Abstract
Social closeness is a potent moderator of vicarious affect and specifically vicarious embarrassment. The neural pathways of how social closeness to another person affects our experience of vicarious embarrassment for the other’s public flaws, failures and norm violations are yet unknown. To bridge this gap, we examined the neural response of participants while witnessing threats to either a friend’s or a stranger’s social integrity. The results show consistent responses of the anterior insula (AI) and anterior cingulate cortex (ACC), shared circuits of the aversive quality of affect, as well as the medial prefrontal cortex and temporal pole, central structures of the mentalizing network. However, the ACC/AI network activation was increased during vicarious embarrassment in response to a friend’s failures. At the same time, the precuneus, a brain region associated with self-related thoughts, showed a specific activation and an increase in functional connectivity with the shared circuits in the frontal lobe while observing friends. This might indicate a neural systems mechanism for greater affective sharing and self-involvement while people interact with close others that are relevant to oneself.
Keywords: vicarious embarrassment, mentalizing, shared circuits, social emotions, social closeness, fMRI
Introduction
Most human affect is rooted in the social ties with the environment that we are living in. This applies for the pain we feel at others’ injuries (Singer et al., 2004), the joy when witnessing the success of a colleague (Mobbs et al., 2009), the disgust when listening to others’ nauseating stories (Wicker et al., 2003; Jabbi et al., 2008) and the embarrassment we feel on behalf of others’ blunders and pratfalls (Krach et al., 2011; Paulus et al., 2015). The social relation to the target person thereby is an important modulator of such vicarious responses to the emotional events in the environment (Singer et al., 2006; Cheng et al., 2010). This is particularly true for vicarious embarrassment as can be illustrated in everyday life situations. For example, parents cringe only if their children, but not others’ children, act out in public or one flushes only to a fellow student’s blatant ignorance while a similar incident happening to an unrelated other rather evokes amusement (see e.g. Fortune and Newby-Clark, 2008). In this study, we investigate how our social ties and friendship in particular affect the experience of vicarious embarrassment and propose novel neural pathways for how vicarious affect is modulated by social relation.
Earlier studies showed that vicarious embarrassment on behalf of unrelated others’ public flaws, blunders or norm violations is a form of vicarious social pain (Krach et al., 2011, 2015; Müller-Pinzler et al., 2012; Paulus et al., 2015; Melchers et al., 2015). Similar to how physical pain signals injuries of the bodily integrity, social pain signals threats to the social integrity—which undoubtedly is in danger in embarrassing moments (Macdonald and Leary, 2005; Eisenberger, 2012; Paulus et al., 2013, 2015). In the case of embarrassment, the integrity threat results from the expected negative evaluations in the eyes of others (Keltner and Buswell, 1997; Müller-Pinzler et al., 2015) and can also be experienced vicariously (Krach et al., 2011; Müller-Pinzler et al., 2012). The vicarious experience of embarrassment is associated with neural activation of the anterior cingulate cortex (ACC), the anterior insula (AI) and, if induced strong enough, higher-order somatosensory cortex areas (Paulus et al., 2015). This network of brain regions is also linked to the experience of vicarious physical pain (De Vignemont and Singer, 2006; Lamm et al., 2011) and other forms of vicarious social pain (Immordino-Yang et al., 2009; Masten et al., 2011). These neural pathways are also fundamentally involved in the first-hand experiences of social and physical pain (Eisenberger et al., 2003; Kross et al., 2011; Wager et al., 2013; Müller-Pinzler et al., 2015), supporting the notion that activity of the AI and ACC network represents consciously accessible bodily affect in shared circuits (Keysers and Gazzola, 2006) that can then somehow be articulated in the course of social interactions (Hein et al., 2010).
Besides the involvement of these shared circuits in vicarious embarrassment, one needs to have the capacity to read and understand the potentially negative evaluations of bystanders. Only then one will be able to understand the threats to another’s social integrity. This process of so-called ‘mentalizing’ about others’ evaluations, thoughts and intentions refers to a rather reflective process of perspective taking (Frith and Frith, 2003). The medial prefrontal cortex (mPFC) and the temporal poles (TP) thereby are part of a well-defined neural pathway that is engaged when we construct internal models about another person’s mental state (Lieberman, 2007; Hein and Singer, 2008) or experience embarrassment on behalf of another’s public flaws, blunders or norm violations (Paulus et al., 2015).
As outlined above, the perceived relation to the social target is a highly potent moderator for one’s vicarious emotional responses (Singer et al., 2006; Cheng et al., 2010). Negative experiences of physical or social pain (Singer et al., 2006; Meyer et al., 2012) as well as positive experiences like vicarious reward are modulated by the relations to the social target (Mobbs et al., 2009; Leng and Zhou, 2010). Behavioral data demonstrated that with increasing closeness to a social target participants showed more intense vicarious experiences of physical (Cheng et al., 2010) and social pain (Meyer et al., 2012) and also embarrassment (Fortune and Newby-Clark, 2008; Chekroun and Nugier, 2011). On the level of neural systems, the effect of social closeness on physical and social pain was accordingly accompanied by increased shared circuit activity within the ACC and AI (Cheng et al., 2010; Beeney et al., 2011; Meyer et al., 2012) and increased mPFC engagement (Meyer et al., 2012). It has been argued that the perceived social closeness strengthens the affective link to the other person, which results in the more intense caring for the other’s affect (Cheng et al., 2010). Further, the mental representations of close others are considered to be more vivid and rich and might therefore boost the affective consequences that follow a friend’s social deconstruction during embarrassing moments (Cheng et al., 2010; Meyer et al., 2012).
Another rationale for the increase of vicarious embarrassment by the perceived social closeness to the target is that observers might be particularly concerned about their own social image. This notion is supported by behavioral studies on vicarious embarrassment, which indicated that social closeness increases the observers’ concerns about their own images while observing inappropriate behaviors of e.g. friends (Fortune and Newby-Clark, 2008). The psychological literature on social identity and group processes has recently characterized comparable effects. There, wrongdoings or inadequate behaviors (e.g. racist attitudes or behaviors) of in-group members pose a threat to one’s own group’s social integrity and yield vicarious shame or guilt to in-group members even in case one is neither involved nor responsible for the other’s norm transgressions (Chekroun and Nugier, 2011; Lickel et al., 2005, 2011). The increased concerns about one’s own social image and the degree of overlap between cognitive representations of self and others (Aron et al., 1991) should thereby enhance self-related thoughts when observing a close other. On the neural systems level, this notion should manifest in greater involvement of the precuneus, a structure known to be involved in self-related thoughts, self-referential processing, representation of the self, as well as the assessment of self-relevant sensations (Cavanna and Trimble, 2006; Northoff et al., 2006; Mazzola et al., 2010). Accordingly, if perceivers are concerned about their friend’s actions to reflect negatively upon themselves, the integration of the precuneus in the neural systems’ pathways that convey vicarious embarrassment should increase.
According to this there are two potential models for how social closeness modulates vicarious embarrassment. In the first model, social closeness strengthens the affective link to the other person leading to richer mental representations of close others and a potential increase in mentalizing about the other person’s mind. In the second model, the perceived social closeness to the target might induce concerns about one’s own social image. Based on the above outlined research and theoretical considerations we hypothesized a greater response within the shared circuits of the AI and ACC when cringing in response to the wrongdoings of friends compared with strangers. Second, as one’s friend’s wrongdoings more strongly reflect on oneself compared with those of a stranger we expected the precuneus, a brain region that is involved in the processing of self-related thoughts, to show greater activity while observing your friends’ misbehaviors.
Materials and Methods
Ethics statement
We confirm that the research has been conducted in compliance with the ethical guidelines of the American Psychological Association. The study protocol was approved by the local ethics committee at the local faculty of medicine at Philipps-University Marburg and all subjects gave written informed consent.
Participants
All participants (N = 64) were fluent in German and had normal or corrected to normal vision. The majority of the participants (87.5%) were university students. A minority of participants worked (7.8%) or attended high school (3.1%). On average, participants had spent 15.85 years in education (range 12–22 years, s.d. = 2.38). Participants were assigned either to a ‘friend’ or a ‘stranger’ condition. Data of the sample in the ‘stranger’ condition have been published previously (Krach et al., 2011; Paulus et al., 2015). These N = 32 participants (17 female; aged 20–28 years; M = 22.81; s.d. = 2.19) were age- and sex-matched to N = 32 participants who received the ‘friend’ instruction (17 female; aged 18–31 years; M = 23.56; s.d. = 3.30, P’s ≥ 0.288).
Stimuli, experimental design and procedure
Social closeness was manipulated in a between-subjects design with one group being assigned to the ‘stranger’ and the other one to the ‘friend’ condition. Both groups viewed a set of 36 previously validated hand-drawn sketches representing vicarious embarrassment situations and nine neutral sketches serving as control situations. Situations that elicited vicarious embarrassment displayed a social target while he or she was violating a social norm in public and thus threatened his or her social integrity. Neutral control stimuli displayed the social target in a public context without violating socially normative standards. For clarification each sketch was accompanied by a two-sentence description of the current situation (e.g. ‘You are at the grocery store: You observe a woman at the cashier realizing that she cannot pay her purchase…’; see Figure 1). Notably, in the ‘stranger’ condition, the social target was introduced as a stranger (e.g. ‘You observe a “person” …’) whereas in the ‘friend’ condition the social target was referred to as a friend (e.g. ‘You observe your “friend” …’). To cover a broad variety of public norm violations that elicit embarrassment on behalf of others in everyday life, situations modelled four different types of scenarios, varying the degree of intentionality and awareness of the social target’s behavior (see Krach et al., 2011 for a detailed description). For the means of the present study, the four types of situations were collapsed into one and compared with the neutral control situations.
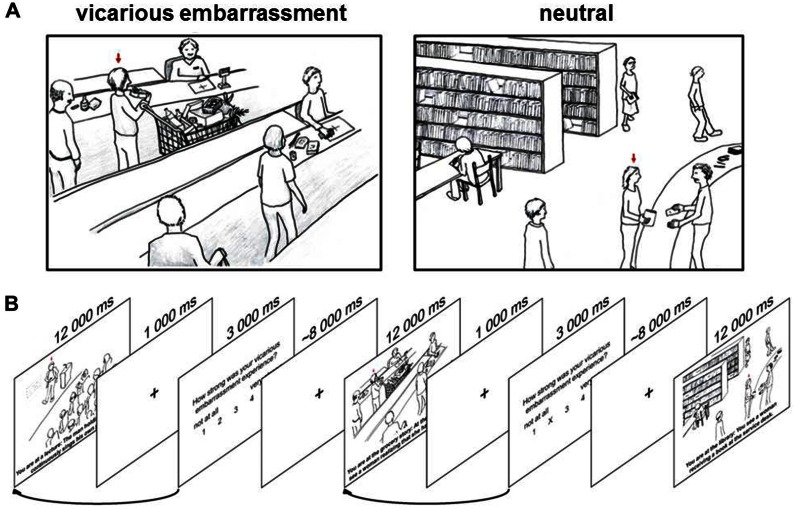
Fig. 1.
Stimulus material and timing of the experimental paradigm. (A) Examples of the presented stimuli. Social targets (friends and stranger) are exposed during public flaws, blunders or norm violations in different situations (vicarious embarrassment, VE) as well as in neutral scenarios (NEUT). The sketches were presented together with a short description of the situation that describes the observed person either as a stranger or a friend. In the ‘friend’ condition, these are ‘You are at the grocery store: your friend realizes at the cashier that she cannot pay her purchase…’ (VE) and ‘You are at the library: your friend returns some books at the service desk…’ (NEUT). In the ‘stranger’ condition, the sentences read ‘You are at the grocery store: a woman realizes at the cashier that she cannot pay her purchase…’ (VE) and ‘You are at the library: a man returns some books at the service desk…’ (NEUT). (B) Timing of the experimental paradigm. The arrows pointing from the rating period to the preceding stimulus presentation indicate that the intensity of the self-report was used as parametric weight for the preceding stimulus.
After providing written informed consent, participants were carefully instructed about the experimental procedure. Therefore, participants received two exemplary situations that were not displayed during the functional magnetic resonance imaging (fMRI) session and asked to mentally visualize the displayed situations as vividly as possible. In the friend condition participants were instructed to imagine that the social target, which was marked by a red arrow above the persons head, is their friend and in the stranger condition they were instructed to imagine that they observe an unknown person. In the MRI, all sketches were presented for 12 s together with the description of the situation. The text was presented in a black 24-point non-serif font (Arial) on a white background in two to three rows below the sketches. The stimulus presentation was followed by a blank screen for 1 s and a subsequent rating period of 3 s.
During the rating period, participants were asked to evaluate the intensity of their preceding vicarious embarrassment experiences (‘How strong was your experience of vicarious embarrassment?’). Responses were made on a scale ranging from 1 (‘not at all’) to 5 (‘very strong’) using a button press of the right hand.
A jittered low-level baseline showing a fixation cross for an average of 8 s was interleaved between the rating period and the following trial. Stimuli were presented in a pseudo-randomized order, ensuring that no class of situation was immediately repeated and different situations had equal frequency throughout the entire fMRI time-series. The total experiment lasted 20.28 min.
Data acquisition
Participants were scanned at 3 T (Siemens Trio, Erlangen) with 36 near-axial slices and a distance factor of 10% providing whole brain coverage. An echo planar imaging (EPI) sequence was used for acquisition of functional volumes during the experiment (repetition time = 2.2 s, echo time = 30 ms, flip angle = 90°, slice thickness = 3 mm, field of view = 192). Stimuli were presented on an LCD screen with Presentation 11.0 software package (Neurobehavioral Systems, Albany, CA, USA).
Data analysis
Behavioral data
Data were analyzed with PASW Statistics 18 (SPSS, 2009, Chicago, IL). The average self-reports in the different vicarious embarrassing (VE) and neutral situations (NEUT) were analyzed using analysis of variance (ANOVA) with Group as a between-subject factor (stranger vs friend) and Condition as a within-subject factor (VE vs NEUT). Main effects and interactions were examined to identify effects of the emotion induction and group differences in the vicarious embarrassment self-report.
fMRI data
fMRI data were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm). Four images were dummy scans and 51 scans comprising the presentation of the first five stimuli (one for each type of VE situation and NEUT) were discarded from further analyses. This had to be done because of delayed onsets of the EPI acquisition in a sub-sample of participants in the ‘friend’ condition. To maintain similar statistical power and within-subject models for all participants and achieve valid group comparisons, we discarded the amount of scans for all participants prior to the analyses. The remaining 494 EPI volumes were corrected for timing differences of the slice acquisitions, motion-corrected and spatially normalized to the standard template of the Montreal Neurological Institute (MNI) using the EPI template. The normalized volumes were resliced with a voxel size of 2 × 2 × 2 mm, smoothed with an 8 mm full-width half-maximum isotropic Gaussian kernel and high-pass filtered at 1/256 Hz to remove low frequency drifts.
Vicarious embarrassment-related activation
Statistical analysis was performed in a two-level, mixed-effects procedure. The fixed-effects generalized linear model (GLM) on the first level included six epoch regressors modeling hemodynamic responses to the vicariously embarrassing situations (4), neutral situations (1) and rating phase (1) with the abovementioned stimulus durations. The vicarious embarrassment ratings after each situation of vicarious embarrassment were entered as parametric modulators to explain additional variance in neural activation due to differences in emotional responses on the within-subject level. Six additional regressors modeling head movement parameters were introduced to account for noise.
Beta-maps of activation in the vicarious embarrassment and the neutral situations were analyzed on the second level. The second-level analysis of activation differences was conducted with a random-effects GLM. The GLM contained one factor for the friend and stranger condition and a second factor for the five dependent levels of VE and the NEUT situations. The second-level analysis was controlled for individual differences in the self-report of vicarious embarrassment within each category and group by introducing subjects’ averaged ratings within each category as covariates in the GLM. To identify brain regions that are generally involved in vicarious embarrassment, we conducted a conjunction-analysis across both groups and contrasted the VE against the NEUT condition (friend_VE–friend_NEUT ∩ stranger_VE–stranger_NEUT). The results of the conjunction-analysis were thresholded at P < 0.05 applying family-wise error (FWE) correction for a whole brain analysis. To find brain regions that show stronger vicarious embarrassment-related activation when observing a friend compared with a stranger, the interaction of both factors was calculated ((friend_VE–friend_NEUT) − (stranger_VE–stranger_NEUT)). The reverse comparison was calculated to investigate increased effects when observing a stranger compared with a friend ((stranger_VE–stranger_NEUT) − (friend_VE–friend_NEUT)). Both analyses were first conducted in the whole brain and in a second step restricted to the regions of interest (ROIs) activated during vicarious embarrassment as defined by the results of the prior conjunction analysis. Finally, to examine how our friends’ misbehaviors could endanger our own social integrity, and accordingly enhance self-reflective thoughts at their wrongdoings, we contrasted the friend with the stranger condition (friend_VE–stranger_VE). For all contrasts that examined differences between groups or the interaction, we applied FWE correction on the cluster extent threshold.
To examine the correspondence between hemodynamic responses and the behavioral reports of vicarious embarrassment, we additionally tested the parametric weights for the different VE facets (Paulus et al., 2015). Therefore, β-images of the four parametric weights were computed and analyzed at the group level. In the random-effects GLM, we implemented a 4 × 2 repeated measures ANOVA with the parametric weights of the four VE facets as repeated factor. We first tested the average effect of the parametric modulators within each group and second calculated the difference between the friend and the stranger condition to identify brain regions that show greater correspondence between brain and behavior in either condition. Both analyses were restricted to the ROIs activated during vicarious embarrassment as defined by the results of the prior conjunction analysis. All results are reported in MNI space.
Functional connectivity analysis of the precuneus
To examine context-dependent modulations of the connectivity pattern of the precuneus, we conducted a functional connectivity analysis in both groups. To do so, we selected the area within the precuneus that had stronger activation in the friend compared with the stranger condition as seed region (see Figure 3; friend_VE–stranger_VE, P < 0.0005, uncorrected, within the anatomical boundaries of precuneus and posterior cingulate cortex). Timeseries were mean centered and task induced hemodynamic responses were removed by applying an effects-of-interest correction with an F-contrast set to the six movement parameters (Paulus et al., 2014). Because we expected differences in connectivity profiles to emerge while participants processed the social stimuli, only those periods of the timeseries that represent hemodynamic signal during stimulus presentation were included in the analyses. To do so, the timeseries when participants did not attend to the stimuli (i.e. low-level baseline, rating period) were fixed to zero, so that variability of blood oxygenation level dependent (BOLD) signal of the precuneus during these periods could not contribute to explain variance in the target timeseries. To account for the delay of the hemodynamic response, valid stimulus intervals of the BOLD timeseries were set to 2.2 s after stimulus onset until 3.4 s after the end of stimulus presentation corresponding to a total duration 13.2 s of BOLD signal per stimulus. The so derived timeseries covered the variability in the BOLD response for all situations where participants observed a social target being it in a VE or NEUT situation. To account for noise, two additional timeseries were extracted for each subject from the first eigenvariates of all voxels within masks covering medial cerebrospinalfluid regions (CSF) or white matter (WM) (Bedenbender et al., 2011). The fixed-effects GLM for the connectivity analysis on the first-level thus included the modified timeseries of the precuneus, the WM and CSF noise regressors, and the above described regressors of the original design matrix including the regressors modeling head movement parameters. Beta-maps of the precuneus timeseries were analyzed on the group-level with a random-effects GLM. Standard deviations of the six head movement parameters of each subject were included as covariates to reduce the influence of different amplitudes of head movement between and within groups on connectivity profiles. In addition, the averaged ratings of vicarious embarrassment within each category were included as covariates as previously described. Differences in functional connectivity profiles of the precuneus between the friend and the stranger group were investigated within the brain regions that were consistently activated during vicarious embarrassment in both conditions. The results were FWE corrected within an ROI of regions consistently activated during vicarious embarrassment defined by the results of the prior conjunction analysis (friend_VE–friend_NEUT ∩ stranger_VE–stranger_NEUT; see Figure 2 and Table 1).
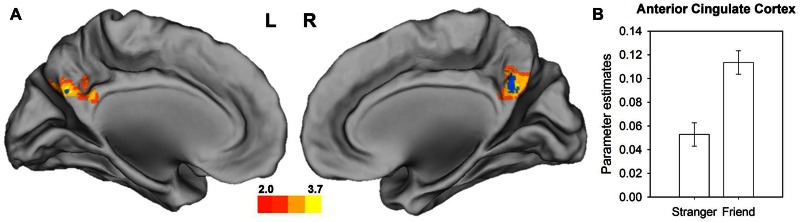
Fig. 3.
Increased activation and functional connectivity while observing a friend vs stranger in vicarious embarrassment situations. (A) Increased activation of the precuneus for friend_VE–stranger_VE is depicted with a yellow color gradient (P < 0.05, corrected on cluster extend level; the colour gradient illustrates the corresponding t-values) and the constricted precuneus area used as seed region for the functional connectivity analysis is depicted in blue (P < 0.0005, uncorrected, masked with an anatomical precuneus with the PCC mask). (B) Increased connectivity of the precuneus and ACC while observing a friend vs stranger during social situations. Bar charts indicate the mean parameter estimates with standard errors at the peak voxel of the connectivity effect within the ACC (−10, 22, 42 mm).
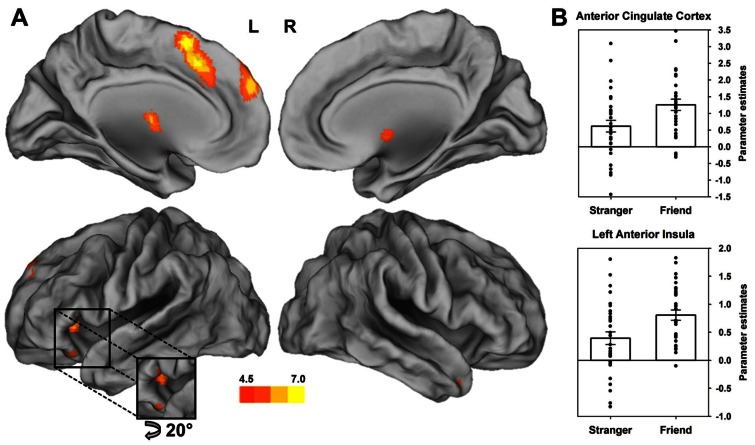
Fig. 2.
Increased activation during the experience of vicarious embarrassment for friends and strangers exposed in public situations. (A) The conjunction analysis [(friend_VEfriend_NEUT ∩ stranger_VEstranger_NEUT] reveals increased activation of the ACC, left anterior insula, mPFC, brainstem and right temporal pole while observing friends and stranger in embarrassing situations (P < 0.05, FWE corrected). The colour gradient illustrates the corresponding t-values. (B) Activation of the ACC and AI is specifically increased during vicarious embarrassment for friends vs strangers (see Results section). Parameter estimates are plotted for the peak voxel of the interaction effect [friend_VEfriend_NEUT − stranger_VEstranger_NEUT] within the ACC (−4, 30, 28 mm) and left anterior insula (−28, 20, 2 mm).
Table 1.
Vicarious embarrassment related activation while observing a friend or a stranger
| Side | MNI coordinates |
Cluster size | T | P | |||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| (friend_VE‐friend_NEUT) ∩ (stranger_VE‐stranger_NEUT) | |||||||
| ACC/SMA | L | −4 | 14 | 56 | 581 | 7.25 | <0.001 |
| −6 | 22 | 42 | 6.57 | <0.001 | |||
| −10 | 26 | 34 | 6.09 | <0.001 | |||
| Anterior insula | L | −32 | 24 | 0 | 146 | 6.34 | <0.001 |
| −40 | 24 | 4 | 5.76 | <0.001 | |||
| mPFC/Superior medial gyrus | L/R | −2 | 56 | 30 | 375 | 6.23 | <0.001 |
| −6 | 52 | 40 | 5.69 | 0.001 | |||
| Thalamus | L/R | −8 | −6 | 4 | 370 | 5.99 | <0.001 |
| Putamen | −14 | 10 | 2 | 5.95 | <0.001 | ||
| −16 | 2 | −6 | 5.24 | 0.004 | |||
| Brainstem | L/R | −2 | −20 | −22 | 144 | 5.96 | <0.001 |
| Nucleus caudatus | R | 12 | 10 | 2 | 163 | 5.54 | 0.001 |
| 10 | 2 | −2 | 5.43 | 0.001 | |||
| 6 | −6 | −12 | 4.88 | 0.013 | |||
| Inferior frontal gyrus | L | −36 | 26 | −18 | 28 | 5.46 | 0.001 |
| Inferior temporal gyrus | L | −42 | −40 | −16 | 20 | 5.06 | 0.006 |
| Medial temporal pole | R | 40 | 14 | −36 | 13 | 4.86 | 0.022 |
Notes. Results for the conjunction analysis (friend_VE‐friend_NEUT) ∩ (stranger_VE‐stranger_NEUT). All P-values are family-wise error corrected for the whole brain. Clusters with less than 10 voxels are not reported.
Results
Behavioral data
As expected, participants self-reported vicarious embarrassment was significantly stronger for all categories of vicarious embarrassment [M = 3.04, s.d. = 0.61 (stranger), M = 3.10, s.d. = 0.50 (friend)] compared with the neutral condition [M = 1.04, s.d. = 0.11 (stranger), M = 1.02, s.d. = 0.07 (friend)] across both groups [F(1, 62) = 861.37, P < 0.001]. There was no main effect of Group indicating overall similar responses [F(1, 62) = 0.12, P = 0.733] and no significant interaction between Group and Condition [F(1, 62) = 0.31, P = 0.578].
Neuroimaging data
According to our expectations and prior studies on vicarious embarrassment (Krach et al., 2011; Paulus et al., 2015), we found stronger shared circuits activity in the ACC [t(302) = 6.09, P < 0.001; corrected for whole brain analyses] and the AI [t(302) = 6.34, P < 0.001; corrected for whole brain analyses] as well as mentalizing regions in the mPFC [t(302) = 6.23, P < 0.001; corrected for whole brain analyses] and the temporal pole [t(302) = 4.86, P = 0.022; corrected for whole brain analyses] during VE compared with NEUT situations for both groups (see also Figure 2 and Table 1). In addition and irrespective of the social relation to the target, the thalamus [t(302) = 5.99, P < 0.001; corrected for whole brain analyses] and brainstem [t(302) = 5.96, P < 0.001; corrected for whole brain analyses] confirmed the typical activation in response to social pain.
In both groups, there was a positive correspondence of vicarious embarrassment ratings with neural activations of the left AI [stranger: t(246) = 5.68, P < 0.001; x = −32, y = 22, z = 6; friend: t(246) = 3.40, P = 0.008; x = −28, y = 26, z = 4] and activation of the ACC was positively associated with vicarious embarrassment ratings in the stranger group [t(246) = 5.06, P < 0.001, x = −8, y = 24, z = 30] but not in the friend group [t(246) = 2.01, P = 0.153, x = −6, y = 28, z = 26]. Comparing both groups revealed a greater correspondence of vicarious embarrassment ratings and neural activations in the stranger group vs friend group for the ACC [t(246) = 2.63, P = 0.041, x = −10, y = 24, z = 30] and trend-wise also for the left AI [t(246) = 2.62, P = 0.070, x = −38, y = 18, z = 6].
As hypothesized, there was an interaction effect revealing that vicarious embarrassment-associated activity was stronger in the friend compared with the stranger condition for the ACC [t(302) = 2.84, P = 0.026; corrected for ROI analyses] and the left AI [t(302) = 2.81, P = 0.047; corrected for ROI analyses]. A whole-brain analysis did not reveal any additional significant clusters. The opposed contrast did not show any significant differences, neither in the ROI analyses nor in the whole-brain analysis.
When directly contrasting the activation while observing friends’ wrongdoings compared with those of a stranger (friend_VE–stranger_VE) we found an increased BOLD response in the precuneus [t(302) = 4.72; k = 1016; P = 0.006; corrected for whole-brain analyses] (see Figure 3). The subsequent functional connectivity analyses showed increased functional integration of the precuneus timeseries with the ACC [t(48) = 4.21, P = 0.046; corrected for ROI analyses] in the friend compared with the stranger condition (see Figure 3B).
Discussion
Is it always favorable to keep your friends close? In the present study we examined how the social ties with others affect the vicarious embarrassment on behalf of their flaws, failures or norm violations and unravel pathways that help to understand vicarious embarrassment on the neural systems level. We predicted that vicarious embarrassment would trigger activity within shared circuits as a measure for an empathic sharing of others’ unbearable moments and that this activation would be modulated by the social closeness to the target person. Further, as humans are concerned about their social images, we expected that the cringe worthy behaviors of close friends would trigger precuneus activity as a neural measure of increased self-referential processing.
Empathizing with others’ predicaments
Confirming earlier studies on the unpleasant emotional experience of vicarious embarrassment (Krach et al., 2011; Paulus et al., 2015), the shared circuits within the AI and ACC (Keysers and Gazzola, 2006), were involved either if participants observed their friends or unrelated strangers in embarrassing conditions. Similarly, activations within the brainstem and thalamus were increased when participants observed a friend or stranger. These activations might reflect how information of bodily arousal going along with vicarious embarrassment is relayed. However, activity within the shared circuits of the AI and ACC was specifically increased if participants observed threats to their friends’ social integrity, supporting the notion that social closeness modulates empathic processes on the level of shared circuits. Further, we corroborated earlier findings that the subjective experience of vicarious embarrassment is indeed linked to AI and ACC activation (Krach et al., 2015; Paulus et al., 2015). However, in the friend condition, the link between neural response and behavior was lower compared with the stranger condition.
In a classic study, Shearn et al. (1999) could nicely demonstrate that participants showed stronger blushing when observing a friend as compared with a stranger in an embarrassing situation and argued that empathic blushing, such as proper empathy (Davis, 1996), would be particularly influenced by the increased empathic accuracy within friendship and kinship relations (Ickes et al., 1990; Ickes, 1997; Zaki et al., 2009). Our findings are also in line with recent studies on the effect of social closeness on vicarious physical (Cheng et al., 2010) and social pain (Beeney et al., 2011; Meyer et al., 2012) that reported increased shared circuit activity within the ACC and AI if friends compared with strangers were observed. Meyer et al. (2012) made participants observe friends and strangers during social exclusion as a form of social pain, which likewise activated affective pain regions in the ACC and AI—the very same regions that were previously shown to be associated with the direct and firsthand experience of social exclusion (Eisenberger et al., 2003; Eisenberger, 2012). In line with the present findings of an upregulation within shared circuits during the observation of friends vs strangers in embarrassing predicaments, Meyer et al. reported a ‘social closeness effect’ on the experience of social exclusion. Similarly, Cheng et al. (2010) found a comparable ‘social closeness effect’ in the ACC and AI for sharing the physical pain of loved ones compared with strangers. Independent of the (social) pain of others being observed the fact that we have more vivid and rich mental representations of our close friends’ feelings compared with unrelated others eases sharing their affect and accordingly could lead to increased activity in empathy networks. This should be particularly true for sharing a close friend’s embarrassing moments, which are triggered by deviations from social norms. Gaining access to a stranger’s emotionality during such moments is much more effortful compared with sharing a close friend’s thoughts and feelings with whom we spend time on a daily basis and where we thus have more intuitive access to the other’s mind (Ickes, 1997; Zaki et al., 2009; Zaki and Ochsner, 2011). Shared past emotional moments or experiences (e.g. shared grief upon the death of a friend or joint cheering in a sports event) as well as common norms or attitudes (e.g. shared rejection of racism) contribute to a better and more accurate mental representation of close others and accordingly ease empathizing with their situations.
Role of mentalizing
To gain access to another person’s affect, participants need to form a representation of the other’s mental states (Frith and Frith, 2006). Thus, apart from the direct sharing of the other person’s affective state through mirroring, participants engage in perspective taking to build a model of another’s mind, a process that is associated with neural activity of the mPFC and the TP. Accordingly, in the present study we could show that the mPFC and TP were consistently involved during vicarious embarrassment, also while witnessing friends. This involvement, however, was not modulated by social closeness. We have previously argued that mentalizing should be viewed as a general underlying process to understand vicarious embarrassment. Without mentalizing on others’ minds it is not possible to discern the evaluations in the eyes of bystanders that constitute the threat to the social integrity of the target (Paulus et al., 2015) and to experience this complex social emotion. This is why it was not surprising that we found an upregulation in the mentalizing network independent of the social relation to the humiliated social target.
Role of self-referential processing
A large body of literature in social psychology has shown that people have a strong motivation to feel good about themselves (Tesser, 1988; Baumeister, 1994) and accordingly are concerned about the portrayal of their social images (Mead, 1934; Leary and Kowalski, 1990). Importantly, the own social images are not only fixed and stable across time, but are also continuously (re)constructed and adjusted to the social context. Self-portrayals can thus be highly heterogeneous and incorporate past, present and future selves (Markus and Nurius, 1986; Wilson and Ross, 2001) or differentiate between private (personal) and public (relational, social, and collective) aspects of the self (Robins et al., 1999) which in total constitute a person’s identity. One component that is continuously shaping our social images is the feedback of others (Leary et al., 1995). People give feedback for one’s behaviors and attitudes and their acceptance or rebuttal shapes the social image that one aims to represent. However, also one’s close friends’ (mis)behaviors can impact and endanger our own social images as the social ties to a person may communicate a sharing of certain attitudes, norms and values (Fortune and Newby-Clark, 2008). Observing a friend’s norm violating behavior in a public context (e.g. yelling at the restaurant waiter) thereby may elicit concerns about one’s own social image (Fortune and Newby-Clark, 2008; Chekroun and Nugier, 2011), however, only if one disagrees with the friend’s behavior in that particular moment. Such concerns about one’s own portrayed social image elicit the emotional reaction of embarrassment (Eisenberg, 2000; Tangney and Dearing, 2002; Macdonald and Leary, 2005). Previous research could show that people react to others’ embarrassing circumstances, which also constituted a threat to their own social integrity, by showing signs of appeasement gestures and restitution behavior with the aim to restore their own social image (Semin and Manstead, 1982; Keltner and Buswell, 1997). Interestingly, concerns about the social image evolve relatively early in the human development. Children have a concept of an extended identity from around 8 years onwards and spontaneously report feeling embarrassed on behalf of another child or toddler in their care who commits a faux pas (Bennett et al., 1998). With increasing age children even appear to have a greater preoccupation with their social images and increasingly make judgments concerning others’ evaluations. Around that time a passive audience is already sufficient to induce first-person experiences of embarrassment following rule violations (Bennett et al., 1998). Interestingly, opposed to vicarious embarrassment, first-person experiences of embarrassment are experienced as less devastating in front family members and friends compared with strangers (Macdonald and Davies, 1983; Lewis et al., 1991; Keltner and Buswell, 1997). Likewise, young children were more likely to show displays of embarrassment when dancing in front of strangers as compared with dancing in front of their mothers (Lewis et al., 1991).
In a similar way, people tend to exert social control over deviant in-group members in order to avoid embarrassment and damage to their own and the group’s social integrity (Chekroun and Nugier, 2011). Studies in the field of group-based emotions have brought up the concept of identity threat which could also mediate the effect of others’ wrongdoings on the personal experience of vicarious embarrassment. Lickel et al. (2004, 2005) showed that group-based emotions such as shame or guilt were elicited when people were afraid of maintaining a positive group identity. Hence, people felt ashamed for another’s wrongdoing when that person’s behavior was relevant to their own social identity and when they feared that the other person’s behavior would negatively reflect upon themselves (Lickel et al., 2005).
Taken together, concerns about one’s own social image might pose a potent additional factor increasing embarrassment-related activation of the ACC and AI in response to a friend’s public failures. The increased activity within the precuneus, which is considered to be a neural correlate of ongoing self-related thoughts and representation of the self (Cavanna and Trimble, 2006; Northoff et al., 2006; Mazzola et al., 2010), might indicate the greater overlap between the cognitive representations of self and others, increased social image concerns and engagement in self-referential processing, while observing a friend’s norm violating behaviors. The increased functional coupling of the precuneus with the ACC could thus represent a neural pathway that further explains, how the affective response in shared circuits is modulated the social relation by own image concerns.
Limitations
One limitation of the present study is the lack of the expected significant differences on the behavioral level, while finding coherent differences on neural activation and connectivity measures. Several factors could explain this observation. First, the lack of findings on the behavioral level might be explained by diminished statistical power for the behavioral effects. The level of neural activation is closer to the actual psycho-physiological process that we are interested in, e.g. the affective arousal of vicarious embarrassment that is mapped in the AI and ACC activation. Second, social desirability might influence the validity of the participants’ self-report on their emotional experiences during the fMRI study. Specifically when it comes to emotions, which are related to judgments of another person’s condition, people tend to adjust their explicit rating, to correspond to societal norms, standards and the expectations of the experimenter. This specifically could have affected the ratings in the friend condition, as participants might rather support friends than strangers and defend their social integrity in front of others in order to decrease negative affect (Cohen and Wills, 1985), maintain self-esteem (Major et al., 1991) and enhance well-being (Cohen and Hoberman, 1983). In case of strangers, it might be easier and more socially accepted to explicitly express negative emotions such as vicarious embarrassment. These thoughts are supported by the correspondence between the behavioral report and the neural activation measures in the AI and ACC. Importantly, the association was not as strong for observing friends compared with strangers. The reduced coupling in the friend condition could be explained by effects of social desirability and adjustments of the behavioral report to align with the expectations of the environment.
Conclusion and outlook
The present data open up new perspectives for how social closeness affects brain processes related to vicarious affect. Earlier studies on empathy for various negative or positive states provided evidence for an involvement of mentalizing areas and shared circuits of affect processing (Mobbs et al., 2009; Leng and Zhou, 2010; Meyer et al., 2012). During social interaction the relationship to the social target modulates cognitive and mentalizing processes that influence shared circuit activations and the empathic response, similar to how the responsibility for another’s suffering or ingroup-membership upregulate shared circuit activity (Hein et al., 2010; Cui et al., 2015). We initially described two models of how social closeness modulates vicarious embarrassment. Based on the present data we, however, cannot prefer one of the two provided models. Precuneus activation might map increased mentalizing about the other person’s mind as well as be related to increased concerns about one’s own social image during vicarious embarrassment for a close other. Nonetheless, the notion of self-referential processing to mediate affect experienced on behalf of others is novel in the neurosciences. Our finding thus might help integrating and informing findings from social psychology on group processes, where the identification of oneself with a social group is the key concept in the phenomenon of vicarious affect (Lickel et al., 2005).
Funding
Research leading to this abstract has been funded by the German Research Foundation (DFG; KR3803/2-1, KR3803/7-1), the Research Foundation of the Philipps-University Marburg and the von Behring-Röntgen-Stiftung (KR 60-0023).
Conflict of interest. None declared.
References
- Aron A., Aron E.N., Tudor M., Nelson G. (1991). Close relationships as including other in the self. Journal of Abnormal and Social Psychology, 60, 241–53. [Google Scholar]
- Baumeister R.F. (1994). Self and identity: a social psychology perspective. In: Tesser A., editor. Advanced Social Psychology. Boston: McGraw-Hill. [Google Scholar]
- Bedenbender J., Paulus F.M., Krach S., et al. (2011). Functional connectivity analyses in imaging genetics: considerations on methods and data interpretation. PLoS One, 6, e26354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeney J.E., Franklin R.G., Levy K.N., Adams R.B. (2011). I feel your pain: emotional closeness modulates neural responses to empathically experienced rejection. Social Neuroscience, 6, 369–76. [DOI] [PubMed] [Google Scholar]
- Bennett M., Yuill N., Banerjee R., Thomson S. (1998). Children’s understanding of extended identity. Developmental Psychology, 34, 322–31. [DOI] [PubMed] [Google Scholar]
- Cavanna A.E., Trimble M.R. (2006). The precuneus: a review of its functional anatomy and behavioural correlates. Brain, 129, 564–83. [DOI] [PubMed] [Google Scholar]
- Chekroun P., Nugier A. (2011). “I’m ashamed because of you, so please, don’t do that!”: reactions to deviance as a protection against a threat to social image. European Journal of Social Psychology, 41, 479–88. [Google Scholar]
- Cheng Y., Chen C., Lin C., Chou K., Decety J. (2010). NeuroImage love hurts : an fMRI study. Neuroimage, 51, 923–9. [DOI] [PubMed] [Google Scholar]
- Cohen S., Hoberman H. (1983). Positive events and social supports as buffers of life change stress. Journal of Applied Social Psychology, 13, 99–125. [Google Scholar]
- Cohen S., Wills T.A. (1985). Stress, social support, and the buffering hypothesis. Psychological Bulletin, 98, 310–57. [PubMed] [Google Scholar]
- Cui F., Abdelgabar A., Keysers C., Gazzola V. (2015). Responsibility modulates pain-matrix activation elicited by the expressions of others in pain. Neuroimage, 114, 371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M.H. (1996). Empathy: A Social-Psychological Approach. Boulder: Westview Press. [Google Scholar]
- De Vignemont F., Singer T. (2006). The empathic brain: how, when and why? Trends in Cognitive Sciences, 10, 435–41. [DOI] [PubMed] [Google Scholar]
- Eisenberg N. (2000). Emotion, regulation, and moral development. Annual Review of Psychology, 51, 665–97. [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I. (2012). Broken hearts and broken bones: a neural perspective on the similarities between social and physical pain. Current Directions in Psychological Science, 21, 42–7. [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. (2003). Does rejection hurt? An FMRI study of social exclusion. Science, 302, 290–2. [DOI] [PubMed] [Google Scholar]
- Fortune J.L., Newby-Clark I.R. (2008). My friend is embarrassing me: exploring the guilty by association effect. Journal of Personality and Social Psychology, 95, 1440–9. [DOI] [PubMed] [Google Scholar]
- Frith C.D., Frith U. (2006). The neural basis of mentalizing. Neuron, 50, 531–4. [DOI] [PubMed] [Google Scholar]
- Frith U., Frith C.D. (2003). Development and neurophysiology of mentalizing. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 358, 459–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., Singer T. (2010). Neural responses to ingroup and outgroup members’ suffering predict individual differences in costly helping. Neuron, 68, 149–60. [DOI] [PubMed] [Google Scholar]
- Hein G., Singer T. (2008). I feel how you feel but not always: the empathic brain and its modulation. Current Opinion in Neurobiology, 18, 153–8. [DOI] [PubMed] [Google Scholar]
- Ickes W. (1997). Empathic Accuracy. New York: Guilford Press. [Google Scholar]
- Ickes W., Stinson L., Bissonnette V., Garcia S. (1990). Naturalistic social cognition: empathic accuracy in mixed-sex dyads. Journal of Personality and Social Psychology, 59, 730–42. [Google Scholar]
- Immordino-Yang M.H., McColl A., Damasio H., Damasio A. (2009). Neural correlates of admiration and compassion. Proceedings of the National Academy of Sciences of the United States of America, 106, 8021–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbi M., Bastiaansen J., Keysers C. (2008). A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One, 3, e2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner D., Buswell B.N. (1997). Embarrassment: its distinct form and appeasement functions. Psychological Bulletin, 122, 250–70. [DOI] [PubMed] [Google Scholar]
- Keysers C., Gazzola V. (2006). Towards a unifying neural theory of social cognition. Progress in Brain Research, 156, 379–401. [DOI] [PubMed] [Google Scholar]
- Krach S., Cohrs J.C., de Echeverria Loebell N.C., et al. (2011). Your flaws are my pain: linking empathy to vicarious embarrassment. PLoS One, 6, e18675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krach S., Kamp-Becker I., Einhäuser W., et al. (2015). Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Human Brain Mapping, 36, 4730–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kross E., Berman M.G., Mischel W., Smith E.E., Wager T.D. (2011). Social rejection shares somatosensory representations with physical pain. Proceedings of the National Academy of Sciences of the United States of America, 108, 6270–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54, 2492–502. [DOI] [PubMed] [Google Scholar]
- Leary M.R., Kowalski R.M. (1990). Impression management: a literature review and two-component model. Psychological Bulletin, 107, 34–47. [Google Scholar]
- Leary M.R., Tambor E.S., Terdal S.K., Downs D.L. (1995). Self-esteem as an interpersonal monitor: the sociometer hypothesis. Journal of Personality and Social Psychology, 68, 518–30. [Google Scholar]
- Leng Y., Zhou X. (2010). Modulation of the brain activity in outcome evaluation by interpersonal relationship: an ERP study. Neuropsychologia, 48, 448–55. [DOI] [PubMed] [Google Scholar]
- Lewis M., Stanger C., Sullivan M.W., Barone P. (1991). Changes in embarrassment as a function of age, sex and situation. The British Journal of Developmental Psychology, 9, 485–92. [Google Scholar]
- Lickel B., Schmader T., Barquissau M. (2004). The evocation of moral emotions in intergroup contexts: the distinction between collective guilt and collective shame. In: Branscombe N.R., Doosje B., editors. Collective Guilt: International Perspectives. New York, NY: Cambridge University Press. [Google Scholar]
- Lickel B., Schmader T., Curtis M., Scarnier M., Ames D.R. (2005). Vicarious shame and guilt. Group Processes Intergroup Relations, 8, 145–57. [Google Scholar]
- Lickel B., Steele R.R., Schmader T. (2011). Group-based shame and guilt: emerging directions in research. Social and Personality Psychology Compass, 5, 153–63. [Google Scholar]
- Lieberman M.D. (2007). Social cognitive neuroscience: a review of core processes. Annual Review of Psychology, 58, 259–89. [DOI] [PubMed] [Google Scholar]
- Macdonald G., Leary M.R. (2005). Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin, 131, 202–23. [DOI] [PubMed] [Google Scholar]
- Macdonald L.M., Davies M.F. (1983). Effects of being observed by a friend or stranger on felt embarrassment and attributions of embarrassment. Journal of Psychology, 113, 171–4. [Google Scholar]
- Major B., Testa M., Bylsma W.H. (1991). Resonance to Upward and Downward Comparisons: The Impact of Esteem Relevance and Perceived Control. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Markus H.R., Nurius P. (1986). Possible selves. American Psychologist, 41, 954–69. [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. (2011). An fMRI investigation of empathy for “social pain” and subsequent prosocial behavior. Neuroimage, 55, 381–8. [DOI] [PubMed] [Google Scholar]
- Mazzola V., Latorre V., Petito A., et al. (2010). Affective response to a loved one’s pain: insula activity as a function of individual differences. PLoS One, 5, e15268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead G.H. (1934). Mind, Self, and Society: From the Standpoint of a Social Behaviorist, Chicago: University of Chicago Press. [Google Scholar]
- Melchers M., Markett S., Montag C., et al. (2015). Reality TV and vicarious embarrassment: an fMRI study. Neuroimage, 109, 109–17. [DOI] [PubMed] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2012). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience, 8, 446-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Meyer M., et al. (2009). A key role for similarity in vicarious reward. Science, 324, 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Pinzler L., Gazzola V., Keysers C., et al. (2015). Neural pathways of embarrassment and their modulation by social anxiety. Neuroimage, 119, 252–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Pinzler L., Paulus F.M., Stemmler G., Krach S. (2012). Increased autonomic activation in vicarious embarrassment. International Journal of Psychophysiology, 86, 74–82. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage, 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Paulus F.M., Bedenbender J., Krach S., et al. (2014). Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Human Brain Mapping, 35, 1190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus F.M., Müller-Pinzler L., Jansen A., Gazzola V., Krach S. (2015). Mentalizing and the role of the posterior superior temporal sulcus in sharing others’ embarrassment. Cerebral Cortex, 25, 2065–75. [DOI] [PubMed] [Google Scholar]
- Paulus F.M., Müller-Pinzler L., Westermann S., Krach S. (2013). On the distinction of empathic and vicarious emotions. Frontiers in Human Neuroscience, 7, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins R.W., Norem J.K., Cheek J.M. (1999). Naturalizing the self. In: Pervin L.A., John O.P., editors. Handbook of personality: Theory and research. 2nd ed New York, NY: Guilford Press, 443–77. [Google Scholar]
- Semin G.R., Manstead A.S.R. (1982). The social implications of embarrassment displays and restitution behaviour. European Journal of Social Psychology, 12, 367–77. [Google Scholar]
- Shearn D., Spellman L., Straley B., Meirick J. (1999). Empathic blushing in friends and strangers. Motivation and Emotion, 23, 307–16. [Google Scholar]
- Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303, 1157–62. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J.P., Stephan K.E., Dolan R.J., Frith C.D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439, 466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangney J.P., Dearing R.L. (2002). Shame and Guilt. New York: Guilford Press. [Google Scholar]
- Tesser A. (1988). Toward a self-evaluation maintenance model of social behavior. In: Berkowitz L., editor. Advances in Experimental Social Psychology. San Diego, CA: Academic Press, 181–227. [Google Scholar]
- Wager T.D., Atlas L.Y., Lindquist M., Roy M., Woo C.-W., Kross E. (2013). An fMRI-based neurologic signature of physical pain. The New England Journal of Medicine, 368, 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. (2003). Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron, 40, 655–64. [DOI] [PubMed] [Google Scholar]
- Wilson A.E., Ross M. (2001). From chump to champ: People's appraisals of their earlier and present selves. Journal of Personality and Social Psychology, 80, 572–84. [DOI] [PubMed] [Google Scholar]
- Zaki J., Ochsner K. (2011). Reintegrating the study of accuracy into social cognition research. Psychological Inquiry, 22, 159–82. [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences of the United States of America, 106, 11382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]