Abstract
Recent evidence indicates that empathic responses to others’ pain are modulated by various situational and individual factors. However, few studies have examined how empathy and underlying brain functions are modulated by social hierarchies, which permeate human society with an enormous impact on social behavior and cognition. In this study, social hierarchies were established based on incidental skill in a perceptual task in which all participants were mediumly ranked. Afterwards, participants were scanned with functional magnetic resonance imaging while watching inferior-status or superior-status targets receiving painful or non-painful stimulation. The results revealed that painful stimulation applied to inferior-status targets induced higher activations in the anterior insula (AI) and anterior medial cingulate cortex (aMCC), whereas these empathic brain activations were significantly attenuated in response to superior-status targets’ pain. Further, this neural empathic bias to inferior-status targets was accompanied by stronger functional couplings of AI with brain regions important in emotional processing (i.e. thalamus) and cognitive control (i.e. middle frontal gyrus). Our findings indicate that emotional sharing with others’ pain is shaped by relative positions in a social hierarchy such that underlying empathic neural responses are biased toward inferior-status compared with superior-status individuals.
Keywords: social hierarchy, empathy, anterior insula (AI), anterior medial cingulate cortex (aMCC), Toronto Alexithymia Scale-20 Items (TAS-20), functional connectivity
Introduction
Empathy reflects the ability to identify and share the emotions and feelings of others (Decety and Jackson, 2004; Zaki, 2014). Numerous functional imaging studies have explored the neural signatures underlying empathy with experimental paradigms in which participants were exposed to the pain experience of others (Singer et al., 2004; Jackson et al., 2005; Lamm and Decety, 2008; Gu et al., 2012, 2013). A recent meta-analysis has revealed that the bilateral anterior insula (AI) and anterior medial cingulate cortex (aMCC) are most consistently involved in the perception of others’ pain, and thereby are identified as the core network of empathy for pain (Lamm et al., 2011). Notably, these brain regions have also been implicated in representing affective-motivational aspects of the first-person physical and social pain experience (Peyron et al., 2000; Rainville, 2002; Eisenberger et al., 2003). Therefore, the engagement of the aMCC and AI in perceiving others’ pain is thought to subserve emotional sharing with others and constitute the affective aspects of empathy (Singer et al., 2004; Xu et al., 2009; Lamm et al., 2011).
Empathic neural responses to others’ pain often occur automatically; however, they are also tremendously modulated by various individual and situational factors (Decety and Jackson, 2004; Goubert et al., 2005; De Vignemont and Singer, 2006; Cheng et al., 2007; Zaki, 2014). For instance, brain activity underlying the perception of others’ pain is modulated according to perceiver’s empathic ability, which is often measured by the interpersonal reactivity index (IRI) (Davis, 1980). The higher perceiver’s scores on dispositional empathy, the stronger aMCC and AI responses to the pain of others (Singer et al., 2004, 2006). Notably, the ability to identify one’s own feelings also modulates empathic neural responses, such that people with difficulties in identifying and describing their own emotions (i.e. alexithymia) show attenuated activations in AI while introspecting their own feelings and while empathizing with others’ pain (Silani et al., 2008; Bird et al., 2010). These findings are consistent with the ‘shared representations’ account of empathy, positing that neural networks engaged by the first-person pain experience also underpin the sharing of others’ pain (De Vignemont and Singer, 2006; Lamm et al., 2011; Rütgen et al., 2015).
Regarding the context-dependent empathic responses, it has been revealed that neural responses of the aMCC and AI to the pain of others are constrained by top-down attention and cognitive reappraisal (Gu and Han, 2007; Lamm et al., 2007a,b). Further, the empathic neural responses of aMCC and AI are modulated by interpersonal relations such that they are attenuated while observing disliked others or out-group members in pain (Singer et al., 2006; Xu et al., 2009). Likewise, Meyer et al. (2012, 2015) recently observed that empathy for the social exclusion (i.e. social pain) of friends as compared with strangers relied more heavily on affective pain regions including aMCC and AI. Taken together, these findings indicate that affective sharing with others’ pain is modulated according to both personal and situational factors. However, specific to situational factors, few studies have examined how empathy and underlying brain functions are modulated by social hierarchies that are ubiquitous to human societies (Cheng et al., 2014).
In human societies, social hierarchies can be readily established according to many dimensions, such as knowledge, skill and physical strength. For instance, Zink et al. (2008) created social hierarchies among experimental participants based on their performances in a simple perceptual task; and they demonstrated that people are strongly engaged in this hierarchical context. Employing similar procedures, previous studies have shown modulations of social hierarchies on human socioemotional functioning (Boksem et al., 2012; Hu et al., 2014, 2015) and attentional/cognitive processes (Santamaría-García et al., 2013; Breton et al., 2014; Feng et al., 2015). More relevant to empathy modulation, the knowledge that others are superior often conflicts with positive self-views and provokes negative feelings due to upward social comparison (Smith et al., 1996; Takahashi et al., 2009). These negative feelings in turn may preclude empathy for superior-status individuals. Indeed, people tend to eliminate emotional sharing or even feel pleasure when imagined misfortune happens to advantaged targets (Smith et al., 1996; Brigham et al., 1997; van Dijk et al., 2006; Takahashi et al., 2009). Therefore, it is likely that empathic neural responses in the aMCC and AI are diminished in response to painful stimulation applied to superior-status compared with inferior-status targets.
To test this hypothesis, we examined empathic neural responses with functional magnetic resonance imaging (fMRI) in a hierarchical context. Prior to fMRI scanning, we followed the procedures of Zink et al. (2008) to create social hierarchies based on incidental skill in a game setting. Specifically, subjects performed a perceptual task and were told that all participants were ranked according to performance on the task. Covertly, all participants were told that they were medially ranked (‘two-star players’). Thus, self-identities of hierarchical positions were set experimentally. During subsequent fMRI scanning, participants were asked to empathize with inferior-status (‘one-star players’) or superior-status (‘three-star players’) targets receiving painful or non-painful stimulation (Xu et al., 2009). With this experimental design, we assessed modulations of social hierarchy on empathic neural responses with both a regions-of-interest (ROIs) analysis and an exploratory voxel-wise whole-brain analysis. The ROI analysis focused on the bilateral AI and aMCC for their consistent involvement in empathy for pain (Lamm et al., 2011).
With these neuroimaging measurements, we first examined whether empathic brain responses were attenuated in perceiving pain of superior-status as compared with inferior-status targets. Furthermore, we investigated whether neural responses of AI and aMCC to others’ pain were modulated according to behavioral measures such as empathy-related personality traits and subjective ratings to others’ pain. For this purpose, personality measures reflecting participants’ ability to understand their own feelings (manifested as low level of alexithymia) and others’ emotions (reflected by high scores on the IRI) were examined due to their demonstrated associations with empathic neural responses (Singer et al., 2004; Bird et al., 2010). Taken together, our study examined both context-specific and person-specific empathic neural responses to others’ pain.
Materials and methods
Subjects
Twenty-two individuals (11 females) (mean age ± s.d.: 22.23 ± 1.85) participated in this study and completed fMRI scanning for monetary compensation. All participants were right-handed, had normal or corrected-to-normal vision, and had no history of neurological or psychiatric disorder. Written informed consents were collected for all participants. The study was conducted according to the ethical guidelines and principles of the Declaration of Helsinki and was approved by the Institutional Review Board at Beijing Normal University.
Stimuli
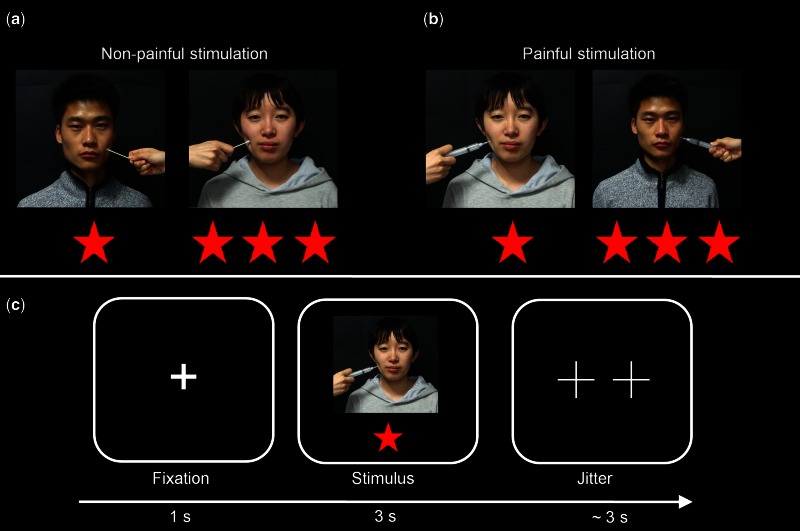
A set of 64 color photographs, showing faces of four targets (two females) unknown to all participants, was employed in this study. These photographs, 16 for each target, were derived from video clips used in a previous study (Xu et al., 2009). Notably, Xu et al. (2009) have demonstrated that these stimuli are adequate to elicit empathy-related brain activity and subjective empathic feelings. For each target, eight photographs depicted faces receiving painful stimulation (needle penetration) to the left or right cheek; and the other eight photographs showed faces receiving non-painful stimulation (Q-tip touch) (Figure 1a and b). Each photograph was set to the same size of 298 × 298 pixels.
Fig. 1.
Illustration of experimental stimuli and procedure. a) Non-painful stimulation to inferior-status and superior-status targets. b) Painful stimulation to inferior-status and superior-status players. c) Experimental procedure. Note: the photographs displayed in the figure were not employed in the experiment but were only used for illustration purpose.
Procedure
Prior to fMRI scanning, participants’ personality traits were measured by the IRI (Davis, 1980) and TAS-20 Items (Bagby et al., 1994). IRI is a self-administered questionnaire measuring the empathetic ability in four aspects: (i) empathic concern, feeling of warmth and concern for others; (ii) perspective taking, adopting the perspective of other people; (iii) fantasy, identifying with fictitious characters in books or movies and (iv) personal distress, feelings of discomfort and anxiety when witnessing the negative experiences of others. TAS-20 is a self-administered questionnaire consisting of 20 items, which are scored on a 5-point scale from ‘strongly disagree’ to ‘strongly agree’, with higher scores indicating greater levels of alexithymia. TAS-20 provides an overall measure of deficiency in understanding, processing or describing emotions (Bagby et al., 1994), and it consists of three subscales: difficulty identifying emotions, difficulty describing emotions and externally oriented thinking. The associations between alexithymia and empathy have been previously observed, suggesting that the awareness of one’s own emotions is a prerequisite to share emotions of others (Moriguchi et al., 2007; Grynberg et al., 2010).
To establish the social hierarchy, all participants were asked to perform a dot-estimation task. In this task, participants were presented with 100 red dots in a white background and asked to judge which side (left or right) of the field had more dots (Feng et al., 2013). Participants were told that their performance in this task would be evaluated by both speed and accuracy and would be compared with other players for ranking purpose. They were also told that more than 650 people had already performed this task as a part of a cognitive ability test, and all of them were ranked as inferior (‘one star players’), medium (‘two star players’) or superior (‘three star players’) status according to their performance. Covertly, outcomes of this dot-estimation task were always fixed, such that all participants were told that they were mediumly ranked based on their performance. Similar procedures to establish social hierarchy were initially employed in a landmark study by Zink et al. (2008), and these authors demonstrated that individuals are strongly engaged in the hierarchical context in this paradigm. Accordingly, this paradigm has been widely employed in the current literature (Boksem et al., 2012; Santamaría-García et al., 2013; Breton et al., 2014).
After the dot-estimation task, participants were told that a fraction of the aforementioned 650 players had agreed to take part in a sensory test in which they had received painful or non-painful stimulation. Participants were then told that they would view faces of four of these players in the other two social positions: two (one female) superior players and two (one female) inferior players. For the four targets employed, the combinations of targets and hierarchies were counterbalanced across subjects to control for potential confounding factors such as attractiveness. To ensure that participants believed that the four players had received both painful and non-painful stimulation, participants were asked to watch video clips that vividly depicted needle penetration or Q-tip touch applied to each target. In cases where a participant questioned about the ranking procedure or stimulation applied, the experiment was terminated and such participants (three females and two males, not included in the present sample of N = 22) were excluded from fMRI scanning.
On the fMRI session, each trial of the fMRI task consisted of a central fixation (1 s) followed by a photograph (3 s) of either inferior or superior player (Figure 1c). On each photograph, participants were instructed to empathize and judge how much pain the depicted target was feeling (de Greck et al., 2012). No overt response was required from participants for minimizing motor-related confounds (Decety et al., 2008; Akitsuki and Decety, 2009). Afterwards, an optimized jitter generated by an fMRI simulator software (http://www.cabiatl.com/CABI/resources/fmrisim/) was presented with minimum of 1 s and average of 3 s. Each scanning run consisted of 64 trials and lasted for 448 s. Each participant completed two scanning runs with each photograph being non-repetitively presented once in each run.
In the post-scan session, participants were asked with two evaluation scales for each photograph presented: (i) ‘how painful do you think the target feels’ (pain intensity: 1 = not at all, 9 = extremely painful) and (ii) ‘how pleasant do you feel when observing the photograph’ (pleasantness: 1 = extremely unpleasant, 9 = extremely pleasant). Finally, participants were asked whether they believed that their own and the four viewed targets’ social positions were based on their performances in the dot-estimation task and whether the painful/non-painful stimulations applied to targets were real. The debriefing received positive confirmations from all participants that completed the fMRI scanning.
Data acquisition
Imaging data were acquired with a 3T Siemens Trio scanner equipped with a 12-channel transmit/receive head coil. A T2-weighted gradient-echo echo-planar-imaging (EPI) sequence was used to acquire functional images (TR/TE = 2000 ms/30 ms, flip angle = 90°, number of axial slices = 33, slice thickness = 3.5 mm, gap between slices = 0.7 mm, matrix size = 64 × 64, FOV = 224 × 224 mm). High-resolution anatomical images covering the entire brain were also obtained by a magnetization prepared rapid acquisition with gradient-echo (MPRAGE) sequence (TR/TE = 2530/3.39 ms, flip angle = 7°, number of sagittal slices = 144, slice thickness = 1.33 mm, matrix size = 256 × 256, FOV = 256 × 256 mm).
Data analysis
Behavioral data analysis
Behavioral data analyses were carried out using SPSS 16.0 (IBM, Somers, USA) with a significance threshold of P < 0.05 (two-tailed). Subjective ratings of pain intensity and pleasantness were submitted to a 2 (social hierarchy: superior status vs inferior status) × 2 (stimulation valence: painful stimulation vs non-painful stimulation) repeated measures analysis of variance (ANOVA).
fMRI data analysis
Functional neuroimaging data analyses were performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Preprocessing of functional data included slice-timing correction, realignment through rigid-body registration to correct for head motion, spatial normalization to the Montreal Neurological Institute (MNI) template, spatial smoothing (FWHM = 5 mm) and temporal high-pass filtering (removal of low frequency drift of T > 80 s).
A two-level general linear model (GLM) was used to analyze functional data. The first-level modeling included regressors defined for each subject. These regressors modeled blood oxygenation level-dependent (BOLD) responses to the fixation and the four task conditions of painful stimulation applied to inferior players (Inferior-Pain), non-painful stimulation applied to inferior players (Inferior-NoPain), painful stimulation applied to superior players (Superior-Pain) and non-painful stimulation applied to superior players (Superior-NoPain). The six movement parameters obtained from the realignment (three translations, three rotations) were also included in the design matrix as nuisance regressors. Each task regressor was generated by convolving the corresponding boxcar stimulus function with a canonical hemodynamic response function (HRF) (Büchel et al., 1998). To improve noise estimation, the GLM also considered signal temporal autocorrelations with a first-order autoregressive model (Bullmore et al., 1996). In the first-level GLM, regression coefficients (or beta values) for each regressor were computed at every voxel within the brain.
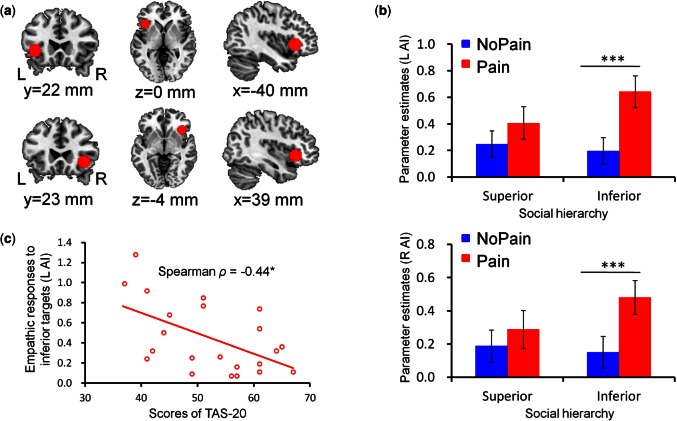
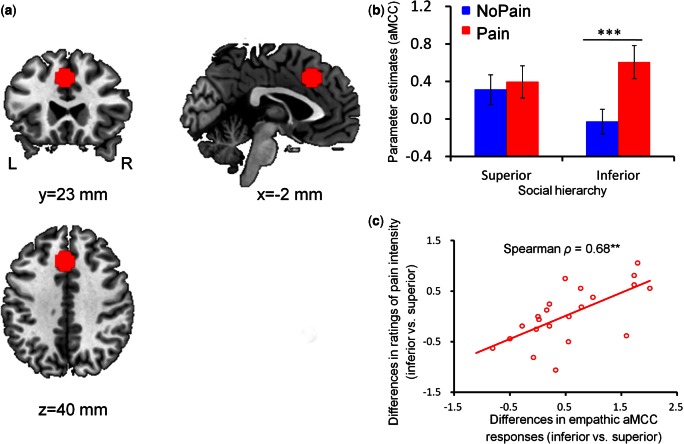
With the obtained parameter estimates, we performed a region-of-interest (ROI) analysis based on a priori hypotheses (Poldrack, 2007; Poldrack and Mumford, 2009). This ROI analysis focused on the aMCC and bilateral AI as they are most consistently implicated in perceiving others’ pain, thereby constituting the core network of empathy for pain (Lamm et al., 2011). To determine the ROIs independently of the present data, the regions of bilateral AI (Figure 2a) and aMCC (Figure 3a) were defined as spheres (radius = 10 mm) centered at MNI coordinates (x/y/z = −40/22/0 mm, 39/23/−4 mm and −2/23/40 mm) reported in a previous meta-analysis (Lamm et al., 2011). The average parameter estimates across all voxels in each ROI were extracted from each subject for all experimental conditions using SPM REX toolbox (https://www.nitrc.org/projects/rex/). These data were then compared between conditions with a 2 (social hierarchy: superior status vs inferior status) × 2 (stimulation valence: painful stimulation vs non-painful stimulation) repeated measures ANOVA.
Fig. 2.
fMRI ROI results of the anterior insula. a) Functional ROI in the bilateral anterior insula. b) ROI analysis of the parameter estimates of the bilateral anterior insula as a function of social hierarchy and stimulation valence. Error bars indicate one standard error. c) The correlation between scores of TAS-20 and empathic responses of the left AI in response to inferior targets (pain vs nopain). L, left; R, right; AI, anterior insula. ***P < 0.0005; *P < 0.05.
Fig. 3.
fMRI ROI results of the aMCC. a) Functional ROI in the aMCC. b) ROI analysis of the parameter estimates of the aMCC as a function of social hierarchy and stimulation valence. Error bars indicate one standard error. c) The correlation between empathic aMCC responses and subjective ratings of pain intensity regarding the differences between inferior-status and superior-status targets [i.e. (Inferior-Pain − Inferior-NoPain) − (Superior-Pain − Superior-NoPain)]. L, left; R, right; aMCC, anterior medial cingulate cortex. ***P < 0.0005; **P < 0.01.
The ROI analysis was supplemented with an exploratory whole-brain analysis using voxel-wise repeated measures ANOVA. This analysis was employed to confirm the interaction between social hierarchy and stimulation valence in the predefined ROIs as well as to explore the same interaction in other brain regions. To correct for false positives yielded by multiple comparisons, statistical maps were clipped with a joint threshold at both the voxel level and the cluster level. The cluster threshold was determined using a Monte Carlo simulation-based estimator implemented in Matlab (Slotnick et al., 2003; Slotnick and Schacter, 2004). On the basis of simulations (5000 iterations) and the estimated spatial smoothness of FWHM = 9 mm, a family-wise error (FWE) correction at P < 0.05 is achieved with a cluster defining threshold of P < 0.005 and a cluster extent of 86 contiguous resampled voxels (688 mm3) (Janes et al., 2010; Dietsche et al., 2014; Abel et al., 2015; Henry et al., 2015; Willems et al., 2015). This joint threshold was applied to all results of whole-brain analyses.
Focusing on the predefined ROIs of AI and aMCC, we also performed an analysis of psychophysiological interaction (PPI) (Friston et al., 1997) to examine how social hierarchy modulates functional connectivity between the AI/aMCC and other regions of the brain. Specifically, we used the generalized PPI toolbox (http://www.nitrc.org/projects/gppi) (McLaren et al., 2012) with fMRI signal time courses individually extracted from the AI and aMCC as the seeding signals. These seeding signals were then deconvolved with the canonical HRF, resulting in estimates of the underlying neuronal activity (Gitelman et al., 2003). Subsequently, the interactions of these estimated neuronal time-series and vectors representing each of the onsets for the fixation and four stimulus types (Inferior-Pain, Inferior-NoPain, Superior-Pain, Superior-NoPain) were computed. Lastly, these interaction terms were re-convolved with the HRF and entered into a new GLM along with the vectors for the onsets of each stimulus type (i.e. the psychological terms), the original average time-series, and nuisance regressors (i.e. six movement parameters derived from realignment corrections). Group level analysis of the PPI data was almost identical to that of activation data except the beta values used were derived from the PPI regressors. In this study, we focused on connections that exhibited a significant interaction effect of social hierarchy × stimulation valence. Namely, those connections with a different painful vs non-painful contrast between the superior and inferior status.
Moreover, given previous reports of differential behavioral responses to superior-status men and women (Maner et al., 2007; DeWall, 2008), potential effects of target gender were explored by adding it as a within-subjects factor to the GLMs described earlier. However, since we did not observe significant modulations of target gender on either subjective ratings or empathic AI and aMCC responses (Supplementary Figures S1 and S2), the current data analyses on the empathic responses were collapsed across gender of targets.
Finally, to examine potential relationships between the internal brain activation and external behavior, Spearman’s Rank non-parametric (i.e. Spearman ρ) correlations that are more robust to outliers than Pearson’s linear correlations (Rousselet and Pernet, 2012) were computed to determine associations among dispositional (personality scores), behavioral (subjective ratings), fMRI (BOLD signal changes) and functional connectivity (connectivity strengths) measures.
Results
Behavioral results
Relative to non-painful stimulation, painful stimulation was rated with higher scores of pain intensity (F(1, 21) = 123.25, P < 0.0005) and lower scores of pleasantness (F(1, 21) = 9.84, P < 0.01). These rating scores of painful and non-painful stimulations did not differ between superior and inferior status (all P > 0.05).
fMRI results: ROI analysis
The analysis of BOLD responses in the left AI (F(1, 21) = 17.42, P < 0.0005), right AI (F(1, 21) = 9.24, P < 0.01) and aMCC (F(1, 21) = 22.20, P < 0.0005) confirmed the augmented activity for painful stimulation as compared with non-painful stimulation; whereas the main effect of social hierarchy was not significant (left AI: F(1, 21) = 2.61, P > 0.05; right AI: F(1, 21) = 1.40, P > 0.05; aMCC: F(1, 21) = 0.71, P > 0.05). Moreover, in supporting our hypothesis, we observed significant interactions of social hierarchy and stimulation valence in all of these three regions (left AI: F(1, 21) = 7.27, P < 0.05, Figure 2b; right AI: F(1, 21) = 5.86, P < 0.05, Figure 2b; aMCC: F(1, 21) = 10.75, P < 0.005, Figure 3b). For superior-status targets, BOLD responses in these areas did not significantly differ between painful and non-painful stimulation (left AI: t(21) = 1.54, P > 0.05; right AI: t(21) = 1.10, P > 0.05; aMCC: t(21) = 0.73, P > 0.05). For inferior-status targets, however, painful stimulation elicited higher BOLD responses than non-painful stimulation (left AI: t(21) = 5.97, P < 0.0005; right AI: t(21) = 4.15, P < 0.0005; aMCC: t(21) = 5.45, P < 0.0005). Noteworthy, to non-painful stimulation, aMCC showed stronger responses to superior-status than inferior-status targets (t(21) = 2.54, P < 0.05).
The correlation analysis revealed that empathic responses of the left AI to inferior targets (i.e. Inferior-Pain vs Inferior-NoPain) were negatively correlated with participants’ scores of TAS-20 (Spearman ρ = −0.44, P < 0.05, Figure 2c); whereas this correlation was not significant in response to superior-status targets (P > 0.05). In addition, empathic aMCC responses and empathic subjective ratings of pain intensity were positively correlated with each other regarding the difference between inferior and superior status [i.e. (Inferior-Pain − Inferior-NoPain) − (Superior-Pain − Superior-NoPain)] (Spearman ρ = −0.68, P < 0.005, Figure 3c).
fMRI results: exploratory whole-brain analysis
Whole-brain analysis of neuroimaging data was detailed in the supplementary materials (Supplementary Figures S3–S5 and Tables S1–S3).
fMRI results: PPI analysis
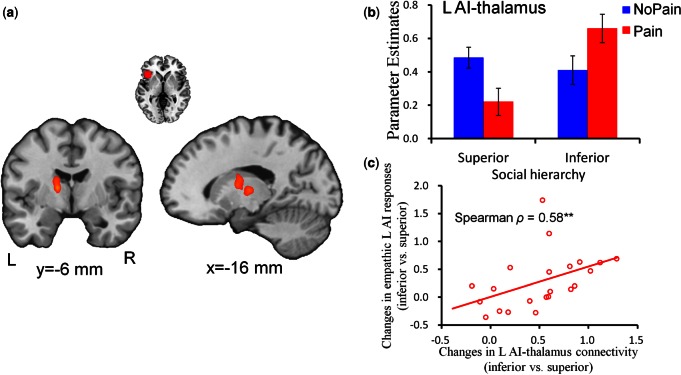
PPI analysis was performed to assess the interaction effects of social hierarchy × stimulation valence on the functional connectivity between AI/aMCC and other brain regions. The connectivity contrast of [(Inferior-Pain vs Inferior-NoPain) > (Superior-Pain vs Superior-NoPain)] for the left AI as a seed region identified the following brain regions (P < 0.05 FWE corrected at the cluster level): the left thalamus (−14/−6/10 mm, cluster size = 182 voxels, T = 5.14) (Figure 4a and b) and the right calcarine (30/−66/8 mm, cluster size = 138 voxels, T = 4.03); and the reverse contrast identified the left middle occipital gyrus (−28/−92/0 mm, cluster size = 126 voxels, T = −4.55). Notably, empathic left AI-thalamus connectivity and empathic left AI responses were positively correlated with each other regarding the difference between inferior and superior status [i.e. (Inferior-Pain − Inferior-NoPain) − (Superior-Pain − Superior-NoPain)] (Spearman ρ = 0.58, P < 0.01, Figure 4c).
Fig. 4.
Interactive effects of social hierarchy and stimulation valence on functional coupling between left anterior insula and thalamus. a) Illustration of the left thalamus showing significant changes in functional coupling with the left anterior insula as revealed by the interaction of social hierarchy and stimulation valence. Images are thresholded at P < 0.05 corrected for multiple comparisons at the cluster level. b) Parameter estimates of the left AI-thalamus connectivity as a function of social hierarchy and stimulation valence. Error bars indicate standard error. c) The correlation between strength of left AI-thalamus connectivity and empathic AI responses regarding the differences between inferior-status and superior-status targets [i.e. (Inferior-Pain − Inferior-NoPain) − (Superior-Pain − Superior-NoPain)]. L, left; R, right; AI, anterior insula. **P < 0.01.
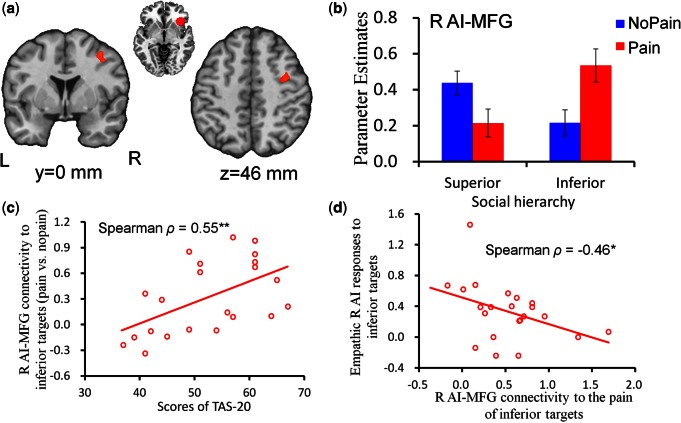
The connectivity contrast of [(Inferior-Pain vs Inferior-NoPain) > (Superior-Pain vs Superior-NoPain)] for the right AI as a seed region identified the right middle frontal gyrus (MFG) (42/−8/32 mm, cluster size = 91 voxels, T = 4.52) (Figure 5a and b); whereas no significant cluster was identified with the reverse contrast. The correlation analysis revealed that scores of TAS-20 were positively correlated with empathic AI-MFG connectivity changes in response to inferior-status targets (Spearman ρ = 0.55, P < 0.01, Figure 5c). Further, the strength of right AI-MFG connectivity in response to the pain of inferior targets showed a negative correlation with empathic AI responses to inferior-status targets (Spearman ρ = −0.46, P < 0.05, Figure 5d). These correlations were not significant in response to superior-status targets (all P > 0.05).
Fig. 5.
Interactive effects of social hierarchy and stimulation valence on functional coupling between right anterior insula and MFG. a) Illustration of the right MFG showing significant changes in functional coupling with the right anterior insula as revealed by the interaction of social hierarchy and stimulation valence. Images are thresholded at P < 0.05 corrected for multiple comparisons at the cluster level. b) Parameter estimates of the right AI-MFG connectivity as a function of social hierarchy and stimulation valence. Error bars indicate standard error. c) The correlation between scores of TAS-20 and strength of right AI-MFG connectivity in response to inferior targets. d) The correlation between strength of right AI-MFG connectivity in response to the pain of inferior targets and empathic AI responses to inferior-status targets. L, left; R, right; AI, anterior insula; MFG, middle frontal gyrus. **P < 0.01; *P < 0.05.
Finally, aMCC showed significant functional covariation with the right precuneus (38/−80/36 mm, cluster size = 91 voxels, T = 4.43) as revealed by the connectivity contrast of [(Inferior-Pain vs Inferior-NoPain) > (Superior-Pain vs Superior-NoPain)], whereas no significant cluster was identified with the reverse contrast.
Discussion
Our study examined the influence of social hierarchy on the neural responses to others’ pain. We identified brain activation to the pain of others in the AI and aMCC that are implicated in affective aspects of empathy for pain (Lamm et al., 2011). The empathic neural responses in the left AI inversely correlated with the alexithymia traits, and neural responses in the aMCC were positively associated with subjective sensitivity to others’ pain. Notably, we observed significant modulations of social hierarchy on these empathic neural responses, such that they were evident in the perception of pain of inferior-status targets but were significantly attenuated in response to the pain of superior-status targets. Finally, we observed stronger functional couplings of AI with brain regions implicated in emotional processing (i.e. thalamus) and cognitive control (i.e. MFG) in response to the pain of inferior-status than superior-status targets. Our findings indicate that brain functions underlying empathy are modulated by the relative positions in a social hierarchy such that empathic neural responses are biased toward inferior-status compared with superior-status targets.
We first replicated previous findings on the neural signatures underlying empathy for others’ pain. Among other brain regions, the AI and aMCC showed stronger responses to the painful than non-painful stimulation applied to others. The AI and aMCC responses to others’ pain are thought to represent feeling states of others (Lamm et al., 2011; Bernhardt and Singer, 2012). This assertion has support from the present and previous observations that empathic aMCC responses were associated with subjective sensitivity to the pain of others (Lamm and Decety, 2008). Furthermore, the AI and aMCC are also engaged in affective and motivational aspects of first-person pain experience (Peyron et al., 2000; Rainville, 2002), leading to the notion that emotional sharing with others’ pain is based on shared neural representations for first-person and vicarious experiences of emotion (Singer et al., 2004; Lamm et al., 2011; Bernhardt and Singer, 2012; Rütgen et al., 2015). This hypothesis is confirmed by our findings that neural responses of AI to the pain of inferior-status targets correlated inversely with alexithymia traits that involve difficulties in understanding one’s own emotions (Silani et al., 2008; Bird et al., 2010). Noteworthy, correlations of the AI and aMCC activations with behavioral measures were different. The AI responses were correlated with alexithymia scores, whereas the aMCC responses with pain intensity ratings. Whether these findings imply different roles of the AI and aMCC remains to be elucidated, since much of the research has focused on the commonality of AI and aMCC (see also Gu et al., 2010; Bernhardt and Singer, 2012). Recent attempts to dissociate functions of these brain regions have not yet provided straightforward predictions on the distinct correlations of their activations with behavioral measures (Gu et al., 2010, 2012, 2013).
We next studied the modulations of social hierarchy on empathic neural activity in the bilateral AI and aMCC. Our findings revealed that empathy-related activations in these brain regions were significantly attenuated in response to the pain of superior-status targets. These results concur with previous observations that empathic neural responses in the aMCC and AI are modulated by interpersonal relationship such that they are remarkably decreased by the knowledge that out-group members or disliked others are in pain (Singer et al., 2006; Xu et al., 2009). The evidence that empathic neural responses are modulated by interpersonal relations supports the context-dependent account of empathy (De Vignemont and Singer, 2006).
Modulations of social hierarchy on empathic neural responses might be mediated by the social comparison processes. For instance, the knowledge that others are better threatens positive self-views and induces negative affect due to upward social comparison (Major et al., 1993). This negative affective link with superior-status targets in turn may dampen empathy (Singer et al., 2006). This is supported by the stronger aMCC responses to superior than inferior targets at baseline in the context of non-painful stimuli. Such an aMCC activation pattern echoes previous observation that upward social comparison with advantaged targets induced enhanced activations in the aMCC, which was thought to reflect painful feelings or conflicts of positive self-concepts (Takahashi et al., 2009). In Takahashi et al.’s (2009) study, negative affective link predicts experienced pleasure and associated brain activations (e.g. ventral striatum) in response to imagined misfortunes on advantaged individuals. We did not identify the involvement of reward neural circuit in response to the pain of superior-status targets. This might be due to the reason that participants were asked to intentionally empathize with the pain of others. Noteworthy, this affective account is very tentative given that we did not collect participants’ attitudes toward superior-status and inferior-status targets. Alternatively, differential empathic neural responses to inferior and superior targets might be attributed to attentional/cognitive processes (Gu and Han, 2007; Lamm et al., 2007b). It has been demonstrated both by previous data (Zink et al., 2008) and by the main effect of social status in the present whole-brain analyses that greater attentional resources are directed to the superior than inferior targets. As such, when viewing superior targets in pain, the status itself may deter attention from allocating to pain so that empathic neural response are dampened (Gu and Han, 2007).
Despite the observed effects of social status on the fMRI responses to others’ pain, social status had no effect on participants’ self-reported ratings of pain intensity. This divergence could be due to attention redirection in the self-paced rating task, which presumably allowed more time for evaluation as well as increased attention to the pain-related features. Another possible account is that subjective ratings of pain intensity for superior-status targets might be based on cognitive evaluations or sensory perception (cf. Xu et al., 2009). It is not without precedent that differential neuropsychological processes are involved in empathizing with different targets. Indeed, Meyer et al. (2012, 2015) have demonstrated that empathy for strangers’ social suffering relies more heavily on mentalizing networks, whereas empathy for friends’ social suffering relies on networks implicated in emotional sharing and self-processing. In line with this account, our whole-brain analysis revealed that empathy for the pain of superior-status relative to inferior-status targets induced stronger responses in the precuneus, which is implicated in mentalizing (Lieberman, 2010).
The neural empathic bias to inferior-status targets was accompanied by enhanced functional connectivity of AI and aMCC with other brain regions including thalamus and MFG. On the one hand, the thalamus plays a critical role in affective aspects of empathy (Nummenmaa et al., 2008; Hillis, 2014). Patients with thalamus lesion have shown lower ability in emotional empathy as measured by the ‘Reading the mind in the Eyes Test’ (Wilkos et al., 2015). The thalamus might contribute to emotional empathy by relaying sensory information about affective experience of others to the insula to shape the representation of others’ emotion (Craig, 2002; Hillis, 2014). In line with this viewpoint, our results revealed that stronger AI-thalamus connectivity strengths predicted higher empathic neural responses of the AI. On the other hand, the MFG is often involved in cognitive control and presumably contributes to emotion regulation of empathy (Decety and Jackson, 2004; Gu and Han, 2007). This is also consistent with our findings that the stronger right AI-MFG connectivity strengths predicted lower empathic AI responses to inferior-status targets. In addition to emotional sharing, emotion regulation constitutes another crucial component of empathy to manage intersubjective transactions between self and other (Decety and Jackson, 2004; Lamm et al., 2010).
Several limitations warrant consideration. First, our study did not consider gender of perceivers as a potential moderators as many previous studies did (Chiao et al., 2008; Zink et al., 2008; de Greck et al., 2012). It is possible that genders of perceivers and targets interact with each other to modulate the processing of social hierarchy and its effects on socio-emotional functioning (Maner et al., 2007; DeWall, 2008). Replication in larger groups of men and women will help to clarify sex differences in the modulation of social hierarchy on empathic neural responses. Second, we deliberately used faces of neutral expressions for both painful and non-painful stimulations to avoid confounding effects of emotional contexts on empathic responses (cf. Han et al., 2009). One may argue that neural responses to painful stimulation were attributed to conflict resolution. This argument is not consistent with our results that empathic neural responses of the AI/aMCC were modulated according to empathy-related personality traits (i.e. alexithymia) and subjective sensitivity to others’ pain. Finally, our design did not include a same-status condition that could help to identify the directions of effects of social hierarchy on empathic responses. For instance, it is possible that empathic responses to inferior targets reflect general affective sharing as in the neutral (e.g. same-status) condition rather than increased empathy for the pain of inferior targets.
In summary, our findings confirmed the hypothesis that empathic neural responses are modulated by relative positions in a social hierarchy. We showed evidence that the affective neuronal network consisting of the aMCC and AI is engaged in empathic responses to the pain of inferior-status but not superior-status individuals. In addition, the AI showed stronger functional couplings with thalamus and MFG that are respectively associated with emotional processing and cognitive control in response to the pain of inferior-status than superior-status targets. These findings indicate a bias of emotional sharing with inferior-status compared with superior-status others and complement previous observations on the effects of social hierarchy on human social behaviors and cognitive functions (Koski et al., 2015).
Supplementary Material
Acknowledgements
The authors thank Dr Shihui Han for generous sharing of experimental stimuli and Dr Mac Merritt for improving language.
Funding
This study was supported by the National Natural Science Foundation of China (31530031, 81471376, 31300869), the National Basic Research Program of China (973 Program: 2014CB744600), the Natural Science Foundation of Jiangsu Province of China (BK20130415) and Natural Science Foundation of SZU (201564).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Abel S., Weiller C., Huber W., Willmes K., Specht K. (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138(4), 1097–112. [DOI] [PubMed] [Google Scholar]
- Akitsuki Y., Decety J. (2009). Social context and perceived agency affects empathy for pain: an event-related fMRI investigation. Neuroimage, 47(2), 722–34. [DOI] [PubMed] [Google Scholar]
- Bagby R.M., Parker J.D., Taylor G.J. (1994). The twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research, 38(1), 23–32. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Singer T. (2012). The neural basis of empathy. Annual Review of Neuroscience, 35, 1–23. [DOI] [PubMed] [Google Scholar]
- Bird G., Silani G., Brindley R., White S., Frith U., Singer T. (2010). Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain, 133(Pt 5), 1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem M.A., Kostermans E., Milivojevic B., De Cremer D. (2012). Social status determines how we monitor and evaluate our performance. Social Cognitive and Affective Neuroscience, 7(3), 304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton A., Jerbi K., Henaff M.-A., et al. (2014). Face the hierarchy: ERP and oscillatory brain responses in social rank processing. PLoS One, 9(3), e91451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham N.L., Kelso K.A., Jackson M.A., Smith R.H. (1997). The roles of invidious comparisons and deservingness in sympathy and schadenfreude. Basic and Applied Social Psychology, 19(3), 363–80. [Google Scholar]
- Büchel C., Holmes A., Rees G., Friston K. (1998). Characterizing stimulus–response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage, 8(2), 140–8. [DOI] [PubMed] [Google Scholar]
- Bullmore E., Brammer M., Williams S.C., et al. (1996). Statistical methods of estimation and inference for functional MR image analysis. Magnetic Resonance in Medicine, 35(2), 261–77. [DOI] [PubMed] [Google Scholar]
- Cheng J.T., Tracy J.L., Anderson C. (2014). The Psychology of Social Status. New York: Springer. [Google Scholar]
- Cheng Y., Lin C.-P., Liu H.-L., et al. (2007). Expertise modulates the perception of pain in others. Current Biology, 17(19), 1708–13. [DOI] [PubMed] [Google Scholar]
- Chiao J.Y., Adams R.B., Peter U.T., Lowenthal W.T., Richeson J.A., Ambady N. (2008). Knowing who’s boss: fMRI and ERP investigations of social dominance perception. Group Processes & Intergroup Relations, 11(2), 201–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. (2002). How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience, 3(8), 655–66. [DOI] [PubMed] [Google Scholar]
- Davis M.H. (1980). A multidimensional approach to individual differences in empathy. JSAS Catalog of Selected Documents in Psychology, 10, 85. [Google Scholar]
- de Greck M., Wang G., Yang X., Wang X., Northoff G., Han S. (2012). Neural substrates underlying intentional empathy. Social Cognitive and Affective Neuroscience, 7(2), 135–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vignemont F., Singer T. (2006). The empathic brain: how, when and why? Trends in Cognitive Sciences, 10(10), 435–41. [DOI] [PubMed] [Google Scholar]
- Decety J., Jackson P.L. (2004). The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 3(2), 71–100. [DOI] [PubMed] [Google Scholar]
- Decety J., Michalska K.J., Akitsuki Y. (2008). Who caused the pain? An fMRI investigation of empathy and intentionality in children. Neuropsychologia, 46(11), 2607–14. [DOI] [PubMed] [Google Scholar]
- DeWall C.N. (2008). High status men (but not women) capture the eye of the beholder. Evolutionary Psychology, 6, 328–41. [Google Scholar]
- Dietsche B., Backes H., Stratmann M., Konrad C., Kircher T., Krug A. (2014). Altered neural function during episodic memory encoding and retrieval in major depression. Human Brain Mapping, 35(9), 4293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. (2003). Does rejection hurt? An fMRI study of social exclusion. Science, 302(5643), 290–2. [DOI] [PubMed] [Google Scholar]
- Feng C., Luo Y., Gu R., et al. (2013). The flexible fairness: equality, earned entitlement, and self-interest. PLoS One, 8(9), e73106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C., Tian T., Feng X., Luo Y.-J. (2015). Attention modulations on the perception of social hierarchy at distinct temporal stages: an electrophysiological investigation. Neuropsychologia, 70, 21–9. [DOI] [PubMed] [Google Scholar]
- Friston K., Buechel C., Fink G., Morris J., Rolls E., Dolan R. (1997). Psychophysiological and modulatory interactions in neuroimaging. Neuroimage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. (2003). Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage, 19(1), 200–7. [DOI] [PubMed] [Google Scholar]
- Goubert L., Craig K.D., Vervoort T., et al. (2005). Facing others in pain: the effects of empathy. Pain, 118(3), 285–8. [DOI] [PubMed] [Google Scholar]
- Grynberg D., Luminet O., Corneille O., Grèzes J., Berthoz S. (2010). Alexithymia in the interpersonal domain: a general deficit of empathy? Personality and Individual Differences, 49(8), 845–50. [Google Scholar]
- Gu X., Gao Z., Wang X., et al. (2012). Anterior insular cortex is necessary for empathetic pain perception. Brain, 135(9), 2726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Han S. (2007). Attention and reality constraints on the neural processes of empathy for pain. Neuroimage, 36(1), 256–67. [DOI] [PubMed] [Google Scholar]
- Gu X., Liu X., Guise K.G., Naidich T.P., Hof P.R., Fan J. (2010). Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. The Journal of Neuroscience, 30(10), 3739–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X., Liu X., Van Dam N.T., Hof P.R., Fan J. (2013). Cognition–emotion integration in the anterior insular cortex. Cerebral Cortex, 23(1), 20–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S., Fan Y., Xu X., et al. (2009). Empathic neural responses to others’ pain are modulated by emotional contexts. Human Brain Mapping, 30, 3227–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry M.J., Herrmann B., Obleser J. (2015). Selective attention to temporal features on nested time scales. Cerebral Cortex, 25(2), 450–9. [DOI] [PubMed] [Google Scholar]
- Hillis A.E. (2014). Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain, 137(4), 981–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Blue P., Yu H., et al. (2015). Social status modulates the neural response to unfairness. Social Cognitive and Affective Neuroscience, doi:10.1093/scan/nsv086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Cao Y., Blue P.R., Zhou X. (2014). Low social status decreases the neural salience of unfairness. Frontiers in Behavioral Neuroscience, 8(402), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.L., Meltzoff A.N., Decety J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage, 24(3), 771–9. [DOI] [PubMed] [Google Scholar]
- Janes A.C., Pizzagalli D.A., Richardt S., et al. (2010). Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry, 67(8), 722–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski J.E., Xie H., Olson I.R. (2015). Understanding social hierarchies: the neural and psychological foundations of status perception. Social Neuroscience, 10(5), 527–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C., Batson C. D., Decety J. (2007a) The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience, 19(1), 42–58. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J. (2008). Is the extrastriate body area (EBA) sensitive to the perception of pain in others. Cerebral Cortex, 18(10), 2369–73. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage, 54(3), 2492–502. [DOI] [PubMed] [Google Scholar]
- Lamm C., Meltzoff A.N., Decety J. (2010). How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience, 22(2), 362–76. [DOI] [PubMed] [Google Scholar]
- Lamm C., Nusbaum H.C., Meltzoff A.N., Decety J. (2007b) What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One, 2(12), e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman M.D. (2010). Social cognitive neuroscience. In: Fiske S.T., Gilbert D.T., Lindzey G. editors. Handbook of Social Psychology, 5th edn New York, NY: McGraw-Hill. [Google Scholar]
- Major B., Sciacchitano A.M., Crocker J. (1993). In-group versus out-group comparisons and self-esteem. Personality and Social Psychology Bulletin, 19(6), 711–21. [Google Scholar]
- Maner J.K., Gailliot M.T., DeWall C.N. (2007). Adaptive attentional attunement: evidence for mating-related perceptual bias. Evolution and Human Behavior, 28(1), 28–36. [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2012). Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Social Cognitive and Affective Neuroscience, 8(4), 446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.L., Masten C.L., Ma Y., et al. (2015). Differential neural activation to friends and strangers links interdependence to empathy. Culture and Brain, 3(1), 21–38. [Google Scholar]
- Moriguchi Y., Decety J., Ohnishi T., et al. (2007). Empathy and judging other’s pain: an fMRI study of alexithymia. Cerebral Cortex, 17(9), 2223–34. [DOI] [PubMed] [Google Scholar]
- Nummenmaa L., Hirvonen J., Parkkola R., Hietanen J.K. (2008). Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage, 43(3), 571–80. [DOI] [PubMed] [Google Scholar]
- Peyron R., Laurent B., Garcia-Larrea L. (2000). Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiologie Clinique, 30(5), 263–88. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A. (2007). Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience, 2(1), 67–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack R.A., Mumford J.A. (2009). Independence in ROI analysis: where is the voodoo? Social Cognitive and Affective Neuroscience, 4(2), 208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P. (2002). Brain mechanisms of pain affect and pain modulation. Current Opinion in Neurobiology, 12(2), 195–204. [DOI] [PubMed] [Google Scholar]
- Rousselet G.A., Pernet C.R. (2012). Improving standards in brain-behavior correlation analyses. Frontiers in Human Neuroscience, 6(119), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rütgen M., Seidel E.-M., Silani G., et al. (2015). Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Sciences, 12(41), E5638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaría-García H., Pannunzi M., Ayneto A., Deco G., Sebastián-Gallés N. (2013). “If you are good, I get better”: the role of social hierarchy in perceptual decision-making. Social Cognitive and Affective Neuroscience, 9(10), 1489–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G., Bird G., Brindley R., Singer T., Frith C., Frith U. (2008). Levels of emotional awareness and autism: an fMRI study. Social Neuroscience, 3(2), 97–112. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O’Doherty J.P., Stephan K.E., Dolan R.J., Frith C.D. (2006). Empathic neural responses are modulated by the perceived fairness of others. Nature, 439(7075), 466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick S.D., Moo L.R., Segal J.B., Hart J. (2003). Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research, 17(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Slotnick S.D., Schacter D.L. (2004). A sensory signature that distinguishes true from false memories. Nature Neuroscience, 7(6), 664–72. [DOI] [PubMed] [Google Scholar]
- Smith R., Turner T., Garonzik R., Leach C., Urch-Druskat V., Weston C. (1996). Envy and schadenfreude. Personality and Social Psychology Bulletin, 22(2), 158–68. [Google Scholar]
- Takahashi H., Kato M., Matsuura M., Mobbs D., Suhara T., Okubo Y. (2009). When your gain is my pain and your pain is my gain: neural correlates of envy and schadenfreude. Science, 323(5916), 937–39. [DOI] [PubMed] [Google Scholar]
- van Dijk W.W., Ouwerkerk J.W., Goslinga S., Nieweg M., Gallucci M. (2006). When people fall from grace: reconsidering the role of envy in schadenfreude. Emotion, 6(1), 156. [DOI] [PubMed] [Google Scholar]
- Wilkos E., Brown T.J., Slawinska K., Kucharska K.A. (2015). Social cognitive and neurocognitive deficits in inpatients with unilateral thalamic lesions–pilot study. Neuropsychiatric Disease and Treatment, 11, 1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems R.M., Frank S.L., Nijhof A.D., Hagoort P., Van den Bosch A. (2015). Prediction during natural language comprehension. Cerebral Cortex, doi: 10.1093/cercor/bhv075. [DOI] [PubMed] [Google Scholar]
- Xu X., Zuo X., Wang X., Han S. (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience, 29(26), 8525–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J. (2014). Empathy: a motivated account. Psychological Bulletin, 140(6), 1608. [DOI] [PubMed] [Google Scholar]
- Zink C.F., Tong Y., Chen Q., Bassett D.S., Stein J.L., Meyer-Lindenberg A. (2008). Know your place: neural processing of social hierarchy in humans. Neuron, 58(2), 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.