Abstract
We report that a regioselective reductive transposition of primary allylic bromides is catalyzed by a biphilic organophosphorus (phosphetane) catalyst. Spectroscopic evidence supports the formation of a pentacoordinate (σ5-P) hydridophosphorane as a key reactive intermediate. Kinetics experiments and computational modeling are consistent with a unimolecular decomposition of the σ5-P hydridophosphorane via a concerted cyclic transition structure that delivers the observed allylic transposition and completes a novel PIII/PV redox catalytic cycle. These results broaden the growing repertoire of reactions catalyzed within the PIII/PV redox couple and suggest additional opportunities for organophosphorus catalysis in a biphilic mode.
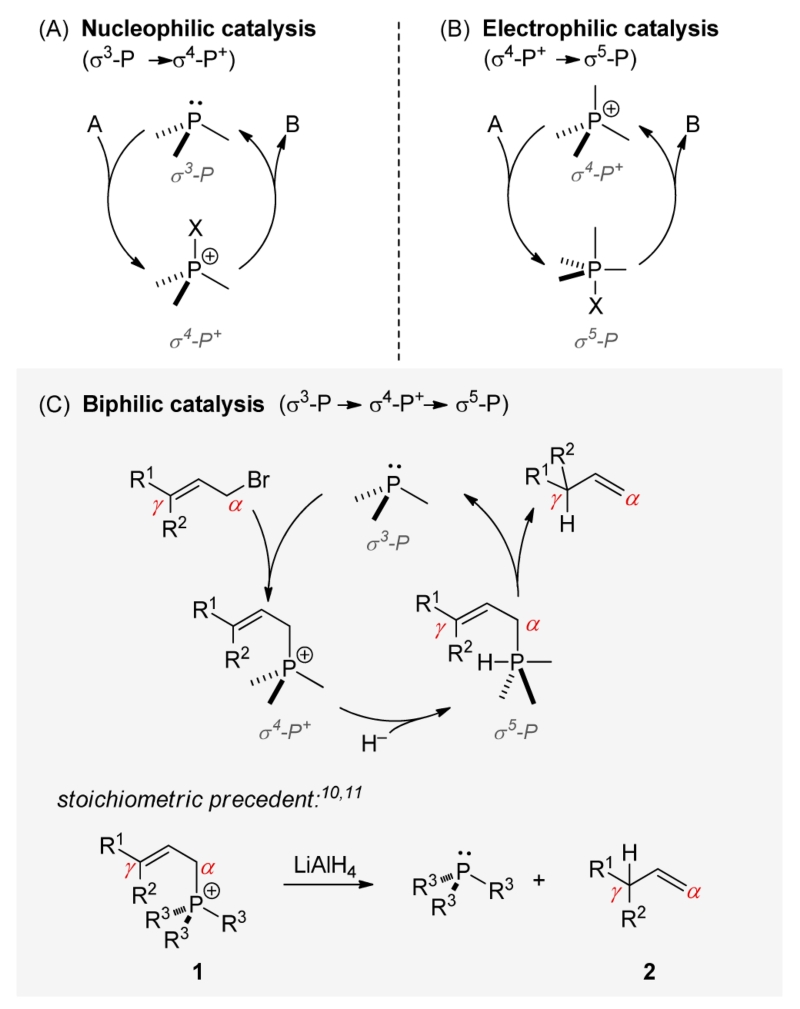
Phosphorus-based compounds figure prominently in a range of catalytic applications. Apart from their role as supporting ligands in transition metal chemistry,1 tricoordinate phosphorus compounds comprise a well-known class of nucleophilic catalysts,2 wherein neutral tricoordinate (σ3-P) phosphines (Figure 1A) behave as electron-pair donors with respect to electron-deficient substrates to elicit catalysis via tetracoordinate (σ4-P+) phosphonium intermediates. More recently, the potential of Lewis acidic σ4-P+ cations themselves to behave as catalysts in an electrophilic mode via intermediate pentacoordinate (σ5-P) phosphoranes has been demonstrated (Figure 1B).3–5 Herein, we demonstrate a biphilic6 mode of organophosphorus catalysis that unifies both nucleophilic (donor) and electrophilic (acceptor) reactivities at a single phosphorus center. Specifically, we show that a small-ring phosphacycle catalyzes the regioselective transpositive reduction of allylic bromides in a manner that involves interconversion of well-characterized σ3-P, σ4-P+, and σ5-P species (Figure 1C). Taken together with ongoing work in the area of phosphine oxide redox catalysis,7 these results further solidify the viability of the PIII/PV oxidation state couple in catalytic chemistry8 and portend new developments in biphilic organophosphorus catalysis that leverage the unique reactivities of σ5-P phosphoranes.9
Figure 1.
Modes of organophosphorus catalysis.
It is known from the work of van Tamelen10 and Nojima11 that stoichiometric reaction of allylic phosphonium cations (e.g., 1, Figure 1C, bottom) with metal hydrides results in reductive deallylation of the σ4-P+ cation to the corresponding σ3-P phosphine.12 With respect to the allylic moiety, the reduction is regiospecific; alkenes 2 resulting from allylic transposition (i.e., γ-reduction) are the sole products observed under these stoichiometric conditions.13 On account of this regiochemical outcome (and by analogy to reduction of related σ4-P+ cations14), the possible intervention of σ5-P hydridophosphorane intermediates was posited by Gallagher,15 although direct experimental evidence for this proposed pathway has not been reported.
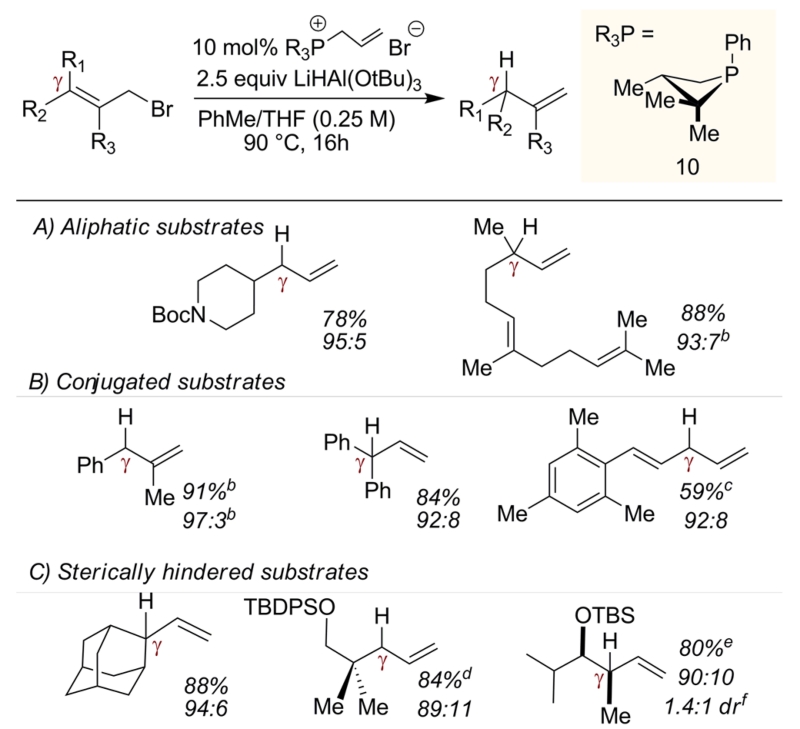
On the basis of the aforementioned stoichiometric precedent, this allylic reduction presented itself as an appealing platform by which to test emerging hypotheses regarding the design features requisite for biphilic organophosphorus catalysis.16 In contrast to the regiospecific transposition of the corresponding stoichiometric reduction (Figure 1C), catalytic loading (10 mol %) of triphenylphosphine-derived ion 617 in the reduction of cinnamyl bromide (3) with LiAlH(OtBu)3 delivers products 4:5 in a 18:82 ratio (Table 1, entry 2); this γ/α-selectivity ratio deviates only marginally from an uncatalyzed control reduction (entry 1). Evidently, direct uncatalyzed reduction of 3 with LiAlH(OtBu)3 outcompetes notional biphilic catalysis by triphenylphosphine. Following on Gallagher’s hypothesis and abundant precedent demonstrating enhanced electrophilic reactivity of cyclic phosphorus-based compounds compared to their acyclic congeners,18 the conversion σ4-P+ → σ5-P was targeted for acceleration. While neither dibenzophosphole 8 nor phospholane 9 derivatives (proven catalyst motifs in several catalytic PIII/PV=O redox methods)19 satisfactorily alter the reduction outcome, catalytic loading of phosphonium salt derived from a four-membered phosphetane 1020,21 delivers reduction products with excellent γ/α-selectivity. Specifically, dropwise addition of LiAlH(OtBu)3 (2.5 equiv, 0.42 M in THF) to a 90 °C mixture of 3 and 10 mol % of allylated 10 in toluene over 15 h results in a combined 96% yield of reduction products as a 94:6 ratio in favor of the γ-reduction product 4. Use of neutral trivalent phosphetane 10 provides similarly high selectivity (entry 7), while the corresponding HBF4 salt results in a slightly diminished 4:5 ratio (entry 8).The identity of the exocyclic P-substituent has only a minor effect on the observed product ratio (compare entries 6 and 9), indicating the dominant role of the phosphetane moiety in controlling the reactivity.
Table 1. Effect of Organophosphorus Catalyst on Regioselectivity of Transpositive Allylic Reductiona.
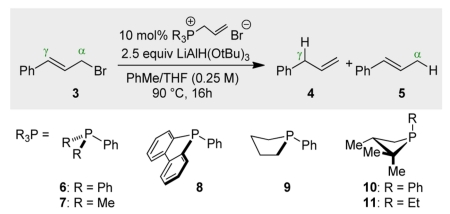
| |||
|---|---|---|---|
|
| |||
| entry | R3Pb | yieldc | ratioc (4:5) |
| 1 | none | 80 | 9:91 |
| 2 | 6 | 78 | 18:82 |
| 3 | 7 | 85 | 11:89 |
| 4 | 8 | 95 | 19:81 |
| 5 | 9 | 95 | 54:46 |
| 6 | 10 | 96 | 94:6 |
| 7 | 10 d | 100 | 98:2 |
| 8 | 10 e | 81 | 88:12 |
| 9 | 11 | 96 | 91:9 |
See Supporting Information for full experimental details.
See ref 17.
The combined yield and ratio of 4 and 5 were determined by GC analysis (dodecane internal standard).
Neutral trivalent 10 as catalyst.
10·HBF4 salt as precatalyst.
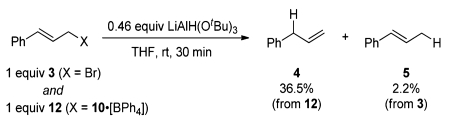
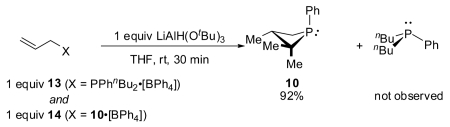
Stoichiometric competition experiments confirm the enhanced reactivity of the strained phosphonium salts derived from four-member phosphacycle 10 toward hydridic reduction. In the reaction of an equimolar mixture of cinnamylphosphetanium salt 12 and cinnamyl bromide 3 with limiting LiAlH(OtBu)3 (eq 1), it is 12 that is preferentially reduced as inferred from the product distribution determined by GC. Similarly, by monitoring the 31P NMR spectra of the reaction of an equimolar mixture of 14 and acyclic phosphonium 13 (eq 2), we estimate a rate difference of >20 in favor of the phosphetanium 14. Qualitatively, these outcomes conform to strain/acceleration arguments advanced in organophosphorus
 |
(1) |
 |
(2) |
chemistry by Westheimer22 and Hudson;23 namely, the strain accrued to the σ4-P+ phosphetanium 12 minimizes the geometric reorganization (and by consequence the free energy) necessary to access a presumed σ5-P hydridophosphorane. As has been noted by Hoz,24 molecular orbital electronic effects may also play a significant role in the rate accelerations in small-ring systems.
Assessment of the phosphetane-catalyzed allylic reduction with respect to a panel of sterically and electronically varied allylic bromides are collected in Table 2. On a 1 mmol scale, good yield and selectivity for the transposed reduction product is observed for γ-alkyl substituted substrates (Table 2A). Regioselective reductive transposition also deconjugates styrenyl and dienyl substrates with high selectivity (Table 2B). Similarly, sterically encumbered allylic bromides are converted with high selectivity to the contrasteric γ reduction products (Table 2C). Additional examples can be found in the Supporting Information (SI) (Table S3). In all instances, control reductions conducted in the absence of the phosphetane catalyst favored direct α-reduction products, confirming that the γ selectivities reported in Table 2 are in fact due to the action of the catalyst.
Table 2. Assessment of Phosphetane-Catalyzed Allylic Reductiona.
See Supporting Information for full experimental details. Isolated yields are reported unless otherwise stated. Ratios (γ:α) determined by 1H NMR integration unless otherwise stated.
Determined by GC analysis.
Reaction time 10 h due to thermal instability of substrate.
TBDPS = tert-butyldiphenylsilyl.
TBS = tert-butyldimethylsilyl.
d.r. and relative stereochemistry of major isomer determined after TBS deprotection.
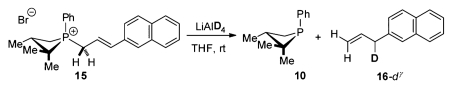
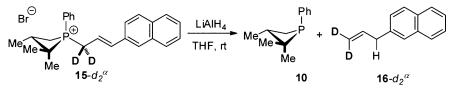
Stoichiometric isotopic labeling experiments designed to establish the provenance of the newly formed γ-allylic C–H were conducted. Treatment of 15 with LiAlD4 results in formation of 16-dγ (eq 3), where deuterium is incorporated
 |
(3) |
 |
(4) |
exclusively at the γ-position.11 Complementarily, treatment of 15-d2α with LiAlH4 results in formation of 16-d2α without loss or scrambling of the geminal deuterium labels (eq 4), indicating the phosphetanium salt is not deprotonated during the course of the reaction.
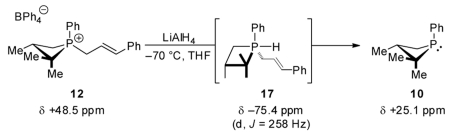
In accord with Gallagher’s proposal and consistent with the notion of biphilic catalysis in this system, in situ spectral data reveal the intermediacy of a σ5-P hydridophosphorane species in the reductive deallylation of σ4-P+ cinammylphosphetanium ion 12 to σ3-P phosphetane 10 (eq 5).25 When monitored by 31P
 |
(5) |
 |
(6) |
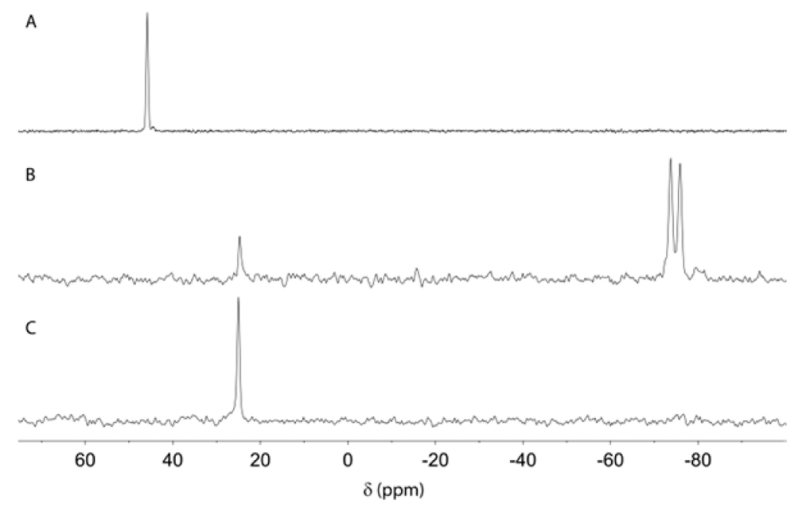
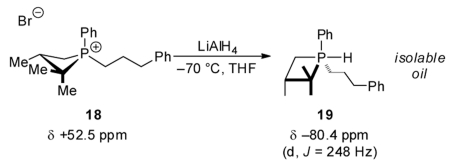
NMR, reaction of 12 (δ +48.5 ppm, Figure 2A) with LiAlH4 at −70 °C results in formation of a new phosphorus species 17 (δ −75.4 ppm, d, J = 258 Hz, Figure 2B), whose chemical shift and multiplicity are consistent with formulation as a σ5-P hydridophosphorane.26 This assignment is further supported by the observation that treatment of the saturated σ4-P+ hydrocinammylphosphetanium ion 18 with LiAlH4 (eq 6) yields an isolable species (19) with similar spectral characteristics (δ −80.4 ppm, d, J = 248 Hz). Subsequent warming of the NMR solution containing 17 results in clean conversion to σ3-P phosphetane 10 (δ +25.1 ppm, Figure 2C) without the intervention of any observable intermediates. Overall, the conversion of 12 → 10 proceeds with retention of configuration at phosphorus. We tentatively assign a trigonal bipyramidal stereochemistry depicted as 17 that minimizes both repulsive steric interactions (i.e., the large gem-dimethyl group is equatorial and the small hydride is apical) and phosphetane ring strain; this assignment conforms to the results of DFT modeling (see Figure S2 in SI).
Figure 2.
In situ 31P NMR spectra for the reaction shown in eq 5. (A) Initial spectrum of phosphetanium 12. (B) Spectrum recorded at −70 °C immediately following addition of LiAlH4 showing formation of 17. (C) Spectrum recorded after warming reaction to −30 °C showing formation of 10. Units are ppm relative to 85% H3PO4 external standard.
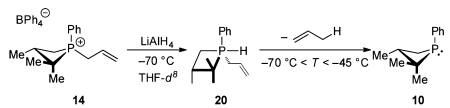
The kinetics of the process σ5-P → σ3-P (i.e., 20 → 10) were monitored by VT-NMR over the temperature range −70 °C < T < −45 °C for the parent allyl system (eq 7), permitting extraction
 |
(7) |
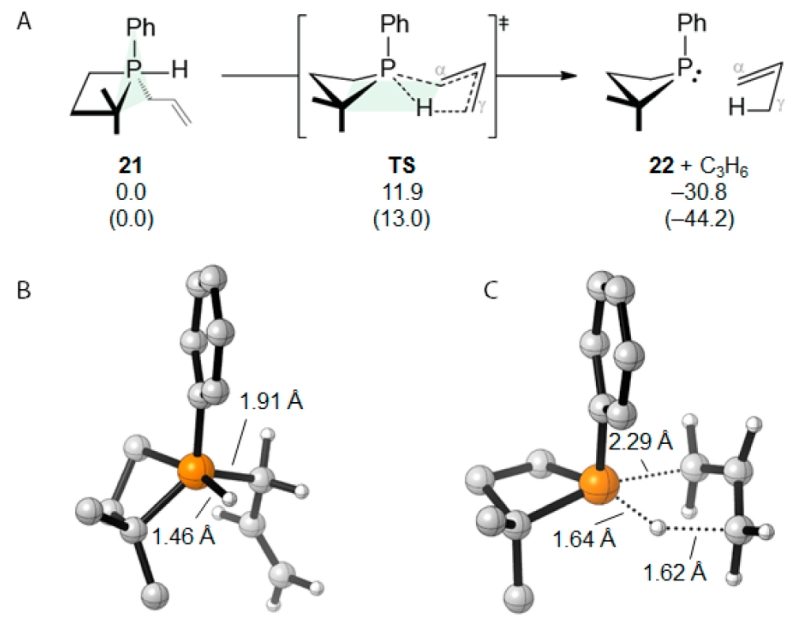
of the activation parameters by Eyring analysis (ΔH‡ = +15.2(8) kcal·mol−1, ΔS‡ = −3(4) cal·mol−1 K−1). DFT calculations (M06-2X/6-311++G(2d,2p)) provide further data regarding the conversion 20 → 10. Structure 21 (Figure 3) represents the global minimum for the suite of polytopal isomers of the σ5-P hydridophosphorane in which the phosphetane ring spans one apical and one equatorial site of a phosphorus-centered trigonal bipyramid.27 The lowest energy pathway connecting hydridophosphorane 21 and phosphetane 22 transits via a single first-order saddle point, whose structure (TS) involves concomitant P–Cα bond cleavage (2.29 Å) and direct transferal of H from P to Cγ (d(P–H) 1.64 Å, d(H–Cγ) 1.62 Å). With respect to geometry, TS is best described as a distorted square pyramid about phosphorus with the P-phenyl moiety occupying the apical site, and both the four-membered phosphetane and five-membered envelope-like cycle spanning cis-basal positions. The computed ΔH‡ (11.9 kcal/mol) is in good agreement with the experimental value, and the cyclic nature of transition structure TS is consistent with the negative value of the experimentally determined activation entropy. We note that this proposed mechanism via the five-center, six-electron transition structure of TS bears a formal orbital equivalency with well-known hydrocarbon-based pericyclic group transfer reactions28 (e.g., ene/retro-ene reactions29).
Figure 3.
Computational modeling of the conversion σ5-P → σ3-P [M06-2X/6-311++G(2d,2p)]. (A) Enthalpy (free energy) for pathway in kcal/mol. (B) Optimized σ5-P hydridophosphorane structure 21. (C) Optimized transition state structure TS.
In summary, we have demonstrated a regioselective reductive transposition of allylic bromides catalyzed by a small-ring phosphacycle. The experimental and computational results implicate the operation of a PIII/PV mechanistic pathway that involves the interconversion of discrete, observable σ3-P, σ4-P+, and σ5-P species. We note that the phosphetane-catalyzed allylic reduction represents a phosphacatalytic complement to known stoichiometric diazene-mediated30 and catalytic Pd π-allyl31 reduction protocols. The biphilic organophosphorus catalysis demonstrated here presents reactivity that merges archetypal nucleophilic and electrophilic manifolds; additional studies, both synthetic and mechanistic, that probe this reactivity are currently underway.
Supplementary Material
ACKNOWLEDGMENTS
Financial support was provided by the Pennsylvania State University and the NIH (GM114547). A.T.R. gratefully acknowledges early career support from the Alfred P. Sloan Foundation.
Footnotes
ASSOCIATED CONTENT
Synthetic procedures, spectral characterization data, kinetics data, Cartesian coordinates for stationary points. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- (1).Kamer PCJ, van Leeuwen PWNM. Phosphorus(III)Ligands in Homogeneous Catalysis: Design and Synthesis. Hoboken, NJ; Wiley: 2012. [Google Scholar]
- (2).(a) Denmark SE, Beutner GL. Angew. Chem., Int. Ed. 2008;47:1560. doi: 10.1002/anie.200604943. [DOI] [PubMed] [Google Scholar]; (b) Fan YC, Kwon O. Chem. Commun. 2013;49:11588. doi: 10.1039/c3cc47368f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Wang Z, Xu X, Kwon O. Chem. Soc. Rev. 2014;43:2927. doi: 10.1039/c4cs00054d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Werner T. Adv. Synth. Catal. 2009;351:1469. [Google Scholar]
- (4).(a) Caputo CB, Hounjet LJ, Dobrovetsky R, Stephan DW. Science. 2013;341:1374. doi: 10.1126/science.1241764. [DOI] [PubMed] [Google Scholar]; (b) Pérez M, Hounjet LJ, Caputo CB, Dobrovetsky R, Stephan DW. J. Am. Chem. Soc. 2013;135:18308. doi: 10.1021/ja410379x. [DOI] [PubMed] [Google Scholar]
- (5).(a) Sereda O, Tabassum S, Wilhelm R. In: Asymmetric Organocatalysis. List B, editor. Topics in Current Chemistry; Springer; Berlin, Heidelberg: 2010. pp. 86–117. [Google Scholar]; (b) Terada M, Kouchi M. Tetrahedron. 2006;62:401. [Google Scholar]
- (6).According to definition, biphilic species are “compounds which can donate electrons to a substrate to form a σ-bond, and also accept them at the same center to form a second σ- or π-bond.” See: Kirby AJ, Warren SG. The Organic Chemistry of Phosphorus. Elsevier; Amsterdam: 1967. p. 20.
- (7).For overviews, see: Marsden SP. In: Sustainable Catalysis. Dunn PJ, Hii KK, Krische MJ, Williams MT, editors. Vol. 339. John Wiley & Sons, Inc.; 2013. van Kalkeren HA, van Delft FL, Rutjes FPJT. ChemSusChem. 2013;6:1615. doi: 10.1002/cssc.201300368. An J, Denton RM, Lambert TH, Nacsa ED. Org. Biomol. Chem. 2014;12:2993. doi: 10.1039/c4ob00032c.
- (8).Dunn NL, Ha M, Radosevich AT. J. Am. Chem. Soc. 2012;134:11330. doi: 10.1021/ja302963p. [DOI] [PubMed] [Google Scholar]
- (9).Burgada R, Setton R. In: The Chemistry of Organophosphorus Compounds. Hartley F, editor. Vol. 3. Wiley; Chichester, U.K.: 1994. pp. 185–272. [Google Scholar]
- (10).Axelrod E, Milne GM, van Tamelen EE. J. Am. Chem. Soc. 1970;92:2139. [Google Scholar]
- (11).Hirabe T, Nojima M, Kusabayashi S. J. Org. Chem. 1984;49:4084. [Google Scholar]
- (12).For related examples, see: Kondo K, Negishi A, Tunemoto D. Angew. Chem., Int. Ed. Engl. 1974;13:407. Utsugi M, Miyano M, Nakada M. Org. Lett. 2006;8:2973. doi: 10.1021/ol0608606.
- (13).For phosphine-catalyzed regioretentive allylic substitution, see: Cho C-W, Kong J-R, Krische MJ. Org. Lett. 2004;6:1337. doi: 10.1021/ol049600j. Cho C-W, Krische MJ. Angew. Chem., Int. Ed. 2004;43:6689. doi: 10.1002/anie.200461381.
- (14).Brophy JJ, Gallagher MJ. Aust. J. Chem. 1969;22:1399. [Google Scholar]
- (15).Donoghue N, Gallagher MJ. Chem. Commun. 1998:1973. [Google Scholar]
- (16).Zhao W, Yan PK, Radosevich AT. J. Am. Chem. Soc. 2015;137:616. doi: 10.1021/ja511889y. [DOI] [PubMed] [Google Scholar]
- (17).The σ4-P+ allylphosphonium salts derived from 6–11 are conveniently manipulated air stable solids that liberate the corresponding free σ3-P phosphines in solution by reduction with loss of propene.
- (18).For early studies of cyclic phosphonium reactivity, see: Aksnes G, Bergesen K, Juvvik P, Thelin H, Sjöberg B, Larsen E. Acta Chem. Scand. 1965;19:931. Cremer S, Trivedi B, Weitl F. J. Org. Chem. 1971;36:3226.
- (19).(a) O’Brien CJ, Tellez JL, Nixon ZS, Kang LJ, Carter AL, Kunkel SR, Przeworski KC, Chass GA. Angew. Chem., Int. Ed. 2009;48:6836. doi: 10.1002/anie.200902525. [DOI] [PubMed] [Google Scholar]; (b) van Kalkeren HA, Leenders SHAM, Hommersom CRA, Rutjes FPJT, van Delft FL. Chem.—Eur. J. 2011;17:11290. doi: 10.1002/chem.201101563. [DOI] [PubMed] [Google Scholar]; (c) O’Brien CJ, Lavigne F, Coyle EE, Holohan AJ, Doonan BJ. Chem.—Eur. J. 2013;19:5854. doi: 10.1002/chem.201300546. [DOI] [PubMed] [Google Scholar]
- (20).Phosphetane 10 was prepared on gram scale via McBride reaction of PhPCl2, tert-butylethylene, and AlCl3 according to: Cremer SE, Chorvat RJ. J. Org. Chem. 1967;32:4066.
- (21).Marinetti A, Carmichael D. Chem. Rev. 2002;102:201. doi: 10.1021/cr990135r. [DOI] [PubMed] [Google Scholar]
- (22).Westheimer FH. Acc. Chem. Res. 1968;1:70. [Google Scholar]
- (23).Hudson RF, Brown C. Acc. Chem. Res. 1972;5:204. [Google Scholar]
- (24).Sella A, Basch H, Hoz S. J. Am. Chem. Soc. 1996;118:416. [Google Scholar]
- (25).The BPh4− counterion was required for low temperature solubility of phosphonium 12 in THF.
- (26).(a) Hellwinkel D. Angew. Chem., Int. Ed. 1966;5:968. [Google Scholar]; (b) Ross MR, Martin JC. J. Am. Chem. Soc. 1981;103:1234. [Google Scholar]; (c) Kojima S, Kajiyama K, Nakamoto M, Akiba K.-y. J. Am. Chem. Soc. 1996;118:12866. [Google Scholar]; (d) Kojima S, Sugino M, Matsukawa S, Nakamoto M, Akiba K.-y. J. Am. Chem. Soc. 2002;124:7674. doi: 10.1021/ja0170145. [DOI] [PubMed] [Google Scholar]
- (27).See SI for full details.
- (28).Woodward RB, Hoffmann R. Angew. Chem., Int. Ed. 1969;8:781–853. [Google Scholar]
- (29).(a) Hoffmann HMR. Angew. Chem., Int. Ed. 1969;8:556. [Google Scholar]; (b) Oppolzer W, Snieckus V. Angew. Chem., Int. Ed. 1978;17:476. [Google Scholar]
- (30).(a) Myers AG, Zheng B. J. Am. Chem. Soc. 1996;118:4492. [Google Scholar]; (b) Myers AG, Zheng B. Tetrahedron Lett. 1996;37:4841. [Google Scholar]; (c) Movassaghi M, Ahmad OK. J. Org. Chem. 2007;72:1838. doi: 10.1021/jo062325p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).(a) Lautens M, Paquin J-F. Org. Lett. 2003;5:3391. doi: 10.1021/ol0350238. [DOI] [PubMed] [Google Scholar]; (b) Chau A, Paquin J-F, Lautens M. J. Org. Chem. 2006;71:1924. doi: 10.1021/jo052267s. [DOI] [PubMed] [Google Scholar]; (c) Movassaghi M, Ahmad OK. Angew. Chem., Int. Ed. 2008;47:8909. doi: 10.1002/anie.200802921. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.