Abstract
Defects in experiencing disgust may contribute to obesity by allowing for the overconsumption of food. However, the relationship of disgust proneness and its associated neural locus has yet to be explored in the context of obesity. Thirty-three participants (17 obese, 16 lean) completed the Disgust Propensity and Sensitivity Scale-Revised and a functional magnetic resonance imaging paradigm where images from 4 categories (food, contaminates, contaminated food or fixation) were randomly presented. Independent two-sample t-tests revealed significantly lower levels of Disgust Sensitivity for the obese group (mean score = 14.7) compared with the lean group (mean score = 17.6, P = 0.026). The obese group had less activation in the right insula than the lean group when viewing contaminated food images. Multiple regression with interaction analysis revealed one left insula region where the association of Disgust Sensitivity scores with activation differed by group when viewing contaminated food images. These interaction effects were driven by the negative correlation of Disgust Sensitivity scores with beta values extracted from the left insula in the obese group (r = −0.59) compared with a positive correlation in the lean group (r = 0.65). Given these body mass index–dependent differences in Disgust Sensitivity and neural responsiveness to disgusting food images, it is likely that altered Disgust Sensitivity may contribute to obesity.
Keywords: disgust, fMRI, obesity, BMI, insula
Introduction
Obesity is a growing public health concern with many contributing factors (Caballero, 2007; Cohen, 2008). Numerous biological, social and learned factors contribute to feeding behavior, including initiation and termination of food intake (Berthoud et al., 2002; Berthoud, 2012). Darwin (2009) initially proposed that experiencing disgust facilitates food rejection (Darwin, 2009). This idea has been corroborated by Rozin and Fallon (1987), who assert that even the physical disgust response (e.g. closing of the nostrils, nausea and gagging) functions to terminate eating. Therefore, deficits in the experience of disgust specifically in relation to food may be one factor that contributes to obesity.
Increased levels of food-related disgust have been associated with eating disorders (Davey et al., 1998; Troop et al., 2002). For example, one study found higher levels of disgust to various stimuli, especially in response to foods, in anorexia nervosa (Aharoni and Hertz, 2012). Because anorexia nervosa is characterized by a decreased body mass index (BMI), it is possible that increased disgust proneness that prevents adaptive eating behavior contributes to reduced BMI. Conversely, a recent study found significantly lower disgust proneness scores in obese compared with lean individuals (Houben and Havermans, 2012). These findings may suggest that increased disgust proneness among individuals with anorexia nervosa may partially account for lower BMI, whereas decreased disgust proneness among obese individuals may be associated with higher BMI.
Recent developments in the psychometric assessment of individual differences in disgust proneness may allow for a more precise delineation of the role of disgust in obesity. Indeed, the development of the Disgust Propensity and Sensitivity Scale-Revised (DPSS-R) allows investigators to distinguish between two characteristics of disgust proneness: how easily people are disgusted (propensity) and how unpleasant the experience of disgust is appraised (sensitivity). The DPSS-R serves as a useful alternative to the Disgust Scale (Haidt et al., 1994) and the Disgust and Contamination Sensitivity Questionnaire (Rozin et al., 1984) in that it does not measure disgust for specific elicitors. This is of particular importance for a study examining differences in food-related disgust because it limits the possibility that between-group differences are due to irrelevant elicitors such as spiders or blood (van Overveld et al., 2006). Given that the neural basis for potentially altered disgust response in obesity is unclear, utilizing the DPSS-R in conjunction with a food-related disgust functional magnetic resonance imaging (fMRI) task is a first step in the exploration of the neurobehavioral disgust response.
Delineation of brain regions associated with disgust responding may offer important insights into eating behaviors that contribute to obesity. Insula activation has been associated with individual differences in disgust proneness when viewing disgust stimuli (Phillips et al., 1997; Mataix-Cols et al., 2008). Baumann and colleagues showed greater insula activation to disgusting images compared with neutral images in normal weight individuals (Wicker et al., 2003; Wright et al., 2004; Baumann and Mattingley, 2012). The insula is thought to regulate interoceptive awareness and may play a role in satiety (Craig, 2003, 2009). Viewing images of disgusting foods, in contrast with images of non-food items or appetizing foods, elicits insula activation (Calder et al., 2007). Therefore, it is likely that the insula actively regulates the disgust response. This pattern of finding raises the possibility that brain regions associated with disgust may be underactive among obese individuals, especially in response to food cues.
Although imaging studies have identified the insula as a primary region implicated in the disgust response, much remains unknown about the neural correlates of the disgust response in obesity. To our knowledge, no studies have examined differences in brain activation in response to disgusting food-related images between lean and obese individuals. Thus, we paired disgusting food-related images with the administration of the DPSS-R to examine the neurobehavioral correlates of the disgust response specifically in the context of food. We hypothesized that obese individuals compared with lean individuals (i) will have decreased disgust proneness scores and (ii) exhibit decreased insula activation during a disgust-probing food-specific fMRI task.
Methods
Participants
Thirty-three participants (17 obese, 16 lean) who met the following eligibility criteria were recruited via e-mail and poster advertisement: no current medical illness, no past brain trauma, no use of psychotropic medications and no current or past drug or alcohol abuse. Height and weight were measured on the initial screening day and BMI was calculated [BMI = weight (kg)/height2 (m)]. Participants with a BMI > 30 were classified as obese and participants with a BMI < 25 were classified as lean. Individuals with a BMI of 25–30 were classified as overweight and excluded from the study.
Study procedures
Each participant completed the DPSS-R before an fMRI task (van Overveld et al., 2006). The DPSS-R distinguishes between two characteristics of disgust proneness: how easily people are disgusted (propensity) and how unpleasant the experience of disgust is appraised (sensitivity). Participants rated how often a statement is true based on a five point Likert ranging from ‘never’ to ‘always’. Example statements include ‘I avoid disgusting things’ (propensity) and ‘When I feel disgusted, I worry that I might pass out’ (sensitivity). All questionnaires were completed in the afternoon after a 4-h fast. The study protocol was approved by the Vanderbilt University Institutional Review Board and the procedures were in accordance with the guidelines of the Helsinki Declaration on human experimentation.
fMRI task
Participants completed a randomized jittered rapid-event-related fMRI paradigm where images (14 per category) from 4 categories, food (e.g. crackers, cookies), contaminates (e.g. mold, toilet), contaminated food (e.g. moldy cracker, cookies on a toilet seat) or fixation (white crosshairs on black background), were randomly presented. The fixation cross was used as the baseline condition. Food, contaminates and contaminated food images were generally matched for color, intensity and brightness but not for additional visual parameters or complexity. The order of images was randomized across all trials. The order of category of image was also randomized to prevent possible order effects. Participants saw each image only once throughout the study. Images from the food category consisted of high and low energy dense foods. These images were not selected to examine differences in caloric content, but instead selected to represent the full spectrum of food choices available across several diets. The images in the contaminated food category were created by combining the images from the food category with the images from the contaminates category. Some of these images were obtained with a digital camera (e.g. pizza left in a dumpster) and the other images were created in Photoshop (e.g. bugs crawling over a filet of salmon). Although the level of disgust elicited by each image was not quantified, the disgust stimuli were modeled after examples listed in various disgust questionnaires and International Affective Picture System (IAPS) images.
The images were presented for 4 s with an inter-stimulus interval presentation of a fixation cross jittered between 2 and 8 s. To enhance the ecological validity of the task, the participants were instructed to think about whether they would eat what was presented in each image. All fMRI scans were conducted in the afternoon after a 4-h fast in order to induce a hunger state in participants. Participant hunger was assessed using a self-report hunger scale that provided a composite hunger index that included individual questions on current hunger and time since last meal (Grand, 1968).
fMRI data acquisition
Participants underwent fMRI on a 3T Intera Achieva MRI scanner (Phillips Medical Systems, Andover, MA). In each 270 s functional run, 28 field echo EPI (128 dynamics, 4.50 mm slice thickness with 0.45 mm gap, 2 s repetition time, 34 ms echo time, 79° flip angle, field of view = 240, matrix = 80 × 78) scans were acquired.
Analyses of normality
Normality was determined by calculating the Fisher Z score (skewness/standard error of skewness). The data were normally distributed if |Fisher Z| < 2.58 (P = 0.01). Pearson’s correlations were used for normally distributed data and Spearman’s Rho correlations were used for non-normally distributed data. One subject was labeled as an outlier and excluded from the dataset because extracted beta values were greater than five standard deviations from the mean (mean = −2.45, s.d. = 9.75, outlier beta value = −56.6).
Self-reported data analysis
Scale score differences between the two groups were conducted using independent t-tests. Statistical significance of the between-group differences was determined at P ≤ 0.05.
fMRI data analysis
Data were analyzed using SPM8 (Wellcome Department of Cognitive Neuroscience, London) utilizing the General Linear Model and a random effects analysis. The functional data were slice-time corrected using the first slice as the reference slice and then motion corrected by being spatially aligned to their mean functional image. Images were stereotactically normalized to the SPM EPI template (Montreal Neurological Institute) and then smoothed with a full-width half maximum 8-mm Gaussian Kernel.
All conditions (i.e. food, contaminates, contaminated foods) were modeled by group (i.e. obese, lean) in a full-factorial model in SPM8. This method allows for the calculation of all possible contrasts of interests (e.g. lean > obese: food, lean > obese: contaminates, lean > obese: contaminated food, obese > lean: food, obese > lean: contaminates, obese > lean: contaminated foods, lean > obese: contaminated food > contaminates + food, obese > lean: contaminated food > contaminates + food). All contrasts displaying significant results were calculated, reported and discussed in future sections of this article.
Regions of interest analysis was restricted to bilateral insula. The insula mask was created using WFUpickatlas. We used the AFNI-based Alphasim program to run a Monte Carlo simulation to determine the extent threshold and voxel cluster size for uncorrected P values to generate a family wise error corrected P ≤ 0.05 for our bilateral insula mask. The extent threshold cluster size for voxel-level P values of P ≤ 0.05 and P ≤ 0.01 were 74 and 35 voxels, respectively.
fMRI regression analysis
Our primary fMRI regression analysis was used to examine the association of the behavioral measure of disgust with the disgust-based fMRI task. We used the multiple regression module in SPM8 to examine the association of Disgust Sensitivity scores with BOLD activation elicited from viewing each image condition (food, contaminate, contaminated food). In order to control for the effect of group (lean vs obese), group was modeled as a condition of no effect in our primary regression model. A subsequent regression model—with the addition of an interaction term of Disgust Sensitivity scores with group—was created to determine where the association of Disgust Sensitivity scores with BOLD activation elicited from viewing each image condition differed between the lean and obese groups. A corrected P value of 0.05 was achieved at a voxel level P ≤ 0.01 and extent threshold voxel cluster size of 35. Beta values were extracted from (region of interest) ROIs utilizing REX. SPSS was used to examine group-level correlations of Disgust Sensitivity scores with extracted beta values.
Results
Demographics
Mean (min, max) BMIs of the lean and obese groups were 21.5 (19.1, 23.7) and 36.4 (30.0, 45.6), respectively. Groups were equivalent on sex (lean men = 7, women = 9; obese men = 7, women = 10) and age (lean = 25.2, obese = 27.7) (Table 1). BMI and age were normally distributed (|Fisher Z| < 2.58).
Table 1.
Subject demographics
| Group | |||
|---|---|---|---|
| Lean (n = 16) | Obese (n = 17) | P value | |
| Male/female (n) | 7/9 | 7/10 | 0.384 (χ2) |
| Mean (min, max) | Mean (min, max) | ||
| Age | 25.2 (20, 32) | 27.7 (21, 35) | 0.077 |
| BMI | 21.5 (19.1, 23.7) | 36.4 (30.0, 45.6) | <0.001 |
BOLD = P < 0.05.
Behavioral
Hunger scores, Disgust Sensitivity scores and Disgust Propensity scores were normally distributed (|Fisher Z| < 2.58). Groups did not differ on the hunger index (P = 0.26; mean lean score = 19.33, 25 percentile = 16.56, 75 percentile = 22.69; mean obese score = 18.28, 25 percentile = 14.56, 75 percentile = 20.19). The obese groups demonstrated statistically significant lower levels of Disgust Sensitivity (mean score = 14.7, s.d. = 3.7, min = 8, max = 22) than the lean group (mean score = 17.6, s.d. = 3.7, min = 12, max = 26; P = 0.026). However, there was no statistically significant difference between the groups in Disgust Propensity (mean obese score = 20.9, s.d. = 4.9, min = 12, max = 32; mean lean score = 21.0, s.d. = 3.1, min = 16, max = 26; P = 0.906) (Table 2).
Table 2.
Disgust Propensity and Sensitivity Scale-Revised scores
| Group | |||
|---|---|---|---|
| Lean (n = 16) | Obese (n = 17) | P value | |
| Mean (min, max) | Mean (min, max) | ||
| Disgust Sensitivity Score | 17.6 (12, 26) | 14.7 (8, 22) | 0.026 |
| Disgust Propensity Score | 21.0 (16, 26) | 20.9 (12, 32) | 0.906 |
Neural activation
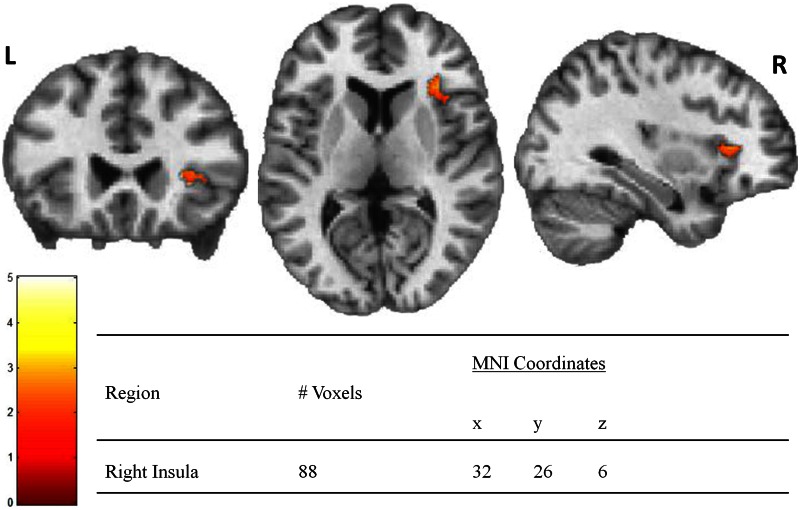
Between groups a priori bilateral insula analysis in SPM8 revealed one cluster in the right anterior insula where obese individuals had less BOLD activation than lean individuals when viewing contaminated food images (lean > obese: contaminated food > baseline) (Figure 1). No significant between-group differences were observed for any other contrast (e.g. obese > lean: food > baseline, lean > obese: contaminate > baseline, lean > obese: food > baseline).
Fig. 1.
Insula activation is lower in obese subjects when viewing contaminated foods. Note: Color bar represents T values for activated voxel group at statistical threshold of P < 0.05 and extent threshold = 74 voxels for corrected family wise error (P < 0.05).
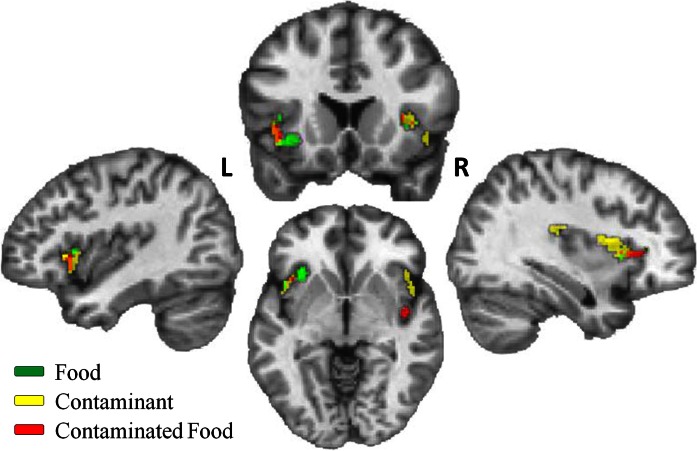
We next examined the association of Disgust Sensitivity scores with BOLD activation while viewing images from each condition (i.e. food, contaminate and contaminated food). Multiple regression analysis revealed several bilateral insula regions where Disgust Sensitivity scores were positively correlated with BOLD activation for each condition (Table 3). There was considerable overlap in BOLD activation across all three conditions. Notably, all right posterior BOLD activation was restricted to the contaminant and contaminated food conditions. All regions with positive associations are displayed on a single subject brain and color coded by condition (Figure 2). There were no negative associations of Disgust Sensitivity scores with BOLD activation in any condition.
Table 3.
Positive association of Disgust Sensitivity scores with activation while viewing food, contaminate and contaminant food images
| Region | No. of voxels | MNI coordinates |
|||
|---|---|---|---|---|---|
| x | y | z | |||
| Food | |||||
| Left insula | 144 | −34 | 24 | 0 | |
| Right insula | 36 | 32 | 16 | 4 | |
| Contaminate | |||||
| Left insula | 73 | −44 | 16 | 0 | |
| Right insula | 135 | 38 | 6 | 14 | |
| Right insula | 39 | 32 | −20 | 22 | |
| Right insula | 62 | 46 | 22 | −6 | |
| Contaminated food | |||||
| Left insula | 48 | −38 | 22 | 0 | |
| Right insula | 45 | 42 | −10 | −10 | |
| Right insula | 71 | 38 | 28 | 6 | |
MNI, Montreal Neurological Institute.
Fig. 2.
Positive association of Disgust Sensitivity scores with activation while viewing food, contaminant and contaminated food images. Note: Activated voxel group at statistical threshold of P < 0.01 and extent threshold = 35 voxels for corrected family wise error (P < 0.01).
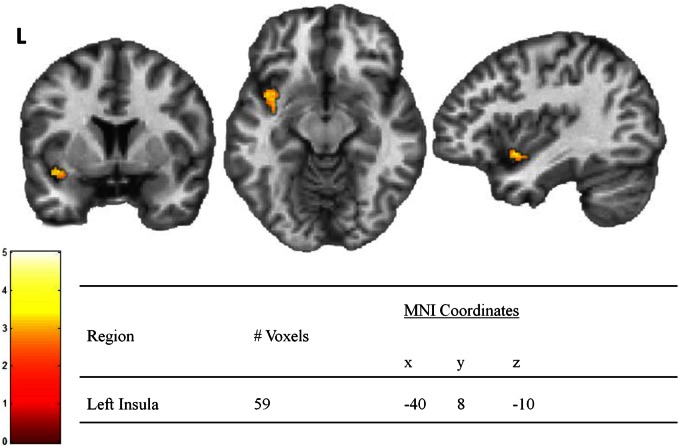
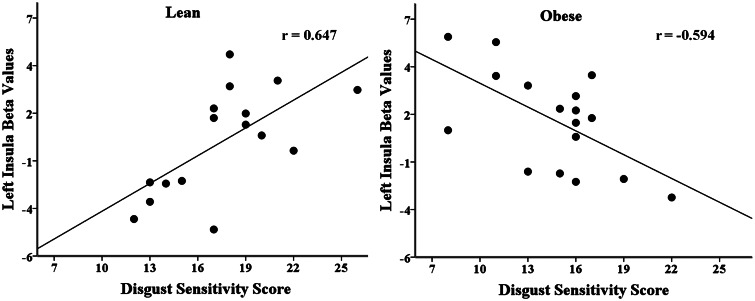
Finally, we determined where the association of Disgust Sensitivity scores with BOLD activation elicited from viewing each image condition differed between the lean and obese groups. Multiple regression with interaction analysis revealed one left insula region where the association of Disgust Sensitivity scores with BOLD activation differed by group when viewing contaminated food images (Figure 3). This effect was not present for the food or contaminant condition. To determine if the level of Disgust Sensitivity scores was associated with insula activation, we examined the group-level correlations of Disgust Sensitivity scores with beta values extracted from the significant left insula region. This analysis revealed a negative correlation of Disgust Sensitivity scores with beta values in the obese group (r = −0.59) and a positive correlation of Disgust Sensitivity scores with beta values in the lean group (r = 0.65, difference: Z = 3.77, P < 0.001) (Figure 4). BMI was not correlated with Disgust Sensitivity scores or extracted beta values, and therefore no mediation analysis was necessary.
Fig. 3.
Activation for the interaction of group (lean vs obese) and Disgust Sensitivity scores when viewing contaminated foods. Note: Color bar represents T values for activated voxel group at statistical threshold of P < 0.01 and extent threshold = 35 voxels for corrected family wise error (P < 0.01).
Fig. 4.
Inverse association of Disgust Sensitivity scores and activation in obese individuals.
Discussion
This study identified differences in disgust proneness and associated neural substrates between obese and lean individuals. Although lean and obese individuals did not significantly differ in how easily they are prone to experiencing disgust (propensity), obese individuals were less likely to appraise the experience of disgust as negative (sensitivity). The finding that obese individuals have lower Disgust Sensitivity scores further supports the possibility that a diminished disgust response may be one factor associated with obesity. Our findings are consistent with the notion that the experience of the disgust response and an associated negative appraisal are factors necessary to inhibit the drive for overconsumption. Although individuals with higher levels of disgust (e.g. individuals with anorexia nervosa) over-inhibit their drive to eat, it is possible that individuals with lower levels of disgust (e.g. obese individuals) may have a reduced ability to inhibit their drive to eat. In other words, obese and lean individuals may recognize that they are consuming excess calories (Disgust Propensity) but obese individuals experience diminished negative appraisal (Disgust Sensitivity), which may contribute to their failure to reduce caloric consumption.
As hypothesized, the insula had decreased activation in the obese group as compared with the lean group when viewing contaminated food images. This is, to our knowledge, the first identification of reduced insula activation to disgust stimuli in obese relative to lean individuals. This difference in insula activation was restricted to the right hemisphere, suggesting that the left insula may be less sensitive to contaminated food stimuli. Alternatively, if the observed findings are a consequence of obesity rather than a risk factor for it, the left insula may be resistant to the effects of obesity. Decreased insula activation was only observed when viewing contaminated foods, and not when viewing food or contaminant alone, suggesting that differences in disgust responses for lean vs obese individuals are specific to the interaction of food and contaminant. These findings raise the possibility that lower Disgust Sensitivity and reduced insula activation may contribute to the tendency to overeat among obese individuals.
The regression analysis revealed several overlapping areas within the insula with a positive association of Disgust Sensitivity scores with BOLD activation while viewing images from each category (food, contaminant, contaminated food). The majority of overlap was found in bilateral anterior insula, a region commonly associated with object valence (Britton et al., 2006; Viinikainen et al., 2010). Therefore, it is possible that the positive association of Disgust Sensitivity scores with BOLD activation within this sub-region of the anterior insula is driven by the need to assign a positive or negative valence to a viewed image. This is especially likely considering the rapid-event-related design of the experiment. When rapidly viewing and appraising whether one would eat various images of food, contaminant and contaminated foods, one must quickly discern whether a food item is edible or contaminated. This concept may be translated to animal foraging behavior, where an animal needs to identify fresh and edible items from rotting or poisonous items.
The regression analysis also revealed some distinct areas within the insula with a positive association of Disgust Sensitivity scores with BOLD activation while viewing images from the contaminant and contaminated food categories. Although one area of activation associated with contaminated food images was only slightly posterior to the primary anterior insula region, an area of activation associated with contaminant images was located in the far posterior insula. This suggests that the insula may be organized as a gradient, where the valence of food-related items is processed in the anterior insula and the valence of pure contaminants is processed in the posterior insula. Further studies are necessary to fully understand the anterior–posterior organization of the insula in terms of valence processing.
The interaction analysis revealed one area of activation within the anterior left insula where the association of Disgust Sensitivity scores with BOLD activation while viewing contaminated food images differed by group. Within this area of activation, there was a positive association of Disgust Sensitivity scores with BOLD activation in the lean group, whereas there was a negative association of Disgust Sensitivity scores with BOLD activation within the obese group. This finding suggests that there is a functional dissociation between self-report of disgust sensitivity (Disgust Sensitivity scores) and neural activation (extracted beta values) in obese individuals. In a typical excitatory neurobehavioral system, neural activation should increase as measurable behavior increases. In this context, this system remains intact in the lean group, but is disrupted in the obese group. This observed uncoupling of disgust-related behavior and neural activation among obese individuals may reflect a fundamental dysregulation in a disgust system that may help mediate appropriate eating behavior.
The behavioral result of dysregulation in the disgust system has been demonstrated in a previous study showing that lower measures for core and contamination disgust predict a greater likelihood of eating high calorie food (Houben and Havermans, 2012). Via this mechanism, lower disgust in obesity might lead to a greater probability of ingesting higher calorie foods, which are associated with obesity. Lower behavioral disgust, paired with a negative association of disgust scores with neural activation in disgust regions, may lead to increased food intake and subsequent weight gain. A reduced disgust response may slightly extend the extent to which obese individuals will consume food. This may present as an increase in the total calories consumed in one meal or as the frequency an individual will consume a high calorie food over a low calorie food (Houben and Havermans, 2012). This can be directly contrasted with individuals with anorexia nervosa—with higher levels of disgust—who are less likely to consume excess calories over an extended timeframe (Hadigan et al., 2000).
Barrett and Simmons (2015) propose that the anterior insula is one region within an overall interoceptive network responsible for a unified homeostatic and allostatic response. This network uses expectations about the world based on past experiences to estimate the body’s upcoming metabolic needs. In this context, any disruption in this system—such as an underactive disgust response—might potentially lead to an imbalance of the predicted need of metabolic resources and slightly bias the individual to choose higher calorie foods. Over the long term, this chronic imbalance may lead to continual intake of excess calories and associated increase in BMI.
There were several limitations to the study. Although stratifying the groups into lean (BMI < 25) and obese (BMI > 30) allows for stronger between-group comparisons, the addition of an overweight group (BMI = 25–30) may yield insight into the differences in disgust response during the transition from a lean BMI to an obese BMI. Because correlative findings do not reveal causative factors, a prospective study would be critical to determine if these differences were the cause or the consequence of obesity. Next, we did not quantify the level of disgust elicited by each image. However, the disgust stimuli were modeled after examples listed in various disgust questionnaires and IAPS images. Furthermore, it seems unlikely that individuals become obese by eating contaminated foods. Instead, the stimuli used in the study were designed to elicit a general disgust response and a food-specific disgust response. Finally, we did not screen individuals for specific dietary restrictions or guidelines (e.g. gluten-intolerance, veganism), allowing for the potential for individual brain changes inconsistent with those of a typical omnivore.
Our results are inconsistent with those reported by Houben et al., showing that obese individuals have lower Disgust Proneness, as measured by the Disgust Scale-Revised (Houben and Havermans, 2012). We observed that obese individuals have Disgust Propensity scores equivalent to lean individuals, but have decreased Disgust Sensitivity scores when compared with lean individuals. We believe that methodological differences may have accounted for the divergent findings—specifically the use of different disgust scales and differences in the BMI of the lean and obese groups. The Disgust Scale-Revised measures only disgust proneness and does not differentiate between two components of disgust proneness (propensity and sensitivity). In addition, Houben et al. divided their sample into low BMI (defined as 1 s.d. below the mean BMI) and high BMI (defined as 1 s.d. above the mean BMI). Their low BMI group consisted of individuals with a BMI range of 13.86–18.73 and their high BMI group consisted of individuals with a BMI range of 28.79–38.96. Therefore, their low BMI group was composed of a mix of underweight individuals (as defined by BMI < 18) or individuals with BMIs that fall in the low range of healthy weight (BMI = 18–24.9). It is not possible to distinguish whether the reported between-group differences for disgust proneness are influenced by the underweight nature of the low BMI group, or if they reflect a difference between lean and obese individuals.
Our results do not exhibit the commonly observed increase in neural activation in obese individuals when viewing food cues (Davids et al., 2009; Dimitropoulos et al., 2012; Martens et al., 2013). This is likely due to our unique task designed to examine the effects of altered disgust rather than altered activation in response to food cues. We did not attempt to stratify the food cues into images of high- and low-calorie foods, and this potentially accounts for the failure to elicit significant differences in food-related activation between the lean and obese groups. Furthermore, the disgust cues and food-related disgust cues were interleaved with the food cues. Because the task was rapid-event-related, it is possible that the intermixed disgust cues had a lingering effect that may have impacted any group differences in food-related neural activation. A future study using both a rapid-event-related design with disgust cues and a block design with only food cues could allow us to address this limitation.
Examination of differences in Disgust Sensitivity and associated neural substrates in this study supports the notion that a reduced disgust response may contribute to obesity. This study offers a dimensional complement to previous studies showing that individuals with anorexia nervosa have significantly elevated disgust response, specifically to food-related items, as compared with controls (Troop et al., 2002; Aharoni and Hertz, 2012). That is, although individuals with anorexia nervosa have a heightened food disgust response, obese individuals have a diminished food disgust response (anorexic > lean > obese). Because this diminished disgust response was observed at both the level of personality and at the level of neural responses, it might be possible that a decreased disgust response could encourage overeating. In the context of obesity, the disgust response may be a potential target for intervention.
Funding
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21HD053766 to R.L.C.); the National Institute of Mental Health (K01-MH083052 to J.U.B.); Resources of the Tennessee Valley Healthcare System and National Institute of Diabetes and Digestive and Kidney Diseases(RO1 DK085712-02 to K.N.); and the National Center for Research Resources, grant UL1 (RR024975-01), now at the National Center for Advancing Translational Sciences, grant 2 (UL1 TR000445-06). Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health.
Conflict of interest. Within the past 3 years, Dr Cowan has received publication royalties from Lippincott Williams and Wilkins, consultant income from the Southwest Michigan First Life Science Fund, the University of West Alabama, and Ascend Learning/Jones & Bartlett Learning and research, and salary support from Shire Pharmaceuticals and Novo Nordisk for projects not overlapping with this report. Dr Niswender receives research support from Novo Nordisk for a project not overlapping with this report. The other authors have no financial relationships relevant to this article to disclose.
References
- Aharoni R., Hertz M.M. (2012). Disgust sensitivity and anorexia nervosa. European Eating Disorders Review , 20, 106–10. [DOI] [PubMed] [Google Scholar]
- Barrett L.F., Simmons W.K. (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience , 16, 419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann O., Mattingley J.B. (2012). Functional topography of primary emotion processing in the human cerebellum. Neuroimage , 61, 805–11. [DOI] [PubMed] [Google Scholar]
- Berthoud H., Woods S., Cowley M., Levin B., Kelley A. (2002). Blaming the brain for obesity: neural control of food intake and energy homeostasis. Society for Neuroscience Abstract Viewer and Itinerary Planner , 2002, 393–428. [Google Scholar]
- Berthoud H.R. (2012). The neurobiology of food intake in an obesogenic environment. Proceedings of the Nutrition Society , 71, 478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J.C., Phan K.L., Taylor S.F., Welsh R.C., Berridge K.C., Liberzon I. (2006). Neural correlates of social and nonsocial emotions: an fMRI study. Neuroimage , 31, 397–409. [DOI] [PubMed] [Google Scholar]
- Caballero B. (2007). The global epidemic of obesity: an overview. Epidemiologic Reviews , 29, 1–5. [DOI] [PubMed] [Google Scholar]
- Calder A.J., Beaver J.D., Davis M.H., Van Ditzhuijzen J., Keane J., Lawrence A.D. (2007). Disgust sensitivity predicts the insula and pallidal response to pictures of disgusting foods. European Journal of Neuroscience , 25, 3422–8. [DOI] [PubMed] [Google Scholar]
- Cohen D.A. (2008). Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes , 57, 1768–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology , 13, 500–5. [DOI] [PubMed] [Google Scholar]
- Craig A.D. (2009). How do you feel—now? The anterior insula and human awareness. Nature Reviews Neuroscience , 10, 59–70. [DOI] [PubMed] [Google Scholar]
- Darwin C. (2009) Special expressions of man: suffering and weeping. In: Darwin F., editor. The Expression of the Emotions in Man and Animals, 2nd edn, Cambridge: Cambridge University Press. [Google Scholar]
- Davey G.C.L., Buckland G., Tantow B., Dallos R. (1998). Disgust and eating disorders. European Eating Disorders Review , 6, 201–11. [Google Scholar]
- Davids S., Lauffer H., Thoms K., et al. (2009). Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. International Journal of Obesity , 34, 94–104. [DOI] [PubMed] [Google Scholar]
- Dimitropoulos A., Tkach J., Ho A., Kennedy J. (2012). Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite , 58, 303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grand S. (1968). Color-word interference. II. An investigation of the role of vocal conflict and hunger in associative priming. Journal of Experimentai Psychology , 77, 31–40. [DOI] [PubMed] [Google Scholar]
- Hadigan C.M., Anderson E.J., Miller K.K., et al. (2000). Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. International Journal of Eating Disorders , 28, 284–92. [DOI] [PubMed] [Google Scholar]
- Haidt J., McCauley C., Rozin P. (1994). Individual differences in sensitivity to disgust: a scale sampling seven domains of digust elicitors. Personality and Individual Differences , 16, 701–13. [Google Scholar]
- Houben K., Havermans R.C. (2012). A delicious fly in the soup. The relationship between disgust, obesity, and restraint. Appetite , 58, 827–30. [DOI] [PubMed] [Google Scholar]
- Martens M.J., Born J.M., Lemmens S.G., et al. (2013). Increased sensitivity to food cues in the fasted state and decreased inhibitory control in the satiated state in the overweight. The American Journal of Clinical Nutrition , 97, 471–9. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D., An S.K., Lawrence N.S., et al. (2008). Individual differences in disgust sensitivity modulate neural responses to aversive/disgusting stimuli. European Journal of Neuroscience , 27, 3050–8. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Senior C., et al. (1997). A specific neural substrate for perceiving facial expressions of disgust. Nature , 389, 495–8. [DOI] [PubMed] [Google Scholar]
- Rozin P., Fallon A. (1987). A perspective on disgust. Psychological Review , 94, 23–41. [PubMed] [Google Scholar]
- Rozin P., Fallon A., Mandell R. (1984). Family resemblance in attitudes to foods. Developmental Psychology , 20, 309–314. [Google Scholar]
- Troop N.A., Treasure J.L., Serpell L. (2002). A further exploration of disgust in eating disorders. European Eating Disorders Review , 10, 218–26. [Google Scholar]
- van Overveld W.J.M., de Jong P.J., Peters M.L., Cavanagh K., Davey G.C.L. (2006). Disgust propensity and disgust sensitivity: separate constructs that are differentially related to specific fears. Personality and Individual Differences , 41, 1241–52. [Google Scholar]
- Viinikainen M., Jääskeläinen I.P., Alexandrov Y., Balk M.H., Autti T., Sams M. (2010). Nonlinear relationship between emotional valence and brain activity: evidence of separate negative and positive valence dimensions. Human Brain Mapping , 31, 1030–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. (2003). Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron , 40, 655–64. [DOI] [PubMed] [Google Scholar]
- Wright P., He G., Shapira N.A., Goodman W.K., Liu Y. (2004). Disgust and the insula: fMRI responses to pictures of mutilation and contamination. Neuroreport , 15, 2347–51. [DOI] [PubMed] [Google Scholar]