Abstract
Background
The differences in gait abnormalities from the earliest to the latter stages of dementia and in the different subtypes of dementia have not been fully examined. This study aims to compare spatio-temporal gait parameters in cognitively healthy individuals, patients with amnestic (aMCI) and non-amnestic (naMCI) MCI, and patients with mild and moderate stages of Alzheimer’s disease (AD) and non-Alzheimer’s disease (non-AD).
Methods
Based on a cross-sectional design, 1719 participants (77.4±7.3 years, 53.9% female) were recruited from cohorts from seven countries participating in the “Gait, cOgnitiOn & Decline” initiative. Mean values and coefficients of variation of spatio-temporal gait parameters were measured during normal pace walking with the GAITRite system at all sites.
Results
Performance of spatio-temporal gait parameters declined in parallel to the stage of cognitive decline from MCI status to moderate dementia. Gait parameters of patients with naMCI were more disturbed compared to patients with aMCI, and MCI subgroups performed better than demented patients. Patients with non-AD dementia had worse gait performance than those with AD dementia. This degradation of the gait parameters was similar between mean values and coefficients of variation of spatio-temporal gait parameters in the earliest stages of cognitive decline, but different in the most advanced stages, especially in the non-AD subtypes.
Conclusions
Spatio-temporal gait parameters were more disturbed in the advanced stages of dementia, and more affected in the non-AD dementias than in AD. These findings suggest that quantitative gait parameters could be used as a surrogate marker for improving the diagnosis of dementia.
Keywords: Dementia, mild cognitive impairment, aging, Alzheimer’s disease, gait disorders, quantitative gait parameters, motor control, cohort studies
INTRODUCTION
Gait abnormalities in dementia are described in the more advanced stages, and are caused by vascular or neurodegenerative subcortical lesions[1]. With the recent advent of instrumental devices that allow easy quantification of spatio-temporal gait parameters, subtle gait abnormalities have been demonstrated at the earliest stages of dementia, and even in the prodromal stage of dementia like mild cognitive impairment (MCI)[2–7]. While gait abnormalities have been associated with neurological conditions[8, 9], the specific profile of quantitative gait parameters has not been defined in the different subtypes and stages of cognitive decline. The use of various gait measurements, the inclusion of patients from different dementia stages, and small sample sizes contribute to this knowledge gap.
Unlike dementia subtypes, specific cognitive domains, like executive function or memory, have been linked to spatio-temporal gait parameters[4, 7, 8, 10]. Based on factor analysis, executive functions have been linked with a pace factor (reflecting gait velocity and length measures), whereas memory functions with a rhythm factor (reflecting cadence and gait timing)[7]. Executive functions, memory and gait parameters have also been linked to different brain systems[11]. Prefrontal regions play a key role in executive functions and gait control using different neuroimaging techniques, including structural[12, 13] and functional[14, 15] MRI, as well as functional near-infrared spectroscopy[16]. Interestingly, memory-related brain structures (i.e. hippocampus) have been linked to both increases[17] and decreases[18] in gait control. The various underlying neuropathologies of the studied population seem to explain these contrasting results that highlight the importance to better define the gait phenotypes of the different subtypes and stages of early cognitive decline.
Thus, to define the spatio-temporal gait parameters in these different subtypes and stages of cognitive decline, we have the opportunity to use the unique setting of the Biomathics consortium combining, in the called “Gait, cOgnitiOn & Decline” (GOOD) initiative[19], several international teams of physicians and researchers studying gait with the GAITRite® system in more than 2700 older individuals with and without dementia. The present study aims to measure and to compare spatio-temporal gait parameters in cognitively healthy individuals (CHI), in patients with the amnestic (aMCI) and the non-amnestic (naMCI) forms of MCI, in patients with mild stage of Alzheimer’s disease (AD) and non-Alzheimer’s disease (non-AD), and in patients with moderate stage of AD and non-AD. Given previous research demonstrating (a) greater gait instabilities in patients with impaired executive functions[8, 10] (i.e. behavioral variant of frontotemporal dementia) in comparison to patients with the classical amnestic form of AD, and (b) greater gait disturbances in patients with more advanced stages of dementia compared to patients with milder forms [1, 4, 9], we hypothesized that patients with more advanced forms of non-AD dementia would present the most disturbed gait parameters in comparison to patients with AD or patients with the milder forms of cognitive disturbances.
METHODS
Study design and population
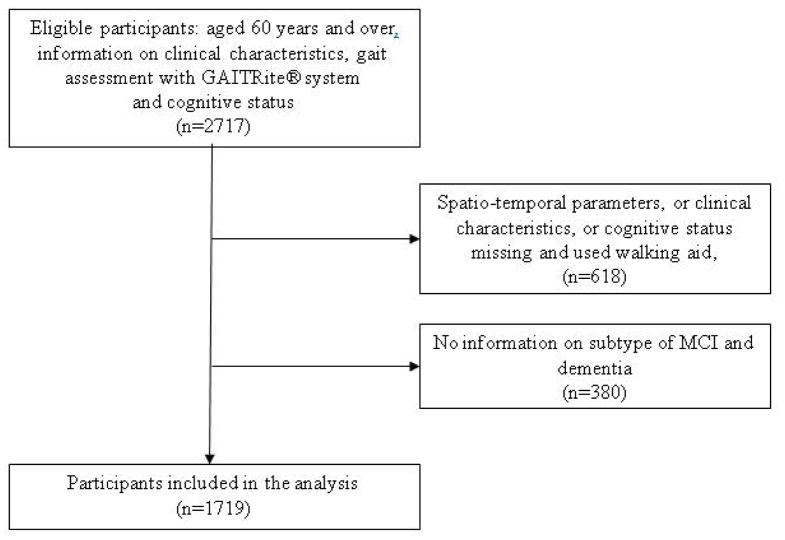
This cross-sectional study used data from the GOOD initiative which combines data from 7 countries (i.e., Australia, Belgium, France, India, Luxembourg, Switzerland and United States) and recruited non-demented and demented older individuals from the “Tasmanian Study of Cognition and Gait” (TASCOG – community-dwellers) (Menzies Research Institute, Hobart, southern Tasmania, Australia), from Mechelen memory clinic (outpatients with cognitive complaints), from the “Gait and Alzheimer Interactions Tracking” (GAIT) study and Angers memory clinic (Angers memory clinic - community-dwellers and outpatients with cognitive complaints), from the “Kerala-Einstein Study” (KES) (Kozhikode city, Kerala, India - community-dwellers), from the Center for Memory and Mobility (CeM2, Luxembourg-city, Luxembourg – outpatients and inpatients with cognitive complaints), from the “Central Control of Mobility in Aging” (CCMA - community-dwellers) (New York, lower Westchester county, US), and from Basel mobility center (University Center for Medicine of Aging Basel, Felix Platter Hospital, Basel - outpatients with cognitive complaints). Participants were included from 2005 (TASCOG study) to 2014 (GAIT study). Inclusion criteria for the present study were aged 60 years and over, participants able to walk without personal assistance, information on clinical characteristics and cognitive status (i.e., CHI, patients with aMCI and naMCI, or mild and moderate AD or non-AD dementia) and gait assessment with the GAITRite® system. From the 2717 participants initially recruited, we excluded 618 because spatio-temporal parameters, clinical characteristics or cognitive status were missing, and we also excluded 380 due to the absence of information on the subtypes or stages of dementia or MCI. After exclusions, a total of 1719 (63.3%) participants (77.4±7.3 years, 53.9% female) were included in the present study (Figure 1). The ethics committee of Angers university hospital approved the GOOD initiative. Furthermore, each center involved in the GOOD initiative obtained individual approval from their local ethics committee. Clinical trials registration number is NCT02350270.
Figure 1.
Flow chart showing the selection of participants included in the analysis.
Gait measurements
Spatio-temporal gait parameters were measured at steady state walking using the GAITRite®-system in each center. All centers followed the European guidelines for spatio-temporal gait analysis in older adults[20]: the participants walked at their usual self-selected walking speed in a quiet, well-lit environment wearing their own footwear. The GAITRite®-System is an electronic walkway-integrated and pressure-sensitive electronic surface providing spatio-temporal gait parameters on a length ranging from 4.6 (TASCOG study) to 7.9 (GAIT study) meters active recording area. Spatio-temporal gait parameters from the GAITRite®-system present excellent test–retest reliability[21]. Participants walked one trial in all cohorts except in TASCOG study, where they walked six trials. In TASCOG study, the mean values of spatio-temporal gait parameters of six trials were used for the analysis. Based on the factor analysis performed in a previous study[7], we focused on the following spatio-temporal parameters: stride length, stride time, swing time, stance time, single support time, double support time, stride width, and stride velocity. Mean values and coefficients of variation (CoV = (standard deviation / mean) x 100) of spatio-temporal gait parameters were the main outcomes.
Dementia and cognitive assessments
Participants from all centers followed a comprehensive neuropsychological assessment that permit to classify them, as CHI, aMCI, naMCI, mild AD, mild non-AD, moderate AD and moderate non-AD (see the supplementary table showing the cognitive tests performed in each cohort involved in GOOD initiative). CHI presented normal cognitive function with all cognitive scores at 1.5 SDs or above the age-appropriate means. Amnestic MCI and naMCI were diagnosed if the participants reported spontaneous cognitive complaints and presented an objective impairment respectively in the memory or the non-memory domains (i.e., defined as a score 1.5 SDs or more below the age-appropriate mean), without impairment into the activities of daily living[22]. We adopted this classification because MCI status presents with a variety of symptoms[23]. Thus, when memory loss was the predominant deficit, patients were classified as aMCI, and when memory loss was not the predominant symptom and/or was combined with other cognitive dysfunctions, patients were classified as naMCI. This classification was done because aMCI is frequently seen as a prodromal stage of Alzheimer disease (AD)[23]. AD and non-AD type dementia were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition at a consensus diagnostic case conferences in all centers, except in TASCOG study: self-report, review of medical history, cognitive testing, and/or clinical interview, followed by interview of proxy if available were used to diagnose dementia. Mild and moderate stages of AD and non-AD were defined by a mini mental status examination (MMSE) score ≥20 and between 19 and 10, respectively, by a Clinical Dementia Rating (CDR) score 1 and 2, respectively, or by review of medical history.
Clinical covariates
Age, gender, body mass index in kg/m2, number of drugs taken per day, use of psychoactive drugs including benzodiazepines or antidepressants or neuroleptics, depressive symptoms assessed using the 4-item, the 15-item, or the 30-item Geriatric depression scale (score≥1 or ≥5 or ≥10 indicated the presence of depressive symptoms, respectively)[24], and history of falls in the past year[25] were collected during the clinical assessment. A fall was defined as unintentionally coming to rest on the ground, floor, or other lower level and not as the result of a major intrinsic event or an overwhelming hazard[25].
Statistics
Baseline characteristics and spatio-temporal gait parameters were summarized using means and standard deviations or frequencies and percentages, as appropriate. Normality of data distribution was checked using the Shapiro-Francia test. Between-group comparisons were performed using unpaired t-test or Chi-square test, as appropriate. Multiple linear regressions exploring the association between each gait parameters (dependent variable) and cognitive status (independent variable) adjusted on participant’s characteristics (i.e., age, gender, number of drugs taken per day, body mass index, use of psychoactive drugs, depression symptoms and history of falls) were performed. P-trends between the seven groups (i.e., MCI amnesic, MCI non-Amnesic, mild dementia AD, mild dementia non-AD, moderate dementia AD, moderate dementia non-AD) are graphed in Figure 2 for each spatio-temporal gait parameter. Subgroups (i.e., aMCI, naMCI, mild and moderate AD and mild and moderate non-AD) and total group “effect size” of spatio-temporal gait parameters are presented in Figure 3 (Review Manager version 5.1, The Nordic Cochrane Centre, Copenhagen, Denmark), in order to identify the largest values of spatio-temporal gait parameters and the homogeneity of spatio-temporal gait values associated with each cognitive status. Finally, in order to quantify the amplitude of spatio-temporal gait values in each one of the six pathological groups, comparisons between mean values and the coefficient of variation of spatio-temporal gait parameters were performed using a paired t-test. P-values <0.05 were considered as statistically significant. All statistics were performed using SPSS (version 15.0; SPSS, Inc., Chicago, IL).
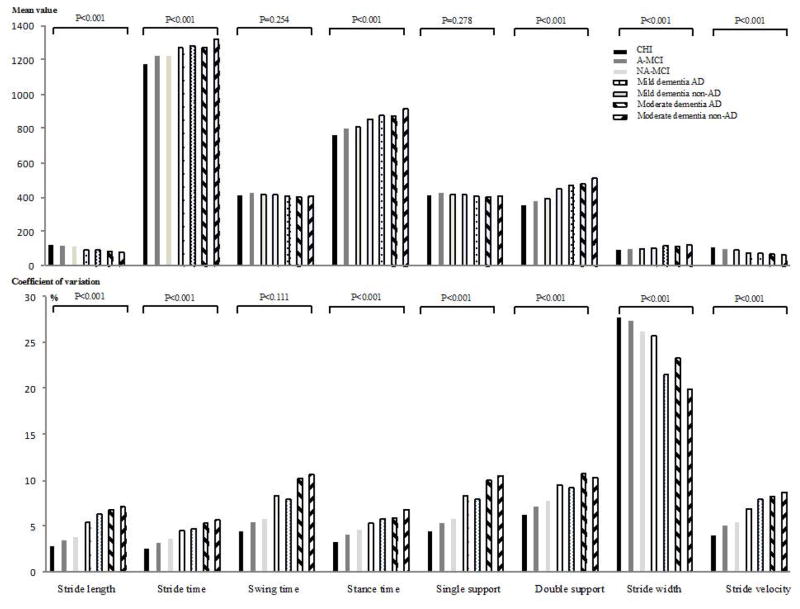
Figure 2.
P-trend between groups of participants categorized on cognitive status (i.e., cognitively healthy individuals, patients with amnestic-mild cognitive impairment, patients with non-amnestic mild cognitive impairment, patients with Alzheimer disease mild dementia and patients with non-Alzheimer disease mild dementia, patients with Alzheimer disease moderate dementia and patients with non-Alzheimer disease moderate dementia) for each spatio-temporal parameter (n=1719).
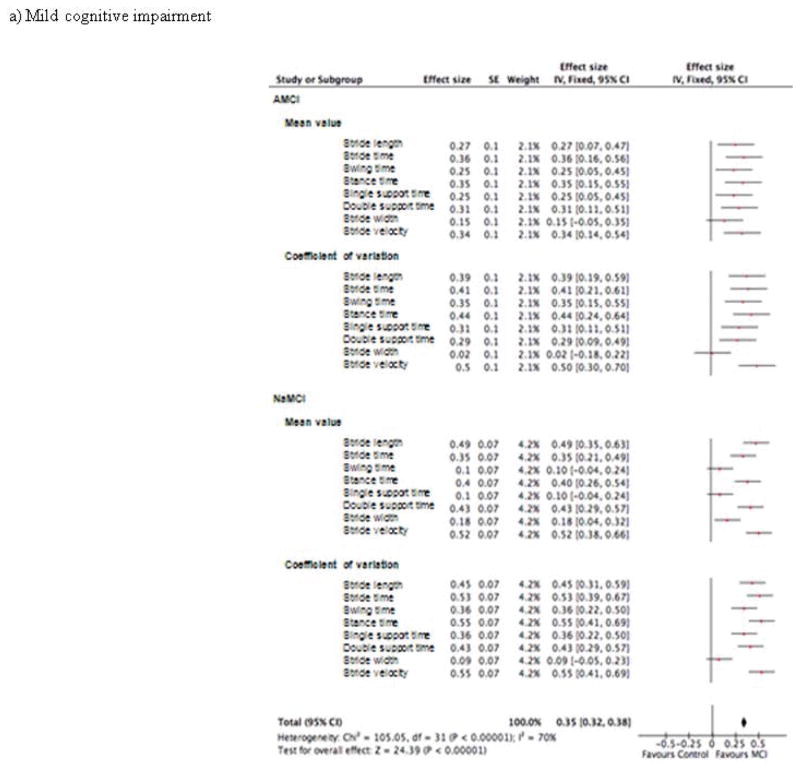
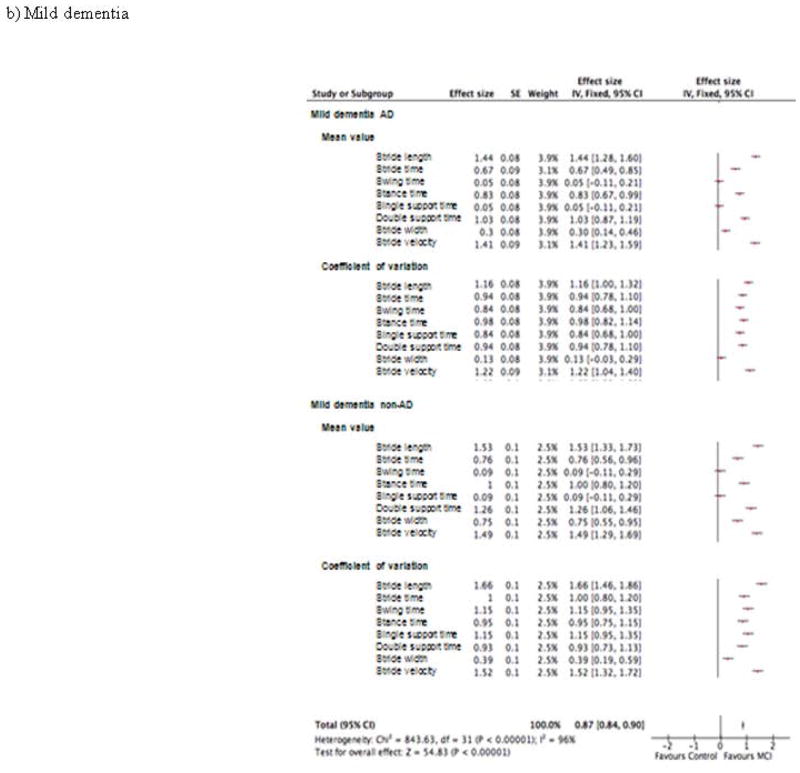
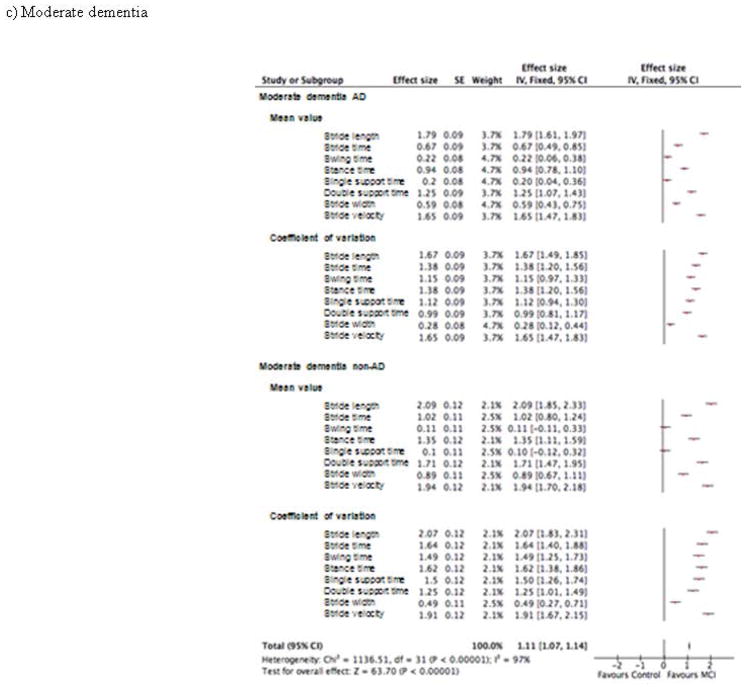
Figure 3.
Effect size of the association of spatio-temporal parameters and cognitive disorders in patient groups with (a) mild cognitive impairment, (b) mild dementia and (c) moderate dementia (n=1719)
RESULTS
Clinical characteristics and spatio-temporal gait parameters are presented in Table 1, by comparing the diagnostic subtypes per type and stages of cognitive decline. Age, number of drugs taken per day, psychoactive drugs and depression symptoms were similar per stages between the 2 profiles (i.e. aMCI vs naMCI and AD vs non-AD). Non-amnestic MCI showed significantly worse gait performances than aMCI for mean value of stride length and CoV of stride length and stride width (after adjustment on participant’s characteristics). For the mild stage of dementia, non-AD walked with significant worse gait performance than AD for CoV of stride length, mean value of stride width, and CoV of stride velocity (after adjustment on participant’s characteristics). For the moderate stage of dementia, non-AD presented significantly more disturbed gait parameters in comparison to AD for walking speed, mean value of stride length, mean value of stance time, and mean value of stride velocity (after adjustment on participant’s characteristics) (Table 1). The decline of gait parameters from normal cognition to the most advanced stages of dementia is illustrated by multivariate regressions examining separately the association between each gait parameter and cognitive status using CHI as the reference group (Table 2). The results showed that naMCI, as well as non-AD dementia, and moderate stages of dementia presented the worse gait performances in comparison to healthy older adults
Table 1.
Comparisons of the participants’ characteristics separated into four groups based on cognitive status (n=1719)
| CHI (n=735) |
MCI | P- value* (P- value†) |
Dementia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Amnestic (n=108) |
Non-amnestic (n=286) |
Mild
|
P- value* (P- value‡) |
Moderate
|
P- value* (P- value¶) |
|||||
| AD (n=196) |
Non-AD (n=126) |
AD (n=177) |
Non-AD (n=91) |
|||||||
| Age (years), mean±SD | 73.9±6.3 | 76.7±7.9 | 75.5±6.6 | 0.117 | 82.5±5.1 | 81.9±5.1 | 0.280 | 83.9±5.6 | 83.3±5.2 | 0.411 |
| Female gender, n (%) | 374 (50.9) | 40 (37.0) | 134 (46.9) | 0.080 | 134 (68.4) | 71 (56.3) | 0.029 | 121 (68.4) | 52 (57.1) | 0.069 |
| Number of drugs taken per day, mean±SD | 1.2±1.4 | 0.7±1.0 | 0.8±1.0 | 0.322 | 4.2±2.3 | 4.6±2.5 | 0.330 | 4.6±2.6 | 4.4±2.3 | 0.147 |
| Body mass index (kg/m2), mean±SD | 27.5±5.3 | 26.4±4.8 | 27.7±5.1 | 0.020 | 26.2±4.4 | 27.2±4.9 | 0.054 | 25.8±4.6 | 26.2±3.8 | 0.069 |
| Use of psychoactive drugs#, n (%) | 93 (12.7) | 24 (22.2) | 70 (24.5) | 0.640 | 85 (43.4) | 60 (47.6) | 0.454 | 79 (44.6) | 46 (50.5) | 0.358 |
| Depression symptoms§, n (%) | 134 (18.2) | 30 (27.8) | 98 (34.3) | 0.220 | 105 (53.6) | 58 (46.0) | 0.187 | 93 (52.5) | 50 (54.9) | 0.709 |
| History of falls**, n (%) | 178 (24.2) | 34 (32.4) | 84 (29.4) | 0.558 | 93 (47.4) | 71 (56.3) | 0.119 | 86 (48.6) | 61 (67.0) | 0.004 |
| Walking speed (cm/s), mean±SD | 104.7±22.2 | 96.7±26.2 | 92.4±25.9 | 0.138 (0.089) | 74.1±18.9 | 71.6±20.4 | 0.245 (0.107) | 68.1±20.6 | 61.7±20.3 | 0.015 (0.005) |
| Stride length | ||||||||||
| Mean value (cm) | 121.9±20.2 | 116.4±23.8 | 111.3±24.7 | 0.067(0.016) | 93.0±19.5 | 90.6±21.7 | 0.298 (0.063) | 85.3±21.6 | 79.6±20.3 | 0.038 (0.008) |
| Cov (%) | 2.8±1.7 | 3.5±2.3 | 3.8±3.2 | 0.265 (0.031) | 5.4±3.6 | 6.3±3.7 | 0.025 (0.015) | 6.8±4.2 | 7.1±4.0 | 0.587 (0.992) |
| Stride time | ||||||||||
| Mean value (ms) | 1175.6±135.2 | 1224.5±153.1 | 1225.1±159.1 | 0.976 (0.818) | 1270.1±163.7 | 1282.6±173.3 | 0.515 (0.793) | 1273.9±187.6 | 1321.1±189.2 | 0.053 (0.058) |
| Cov (%) | 2.6±1.4 | 3.2±1.9 | 3.6±2.8 | 0.196 (0.076) | 4.5±3.5 | 4.7±4.3 | 0.641 (0.313) | 5.3±3.4 | 5.7±4.1 | 0.486 (0.952) |
| Swing time | ||||||||||
| Mean value (ms) | 411.7±45.4 | 422.8±41.8 | 416.3±51.7 | 0.242 (0.382) | 414.1±49.4 | 407.3±57.3 | 0.260 (0.465) | 401.3±60.7 | 406.5±55.0 | 0.489 (0.271) |
| Cov (%) | 4.4±2.7 | 5.4±3.9 | 5.8±6.0 | 0.540 (0.182) | 8.3±8.7 | 7.9±4.6 | 0.677 (0.290) | 10.2±10.1 | 10.6±9.9 | 0.742 (0.782) |
| Stance time | ||||||||||
| Mean value (ms) | 764.0±103.8 | 801.8±128.9 | 808.8±131.1 | 0.633 (0.970) | 856.1±135.1 | 875.3±145.0 | 0.227 (0.841) | 872.6±156.8 | 914.6±159.1 | 0.040 (0.043) |
| Cov (%) | 3.3±1.7 | 4.1±2.4 | 4.6±3.5 | 0.229 (0.135) | 5.3±3.0 | 5.8±5.5 | 0.327 (0.222) | 5.9±2.5 | 6.8±4.4 | 0.040 (0.458) |
| Single support time | ||||||||||
| Mean value (cm) | 411.7±45.4 | 423.0±41.2 | 416.4±51.4 | 0.229 (0.367) | 414.1±49.2 | 407.3±57.3 | 0.255 (0.464) | 401.9±59.8 | 407.2±54.6 | 0.483 (0.357) |
| Cov (%) | 4.4±2.7 | 5.3±3.8 | 5.8±5.9 | 0.503 (0.165) | 8.3±8.7 | 7.9±4.6 | 0.644 (0.299) | 10.0±9.9 | 10.5±9.6 | 0.692 (0.923) |
| Double support time | ||||||||||
| Mean value (cm) | 350.7±85.1 | 378.6±116.8 | 392.1±121.4 | 0.319 (0.604) | 447.3±121.1 | 468.9±134.9 | 0.137 (0.708) | 477.7±150.7 | 512.3±148.7 | 0.075 (0.075) |
| Cov (%) | 6.2±2.9 | 7.1±4.2 | 7.8±5.3 | 0.801 (0.059) | 9.5±5.2 | 9.2±4.7 | 0.644 (0.754) | 10.7±8.4 | 10.3±5.4 | 0.749 (0.385) |
| Stride width | ||||||||||
| Mean value (cm) | 9.3±3.3 | 9.8±3.6 | 9.9±3.7 | 0.775 (0.175) | 10.3±3.4 | 11.8±3.6 | <0.001 (0.003) | 11.3±3.7 | 12.3±3.8 | 0.031 (0.070) |
| Cov (%) | 27.7±16.1 | 27.4±17.9 | 26.2±15.2 | 0.551 (0.026) | 25.7±14.2 | 21.5±13.0 | 0.007 (0.424) | 23.3±14.4 | 19.9±13.0 | 0.065 (0.422) |
| Stride velocity | ||||||||||
| Mean value (cm | 105.5±22.4 | 97.7±26.4 | 93.2±26.1 | 0.137 (0.088) | 74.9±19.0 | 72.4±20.5 | 0.254 (0.110) | 68.9±20.8 | 62.4±20.4 | 0.015 (0.005) |
| Cov (%) | 4.0±2.1 | 5.1±2.9 | 5.4±3.4 | 0.288 (0.055) | 6.9±3.2 | 7.9±4.4 | 0.022 (0.010) | 8.2±3.9 | 8.7±4.4 | 0.257 (0.204) |
CHI: cognitively healthy individuals; MCI: mild cognitive impairment; AD: Alzheimer’s disease; CoV: coefficient of variation = (standard deviation/mean value) x 100;
Comparison between groups based on unpaired t-test or Chi-square test, as appropriate;
based on multiple linear regression exploring the association between each spatio-temporal gait parameters* (dependent variable) and cognitive status (independent variable; MCI) using amnestic mild cognitive impairment as the reference group, adjusted on participant’s characteristics (age, gender, number of drugs taken per day, body mass index, use of psychoactive drugs, depression symptoms and history of falls);
based on multiple linear regression exploring the association between each spatio-temporal gait parameters* (dependent variable) and cognitive status (independent variable; mild dementia) using mild Alzheimer disease as the reference group, adjusted on participant’s characteristics (age, gender, number of drugs taken per day, body mass index, use of psychoactive drugs, depression symptoms and history of falls);
based on multiple linear regression exploring the association between each spatio-temporal gait parameters* (dependent variable) and cognitive status (independent variable; moderate dementia) using moderate Alzheimer disease as the reference group, adjusted on participant’s characteristics (age, gender, number of drugs taken per day, body mass index, use of psychoactive drugs, depression symptoms and history of falls);
Use of benzodiazepines or antidepressants or neuroleptics;
4-items or 15-items or 30-items Geriatric depression scale with abnormal score (≥1, ≥5 and ≥10, respectively);
Fall in the past year of assessment; P-value significant (i.e., P < 0.05) indicated in bold.
Table 2.
Multiple linear regressions showing the association between each spatio-temporal gait parameters* (dependent variable) and cognitive status (independent variable) adjusted on participant’s characteristics (n=1719)
| MCI | Dementia | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Amnestic β [95%CI] (P-value) |
Non-amnestic β [95%CI] (P-value) |
Mild | Moderate | |||
|
| ||||||
| AD β [95%CI] (P-value) |
Non-AD β [95%CI] (P-value) |
AD β [95%CI] (P-value) |
Non-AD β [95%CI] (P-value) |
|||
| Walking speed | −4.70 [−8.65; −0.75] (0.020) | −8.97 [−11.65; −6.29] (<0.001) | −13.54 [−17.28; −9.79] (<0.001) | −16.23 [−20.50; −11.96] (<0.001) | −17.76 [−21.73; −13.79] (<0.001) | −23.32 [−28.14; −18.50] (<0.001) |
| Stride length | ||||||
| Mean value | −3.19 [−6.66;0.28] (0.072) | −7.65 [−10.00; −5.30] (<0.001) | −11.16 [−14.45; −7.87] (<0.001) | −14.85 [−18.56; −11.10] (<0.001) | −17.09 [−20.56; −13.60] (<0.001) | −22.88 [−27.11; −18.65] (<0.001) |
| Cov | 0.30 [−0.27;0.86] (0.301) | 0.78 [0.40;1.16] (<0.001) | 1.29 [0.76;1.82] (<0.001) | 2.26 [1.65;1.87] (<0.001) | 2.57 [2.01;3.14] (<0.001) | 2.78 [2.09;3.46] (<0.001) |
| Stride time | ||||||
| Mean value | 0.03 [0.00;0.06] (0.050) | 0.04 [0.02;0.06] (0.001) | 0.06 [0.03;0.09] (<0.001) | 0.06 [0.02;0.09] (0.001) | 0.06 [0.02;0.09] (<0.001) | 0.09 [0.05;0.013] (<0.001) |
| Cov | 0.43 [−0.11;0.96] (0.115) | 0.83 [0.47;1.19] (<0.001) | 1.06 [0.55;1.56] (<0.001) | 1.31 [0.73;1.89] (<0.001) | 1.75 [1.21;2.28] (<0.001) | 2.07 [1.42;2.72] (<0.001) |
| Swing time | ||||||
| Mean value | 0.01 [−0.00;0.02] (0.112) | 0.01 [−0.00;.0.01] (0.128) | 0.01 [0.00;0.02] (0.007) | 0.00 [−0.01;0.01] (0.586) | 0.00 [−0.00;0.02] (0.977) | 0.00 [−0.01;0.02] (0.559) |
| Cov | 0.40 [−0.78;1.57] (0.507) | 0.089 [0.09;1.68] (0.028) | 1.70 [0.59;2.81] (0.003) | 1.43 [0.17;2.70] (0.027) | 3.35 [2.17;4.52] (<0.001) | 3.61 [2.18;50.4] (<0.001) |
| Stance time | ||||||
| Mean value | 0.02 [−0.00;0.05] (0.064) | 0.03 [0.01;0.05] (<0.001) | 0.04 [0.02;0.07] (<0.001) | 0.06 [0.03;0.08] (<0.001) | 0.06 [0.03;0.08] (<0.001) | 0.09 [0.06;0.12] (<0.001) |
| Cov | 0.61 [0.03;1.20] (0.039) | 1.08 [0.69;1.48] (<0.001) | 0.97 [0.42;1.52] (0.001) | 1.57 [0.94;2.20] (<0.001) | 1.48 [0.89;2.06] (<0.001) | 2.34 [1.63;3.05] (<0.001) |
| Single support time | ||||||
| Mean value | 0.01 [−0.00;0.02] (0.100) | 0.01 [−0.00;0.01] (0.122) | 0.01 [0.00;0.02] (0.007) | 0.00 [−0.01;0.01] (0.579) | 0.00 [−0.01;0.01] (0.873) | 0.00 [−0.01;0.02] (0.480) |
| Cov | 0.33 [−0.82;1.49] (0.569) | 0.87 [0.09;1.65] (0.029) | 1.66 [0.56;2.75] (0.003) | 1.38 [0.13;2.62] (0.030) | 3.12 [1.97;4.28] (<0.001) | 3.45 [20.5;4.86] (<0.001) |
| Double support time | ||||||
| Mean value | 0.02 [−0.01;0.04] (0.132) | 0.03 [0.02;0.06] (<0.001) | 0.04 [0.02;0.06] (<0.001) | 0.05 [0.03;0.08] (<0.001) | 0.06 [0.04;0.08] (<0.001) | 0.09 [0.06;0.11] (<0.001) |
| Cov | 0.54 [−0.43;1.51] (0.276) | 1.37 [0.71;2.02] (<0.001) | 2.14 [1.22;30.6] (<0.001) | 2.02 [0.98;3.07] (<0.001) | 3.20 [2.22;4.17] (<0.001) | 2.90 [1.72;4.08] (<0.001) |
| Stride width | ||||||
| Mean value | 0.12 [−0.54;0.78] (0.724) | 0.35 [−0.10;0.80] (0.122) | 0.13 [−0.49;0.76] (0.676) | 1.36 [0.64;2.07] (<0.001) | 1.02 [0.36;1.68] (0.003) | 1.68 [0.88;2.49] (<0.001) |
| Cov | 0.64 [−2.43;3.72] (0.682) | −0.72 [−2.81;1.37] (0.499) | −0.90 [−3.82;2.01] (0.544) | −4.10 [−7.42; −0.77] (0.016) | −3.14 [−6.23; −0.05] (0.046) | −5.32 [−9.08; −1.58] (0.005) |
| Stride velocity | ||||||
| Mean value | −4.54 [−8.53; −0.56] (0.025) | −8.87 [−11.56; −6.16] (<0.001) | −13.40 [−17.17; −9.62] (<0.001) | −16.04 [−20.34; −11.73] (<0.001) | −17.62 [−21.62; −13.62] (<0.001) | −23.19 [−28.05; −18.33] (<0.001) |
| Cov | 0.73 [0.13;1.34] (0.018) | 1.18 [0.77;1.59] (<0.001) | 1.73 [1.16;2.31] (<0.001) | 2.79 [2.14;3.45] (<0.001) | 2.85 [2.25;3.46] (<0.001) | 3.38 [2.64;4.12] (<0.001) |
CHI cognitively healthy individuals; MCI: mild cognitive impairment; AD: Alzheimer’s disease; CoV: coefficient of variation; CI: confidence interval;
separated model for each gait parameter with each model adjusted on age, gender, number of drugs taken per day, body mass index, use of psychoactive drugs, depression symptoms and history of falls; β: coefficient of regression indicating a decrease or increase in value of gait parameter associated with cognitive decline, the reference group being cognitively healthy individuals; P-value significant (i.e., P < 0.05) indicated in bold.
This degradation of performance of gait parameters from aMCI to naMCI and from AD to non-AD across stages of cognition, and from CHI to moderate dementia, showed that the P-trends between groups were significant for all mean values and coefficients of variation of gait parameters, except for the mean values of the swing time (p=0.254) and the single support (p=0.278) and the CoV of swing time (Figure 2).
The effect sizes of the association of gait parameters and cognitive status are illustrated in Figure 3. The overall effect size for the MCI groups was 0.35 (p<0.001) with the largest effect sizes for the CoV of stride velocity (0.5) in the aMCI group; and for the CoV of stance time and the CoV of stride velocity (0.55) in the naMCI group (Figure 3a). The overall effect size for the mild dementia groups was 0.87 (p<0.001) with the largest effect sizes for the mean value of stride length (1.44) in the mild AD group; and for the CoV of stride length (1.66) in the mild non-AD group (Figure 3b). The overall effect size for the moderate dementia groups was 1.11 (p<0.001) with the largest effect sizes for the mean value of stride length (1.79) in the moderate AD group; and for the mean value of stride length (2.09) in the moderate non-AD group (Figure 3c).
The amplitude (mean value) and the homogeneity (coefficient of variation) of individual mean values and individual coefficients of variation of spatio-temporal gait parameter are illustrated in Table 2. The amplitude of both mean values and coefficient of variation increases with the progression of cognitive decline with less amplitude for the aMCI in comparison to the naMCI and with less amplitude for AD in comparison to non-AD. The amplitude of the CoV of spatiotemporal gait parameters was more affected than the mean value for the aMCI, the naMCI and the moderate AD groups. But, they were affected similarly for the other groups, with a tendency in favor of greater amplitude for the CoVs than the mean values. We observed similarities (i.e. non-significant differences) between mean values and CoV of gait parameters for aMCI and mild AD groups, whereas we found dissimilarities (i.e. significant differences) for the other groups.
DISCUSSION
The findings of the present study showed that spatio-temporal gait parameters are affected in parallel to the type and stage of cognitive impairment from MCI to moderate dementia. Indeed, patients with naMCI presented more disturbed gait parameters than aMCI. Whatever the MCI subgroup, they performed better than patients with dementia. In addition, patients with non-AD dementia had worse gait performance than AD dementia. Furthermore, we showed that this pattern of degradation of gait parameters is homogeneous in the earliest stages of cognitive decline, but becomes heterogeneous in the most advanced stages, especially in the non-AD subtypes.
Gait parameters presented a progressive degradation from normal aging to moderate stage of dementia - with a walking speed declining from 104.7±22.2 cm/s in CHI to 61.7±20.3 cm/s in the moderate stage of non-AD patients, and with a progressive increase of the magnitude of the effect sizes of the gait parameters from aMCI to moderate stage of non-AD dementia. This progressive decline of gait parameters paralleling the evolution of dementia confirms the findings of previous studies[1, 9]. Different factors have been suggested to explain this parallel decline between cognition and gait: the progressive decline of the frontal lobe functions[26, 27], the involvement of the basal ganglia[28] or the accumulation of vascular lesions[13, 29]. This progressive gait degradation with the course of dementia has been associated with poor clinical outcomes such as falls[30], institutionalization[31] and mortality[31]. The differences between the diagnostic subgroups (aMCI versus naMCI or AD versus non-AD) are more pronounced in the latest stage of dementia for the majority of the gait parameters, such as walking speed, stride length stance time, or stride width. These gait parameters reflects the various components of gait, including rhythmic control and dynamic postural control[32]. Among the spatio-temporal parameters, stride length (mean value) presented the highest effect size in both subtypes of dementia in the mild and the moderate stages. This parameter has been specifically associated with disruption of white matter integrity in patients with cerebral small vessel disease[33], subcortical hyperintensities in AD[34] and with hippocampal volume in non-demented older adults[18], suggesting that stride length seems to track both vascular and neurodegenerative components.
Non-amnestic MCI, like non-AD dementia, presented with greater gait decline in comparison to aMCI and AD respectively. These quantitative findings are supported by the observations that clinical gait abnormalities are more prevalent in non-AD dementia than in AD[35], and that they predict non-AD dementia[36]. It is possible that BMI could contribute in part to these disturbed quantitative gait parameters in naMCI and non-AD dementia, as higher BMI was noticed in naMCI in comparison to MCI and a tendency to higher BMI was also noticed between AD and non-AD dementia. The different cognitive profiles between aMCI and naMCI, as well as AD and non-AD could also explain this observation. Memory decline is the hallmark of aMCI and AD, whereas non-memory functions, and mainly executive function, are usually affected in naMCI and non-AD dementia[37]. Increased stride time variability – a marker of disturbed gait rhythmicity[32] - has been associated with executive functioning in healthy older adults[10] and in patients with dementia[38]. In addition, by contrasting executive function and memory performances in non-demented older adults, only executive function has been linked to stride time variability[39]. From a functional perspective, this strong link between executive functioning and gait in aging has been previously illustrated by a high correlation between the activation of the primary motor cortex during a mental imagery task of gait and the performance to the Stroop task in healthy older adults[14] and also by a greater activation of the prefrontal cortex shown by healthy older adults during a mental imagery task of gait in comparison to younger adults[14].
The heterogeneity of gait changes increases in parallel with the progression of cognitive deterioration with a decline in performances being more marked in non-AD dementia than in AD, and more marked in naMCI than in aMCI. In aMCI, we observed a similar variability of changes between mean values and coefficients of variation of gait parameters, whereas in naMCI and more advanced stages of dementia, the variability of changes in mean values of gait parameters was greater than the variability of changes in coefficients of variation of gait changes, suggesting a chaotic modification of gait parameters. With the progression of cognitive decline due to the progression of neuropathological or vascular lesions, we can interpret this chaotic modification of gait parameters by the diffuse spreading of these lesions affecting in a non-harmonic way the brain regions controlling the various steps involved in the goal-directed action of walking: integration of the sensory afferents, motor programming and generation of accurate foot movement[40]. Interestingly, in the earliest stages of cognitive decline, the AD-related profiles (aMCI and mild AD) affect gait parameters in a more homogeneous way than non-AD. In such earlier stages, hippocampal functioning is typically affected in AD[41]. Hippocampus constitutes a key region for gait control[12, 17, 18] that is mainly due to its essential role in spatial navigation[42]. The disturbance of this higher-level functional control affecting spatial navigation acts as a central supervisor that explains its homogeneous effect on the different substructures controlling gait – reflected by a similar effect on mean values and coefficients of variation of spatio-temporal gait parameters.
Comparing quantitative gait parameters collected using similar methods and equipment in a large sample of MCI and demented patients from multiple nations, and divided on cognitive subtypes and stages represent the main strengths of this study. However, the absence of autopsy-confirmed diagnoses constitutes a main limitation. Indeed, although the diagnoses of dementia were made according to standardized criteria, clinical misclassification of subtypes of dementia is still possible. Although the associations between cognitive status and gait parameters were controlled for many covariates, information on some clinical characteristics, such as presence of major outcomes following falls or presence of any prosthesis that could influence gait parameters, is lacking. Furthermore, the absence of information concerning major comorbid conditions affecting gait, such as osteoarthritis or peripheral neuropathy, represents another limitation of this study. Although our findings are controlled for medication, we do not know if demented patients are taken any specific anti-dementia drugs. Finally, identifying stages of dementia based on the MMSE or the CDR represents also a limitation, because both scores did not take into account the level of education that could induce misclassification between mild and moderate stages of dementia.
In conclusion, this study found that spatio-temporal gait parameters deteriorated with the progression of dementia from MCI to moderate dementia, and that they are more affected in the non-AD dementias than in AD. Furthermore, in the most advanced stages and in the non-AD subtypes, this deterioration is more heterogeneous than in the earlier stages and in AD subtype. These findings suggest that quantitative gait parameters could be used as a surrogate marker improving the diagnosis process of dementia. Future analyses need to further examine the respective gait characteristics among the non-AD subtypes of dementia and to study the respective contributions of clinical covariates to gait deterioration in the course of dementia.
Supplementary Material
Table 3.
Mean and coefficient of variation of magnitude* of decline in spatio-tempral gait parameters using cognitively healthy individuals as the reference value among participants categorized on cognitive disorders (i.e., amnestic mild cognitive impairment, non-amnestic mild cognitive impairment, Alzheimer disease mild dementia, non-Alzheimer disease mild dementia, Alzheimer disease moderate dementia, non-Alzheimer disease moderate dementia)
| Mean value
|
P-Value† | Coefficient of variation (%)
|
P-value† | |||
|---|---|---|---|---|---|---|
| Mean value | Coefficient of variation | Mean value | Coefficient of variation | |||
| Amnesic mild cognitive impairment | 0.28±0.07 | 0.34±0.15 | <0.001 | 39.08±13.11 | 94.45±188.70 | 0.201 |
| Non-amnesic mild cognitive impairment | 0.32±0.17 | 0.42±0.15 | 0.008 | 32.9±24.8 | 22.97±20.68 | 0.018 |
| Alzheimer disease mild dementia | 0.72±0.56 | 0.88±0.34 | 0.090 | 47.40±66.69 | 15.43±19.33 | 0.074 |
| Non-Alzheimer disease mild dementia | 0.87±0.57 | 1.09±0.39 | 0.077 | 33.14±43.63 | 10.75±5.78 | 0.003 |
| Alzheimer disease moderate dementia | 0.92±0.61 | 1.20±0.44 | 0.022 | 16.78±14.73 | 9.81±8.29 | 0.027 |
| Non-Alzheimer disease moderate dementia | 1.15±0.77 | 1.50±0.48 | 0.092 | 33.22±45.89 | 9.37±5.49 | 0.008 |
calculated with effect size mean value and standard deviation and pooling all spatio-temporal parameters;
based on paired t-test
Acknowledgments
Funding: G. Allali is supported by a grant from the Geneva University Hospitals and the Resnick Gerontology Center, Albert Einstein College of Medicine, Yeshiva University. The Kerala-Einstein Study was funded by the National Institutes of Health, USA (R01 AG039330). The CCMA study was funded by the National Institutes of Health, USA (R01AG036921, RO1AGO44007-01A1). TASCOG was funded by the National Health and Medical Research Council (NHMRC grant number 403000 and 491109) and the Royal Hobart Hospital Research Foundation. M.L. Callisaya is funded by an NHMRC Early Career Fellowship (1034483); V. Srikanth is funded by an NHRMC CDF/HF Future Leader fellowship.
Footnotes
Institution where the work was carried out: Department of Neurology, Division of Cognitive & Motor Aging, Albert Einstein College of Medicine, Yeshiva University, Bronx, New York, USA.
Conflict of Interest: None
References
- 1.Scherder E, Eggermont L, Swaab D, et al. Gait in ageing and associated dementias; its relationship with cognition. Neurosci Biobehav Rev. 2007;31:485–497. doi: 10.1016/j.neubiorev.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc. 2012;60:2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridenbaugh SA, Kressig RW. Quantitative gait disturbances in older adults with cognitive impairments. Curr Pharm Des. 2014;20:3165–3172. doi: 10.2174/13816128113196660688. [DOI] [PubMed] [Google Scholar]
- 4.Beauchet O, Allali G, Montero-Odasso M, Sejdic E, Fantino B, Annweiler C. Motor phenotype of decline in cognitive performance among community-dwellers without dementia: population-based study and meta-analysis. PLoS One. 2014;9:e99318. doi: 10.1371/journal.pone.0099318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Odasso M, Oteng-Amoako A, Speechley M, et al. The motor signature of mild cognitive impairment: results from the gait and brain study. J Gerontol A Biol Sci Med Sci. 2014;69:1415–1421. doi: 10.1093/gerona/glu155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muir SW, Speechley M, Wells J, Borrie M, Gopaul K, Montero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: the effect of dual-task challenges across the cognitive spectrum. Gait Posture. 2012;35:96–100. doi: 10.1016/j.gaitpost.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry. 2007;78:929–935. doi: 10.1136/jnnp.2006.106914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allali G, Dubois B, Assal F, et al. Frontotemporal dementia: pathology of gait? Mov Disord. 2010;25:731–737. doi: 10.1002/mds.22927. [DOI] [PubMed] [Google Scholar]
- 9.Beauchet O, Allali G, Berrut G, Hommet C, Dubost V, Assal F. Gait analysis in demented subjects: Interests and perspectives. Neuropsychiatr Dis Treat. 2008;4:155–160. doi: 10.2147/ndt.s2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beauchet O, Annweiler C, Montero-Odasso M, Fantino B, Herrmann FR, Allali G. Gait control: a specific subdomain of executive function? J Neuroeng Rehabil. 2012;9:12. doi: 10.1186/1743-0003-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holtzer R, Epstein N, Mahoney JR, Izzetoglu M, Blumen HM. Neuroimaging of mobility in aging: a targeted review. J Gerontol A Biol Sci Med Sci. 2014;69:1375–1388. doi: 10.1093/gerona/glu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc. 2013;61:1074–1079. doi: 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- 13.Srikanth V, Beare R, Blizzard L, et al. Cerebral white matter lesions, gait, and the risk of incident falls: a prospective population-based study. Stroke. 2009;40:175–180. doi: 10.1161/STROKEAHA.108.524355. [DOI] [PubMed] [Google Scholar]
- 14.Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol A Biol Sci Med Sci. 2014;69:1389–1398. doi: 10.1093/gerona/glt207. [DOI] [PubMed] [Google Scholar]
- 15.Blumen HM, Holtzer R, Brown LL, Gazes Y, Verghese J. Behavioral and neural correlates of imagined walking and walking-while-talking in the elderly. Hum Brain Mapp. 2014;35:4090–4104. doi: 10.1002/hbm.22461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzer R, Mahoney JR, Izzetoglu M, Wang C, England S, Verghese J. Online fronto-cortical control of simple and attention-demanding locomotion in humans. Neuroimage. 2015 doi: 10.1016/j.neuroimage.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beauchet O, Launay CP, Annweiler C, Allali G. Hippocampal volume, early cognitive decline and gait variability: which association? Exp Gerontol. 2015;61:98–104. doi: 10.1016/j.exger.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Zimmerman ME, Lipton RB, Pan JW, Hetherington HP, Verghese J. MRI- and MRS-derived hippocampal correlates of quantitative locomotor function in older adults. Brain Res. 2009;1291:73–81. doi: 10.1016/j.brainres.2009.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beauchet O, Merjagnan-Vilcoq C, Annweiler C. From industrial research to academic discoveries, toward a new concept of partnership: the Biomathics model. Front Pharmacol. 2014;5:166. doi: 10.3389/fphar.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kressig RW, Beauchet O, European GNG. Guidelines for clinical applications of spatio-temporal gait analysis in older adults. Aging Clin Exp Res. 2006;18:174–176. doi: 10.1007/BF03327437. [DOI] [PubMed] [Google Scholar]
- 21.Brach JS, Perera S, Studenski S, Newman AB. The reliability and validity of measures of gait variability in community-dwelling older adults. Arch Phys Med Rehabil. 2008;89:2293–2296. doi: 10.1016/j.apmr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 23.Cummings JL, Dubois B, Molinuevo JL, Scheltens P. International Work Group criteria for the diagnosis of Alzheimer disease. Med Clin North Am. 2013;97:363–368. doi: 10.1016/j.mcna.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Pomeroy IM, Clark CR, Philp I. The effectiveness of very short scales for depression screening in elderly medical patients. Int J Geriatr Psychiatry. 2001;16:321–326. doi: 10.1002/gps.344. [DOI] [PubMed] [Google Scholar]
- 25.Beauchet O, Dubost V, Revel Delhom C, et al. How to manage recurrent falls in clinical practice: guidelines of the French Society of Geriatrics and Gerontology. J Nutr Health Aging. 2011;15:79–84. doi: 10.1007/s12603-011-0016-6. [DOI] [PubMed] [Google Scholar]
- 26.Coelho FG, Stella F, de Andrade LP, et al. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2012;19:644–656. doi: 10.1080/13825585.2012.661398. [DOI] [PubMed] [Google Scholar]
- 27.Sheridan PL, Hausdorff JM. The role of higher-level cognitive function in gait: executive dysfunction contributes to fall risk in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:125–137. doi: 10.1159/000105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns JM, Galvin JE, Roe CM, Morris JC, McKeel DW. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology. 2005;64:1397–1403. doi: 10.1212/01.WNL.0000158423.05224.7F. [DOI] [PubMed] [Google Scholar]
- 29.Inzitari M, Gine-Garriga M, Martinez B, et al. Cerebrovascular disease and gait and balance impairment in mild to moderate Alzheimer’s disease. J Nutr Health Aging. 2013;17:45–48. doi: 10.1007/s12603-012-0091-3. [DOI] [PubMed] [Google Scholar]
- 30.Camicioli R, Licis L. Motor impairment predicts falls in specialized Alzheimer care units. Alzheimer Dis Assoc Disord. 2004;18:214–218. [PubMed] [Google Scholar]
- 31.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beauchet O, Allali G, Annweiler C, et al. Gait variability among healthy adults: low and high stride-to-stride variability are both a reflection of gait stability. Gerontology. 2009;55:702–706. doi: 10.1159/000235905. [DOI] [PubMed] [Google Scholar]
- 33.de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain. 2011;134:73–83. doi: 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- 34.Nadkarni NK, McIlroy WE, Mawji E, Black SE. Gait and subcortical hyperintensities in mild Alzheimer’s disease and aging. Dement Geriatr Cogn Disord. 2009;28:295–301. doi: 10.1159/000245158. [DOI] [PubMed] [Google Scholar]
- 35.Allan LM, Ballard CG, Burn DJ, Kenny RA. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc. 2005;53:1681–1687. doi: 10.1111/j.1532-5415.2005.53552.x. [DOI] [PubMed] [Google Scholar]
- 36.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 37.Weakley A, Schmitter-Edgecombe M, Anderson J. Analysis of verbal fluency ability in amnestic and non-amnestic mild cognitive impairment. Arch Clin Neuropsychol. 2013;28:721–731. doi: 10.1093/arclin/act058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc. 2003;51:1633–1637. doi: 10.1046/j.1532-5415.2003.51516.x. [DOI] [PubMed] [Google Scholar]
- 39.Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res. 2005;164:541–548. doi: 10.1007/s00221-005-2280-3. [DOI] [PubMed] [Google Scholar]
- 40.Scherder E, Eggermont L, Visscher C, Scheltens P, Swaab D. Understanding higher level gait disturbances in mild dementia in order to improve rehabilitation: ‘last in-first out’. Neurosci Biobehav Rev. 2011;35:699–714. doi: 10.1016/j.neubiorev.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 41.Sarazin M, Berr C, De Rotrou J, et al. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 42.Ekstrom AD, Kahana MJ, Caplan JB, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.