Abstract
The vicarious reward we receive from watching likable others obtaining a positive outcome is a pervasive phenomenon, yet its neural correlates are poorly understood. Here, we conducted a series of functional magnetic resonance imaging experiments to test the hypothesis that the brain areas responsible for action observation and reward processing work in a coordinated fashion during vicarious reward. In the first experiment (manipulation phase), the participant was instructed to cheer for a particular player in a two-player competitive game (Rock–Paper–Scissors). This manipulation made participants feel more unity with that player and resulted in unity-related activation in the premotor area during action observation. In the following main experiment, the participant witnessed the previously cheered-for or non-cheered-for player succeed in a new solitary game (a stopwatch game). The ventromedial prefrontal cortex (vmPFC) was activated when the cheered-for player succeeded in the game but not when the other player did. Interestingly, this vmPFC activation was functionally connected with premotor activation only during the cheered-for player’s success. These results suggest that vicarious reward is processed in the vmPFC-premotor network, which is activated specifically by the success of the other person with whom the individual feels unity and closeness.
Keywords: action observation network (AON), reward system, vicarious reward, functional magnetic resonance imaging (fMRI), functional connectivity
Introduction
Humans are endowed with the ability to appreciate positive feelings or pleasure by observing likable others achieving positive outcomes, even when there is no direct relationship between the observer and the observed person. This phenomenon, called vicarious reward, is pervasive in daily life. For example, we like to watch professional sports games and TV dramas, or to read autobiographical novels or fiction, cheering on a certain player or character who is engaging in some activity in that situation to be successful. Moreover, children imitate an adult’s behavior more frequently when they observe that the behavior was praised by others than when it was punished (Bandura, 1965).
A set of cortical and subcortical areas, including the ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex and ventral striatum, are involved in processing reward information when participants themselves receive a reward (Rushworth et al., 2007; Kennerley et al., 2011; Klein-Flugge et al., 2013). Although only a few studies have directly addressed the activity of the reward system for vicarious reward, involvement of the similar reward system for vicarious reward has recently become apparent (Mobbs et al., 2009; Fareri et al., 2012; Monfardini et al., 2013; Braams et al., 2014; Apps and Ramnani, 2014; Varnum et al., 2014). For example, Mobbs et al. (2009) found higher activation of the observer’s reward system when a socially desirable player won in a quiz game compared with when a socially undesirable player won. This enhanced activity was also correlated with the subjective feeling of similarity to the player, indicating that observers are more likely to experience vicarious reward when they have a feeling of closeness with the observed person. In addition to these studies, several other studies have reported vmPFC involvement in estimating rewards that others receive (Burke et al., 2010; Suzuki et al., 2012; Kang et al., 2013; Aoki et al., 2014).
It is also reasonable to assume that, to maximally appreciate another’s reward, the observer internally simulates the other’s action, intention and feelings when observing that person’s action and receipt of reward. The action observation network (AON) refers to the brain areas activated when observing another’s action (Grafton, 2009; Kilner, 2011) and includes the frontoparietal sensorimotor network. The definition of the AON is similar to that of the putative mirror neuron system (pMNS) (Rizzolatti and Sinigaglia, 2010), but the AON does not require activation when one performs an action by oneself, as the pMNS does. Our previous studies using functional near-infrared spectroscopy revealed that primary sensorimotor areas (near C3 of the international 10/20 system), a main component of the AON, were more activated when the cheered-for player won than when they lost in a simple competitive game (Shimada and Abe, 2009, 2010). Monfardini et al. (2013) reported that both the AON and the reward system are involved in observational learning of a simple visuomotor task, showing modulation by the other person’s outcome. These studies indicate that the AON is sensitive to the outcome of the other person’s action, which likely induces vicarious reward.
Despite the suggestion from these accumulating findings that the AON and the reward system work together in the processing of vicarious reward, investigation of the functional relationship between the two systems is largely lacking. In this study, we conducted a series of functional magnetic resonance imaging (fMRI) experiments to examine whether and how the reward system (particularly the vmPFC) is functionally associated with the AON during vicarious reward. In the first experiment (manipulation phase), the participant was instructed to cheer for one of two players competing with each other in the popular game Rock–Paper–Scissors (RPS). The main purpose of this first experiment was to manipulate the stance of the participant to the observed players: to make the participant feel more unity with the ‘cheered-for’ player than the ‘non-cheered-for’ player to effectively receive vicarious reward through the cheered-for player thereafter. In the second (main) experiment, the participant observed a new solitary game, the stopwatch (SW) task (Murayama et al., 2010), played by the previously cheered-for or non-cheered-for player. We employed a different task in the second experiment (SW rather than RPS) to avoid any confounding brain activity related to action observation itself (habituation, preference, prior knowledge, etc.) in the processing of vicarious reward. The participant was instructed to attend equally to both players’ game play and to no longer cheer for either player in this task, to avoid attentional contamination of brain activations. The aim of the second experiment was to examine whether the vicarious reward comes from a particular likable player rather than from a task itself. We hypothesized that the reward system processes the vicarious reward profoundly when a likable other’s action was simulated internally by virtue of the AON, which would likely result in functional connectivity between these brain areas.
Materials and methods
Participants
Thirty-two healthy right-handed participants (16 male; mean age, 20.1 ± 1.7 s.d.) recruited from a pool of Tamagawa University (Tokyo, Japan) students took part in the experiment. Participants were randomly assigned to the tend-to-win (TW) group or the tend-to-lose (TL) group (see later). Three participants were excluded from the analyses owing to poor behavioral performance (i.e. more than five trials without a button-pressing response within two seconds of the stimulus ending; see later). The numbers of participants included in the analyses were 16 and 13 for the TW and TL groups, respectively. All participants gave informed consent, and the protocol was approved by the Research Ethics Committee of Tamagawa University, and conducted according to the principles and guidelines of the Declaration of Helsinki.
Experimental tasks
The experiment comprised three fMRI scanning runs: the first for the RPS task and the second and third for the SW task. After the RPS task, the participant was removed from the scanner for about 15 min to fill out a questionnaire for the RPS task and receive instructions for the SW task. The second and third runs were separated by a 2 min break, during which the participant remained inside the scanner. The participant underwent a few practice trials before entering the scanner for both the RPS and SW tasks.
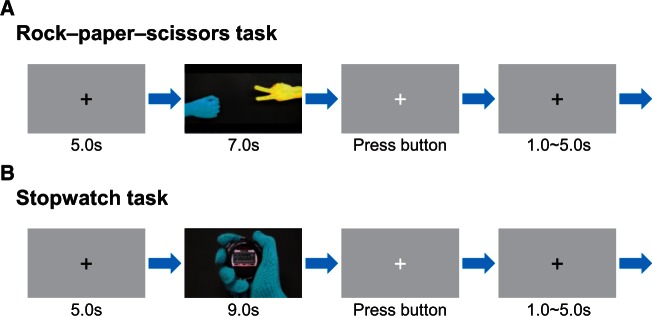
In the RPS task (Figure 1A), movie clips in which two players played the RPS game were presented in every trial. In the clips, the right hand of a player, wearing a blue glove, appeared on one side of the screen and the opponent’s yellow-gloved right hand appeared on the other side. After swinging their hands down twice, the two players’ hands simultaneously each formed a hand gesture of rock, paper or scissors. In this game, rock defeats scissors, scissors defeats paper and paper defeats rock. If both players make the same hand gesture, the outcome is a draw. During the game, the participant was instructed to cheer for whichever player (blue or yellow) the experimenter assigned. This manipulation aimed to enhance the participant’s emotional involvement in the player’s action and therefore to facilitate the experience of vicarious reward by the cheered-for player’s win. At 4 s after the onset of the movie, the outcome of the game was apparent and lasted for 3 s. After the end of the movie, the participant was required to press a button within 2 s to report the outcome for the cheered-for player (WIN, LOSS or DRAW). The inter trial interval was 6–10 s (jittered). The glove color of the cheered-for player (blue or yellow), the location of the cheered-for hand on the screen (left or right) and the assignment of the three buttons (WIN, LOSS and DRAW) to three fingers (index, middle or ring) were counterbalanced across participants.
Fig. 1.
Schematic illustration of the experimental procedure of the RPS task (A) and the SW task (B). (A) The participant watched a movie clip (7 s) of a game of RPS while cheering for one of the players (blue or yellow). The outcome of the game was apparent 4 s after the movie began. After the movie presentation, a white fixation cross was displayed and the participant was required to answer the outcome of the game (WIN, LOSS or DRAW) by pressing a button. (B) The participant watched a movie clip (9 s) of a SW task. The goal of the game was to press a button within ± 0.05 s of the 5.00 s time point of the SW. The outcome of the game was apparent 6 s after the movie began. The participant was required to answer the outcome (success or failure) of the game by pressing a button while a white fixation cross was displayed.
We defined three conditions according to the outcome for the cheered-for player: those in which the cheered-for player won (WIN), lost (LOSS) or when the game ended in a draw with the opponent (DRAW). The participants in the TW group experienced 20 WIN, 10 LOSS and 5 DRAW trials, and those in the TL group experienced 10 WIN, 20 LOSS and 5 DRAW trials. The trials were presented in pseudorandom order. The movie stimuli were highly similar across the conditions, in that the same gesture appeared in different conditions with the same probability (≈1/3). After the experiment, the participant was removed from the scanner and completed a 7-scale questionnaire that assessed their subjective feelings of unity with the cheered-for player (1–7) and the pleasantness associated with observing this player win or lose (−3 to +3).
In the SW task that followed, the participant observed movie clips in which one of the players, wearing a blue or yellow glove, played a solitary SW game (Figure 1B). The order in which a player’s hand appeared was pseudorandomized. In the SW game, the goal for the players was to press a button on a SW so that the button press fell within ± 0.05 s of the 5.00 s time point. The participant was instructed to observe the SW task played using the same hand as in the RPS task (the colors of the gloves were matched between the RPS and SW tasks) but, unlike the RPS task, to no longer cheer for either player. This instruction allows us to examine the effects of implicit unity with the player on vicarious reward in the SW task, yet avoids the effect of explicit cheering. Each movie clip lasted 9 s. At 6 s after the onset of the movie, the outcome of the trial was apparent and lasted for 3 s. After viewing a movie clip, the participant was required to press the button within 2 s to indicate the player’s outcome. The assignment of the two buttons (success and failure) to two fingers (index and middle) was counterbalanced across participants. The inter trial interval was jittered between 6 and 10 s.
We defined four conditions according to the outcome obtained by the player (success or failure) and the player type in the preceding RPS task (the cheered-for or non-cheered-for player): success by the player cheered for in the RPS task (C_Success), failure by the player cheered for in the RPS task (C_Failure), success by the player for whom the participant did not cheer in the RPS task (NC_Success) and failure by the player for whom the participant did not cheer in the RPS task (NC_Failure). Each run consisted of 32 trials, with eight trials in each of the four conditions. The four conditions were presented in pseudorandom order. The participant completed the 7-scale questionnaire (−3 to +3) to assess the pleasantness of observing the player succeeding or failing in the SW task after the experiment, outside the scanner.
The participant was instructed not to make any utterance or body movement except the required button presses during the fMRI scanning.
fMRI data acquisition
The functional imaging was conducted using a 3-T Trio A Tim MRI scanner (Siemens) to acquire gradient echo T2*-weighted echo-planar images (EPIs) with blood oxygenation level-dependent (BOLD) contrast. Thirty-three images were acquired in each volume [repetition time (TR) = 2000 ms; echo time = 25 ms; flip angle = 76°; slice thickness = 3 mm; slice gap = 0.9 mm; field of view = 192 mm2; matrix size = 64 × 64]. Slice orientation was tilted −30° from the anterior commissure (AC) - posterior commissure (PC) line. The superior part of the parietal cortex was out of range of the scan with this setting because we intended to include the entire vmPFC regions and potential subcortical reward areas in the field-of-view. We discarded the first three images before data processing and used statistical analysis to compensate for the T1 saturation effects.
fMRI data analysis
Image analysis was performed using Statistical Parametric Mapping software (SPM version 8; Wellcome Department of Cognitive Neurology, London, UK). Images were corrected for slice acquisition time within each volume, motion corrected with realignment to the first volume, spatially normalized to the standard Montreal Neurological Institute (MNI) EPI template and spatially smoothed using a Gaussian kernel with a full width at half maximum of 8 mm.
For each participant, the BOLD responses across the scanning runs were modeled with a general linear model (GLM). For the RPS task, the GLM included the following event (1 TR duration) regressors: the onset of the movie stimulus, the onset of the outcome (WIN, LOSS and DRAW; three separate regressors) and the button pressing. We assumed that the AON activation irrespective of outcome was well reflected in the coefficients (beta) for the movie onset and that activations relevant to reward processing were reflected in WIN–LOSS contrasts. For the SW task, the GLM included the following event regressors: the onset of movie stimulus (cheered-for, non-cheered-for; two separate regressors), the onset of the outcome (C_Success, C_Failure, NC_Success and NC_Failure; four separate regressors) and the button pressing. We investigated the activity reflecting vicarious reward by analyzing the interaction contrast [C_Success − C_Failure] − [NC_Success − NC_Failure], assuming that vicarious reward comes specifically from the cheered-for player. The motion parameters and session (run) effects were also included as regressors of no interest. The regressors (except for the motion parameters and the session effects) were convolved with a hemodynamic-response function. The estimates were corrected for temporal autocorrelation using a first-order autoregressive model. A second-level whole-brain one-sample t-test was then performed for each condition and contrast. The significance level was set at P < 0.05 family-wise error (FWE) corrected. However, given the strong priors from the previous literature about the role of the vmPFC in processing other’s reward, we also report activity in vmPFC that survived FWE small-volume corrections (SVCs) at P < 0.05. The mask for the SVC in the vmPFC was taken using a sphere of 10 mm radius defined around the peak activation coordinates for reward processing (x = 9, y = 44, z = −14) reported in our previous work (Aoki et al., 2014), in which a highly stringent region of interest selection process was employed: first, the fMRI data were masked anatomically using the Automated Anatomical Labeling atlas of the WFU (Wake Forest University) Pickatlas toolbox for SPM (Tzourio-Mazoyer et al., 2002). The mask for the vmPFC consisted of the bilateral medial orbitofrontal gyrus and gyrus rectus. Then, the peak activation voxel responding to reward (gain–no-gain) during a monetary incentive delay task was selected. This procedure ensures that this coordinate is located within the vmPFC and is strongly involved in reward processing. It is also worth mentioning that participants in this study were recruited from the same pool of university students as the previous study. For illustrative purposes only, activity in selected SPM data is reported at P < 0.005 uncorrected with an extent threshold of 10 voxels.
To assess changes in functional connectivity between the premotor cortex and vmPFC during vicarious reward, we carried out a psychophysiological interaction (PPI) analysis (Friston et al., 1997) for the RPS and SW tasks separately. PPI assesses whether the functional connectivity between a seed (premotor or vmPFC) region and all other voxels in the brain is changed by an experimental condition but it does not require a specific anatomical model. Using SPM, we extracted the BOLD time series (the first eigenvariate) from the voxels within a 6 mm radius sphere surrounding the peak (seed) voxel in the premotor cortex and vmPFC that we isolated in the original SPM analysis. The BOLD time course was deconvolved using the canonical hemodynamic-response function to obtain a time series of neural activity in the seed region (Gitelman et al., 2003). We estimated a GLM with the following regressors: (i) an interaction between the neural activity in the seed region and a variable indicating the WIN/success (+1) and LOSS/failure (−1) convolved with the hemodynamic-response function (PPI regressor, interaction of psychological and physiological variable), (ii) the variable indicating WIN/success (+1) or LOSS/failure (−1) convolved with the hemodynamic-response function (P regressor, psychological variable) and (iii) the original BOLD eigenvariate in the seed region before the deconvolution (Y regressor, physiological variable). Inclusion of the psychological and physiological regressors ensures that any observed effect of the PPI regressor is specific to the task-induced changes in functional connectivity (O’Reilly et al., 2012). The six motion parameters and the regressors of no-interest were also included in the model. The second-level random effects analysis for PPI was first FWE corrected at P < 0.05. However, because we did not find any significant functional connectivity between brain regions at this threshold level, we decided to apply a more lenient threshold for PPI analysis to investigate any possible connectivity during vicarious reward (for discussion of employing a lenient threshold in social neuroscience studies, see Lieberman and Cunningham, 2009): the t-images were first thresholded at t = 2.47 and then underwent cluster-level FWE correction at P < 0.05.
Results
Behavioral data
In the RPS task, the averaged correct rates of button pressing reporting the outcome of the cheered-for player were 98.9 ± 0.5% (mean ± SEM) in the TW group and 97.1 ± 1.1% in the TL group. The difference between groups was not statistically significant (t27 = 1.595, P = 0.122). In the SW task, the averaged correctly reported outcome of the players was 98.4 ± 0.7 (mean ± SEM) % in the TW group and 98.2 ± 0.6 % in the TL group. The difference between groups was not statistically significant (t27 = 0.256, P = 0.800). These results indicate that both groups attended sufficiently to the RPS and SW tasks. Although we found a significant difference between the two groups in the subjective pleasantness of observing the player’s win vs loss (t27 = 3.09, P = 0.02; TW = 2.24 ± 0.30, TL = 0.87 ± 0.48, mean ± SEM) and in the subjective feeling of unity with the player in the RPS task (t27 = 2.37, P = 0.03; TW = 4.12 ± 0.28, TL = 5.07 ± 0.28, mean ± SEM), there was no significant difference in the subjective pleasantness of the player’s success in the SW task (t27 = 0.725, P = 0.474; TW = 1.24 ± 0.43, TL = 1.20 ± 0.31, mean ± SEM). In addition, the groups did not differ in the brain activation results of the WIN–LOSS contrast in the RPS task or in the interaction ([C_Success − C_Failure] − [NC_Success − NC_Failure]) in the SW task even at a lenient threshold level (P < 0.005, uncorrected). Therefore, we disregarded the difference in questionnaire results in the RPS task and merged the behavioral and brain activation data of these two groups in the analyses hereafter.
After merging the TW and TL data, the questionnaire results showed that participants felt more unity with the cheered-for player than with the non-cheered-for player (t28 = 9.06, P < 0.001; cheered-for: 4.56 ± 0.22, non-cheered-for: 1.91 ± 0.18, mean ± SEM). The participants felt more pleasantness when their cheered-for player won against the non-cheered-for player than when the player lost in the RPS task (t28 = 11.7, P < 0.001; win: 2.28 ± 0.13, loss:−0.69 ± 0.26, mean ± SEM). Similarly, the questionnaire results in the SW task showed that the participant felt greater pleasantness when the cheered-for player in the RPS task (note that the participant was not instructed to cheer for any particular player in the SW task) succeeded in the SW task than when the non-cheered-for player did (t28 = 2.34, P = 0.03; cheered-for: 1.22 ± 0.28, non-cheered-for: 0.94 ± 0.26, mean ± SEM). This difference was not observed in the failure trials (t28 = 1.14, P = 0.3; cheered-for:−0.19 ± 0.24, non-cheered-for: 0.09 ± 0.21, mean ± SEM). These results indicate that the participants indeed obtained vicarious reward from the success of the cheered-for player in both the RPS and SW tasks.
fMRI data during RPS task (manipulation phase)
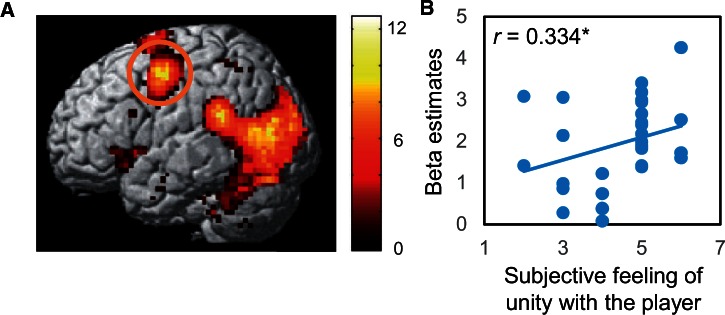
We examined brain activation during observation of the cheered-for player’s action (hand movement) in the RPS task and found significant activation in the premotor cortex as well as in visual-related areas (Table 1). Importantly, activation in the left premotor cortex (x = −36, y = −4, z = 46; Figure 2A) was significantly positively correlated with the subjective feeling of unity with the cheered-for player in the RPS task (r = 0.334, P < 0.05, one-tailed, Spearman’s rank correlation; Figure 2B). No such correlation was observed with the pleasantness measure (P > 0.1).
Table 1.
Brain regions exhibiting significant activation when observing the player’s hand action in the RPS game
| Region | Peak MNI coordinates |
k | t value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| L/R occipital cortex | −12 | −76 | −5 | 5381 | 12.63 |
| 9 | −70 | −2 | 11.62 | ||
| R fusiform gyrus | 36 | −49 | −14 | 11.56 | |
| L premotor cortex | −36 | −4 | 46 | 185 | 10.36 |
| R premotor cortex | 36 | −4 | 43 | 232 | 8.38 |
| 36 | 14 | 25 | 7.09 | ||
| L premotor cortex | −9 | 2 | 61 | 136 | 7.47 |
| Cingulate gyrus | −9 | 17 | 34 | 5.98 | |
| R premotor cortex | 9 | 8 | 55 | 5.92 | |
| R precuneus | 30 | −52 | 52 | 30 | 6.6 |
| L precuneus | −27 | 49 | 52 | 25 | 6.5 |
Fig. 2.
(A) Activation while observing the hand action in the RPS game. The left premotor cortex (peak at −36 −4 46) was strongly activated during action observation. (B) The beta value of the left premotor cortex was significantly correlated with the subjective feeling (questionnaire) of unity with the cheered-for player (r = 0.334, P < 0.05, Spearman’s rank correlation).
We examined brain activation when the outcome of the trial (WIN, LOSS or DRAW) became apparent after the players’ hand movements. The contrast between WIN and LOSS conditions (WIN − LOSS) resulted in no significant difference in the measured area (by FWE-corrected whole-brain analysis or SVC at the vmPFC). We then tested using a PPIs analysis with the premotor cortex as a seed. No region showed a significant functional connectivity with the premotor cortex (P > 0.05, cluster corrected).
fMRI data during SW task
We further examined whether cheering for a particular player in the RPS task has an effect on the premotor-vmPFC activations in the subsequent SW task, where the participant observed either the cheered-for player or the non-cheered-for (opponent) player in the RPS task performing the SW task. We did not find any activation in the whole-brain analysis with the defined criteria. However, the SVC analysis revealed that the vmPFC showed a significant interaction of player × outcome [(C_Success − C_Failure) − (NC_Success − NC_Failure)] (x = 15, y = 38, z = −17; k = 8; P < 0.05; FWE small-volume corrected; Figure 3A). Subsequent simple main effect analyses showed that vmPFC activation was greater for the player’s success than the loss for the cheered-for-player (t28 = 3.10, P < 0.005, two tailed; β_success = 0.537 ± 0.177, β_failure = −0.220 ± 0.124, mean ± SEM; Figure 3B), while the opposite tendency was observed for the non-cheered-for player (t28 = −1.87, P = 0.07, two tailed; β_success = 0.129 ± 0.085, β_failure = 0.478 ± 0.293, mean ± SEM). We did not find any activation for the inverse interaction ([(C_Failure − C_Success) − (NC_Failure − NC_Success)]; P > 0.05, FWE small-volume corrected).
Fig. 3.
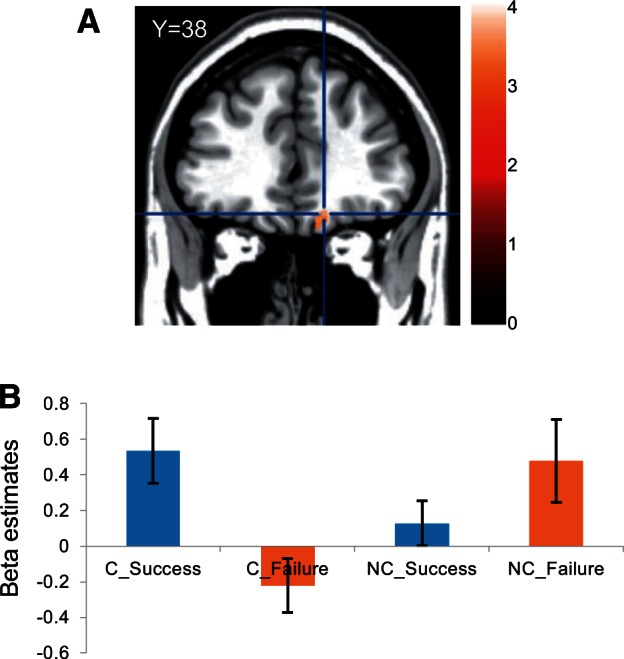
(A) Interaction between the outcome (success and failure) and the player (previously cheered-for and previously non-cheered-for) in the SW game was found in vmPFC (peak at 15 38 − 17) ([C_Success − C_Failure] − [NC_Success − NC_Failure]). (B) Beta estimates for each condition in the vmPFC.
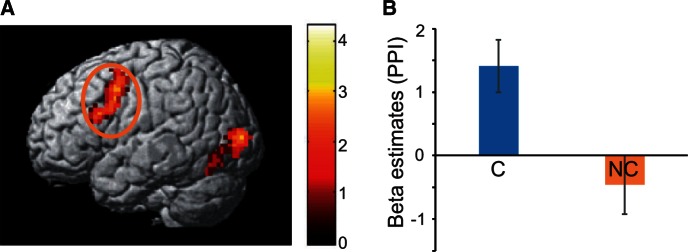
We then applied PPI analyses using the contrast between the success and failure conditions (success − failure) with the vmPFC as a seed. Because we found a significant interaction (player × outcome) in vmPFC activation during the outcome period (see earlier), we applied PPI analyses separately to the participant’s cheered-for and non-cheered-for players. We found a significant functional connectivity between the vmPFC and the premotor cortex (x = −51, y = 20, z = 25) only for the previously cheered-for player (P < 0.05, cluster corrected; Figure 4A; Table 2) but not for the non-cheered-for player (P > 0.1). The connectivity (the beta value of the PPI analysis) was significantly stronger for the cheered-for player than that for the non-cheered-for player (t28 = 2.63, P < 0.01; β_root-for = 1.412 ± 0.910, β_non-rooted-for = −0.457 ± 1.157, mean ± SEM; Figure 4B).
Fig. 4.
(A) PPI analysis showed a significant functional connectivity between the vmPFC and the premotor cortex (peak at − 51 20 25) using the contrast of C_Success vs C_Failure in the SW game. (B) The PPI beta value was significantly larger for the cheered-for player (C) than for the non-cheered-for player (NC).
Table 2.
Brain regions exhibiting significant functional connectivity using the contrast between C_Success vs C_Failure with the vmPFC as a seed
| Region | Peak MNI coordinates |
k | t value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R occipital cortex | 24 | −82 | −5 | 1983 | 4.47 |
| 15 | −79 | −5 | 4.43 | ||
| 9 | −82 | 1 | 4.35 | ||
| L premotor cortex | −48 | 5 | 37 | 370 | 3.42 |
| −51 | 20 | 25 | 3.37 | ||
| −33 | 5 | 58 | 3.29 | ||
To show the specificity of the vmPFC-premotor coupling, we further investigated two regions as candidates for the PPI seed. The first was the dorsolateral PFC (DLPFC), as a previous study reported, using PPI analysis, that the DLPFC and MNS form a dynamic link during imitation learning (Higuchi et al., 2012). The DLPFC is generally considered to play a role in higher-order supervisory and monitoring functions. According to this previous study, we took two coordinates ([−28, 52, 18] and [−32, 38, 32]) in the left middle frontal gyrus as a seed region with an equivalent size to ours (6 mm spheres around the peak). We also investigated the dorsomedial PFC (dmPFC). A previous study had shown that the dmPFC is involved in calculating error signals in prediction of other’s action that can be dissociated from reward prediction error for the other (Suzuki et al., 2012). Although the previous study did not conduct PPI analysis, we considered it worthwhile to take this coordinate ([6, 14, 52]) as a seed to check the functional connectivity with the premotor cortex. By showing these regions, which are involved in other functional aspects of observation of other’s actions, do not have connections with the premotor cortex, we can strengthen our finding that the vmPFC and the premotor cortex are functionally coupled when experiencing vicarious reward. We conducted the same PPI analyses as described earlier and found that neither of these regions showed significant functional connectivity with the premotor regions (P > 0.05, cluster corrected).
Discussion
This study investigated the functional relationship between the AON and the reward system during vicarious reward. The AON was activated during observations of the cheered-for player executing a motor action (RPS game) and significantly correlated with subjective ratings of unity with the player, confirming the unity between the participant and the player both in subjective experience and brain activation. In the following SW task, vicarious reward occurred selectively for that particular cheered-for player, even though the participant was no longer instructed to cheer for any particular player, with significantly greater vmPFC activation for the player’s success than failure. The opposite vmPFC activation pattern was observed for the non-cheered-for player. We also found significant functional connectivity between the premotor area and vmPFC in success (C_Success) compared with failure trials (C_Failure). These results demonstrate that the vicarious reward experience is processed within the coordinated activation of the AON and the reward system. We further suggest that vicarious reward is facilitated by cheering, and occurs in a person-specific and task-independent manner.
Our result, that the vmPFC is critically involved in evaluating another person’s reward, is consistent with previous studies. Suzuki et al. (2012) conducted a simple decision-making task that was designed to dissociate the reward prediction error from the action prediction error of another’s action. They found that vmPFC activity was significantly correlated with the reward prediction error, while dorsomedial and lateral PFC were more related to the action prediction error, suggesting the specific role of the vmPFC in evaluating another person’s reward. Burke et al. (2010) also showed a similar result with an observational learning paradigm. With a simple two-armed-bandit task, they demonstrated that the vmPFC was responsible for the reward prediction error of another person’s decision, whereas the ventral striatum was more relevant in representing the reward prediction error of the participant’s own decision. Behrens et al. (2008) demonstrated, in a decision-making task with a confederate’s advice, that the ventral striatum and vmPFC were related to reward prediction error processing, whereas only the vmPFC was further involved in integration of the reward and the confederate’s fidelity information. Finally, a recent study clearly showed that the vmPFC is so flexible that both the self and other’s subjective values were interchangeably represented depending on whether the choice selection was taken based on participants’ own preference or the estimated preference of another person (Nicolle et al., 2012). These studies cumulatively show that the vmPFC is the most likely brain region involved in evaluating and representing another person’s reward so that it can be incorporated into the self’s reward system. It is worth mentioning that we obtained negative results for vmPFC activation to the WIN–LOSS contrast, as well as its functional connectivity with the premotor cortex during the RPS task. We consider the reason for this to be that the RPS task was employed as a manipulation task to facilitate the ‘unity’ between the observer and the player, so that the vicarious reward had not yet been received adequately at this stage owing to immature assimilation between the observer and the cheered-for player.
Our results show that vicarious reward is specifically derived from the previous cheered-for player in the SW task, which was facilitated by cheering in the previous RPS task. In our experiment, we explicitly asked the participant to cheer for one particular player. This manipulation likely facilitated the participant’s implicit assumption that the participant and the player share the goal of the player winning, and hence the player became personally closer (Tajfel, 1966; Aron et al., 1991). The notion that vicarious experience and brain activation are facilitated by a subjective implicit/explicit attitude toward the observed person has support in the literature. For example, recent studies have shown that vicarious reward is pronounced for a socially desirable person to whom the participant feels great similarity (Mobbs et al., 2009), a participant’s friend compared with unfamiliar others (Fareri et al., 2012), or when interdependence between the participant and their friend was primed (Varnum et al., 2014). Similarly, the participant felt more empathy for pain inflicted on a fair than on an unfair person (Singer et al., 2006), and on an ingroup compared with on an outgroup member (Hein et al., 2010). Interestingly, in the SW task in this study, the vmPFC activation was marginally greater in failure trials than in success trials for the non-cheered-for player (P = 0.07; Figure 3B). This result is consistent with previous studies that have examined reward system activity during ‘Schadenfreude’, in which the participant feels pleasure when the unlikable other suffers something undesirable (Singer et al., 2006; Takahashi et al., 2009). This evidence supports the idea that whether the participant receives vicarious reward from the observed person’s success depends on whether the participant regards them as similar/close/likable. Our result additively suggests that cheering for another’s success is an effective way of facilitating similarity/closeness with the other, even when who to cheer for is randomly assigned by an experimenter.
Our finding of functional connectivity between the premotor area, which is a principal component of the AON, and the vmPFC in the processing of vicarious reward, has considerable empirical significance. Functional connectivity between the vmPFC and the inferior parietal lobule, which is a part of the AON, has also been reported in a task where the participant vicariously buys an item on behalf of another (Janowski et al., 2013). Monfardini et al. (2013) showed that the AON and the reward system were activated during both observational learning, and actual trial and error learning of a simple visually guided motor decision task. They further showed that the posterior superior temporal sulcus and dorsomedial PFC (dmPFC) were coactivated during the observation of an incorrect outcome of another’s action. These results suggest that the AON and PFC are functionally connected in situations where the participant is involved in simulating another person’s internal value representation to make decisions for them. Recently, Ruff and Fehr (2014) proposed a ‘common currency schema’ in which social and non-social rewards are processed in the same reward circuit with differential functional connectivity to other domain-specific brain regions. Our findings are in line with this model, in that vicarious reward experienced by observing another person’s successful action is processed by functionally connected reward-AON circuits. Taken together, we suggest that vicarious reward is established by the vmPFC, which is flexible enough to incorporate another’s reward representation, to assimilate it with one’s own reward representation and to function coordinately with the bodily simulation of another’s action that is accomplished by the AON.
Funding
This work was supported by Grants-in-Aid for Scientific Research (25700015 and 22240026) from the Japan Society for the Promotion of Science (JSPS).
Conflict of interest. None declared.
References
- Aoki R., Matsumoto M., Yomogida Y., et al. (2014). Social equality in the number of choice options is represented in the ventromedial prefrontal cortex. The Journal of Neuroscience, 34, 6413–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps M.A.J., Ramnani N. (2014). The anterior cingulate gyrus signals the net value of others’ rewards. The Journal of Neuroscience, 34, 6190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A., Aron E.N., Tudor M., Nelson G. (1991). Close relationships as including other in the self. Journal of Personality and Social Psychology, 60, 241–53. [Google Scholar]
- Bandura A. (1965). Influence of models’ reinforcement contingencies on the acquisition of imitative response. Journal of Personality and Social Psychology, 1, 589–95. [DOI] [PubMed] [Google Scholar]
- Behrens T.E.J., Hunt L.T., Woolrich M.W., Rushworth M.F.S. (2008). Associative learning of social value. Nature, 456, 245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Guroglu B., de Water E., et al. (2014). Reward-related neural responses are dependent on the beneficiary. Social Cognitive and Affctive Neuroscience, 9, 1030–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke C.J., Tobler P.N., Baddeley M., Schultz W. (2010). Neural mechanisms of observational learning. Proceedings of the National Academy of Sciences of the United States of America, 107, 14431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri D.S., Niznikiewicz M.A., Lee V.K., Delgado M.R. (2012). Social network modulation of reward-related signals. The Journal of Neuroscience, 32, 9045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., et al. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6, 218–29. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage, 19, 200–7. [DOI] [PubMed] [Google Scholar]
- Grafton S.T. (2009). Embodied cognition and the simulation of action to understand others. Annals of the New York Academy of Sciences, 1156, 97–117. [DOI] [PubMed] [Google Scholar]
- Hein G., Silani G., Preuschoff K., Batson C.D., Singer T. (2010). Neural responses to ingroup and outgroup menbers’ suffering predict individual differences in costly helping. Neuron, 68, 149–60. [DOI] [PubMed] [Google Scholar]
- Higuchi S., Holle H., Roberts N., Eickhoff S.B., Vogt S. (2012). Imitation and observational learning of hand actions: prefrontal involvement and connectivity. NeuroImage, 59, 1668–83. [DOI] [PubMed] [Google Scholar]
- Janowski V., Camerer C., Rangel A. (2013). Empathic choice involves vmPFC value signals that are modulated by social processing implemented in IPL. Social Cognitive and Affctive Neuroscience, 8, 201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang P., Lee Y., Choi I., Kim H. (2013). Neural evidence for individual and cultural variability in the social comparison effect. The Journal of Neuroscience, 33, 16200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerley S.W., Behrens T.E.J., Wallis J.D. (2011). Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nature Neuroscience, 14, 1581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner J.M. (2011). More than one pathway to action understanding. Trends in Cognitive Science, 15, 352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flugge M.C., Barron H.C., Brodersen K.H., Dolan R.J., Behrens T.E.J. (2013). Segregated encoding of reward-identity and stimulus-reward associations in human orbitofrontal cortex. The Journal of Neuroscience, 33, 3202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. (2009). Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affctive Neuroscience, 4, 423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D., Yu R., Meyer M., et al. (2009). A key role for similarity in vicarious reward. Science, 324, 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama K., Matsumoto M., Izuma K., Matsumoto K. (2010). Neural basis of the undermining effect of monetary reward on intrinsic motivation. Proceedings of the National Academy of Sciences of the United States of America, 107, 20911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfardini E., Gazzola V., Boussaoud D., et al. (2013). Vicarious neural processing of outcomes during observational learning. PLoS One, 8, e73879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A., Klein-Flugge M.C., Hunt L.T., et al. (2012). An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron, 75, 1114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affctive Neuroscience, 7, 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G., Sinigaglia C. (2010). The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Reviews Neuroscience, 11, 264–74. [DOI] [PubMed] [Google Scholar]
- Ruff C.C., Fehr E. (2014). The neurobiology of rewards and values in social decision making. Nature Reviews Neuroscience, 15, 549–62. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F.S., Behrens T.E.J., Rudebeck P.H., Walton M.E. (2007). Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behavior. Trends in Cognitive Science, 11, 168–76. [DOI] [PubMed] [Google Scholar]
- Shimada S., Abe R. (2009). Modulation of the motor area activity during observation of a competitive game. NeuroReport, 20, 979–83. [DOI] [PubMed] [Google Scholar]
- Shimada S., Abe R. (2010). Outcome and view of the player modulate motor area activity during observation of a competitive game. Neuropsychologia, 48, 1930–4. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J.P., et al. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439, 466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Harasawa N., Ueno K., et al. (2012). Learning to simulate others’ decisions. Neuron, 74, 1125–37. [DOI] [PubMed] [Google Scholar]
- Tajfel H. (1966). Co-operation between human groups. The Eugenics Review, 58, 77–84. [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Kato M., Matsuura M., et al. (2009). When your gain is my pain and your pain is my gain: neural correlates of envy and shadenfreude. Science, 323, 937–9. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage, 15, 273–89. [DOI] [PubMed] [Google Scholar]
- Varnum M.E.W., Shi Z., Chen A., Qiu J., Han S. (2014). When “Your” reward is the same as “My” reward: self-construal priming shifts neural responses to own vs. friends’ rewards. NeuroImage, 87, 164–9. [DOI] [PubMed] [Google Scholar]