Abstract
Happiness refers to people’s cognitive and affective evaluation of their life. Why are some people happier than others? One reason might be that unhappy people are prone to ruminate more than happy people. The default mode network (DMN) is normally active during rest and is implicated in rumination. We hypothesized that unhappiness may be associated with increased default-mode functional connectivity during rest, including the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC) and inferior parietal lobule (IPL). The hyperconnectivity of these areas may be associated with higher levels of rumination. One hundred forty-eight healthy participants underwent a resting-state fMRI scan. A group-independent component analysis identified the DMNs. Results indicated increased functional connectivity in the DMN was associated with lower levels of happiness. Specifically, relative to happy people, unhappy people exhibited greater functional connectivity in the anterior medial cortex (bilateral MPFC), posterior medial cortex regions (bilateral PCC) and posterior parietal cortex (left IPL). Moreover, the increased functional connectivity of the MPFC, PCC and IPL, correlated positively with the inclination to ruminate. These results highlight the important role of the DMN in the neural correlates of happiness, and suggest that rumination may play an important role in people’s perceived happiness.
Keywords: happiness, default mode network, rumination, functional connectivity, resting-state fMRI
Introduction
Happiness is a fundamental human pursuit (Diener, 2000; Parks et al., 2012). However, not everyone is equipped with the same ability to be happy (Lyubomirsky, 2001; Diener and Seligman, 2002). In our daily lives, it is common for some people to be happy while others often experience fits of gloom. So, why are some people happier than others? There has been considerable interest in the sources of individual differences in happiness. A great deal of research has shown that both internal factors (e.g. self-reference, emotion regulation, and social comparison) and external factors (e.g. age, gender, education, marriage and income) influence happiness (Diener et al., 1999; Lyubomirsky, 2001; Diener, 2013). However, little work has been done to investigate the neurobiological sources of these individual differences (Kringelbach and Berridge, 2009); doing so may provide new insights into our understanding of happiness.
A starting point for this study is the observation that happiness is often negatively associated with excessive, negative, self-focused processing; i.e. rumination. Emerging evidence shows that unhappy people are inclined to dwell on their negative life events, focus on their self-emotions and feel self-conscious, which results in a variety of adverse consequences (Lyubomirsky et al., 2003, 2011; Nolen-Hoeksema et al., 2008; Killingsworth and Gilbert, 2010; Stawarczyk et al., 2012; Andrews-Hanna et al., 2013; Mason et al., 2013). For instance, Killingsworth and Gilbert (2010) collected real-time self-reported instances of mind wandering and happiness from 2250 participants and found that people were less happy when their minds wandered than when not. Unhappy individuals, unlike their happy peers, showed increased self-focused thoughts and were more sensitive to unfavorable achievement feedback, which adversely affected their performance on important academic tasks (Lyubomirsky et al., 2011). In addition, excessive self-focused cognition (rumination) is implicated in depressive and anxiety symptoms (Nolen-Hoeksema et al., 2008). Finally, recent work utilizing a longitudinal design reported that low self-esteem predicted subsequent rumination, which in turn predicted subsequent depression, and that rumination partially mediated the prospective effect of low self-esteem on depression (Kuster et al., 2012).
In recent years, neuroimaging studies on happiness have advanced our understanding of its neural underpinnings (Urry et al., 2004; van Reekum et al., 2007; Heller et al., 2013; Cunningham and Kirkland, 2014; Lewis et al., 2014; Luo et al., 2014, 2015; Kong et al., 2015a,b,c,d). For example, one resting-state electroencephalography (EEG) study revealed that individuals with greater activation in the left superior prefrontal cortex were happier (compared with the right superior prefrontal cortex (Urry et al., 2004). Structural magnetic resonance imaging (MRI) studies have reported gray matter volume associations with aspects of happiness; eudaimonic happiness, characterized by meaning and self-realization, was shown to be positively associated with gray matter volume in the right insular cortex (Lewis et al., 2014). Additionally, global life satisfaction has been linked to the parahippocampal gyrus (Kong et al., 2015a). More recently, two task-based functional MRI studies found that happier individuals showed greater amygdala responses to positive stimuli (Cunningham and Kirkland, 2014), as well as sustained activity in the striatum and dorsolateral prefrontal cortex in response to positive stimuli (Heller et al., 2013). However, none of these studies has looked at how a specific psychological process, such as rumination, relates to the neural basis of happiness.
As a complement to the EEG and task-based studies mentioned earlier, the disposition of happiness warrants investigation from the perspective of resting-state functional connectivity (Biswal et al., 1995; Fox and Raichle, 2007). This method may provide insight into individual differences in happiness as they relate to spontaneous self-referential processing. Functional connectivity refers to the temporal correlations of spontaneous brain activity in spatially remote areas (Friston et al., 1993). These connectivity patterns, namely resting-state networks (RSNs), are thought to characterize the individual differences in intrinsic brain functions (Fox and Raichle, 2007). One such RSN, the default mode network (DMN) includes the medial prefrontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus (PCU) and inferior parietal lobule (IPL). The DMN is active during introspectively oriented mental activity at rest (e.g. self-reflection, theory of mind or mind wandering), and is suppressed in the presence of an external task (Raichle et al., 2001; Buckner et al., 2008). The DMN has been theorized to play a pivotal role in unconstrained, self-referential cognition (Raichle et al., 2001; Weissman et al., 2006; Mason et al., 2007; Buckner et al., 2008; Andrews-Hanna et al., 2014). In our previous study, we reported that local functional homogeneity of intrinsic brain activity was altered within DMN areas (e.g. the MPFC and PCC) among happy and unhappy individuals (Luo et al., 2014). In this study, we sought to extend our previous findings by directly investigating how the functional connectivity of DMN is associated with happiness.
Although the relationship between happiness and the DMN has yet to be examined, previous research has examined the relationship between emotion-related disorders (e.g. depression) and the DMN (Greicius, 2008; Broyd et al., 2009; Whitfield-Gabrieli and Ford, 2012). For example, hyperactivation and hyperconnectivity of the DMN has been found in patients with depression who were characterized by high levels of rumination (Whitfield-Gabrieli and Ford, 2012). Moreover, during both passive viewing and actively appraising negative images, individuals with depression exhibited an overactive DMN (Sheline et al., 2009). Additional studies have reported that participants with depression showed increased resting-state functional connectivity of the MPFC, PCC, and thalamus within the DMN compared with the healthy control participants (Greicius et al., 2007; Berman et al., 2011; Sheline et al., 2010; Zhou et al., 2010). Moreover, the increased level of DMN dominance (greater DMN activity relative to the task-positive network) was also found in people with depression (Hamilton et al., 2011). Notably, the degree of resting-state functional connectivity correlated with the person’s tendency to ruminate (Greicius et al., 2007; Sheline et al., 2010; Berman et al., 2011; Hamilton et al., 2011). However, an overactive DMN in patients with depression is not always observed. For example, decreased functional connectivity in patients with major depressive disorder has been reported (Veer et al., 2010). In addition, an independent study reported evidence for the dissociation between anterior and posterior functional connectivity of the resting-state DMN in people with depression, with an increase in functional connectivity in the anterior medial cortical regions and a decreased functional connectivity in the posterior medial cortical regions (Zhu et al., 2012). Therefore, the relationship between functional connectivity in the resting-state DMN and depression remains unclear.
Although there is considerable evidence for abnormal patterns of DMN activity in people with depression, it is important to investigate the relationship between DMN activities and happiness in non-clinical samples. We treat happiness and subjective well-being as interchangeable terms in this study. Happiness refers to people’s cognitive and affective evaluation of their life. Thus, happiness is a broad construct that encompasses frequent positive affects, occasional negative affects and high level of life satisfaction (Diener et al., 1999, 2002). In addition, we conceptualized happiness as a trait rather than a transient emotion state (Lyubomirsky et al., 2005). In this study, happiness was measured by the Chinese Happiness Inventory (CHI) (Lu and Shih, 1997; based on the Oxford Happiness Inventory) with 28 items (Argyle et al., 1989) alongside an additional 20 items to accurately cover the sources of happiness for Chinese people. These Chinese sources of happiness include ‘harmony of interpersonal relationships’, ‘being praised and respected by others’, ‘satisfaction of material needs’, ‘achievement at work’, ‘downward social comparisons’ and ‘peace of mind’ (Lu and Shih, 1997).
There are several concepts related to happiness, such as depression and self-esteem. Although those concepts may partly overlap and be related, they are distinct constructs (Lyubomirsky et al., 2006; Ryff et al., 2006). Given that the absence of depression and high self-esteem does not guarantee happiness, they are therefore not sufficient conditions for happiness (Lyubomirsky et al., 2006). Furthermore, more and more researchers argue that happiness and unhappiness are not opposite ends of a bipolar continuum, but rather are distinct domains of mental functioning (Ryff et al., 2006). Despite the considerable amount of neural evidence and research on depression (Greicius et al., 2007; Sheline et al., 2010; Hamilton et al., 2011) and self-esteem (Chavez and Heatherton, 2015), it is still necessary to investigate the associations between DMN and happiness.
In this study, we investigated whether happiness is associated with the DMN and whether this relationship is associated with rumination. Our hypotheses were derived from the conclusion that depression is associated with hyperconnectivity of the DMN (Whitfield-Gabrieli and Ford, 2012), and that within the DMN, the MPFC, PCC and IPL are theorized to be involved in self-referential processes (Northoff et al., 2006; Andrews-Hanna et al., 2010). Therefore, it is possible that unhappiness is associated with increased functional connectivity between the MPFC, PCC and IPL in a non-clinical population. Furthermore, the inclination to ruminate may be related to the hypothesized increased functional connectivity between the aforementioned areas when at rest.
Methods
Participants and behavioral measures
Participants included 168 healthy young adults, who volunteered as part of an ongoing project investigating the association between brain imaging and mental health. Data from 51 of these participants have been previously published in a study that investigated the association between regional homogeneity and happiness (Luo et al., 2014). Eighteen participants were excluded due to excessive head motion (see below for details) and another two participants were excluded because they failed to complete self-report measures. The results reported here are from the remaining 148 participants (61 men, 81 women; mean age = 20.90 years, SD = 1.65 years). They were healthy undergraduate and postgraduate students with no history of neurological conditions or psychiatric episodes. In accordance with the Declaration of Helsinki, written informed consent was obtained from all participants. The study protocol was approved by the local ethics committee.
All participants’ happiness levels were assessed with the CHI, full version (Lu et al., 1997) (alpha = 0.94). The CHI is a standardized measure of 48 items; higher total scores indicate a higher level of overall happiness. The CHI is composed of a universal component of happiness and a specific component of the Chinese conceptualization of happiness (Lu and Shih, 1997).
Rumination was measured with the Ruminative Responses Scale (RRS) (Treynor et al., 2003). We did not measure the rumination at first (78 participants), and the RRS was included for the final 70 participants. The RRS can be divided into three components: depression, brooding and reflection. According to Treynor et al. (2003), rumination is composed of two components: brooding and reflection. Therefore, we focused on the Brooding and Reflection subscales of RRS. The Brooding subscale assesses the tendency to passively compare current mood with ideal standards, and the Reflection subscale assesses the tendency to consider why one is depressed (Treynor et al., 2003). The Chinese version of the RRS has 21 items (rated on 1 = Never to 4 = Always) (Yang et al., 2009) (total RRS alpha = 0.89; Brooding alpha = 0.65 and Reflection alpha = 0.78).
Data acquisition
The experiment was performed on a 3.0-T scanner (Magnetom Trio, Siemens, Erlangen, Germany). Functional images were acquired using a single-shot, gradient-recalled echo planar imaging sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, 32 axial slices, FOV = 192 × 192 cm, acquisition matrix = 64 × 64, slice thickness = 3 mm, without gap, voxel size = 3 × 3 × 4 mm). For each participant, a total of 8 min resting data were acquired. Participants were instructed to simply rest with their eyes closed, not to think of anything in particular, and not to fall asleep. To minimize head motion, the participants’ heads were restricted with foam cushions. For spatial normalization and localization, high-resolution T1-weighted anatomical images were also acquired along the sagittal orientation using a 3D magnetization prepared rapid gradient-echo sequence (176 slices, TR = 1900 ms, TE = 2.53 ms, flip angle = 9°, resolution = 256 × 256 and voxel size = 1 × 1 × 1 mm) on each participant.
Data pre-processing
Data were pre-processed using DPARSF (Data Processing Assistant and Resting-state FMRI, version 2.3, http://www.restfmri.net/forum/DPARSF) (Chao-Gan and Yu-Feng, 2010). The first 10 volumes were removed to account for the T1 equilibrium effect, leaving 230 volumes for the final analysis. The functional images were sinc interpolated in time to correct for slice time differences. Then, the images were realigned with a six-parameter (rigid body) linear transformation to correct for head movements. Next, the functional images were co-registered with the corresponding T1-volume and warped into the Montreal Neurological Institute (MNI) space at a resolution of 3 × 3 × 3 mm, using Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra in SPM8 (Ashburner, 2007). Finally, the images were spatially smoothed using a Gaussian filter of 4-mm full width at half maximum (FWHM) to reduce noise.
Head motion
To rule out the confounding effect of head motion, we computed frame-wise displacement (FD) for our data (Power et al., 2012, 2014). We used a ‘scrubbing’ method, as recent studies have emphasized the confounding effect of transient head motion on the time course of resting-state data (Power et al., 2012, 2015; Van Dijk et al., 2012; Yan et al., 2013a,b). For each participant, the volumes with a FD above 0.2 mm, and the 1-back and 2-forward neighbors, were scrubbed (Power et al., 2012, 2013). Participants with excessive motion (<5 min of useable data) were excluded as an outlier (Power et al., 2014). A total of 18 participants were identified as outliers and eliminated from the final analyses. The FD of the remaining data was low (M = 0.11, SD = 0.04), and there were no correlations between FD and behavioral measures (rs < 0.08, Ps > 0.36). Lastly, mean FD of each participant was included in the multiple regression models as nuisance covariates to remove the residual effect of motion effect at the group level.
Independent component analysis
Group spatial independent component analysis (ICA) was carried out by the Group ICA for fMRI Toolbox (GIFT v2.0a, icatb.sourceforge.net) (Calhoun et al., 2001) for 148 participants. The number of components was set to 20 (Smith et al., 2009). To perform group ICA, dimensionality of the data was first reduced using principle component analysis (PCA), then the reduced data were concatenated over the time domain using the infomax algorithm. This algorithm was repeated 20 times by running the ICASSO toolbox, using both ‘randinit’ and ‘bootstrap’ methods, to ensure the reliability of the derived components (Himberg et al., 2004; Correa et al., 2007; Das et al., 2014). Afterward, individual image maps were reconstructed from the aggregated data based on matrices stored during PCA. The resulting image maps and time courses were then back-reconstructed and calibrated using Z values to normalize the signal. It has been reported that Z values can be indirectly used to measure the functional connectivity within a network, since Z values represented the contribution of the voxels to the independent components (Beckmann et al., 2005; Liao et al., 2010). The ICASSSO results showed that the component stability ranged above 0.95 for all included independent components.
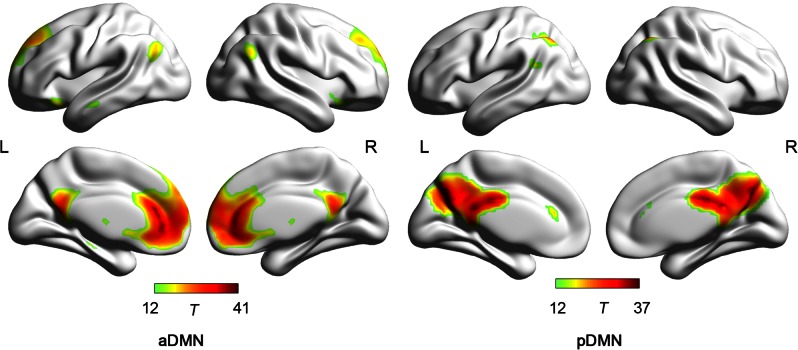
Components corresponding to the DMN were then identified. Components were identified by template matching and visual inspection. First, using the DMN template according to Smith et al. (2009), we performed a spatial template matching procedure (von dem Hagen et al., 2013). Two components were identified as having a highest spatial overlap with the template (correlation values: 0.32, 0.19). Then, two components matching the DMN were further conformed by visual inspection. According to the group ICA results, the DMN were split into the anterior DMN (aDMN) and the posterior DMN (pDMN), which was consistent with previous research (Liemburg et al., 2012; Ding and Lee, 2013; von dem Hagen et al., 2013).
Statistical analysis of independent components
Statistical analyses were performed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). For each DMN component, random-effect analyses using one-sample t-tests were performed respectively. We set a stringent threshold criterion to examine regions typically found in the DMN (T > 12, k > 20). The resulting statistical maps were used as masks in following analysis to restrict the analysis within DMN areas.
Multiple regression analyses with happiness, gender (covariate of no interest), age (covariate of no interest), FD (covariate of no interest) and the Z values were performed. The results of multiple regressions were corrected using a Monte Carlo simulation (Ledberg et al., 1998), resulting in a corrected threshold of P < 0.05 (AlphaSim program within AFNI (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html). The following parameters were used: single voxel P = 0.05, 10 000 simulations, FWHM = 4 mm, cluster connection radius r = 5 mm). Minimum cluster sizes of aDMN and pDMN were 30 voxels (810 mm3) and 27 voxels (729 mm3), respectively.
Connectivity-behavior analysis
To investigate the relationship between Z values in the network maps and the level of rumination, a post-hoc Pearson correlation analysis was performed. The voxels in the DMN showing significant correlations between Z values and happiness were extracted as a mask consisting of several regions of interests (ROIs). These averaged masks (ROIs) (at group level) were applied to all participants. The mean Z values for each individual within these ROIs were correlated with the scores on the Brooding and Reflection subscales of the RRS. Considering these analyses were exploratory in nature, a statistical significance level of P < 0.05 uncorrected and P < 0.01 uncorrected, were used.
Results
Demographic results
Spatial pattern of DMN
The results of the Group ICA analyses are shown in Figure 1 and visualized with BrainNet Viewer (Xia et al., 2013). Visual inspection indicated that the aDMN was mainly composed of the MPFC and anterior cingulate cortices, the PCC/PCU, and the bilateral IPL. The pDMN included the PCC, retrosplenial cortex and PCU (Table 1).
Fig. 1.
The spatial pattern in the aDMN and pDMN
Note: aDMN, anterior default mode network, pDMN, posterior default mode network. L, left; R, Right.
Table1.
Demographics
| Mean | SD | Range | |
|---|---|---|---|
| Age (years) | 20.90 | 1.65 | 18–26 |
| Head Motion (mm) | 0.11 | 0.038 | 0.023–0.142 |
| CHI (n = 148) | 115.96 | 18.04 | 73–167 |
| RRS-Brooding (n = 70) | 10.27 | 2.45 | 5–18 |
| RRS-Reflection (n = 70) | 11.01 | 2.76 | 6–20 |
Note: CHI, Chinese Happiness Inventory; RRS, Ruminative Responses Scale.
DMN and happiness
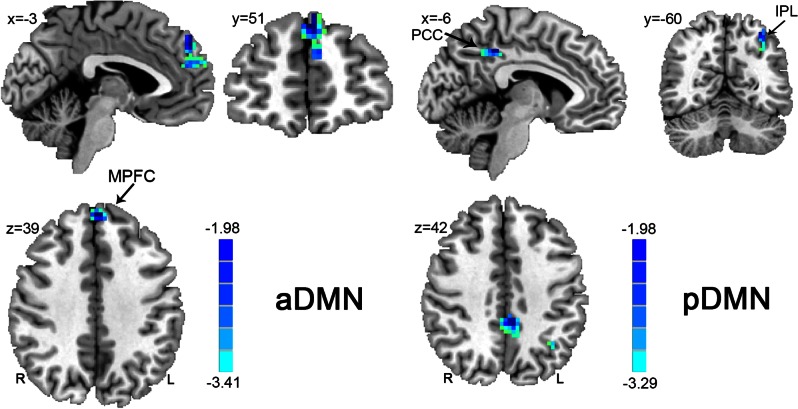
Multiple regression analysis (P < 0.05, Alphasim corrected) indicated that Z values of some areas within the DMN were negatively correlated with happiness (see Figure 2, Table 2). More specifically, within the aDMN, the Z values of the bilateral MPFC were negatively correlated with happiness. Similarly, within the pDMN, the z values of the bilateral PCC, and left IPL were negatively correlated with happiness.
Fig. 2.
Regions showing significant correlations between the DMNs and happiness. The left panel shows regions with significant correlations between the aDMN and happiness; the right panel shows regions with significant correlations between the pDMN and happiness. Numbers in the upper left corner of each image refer to the x-, y- or z-plane coordinates of the MNI space (R, right; L, left). Abbreviations: MPFC, medial prefrontal cortex; PCC, poster cingulate cortex; IPL, inferior parietal lobule.
Table 2.
Brain regions demonstrating significant correlations between the functional connectivity of the DMN and happiness.
| Anatomical region | Side | Bas | MNI |
Voxel | Peak | ||
|---|---|---|---|---|---|---|---|
| x | y | z | Size | T-value | |||
| aDMN | |||||||
| MPFC | B | 9/10/32 | 0 | 51 | 39 | 82 | −3.41 |
| pDMN | |||||||
| PCC | B | 31 | −6 | −42 | 42 | 42 | −3.17 |
| IPL | L | 7/40 | −33 | −60 | 54 | 29 | −3.13 |
Note: Side refers to the hemisphere (B, bilateral; R, right; and L, left). Brodmann areas (BAs), coordinates of peak t-value in MNI space, volume in voxels, and peak t-values are specified for each region showing significant correlations.
Connectivity-behavior results
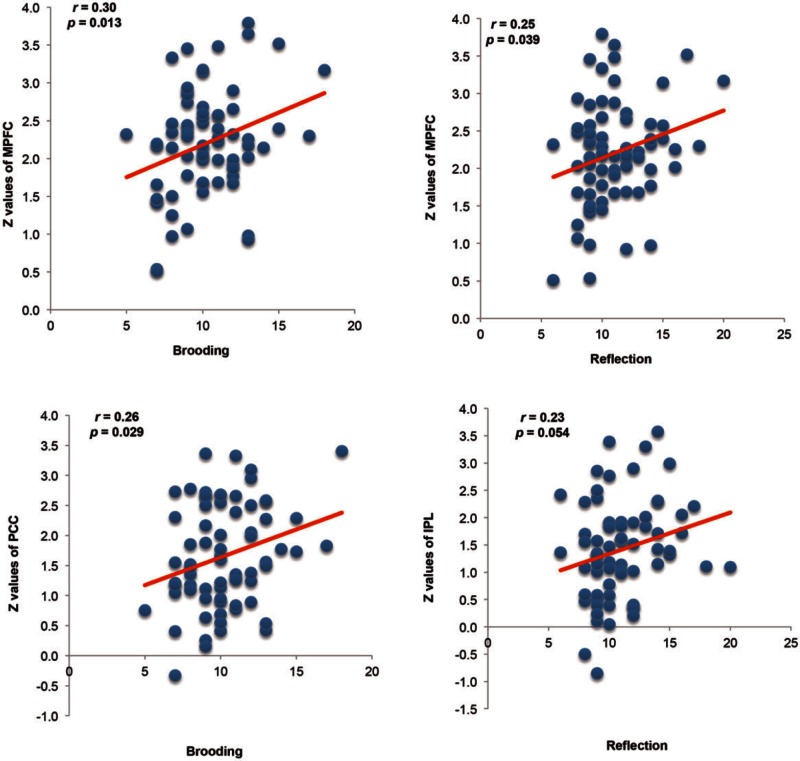
There were significant positive correlations between the RRS subscale scores and the Z values of the MPFC within the aDMN, and between the Z values of the PCC and IPL within the pDMN (Figure 3). Specifically, the mean Z values of the MPFC were positively correlated with the scores on the two RRS subscales [Brooding (r = 0.30, P = 0.013) and Reflection (r = 0.25, P = 0.039)]. In addition, the mean Z values of the PCC were positively correlated with scores for Brooding (r = 0.26, P = 0.029), and the mean Z values of the IPL was marginally correlated with scores for Reflection (r = 0.23, P = 0.054).
Fig. 3.
Correlations between scores on the RRS subscales and mean Z values of the MPFC, PCC and IPL. MPFC, medial prefrontal cortex; PCC, poster cingulate cortex; IPL, inferior parietal lobule.
Discussion
Our results provide neural evidence that rumination may underlie the association between DMN activities and individual differences in happiness within a healthy population. Consistent with our hypothesis, a group ICA approach found that the increased functional connectivity of the DMN was associated with lower levels of happiness. Compared with their happy peers, individuals reporting greater levels of unhappiness showed increased functional connectivity in core DMN areas, including the MPFC, PCC and IPL. Furthermore, the strength of functional connectivity in the MPFC, PCC and IPL was positively correlated with scores on the two subscales of rumination: brooding and reflection. The increased functional connectivity within the DMN among unhappy people may suggest the unhappy people are associated with excessive negative self-reflection.
The current results provide further evidence that unhappiness is associated with hyperconnectivity of DMN areas in a non-clinical sample, and that the strength of the hyperconnectivity between the MPFC, PCC and IPL is associated with the trait of rumination. Unhappy people may spend more time ruminating on negative self-feelings, thoughts and emotions (Lyubomirsky et al., 2003, 2011; Nolen-Hoeksema et al., 2008; Killingsworth and Gilbert, 2010; Stawarczyk et al., 2012; Andrews-Hanna et al., 2013; Mason et al., 2013). The DMN is active when people are engaged in unconstrained self-referential processing and deactivates during goal-oriented activity. As the midline hubs of the DMN (MPFC and PCC) are highly implicated in personally significant evaluations, e.g. during self-relevant affective decision-making (Andrews-Hanna et al., 2010), it is not surprising that unhappy people showed increased functional connectivity in the DMN. Prior studies also found the intrinsic activities of DMN areas were associated with happiness (Luo et al., 2014, 2015). For example, our prior resting-state study found the local synchronization of intrinsic brain activities in the MPFC and PCC were altered between happy and unhappy people (Luo et al., 2014). However, although previous studies speculated as to the existence of an association between the altered intrinsic activities of DMN areas and rumination (Luo et al., 2014, 2015), no prior research had directly tested this speculation. The present results provide direct novel evidence that the increased functional connectivity of DMN areas among unhappy people is associated with the inclination to ruminate.
More specifically, we found that unhappy people exhibited increased functional connectivity of the bilateral dorsal MPFC, within the anterior DMN, relative to happy people. Moreover, the Z values of the dorsal MPFC were positively correlated with rumination. The MPFC, consisting of the midline core areas of the DMN, are highly implicated in self-referential and emotional processing (Andrews-Hanna et al., 2010). Importantly, the dorsal MPFC has been termed the ‘dorsal nexus’ because it connects the cognitive control network, DMN and affective network (Sheline et al., 2010). In clinical samples, patients with depression demonstrated the hyperconnectivity of dorsal MPFC more so than healthy people (Sheline et al., 2010; Zhu et al., 2012). Moreover, experienced meditators, people who are characterized by attention to the present and less mind wandering, demonstrated relatively deactivated MPFC compared with matched controls (Brewer et al., 2011). Taken together, our study provides novel evidence that demonstrates that the MPFC is involved in unhappy individual’s rumination, in a non-clinical sample, which may indicate that individuals who report greater levels of unhappiness spend excessive amounts of time think about how they feel about themselves and find it is difficult to disengage from these thoughts.
Furthermore, the posterior DMN areas, including the PCC and IPL, showed increased functional connectivity among unhappy people. Additionally, the Z values of the PCC were positively correlated with scores for brooding, and those of the IPL were positively correlated with scores for reflection, two subscales of rumination. The results were consistent with previous studies, which found increased functional connectivity in the PCC of patients with depression (Greicius et al., 2007; Berman et al., 2011; but see Zhu et al., 2012) and recovered anorexia nervosa (Cowdrey et al., 2014), relative to healthy controls. Those posterior parietal regions play an important role in episodic memory, especially when recalling the past or anticipating the future (Wagner et al., 2005; Fransson and Marrelec, 2008). This raises the possibility that the increased connectivity of the PCC and IPL indicates that unhappy individuals may more frequently engage in episodic memory retrieval compared with happy individuals. However, further studies are needed to test this speculation.
Although our results provide direct evidence that the functional connectivity of the DMN is associated with happiness, it is worth noting the limitations and future directions of this study. First, due to the cross-sectional nature of this research, the current study is unable to establish causal connections between happiness and brain activity. Prospective longitudinal imaging studies will be necessary to address this question. Second, recent work indicates that dynamic functional connectivity may greatly enhance our understanding of the fundamental features of brain networks (Hutchison et al., 2013). Thus, future research may provide insight into the neural correlates of happiness by correlating the dynamic functional connectivity of the DMN and the related real-time mental contents among happy and unhappy people.
Conclusion
In summary, this study provides insights into the associations between happiness and the functional connectivity of the DMN with resting-state fMRI. When compared with their happy peers, unhappy people showed increased functional connectivity of the MPFC, PCC, and IPL within the DMN. Furthermore, increased connectivity in these core areas within the DMN was associated with an increased inclination to ruminate. Differences in happiness were reflected in differential functional connectivity strengths within the DMN; rumination may underline this relationship. Based on the current findings, future research towards determining the direction of causation between happiness, DMN and rumination are needed.
Acknowledgements
The authors would like to thank Dr Jennifer Beer from University of Texas at Austin and Dr Gary Lewis from University of York for their proofreading and valuable comments on the article. We also thank Baolin Li, Jie Liu and Cuihua Bi for their assistance in fMRI data collection.
Funding
This work was supported by ‘the Research Team’s Construction Project from the Faculty of Psychology in Southwest University (2012) [TR201201-1 to X.H.]’; ‘the Youth foundation for Humanities and Social Science Research of Ministry of Education’ [15XJC190001 to Y.L.]; and ‘the Fundamental Research Funds for the Central Universities’ [GK201503009 to Y. L.]’; ‘the Youth foundation for Humanities and Social Science Research of Ministry of Education’ [15YJC190016 to S.Q.]; ‘the Fundamental Research Funds for the Central Universities’ [GK201503006 to S.Q.].
References
- Andrews-Hanna J.R., Kaiser R.H., Turner A.E.J., et al. (2013). A penny for your thoughts: dimensions of self-generated thought content and relationships with individual differences in emotional wellbeing. Frontiers in Psychology , 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Reidler J.S., Sepulcre J., Poulin R., Buckner R.L. (2010). Functional-anatomic fractionation of the brain’s default network. Neuron , 65(4), 550–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Annals of the New York Academy of Sciences , 1326(5), 29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyle M., Martin M., Crossland J. (1989). Happiness as a function of personality and social encounters. In Forgas J.P., Innes J.M., editors. Recent Advances in Social Psychology: An International Perspective, pp. 189–203. North-Holland: Elsevier. [Google Scholar]
- Ashburner J. (2007). A fast diffeomorphic image registration algorithm. NeuroImage , 38(1), 95–113. [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. (2005). Investigations into resting-state connectivity using independent component analysis. Philosophical Transactions of the Royal Society B: Biological Sciences , 360(1457), 1001–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman M.G., Peltier S., Nee D.E., Kross E., Deldin P.J., Jonides J. (2011). Depression, rumination and the default network. Social Cognitive and Affective Neuroscience , 6(5), 548–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine , 34(4), 537–41. [DOI] [PubMed] [Google Scholar]
- Brewer J.A., Worhunsky P.D., Gray J.R., Tang Y.Y., Weber J., Kober H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America , 108(50), 20254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broyd S.J., Demanuele C., Debener S., Helps S.K., James C.J., Sonuga-Barke E.J.S. (2009). Default-mode brain dysfunction in mental disorders: a systematic review. Neuroscience And Biobehavioral Reviews , 33(3), 279–96. [DOI] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain’s default network - anatomy, function, and relevance to disease. Year in Cognitive Neuroscience , 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Adali T., Pearlson G.D., Pekar J.J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping , 14(3), 140–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao-Gan Y., Yu-Feng Z. (2010). DPARSF: a MATLAB Toolbox for “Pipeline” data analysis of resting-state fMRI. Frontiers in Systems Neuroscience , 4, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez R.S., Heatherton T.F. (2015). Multimodal frontostriatal connectivity underlies individual differences in self-esteem. Social Cognitive and Affective Neurosciece , 10(3), 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa N., Adali T., Calhoun V.D. (2007). Performance of blind source separation algorithms for fMRI analysis using a group ICA method. Magnetic Resonance Imaging , 25(5), 684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdrey F.A., Filippini N., Park R.J., Smith S.M., McCabe C. (2014). Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Human Brain Mapping , 35(2), 483–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Kirkland T. (2014). The joyful, yet balanced, amygdala: moderated responses to positive but not negative stimuli in trait happiness. Social Cognitive and Affective Neurosciece , 9(6), 760–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Calhoun V., Malhi G.S. (2014). Bipolar and borderline patients display differential patterns of functional connectivity among resting state networks. NeuroImage , 98(4), 73–81. [DOI] [PubMed] [Google Scholar]
- Diener E. (2000). Subjective well-being: The science of happiness and a proposal for a national index. American Psychologist , 55(1), 34–43. [PubMed] [Google Scholar]
- Diener E. (2013). The remarkable changes in the science of subjective well-being. Perspectives on Psychological Science , 8(6), 663–66. [DOI] [PubMed] [Google Scholar]
- Diener E., Lucas R.E., Oishi S. (2002). Subjective well-being: the science of happiness and life satisfaction. In: Snyder C.R., Lopez S.J., editors. The Handbook of Positive Psychology, pp. 63–73. New York: Oxford University Press. [Google Scholar]
- Diener E., Seligman M.E.P. (2002). Very happy people. Psychological Science , 13(1), 81–4. [DOI] [PubMed] [Google Scholar]
- Diener E., Suh E.M., Lucas R.E., Smith H.L. (1999). Subjective well-being: three decades of progress. Psychological Bulletin , 125(2), 276–302. [Google Scholar]
- Ding X., Lee S.-W. (2013). Cocaine addiction related reproducible brain regions of abnormal default-mode network functional connectivity: a group ICA study with different model orders. Neuroscience Letters , 548, 110–4. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. (2007). Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience , 8(9), 700–11. [DOI] [PubMed] [Google Scholar]
- Fransson P., Marrelec G. (2008). The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. NeuroImage , 42(3), 1178–84. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D., Liddle P.F., Frackowiak R.S.J. (1993). Functional connectivity—the principal-component analysis of large (pet) data sets. Journal of Cerebral Blood Flow and Metabolism , 13(1), 5–14. [DOI] [PubMed] [Google Scholar]
- Greicius M.D. (2008). Resting-state functional connectivity in neuropsychiatric disorders. Current Opinion in Neurology , 21(4), 424–30. [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Flores B.H., Menon V., et al. (2007). Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry , 62(5), 429–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Furman D.J., Chang C., Thomason M.E., Dennis E., Gotlib I.H. (2011). Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biological Psychiatry , 70(4), 327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller A.S., van Reekum C.M., Schaefer S.M., et al. (2013). Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science , 24(11), 2191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himberg J., Hyvarinen A., Esposito F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage , 22(3), 1214–22. [DOI] [PubMed] [Google Scholar]
- Hutchison R.M., Womelsdorf T., Allen E.A., et al. (2013). Dynamic functional connectivity: promise, issues, and interpretations. NeuroImage , 80, 360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth M.A., Gilbert D.T. (2010). A wandering mind is an unhappy mind. Science , 330(6006), 932. [DOI] [PubMed] [Google Scholar]
- Kong F., Ding K., Yang Z., et al. (2015a). Examining gray matter structures associated with individual differences in global life satisfaction in a large sample of young adults. Social Cognitive and Affective Neuroscience, 10(7), 952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong F., Hu S., Wang X., Song Y., Liu J. (2015b). Neural correlates of the happy life: the amplitude of spontaneous low frequency fluctuations predicts subjective well-being. NeuroImage , 107, 136–45. [DOI] [PubMed] [Google Scholar]
- Kong F., Hu S., Xue S., Song Y., Liu J. (2015c). Extraversion mediates the relationship between structural variations in the dorsolateral prefrontal cortex and social well-being. NeuroImage , 105, 269–75. [DOI] [PubMed] [Google Scholar]
- Kong F., Liu L., Wang X., Hu S., Song Y., Liu J. (2015d). Different neural pathways linking personality traits and eudaimonic well-being: a resting-state functional magnetic resonance imaging study. Cognitive, Affective, and Behavioral Neuroscience , 15(2), 299–309. [DOI] [PubMed] [Google Scholar]
- Kringelbach M.L., Berridge K.C. (2009). Towards a functional neuroanatomy of pleasure and happiness. Trends in Cognitive Sciences , 13(11), 479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster F., Orth U., Meier L.L. (2012). Rumination mediates the prospective effect of low self-esteem on depression: a five-wave longitudinal study. Personality and Social Psychology Bulletin , 38(6), 747–59. [DOI] [PubMed] [Google Scholar]
- Ledberg A., Akerman S., Poland P.F. (1998). Estimation of the probabilities of 3D clusters in functional brain images. NeuroImage , 8(2), 113–28. [DOI] [PubMed] [Google Scholar]
- Lewis G.J., Kanai R., Rees G., Bates T.C. (2014). Neural correlates of the ‘good life’: eudaimonic well-being is associated with insular cortex volume. Social Cognitive and Affective Neuroscience , 9(5), 615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao W., Chen H., Feng Y., et al. (2010). Selective aberrant functional connectivity of resting state networks in social anxiety disorder. NeuroImage , 52(4), 1549–58. [DOI] [PubMed] [Google Scholar]
- Liemburg E.J., Swart M., Bruggeman R., et al. (2012). Altered resting state connectivity of the default mode network in alexithymia. Social Cognitive and Affective Neuroscience , 7(6), 660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L., Shih J.B. (1997). Personality and happiness: Is mental health a mediator?. Personality and Individual Differences , 22, 249–56. [Google Scholar]
- Lu L., Shih J.B., Lin Y.Y., Ju L.S. (1997). Personal and environmental correlates of happiness. Personality and Individual Differences , 23(3), 453–62. [Google Scholar]
- Luo Y., Huang X., Yang Z., Li B., Liu J., Wei D. (2014). Regional homogeneity of intrinsic brain activity in happy and unhappy individuals. PLoS One , 9(1), e85181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Li B., Liu J., Bi C., Huang X. (2015). Amplitude of low-frequency fluctuations in happiness: A resting-state fMRI study (In Chinese). Chinese Science Bulliten , 60, 170–8. [Google Scholar]
- Lyubomirsky S. (2001). Why are some people happier than others? The role of cognitive and motivational processes in well-being. American Psychologist , 56(3), 239–49. [PubMed] [Google Scholar]
- Lyubomirsky S., Boehm J.K., Kasri F., Zehm K. (2011). The cognitive and hedonic costs of dwelling on achievement-related negative experiences: implications for enduring happiness and unhappiness. Emotion , 11(5), 1152–67. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S., Kasri F., Zehm K. (2003). Dysphoric rumination impairs concentration on academic tasks. Cognitive Therapy and Research , 27(3), 309–30. [Google Scholar]
- Lyubomirsky S., Sheldon K.M., Schkade D. (2005). Pursuing happiness: the architecture of sustainable change. Review of General Psychology , 9(2), 111–31. [Google Scholar]
- Lyubomirsky S., Tkach C., DiMatteo M.R. (2006). What are the differences between happiness and self-esteem. Social Indicators Research , 78(3), 363–404. [Google Scholar]
- Mason M.F., Brown K., Mar R.A., Smallwood J. (2013). Driver of discontent or escape vehicle: the affective consequences of mindwandering . Frontiers in Psychology , 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M.F., Norton M.I., Van Horn J.D., Wegner D.M., Grafton S.T., Macrae C.N. (2007). Wandering minds: the default network and stimulus-independent thought. Science , 315(5810), 393–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S., Wisco B.E., Lyubomirsky S. (2008). Rethinking rumination. Perspectives on Psychological Science , 3(5), 400–24. [DOI] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., Greck M., Bennpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain - a meta-analysis of imaging studies on the self. NeuroImage , 31(1), 440–57. [DOI] [PubMed] [Google Scholar]
- Parks A.C., Della Porta M.D., Pierce R.S., Zilca R., Lyubomirsky S. (2012). Pursuing happiness in everyday life: the characteristics and behaviors of online happiness seekers. Emotion , 12(6), 1222–34. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage , 59(3), 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2013). Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. NeuroImage , 76, 439–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. (2014). Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage , 84, 320–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Schlaggar B.L., Petersen S.E. (2015). Recent progress and outstanding issues in motion correction in resting state fMRI. NeuroImage , 105, 536–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America , 98(2), 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryff C.D., Dienberg Love G., Urry H.L., et al. (2006). Psychological well-being and ill-being: do they have distinct or mirrored biological correlates?. Psychotherapy and Psychosomatics , 75(2), 85–95. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America , 106(6), 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline Y.I., Price J.L., Yan Z.Z., Mintun M.A. (2010). Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences of the United States of America , 107(24), 11020–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America , 106(31), 13040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D., Majerus S., Van der Linden M., D’Argembeau A. (2012). Using the daydreaming frequency scale to investigate the relationships between mind-wandering, psychological well-being, and present-moment awareness. Frontiers in Psychology , 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treynor W., Gonzalez R., Nolen-Hoeksema S. (2003). Rumination reconsidered: a psychometric analysis. Cognitive Therapy and Research , 27(3), 247–59. [Google Scholar]
- Urry H.L., Nitschke J.B., Dolski I., et al. (2004). Making a life worth living - neural correlates of well-being. Psychological Science , 15(6), 367–72. [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. (2012). The influence of head motion on intrinsic functional connectivity MRI. NeuroImage , 59(1), 431–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum C.M., Urry H.L., Johnstone T., et al. (2007). Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience , 19(2), 237–48. [DOI] [PubMed] [Google Scholar]
- Veer I.M., Beckmann C.F., van Tol M.J., et al. (2010). Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Frontiers in Systems Neuroscience , 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von dem Hagen E.A.H., Stoyanova R.S., Baron-Cohen S., Calder A.J. (2013). Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience , 8(6), 694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A.D., Shannon B.J., Kahn I., Buckner R.L. (2005). Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences , 9(9), 445–53. [DOI] [PubMed] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. (2006). The neural bases of momentary lapses in attention. Nature Neuroscience , 9(7), 971–8. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Ford J.M. (2012). Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology , 8, 49–76. [DOI] [PubMed] [Google Scholar]
- Xia M., Wang J., He Y. (2013). BrainNet Viewer: a network visualization tool for human brain connectomics. PloS one , 8(7), e68910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Cheung B., Kelly C., et al. (2013a). A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. NeuroImage , 76(1), 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C.G., Craddock R.C., Zuo X.N., Zang Y.F., Milham M.P. (2013b). Standardizing the intrinsic brain: towards robust measurement of inter-individual variation in 1000 functional connectomes. NeuroImage , 80, 246–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Ling Y., Xiao J., Yao S. (2009). The Chinese version of ruminative responses scale in high school students: its reliability and validity. Chinese Journal of Clinical Psychology , 17(1), 27–31. [Google Scholar]
- Zhou Y., Yu C., Zheng H., et al. (2010). Increased neural resources recruitment in the intrinsic organization in major depression. Journal of Affective Disorders , 121(3), 220–30. [DOI] [PubMed] [Google Scholar]
- Zhu X.L., Wang X., Xiao J., et al. (2012). Evidence of a dissociation pattern in resting-state default mode network connectivity in first-episode, treatment-naive major depression patients. Biological Psychiatry , 71(7), 611–7. [DOI] [PubMed] [Google Scholar]