Abstract
Previously, experimentally induced flow experiences have been demonstrated with perfusion imaging during activation blocks of 3 min length to accommodate with the putatively slowly evolving “mood” characteristics of flow. Here, we used functional magnetic resonance imaging (fMRI) in a sample of 23 healthy, male participants to investigate flow in the context of a typical fMRI block design with block lengths as short as 30 s. To induce flow, demands of arithmetic tasks were automatically and continuously adjusted to the individual skill level. Compared against conditions of boredom and overload, experience of flow was evident from individuals’ reported subjective experiences and changes in electrodermal activity. Neural activation was relatively increased during flow, particularly in the anterior insula, inferior frontal gyri, basal ganglia and midbrain. Relative activation decreases during flow were observed in medial prefrontal and posterior cingulate cortex, and in the medial temporal lobe including the amygdala. Present findings suggest that even in the context of comparably short activation blocks flow can be reliably experienced and is associated with changes in neural activation of brain regions previously described. Possible mechanisms of interacting brain regions are outlined, awaiting further investigation which should now be possible given the greater temporal resolution compared with previous perfusion imaging.
Keywords: flow experience, multiple-demand system, amygdala, medial prefrontal cortex, dorsal raphe nucleus
Introduction
When engaging in a demanding activity, an optimal balance between task demands and individual skill level may give rise to the experience of “flow”. Flow is characterized by subjective experiences of effortless involvement, a high degree of task-related, focused attention, a deep sense of control and suspension of self-reflective thoughts contributing to a positive experience of pleasantness and intrinsic motivation (Csikszentmihalyi, 1975, 1990, 2000).
Recently, we have investigated the neural correlates of flow experiences (Ulrich et al., 2014) using perfusion magnetic resonance imaging (MRI) which is particularly suitable for experimental paradigms with rather low frequencies of alternating conditions (Wang et al., 2003). During blocks of 3 min duration, participants were asked to solve mental arithmetic tasks of low task difficulty (“boredom”), high task difficulty (“overload”) and task difficulty optimally fitting individual participants’ skills, achieved by continuous and automatic adjustment of the task difficulty level (“flow” condition). Compared with control conditions, the flow condition was associated with higher ratings on items assessing the degree of subjectively experienced flow documenting involvement and enjoyment, intrinsic motivation and perceived fit of skills and task demands. Analysis of neuroimaging data revealed two patterns of brain activation: In the left inferior frontal gyrus (IFG) and left basal ganglia, regional cerebral blood flow (rCBF) was significantly higher during flow than during boredom and overload. Given previous research, engagement of these regions was interpreted to contribute to the experience of flow by coding increased outcome probability (basal ganglia) and a deeper sense of cognitive control (IFG). Arranging the three conditions according to the ratio of demands to skills (increasing from boredom to flow to overload), this pattern reflected an inverted U (invU) effect. In contrast, brain regions previously described as parts of the “default-mode network” (DMN; Shulman et al., 1997; Raichle et al., 2001; Buckner et al., 2008; Sheline et al., 2009; Andrews-Hanna, 2012) such as the bilateral medial prefrontal cortex (MPFC), the angular gyri and amygdala were associated with significant rCBF decreases during flow compared with the control conditions (a so-called U effect). Given the proposed role of the MPFC as part of the DMN in self-referential processing (e.g. Gusnard et al., 2001; Zysset et al., 2003; D’Argembeau et al., 2007; Johnson et al., 2009; Jenkins and Mitchell, 2011), our previous finding of diminished DMN activity was interpreted to reflect one of the defining aspects of flow, that is, reduction of self-reflection (Csikszentmihalyi, 1990; Peifer, 2012). The temporary reduction of amygdala activity during flow was interpreted to align with this temporary reduction in self-referential processing since self-referential processing has been reported to correlate with unhappiness (Brewer et al., 2011), negative affectivity (Lemogne et al., 2011) and attribution of negative emotions to oneself (Grimm et al., 2009; Lemogne et al., 2009, 2012), and it has been shown in previous studies with direct emotional challenges that negative emotions were associated with increased activation of the amygdala while positive emotions aligned with relatively reduced activity (Morris et al., 1996; Whalen et al., 1998; Kim et al., 2004; Straube et al., 2008).
However, while functional features of previously observed brain regions render them likely to contribute to the experience of flow, we have also concluded that the pattern of increased and decreased neural activation associated with experiencing flow may indicate a specific interaction of brain regions that, similar to the different defining features of flow as psychological construct, may contribute to the final subjective experience. In other words, it is tempting to assume that it is not only the availability of optimally functioning single processes or brain regions working at optimal efficiency, but their effective interaction that finally leads to the experience of flow.
Unfortunately, the temporal resolution of previous perfusion imaging, though advantageous for low-frequency paradigms, did not permit a test of these interactions in the sense of effective functional connectivity. Therefore, the major motivation for this study was to investigate whether it is possible to experimentally induce flow experience at higher temporal resolution in order to measure its neural effects using a typical functional MRI (fMRI) block design with blocks of activation as short as 30 s to pave the way for ensuing analyses of effectively interacting brain regions. This requirement posed a challenge to the present investigation since it was not clear whether subjects would be able to experience flow in such short periods of time. We therefore further optimized our previous procedure of pre-adjustment of task difficulty to participants’ individual level of skill to facilitate their ability to enter a state of flow rather rapidly, and employed three different lines of evidence to support that it was the experience of flow which was measured. Functionally, we predicted a replication of at least those brain regions that have emerged as neural correlates of flow in our previous study. Specifically, we expected to observe inverted U-shaped effects in neural activation in the IFG and basal ganglia, and U-shaped effects were expected in MPFC and amygdala.
To further bolster the observance of experimentally induced flow we also measured participants’ electrodermal activity (EDA). Although scarcely used in the context of empirical flow research so far (e.g. Nacke and Lindley, 2009), EDA has been established in other fields as a sensitive and reliable psychophysiological index of changes in autonomic sympathetic arousal that are integrated with emotional and cognitive states (Critchley, 2002; Dawson et al., 2007; Boucsein et al., 2012). Given the novelty of the present approach there was no clear prediction as to whether increases or decreases in EDA would associate with the experience of flow. Intuitively, one might expect that the flow feature of subjective effortlessness would trigger a decrease in sympathetic arousal and hence lower EDA during flow compared with boredom and overload conditions. On the other hand, previous research has shown that EDA was increased during flow when compared with control conditions (Nacke and Lindley, 2009) as were other parameters of sympathetic activity (Peifer et al., 2014; Harmat et al., 2015), and it was speculated that down-regulation of irrelevant brain processes may come along with sympathetic activation as indicated by an increase in EDA (Peifer, 2012) reflecting enhanced attention on task fulfillment. This speculation is further supported by previous research showing a dynamic relationship between externally and internally focused attention (Critchley et al., 2011).
While objective measures of ongoing brain activation and EDA appear ideally suited to align with one of flow’s key characteristics to appear during activity, flow is also a subjective experience. Hence, physiological measures were supplemented by self-report measures and it was hypothesized that the subjectively experienced degree of flow was highest during the blocks of the flow condition compared with the boredom and overload conditions.
Also, to control for putative habitual or trait influences we included an assessment of participants' preference for mental arithmetic and reading as in our previous study, and also used the Swedish Flow Proneness Questionnaire (SFPQ; Ullén et al., 2012) to assess participants’ propensity to experience flow in different domains of everyday living.
Materials and methods
Participants
As in our previous perfusion MRI study on flow experience (Ulrich et al., 2014), the present sample of participants was restricted to males only in order to control for gender differences as a potential source of variation. Twenty-three right-handed students aged 24.0 years on average (standard deviation (SD) = 2.7) were recruited from the local university and were paid 20 EUR for participation. Neither psychiatric nor neurological disorders nor any contraindications regarding the fMRI procedure were reported. All subjects had normal or corrected-to-normal vision. The study was in accordance with the ethics committee at the University of Ulm and with the Declaration of Helsinki. Written informed consent was obtained prior to the experiment.
Experimental design
The present experimental design resembled that of our previous study (Ulrich et al., 2014). Major changes, however, applied to the length and number of blocks to meet the requirements imposed by the experimental realization as an fMRI block design to measure the blood oxygen level-dependent (BOLD) signal. Subjects performed visually presented mental arithmetic tasks. On each trial, a math expression appeared in black font above an on-screen keyboard in the center of the screen. Stimuli were presented on a white background on MRI compatible video goggles (VisuaStim Digital, Resonance Technology Inc., Northridge, CA, USA) at a resolution of 800 × 600 pixels. The on-screen keyboard was controlled by an MRI compatible trackball (NAtA TECHNOLOGIES, Coquitlam, Canada). Participants were asked to sum the presented two or more numbers in their mind and to enter the result as accurately and as fast as possible using the on-screen keyboard. After a period of 10 s per each math calculation, or when participants had submitted the result, there was a break of 1 s during which the string “xxx + x” appeared on the screen. Calculations and intermitting breaks were presented until the block length of 30 s had elapsed.
Depending on the specific experimental condition, task difficulty was either very low (boredom or “B” condition) or was challenging with dynamic adjustment of task difficulty to participants’ individual skill level (flow or “F” condition) or was overwhelming due to very high task difficulty (overload or “O” condition). In the boredom condition, subjects performed addition tasks involving always two numbers only, which were randomly drawn with the following restrictions: The first summand belonged to the interval 100–109, the second summand to the interval 1–9 and the result was between 101 and 110. In the flow condition, task demands adapted automatically and continuously to participants’ level of skill which had been estimated beforehand in a preparation phase (see below), and used as starting level. When the result was correct, task difficulty increased by one level, and when the result was incorrect, the level decreased by one level. Adjustment of task difficulty was achieved by two alternate mechanisms: In case the last summand had only one digit, the level increased by changing the last summand to a two-digit number in the next calculation. A further increase was accomplished by adding an additional one-digit number to the mathematical expression, etc. Analogously, a decrease in task difficulty was achieved by the two mechanisms outlined above but in reverse order. In the overload condition, the starting level of task difficulty was set three levels higher than the starting level of the flow condition. Levels were adjusted in the manner described above, but were not permitted to fall below the starting level. Our previous study (Ulrich et al., 2014) had confirmed that task demands in the overload condition are sufficiently higher than participants’ average level of skill, while minimizing the probability of permanent frustration. Subjects did not receive explicit feedback about response accuracy in any of the conditions.
The experiment began with a block of a rest condition where subjects were asked to fixate a black cross centered on a white screen while avoiding unnecessary movements. Afterwards, nine blocks per task condition, with intermitting rest blocks, were performed. Both task and rest blocks lasted 30 s. There were two predefined block sequences counterbalanced across participants: –B–F–O–B–F–B–O–F–O–F–B–O–B–F–O–B–O–F–B–F–O–F–B–O–F–O–B– and –B–O–F–B–O–B–F–O–F–O–B–F–B–O–F–B–F–O–B–O–F–O–B–F–O–F–B– (rest blocks are reflected by the hyphen).
In the preparation phase, prior to scanning, participants underwent two practice blocks. The first block consisted of an extended version of the B condition, lasting 3 min, to make participants familiar with the trackball. After a short break of 15 s, a modified version of the flow condition was performed aimed at determining participants’ individual level of skill: Starting at the lowest possible level, in the course of 4 min task demands were automatically and continuously adapted to participants’ skills. The average task level pertaining to the last 25 % of results was used as the starting level for the flow condition in the experiment.
The computer algorithms generating and presenting the math calculations, analyzing the results and performing the level adjustments were programmed in Scala version 2.9.01 and run in Java Runtime Environment version 6.0.05 on a standard PC with Windows XP Professional version 2002 Service Pack 3.
Post-scan questionnaires
After the experiment, outside the MR scanner, participants completed questions on their subjective experiences during the fMRI experiment and questions regarding their preference for math. Since it is known that flow proneness (FP) varies widely between individuals (Csikszentmihalyi and Csikszentmihalyi, 1988; Csikszentmihalyi, 2000), we also included a German version of the SFPQ, developed by Ullén et al. (2012), which measures how prone participants are to experience flow in three everyday activities (i.e. work, maintenance and leisure). All questions were presented in written form on a computer screen, and responses (mouse clicks) were automatically recorded by the computer program.
First, the following three Likert-scaled statements were presented: “I would love to solve math calculations of that kind again", “Task demands were well matched to my ability" and “I was thrilled". Participants’ responses could range from 1 (“I do not agree at all”) to 7 (“I completely agree”). In our previous investigation (Ulrich et al., 2014), those three items discriminated best between the flow condition and both control conditions. Also, these items have been included in several previous experimental flow studies and consistently represented core elements of the flow assessment based on instruments including a larger set of items (Keller and Bless, 2008; Keller and Blomann, 2008; Keller et al., 2011b). Since in this study the questions were presented after the experiment, we first informed participants about the presence of three basic conditions varying in the level of task difficulty (labeled “easy”, “moderate” and “difficult” for B, F and O, respectively) and then asked subjects to retrospectively respond to “some questions on their subjective experiences” related to each of the three conditions (in the order B–F–O).
Since one may speculate that individuals who strongly dislike mental arithmetic will show a limited likelihood to enter a state of flow in the paradigm applied in this study, we examined whether participants’ attitude toward mental arithmetic actually affects their reactions to the task. Three questions were used to assess participants’ preferences for mental arithmetic, and for reading, as a control: “How much do you like performing mental arithmetic?”, “How much do you like reading?” and “To what extent do you prefer mental arithmetic over reading?”. Responses were given on visual analog scales ranging from “not at all” to “as much as possible” (items 1 and 2) and “much less than reading” to “much more than reading” (item 3), respectively.
In order to test whether habitual FP actually affects reactions to the task, we also administered a German version of the SFPQ. Upon each of the seven Likert-scaled questions in the domains “Work”, “Maintenance” and “Leisure”, participants were forced to choose between one of five response alternatives (“Never”, “Rarely”, “Sometimes”, “Often” or “Everyday, or almost everyday”). Since all participants were students, for the domain “Work”, the statement “When you do something at work, how often does it happen that…” was replaced by “When you study for exams, prepare a presentation, or write a research paper, how often does it happen that…”.
MRI data acquisition
Functional images were acquired on a 3-Tesla magnetic resonance scanner (MAGNETOM Allegra, Siemens AG, Erlangen, Germany) in combination with a single channel transmit/receive head coil (RAPID Biomedical GmbH, Rimpar, Germany). During the experiment, an echo-planar pulse sequence (EPI) was applied to measure the T2*-weighted BOLD signal. The following parameters were used: repetition time (TR) = 2000 ms, echo time (TE) = 35 ms, bandwidth = 3396 Hz/Px, flip angle = 90°, field of view (FOV) = 228 mm, matrix size = 64 × 64, number of slices = 33, slice thickness = 3.0 mm, interslice gap = 0.6 mm, isotropic voxel size of 3.6 mm3. Ascending slice acquisition was parallel to the AC-PC line. Scan time was about 27 min, corresponding to 825 EPI volumes. To obtain a high-resolution T1-weighted structural image for later coregistration purposes, a magnetization prepared rapid acquisition gradient echo sequence was employed (TR = 2500 ms, TE = 4.57 ms, inversion time = 1100 ms, bandwidth = 130 Hz/Px, flip angle = 12°, FOV = 256 mm, matrix size = 256 × 256, voxel volume = 1 mm3, slice orientation: sagittal; scan time about 9 min).
Measurement of EDA
To obtain a marker of sympathetic activity in response to the task conditions participants’ EDA was measured during the fMRI experiment. Except for the electrode paste, hardware and software described below were from Brain Products GmbH (Gilching, Germany). Before entering the MR scanner, participants were asked to wash their hands with lukewarm water without soap (Boucsein et al., 2012). Then, two reusable sintered silver/silver chloride electrodes (as part of the “GSR MR Set”) were prepared with TD-246 Skin Conductance Electrode Paste (0.5 % saline; Discount Disposables, St. Albans, VT, USA) and placed on the volar middle phalanges of the left index and middle fingers. The electrodes were connected to the “Galvanic Skin Response (GSR) sensor for fMRI” relying on the constant voltage (0.5 V) measuring principle. The signal was passed on to the “ExG AUX Box” before entering the amplifier “BrainAmp ExG-MR-16”. Power was supplied by a rechargeable 5.6 V “PowerPack” battery. The amplified signal left the MR cabin through a fiber optic cable to undergo opto-electric conversion by the “USB 2 Adapter” connected to a standard laptop. The EDA signal was recorded at a sampling rate of 5000 Hz, and low-pass filtered (cutoff at 250 Hz) using the software BrainVision Recorder 1.20.0506 running under Windows XP Professional version 2002 Service Pack 3.
Data analysis
Performance data and post-scan questionnaires
Task performance as the percentage of correctly solved math calculations per condition, and ratings associated with the three items assessing subjects’ flow experience during the experiment were analyzed using repeated measurement analyses of variance (ANOVA) to test for significant differences between boredom, flow and overload conditions (averaged across block repetitions of each condition). Significant differences were further investigated using post-hoc Newman–Keuls tests. For the purpose of correlation analyses, an index of the individually experienced level of flow was obtained by computing the difference “−B + 2F − O” from each item’s rating scores and summing up these differences across items. Like in our previous study we refer to that measure as “flow index” (Ulrich et al., 2014). Ratings on SFPQ questions were averaged within each domain to obtain domain-specific measures (FP-Work, FP-Maintenance and FP-Leisure). In addition, the mean score across domains was computed (FP-total). Across subjects it was tested whether the flow index correlated with task performance, with preference for mental arithmetic or with FP. All performance and self-report results were assessed at a significance level of P < 0.050.
MRI data
Imaging data preprocessing and statistical analyses were performed with the software package Statistical Parametric Mapping (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included discarding the first four EPI volumes, spatial realignment of the EPIs to the session mean and coregistration of the individual T1 image to the mean EPI. Thereafter, the T1 image was segmented into grey and white matter using SPM8’s “new segment” routine. Spatial normalization to the Montreal Neurological Institute (MNI) space was achieved by applying the resulting deformation field to the T1 image and the EPI images. Before smoothing all EPI images using a Gaussian kernel with 8 mm full width at half maximum, voxels were resampled to 2 mm × 2 mm × 2 mm.
After data preprocessing, a hierarchical standard modeling approach was used. For each subject a General Linear Model (GLM) was set up by specifying the onsets and durations (30 s) of the task blocks for B, F and O as separate regressors. The spatial realignment parameters were added to the design matrix as conditions of no interest. Resulting box–car functions were convolved with the canonical hemodynamic response function. To remove low-frequency scanner drifts, data were high-pass filtered with a frequency cutoff at 128 s, and an autoregression model of polynomial order 1 was used to account for temporally correlated residual errors. After model estimation, one-sided t-contrast images representing the averaged, estimated magnitude of neural activation associated with B, F and O, vs baseline were computed for each participant and then submitted to a flexible factorial random-effects analysis with factors Condition (B vs F vs O) and Subject. Upon estimation, two one-sided t-contrasts were used to test for inverted U (invU)-shaped [−B + 2F − O], and U-shaped [B − 2F + O] differences between conditions. Statistical parametric maps were assessed at a voxel height threshold of P < 0.001 and a family-wise error (FWE)-corrected cluster threshold of P < 0.050 [corresponding to 257 (invU) or 214 (U) contiguously significant voxels]. Significant clusters were saved as masks for use in follow-up analyses testing for significant correlations of neural invU- or U-shaped differences with the flow index, and with the invU-shaped EDA response pattern. Beforehand, respective one-sided t-contrast images representing neural invU [ − B + 2F − O], and U differences [B − 2F + O] had been computed at the single-subject level to be entered into separate SPM8 regression analyses. Results based on invU contrast images were assessed within clusters showing a significant categorical invU effect (shown in Figure 1). Analogously, correlation analyses involving U-shaped contrast images were restricted to clusters with a significant categorical U effect (see Figure 2). Significance of correlations was assessed at an uncorrected voxel height threshold of P < 0.050 combined with a cluster extent threshold of 10 contiguous voxels.
Fig. 1.
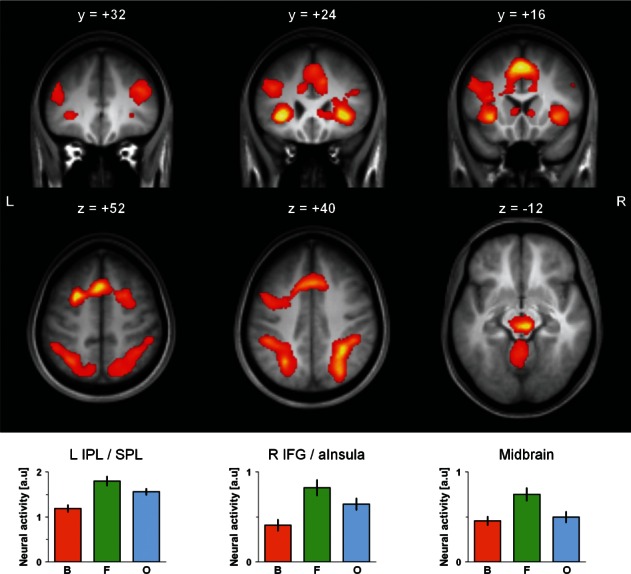
Brain regions showing invU-shaped responses, i.e. significantly higher brain activation during flow (F) than during boredom (B) and overload (O), revealed by the contrast [−B + 2F − O]. Effects were overlaid on coronal and transverse sections of the group averaged T1 image using MRIcron (http://www.mccauslandcenter.sc.edu/mricro/mricron/). Coordinates given above each section refer to MNI space. Averaged parameter estimates representing neural activity during B, F and O are shown for selected brain regions (bottom panel). Estimates were extracted from and averaged across the entire respective cluster listed in Table 1 except for the midbrain where averaging was, in addition, restricted to a region of interest (639 voxels) defined by the WFU Pickatlas v2.4 (Maldjian et al., 2003, 2004). Error bars represent standard error of the mean (23 subjects). a.u.: arbitrary unit, L: left, R: right, aInsula: anterior insula, IFG: inferior frontal gyrus, IPL: inferior parietal lobule, SPL: superior parietal lobule.
Fig. 2.
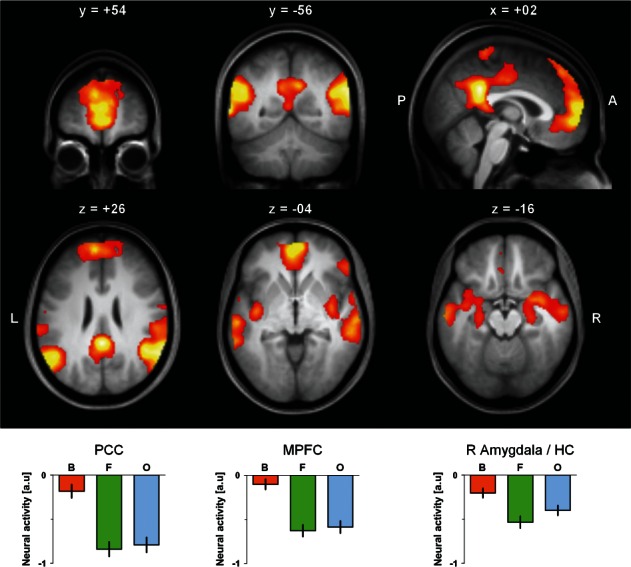
Brain regions that were less active in the flow (F) condition than in the boredom (B) and overload (O) conditions, resulting in a U-shaped response pattern, detected by the contrast [B − 2F + O]. Statistical parametric maps were overlaid on the group averaged T1 image using MRIcron. Averaged parameter estimates derived from the medial prefrontal cortex (MPFC) refer to the entire cluster listed in Table 2. Estimates from the posterior cingulate cortex (PCC), and from the right amygdala/hippocampus (HC) were additionally restricted (636 voxels and 304 voxels, respectively) to anatomical masks created using the WFU Pickatlas v2.4 (Maldjian et al., 2003, 2004). Error bars represent standard error of the mean (23 subjects). a.u.: arbitrary unit, A: anterior, L: left, P: posterior, R: right.
EDA data
Raw data were converted into the Brain Vision Data Exchange Marker File format using BrainVision Analyzer 2.0.4.368 (Brain Products GmbH, Gilching, Germany). Using in-house written software under MATLAB (Version 7.9.0.529) data were downsampled to a rate of 5 Hz, bandpass filtered with a third-order Butterworth filter and cutoff frequencies of 0.083 and 0.0078 Hz, respectively, to remove fast and slow changes in skin conductance level. Data were then averaged in the interval between two consecutive TTL triggers to temporally align with the MR sampling rate of 2 s (TR) and transformed into binary ANALYZE (7.5) file format to be further processed within the software framework of SPM8. Akin to the analysis of fMRI data we used a temporally extended response function as predictor in a GLM approach. The underlying canonical skin conductance response function (SCRF) was set up as described in Bach et al. (2010), which is basically a modeled Gaussian smoothed exponential function with empirically derived estimates for event-to-peak time, rise time and two exponential constants approximately defining the decay. This canonical SCRF was convolved with typical box–car functions starting at the onset of each of the nine repetitions per each condition with duration of 30 s each. From individual first-level analysis averaged contrast estimates of the magnitude of skin conductance change against implicit baseline (fixation cross) were computed per each condition and propagated to a random-effects group analysis. A one-way ANOVA model for repeated measures was set-up as a flexible factorial design within SPM8, with Condition (B, F and O) as within-subjects factor, and an additional factor Subject to account for between-subjects variance. Akin to present fMRI data analysis linear contrasts (Friston et al., 1995) were set up to test for inverted U-shaped [−B + 2F − O], and U-shaped [B − 2F + O] differences between conditions. Differences were inferred significant at a nominal level of P < 0.050.
Results
Performance data and post-scan questionnaires
On average participants correctly solved 96.2 % of all math expressions in the boredom condition, 56 % in the flow condition and 3.8 % in the overload condition. Differences between conditions were significant (F(2, 44) = 788.40, P < 0.001), and post-hoc Newman–Keuls tests showed pair-wise differences between all conditions (all P < 0.001).
Participants’ average ratings on the questions assessing subjective flow experiences during the task blocks are presented in Figure 3A. The pattern across B, F and O followed an invU shape. The differences between conditions were significant for all three items, and also for the sum of items (all F(2, 44) > 6.25, all P < 0.005). Pair-wise differences between F and B, and F and O were significant for all items and the sum of items (all P < 0.050) as revealed by post-hoc Newman–Keuls tests. Correlation analyses showed that there were no significant positive relationships between participants’ flow index and their preference for mental arithmetic (r = −0.13, t(22) = 0.61, P = 0.274, one-sided), preference for reading (r = 0.07, t(22) = 0.30, P = 0.383, one-sided), or greater preference for mental arithmetic than for reading (r = 0.02, t(22) = 0.10, P = 0.461, one-sided). It was also tested whether in the flow condition task performance positively correlated with individuals’ ratings. There was no significant correlation for any one of the three items (all r < 0.13, t(22) < 0.59, all P > 0.282).
Fig. 3.
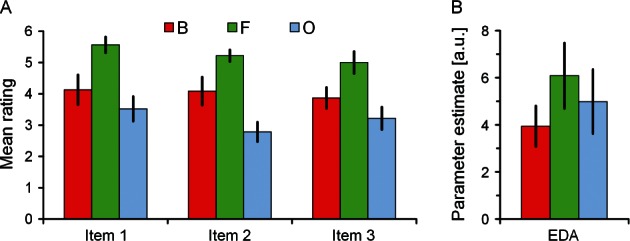
(A) Mean ratings (23 participants) associated with the boredom (B), flow (F) and overload (O) conditions on post-scan questions aimed at assessing the subjectively experienced degree of flow. Item description: 1: “I would love to solve math calculations of that kind again”, 2: “Task demands were well matched to my ability”, 3: “I was thrilled”. B. GLM parameter estimates representing EDA averaged over 22 participants. Error bars indicate standard error of the mean. a.u.: arbitrary unit.
On the SFPQ measuring participants’ proneness to experience flow (FP), the following mean scores were obtained which were in agreement with previously reported neuroimaging data on the same questionnaire (de Manzano et al., 2013): FP-Work = 3.52 (SD = 0.28), FP-Maintenance = 3.46 (SD = 0.33), FP-Leisure = 3.76 (SD = 0.31), and FP-Total = 3.58 (SD = 0.22). A significant positive correlation with the flow index was found for FP-Maintenance (r = 0.45, t(22) = 2.29, P = 0.016, one-sided), whereas correlations between the flow index and the scores of FP-Work, FP-Leisure and FP-Total were not robust (all r < 0.26, all t(22) < 1.21, all P > 0.12, one-sided).
EDA data
The GLM parameter estimates representing average EDA during the fMRI tasks under conditions B, F and O are summarized in Figure 3B. Resembling the invU patterns observed for subjective experiences, EDA was significantly greater in the flow condition than in both control conditions (z-score = 3.51, P < 0.001). A positive correlation between participants’ invU (−B + 2F − O) shaped EDA difference and the flow index initially failed significance (r = 0.25, t(22) = 1.17, p = 0.128, one-sided) owing to one obvious outlier. After its removal, the correlation was r = 0.36, just above the nominal significance level (t(21) = 1.70, P = 0.052, one-sided).
Neuroimaging data
As depicted in Figure 1 and summarized in Table 1, significantly higher neural activation during the flow condition than during the boredom and overload conditions (−B + 2F − O) was observed bilaterally in the triangular (Brodmann area (BA) 45) and opercular (BA 44) parts of the IFG, the anterior insula (BA 47), the middle cingulate cortex (BA 24), the pre-supplementary motor area (pre-SMA; BA 6), and adjacently, in the superior and middle frontal gyri (BA 8). Further cortical areas showing an invU pattern of neural activation were the bilateral inferior (IPL) and superior (SPL) parietal lobules (BA 40, and 7, respectively). Subcortically, the invU-shaped pattern of neural activation was found in the cerebellum, the thalami, the head and body of the caudate nuclei and in the midbrain bilaterally, most likely including the dorsal raphe nuclei (DRN) and the red nuclei.
Table 1.
Brain regions showing higher neural activation during the flow condition than during the boredom and overload conditions, revealed by the contrast [ − B + 2F − O] at a threshold of P < 0.001 (voxel level) and P < 0.050 (cluster level, FWE-corrected)
| Brain region | BA | Number of voxels | Peak voxel (MNI space) |
||||
|---|---|---|---|---|---|---|---|
| x | y | z | z-score | ||||
| L | Anterior insula | 47 | 7591 | −30 | 22 | −2 | 7.23 |
| L | Pre-SMA | 6 | −2 | 16 | 48 | 6.88 | |
| L | Middle frontal gyrus | 8 | −24 | 6 | 52 | 6.39 | |
| L | Middle cingulate cortex | 24 | −10 | 14 | 44 | 5.91 | |
| L | IFG, opercular part | 44 | −38 | 8 | 26 | 5.70 | |
| R | Superior frontal gyrus | 8 | 26 | 6 | 56 | 5.37 | |
| L | Thalamus | – | −18 | −10 | 0 | 5.33 | |
| R | Middle cingulate cortex | 32 | 10 | 24 | 32 | 5.09 | |
| L | IFG, triangular part | 45 | −40 | 24 | 26 | 4.84 | |
| L | Thalamus | – | −14 | −8 | 10 | 4.75 | |
| L | Caudate nucleus | – | −18 | −6 | 24 | 4.31 | |
| R | Thalamus | – | 12 | −4 | 2 | 4.25 | |
| R | Caudate nucleus | – | 16 | 0 | 24 | 4.11 | |
| L | Caudate nucleus | – | −16 | −20 | 20 | 4.10 | |
| L | IFG, triangular part | 45 | −44 | 34 | 16 | 3.98 | |
| R | Anterior insula | 47 | 1601 | 30 | 22 | 0 | 6.48 |
| R | IFG, triangular part | 45 | 40 | 32 | 24 | 4.94 | |
| R | Caudate nucleus | – | 10 | 20 | 0 | 4.59 | |
| L | Caudate nucleus | – | −8 | 16 | 2 | 3.80 | |
| L | Superior parietal lobule | 7 | 2298 | −28 | −60 | 46 | 6.42 |
| L | Inferior parietal lobule | 40 | −44 | −40 | 40 | 5.18 | |
| R | Midbrain | – | 3116 | 4 | −24 | −12 | 6.24 |
| L | Midbrain | – | −2 | −24 | −14 | 5.83 | |
| R | Cerebellum | – | 30 | −58 | −28 | 5.63 | |
| R | Cerebellum | – | 2 | −46 | −18 | 5.16 | |
| R | Inferior parietal lobule | 40 | 2875 | 32 | −50 | 42 | 6.18 |
| R | Inferior parietal lobule | 40 | 40 | −40 | 40 | 5.16 | |
| R | Superior parietal lobule | 7 | 26 | −68 | 48 | 4.89 | |
| R | IFG, opercular part | 44 | 257 | 46 | 8 | 28 | 4.63 |
Figure 2 and Table 2 summarize locations where significant U-shaped differences (B − 2F + O) in brain activation emerged. This was observed in dorsal and ventral regions of the MPFC, precisely, in the medial superior and medial orbital frontal gyri (BA 9/10), and in the anterior cingulate cortex (including both subgenual and pregenual subregions). A separate frontal cluster appeared in the orbital part of the right IFG (BA 47). Posteriorly, U-shaped patterns were detected in the posterior (PCC) and middle cingulate cortex (BA 26, and 23, respectively), superiorly extending into the paracentral lobules (BA 4). Another cluster was located in the right temporal cortex including anterior, middle and posterior portions of the middle and superior temporal gyri (BA 22/42/20). It also encompassed parts of the postcentral gyrus (BA 3), the angular gyrus (BA 39), the middle insula, the hippocampus (HC) and of the amygdala. Homologous temporal, parietal, and insular activation differences were found in the left hemisphere, which also applies to the HC and to the amygdala.
Table 2.
Brain regions showing lower neural activation during the flow condition than during the boredom and overload conditions, revealed by the contrast [B − 2F + O] at a threshold of P < 0.001 (voxel level) and P < 0.050 (cluster level, FWE-corrected)
| Brain region | BA | Number of voxels | Peak voxel (MNI space) |
||||
|---|---|---|---|---|---|---|---|
| x | y | z | z-score | ||||
| R | Posterior cingulate cortex | 26 | 3926 | 2 | −44 | 30 | 6.58 |
| L | Middle cingulate cortex | 23 | −4 | −24 | 38 | 5.32 | |
| L | Paracentral lobule | 4 | −6 | −36 | 70 | 4.58 | |
| R | Paracentral lobule | 4 | 4 | −38 | 70 | 4.30 | |
| R | Middle temporal gyrus | 22 | 7939 | 62 | −46 | 10 | 6.53 |
| R | Angular gyrus | 39 | 56 | −56 | 30 | 6.35 | |
| R | Superior temporal gyrus | 42 | 58 | −24 | 20 | 5.66 | |
| R | Amygdala | – | 30 | 0 | −18 | 4.98 | |
| R | Middle insula | – | 38 | −8 | −4 | 4.71 | |
| R | Postcentral gyrus | 3 | 60 | −10 | 40 | 4.61 | |
| R | HC | – | 26 | −16 | −18 | 4.51 | |
| R | Temporal pole | 20 | 46 | 10 | −34 | 4.49 | |
| R | Amygdala | – | 20 | −4 | −20 | 4.11 | |
| L | Middle temporal gyrus | 37 | 4665 | −62 | −56 | 8 | 6.37 |
| L | Angular gyrus | 39 | −48 | −58 | 26 | 6.12 | |
| L | Middle temporal gyrus | 21 | −60 | −22 | −6 | 5.30 | |
| L | Superior temporal gyrus | 42 | −60 | −28 | 20 | 5.20 | |
| L | Middle insula | – | −40 | −12 | −4 | 4.80 | |
| L | HC | – | −26 | −16 | −20 | 4.33 | |
| L | Amygdala | – | −26 | −2 | −16 | 3.85 | |
| L | Medial superior frontal gyrus | 10 | 5449 | −6 | 60 | 10 | 6.30 |
| R | Medial orbital frontal gyrus | 10 | 6 | 54 | −4 | 5.89 | |
| L | Medial superior frontal gyrus | 10 | −8 | 52 | 24 | 5.63 | |
| R | Medial superior frontal gyrus | 9 | 12 | 40 | 48 | 5.39 | |
| R | Anterior cingulate cortex | 32 | 6 | 50 | 24 | 4.76 | |
| R | IFG, orbital part | 47 | 297 | 48 | 34 | −2 | 4.43 |
| R | IFG, orbital part | 47 | 56 | 26 | −2 | 4.05 | |
| L | Postcentral gyrus | 3 | 214 | −62 | −14 | 36 | 4.00 |
| L | Postcentral gyrus | 3 | −46 | −26 | 58 | 3.94 | |
| L | Postcentral gyrus | 3 | −56 | −18 | 44 | 3.60 | |
As summarized in Table 3, the neural invU-shaped pattern observed in the left caudate nucleus significantly correlated with the subjectively experienced degree of flow. There were also robust correlations between the flow index and U-shaped neural modulation of the MPFC and of the right amygdala (see Table 3, and Figure 4).
Table 3.
Brain regions showing a positive correlation between neural activation differences (invU, or U) and subjectively experienced degree of flow (flow index)
| Brain region | Number of voxels | Peak voxel |
Peak statistics |
Cluster statistics |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | r | t(22) | P | r | t(22) | P | |||
| Correlation between invU-shaped modulation of neural activation and the flow index | |||||||||||
| L | Caudate | 42 | −18 | −12 | 28 | 0.51 | 2.72 | 0.006 | 0.42 | 2.14 | 0.022 |
| Correlation between U-shaped modulation of neural activation and the flow index | |||||||||||
| R | Amygdala | 70 | 32 | 0 | −20 | 0.52 | 2.76 | 0.006 | 0.42 | 2.11 | 0.023 |
| R | MPFC | 10 | 12 | 52 | −4 | 0.39 | 1.96 | 0.031 | 0.38 | 1.86 | 0.038 |
| L | MPFC | 21 | −10 | 58 | 8 | 0.39 | 1.93 | 0.033 | 0.37 | 1.83 | 0.041 |
P-values one-sided.
Fig. 4.
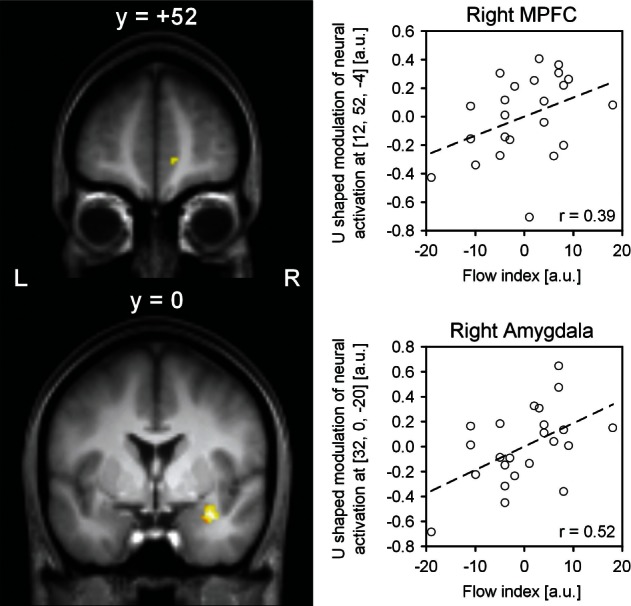
Significant positive correlations between the subjectively experienced degree of flow, represented by the flow index, and the magnitude of U-shaped modulation of neural activation. Statistical parametric maps were superimposed on coronal sections of the group averaged T1 image. Scatter plots were derived from the voxel with the highest correlation coefficient. Data are mean-centered. MPFC: medial prefrontal cortex, a.u.: arbitrary unit, L: left, R: right.
Discussion
The experience of flow ensues when humans engage in a demanding task while task demands are balanced with the individual’s level of skill. In this fMRI study with 23 healthy, male participants, flow was experimentally induced in a mental arithmetic task by way of automatically and continuously adjusting task difficulty to participants’ individual skill level. This “flow condition” was compared with conditions of boredom and overload. Post-scan assessments revealed significantly greater subjective flow experience during the flow condition compared with control conditions (inverted U effect). As a simplified measure of the degree of experienced flow, the “flow index” (introduced in Ulrich et al., 2014) was determined which significantly correlated with the FP Questionnaire (Maintenance subscale), and with participants’ invU-shaped EDA, that is, greater activity under flow relative to both control conditions. Notably, the flow experience did not depend on participants’ task performance, and not on their preference for mental arithmetic. In the brain, differences between conditions following an invU-shaped pattern were detected in several mostly lateral frontal and posterior parietal areas, the thalamus, the basal ganglia and in the midbrain. In contrast, MPFC, posterior cingulate (PCC), lateral temporoparietal, and medial temporal regions including the amygdala, were less active during the flow condition compared with control conditions (U effect). Moreover, the U-shaped response profiles of the MPFC and the amygdala under flow were robustly related to participants’ experienced degree of flow, as expressed by the flow index.
Relative increases in neural activation during the experience of flow
Most brain regions showing higher activation during the flow than during the control conditions closely correspond to what has been termed “multiple-demand (MD) system” (Duncan, 2010; Fedorenko et al., 2013), including parts of the anterior insula, the IFG, the pre-SMA, the IPL/SPL, the thalamus and the cerebellum. The MD system has been suggested to operate functionally general, that is, to represent domain- and process-general functions in a wide array of demanding cognitive tasks, including mental arithmetic. During processing a cognitive task the MD system may divide the problem at hand into smaller sub-parts that can be solved independently (i.e. sequential mental programming; Duncan, 2010). This general functional feature might explain why engagement of the MD system was highest during flow, but not during overload, although the latter condition was associated with the highest nominal level of task difficulty: As indicated by the performance data, during flow participants were much more successful in performing the necessary sequential arithmetic operations to arrive at a correct solution, which suggests that the magnitude of activity of MD brain regions may be better accounted for by the product of task difficulty and successful performance, rather than by mere task difficulty. Whether this putative mechanism is specific to the present task with its inherent feature of providing sequential steps associated with successful solutions remains an open issue. Experimentally induced flow experiences have been reported across a variety of activities such as playing a computer game (Tetris; Keller and Bless, 2008; Harmat et al., 2015) or answering knowledge questions (Keller et al., 2011a). With some face validity a serial structure of task affordances appears likely in the Tetris game. Even when probing long-term memory to select among various alternatives in general knowledge games, one can speculate that this procedure can be fragmented into systematic serial queries to reach the final solution. However, the question of how experience of flow relates to the inherent structure of task affordances and related mental operations is an intriguing issue for future investigations.
Relative decreases in neural activation during the experience of flow
Mirroring the invU-shaped response profile of MD regions, another set of brain regions showed the opposite pattern, that is, relatively greater decreases in brain activation during the experience of flow compared with conditions of boredom and overload. These brain regions, i.e. the MPFC, the PCC, the lateral temporoparietal cortex and regions of the medial temporal lobe, have been suggested to constitute the brain’s DMN. The DMN usually shows greater activity during passive states than during goal-directed behaviors (Shulman et al., 1997; Raichle et al., 2001; Buckner et al., 2008; Sheline et al., 2009; Andrews-Hanna, 2012). An important function attributed to DMN activity is self-referential processing which appears to be particularly tied to the MPFC and the PCC (Gusnard et al., 2001; Zysset et al., 2003; Northoff et al., 2006; D'Argembeau et al., 2007; Johnson et al., 2009; van Buuren et al., 2010; Brewer et al., 2011; Jenkins and Mitchell, 2011; Whitfield-Gabrieli et al., 2011; Philippi et al., 2012), and studies in healthy controls and patients with major depression have associated increased activity of the MPFC with unhappiness (Brewer et al., 2011), negative affectivity (Lemogne et al., 2011) and increased depressive self-focus associated with increased attribution of negative emotions to oneself (Grimm et al., 2009; Lemogne et al., 2009, 2012). Therefore, given the congruency of present and previous results (Ulrich et al., 2014), it appears plausible to assume that absence of self-reflective thoughts as one of the hallmarks of flow together with a putative reduction of negative affectivity is associated with decreased MPFC activity.
Different from our previous study, an additional U effect can now be reported in the posterior cingulate cortex. Although this region had already been identified in our previous study (Ulrich et al., 2014) it could not be reported because its cluster size had failed the cluster extent threshold required. Together with the MPFC, meditation techniques aimed at focusing attention away from self-referential processing were related to decreased PCC activity in experienced meditators (Farb et al., 2007; Brewer et al., 2011). Moreover, a recent fMRI study applying real-time neurofeedback to experienced meditators has linked decreases in PCC activity to subjective experiences of undistracted awareness (concentration), effortless doing and contentment (Garrison et al., 2013). This is in good agreement with the present result of decreased PCC activity during flow, since deep concentration on the task at hand and subjective feelings of effortless involvement are defining characteristics of experiencing flow.
Differential modulation of sympathetic arousal and emotional arousal during flow
EDA indexing sympathetic arousal was highest during flow, and lower during boredom and overload. This result is in agreement with the proposal that the experience of flow is accompanied by a moderate level of sympathetic arousal (Peifer, 2012), it aligns with a previous report (Nacke and Lindley, 2009) using the same measure during flow experiences while playing a computer-game, and it is in agreement with other studies using heart-rate variability as a measure of sympathetic arousal to show that the subjective feeling of effortlessness (Bruya, 2010) dissociates from objective measures of sympathetic arousal.
Together with the particularly U-shaped brain imaging result pattern in the ventral MPFC, it is tempting to speculate on putative mechanisms that gave rise to increased EDA during flow. Recent work has suggested that the right fronto-insular cortex, which was most active during flow in this study, initiates control signals that causally disengage activity of task-irrelevant DMN regions (Sridharan et al., 2008), and that this shift in engagement is accompanied by a shift from internally to externally focused attention (Critchley et al., 2011) indexed by increased sympathetic arousal. Corresponding to this view is the observation from recent neuroimaging studies of an association between ventral MPFC and EDA, where decreases in ventral MPFC activity were correlated with increased EDA during functional challenges (Nagai et al., 2004; Zhang et al., 2013), and also during resting-state fMRI (Fan et al., 2012).
In this context, it is intriguing that in this study a region in the midbrain, most likely representing the DRN was highly activated during flow, showing the greatest invU effect. Serotonergic DRN projections to the MPFC, despite involving both excitatory and inhibitory effects, have been reported to result in a net decrease in neural activity of the MPFC (Hajós et al., 2003; Puig et al., 2005; Hahn et al., 2012). Furthermore, psychopharmacological studies could show that the effects of selective serotonin reuptake inhibitors resulted in lowering DMN hyperactivity which associated with amelioration of depressive symptoms (e.g. Drevets, 2000; Mayberg et al., 2000; Brody et al., 2001; Drevets et al., 2002; Sheline et al., 2009; Cooney et al., 2010; Nejad et al., 2013; van de Ven et al., 2013). Thus, albeit speculative, the relative down-regulation of MPFC activity during flow might have been an immediate consequence of the flow-associated relative increase in DRN activity, which in turn may also have motivated the observed increase in EDA.
Alternatively, though, increased activity of the DRN can also have more direct influences on increasing EDA. An increase in EDA results from slight increases in eccrine sweat gland activity which is directly controlled by the hypothalamus (Davison and Koss, 1975; Shibasaki and Crandall, 2010). Since most areas of the hypothalamus receive serotonergic inputs from the DRN (Sawchenko et al., 1983; Willoughby and Blessing, 1987; Petrov et al., 1992), it is therefore also possible that increases in EDA during flow were conditioned by rather direct modulations of hypothalamic influences on sympathetic sudomotor nerve activation.
While EDA was invU-shaped, the amygdala showed a U-shaped response profile. Given that the amygdala receives serotonergic inputs (Graeff et al., 1996; Barnes and Sharp, 1999; Lowry et al., 2005; Celada et al., 2013) its U-shaped response pattern is suggestive to represent the most adequate serotonergic innervation that can be achieved by an optimal fit between task demands and the individual skill level during flow, while the boredom and overload conditions cannot trigger this optimal point of action. Given the amygdala’s role in mediating emotional arousal (Canli et al., 2002; Hamann et al., 2002; McGaugh, 2004; Lewis et al., 2007; Anders et al., 2008; Colibazzi et al., 2010; McReynolds and McIntyre, 2012) it is not unlikely that this “optimal” innervation may condition the positive feeling during flow, since it has been reported that positive emotions align with relatively reduced activity of the amygdala (Morris et al., 1996; Whalen et al., 1998; Kim et al., 2004; Straube et al., 2008).
One of the most intriguing and novel results of this study was the opposite response pattern of EDA and amygdala activation, which suggests that during the experience of flow two types of arousal may undergo opposite modulation: Sympathetic arousal is up-modulated, while, at the same time, emotional arousal mediated by the amygdala is down-modulated (see also Ulrich et al., 2014). This suggests that one of the distinct neural features of experiencing flow might be this seemingly paradoxical differential modulation of sympathetic and positive emotional arousal.
Comparison of the present BOLD results with our previous findings from MR-based perfusion imaging
Except for one specific region (left putamen) the present BOLD imaging study replicated our previous findings (Ulrich et al., 2014) of invU-shaped neural activation in the IFG, and U-shaped response profiles in the MPFC, the lateral temporoparietal cortex, the HC and amygdala. This study also revealed that, in addition to the IFG, other brain regions of the multiple demand system showed an invU-shaped pattern. As an explanation for the discrepancies it seems most plausible to assume that it is the difference in contrast-to-noise ratio between MR-based perfusion and BOLD imaging which has been described previously (Yang et al., 2005; Liu and Brown, 2007; Viviani et al., 2011; Wang et al., 2011; Detre et al., 2012; Donahue and Jezzard, 2013). Increased sensitivity of BOLD imaging might also explain why clusters found in this study involved both hemispheres while in our previous study the invU clusters were left-sided, and regions showing a U pattern of activation were mostly located in the right hemisphere. Still, beyond these observations the replication as reported above also suggests that it is indeed possible to induce experiences of flow even in a typical functional MRI block design with blocks of functional challenges of durations as short as 30 s. However, whether the intensity of present experiences of flow corresponds to intensities experienced in everyday situations cannot be answered with present data. Particularly, altered experiences of time spent during task processing and the feeling of absorption or immersion are likely to differ between experimental and everyday situations. Insofar, our results here should be understood as a more abstract approximation to the experience of flow, a price to pay when a complex construct is to be brought under rigorous experimental control in a laboratory setting.
Limitations and future directions
Given the temporal restrictions of present experimental settings we did not ask participants to rate aspects of altered time perception, absorption or immersion which certainly add to the multi-dimensionality of everyday flow experiences. This was mainly because corresponding items were not judged appropriate during pilot experiments with respect to the rather short block duration of 30 s, and imposed the risk that participants would perhaps discount the validity of the other better suited items on subjective experiences. From our previous study, we had also learned that items related to aspects of changes in time perception come with semantic ambiguity which renders them less discriminative, particularly with respect to the overload condition. Still, we acknowledge that our present selection of items was essentially driven by pragmatic considerations, and alternative ways to assess the subjectively experienced degree of flow should be tested in future studies to add to the multi-dimensionality of subjective flow experiences. In this context, it is also worth mentioning that asking for subjective experiences upon the various conditions with a temporal gap (i.e. after scanning) may not be ideal in obtaining participants’ experiences as lively as possible, while presentation of questionnaires during scanning is also not an optimal solution due to time restrictions and physical circumstances in the scanner. An alternative for future studies could be to replicate at least parts of the task offline and obtaining subjects’ responses immediately afterwards.
Another limiting aspect refers to the present independence between task performance and the experience of flow, which should be treated with some caution since due to the necessary and continuous adjustment of task difficulty in the flow condition the distribution of correct and incorrect solutions is limited and may therefore artificially shrink correlation coefficients.
Like in our previous study, the present sample of participants was restricted to male volunteers for reasons discussed in Ulrich et al. (2014). Although this feature reduces generalizability of the present findings, we accepted this limitation in order to keep results comparable between studies.
Present fMRI data analysis focused exclusively on the aspect of segregation. Thus, it still remains an open question how the identified brain regions showing invU- and U-shaped hemodynamic responses might causally interact to give rise to the experience of flow. However, with this new methodological approach a framework can be suggested that should open new avenues for research designed to better understand the neurobiological mechanisms of flow and to make the different mechanistic speculations above testable. Especially, modulation of serotonergic projection originating in the midbrains’ DRN was not evident in the previous perfusion study, and appears to bear novel and possibly crucial information as a promising starting point to track the mechanism which finally effects down-regulation of the MPFC and the amygdala, or may even mediate the interaction between both structures (Quirk and Beer, 2006; Price and Drevets, 2012; Robinson et al., 2013) that at present appear as the most relevant neural players in causing the experience of flow.
Acknowledgements
We thank all volunteers for their participation in this study, Sonja Illek and Kathrin Brändle for their great help in data collection, and Bärbel Herrnberger for technical assistance. We would also like to express our sincere appreciation to Haluk Erce Rodopman for programming the experiments of the present and our previous perfusion studies.
Funding
This work was supported by a grant from the German Research Foundation (DFG) to Johannes Keller [grant number KE 913/5-1].
Conflict of interest. None declared.
References
- Anders S., Eippert F., Weiskopf N., Veit R. (2008). The human amygdala is sensitive to the valence of pictures and sounds irrespective of arousal: an fMRI study. Social Cognitive and Affective Neuroscience, 3, 233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R. (2012). The brain's default network and its adaptive role in internal mentation. Neuroscientist, 18, 251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach D.R., Flandin G., Friston K.J., Dolan R.J. (2010). Modelling event-related skin conductance responses. International Journal of Psychophysiology, 75, 349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes N.M., Sharp T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology, 38, 1083–152. [DOI] [PubMed] [Google Scholar]
- Boucsein W., Fowles D.C., Grimnes S., et al. (2012). Publication recommendations for electrodermal measurements. Psychophysiology, 49, 1017–34. [DOI] [PubMed] [Google Scholar]
- Brewer J.A., Worhunsky P.D., Gray J.R., Tang Y.Y., Weber J., Kober H. (2011). Meditation experience is associated with differences in default mode network activity and connectivity. Proceedings of the National Academy of Sciences of the United States of America, 108, 20254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody A.L., Saxena S., Stoessel P., et al. (2001). Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Archives of General Psychiatry, 58, 631–40. [DOI] [PubMed] [Google Scholar]
- Bruya B. (2010). Introduction. In: Bruya B., editor. Effortless Attention. A new Perspective in the Cognitive Science of Attention and Action. Cambridge: MIT Press, pp. 1–28. [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. (2008). The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences, 1124, 1–38. [DOI] [PubMed] [Google Scholar]
- Canli T., Desmond J.E., Zhao Z., Gabrieli J.D. (2002). Sex differences in the neural basis of emotional memories. Proceedings of the National Academy of Sciences of the United States of America, 99, 10789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P., Puig M.V., Artigas F. (2013). Serotonin modulation of cortical neurons and networks. Frontiers in Integrative Neuroscience, 7, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colibazzi T., Posner J., Wang Z., et al. (2010). Neural systems subserving valence and arousal during the experience of induced emotions. Emotion, 10, 377–89. [DOI] [PubMed] [Google Scholar]
- Cooney R.E., Joormann J., Eugene F., Dennis E.L., Gotlib I.H. (2010). Neural correlates of rumination in depression. Cognitive, Affective and Behavioral Neuroscience, 10, 470–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D. (2002). Electrodermal responses: what happens in the brain. Neuroscientist, 8, 132–42. [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Nagai Y., Gray M.A., Mathias C.J. (2011). Dissecting axes of autonomic control in humans: insights from neuroimaging. Autonomic Neuroscience, 161, 34–42. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M. (1975) Beyond Boredom and Anxiety: Experiencing Flow in Work and Play. San Francisco, CA: Jossey-Bass. [Google Scholar]
- Csikszentmihalyi M. (1990). Flow: The Psychology of Optimal Experience. New York: Harper & Row. [Google Scholar]
- Csikszentmihalyi M. (2000). Happiness, flow, and economic equality. American Psychologist, 55, 1163–4. [PubMed] [Google Scholar]
- Csikszentmihalyi M., Csikszentmihalyi I.S. (1988). Optimal Experience: Psychological Studies of Flow in Consciousness. Cambridge: Cambridge University Press. [Google Scholar]
- D’Argembeau A., Ruby P., Collette F., et al. (2007). Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience, 19, 935–44. [DOI] [PubMed] [Google Scholar]
- Davison M.A., Koss M.C. (1975). Brainstem loci for activation of electrodermal response in the cat. American Journal of Physiology, 229, 930–4. [DOI] [PubMed] [Google Scholar]
- Dawson M.E., Schell A.M., Filion D.L. (2007). The electrodermal system. In: Cacioppo J.T., Tassinary L.G., Berntson. G.G., editors. Handbook of Psychophysiology. New York: Cambridge University Press, pp. 159–81. [Google Scholar]
- de Manzano O., Cervenka S., Jucaite A., Hellenäs O., Farde L., Ullén F. (2013). Individual differences in the proneness to have flow experiences are linked to dopamine D2-receptor availability in the dorsal striatum. Neuroimage, 67, 1–6. [DOI] [PubMed] [Google Scholar]
- Detre J.A., Rao H., Wang D.J., Chen Y.F., Wang Z. (2012). Applications of arterial spin labeled MRI in the brain. Journal of Magnetic Resonance Imaging, 35, 1026–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue M.J., Jezzard P. (2013) MR perfusion imaging in neuroscience. In: Barker P.B., Golay X., Zaharchuk G., editors. Clinical Perfusion MRI: Techniques and Applications. Cambridge: Cambridge University Press, pp. 103–26. [Google Scholar]
- Drevets W.C. (2000). Neuroimaging studies of mood disorders. Biological Psychiatry, 48, 813–29. [DOI] [PubMed] [Google Scholar]
- Drevets W.C., Bogers W., Raichle M.E. (2002). Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. European Neuropsychopharmacology, 12, 527–44. [DOI] [PubMed] [Google Scholar]
- Duncan J. (2010). The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14, 172–9. [DOI] [PubMed] [Google Scholar]
- Fan J., Xu P., Van Dam N.T., et al. (2012). Spontaneous brain activity relates to autonomic arousal. Journal of Neuroscience, 32, 11176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb N.A., Segal Z.V., Mayberg H., et al. (2007). Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience, 2, 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., Kanwisher N. (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences of the United States of America, 110, 16616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-B., Frith C.D., Frackowiak R.S.J. (1995). Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping, 2, 189–210. [Google Scholar]
- Garrison K.A., Santoyo J.F., Davis J.H., Thornhill T.A.t., Kerr C.E., Brewer J.A. (2013). Effortless awareness: using real time neurofeedback to investigate correlates of posterior cingulate cortex activity in meditators' self-report. Frontiers in Human Neuroscience, 7, 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeff F.G., Guimaraes F.S., De Andrade T.G., Deakin J.F. (1996). Role of 5-HT in stress, anxiety, and depression. Pharmacology, Biochemistry and Behavior, 54, 129–41. [DOI] [PubMed] [Google Scholar]
- Grimm S., Ernst J., Boesiger P., et al. (2009). Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical-cortical midline structures. Human Brain Mapping, 30, 2617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. (2001). Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A., Wadsak W., Windischberger C., et al. (2012). Differential modulation of the default mode network via serotonin-1A receptors. Proceedings of the National Academy of Sciences of the United States of America, 109, 2619–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M., Gartside S.E., Varga V., Sharp T. (2003). In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology, 45, 72–81. [DOI] [PubMed] [Google Scholar]
- Hamann S.B., Ely T.D., Hoffman J.M., Kilts C.D. (2002). Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science, 13, 135–41. [DOI] [PubMed] [Google Scholar]
- Harmat L., de Manzano O., Theorell T., Högman L., Fischer H., Ullén F. (2015). Physiological correlates of the flow experience during computer game playing. International Journal of Psychophysiology, 97, 1–7. [DOI] [PubMed] [Google Scholar]
- Jenkins A.C., Mitchell J.P. (2011). Medial prefrontal cortex subserves diverse forms of self-reflection. Social Neuroscience, 6, 211–8. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Nolen-Hoeksema S., Mitchell K.J., Levin Y. (2009). Medial cortex activity, self-reflection and depression. Social Cognitive and Affective Neuroscience, 4, 313–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J., Bless H. (2008). Flow and regulatory compatibility: an experimental approach to the flow model of intrinsic motivation. Personality and Social Psychology Bulletin, 34, 196–209. [DOI] [PubMed] [Google Scholar]
- Keller J., Bless H., Blomann F., Kleinböhl D. (2011a). Physiological aspects of flow experiences: skills-demand-compatibility effects on heart rate variability and salivary cortisol. Journal of Experimental Social Psychology, 47, 849–52. [Google Scholar]
- Keller J., Blomann F. (2008). Locus of control and the flow experience: an experimental analysis. European Journal of Personality, 22, 589–607. [Google Scholar]
- Keller J., Ringelhan S., Blomann F. (2011b). Does skills-demands-compatibility result in intrinsic motivation? Experimental test of a basic notion proposed in the theory of flow-experiences. Journal of Positive Psychology, 6, 408–17. [Google Scholar]
- Kim H., Somerville L.H., Johnstone T., et al. (2004). Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience, 16, 1730–45. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Delaveau P., Freton M., Guionnet S., Fossati P. (2012). Medial prefrontal cortex and the self in major depression. Journal of Affective Disorders, 136, e1–e11. [DOI] [PubMed] [Google Scholar]
- Lemogne C., Gorwood P., Bergouignan L., Pelissolo A., Lehericy S., Fossati P. (2011). Negative affectivity, self-referential processing and the cortical midline structures. Social Cognitive and Affective Neuroscience, 6, 426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemogne C., le Bastard G., Mayberg H., et al. (2009). In search of the depressive self: extended medial prefrontal network during self-referential processing in major depression. Social Cognitive and Affective Neuroscience, 4, 305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P.A., Critchley H.D., Rotshtein P., Dolan R.J. (2007). Neural correlates of processing valence and arousal in affective words. Cerebral Cortex, 17, 742–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.T., Brown G.G. (2007). Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. Journal of the International Neuropsychological Society, 13, 517–25. [DOI] [PubMed] [Google Scholar]
- Lowry C.A., Johnson P.L., Hay-Schmidt A., Mikkelsen J., Shekhar A. (2005). Modulation of anxiety circuits by serotonergic systems. Stress, 8, 233–46. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. (2004). Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage, 21, 450–5. [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mayberg H.S., Brannan S.K., Tekell J.L., et al. (2000). Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry, 48, 830–43. [DOI] [PubMed] [Google Scholar]
- McGaugh J.L. (2004). The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience, 27, 1–28. [DOI] [PubMed] [Google Scholar]
- McReynolds J.R., McIntyre C.K. (2012). Emotional modulation of the synapse. Reviews in the Neurosciences, 23, 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J.S., Frith C.D., Perrett D.I., et al. (1996). A differential neural response in the human amygdala to fearful and happy facial expressions. Nature, 383, 812–5. [DOI] [PubMed] [Google Scholar]
- Nacke L., Lindley C.A. (2009). Affective ludology, flow and immersion in a first-person shooter: measurement of player experience. Loading…: The Journal of the Canadian Game Studies Association, 3 Available at : http://arxiv.org/ftp/arxiv/papers/1004/1004.0248.pdf. [Google Scholar]
- Nagai Y., Critchley H.D., Featherstone E., Trimble M.R., Dolan R.J. (2004). Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage, 22, 243–51. [DOI] [PubMed] [Google Scholar]
- Nejad A.B., Fossati P., Lemogne C. (2013). Self-referential processing, rumination, and cortical midline structures in major depression. Frontiers in Human Neuroscience, 7, 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. (2006). Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage, 31, 440–57. [DOI] [PubMed] [Google Scholar]
- Peifer C. (2012). Psychophysiological correlates of flow-experience. In: Engeser S., editor. Advances in Flow Research. New York: Springer, pp. 139–64. [Google Scholar]
- Peifer C., Schulz A., Schachinger H., Baumann N., Antoni C.H. (2014). The relation of flow-experience and physiological arousal under stress—can u shape it? Journal of Experimental Social Psychology, 53, 62–9. [Google Scholar]
- Petrov T., Krukoff T.L., Jhamandas J.H. (1992). The hypothalamic paraventricular and lateral parabrachial nuclei receive collaterals from raphe nucleus neurons: a combined double retrograde and immunocytochemical study. Journal of Comparative Neurology, 318, 18–26. [DOI] [PubMed] [Google Scholar]
- Philippi C.L., Duff M.C., Denburg N.L., Tranel D., Rudrauf D. (2012). Medial PFC damage abolishes the self-reference effect. Journal of Cognitive Neuroscience, 24, 475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price J.L., Drevets W.C. (2012). Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Sciences, 16, 61–71. [DOI] [PubMed] [Google Scholar]
- Puig M.V., Artigas F., Celada P. (2005). Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cerebral Cortex, 15, 1–14. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. (2006). Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology, 16, 723–7. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. (2001). A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America, 98, 676–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson O.J., Overstreet C., Allen P.S., et al. (2013). The role of serotonin in the neurocircuitry of negative affective bias: serotonergic modulation of the dorsal medial prefrontal-amygdala ‘aversive amplification' circuit. Neuroimage, 78, 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawchenko P.E., Swanson L.W., Steinbusch H.W., Verhofstad A.A. (1983). The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Research, 277, 355–60. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Barch D.M., Price J.L., et al. (2009). The default mode network and self-referential processes in depression. Proceedings of the National Academy of Sciences of the United States of America, 106, 1942–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M., Crandall C.G. (2010). Mechanisms and controllers of eccrine sweating in humans. Frontiers in bioscience (Scholar edition), 2, 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G.L., Fiez J.A., Corbetta M., et al. (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience, 9, 648–63. [DOI] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America, 105, 12569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T., Pohlack S., Mentzel H.J., Miltner W.H. (2008). Differential amygdala activation to negative and positive emotional pictures during an indirect task. Behavioural Brain Research, 191, 285–8. [DOI] [PubMed] [Google Scholar]
- Ullén F., de Manzano Ö., Almeida R., et al. (2012). Proneness for psychological flow in everyday life: associations with personality and intelligence. Personality and Individual Differences, 52, 167–72. [Google Scholar]
- Ulrich M., Keller J., Hoenig K., Waller C., Grön G. (2014). Neural correlates of experimentally induced flow experiences. Neuroimage, 86, 194–202. [DOI] [PubMed] [Google Scholar]
- van Buuren M., Gladwin T.E., Zandbelt B.B., Kahn R.S., Vink M. (2010). Reduced functional coupling in the default-mode network during self-referential processing. Human Brain Mapping, 31, 1117–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven V., Wingen M., Kuypers K.P., Ramaekers J.G., Formisano E. (2013). Escitalopram decreases cross-regional functional connectivity within the default-mode network. PLoS One, 8, e68355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viviani R., Messina I., Walter M. (2011). Resting state functional connectivity in perfusion imaging: correlation maps with BOLD connectivity and resting state perfusion. PLoS One, 6, e27050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.J., Chen Y., Fernandez-Seara M.A., Detre J.A. (2011). Potentials and challenges for arterial spin labeling in pharmacological magnetic resonance imaging. Journal of Pharmacology and Experimental Therapeutics, 337, 359–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Aguirre G.K., Kimberg D.Y., Roc A.C., Li L., Detre J.A. (2003). Arterial spin labeling perfusion fMRI with very low task frequency. Magnetic Resonance in Medicine, 49, 796–802. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Rauch S.L., Etcoff N.L., McInerney S.C., Lee M.B., Jenike M.A. (1998). Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience, 18, 411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J.D. (2011). Associations and dissociations between default and self-reference networks in the human brain. Neuroimage, 55, 225–32. [DOI] [PubMed] [Google Scholar]
- Willoughby J.O., Blessing W.W. (1987). Origin of serotonin innervation of the arcuate and ventromedial hypothalamic region. Brain Research, 418, 170–3. [DOI] [PubMed] [Google Scholar]
- Yang Y., Gu H., Ross T.J., Zhan W., Yang S. (2005). Single-shot magnetic resonance imaging (MRI) techniques and their applications. In: Leondes C.T., editor. Medical Imaging Systems Technology: Modalities. Hackensack, NJ: World Scientific Publishing Co., pp. 241–80. [Google Scholar]
- Zhang S., Hu S., Chao H.H., et al. (2013). Ventromedial prefrontal cortex and the regulation of physiological arousal. Social Cognitive and Affective Neuroscience, 9, 900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zysset S., Huber O., Samson A., Ferstl E.C., von Cramon D.Y. (2003). Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neuroscience Letters, 335, 183–6. [DOI] [PubMed] [Google Scholar]


