mood (Watson et al., 2009; Vallender et al., 2009). SERT is a monoamine protein located within axons and pre-synaptic terminals and serves to terminate the action of 5-HT by uptake from the synaptic cleft for recycling (Sur et al., 1996; Murphy et al., 1998; Zhou et al., 1998). In humans and rhesus macaque monkeys, allelic variants in the SERT-linked polymorphic region (5-HTTLPR) have been shown to regulate the expression of the SERT gene (SLC6A4) (Lesch et al., 1997). Notably, in a functional magnetic resonance imaging (fMRI) study of humans, Hariri et al. (2002) showed that individuals carrying one or two copies of the short allele of 5-HTTLPR exhibited greater amygdala activity in response to the presentation of images of angry or fearful facial expressions compared with individuals homozygous for the long allele variant.
Given the importance of both the serotonergic system and amygdala in orchestrating social behavior, this study sought to compare bonobos and chimpanzees with respect to the volume of regions and serotonergic innervation in the whole amygdala, as well as lateral, basal, accessory basal and central nuclei. We used immunohistochemistry against the SERT protein to label serotonergic axons in post-mortem brains of bonobos and chimpanzees. We adopted this experimental approach because SERT is expressed at high levels in the majority of serotonergic afferents in the forebrain, including the amygdala (Qian et al., 1995; Freedman and Shi, 2001; Asan et al., 2013) and is more stable during the post-mortem period than 5-HT (Verney et al., 2002). Considering the limited availability of biomaterials from these endangered species, this strategy allowed us to investigate the anatomical distribution of serotonergic innervation in brains that were obtained opportunistically at necropsy. In light of the variation in bonobo and chimpanzee socio-affective behavior, we hypothesized that amygdala nuclear size and serotonergic axon density would differ between species. In particular, we predicted that bonobos would exhibit greater serotonergic innervation of the amygdala than chimpanzees.
Materials and methods
Sample, tissue preparation and immunohistochemistry
Brains were collected from apes following death unrelated to this study. Chimpanzee brains (N = 7) were obtained from the Yerkes National Primate Research Center. Bonobo brains (N = 7) came from Milwaukee County Zoo (N = 4), Jacksonville Zoo (N = 2) and the Ape Cognition and Conservation Initiative (N = 1). The apes were maintained in accordance with each institution’s animal care and use guidelines. Supplementary Table S1 contains sex and age information for the sample. Left hemispheres were used for all individuals. Within 14 h of each subject’s death, the brain was removed and immersed in 10% formalin. In most cases, the brain was transferred to 0.1 M phosphate buffered saline (PBS) with 0.1% sodium azide solution after 10–14 days and stored at 4°C. Temporal lobe blocks which included the amygdala were cryoprotected by immersion in buffered sucrose solutions up to 30%, embedded in TBS freezing medium, frozen in a slurry of dry ice and isopentane and sectioned at 40 µm with a sliding microtome in the coronal plane, with the exception of one chimpanzee brain that was sectioned at 50 µm.
A one-in-ten series of free-floating sections were stained with mouse monoclonal IgG1 antibody against SERT (1: 100 000 dilution, MAB5618, EMD Millipore, Billerica, MA). Prior studies of the neocortex in humans, chimpanzees and macaques (Raghanti et al., 2008) have demonstrated the selectivity of this antibody using immunohistochemistry on formalin-fixed tissue. Furthermore, antibody specificity has been reported previously in Western blot studies, showing a molecular weight of 60–70 kDa (Ramsey and DeFelice, 2002; Serafeim et al., 2002). Prior to immunostaining, sections were rinsed thoroughly in PBS and pre-treated for antigen retrieval by incubation in 10 mM sodium citrate buffer (pH 3.5) at 37°C in an oven for 30 min. Sections were then rinsed and immersed in a solution of 2.5% hydrogen peroxide in 75% methanol to eliminate endogenous peroxidase activity. After rinsing again, sections were incubated in solution containing PBS with 2% normal horse serum and 0.1% Triton X-100 detergent for 1 h and then incubated in primary antibody diluted in PBS for ∼24 h on a rotator at 4°C. After rinsing in PBS, sections were incubated in biotinylated anti-mouse IgG (1: 200 dilution, BA-2000, Vector Laboratories, Burlingame, CA) and processed with the avidin-biotin-peroxidase method using a Vectastain Elite ABC kit (pk-6100, Vector Laboratories). Sections were rinsed again in PBS, followed by a rinse in sodium acetate buffer. Immunoreactivity was revealed using 3,3′-diaminobenzidine and nickel enhancement according to a modification of the methods in Shu et al. (1988) as described in Van der Gucht et al. (2001). When the primary antibody was excluded in control experiments, no immunostaining was observed.
Cytoarchitecture
In each specimen, an adjacent 1: 10 series of sections through the amygdala was Nissl-stained with 0.5% cresyl violet to reveal cytoarchitecture. Prior to stereologic measurement of SERT-immunoreactive (-ir) axons, Nissl-stained sections were used to identify boundaries of four main nuclei, as described later. Training and interrater reliability was performed between two investigators (NB and CDS). All contours were drawn and measurements were collected by CDS. As control regions, SERT-ir axon density in three equidistantly spaced sections were also measured within the anterior portion of the middle temporal cortex (corresponding to Brodmann’s area 21), a region involved in memory and auditory processing, as well as the head of the caudate nucleus, which is associated with motor and cognitive functions.
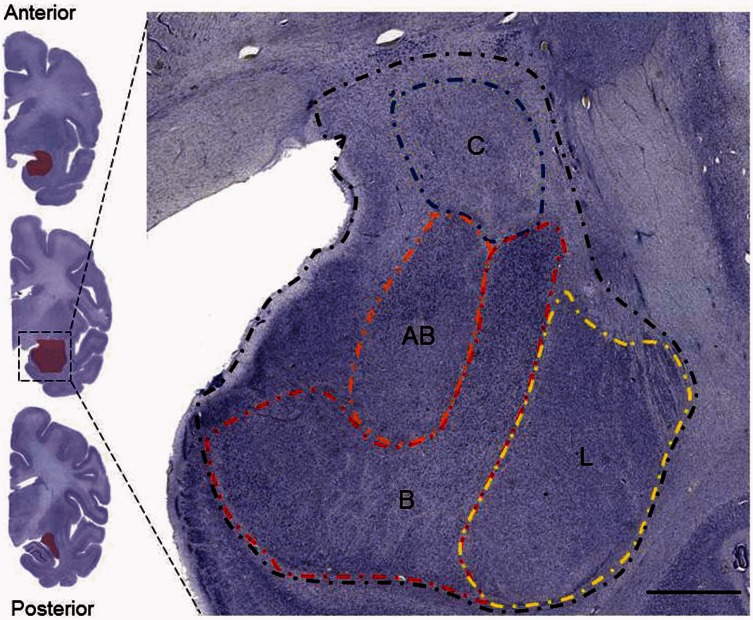
Our definitions of great ape amygdala cytoarchitecture have been published in detail previously (Barger et al., 2007, 2012) and are consistent with descriptions from humans and macaque monkeys (Amaral and Bassett, 1989; Amaral et al., 1992; Heimer et al., 1999; Barger et al., 2012) (Figure 1). Briefly, the anterior and posterior extents of the whole amygdala were defined, respectively, as the first section containing the lateral or basal nuclei and the last section containing the central nucleus. The medial wall of the hemisphere forms the medial border of the amygdala throughout its anteroposterior extent. Anteriorly, the lateral nucleus forms the lateral border of the amygdala, while the central nucleus or the intercalated nuclei surrounding it form the lateral border in the most posterior sections. The lateral, basal and accessory basal nuclei are separated from one another by two longitudinal association fiber bundles (Price et al., 1987). Located medial to the lateral nucleus, the basal nucleus, defined here to include the large-celled magnocellular, intermediate and smaller-celled parvocellular divisions (Price et al., 1987), contains the largest neurons in the amygdala and is easily distinguished. A small notch is visible at the ventromedial boundary between the lateral and basal nuclei, further clarifying its location. The basal nucleus forms the lateral border of the accessory basal nucleus, defined as including the magnocellular, parvocellular and dense ventromedial division (Price et al., 1987; Barger et al., 2012). It is separated from the superficial nuclei comprising the medial surface of the amygdala by an additional association fiber bundle. The central nucleus is located dorsal to the accessory basal and basal nuclei and appears more posteriorly. It is completely surrounded by white matter and is often adjacent to the darkly staining and dense intercalated nuclei, which help to further identify its boundaries. It consists of two subnuclei, the lateral half being a dense cluster of small cells and the medial half composed of lighter-stained, less densely packed heterogeneous cells (Price et al., 1987) (Figure 1).
Fig. 1.
Nissl-stained section of the amygdala in a bonobo, with contours of the whole amygdala, lateral (L), basal (B), accessory basal (AB) and central (C) nuclei drawn. Scale bar = 250 µm.
Quantitative measurement of nucleus volumes and SERT-ir axon density
Quantification of the volume of regions of interest and the density of SERT-ir axons in the amygdala was performed using a Zeiss Axioplan 2 photomicroscope (Zeiss, Thornwood, NY) equipped with a Ludl XY motorized stage (Ludl Electronics, Hawthorne, NY), Heidenhain z-axis encoder and an Optronics MicroFire color video camera (Optronics, Golenta, CA) coupled to a Dell PC workstation running StereoInvestigator software (MBF Bioscience, Williston, VT). A 1: 10 series including the entire rostrocaudal extent of the amygdala was used for the analysis. In StereoInvestigator, contours were drawn around each of the regions in every section in which they appeared under 4× magnification. The volume was calculated as the sum of contour areas multiplied by section thickness. This volume was then corrected for histological shrinkage in each brain by a correction factor that was determined based on the methods described in Bauernfeind et al. (2013).
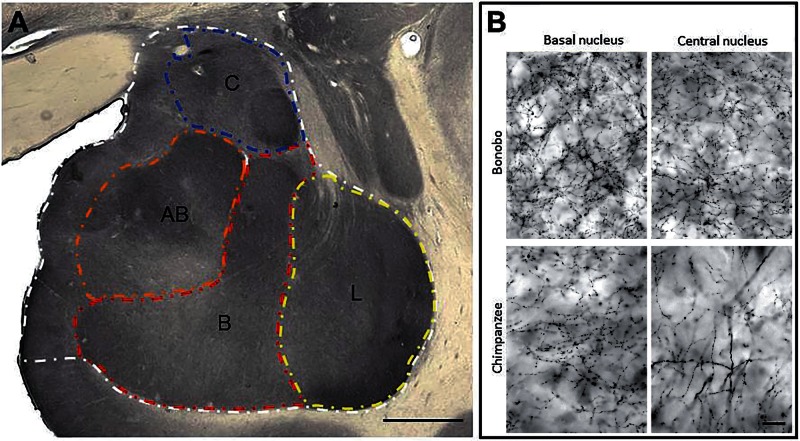
Stereologic sampling was performed to measure length density of SERT-ir axons in the whole amygdala as well as the four nuclei. To determine the anatomical boundaries for SERT-ir axon measurements, first we collected photomicrographs of adjacent Nissl-stained sections in each specimen under 4× magnification (Figure 1). The micrographs were used to outline anatomical boundaries according to the criteria as described earlier. The contours were then opened in the StereoInvestigator software as an overlay while live video of the corresponding SERT-ir section was viewed. The anatomical boundaries drawn from cytoarchitecture were adjusted for scaling and rotated as needed to match the SERT-stained sections using landmarks external to the amygdala (i.e. the optic tract and hypothalamus) (Figure 2). Spaceballs analysis was performed under Koehler illumination using a 63× objective lens (Zeiss Plan-Apochromat, N.A. 1.4). The radius of the probe was 10 µm, with a 1 µm guard zone at the top of the section. Virtual isotropic hemispheres sampled the tissue in a systematic random fashion with the grid spacing optimized according to nucleus size. The sampling design was implemented with the goal of obtaining 100 sampling sites per individual per measured area - lateral and basal nuclei were sampled with a 1000 × 1000 µm grid, accessory basal with a 700 × 700 µm grid and central nucleus with a 600 × 600 µm grid. Measurement within the middle temporal cortex used the same parameters on a 600 × 600 µm grid. The sampling grid used for measurements in the caudate nucleus ranged between 300 × 300 µm and 600 × 600 µm, depending on size of the region. Total axon density was calculated as the total axon length over the planimetric reference volume as obtained from the StereoInvestigator software (Raghanti et al., 2008). All numerical densities of axons derived from these counts were corrected by the number-weighted mean section thickness as described in Sherwood et al. (2007).
Fig. 2.
(A) SERT immunostaining in a chimpanzee with contours of the whole amygdala, lateral (L), basal (B), accessory basal (AB) and central (C) nuclei. Scale bar = 250 µm. (B) Comparison of SERT-ir axons in the basal and central nuclei of bonobo and chimpanzee. Scale bar = 25 µm.
Due to the relatively small sample sizes in this study, we employed non-parametric analyses to compare the species. Differences in overall brain size, amygdala and its nuclei volumes, and SERT-ir axon density were examined using the Mann-Whitney U test. As there was a significant difference in brain size between species, we assessed variation in the volume of amygdala regions with the Quade’s rank analysis of covariance using total brain mass as a covariate. For the analysis of amygdala volumes, one bonobo had to be removed from the sample due to an incomplete anterior amygdala in the block that was provided by the zoo for this research project. A different bonobo was excluded from quantification of SERT-ir axon density due to overfixation and lack of fiber staining. The bonobo sample included a 5-year-old juvenile, so data were initially analyzed both with and without this individual. Results did not differ by excluding this individual, therefore we report results based on the full dataset. SPSS software v. 21 (Armonk, NY) was used for all statistical analyses.
Results
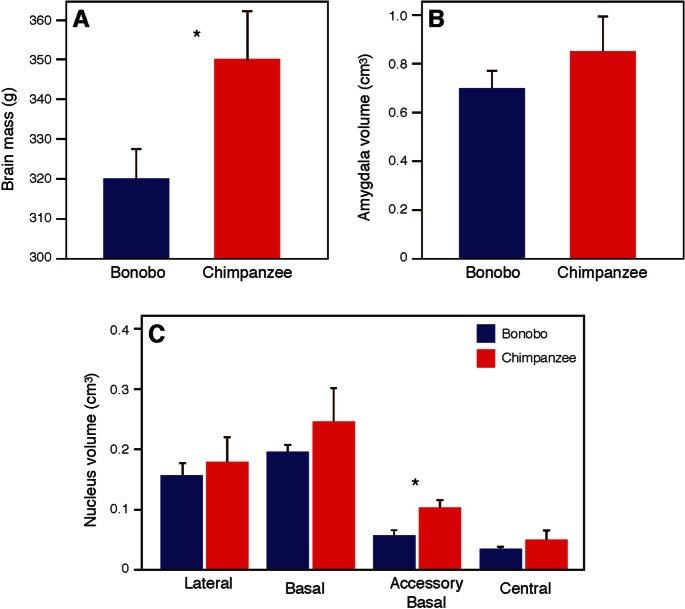
Chimpanzees had significantly larger overall brain masses than bonobos in our sample (Mann-Whitney U test = 2.108, P = 0.038) (Figure 3). Quade’s rank analysis controlling for brain mass as a covariate revealed a trend for whole amygdala volume to be relatively larger in chimpanzee than bonobos (F1,11 = 3.95, P = 0.072; Figure 3). Among the four amygdala nuclei, after controlling for brain mass there was a significant difference between species only for the accessory basal nucleus (F1,11 = 8.69, P = 0.013; Figure 3). The accessory basal nucleus in chimpanzees (volume = 0.1030 ± 0.0116 µm3) was larger than in bonobos (volume = 0.0566 ± 0.0076 µm3; Supplementary Table S2). No sex differences were found in either species for brain mass or the volumes of the amygdala and its nuclei.
Fig. 3.
Comparison between bonobos and chimpanzees in overall brain mass (A), amygdala volume (B) and amygdala nuclei volumes (C). Asterisks indicate P < 0.05. Error bars represent 95% confidence intervals.
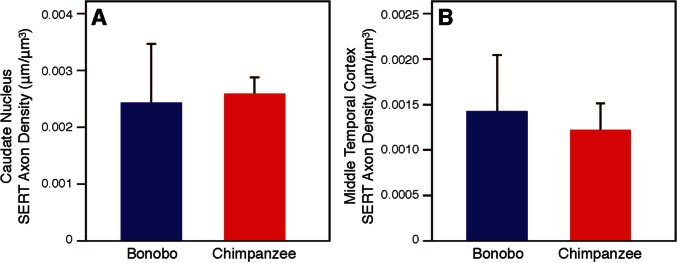
The density of SERT-ir axons in the amygdala of bonobos and chimpanzees is shown in Supplementary Table S2. The whole amygdala of bonobos contained a higher density of SERT-ir axons (0.00521 ± 0.0019 µm/µm3) than in chimpanzees (0.00255 ± 0.0008 µm/µm3) (Mann-Whitney U test: Z = −2.86, P = 0.004; Figure 4). Among the four amygdala nuclei, there were significant differences between species in the basal and central nuclei (Figure 4). The density of SERT-ir axons in the basal nucleus of bonobos (0.00543 ± 0.0013 µm/µm3) was nearly 2-fold greater than in chimpanzees (0.00279 ± 0.0003 µm/µm3; Z = −3.00, P = 0.003). The density of SERT-ir section axons in the central nucleus was also significantly greater in bonobos (0.00481 ± 0.0010 µm/µm3) than in chimpanzees (0.00288 ± 0.0005 µm/µm3; Z = −3.00, P = 0.003). The central nucleus of bonobos was also the only region in which there were any statistically significant sex differences (N = 3 for each sex, Mann-Whitney U test: Z = −1.964, P = 0.05), with bonobo males showing a higher density of SERT-ir axon fibers than bonobo females. There were no differences in SERT-ir axon density between bonobos and chimpanzees in either the middle temporal cortex (Mann-Whitney U-test: Z = −1.143, P = 0.295) or the caudate nucleus (Mann-Whitney U-test: Z = −0.429, P = 0.731; Figure 5).
Fig. 4.
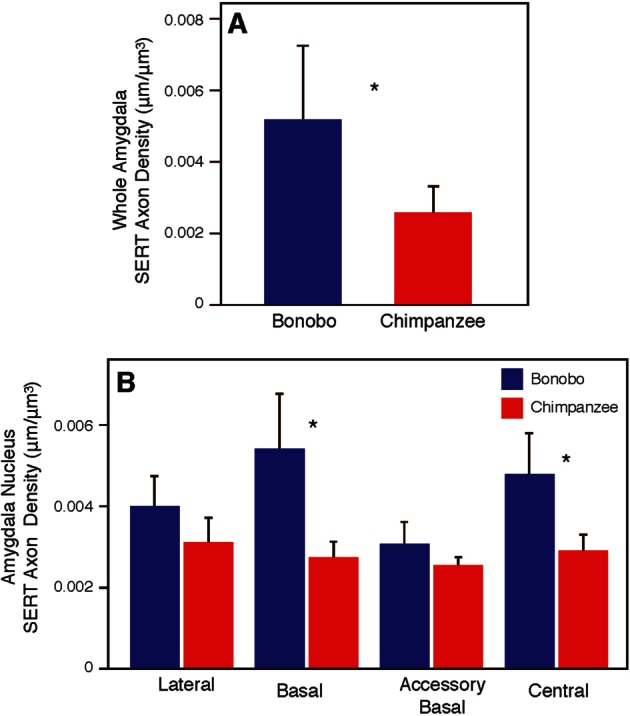
Comparison between bonobos and chimpanzees in SERT-ir axon density in the whole amygdala (A) and amygdala nuclei (B). Asterisks indicate P < 0.05. Error bars represent 95% confidence intervals.
Fig. 5.
Comparisons between bonobos and chimpanzees in SERT-ir axon density in control regions, caudate nucleus (A) and middle temporal cortex (B). Error bars represent 95% confidence intervals.
Discussion
Given the variation in socio-affective behavior between bonobos and chimpanzees, we tested the hypothesis that the amygdala differs between species in its size and serotonergic innervation. We found that there was approximately twice the density of serotonergic axons in the bonobo amygdala compared with chimpanzees, with the most significant differences in the central and basal nuclei. In addition, the accessory basal nucleus was larger in chimpanzees than in bonobos. The precise function of the accessory basal nucleus is unknown, but its connectivity in primates appears to be widespread across multiple brain regions (Stefanacci and Amaral, 2002). Notably, differences between bonobos and chimpanzees in serotonergic axon density were exclusive to the amygdala, as the middle temporal cortex and caudate nucleus did not show variation between species. Overall, these findings highlight the potential for modifications of neuromodulatory systems within specific networks to serve as a substrate for evolutionary change in behavior among closely related species.
Serotonergic innervation was significantly increased in the basal and central nuclei of bonobos compared with chimpanzees, indicating that heightened modulation of outputs from the amygdala may be especially relevant to the appearance of differences in behavior between these species. The basal nucleus is the largest amygdaloid nucleus in apes and it is important for integrating information, exchanging connections with thalamic and hypothalamic nuclei and projecting to numerous cortical territories (Ragsdale and Garybiel, 1991; Everitt and Robbins, 1992; Phillips and LeDoux, 1992; LeDoux, 1997, 2007; Stefanacci and Amaral, 2002; Amaral, 2003; Barger et al., 2007; Euston et al., 2012; Cavallari et al., 2014). Functionally, the basal nucleus has been shown to contribute to contextual aspects of fear conditioning fundamental to environmental threat appraisal (Calandreau et al., 2005). Integrated into the ‘social brain circuit’ (Kling and Steklis, 1976; Brothers, 1990; Adolphs, 2010), the basal nucleus shares strong reciprocal connections with the orbitofrontal cortex, which contribute to appraisal of the emotional context and significance of the environment (Barbas, 2007; Freese and Amaral, 2009). For example, disinhibition of the basal nucleus has been shown to increase social avoidance to a novel partner in rodents (Truitt et al., 2007). The central nucleus is another major source of amygdala output. However, it primarily modulates brain areas that are part of the sympathetic autonomic nervous system, such as hypothalamic and brainstem regions, rather than cortical territories (Freese and Amaral, 2009). The central nucleus is involved in enacting physiological responses to stimuli interpreted by the rest of the amygdala through its connections with autonomic centers (Amaral et al., 1992; Davis, 1992; Pitkänen et al., 1997). Taken together, these observations support a model of amygdala function where species-specific variation in serotonergic innervation may affect reactivity to the social environment through modulation of major output nuclei that send projections to cortical and autonomic centers. Consequently, our findings suggest that higher 5-HT levels in these amygdala regions of bonobos compared with chimpanzees lead to relatively lower neuronal excitability in response to emotionally arousing stimuli and reduced activation of targets that control the sympathetic nervous system (Asan et al., 2013).
Affective responses to social stimuli, which are dependent on amygdala signaling, characterize major differences in behavior observed between bonobos and chimpanzees (Hare et al., 2007, Rilling et al., 2012). Bonobos tend to exhibit more cautious temperaments (Herrmann et al., 2010), decreased risk preference (Heilbronner et al., 2008), reduced ‘emotional reactivity’ (Hare et al., 2007) and better performance on ‘theory of mind’ tasks that require attention to social cues (Herrmann et al., 2010). In contrast, chimpanzees show a greater preference for novel objects and individuals (Herrmann et al., 2011), more tolerance of risk (Heilbronner et al., 2008) and tend to display more aggressive behaviors (Goodall, 1986; Hare et al., 2007). Differential serotonergic innervation in the basal nucleus might contribute to variation in social and environmental appraisal of threats and vigilance. Concomitantly, the observed interspecific differences in serotonergic axon density in the central nucleus may modulate emotional arousal through autonomic channels. In this regard, it is notable that the central nucleus of male bonobos contained not only significantly more serotonergic axons than chimpanzees but also than bonobo females, whereas no sexual dimorphism was evident in the serotonergic innervation of the amygdala in chimpanzees. Although these results are based on relatively small sex-specific sample sizes, we speculate that inhibiting aggressive reactivity may provide benefits to bonobo males in a social context where males and females are co-dominant. This is consistent with data demonstrating that male bonobos show a rise in cortisol prior to food competition with a dominant individual, whereas in the same dyadic food contests, chimpanzee males exhibit an anticipatory increase in testosterone (Wobber et al., 2010a). This indicates that there are physiological differences between bonobo and chimpanzee males in their appraisal of competition, with bonobos exhibiting a passive coping style, which is correlated with more pronounced stress, during episodes of social uncertainty.
Bonobos are reported to engage in socio-sexual activity at a greater rate than chimpanzees (Woods and Hare, 2011). Together with dopamine and reproductive hormones, 5-HT neurotransmission in the amygdala has been shown to regulate sexual behavior (Angoa-Pérez and Kuhn, 2015). However, it is difficult to define the precise role of serotonergic innervation in the increased sexuality of bonobos. In human patients and rodent models, treatment with selective serotonin reuptake inhibitors, which act by increasing synaptically available 5-HT, is associated with male sexual dysfunction but no change in sexual motivation (i.e. libido) has been demonstrated (Barrett et al., 2006; Angoa-Pérez and Kuhn, 2015). The role of 5-HT in regulating sexual behavior in primates is known mostly from pharmacological manipulation of 5-HT neurotransmission, with both facultative and inhibitory responses observed depending on the receptor subtype that is activated (Barrett et al., 2006). Additionally, bonobos engage in socio-sexual behavior for a variety of non-reproductive purposes, such as reconciliation after an aggressive encounter, bonding between females and relieving tension during social feeding (Woods and Hare, 2011).
Variation related to the serotonergic system has been shown to influence the regulation of social behaviors as well as amygdala function in other species. For example, the brains of foxes and rats selectively bred for reduced impulsive aggression exhibited elevated levels of serotonin and tryptophan hydroxylase (an enzyme involved in serotonin synthesis) relative to unselected populations (reviewed in Hare et al., 2012). Polymorphisms of the SERT gene 5-HTTLPR are associated with anxiety, depression and aggression in humans and macaque monkeys (Lesch et al., 1997; Pezawas et al., 2005; Wendland et al., 2005) as well as vulnerability to neuropsychiatric disorders (Søeby et al., 2005; Wendland et al., 2005; Jedema et al., 2010). Individuals carrying a variant of the polymorphism with a shorter repeat sequence have been found to be more exploratory, but also more hesitant when making decisions in reward tasks, potentially an adaptive behavioral strategy during conditions of social and environmental unpredictability (Lesch et al., 1996; Heils et al., 1997; Greenberg et al., 2000; Barr et al., 2005). Congruent with this idea, more tolerant species of macaque monkeys, for which social interactions are more predictable, carry a single allele of 5-HTTLPR, whereas species of macaques with a more despotic, hierarchical social structure display an additional short variant (Wendland et al., 2005). In the brain, the short allelic variant has been linked to heightened amygdala response to social threats such as angry faces, reduced amygdala volume, reduced anterior cingulate cortex volume and functional decoupling of these two structures (Hariri et al., 2002; Pezawas et al., 2005). Although these studies suggest a link between serotonergic innervation in the amygdala and adaptive social behaviors, the neural correlates of 5-HT system gene polymorphisms in primates remain poorly understood. Of relevance to our findings, it is notable that the 5-HTTLPR gene has been sequenced in only a very small number of bonobo and chimpanzee individuals to date (Dobson and Brent, 2013). Current data indicates that there are polymorphisms in the number of repeat elements (between 18 and 20) in 5-HTTLPR sequences (Lesch et al. 1997; Inoue Murayam et al., 2000) but it is uncertain if these differ in their frequency between bonobos and chimpanzees. Given that our results show such striking differences in the phenotype of serotonergic innervation in the amygdala, we predict that further genetic analyses will reveal species-specific variability in the frequencies of 5-HTTLPR alleles or in other serotonergic system genes, such as HTR1A, HTR1B and TPH2.
Other recent studies have also identified differences between bonobos and chimpanzees in the neural systems that regulate social and emotional processing, including variation in the amygdala. Notably, chimpanzees were shown to have a greater number of neurons in the central nucleus of the amygdala than bonobos and also larger numbers of neurons in the basal nucleus than predicted by allometric scaling across the great apes (Barger et al., 2012). Furthermore, diffusion tensor imaging demonstrated that, in bonobos compared with chimpanzees, the pathways connecting the amygdala with other brain regions were expanded and voxel-based morphometry showed relatively enlarged volume of the anterior cingulate cortex and dorsal portions of the amygdala (Rilling et al., 2012). Additionally, differences in the volume and asymmetry of insular cortex and striatum have also been observed between the species (Hopkins et al., 2009; Bauernfeind et al., 2013). Taken together with our results, it is evident that despite the close relatedness of bonobos and chimpanzees, evolution has shaped the distinctive morphology, connectivity and molecular biology of the brain in each lineage to construct species-specific networks for emotional responses and social interactions.
Supplementary Material
Acknowledgements
The authors acknowledge the Milwaukee Country Zoo, the Jacksonville Zoo, the Ape Cognition and Conservation Initiative, Yerkes National Primate Research Center (supported by National Institutes of Health grant ORIP/OD P51 OD011132) and all veterinary staff for donation of specimens. We thank Dr. Mary Ann Raghanti for helpful discussion.
Funding
This work was supported by the National Science Foundation (SBE-1542848), National Institutes of Health (NS042867 NS073134, and NS092988) and the James S. McDonnell Foundation (220020293).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Adolphs R. (1999). The human amygdala and emotion. Neuroscientist, 5(2), 125–37. [Google Scholar]
- Adolphs R. (2010). What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences, 1191, 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D., Damasio A.R. (1998). The human amygdala in social judgement. Nature, 393, 470–4. [DOI] [PubMed] [Google Scholar]
- Albert P.R., Vahid-Ansari F., Luckhart C. (2014). Serotonin-prefrontal cortical circuitry in anxiety and depression phenotypes: pivotal role of pre- and post-synaptic 5-HT1A receptor expression. Frontiers in Behavioral Neuroscience, 8, 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral D. (2003). Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience, 118, 1099–120. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Bassett J.L. (1989). Cholinergic innervation of the monkey amygdala: an immunohistochemical analysis with antisera to choline acetyltransferase. The Journal of Comparative Neurology, 281, 337–61. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L., Pitkänen A., Carmichael S.T. (1992). Anatomical organization of the primate amydaloid complex. In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. New York: Wiley-Liss, 1–66. [Google Scholar]
- Angoa-Pérez M., Kuhn D.M. (2015). Neuroanatomical dichotomy of sexual behaviors in rodents: a special emphasis on brain serotonin. Behavioral Pharmacology, 26(6), 595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asan E., Steinke M., Lesch K-P. (2013). Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochemistry and Cell Biology, 139, 785–813. [DOI] [PubMed] [Google Scholar]
- Barbas H. (2007). Flow of information for emotions through temporal and orbitofrontal pathways. Journal of Anatomy, 211(2), 237–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger N., Stefanacci L., Schumann C.M., et al. (2012). Neuronal populations in the basolateral nuclei of the amygdala are differentially increased in humans compared with apes: a stereological study. The Journal of Comparative Neurology, 520, 3035–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger N., Stefanacci L., Semendeferi K. (2007). A comparative volumetric analysis of the amygdaloid complex and basolateral division in the human and ape brain. American Journal of Physical Anthropology, 134, 392–403. [DOI] [PubMed] [Google Scholar]
- Barr C.S., Newman T.K., Becker M.L., et al. (2003). The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain, and Behavior, 2, 336–40. [DOI] [PubMed] [Google Scholar]
- Barrett G.M., Bardi M., Guillén A.K., Mori A., Shimizu K. 2006. Regulation of sexual behavior in male macaques by sex steroid modulation of the serotonergic system. Experimental Physiology, 91(2): 445–56. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A.L., de Sousa A.A., Avasthi T., et al. (2013). A volumetric comparison of the insular cortex and its subregions in primates. Journal of Human Evolution, 64(4), 263–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers L. (1990). The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts of Neuroscience, 1, 27–51. [Google Scholar]
- Calandreau L., Desmedt A., Decorte L., Jaffard R. (2005). A different recruitment of the lateral and basolateral amygdala promotes contextual or elemental conditioned association in Pavlovian fear conditioning. Learning and Memory, 12(4), 383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari M., Ceccarelli A., Wang G., et al. (2014). Microstructural changes in the striatum and their impact on motor and neuropsychological performance in patients with multiple sclerosis. PLoS One, 9(7), e101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes M.C., Delville Y. (2007). Individual differences in offensive aggression in golden hamsters: a model of reactive and impulsive aggression? Neuroscience, 150, 511–21. [DOI] [PubMed] [Google Scholar]
- Cervantes M.C., Delville Y. (2009). Serotonin 5-HT1A and 5-HT3 receptors in an impulsive-aggressive phenotype. Behavioral Neuroscience, 123(3), 589–98. [DOI] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson R.J. (2000). Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science, 289, 591–94. [DOI] [PubMed] [Google Scholar]
- Davis M. (1992). The role of the amygdala in fear and anxiety. Annual Review of Neuroscience, 15, 353–75. [DOI] [PubMed] [Google Scholar]
- de Waal F. (1997). Bonobo: The Forgotten Ape. Berkley: University of California Press. [Google Scholar]
- Dobson S.D., Brent L.J.N. (2013). On the evolution of the serotonin transporter linked polymorphic region (5-HTTLPR) in primates. Frontiers in Human Neuroscience, 7, 588. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston D.R., Gruber A.J., McNaughton B.L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron, 76, 1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. (1992). Amygdala-ventral striatal interactions and reward-related processes. In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfuntion. New York: Wiley-Liss, 401–42. [Google Scholar]
- Freedman L.J., Shi C. (2001). Monoaminergic innervation of the macaque extended amygdala. Neuroscience, 104, 1067–84. [DOI] [PubMed] [Google Scholar]
- Freese J.L., Amaral D.G. (2009). Neuroanatomy of the primate amygdala. In: Whalen P.J., Phelps E.A., editors. The Human Amygdala. New York, NY: The Guilford Press, 3–42. [Google Scholar]
- Fried I., MacDonald K.A., Wilson C.L. (1997). Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron, 18, 753–65. [DOI] [PubMed] [Google Scholar]
- Goodall J. (1986). The Chimpanzees of Gombe. Cambridge, MA: Harvard University Press. [Google Scholar]
- Greenberg B.D., Li Q., Lucas F.R., et al. (2000). Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population study. American Journal of Medical Genetics, 96(2), 202–16. [DOI] [PubMed] [Google Scholar]
- Hare B., Melis A.P., Woods V., Hastings S., Wrangham R. (2007). Tolerance allows bonobos to outperform chimpanzees on a cooperative task. Current Biology, 17, 619–23. [DOI] [PubMed] [Google Scholar]
- Hare B., Wobber V., Wrangham R. (2012). The self-domestication hypothesis: evolution of bonobo psychology is due to selection against aggression. Animal Behavior, 83, 573–85. [Google Scholar]
- Hariri A.R., Mattay V.S., Tessitore A., et al. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297, 400–3. [DOI] [PubMed] [Google Scholar]
- Hassanain M., Bhatt S., Siegel A. (2003). Differential modulation of feline defensive rage behavior in the medial hypothalamus by 5-HT1a and 5-HT2 receptors. Brain Research, 981, 201–9. [DOI] [PubMed] [Google Scholar]
- Heilbronner S.R., Rosati A.G., Stevens J.R., Hare B., Hauser M.D. (2008). A fruit in the hand or two in the bush? Divergent risk preferences in chimpanzees and bonobos. Biology Letters, 4(3), 246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A., Mössner R., Lesch K.P. (1997). The human serotonin transporter gene polymorphism – basic research and clinical implications. Journal of Neural Transmission, 104(10), 1005–14. [DOI] [PubMed] [Google Scholar]
- Heimer L., de Olmos J.S., Alherd G.F., et al. (1999). The human basal forebrain. In: Bloom F.E., Björklund A., Hökfelt T., editors. Handbook of Chemical Neuroanatomy: The Primate Nervous System, Part III. Amsterdam: Elsevier, 57–226. [Google Scholar]
- Herrmann E., Hare B., Call J., Tomasello M. (2010). Differences in cognitive skills of bonobos and chimpanzees. PLoS One, 5(8), e12438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann E., Hare B., Cissewski J., Tomasello M. (2011). A comparison of temperament in nonhuman apes and human infants. Developmental Science, 14, 1393–405. [DOI] [PubMed] [Google Scholar]
- Hopkins W.D., Lyn H., Cantalupo C. (2009). Volumetric and lateralized differences in selected brain regions of chimpanzees (Pan troglodytes) and bonobos (Pan paniscus). American Journal of Primatology, 71, 988–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue-Murayam M., Niimi Y., Takenaka O., Okada K., Matsuzaki I., Ito S., Murayama Y. (2000). Allelic variation of the serotonin transporter gene polymorphic region in apes. Primates, 41, 267–73. [DOI] [PubMed] [Google Scholar]
- Jacobs C., van der Broeck W., Simoens P. (2007). Neurons expressing serotonin-1B receptor in the basolateral nuclear group of the amygdala in normally behaving and aggressive dogs. Brain Research, 1136, 102–9. [DOI] [PubMed] [Google Scholar]
- Jedema H.P., Gianaros P.J., Greer P.J., et al. (2010). Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin transmission. Molecular Psychiatry, 15, 512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Adolphs R. (2012). Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia, 50(14), 3313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.P., Gläscher J., Tyszka J., Adolphs R. (2009). Personal space regulation by the human amygdala. Nature Neuroscience, 12, 1226–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling A.S., Brothers L.A. (1992). The amygdala and social behavior. In: Aggleton J.P., editor. The Amygdala, Neurobiological Aspects of Emotion, Memory and Mental Dysfuntion. New York: Wiley-Liss, 353–77. [Google Scholar]
- Kling A.S., Steklis H.D. (1976). A neural substrate for affiliative behavior in nonhuman primates. Brain, Behavior and Evolution, 13, 216–38. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (1996). The Emotional Brain. New York, NY: Simon and Schuster. [Google Scholar]
- Ledoux J. (2007). The amygdala. Current Biology, 17(20), R868–74. [DOI] [PubMed] [Google Scholar]
- Lesch K.P., Araragi N., Waider J., van den Hove D., Gutknecht L. (2012). Targeting brain serotonin synthesis, insights into neurodevelopmental disorders with long-term outcomes related to negative emotionality, aggression and antisocial behavior. Philosophical Transactions of the Royal Society of London Series B, Biological Sciences, 367, 2426–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K.P., Bengel D., Helis A., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science, 274(5292), 1527–31. [DOI] [PubMed] [Google Scholar]
- Lesch K.P., Meyer J., Glatz K., et al. (1997). The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective, alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission, 104, 1259–66. [DOI] [PubMed] [Google Scholar]
- Markota M., Sin J., Pantazopoulos H., Jonilionis R., Berretta S. (2014). Reduced dopamine transporter expression in the amygdala of subjects with schizophrenia. Schizophrenia Bulletin, 40(5), 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Buckholtz J.W., Kolachana B., (2006). Neural mechanisms of genetic risk for impulsivity and violence in humans. Proceedings of the National Academy of Sciences of the United States of America, 103(16), 6269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D.L., Andrews A.M., Wichems C.H., Li Q., Tohda M., Greenberg B. (1998). Brain serotonin neurotransmission, an overview and update with an emphasis on serotonin subsystem heterogeneity, multiple receptors, interactions with other neurotransmitter systems, and consequent implications for understanding the actions of serotonergic drugs. Journal of Clinical Psychology, 59(S15), 4–12. [PubMed] [Google Scholar]
- Packard M.G., Cahill L., McGaugh J.L. (1994). Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proceedings of the National Academy of Sciences of the United States of America, 91, 8477–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L., Meyer-Lindenberg A., Drabant E.M., et al. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience, 8, 828–34. [DOI] [PubMed] [Google Scholar]
- Phillips R.G., LeDoux J.E. (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–85. [DOI] [PubMed] [Google Scholar]
- Pitkänen A., Savander V., LeDoux J.E. (1997). Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends in Neurosciences, 20, 517–23. [DOI] [PubMed] [Google Scholar]
- Price J.L., Russchen F., Amaral D.G. (1987). The limbic region: II. The amygdaloid complex. In: Björklund L.W.S.A., Hökfelt T.,editors. Handbook of Chemical Neuroanatomy, Vol. 5, Integrated Systems of the CNS. Amsterdam: Elsevier Science, 279–388. [Google Scholar]
- Prüfer K., Munch K., Hellmann I., et al. (2012). The bonobo genome compared with the chimpanzee and human genomes. Nature, 486(7), 527–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Melikian H.E., Rye D.B., Levey A.I., Blakley R.D. (1995). Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. Journal of Neuroscience, 15, 1261–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghanti M.A., Stimpson C.D., Marcinkieqicz J.L., Erwin J.M., Hof. P.R., Sherwood C.C. (2008). Differences in cortical serotonergic innervation among humans, chimpanzees, and macaque monkeys: a comparative study. Cerebral Cortex, 18(3), 584–97. [DOI] [PubMed] [Google Scholar]
- Ragsdale C.W., Graybiel A.M. (1991). Compartmental organization of the thalamostriatal connection in the cat. The Journal of Comparative Neurology, 311, 134–67. [DOI] [PubMed] [Google Scholar]
- Ramsey I.S., DeFelice L.J. (2002). Serotonin transporter function and pharmacology are sensitive to expression level: evidence for an endogenous regulatory factor. Journal of Biological Chemistry, 277(17), 14475–82. [DOI] [PubMed] [Google Scholar]
- Reynolds G.P. (1992). The amygdala and the neurochemistry of schizophrenia. In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfuntion. New York: Wiley-Liss, 561–74. [Google Scholar]
- Rilling J.K., Scholz J., Preuss T.M., Glasser M.F., Errangi B.K., Behrens T.E. (2012). Differences between chimpanzees and bonobos in neural systems supporting social cognition. Social Cognitive and Affective Neuroscience, 7, 369–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. (1992). Neurophysiology and functions of the primate amygdala. In: Aggleton J.P., editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfuntion. New York: Wiley-Liss, 143–165. [Google Scholar]
- Schumann C., Amaral D.G. (2006). Stereological analysis of amygdala neuron number in autism. Journal of Neuroscience, 26(29), 7674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafeim A., Grafton G., Chamba A., et al. (2002). 5-Hydroxytrptamine drives apoptosis in biopsydlike Burkitt lymphoma cells: reversal by selective serotonin reuptake inhibitors. Blood, 99(7), 2545–53. [DOI] [PubMed] [Google Scholar]
- Sherwood C.C., Raghanti M.A., Stimpson C.D., et al. (2007). Scaling of inhibitory interneurons in areas V1 and V2 of anthropoid primates as revealed by calcium-binding protein immunohistochemistry. Brain, Behavior and Evolution, 69, 176–95. [DOI] [PubMed] [Google Scholar]
- Shu S., Ju G., Fan L. (1988). The glucose-oxidase-DAB-nickel method in peroxidase histochemistry of the nervous system. Neuroscience Letters, 85, 169–71. [DOI] [PubMed] [Google Scholar]
- Søeby K., Larsen S.A., Olsen L., Rasmussen H.B., Werge T. (2005). Serotonin transporter: evolution and impact of polymorphic transcriptional regulation. American Journal of Medical Genetics Part B, 136B, 53–7. [DOI] [PubMed] [Google Scholar]
- Stefanacci L., Amaral D.G. (2002). Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. The Journal of Comparative Neurology, 451, 301–23. [DOI] [PubMed] [Google Scholar]
- Stone V.E., Baron-Cohen S., Calder A., Keane J., Young A. (2003). Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychologia, 41, 209–20. [DOI] [PubMed] [Google Scholar]
- Stone V.E., Cosmides L., Tooby J., Kroll N., Knight R.T. (2002). Selective impairment of reasoning about social exchange in a patient with bilateral limbic system damage. Proceedings of the National Academy of Sciences of the United States of America, 99, 11531–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart A.M., Mitchell I.J., Slater P., Unwin H.L., Crossman A.R. (1986). A semi-quantitative atlas of 5-hudroxtryptamine-1 receptors in the primate brain. Neuroscience, 18(3), 619–39. [DOI] [PubMed] [Google Scholar]
- Sur C., Betz H., Schloss P. (1996). Immunocyctochemical detection of the serotonin transporter in rat brain. Neuroscience, 73 (1), 217–31. [DOI] [PubMed] [Google Scholar]
- Truitt W.A., Sajdyk T.J., Dietrich A.D., Oberlin B., McDougle C.J., Shekhar A. (2007). From anxiety to autism: spectrum of abnormal social behaviors modeled by progressive disruption of inhibitory neuronal function in the basolateral amygdala in Wistar rats. Psychopharmacology, 191, 107–18. [DOI] [PubMed] [Google Scholar]
- Vallender E.J., Lynch L., Novak M.A., Miller G.M. (2009). Polymorphisms in the 3’ UTR of the serotonin transporter are associated with cognitive flexibility in rhesus macaques. American Journal of Medical Genetics Part B, 150B, 467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Gucht E., Vandesande F., Arckens L. (2001). Neurofilament protein: a selective marker for the architectonic parcellation of the visual cortex in adult cat brain. The Journal of Comparative Neurology, 441, 345–68. [DOI] [PubMed] [Google Scholar]
- Verney C., Lebrand C., Gaspar P. (2002). Changing distribution of monoaminergic markers in the developing human cerebral cortex with special emphasis on the serotonin transporter. The Anatomical Record, 267, 87–93. [DOI] [PubMed] [Google Scholar]
- Wang C.C., Lin H.C., Chan Y.H., Gean P.W., Yang Y.K., Chen P.S. (2013). 5-HT1A-recptor agonist modified amygdala activity and amygdala-associated social behavior in a valproate-induced rat autism model. The International Journal of Neuropsychopharmacology, 16, 2027–39. [DOI] [PubMed] [Google Scholar]
- Watson K.K., Ghodasra J.H., Platt M.L. (2009). Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS One, 4, e4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D.P., Muller M., Amsler S.J., Mbabazi G., Mitani J.C. (2006). Lethal intergroup aggression by chimpanzees in Kibale National Park, Uganda. American Journal of Primatology, 68(2), 161–80. [DOI] [PubMed] [Google Scholar]
- Wendland J.R., Lesch K.P., Newman T.K., et al. (2005) Differential functional variability of serotonin transporter and monoamine oxidase A genes in macaque species displaying contrasting levels of aggression-related behavior. Behavior Genetics, 36(2), 163–72.. [DOI] [PubMed] [Google Scholar]
- Wilson M.L., Boesch C., Fruth B., et al. (2014). Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature, 513, 414–7. [DOI] [PubMed] [Google Scholar]
- Wobber V., Hare B., Maboto J., Lipson S., Wrangham R., Ellison P.T. (2010a). Differential changes in steroid hormones before competition in bonobos and chimpanzees. Proceedings of the National Academy of Sciences of the United States of America, 107, 12457–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobber V., Wrangham R., Hare B. (2010. b). Bonobos exhibit delayed development of social behavior and cognition relative to chimpanzees. Current Biology, 20, 226–30. [DOI] [PubMed] [Google Scholar]
- Woods V., Hare B. (2011). Bonobo but not chimpanzee infants use socio-sexual contact with peers. Primates, 52, 111–6. [DOI] [PubMed] [Google Scholar]
- Wrangham R.W. (1993). The evolution of sexuality in chimpanzees and bonobos. Human Nature, 4(1), 47–79. [DOI] [PubMed] [Google Scholar]
- Wrangham R.W. (1999). Evolution of coalitionary killing. Yearbook of Physical Anthropology, 42, 1-30. [DOI] [PubMed] [Google Scholar]
- Zhou F.C., Tao-Cheng J.H., Segu L., Patel T., Wang Y. (1998). Serotonin transporters are located on the axons beyond the synaptic junctions, anatomical and functional evidence. Brain Research, 805, 241–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.